- 1Division of Clinical Rheumatology, Gaetano Pini Hospital, Milan, Italy
- 2Department of Clinical Sciences and Community Health, Research Center for Adult and Pediatric Rheumatic Diseases, Università degli Studi di Milano, Milan, Italy
- 3Department of Neurosciences and Mental Health, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca'Granda Ospedale Maggiore Policlinico, Milan, Italy
- 4Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
- 5Department of Medical Biotechnology and Translational Medicine (BIOMETRA), Università degli Studi di Milano, Milan, Italy
Neuronal stimulation is an emerging field of research focused on the management and treatment of various diseases through the reestablishment of physiological homeostasis. Electrical vagus nerve stimulation has recently been proposed as a revolutionary therapeutic option for rheumatoid arthritis (RA) in combination with or even as a replacement for conventional and biological drugs. In the past few years, disruption of the autonomic system has been linked to RA onset and activity. Novel research on the link between the autonomic nervous system and the immune system (immune-autonomics) has paved the way for the development of innovative RA management strategies. Clinical evidence supports this approach. Cardiovascular involvement, in terms of reduced baroreflex sensitivity and heart rate variability-derived indices, and mood disorders, common comorbidities in patients with RA, have been linked to autonomic nervous system dysfunction, which in turn is influenced by increased levels of circulating pro-inflammatory cytokines. This narrative review provides an overview of the autonomic nervous system and RA connection, discussing most of the common cardiac and mental health-related RA comorbidities and their potential relationships to systemic and joint inflammation.
Introduction
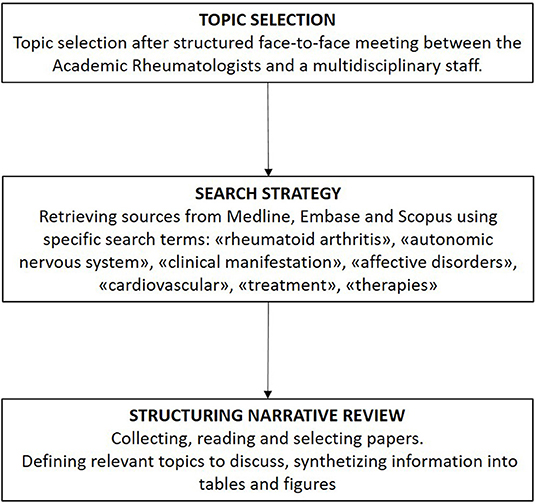
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease leading to progressive joint damage and associated with vascular, metabolic, and psychological comorbidities. RA is considered to pose a major global public health challenge, as its overall prevalence and incidence rates are increasing worldwide (1). Fundamentally, it is of paramount importance to reduce the future burden of this disease through the pursuit of innovative RA treatments that are driven mainly by increasing knowledge of its pathophysiology. An emergent field in this context is the study of autonomic nervous system (ANS) imbalance observed in association with many immune-mediated inflammatory diseases, comprising RA, systemic lupus erythematosus, systemic sclerosis, and inflammatory bowel diseases (2–4). The immune system and ANS can express and respond to numerous common regulatory molecules (e.g., glucocorticoids, cytokines, neuropeptides, and neurotransmitters), which constitute the molecular basis of a complex bidirectional response to homeostasis perturbations induced by infection or inflammation. These findings have led to an innovative research field in RA focused on the emerging concept of “immuno-autonomics,” reflecting the anatomical and functional linkage of the immune system to the nervous system (5). With the understanding of the relevance of this connection, we may consider if and where the ANS is disrupted in RA and hence which therapeutic implications might be relevant. Whether ANS impairment is the result of chronic inflammation or a primitive alteration that affects immune system functioning, disease onset, and severity remains to be established. In this narrative review (Figure 1) (6), we discuss and review the ongoing progress in this field, focusing mainly on current knowledge of the potential connections between ANS and the pathogenesis and clinical manifestations of RA. We then consider the potential innovative therapeutic prospects that target this pathway.
