- 1Key Laboratory of Infectious Diseases, Department of Epidemiology and Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, China
- 2Department of Information, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Critical Care Medicine, The Affiliated Yixing Hospital of Jiangsu University, Yixing, China
- 4Department of General Surgery, Third Affiliated Hospital of Nantong University, Nantong, China
- 5Department of Digestive Medicine, Third Affiliated Hospital of Nantong University, Nantong, China
- 6Department of Epidemiology and Statistics, School of Public Health, Medical College of Soochow University, Suzhou, China
- 7Department of Severe Infectious Diseases, Third Affiliated Hospital of Nantong University, Nantong, China
- 8Department of Infectious Diseases, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 9Institute of Epidemiology and Microbiology, Eastern Theater Command Centers for Disease Prevention and Control, Nanjing, China
Background: Since a greater number of hepatitis C virus (HCV) patients have access to direct-acting antiviral (DAA) based therapies, the number of patients not properly responding to prior DAA regimens is increasing. The objective of this comprehensive analysis was to assess the efficacy and safety of glecaprevir/pibrentasvir (GLE/PIB) in HCV patients who experienced previous DAA therapy failures.
Methods: Bibliographic databases were systematically searched for relevant articles published by November 2020. The main endpoints were sustained viral response after 12 weeks (SVR12), adverse events (AEs; any grade) and severe adverse events (SAEs). Publication bias assessment was performed using funnel plots and the Egger's test.
Results: Fourteen studies consisting of a total of 1,294 subjects were included in this study and the pooled estimate of SVR12, AEs and SAEs rates were 96.8% (95%CI: 95.1–98.2), 47.1% (95%CI: 26.0–69.3), and 1.8% (95%CI: 0.7–3.4), respectively. Subgroup analysis showed that pooled SVR12 rates were 97.9% (95%CI: 96.7–98.9) for Japan and 91.1% (95%CI: 87.3–94.3) for the United States; 95.8% (95%CI: 93.9–97.4) for genotype (GT)1 and 100.0% (95%CI: 99.6–100.0) for GT2; 95.3% (95%CI: 92.4–97.2) for cirrhosis and 96.3% (95%CI: 94.2–97.7) for non-cirrhosis cases. There was no publication bias included this study.
Conclusion: This comprehensive analysis revealed that GLE/PIB is an effective and secure retreatment option for patients who did not optimally respond to DAA treatment, especially the Asian population with GT1-2.
Introduction
Hepatitis C virus infection is a common disease affecting ~180 million individuals worldwide (1, 2). According to the World Health Organization (WHO), ~71 million people develop chronic HCV infections that may lead to cirrhosis, hepatocellular carcinoma (HCC) and liver-related deaths (3, 4). In addition to prevention, effective regimens are critical to achieve the WHO goal of eliminating HCV as a major global public health threat by 2030 (5).
Treatment of HCV has evolved over the past decade. Before direct-acting antiviral, interferon (IFN)-based regimens were the main method of HCV, but the cure rate using this regimen was only 40–65% (6). In addition, the incidence of SAEs and discontinuation of these treatments were both frequent (7). Compared with IFN-based regimens, the efficacy and safety of DAA-based therapies for HCV resulted in dramatical improvements, including high sustained virologic response (SVR) rates, shorter treatment duration, better tolerability, and less SAEs. Despite excellent efficacy of DAA-based regimens, about 5% of patients still failed to achieve SVR and have just drawn public attention in recent years (8). Given the size of the HCV infected population, the absolute number of patients with DAA treatment failure is substantial and increasing as more patients have access to DAA-based therapies. Thus, effective and alternative treatment strategies for these individuals are particularly important.
In 2017, the combination of glecaprevir (GLE; a second-generation NS3/4 protease inhibitor) and pibrentasvir (PIB; a second-generation NS5A inhibitor) was approved and this combination shows high anti-HCV activity across genotypes 1–6 with a high in vitro barrier to resistance (9, 10). In clinical trials, GLE/PIB regimens showed high efficacy and favorable safety for all six major HCV genotypes (11, 12). In addition, GLE/PIB treatments were also effective and well-tolerated in patients with compensated cirrhosis or those with severe renal impairment (13, 14). Furthermore, recent studies uncovered that GLE/PIB regimens are highly effective in patients who failed to achieve SVR after prior DAA therapies (15, 16). However, the relevant researches were just conducted recently and there haven't been a lot of researches in this population. The European Association for the Study of the Liver (EASL) recommended the GLE/PIB regimen to treat treatment-experienced (pegylated IFN-a and ribavirin, pegylated IFN-a, ribavirin, and sofosbuvir, or sofosbuvir and ribavirin) HCV patients, but did not explicitly recommended this regimen to retreat patients with DAA treatment failure on account of insufficient supporting evidences.
Even though the latest guidelines from China recommended using GLE/PIB to retreat patients with prior DAA failure (17), further research is needed to increase the confidence of this recommendation. The aim of this systematic review was to assess the efficacy and safety of GLE/PIB regimens for patients who experienced DAA treatment failure.
Method
Search Strategy
Preferred reporting items for systematic review and meta-analyses (PRISMA) were followed to conduct this study (18). Two investigators independently performed a systematic and comprehensive literature search using multiple databases including PubMed, Embase, Web of Science, Cochrane Library, CNKI, and WanFang Data. Key search terms included (Hepacivirus OR Hepaciviruses OR Hepatitis C-Like Viruses OR Hepatitis C Like Viruses OR Hepatitis C virus OR Hepatitis C viruses OR HCV) AND (glecaprevir OR ABT-493) AND (pibrentasvir OR ABT-530). A manual search was also performed by checking related references and reviewing citations included in the selected publications. There were no language restrictions. The literature search was last updated in November 2020.
Selection Criteria
Studies were be included if they met all the following criteria: (1) HCV patients with previous DAA therapy failures (defined as failure to achieve SVR12 after DAA treatment); (2) retreatment with GLE/PIB; (3) the primary endpoint was SVR12.
Studies were excluded if they met any of the following criteria: (1) patients without a DAA treatment history; (2) patients with a DAA treatment history, but unclear information as to whether they experienced DAA therapy failure; (3) patients who were liver transplant recipients with recurrent hepatitis C; (4) a sample size <10; (5) reports that did not provide the primary endpoint (SVR12); (6) case reports, letters, meta-analysis, editorials or reviews; (7) pharmacokinetics or pharmacodynamics studies.