Relevance of the Autonomic Nervous System to the Pathogenesis of Rheumatoid Arthritis
The ANS operates through visceral reflex arcs mediated by cholinergic and catecholaminergic signaling (Figure 2). Communication between the ANS and the immune system occurs in two main ways: (i) via direct innervation of the lymphoid organs by the efferent sympathetic nervous system (SNS) [postganglionic noradrenergic fibers innervate the bone marrow, thymus, spleen, and lymph nodes by liberating noradrenaline (NA), neuropeptides (substance P, somatostatin, vasoactive intestinal peptide, and neuropeptide Y), neurokinins, and opioids whose receptors are expressed on immune cells] and (ii) by indirect humoral action mediated by NA (liberated into the bloodstream after medullary activation by the SNS) and steroids [due to activation of the hypothalamic–pituitary–adrenal (HPA) axis neuroendocrine response] (7, 8).
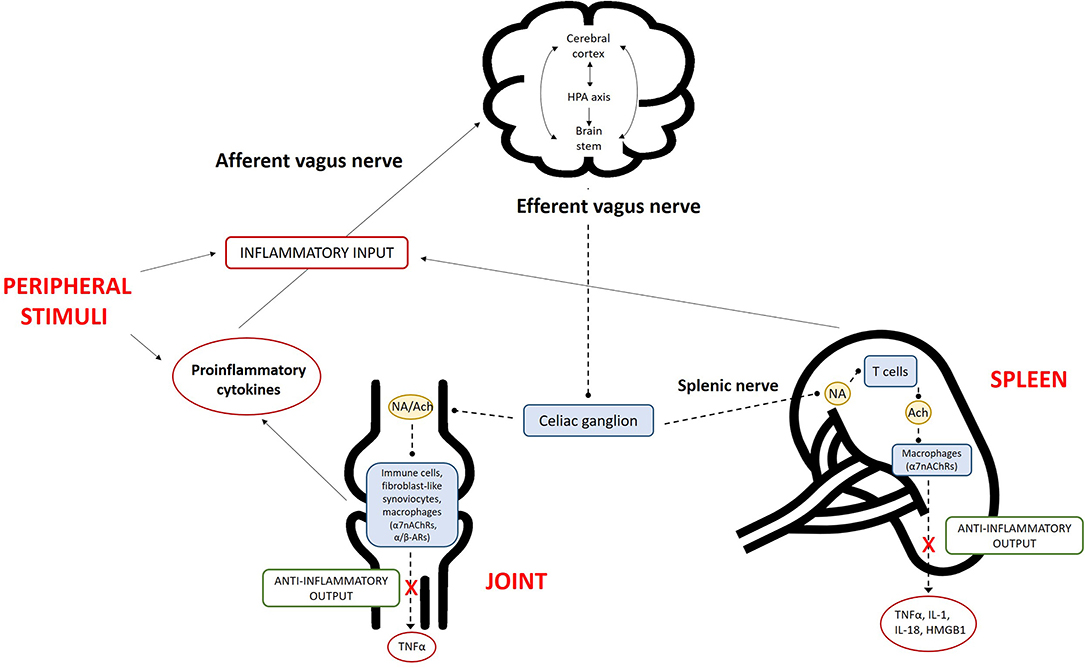
Figure 2. Overview of the inflammatory reflex in rheumatoid arthritis (RA). The central nervous system (CNS) detects peripheral inflammation through the afferent vagus nerve and pro-inflammatory cytokines. The signals act at different levels via (i) activation of the hypothalamic–pituitary–adrenal (HPA) axis, which reduces inflammation; (ii) the cerebral cortex; and (iii) nuclei in the brain stem. The efferent branch of the vagus nerve is activated via the activation of alpha-7 subunit of nicotinic acetylcholine receptors (α7nAChRs) on macrophages, resulting in decreased cytokine production. This mechanism is called the “cholinergic anti-inflammatory pathway.” The combination of inflammation in peripheral tissues, signaling of this information to the brain, and the subsequent efferent neuronal response comprises the “inflammatory reflex.” The activation of α7nAChRs on macrophages can occur locally in the RA joint or in the spleen. The sympathetic fibers of the splenic nerve are activated in the celiac ganglion, leading to noradrenaline (NA) production in the spleen. NA binds to α/β-adrenergic receptors (α/β-ARs) on choline acetyltransferase-positive T cells, which then produce the anti-inflammatory parasympathetic neurotransmitter acetylcholine (Ach), in turn activating α7nAChRs and leading to the reduced production of pro-inflammatory cytokines. In the joint, the activation of a7nAChRs on fibroblast-like synoviocytes or other immune cells also reduces cytokine production. TNF, tumor necrosis factor; IL, interleukin; HMGB1, high mobility group box 1.