Outcome Measures
The primary outcome was the percentage of SVR12, which was defined as plasma HCV RNA below the lower limit of quantification (LLOQ) 12 weeks after end of treatment (EOT). Additional secondary primary outcomes included the percentage of patients with on-treatment breakthrough and post-treatment relapse. Breakthrough was defined as HCV RNA becoming detectable after HCV RNA below LLOQ during the treatment period. Relapse was defined as undetectable HCV RNA at EOT but became detectable within 12 weeks after. We assessed safety in terms of the incidence and intensity of AEs (any grade), common AEs (CAEs), SAEs, and discontinuation due to AEs. Analyses of secondary primary and safety outcomes included only studies reporting these data.
Study Selection and Data Extraction
Study selection and data extraction performed by two independent researchers (CS and HZF). Study selection followed the predetermined selection criteria. Records found through primary search were initially reviewed by title and abstract. The full texts of potentially eligible studies were reviewed and eligible studies were included.
Required data were extracted from eligible studies and respected the original description. The extracted data included study characteristics (the first author's name, year of publication, region, study design, setting, publication type, sample size, subgroup number of patients, regimen, and treatment duration); patient characteristics (age, sex, HCV genotype, treatment history, resistance-associated substitutions, and presence of cirrhosis) and study outcomes (SVR12, on-treatment breakthrough, post-treatment relapse, AEs, CAEs, SAEs, and discontinuation due to AEs).
During this process, any conflicts arising between the two reviewers were resolved by consensus with the help of a third researcher (ZQF).
Quality Assessment
The quality of studies included was assessed using the Newcastle-Ottawa quality assessment scale (NOS) for observational studies, including eight items with a total score of nine. Low quality was scored as 0–5 points, moderate quality as 6–7 points, and high quality as 8–9 points (19). The quality of randomized studies was assessed using the Cochrane Collaboration's tool. The Cochrane Collaboration's tool addresses seven specific domains including randomization, allocation concealment, blinding of subjects, blinding of outcome assessors, reporting of incomplete outcome data, selective outcome reporting, and other potential sources of bias. In each domain, every study took one of three categories: “low risk,” “high risk,” or “unclear risk” for bias (20). The quality of each included study was independently assessed by two investigators.
Statistical Analysis
Effect sizes were collected as pooled event incidences with corresponding 95% confidence intervals (95% CI) using the inverse variance method. Zero events were estimated using Freeman-Tukey double arcsine transformation. Heterogeneity between studies was assessed using Cochran Q-statistics and I2 statistics. An I2 <50% indicated little or no heterogeneity and then the fixed-effects model was used; When the I2 ≥ 50%, this indicated moderate or substantial heterogeneity and the random-effects model was used. To effectively evaluate the efficacy and safety of GLE/PIB, we conducted subgroup analyses of SVR12 by region, setting, duration of treatment, HCV genotype, treatment history, and presence of cirrhosis. Publication bias was explored using funnel plots and the Egger's test. All statistical tests were two-sided, with a p < 0.05 considered as statistically significant. All statistical analyses were conducted using R version 3.6.3.
Results
Study Selection and Basic Information
Our initial search retrieved 1,500 records. After removing 770 overlapping studies, the titles and abstracts of 730 articles were screened. After assessing the full text of 63 articles, 49 articles were excluded for various reasons, and 14 articles (21–34) were eventually added to this study (Figure 1).
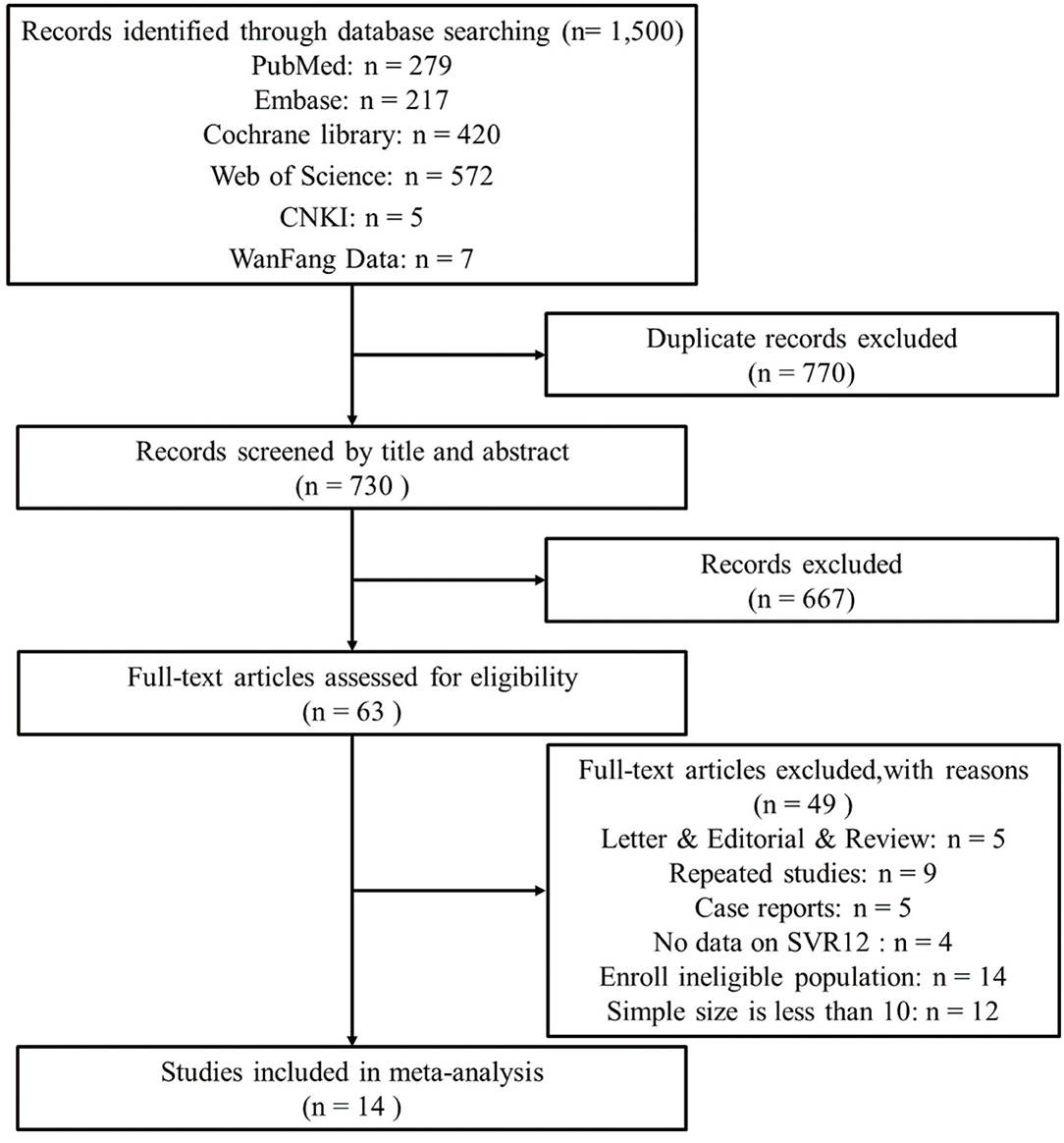
Figure 1. The flow diagram of literature screening and following the preferred reporting items of systematic reviews and meta-analyses (PRISMA).
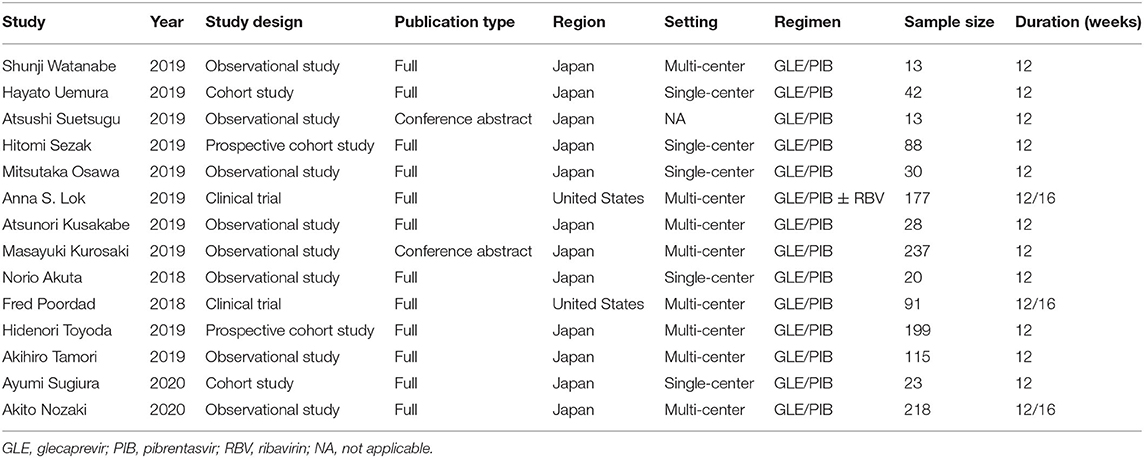
The 14 studies were all published since 2018, including 12 full-articles and two conference abstracts. All studies came from two regions: 12 from Japan and two from the United States. Overall 1,294 subjects showed chronic HCV infection with GT1-3, DAA treatment experience and some were presented with cirrhosis. The treatment regimens were fixed-dose combinations of GLE (300 mg/d) and PIB (120 mg/d) with or without ribavirin (RBV). Treatment courses included 12 and 16 weeks, respectively. Details of study and patient characteristics are shown in Tables 1, 2.
Quality of the Included Studies
Twelve observational studies were assessed by NOS. Among these studies, three were of high quality, six were of moderate quality and the others were of low quality. The quality assessment scores are shown in Supplementary Table 1.
Two clinical trials (26, 32) were assessed using the Cochrane Collaboration's tool. Among these assessed items, randomization, allocation concealment, reporting of incomplete outcome data, selective outcome reporting, and other potential sources of bias were reported in these two different studies. These results are represented in Supplementary Figure 1.
Efficacy of Outcomes
SVR12
Data on SVR12 rates of GLE/PIB retreatment for HCV infection were available in all studies (1,294 cases). The pooled estimation of the SVR12 rate from the random-effect model was 96.8% (95%CI: 95.1–98.2, I2 = 37.1%, P = 0.08) (Figure 2A).
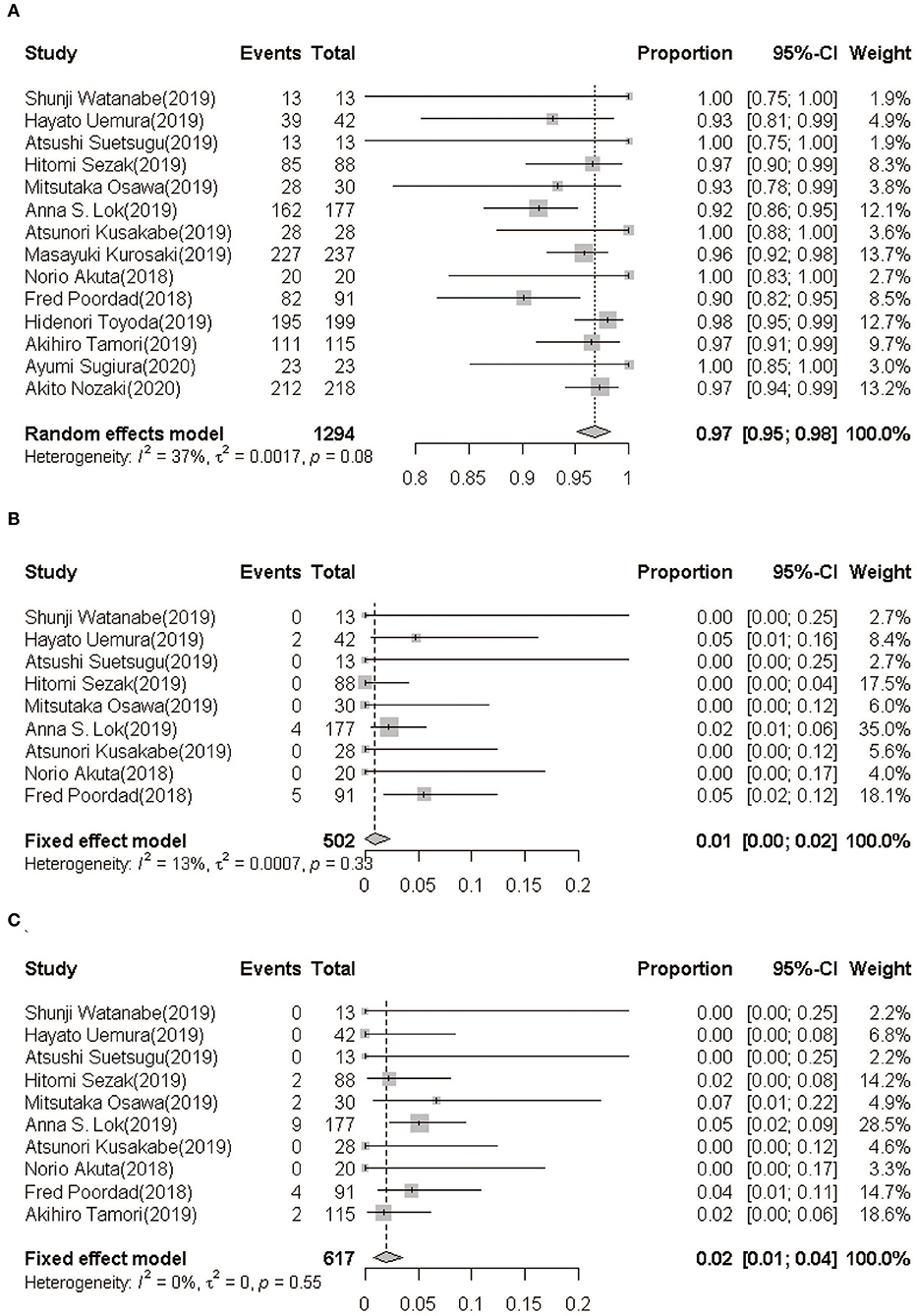
Figure 2. (A) SVR12 rate following GLE/PIB treatment. (B) On-treatment breakthrough rate following GLE/PIB treatment. (C) Post-treatment relapse rate following GLE/PIB treatment. GLE/PIB, glecaprevir/pibrentasvir; CI, confidence interval.