Via beta-2 adrenergic receptors, NA inhibits lymphocyte proliferation, the release of pro-inflammatory cytokines [interleukin (IL)-2, IL-12, interferon gamma, and tumor necrosis factor (TNF)-α], chemotaxis, the activity of natural killer cells and T lymphocytes, and the production of antibodies by B cells. It also stimulates anti-inflammatory cytokines (IL-4, IL-5, and IL-10). Not only postganglionic fibers but also lymphocytes can release NA and acetylcholine, thereby regulating some immune functions in autocrine and paracrine ways (7, 8).
Conversely, the immune system is capable of communicating with the nervous system, mainly via HPA axis activation by pro-inflammatory cytokines and afferent vagal fibers. Neurons express pattern recognition receptors, including toll-like and cytokine receptors (e.g., TNF-R and type 1 IL-1 receptors) (9, 10). This dialog extends beyond the HPA axis; cytokines also directly influence the cerebral cortex and primitive brain (the limbic system, brain stem, and hypothalamus). Cerebral manifestations such as fever, illness, attention deficit, anorexia, and disrupted interaction with external stimuli are examples of their effects during the acute phase of immune activation. In efforts to discern the physiology and pathology of RA, the identification of specific reflexes and pathways is fundamental. A “cholinergic anti-inflammatory pathway,” named after its principal mediator acetylcholine, has been described; its identification has led to the understanding of the “inflammatory reflex” (11). This neural circuit, like other visceral reflex arcs, includes afferent and efferent arms. Vagus afferent fibers are activated peripherally by products of inflammation and then the signal travels to the nucleus tractus solitarius; once the stimulus has been relayed and integrated by other nuclei in the brain stem or hypothalamus (e.g., the rostral ventrolateral medulla and locus coeruleus) (12, 13), the efferent signal leaves the nucleus ambiguus and dorsal motor nucleus and travels back through the vagus nerve until it reaches the celiac ganglia (13, 14). There, preganglionic cholinergic fibers form synapses with adrenergic interneurons, whose splenic fibers end up in the white pulp of the spleen and interact, via NA release, with beta-2 adrenergic receptors of a specific T cell subset. The latter is distinguished by the expression of choline acetyltransferase and when activated induces acetylcholine biosynthesis and release, which in turn stimulates macrophages of the red pulp binding alpha-7 subunit of nicotinic acetylcholine receptors (α7nAChRs) toward an anti-inflammatory immune response (15, 16). The anti-inflammatory signal may bypass the vagus efferent fibers, traveling from the preganglionic fibers of the sympathetic chain directly to the interneurons of the celiac ganglia.
Thus, under basal conditions, the vagus nerve senses the precise location of nascent inflammation and rapidly tonically dampens the overactivation of the innate immune response (17). As the major regulator of the peripheral nervous system (PNS), the vagus modulates inflammation in many anatomical regions, comprising the liver (14), heart (18), pancreas (19), and gastrointestinal tract (20, 21).
The establishment and maintenance of a chronic response involves humoral neuroendocrine mechanisms. This cooperation not only affects innate immunity but also modulates cell trafficking and the lymphoid architecture, ultimately leading to the regulation of humoral immunity and possibly the prevention of T cell-mediated tissue damage (22, 23).
Autonomic imbalance seems to be an early finding, rather than the result of chronic inflammation, in patients with RA. In a prospective cohort study, in which ANS activity was assessed using a validated method via the measurement of subjects' resting heart rate (HR) and heart rate variability (HRV), individuals at risk of RA who subsequently developed arthritis had significantly higher resting HRs than healthy subjects (24). This finding is in agreement with those from other studies, which reflect reduced PNS activity and hence an impaired inflammatory reflex, in patients with RA (25, 26). The early impairment of the PNS in RA, even before the fulfillment of the disease classification criteria (27), is consistent with the correlation between increased inflammatory status and decreased parasympathetic activity observed in healthy subjects in large observational studies (28–30). In detail, levels of inflammatory markers [C-reactive protein (CRP) and IL-6] are inversely related to HRV in young adults (29), and circulating TNF level is an independent predictor of depressed HRV (31), reinforcing the concept of a complex dialog between immunity and the ANS.