Breakthroughs and Relapses
In nine studies (21–27, 29, 32), only 11 of the 503 HCV patients retreated with GLE/PIB showed an on-treatment breakthrough with a pooled rate being 0.92% (95%CI: 0.08–2.32, I2 = 13.1%, P = 0.33) (Figure 2B). Furthermore, 19 of the 617 patients from 10 studies (21–27, 29, 31, 32) showed a post-treatment relapse with a pooled rate being 1.96% (95%CI: 0.78–3.50, I2 = 0.0%, P = 0.55) (Figure 2C).
Subgroup Analysis of the SVR12 Rate
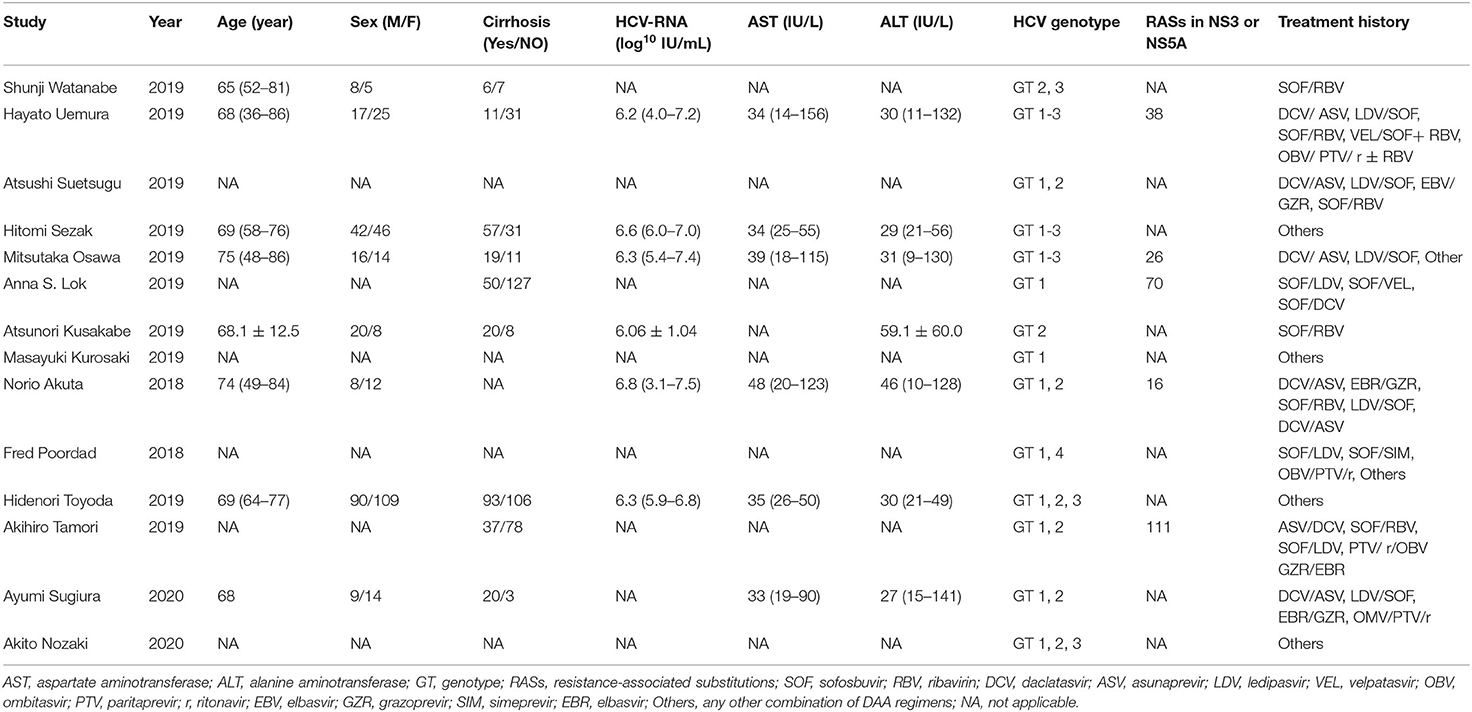
Based on settings, regions, genotypes, treatment history, treatment durations and the presence or absence of cirrhosis, we conducted subgroup analyses as detailed in Table 3. The rate of SVR12 was 96.0% (95%CI: 93.4–97.5) in multi-center and 96.1% (95%CI: 92.3–98.0) in single-center studies. Approximately 97.9% of patients (95%CI: 96.7–98.9) in Japan presented achieved SVR12 rate, while the SVR12 rate of the United States subgroup was 91.1% (95%CI: 87.3–94.3). As for genotypes, the pooled SVR12 rates in GT1, GT2, and GT3 were 95.8% (95%CI: 93.9–97.4), 100.0% (95%CI: 99.6–100.0), and 100.0% (95%CI:66.74–100.0). In subgenotype subgroups, GT1a, GT1b, GT2a, and GT2b were 90.3% (95%CI: 84.4–94.2), 94.8% (95%CI: 90.3–97.3), 100.0% (95%CI: 99.7–100.0), and 100.0% (95%CI: 98.3–100.0), respectively. Among the patients who had treatment history available for analysis, the SVR12 rates of sofosbuvir/ribavirin (SOF/RBV), daclatasvir/asunaprevir (DCV/ASV), ledipasvir/sofosbuvir (LDV/SOF), ombitasvir/paritaprevir/ritonavir (OBV/PTV/r), elbasvir/grazoprevir (EBV/GZR), and other DAAs were 100.0% (95%CI: 99.2–100.0), 97.6% (95%CI: 92.9–100.0), 99.3% (95%CI: 86.0–100.0), 100.0% (95%CI: 73.9–100.0), 97.4% (95%CI: 62.0–100.0), and 96.5% (95%CI: 95.1–97.7), respectively. Nine studies including 784 patients provided data for subgroup analysis with the presence or absence of cirrhosis. The SVR12 rates for patients with or without cirrhosis were 95.3% (95% CI: 92.4–97.2) and 96.3% (95% CI: 94.2–97.7), respectively.