As the PNS and SNS work together, we would expect the SNS to be less active when the PNS is impaired. Contrary to this expectation, the SNS has been found to be overactive in patients with RA and high NA levels (25), rendering comprehension of its pathogenetic role difficult (32). One explanation could involve a difference in intracellular signaling (5). Splenic beta-2 adrenergic receptors usually promote a T helper (Th) 2 and T regulatory (Treg) response via G-coupled proteins and protein kinase A pathways. However, the scenario in which the SNS seems to be chronically activated may entail the downregulation of beta-2 adrenergic receptors and a shift toward Th1 and/or Th17 immune responses via mitogen-activated protein kinase pathways (5).
Collectively taken, these data support the critical role of ANS in RA pathogenic network.
Autonomic Nervous System and Affective Disorders in Rheumatoid Arthritis
Peripheral cytokines profoundly influence neuronal function and brain circuitry. As briefly mentioned above, they reach the brain by different routes and, once there, affect brain function through several mechanisms. They may directly stimulate (i) the central nervous system (CNS) cell population (microglia, astrocytes, and neurons), producing additional cytokines (33); (ii) the HPA axis, resulting in the production of corticotropin-releasing factor and adrenocorticotropic hormone; and (iii) cortisol, influencing many other physiological processes in the CNS. Cytokines alter the metabolism of several neurotransmitters, including serotonin (34, 35), dopamine (35), and glutamate (36, 37), leading to the decreased production of norepinephrine and the trophic or growth factors that are essential for neurogenesis and neuroplasticity (38–40). Changes in all of these factors and amines may lead to the development of psychiatric disorders, further corroborating the link between cytokine increments and mental health. In many studies, the continuous elevation of IL levels has been correlated with impairments in structures profoundly affected in mood disorders, such as the hippocampal region (41, 42) and other areas of the brain (43–45), as well as changes in functional connectivity (46, 47).
In the CIA model, researchers observed exacerbated symptomatology in association with greater cytokine production in mice lacking α7nAChR, suggesting that nicotinic receptor expression is relevant in RA and that the activation of these receptors would have beneficial effects. Interestingly, cholinergic agonists suppressed inflammatory cytokine production in RA whole blood cultures (48). Choline acetyltransferase expression was observed in fibroblast-like synoviocytes and mononuclear cells in RA and osteoarthritis synovial biopsy samples, suggesting that local acetylcholine production contributes to the regulation of joint inflammation by the aforementioned “cholinergic anti-inflammatory pathway” (49). Although the activation of α7nAChR leads to control of the degree of inflammation, the effects of this receptor's action on central neurons, brain functionality, and related cognitive behaviors have not been examined. In general, results indicate that (i) muscarinic agonist administration, (ii) electrical vagus nerve stimulation (VNS) to activate preganglionic parasympathetic nerves, and (iii) treatment with nAChR agonists can all act systemically (although not necessarily identically) to reduce the production of inflammatory cytokines (presumably mostly by macrophages).
Abundant data support associations between autoimmune diseases and psychiatric disorders, with immune activation identified as the common core feature (50, 51). The prominence of psychiatric comorbidities, including major depressive disorder (MDD), bipolar disorder, and anxiety disorders (ADs), in the presence of autoimmune diseases such as RA supports the theory that affective disorders can be considered to be inflammatory conditions (52).
From an epidemiological point of view, concomitant RA and depression has been reported in 6.8–66.2% of patients (53–62). Risk factors for MDD onset in patients with RA include female sex (63, 64), unmarried status, the lack of sufficient social support (65), a high rate of disability (66), and chronic pain (67). Moreover, the presence of depressive symptoms increases the risk of suicide in subjects, and especially women, with RA (68).