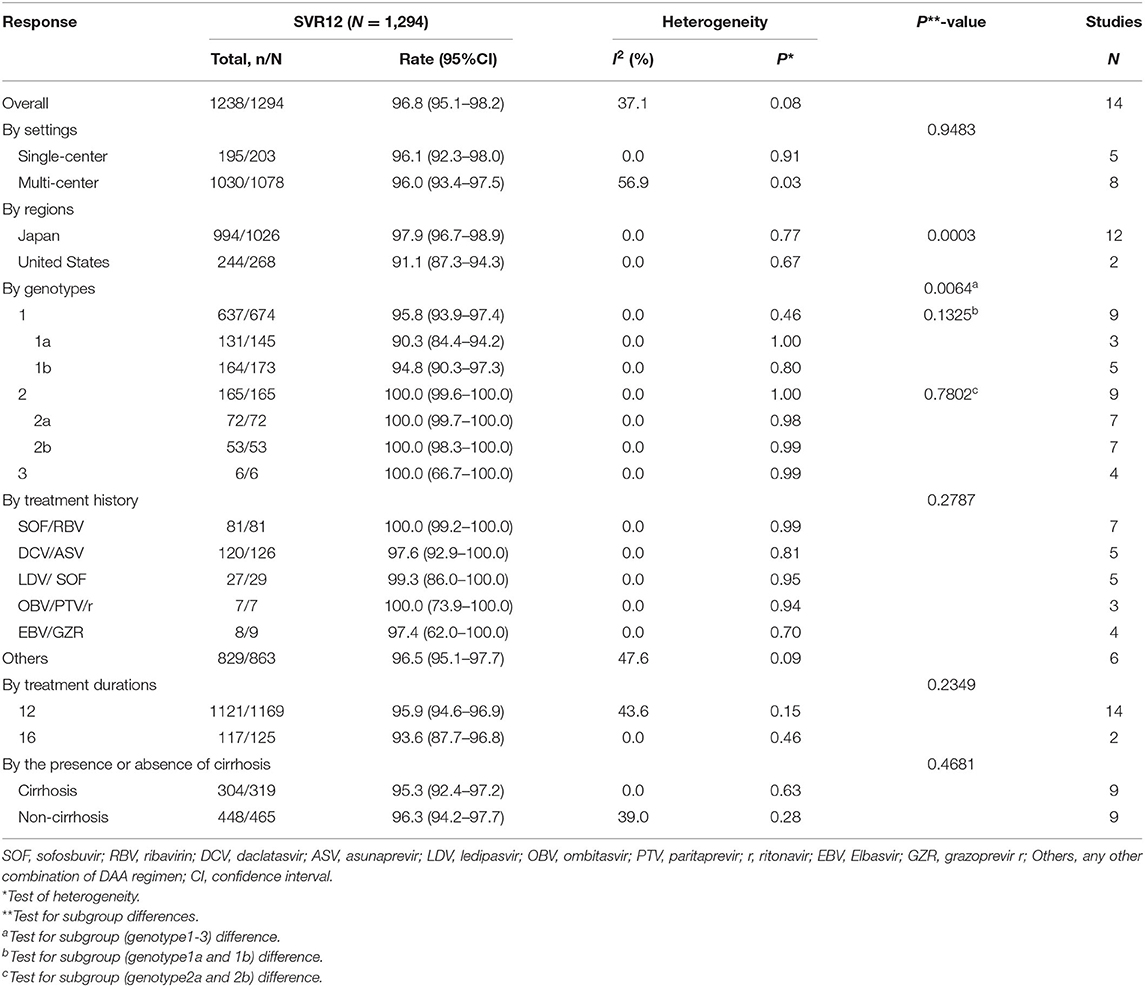
Table 3. SVR12 by settings, regions, genotypes, treatment history, treatment durations, and the presence or absence of cirrhosis.
Additionally, the SVR12 rate of the Japan subgroup was higher than the United States subgroup (P = 0.0003) and the GT1 was lower than the GT2-3 (P = 0.0064). However, there were no significant differences between the other subgroups analyzed.
Safety
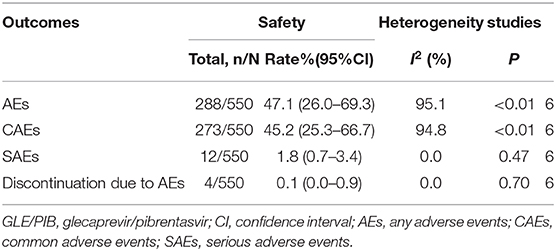
Six studies reported numbers for AEs, CAEs, SAEs, and discontinuation due to AEs were 288, 273, 12, and 4, respectively. The pooled rates of AEs, CAEs, SAEs, and discontinuation due to AEs were 47.1% (95%CI: 26.0–69.3), 45.2% (95%CI: 25.3–66.7), 1.8% (95%CI: 0.7–3.4), and 0.1% (95%CI: 0.0–0.9), respectively (Table 4). The main CAEs were fatigue (6.8%), headache (8.1%), nausea (4.1%), pruritus (11.8%), and appetite loss (1.3%). Furthermore, three studies observed treatment-related laboratory abnormalities in seven patients, including elevation of total bilirubin (5/254) and serum ALT levels (2/254).
Publication Bias and Sensitivity Analysis
Funnel plots for the SVR12 rate are shown in Supplementary Figures 2, 3. The Egger's test for evaluating publication bias showed that no publication bias was identified in these studies (t = 1.72, P = 0.11). Furthermore, the results from the sensitivity analysis manifested that the pooled estimate of SVR12 did not depend on a single study (Supplementary Figure 4).
Discussion
This study provided estimates regarding the efficacy and safety following GLE/PIB retreatment for patients who experienced prior DAA treatment failure. These results indicated that GLE/PIB for patients experiencing DAA therapy failure can achieve high SVR12 rates at 12 and 16 weeks, regardless of sex, age, genotype, the presence or absence of cirrhosis or other demographic factors. The rates of SAEs and discontinuation due to adverse events were minimal in GLE/PIB. Thus, GLE/PIB is an effective and secure retreatment option for patients who experience DAA treatment failure and this is critical information for global HCV treatment guidelines.