In most described cases, depression appears secondary to RA development (69–72). Several pathogenetic mechanisms have been proposed to explain this association. From a neurobiological perspective, reduced expression of BDNF, but not serum levels of pro-inflammatory cytokines (59) or TNF-α (57), has been found in depressed patients with RA with respect to subjects without depression. In contrast, other studies have documented a relationship between MDD and the inflammatory state, defined by plasma CRP levels (73) or indirectly via clinical indices of RA activity (74). The implication of over-inflammation in the onset of mood symptoms in RA is also supported by the evidence of alterations in the micro-structure of brain white matter in subjects with RA and comorbid depression as a result of vasculitis, ischemic brain lesions, and dots of demyelination (75). In contrast, one study failed to identify shared genetic vulnerability to RA and MDD (76). From a psychological perspective, the RA self-schema construct seems to predict the onset of depression. Interpersonal conflicts seem to increase the risk of depression in patients with RA. Vulnerability to depressive symptoms in patients with RA appears to be related to functional disabilities (59, 77–79) and pain (78, 80–82) caused by the disease, although some researchers failed to find an association between RA activity or severity and depression (56, 81, 83).
Comorbid depression increases social impairment (84, 85) and disability (86) in patients with RA. Functional capacity seems to be more compromised in women than in men with concomitant RA and MDD (63). The occurrence of depression in patients with RA worsens the subjective sensation of pain (87–90), thereby encouraging the abuse of analgesic compounds (91, 92). After all, the association between depression and pain sensitivity is already known within the framework of fibromyalgia (93). Of note, the presence of depressive symptoms in musculoskeletal pathologies even reach 81.8% as reported in a recent study (94), where a correlation between the severity of depressive symptoms and fibromyalgia was also identified. Although most studies have focused on the effects of RA on mental health, some authors have hypothesized conversely that depression directly influences RA activity and the number of compromised joints (95–97). In addition, comorbid depression in patients with RA favors the onset of medical complications such as atherosclerosis (98) and myocardial infarction (99), resulting in increased lethality (100, 101). Depressive symptoms also may prevent patients' treatment adherence, thereby worsening the prognosis of RA (96, 102). Taken as a whole, these data indicate that depression worsens the quality of life (90, 103) and general health status (104) of subjects with RA.
Depression is often underestimated in patients with RA, delaying its proper management (94, 105). The treatment of depressive symptoms seems to ameliorate the clinical symptoms of RA (106), although some data contradict the existence of this effect (107). Antidepressants demonstrated to be effective in cases of RA-MDD comorbidity include dothiepin (108) and sertraline (109), eventually combined with cognitive behavioral treatment (107). Non-pharmacological strategies, including yoga, mindfulness meditation, and emotion regulation therapy, have been shown to significantly ameliorate mood symptoms and inflammatory status in patients with RA (110, 111). Finally, immunomodulating drugs, such as anti-TNF-α drugs (105, 112), anti-IL-6 monoclonal antibodies (113), and other biologics (114), appear to ameliorate anxiety and depressive symptoms in patients with RA. Of note, in line with the autonomic dysfunctions already mentioned for RA, VNS was proposed as a treatment for depression in the light of its anti-inflammatory effect (115). This convergence of the efficacy of VNS both for RA and depression opens up extremely promising therapeutic prospective for the treatment of subjects suffering from RA and mood symptoms (116).
ADs have the common psychopathological nucleus of anxiety and partly shared neurobiological abnormalities. They include panic disorder, social phobia (SP) (also known as social anxiety disorder), and generalized anxiety disorder (GAD). Their prevalence in RA ranges from 13 to 70% (53, 55, 65, 78, 117–119) with higher incidence and prevalence compared to the general population (120). Anxious states often occur concomitantly with depressive symptoms (54, 65). SP seems to be more common in individuals with RA than in those with other autoimmune conditions (121, 122). ADs are considered to be a risk factor for the future development of RA (120) and appear to negatively affect its course, worsening functional disabilities (78, 90, 123) and quality of life (90, 103, 124, 125) and changing pain perceptions (82, 89, 126). Moreover, psychological stress and anxiety symptoms were reported to be the most frequent causes of joint symptom exacerbation (127). In support of these clinical data, patients with RA and anxiety were found to have higher serum levels of IL-17 than those without AD (128).