In this meta-analysis, the pooled SVR12, breakthrough, and relapse rates were 96.8% (95%CI: 95.1–98.2), 0.92% (95%CI: 0.08–2.32), and 1.96% (95%CI: 0.78–3.50), respectively. Compared with sofosbuvir/velpatasvir/voxilaprevir (35), and sofosbuvir/elbasvir/grazoprevirs ± ribavirin (36) retreatments, their SVR12 rates were similar. The incidence of failure of GLE/PIB was lower than other regimens, such as sofosbuvir/daclatasvir (5.7%) and sofosbuvir/velpatasvir (3.4%) (37). Although sofosbuvir/velpatasvir/ voxilaprevir was considered as a highly effective option for the re-treatment of HCV patients, the AEs (100%), SAEs (6.5%), and discontinuation due to AEs rates (5.2%) rates were higher than what was observed for GLE/PIB (AEs = 47.1%, SAEs = 1.8%, and discontinuation due to AEs = 0.1%) (35, 38), which explained an advantage for the GLE/PIB regimen.
Our findings revealed that the SVR12 rate among individuals in Japan was significantly higher than individuals in the United States. Only two studies (n = 268) were derived from the United States, so this analysis may be restricted by a finite sample size. Alternatively, a possible explanation was that therapy efficacy was related to race. Kanwal et al. reported differences among gender and race subgroups in the DAA treatment group (39). Most patients in Japan were Asians while most patients in the United States were White, Hispanic or Black. In addition, retreatment data from Asians were limited and available research showed that the SVR24 rate was 91.2% in patients with previous therapy failures (40). Therefore, our study suggested that GLE/PIB was of great significance for the retreatment of Asians with HCV therapy failures and more studies are needed for further evaluation in the United States.
In terms of genotype, there were significant differences among GTs 1-3 while no significant differences were in subgenotype subgroups (GT1a vs. GT1b; GT2a vs. GT2b). In previous studies, patients with GT3 infection showed lower SVR rates compared with other GTs. However, our data were inconsistent with previous studies since the SVR12 rates of GTs2-3 were 100% higher than GT1 (95.4%). On one hand, the sample size of GT3 was small (n = 6) which caused poor accuracy and reliability so more GT3 cases should be included to obtain enough evidence. We inferred that GLE/PIB still had a high efficacy for GT3 in patients with previous DAA therapy failures, considering that a systematic review demonstrated that GLE/PIB had distinct performance (SVR12 rate = 96.1%) when it came to the treatment of GT3 (41). On the other hand, five studies have found baseline resistance-associated substitutions (RASs) in NS3 or NS5A region and these RASs were mostly in subjects with GT1 (261/384). Moreover, at least 25 of 37 patients with GT1 who failed to achieve SVR12 had detected RASs in the NS3 or NS5A region. RASs were produced by the error-prone replication of HCV that could decrease efficacy of the DAA regimens (42). We suspected that the existence of RASs caused the decrease of the SVR12 rate in GT1. Even so, the SVR12 rate was still ≥ 95% in GT1, which suggested an ideal curative effect. Thus, GLE/PIB, one of the NS3/4/NS5A combination regimens, is extremely effective and a strong choice for the HCV population with RASs.
Currently, there are three major classes of antiviral HCV drugs including: inhibitors of the NS3/NS4A protease (PIs), inhibitors of the NS5A complex and inhibitors of the NS5B polymerase (43). In our study, main DAA treatment histories included SOF/RBV and DCV/ASV, belonging to the three classes of DAAs mentioned. As the first pan-genotypic DAA agent that was approved, SOF was widely used in many countries, but about 10% of people treated with SOF-containing regimens did not achieve SVR (44). DCV is a DAA agent that was approved by the European Medicines Agency for combination with other medicinal products for treating chronic HCV genotype 1, 3, or 4 infections (45). Administered with an NS3 protease inhibitor (ASV), DCV achieves greater than a 90% HCV eradication rate, while around 5–10% will not be cured (46). Furthermore, except for four studies without a clear DAA treatment history, these prior DAA treatments mostly were PIs and NS5A or NS5B inhibitor-containing regimens. Although DAA treatment histories were varied, GLE/PIB obtained favorable SVR rates (>95%), especially sofosbuvir-containing regimens (100%), which implied its wide application and fantastic efficacy.
Interestingly, treatment duration did not increase the response rate of GLE/PIB in our subgroup analysis. However, only two studies contained a 16-weeks GLE/PIB therapy period and showed that the SVR rates of the 16-weeks treatment subgroup were higher than the 12-weeks treatment subgroup. These two studies came from the United States with relatively low SVR12 rates. This may explain why the 16-weeks treatment SVR rate did not increase. In addition, there was no significant observed difference between patients with or without cirrhosis. It can be inferred that re-treatment with 12 weeks of GLE/PIB is highly effective in HCV patients with or without cirrhosis and future guidelines should consider recommending a 12-weeks therapy.
Our comprehensive analysis exhibited several strengths. First, our study was the first to evaluate the efficacy and safety of GLE/PIB for HCV patients with previous DAA therapy failures. We screened 14 studies including 1,294 individuals, which allowed us to accurately assess the pooled SVR12 rate, breakthrough, relapse, AE and SAE rates of populations who had previous DAA treatment failure. In addition, the heterogeneity among the included studies for most analyses was small, which indicated that this study is reliable and may help clinicians effectively retreat HCV subjects.
However, despite these strengths, this study still contained several limitations. First, only the efficacy and safety rates were analyzed along with the 95% CI. The relative risk (RR) for the various subgroups was not analyzed due to the absence of a control group. Second, most included studies were from Japan and focused on patients with GT1-3. Our data may not be relatable to other nations and genotypes. Third, some studies offered the frequency of prior DAA treatment but we did not perform subgroup analysis on this subject due to insufficient data.
Conclusion
This comprehensive analysis supports that the GLE/PIB regimen has strong efficacy and increased safety for HCV patients with previous DAAs therapy failures, especially the Asian population with GT1-2 regardless of treatment duration, and the presence or absence of cirrhosis. Furthermore, GLE/PIB is appropriate for subjects with various DAA treatment failures, such as sofosbuvir-containing regimens.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
CS, MY, and HF participated in the design of the study. CS, HF, ZG, WC, and ZF took charge of literature retrieval, data collection, and quality control. CS, JS, and JL performed the statistical analysis. HX, HF, CD, and MY contributed to analysis. CS, HF, and WC wrote the paper. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81773499), Open Research Fund Program of the State Key Laboratory of Virology of China (2019KF005), Key Project of Natural Science Foundation of Yunnan Province (2019FA005), Science Foundation for Distinguished Young Scholars of Jiangsu Province (BK20190106), Jiangsu Program for Young Medical Talents (QNRC2016616), and Novel Coronavirus Pneumonia Project of Nantong (HS2020002).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.592472/full#supplementary-material
References
1. Manns MP, Buti M, Gane E, Pawlotsky JM, Razavi H, Terrault N, et al. Hepatitis C virus infection. Nat Rev Dis Primers. (2017) 3:17006. doi: 10.1038/nrdp.2017.6
2. Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. (2015) 61:77–87. doi: 10.1002/hep.27259
3. Collaborators TPOH. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. (2017) 2:161–76. doi: 10.1016/S2468-1253(16)30181-9
4. WHO. Hepatitis C. (2019). Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed 9 July, 2020).
5. Organization WH. Global Heath Strategy on Viral Hepatitis 2016–2021 Towards Ending Viral Hepatitis. (2016). Geneva: World Health Organization.
6. Chao DT, Abe K, Nguyen MH. Systematic review: epidemiology of hepatitis C genotype 6 and its management. Aliment Pharmacol Ther. (2011) 34:286–96. doi: 10.1111/j.1365-2036.2011.04714.x
7. Sulkowski MS, Cooper C, Hunyady B, Jia J, Ogurtsov P, Peck-Radosavljevic M, et al. Management of adverse effects of Peg-IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol. (2011) 8:212–23. doi: 10.1038/nrgastro.2011.21
8. Naggie S, Muir AJ. Oral combination therapies for hepatitis C virus infection: successes, challenges, and unmet needs. Annu Rev Med. (2017) 68:345–58. doi: 10.1146/annurev-med-052915-015720
9. Ng TI, Tripathi R, Reisch T, Lu L, Middleton T, Hopkins TA, et al. In vitro antiviral activity and resistance profile of the next-generation hepatitis C virus NS3/4A protease inhibitor glecaprevir. Antimicrob Agents Chemother. (2018) 62:17. doi: 10.1128/AAC.01620-17
10. Ng TI, Krishnan P, Pilot-Matias T, Kati W, Schnell G, Beyer J, et al. In vitro antiviral activity and resistance profile of the next-generation hepatitis C virus NS5A inhibitor pibrentasvir. Antimicrob Agents Chemother. (2017) 61:16. doi: 10.1128/AAC.02558-16
11. Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol. (2017) 67:263–71. doi: 10.1016/j.jhep.2017.03.039
12. Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. (2018) 16:417–26. doi: 10.1016/j.cgh.2017.09.027
13. Forns X, Lee SS, Valdes J, Lens S, Ghalib R, Aguilar H, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. (2017) 17:1062–8. doi: 10.1016/S1473-3099(17)30496-6
14. Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Bräu N, Brown A, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. (2017) 377:1448–55. doi: 10.1056/NEJMoa1704053
15. Puoti M, Foster GR, Wang S, Mutimer D, Gane E, Moreno C, et al. High SVR12 with 8-weeks and 12-weeks glecaprevir/pibrentasvir therapy: an integrated analysis of HCV genotype 1–6 patients without cirrhosis. J Hepatol. (2018) 69:293–300. doi: 10.1016/j.jhep.2018.03.007
16. Wyles D, Poordad F, Wang S, Alric L, Felizarta F, Kwo PY, et al. Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: a partially randomized phase 3 clinical trial. Hepatology. (2018) 67:514–23. doi: 10.1002/hep.29541
17. Chinese Society of Hepatology, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C (2019 version). Zhonghua Gan Zang Bing Za Zhi. (2019) 27:962–79. doi: 10.3760/cma.j.issn.1007-3418.2019.12.008
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
19. Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol. (2014) 6:359–68. doi: 10.2147/CLEP.S66677
20. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
21. Watanabe S, Miura K, Morimoto N, Murohisa T, Tano S, Tahara T, et al. Efficacy and safety of glecaprevir/pibrentasvir combination therapy for patients with genotype 2 chronic hepatitis C infection who failed HCV eradication by SOF+RBV treatment. Acta Hepatol Japonica. (2019) 60:77–9. doi: 10.2957/kanzo.60.77
22. Uemura H, Uchida Y, Kouyama J-i, Naiki K, Tsuji S, Sugawara K, et al. NS5A-P32 deletion as a factor involved in virologic failure in patients receiving glecaprevir and pibrentasvir. J Gastroenterol. (2019) 54:459–70. doi: 10.1007/s00535-018-01543-9
23. Suetsugu A, Naiki T, Shimizu S, Sugihara J, Tomita E, Shimizu M. Efficacy of Glecaprevir/Pibrentasvir in patients with hepatitis C virus genotype 1 or 2 and past direct-acting antiviral treatment failure. Hepatol Int. (2019) 13:S92–3. doi: 10.1007/s12072-019-09936-5
24. Sezaki H, Suzuki F, Hosaka T, Fujiyama S, Kawamura Y, Akuta N, et al. Initial- and re-treatment effectiveness of glecaprevir and pibrentasvir for Japanese patients with chronic hepatitis C virus-genotype 1/2/3 infections. J Gastroenterol. (2019) 54:916–27. doi: 10.1007/s00535-019-01575-9
25. Osawa M, Imamura M, Teraoka Y, Uchida T, Morio K, Fujino H, et al. Real-world efficacy of glecaprevir plus pibrentasvir for chronic hepatitis C patient with previous direct-acting antiviral therapy failures. J Gastroenterol. (2019) 54:291–6. doi: 10.1007/s00535-018-1520-9
26. Lok AS, Sulkowski MS, Kort JJ, Willner I, Reddy KR, Shiffman ML, et al. Efficacy of glecaprevir and pibrentasvir in patients with genotype 1 hepatitis C virus infection with treatment failure after NS5A Inhibitor plus sofosbuvir therapy. Gastroenterology. (2019) 157:1506. doi: 10.1053/j.gastro.2019.08.008
27. Kusakabe A, Kurosaki M, Itakura J, Joko K, Akahane T, Tsuji K, et al. Efficacy and safety of glecaprevir/pibrentasvir as retreatment therapy for patients with genotype 2 chronic hepatitis C who failed prior sofosbuvir plus ribavirin regimen. Hepatol Res. (2019) 49:1121–6. doi: 10.1111/hepr.13387
28. Kurosaki M, Itakura J, Izumi N. ns5a resistance profile of genotype 1b virological failures that impacts outcome of re-treatment by glecaprevir/pibrentasvir: nation-wide real world study. J Hepatol. (2019) 70:E111. doi: 10.1016/S0618-8278(19)30198-7
29. Akuta N, Sezaki H, Suzuki F, Fujiyama S, Kawamura Y, Hosaka T, et al. Favorable efficacy of glecaprevir plus pibrentasvir as salvage therapy for HCV failures to prior direct-acting antivirals regimens. J Med Virol. (2019) 91:102–6. doi: 10.1002/jmv.25278
30. Toyoda H, Atsukawa M, Watanabe T, Nakamuta M, Uojima H, Nozaki A, et al. Real-world experience of 12-weeks direct-acting antiviral regimen of glecaprevir and pibrentasvir in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. (2020) 35:855–61. doi: 10.1111/jgh.14874
31. Tamori A, Inoue K, Kagawa T, Takaguchi K, Nouso K, Iwasaki Y, et al. Intention-to-treat assessment of glecaprevir + pibrentasvir combination therapy for patients with chronic hepatitis C in the real world. Hepatol Res. (2019) 49:1365–73. doi: 10.1111/hepr.13410
32. Poordad F, Pol S, Asatryan A, Buti M, Shaw D, Hézode C, et al. Glecaprevir/Pibrentasvir in patients with hepatitis C virus genotype 1 or 4 and past direct-acting antiviral treatment failure. Hepatology. (2018) 67:1253–60. doi: 10.1002/hep.29671
33. Nozaki A, Atsukawa M, Kondo C, Toyoda H, Chuma M, Nakamuta M, et al. The effectiveness and safety of glecaprevir/pibrentasvir in chronic hepatitis C patients with refractory factors in the real world: a comprehensive analysis of a prospective multicenter study. Hepatol Int. (2020) 14:225–38. doi: 10.1007/s12072-020-10019-z
34. Sugiura A, Joshita S, Yamashita Y, Yamazaki T, Fujimori N, Kimura T, et al. Effectiveness of glecaprevir/pibrentasvir for hepatitis C: real-world experience and clinical features of retreatment cases. Biomedicines. (2020) 8:74. doi: 10.3390/biomedicines8040074
35. Wilson E, Covert E, Hoffmann J, Comstock E, Emmanuel B, Tang L, et al. A pilot study of safety and efficacy of HCV retreatment with sofosbuvir/velpatasvir/voxilaprevir in patients with or without HIV (RESOLVE STUDY). J Hepatol. (2019) 71:498–504. doi: 10.1016/j.jhep.2019.05.021
36. Papaluca T, Sinclair M, Gow P, Pianko S, Sievert W, Arachchi N, et al. Retreatment with elbasvir, grazoprevir, sofosbuvir ± ribavirin is effective for GT3 and GT1/4/6 HCV infection after relapse. Liver Int. (2019) 39:2285–90. doi: 10.1111/liv.14201
37. Pawlotsky JM. Retreatment of hepatitis C virus-infected patients with direct-acting antiviral failures. Semin Liver Dis. (2019) 39:354–68. doi: 10.1055/s-0039-1687823
38. Mathur P, Kottilil S, Wilson E. Sofosbuvir/velpatasvir/voxilaprevir: a highly effective option for retreatment of hepatitis C in difficult-to-treat patients. Antivir Ther. (2019) 24:1–10. doi: 10.3851/IMP3264
39. Kanwal F, Kramer JR, El-Serag HB, Frayne S, Clark J, Cao Y, et al. Race and gender differences in the use of direct acting antiviral agents for hepatitis C virus. Clin Infect Dis. (2016) 63:291–9. doi: 10.1093/cid/ciw249
40. Wei L, Zhang M, Xu M, Chuang WL, Lu W, Xie W, et al. A phase 3, open-label study of daclatasvir plus asunaprevir in Asian patients with chronic hepatitis C virus genotype 1b infection who are ineligible for or intolerant to interferon alfa therapies with or without ribavirin. J Gastroenterol Hepatol. (2016) 31:1860–7. doi: 10.1111/jgh.13379
41. Wang X, Fan X, Deng H, Zhang X, Zhang K, Li N, et al. Efficacy and safety of glecaprevir/pibrentasvir for chronic hepatitis C virus genotypes 1-6 infection: a systematic review and meta-analysis. Int J Antimicrob Agents. (2019) 54:780–9. doi: 10.1016/j.ijantimicag.2019.07.005
42. Sarrazin C, Dvory-Sobol H, Svarovskaia ES, Doehle BP, Pang PS, Chuang SM, et al. Prevalence of resistance-associated substitutions in HCV NS5A, NS5B, or NS3 and outcomes of treatment with ledipasvir and sofosbuvir. Gastroenterology. (2016) 151:501–12.e1. doi: 10.1053/j.gastro.2016.06.002
43. Spengler U. Direct antiviral agents (DAAs)—a new age in the treatment of hepatitis C virus infection. Pharmacol Ther. (2018) 183:118–26. doi: 10.1016/j.pharmthera.2017.10.009
44. Tang L, Ward H, Kattakuzhy S, Wilson E, Kottilil S. Dual sofosbuvir and ribavirin therapy for chronic hepatitis C infection. Expert Rev Gastroenterol Hepatol. (2016) 10:21–36. doi: 10.1586/17474124.2016.1119042
45. Agency EM. Daklinza (Daclatasvir): EU Summmary of Product Characteristics. (2016). Available online at: http://www.ema.europa.eu/ (accessed November 20, 2020).
Keywords: pibrentasvir, retreatment, DAAs therapy failures, meta-analysis, glecaprevir
Citation: Shen C, Fan H, Ge Z, Cai W, Shao J, Dong C, Xue H, Fu Z, Li J, Zhang Y and Yue M (2020) Efficacy and Safety of Glecaprevir/Pibrentasvir in HCV Patients With Previous Direct-Acting Antiviral Therapy Failures: A Meta-Analysis. Front. Med. 7:592472. doi: 10.3389/fmed.2020.592472
Received: 07 August 2020; Accepted: 13 November 2020;
Published: 03 December 2020.
Edited by:
Chao Yan, Xuzhou Medical University, ChinaReviewed by:
Hui Jin, Southeast University, ChinaMousumi Chaudhury, Arkansas Children's Nutrition Center, United States
Wenshi Wang, Heidelberg University Hospital, Germany
Copyright © 2020 Shen, Fan, Ge, Cai, Shao, Dong, Xue, Fu, Li, Zhang and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Yue, eXVlbWluZ0Buam11LmVkdS5jbg==
†These authors have contributed equally to this work
 Chao Shen1†
Chao Shen1† Haozhi Fan
Haozhi Fan Ming Yue
Ming Yue