Some authors have hypothesized that ADs in patients with RA are triggered by psychosocial factors, such as pronounced neuroticism, a lower educational level, and poor social support (69). According to this explanation, RA-associated disability in case of AD comorbidity is attributable more to psychological factors implicated in all chronic diseases than to the worsening of symptoms as a result of an increased pro-inflammatory state (129).
Proper AD treatment in patients with RA is essential to prevent functional decline (130) and poor adherence to treatment (131). Some drugs prescribed for RA, such as anti-TNF-α medications, seem to have beneficial effects on anxiety symptoms (112), and their discontinuation is related to the worsening of anxiety (132).
Autonomic Nervous System and Cardiovascular Manifestations in Rheumatoid Arthritis
Cardiovascular autonomic dysfunction in RA has been investigated by measuring heart rate (HR) and heart rate variability (HRV) (24). The link between the cardiovascular system and ANS imbalance in RA could partly explain the well-documented augmented cardiovascular disease and RA-related mortality, not fully justified by traditional risk factors.
It is well known how chronic inflammation influences the development of cardiovascular disease (CVD) by promoting atherosclerosis, myocardial remodeling, and insulin resistance and by modifying lipid levels and function and oxidative stress (133). Furthermore, multimodality imaging is useful to identify high-risk patients who benefit from preventive strategies or treatment intervention (134); the link between brain and heart damage in RA has been documented by magnetic resonance imaging (135).
Fundamentally, we have limited understanding of the mechanisms underpinning the connections between ANS and CVD. An extensive assessment of ANS by means of HR, cardiac/sympathetic baroreflex, and muscle sympathetic nerve activity (MSNA) was carried out in 30 RA patients (normo- and hypertensive) matched with a control group. Regardless the presence of hypertension, HR and sympathetic activity were increased and cardiac baroreflex sensitivity was reduced in RA patients, while sympathetic baroreflex sensitivity was preserved. Moreover, these findings were correlated with pain (VAS) and inflammation (CPR). Hence, a heightened sympathetic outflow was confirmed along with a reduced arterial baroreflex control of the heart both linked directly to RA symptoms, excluding the bias hypertension, a common cardiovascular risk factor (136).
Although data suggest that ANS imbalance is detrimental for cardiovascular disease, including cardiac arrhythmias, hypertension (137), and increased mortality (138, 139), breaking through the original trigger of this vicious circle with ultimate damage of the cardiovascular system is particularly difficult. Moreover, patients with RA without clinical cardiovascular disease have reduced left ventricular systolic function assessed by global longitudinal strain by speckle-tracking echocardiography, and it is related with disease activity (140).
Another study showed how a reduced coronary microvascular perfusion in RA patients, in terms of subendocardial viability ratio, associates with markers of disease activity and classical CVD risk factors, including heart rate. This reinforces the hypothesis that inflammation, by the stimulation of ANS, may impair myocardial perfusion (141).
Finally, according to previous literature, the evidence of cardiovascular autonomic dysfunction might be used to identify patients with RA who respond better to TNF inhibitors (25, 142). In particular, a low parasympathetic outflow and a high sympathetic outflow were associated with a poor anti-TNF response, making HRV a promising useful predictor of outcome, with great benefits for overall costs and quality-adjusted life-years (QALYs) (143).
Furthermore, cardiovascular autonomic imbalance associates with comorbidities including depressive and anxiety disturbances compared to healthy controls (144). Finally, looking at a therapeutic prospective, non-pharmacological intervention such as exercise training, given its well-known benefits on cardiovascular autonomic function, might help restore ANS imbalance, increasing vagal-related indices in RA with consequent potential benefit on pain and inflammation (145). Likewise, ANS imbalance could be partially restored by optimizing pain management (146).
Implications for Treatment and Future Perspectives
Altered ANS parameters have been associated with increased Disease Activity Score-28 (DAS-28), CRP, and erythrocyte sedimentation rate (25, 147). Seropositive RA patients are more prone to ANS dysfunction and more likely to experience the amelioration of cardiovascular autonomic neuropathy when treated with synthetic and biologic disease-modifying antirheumatic drugs (DMARDS) than seronegative patients with RA (148, 149). Thus, seropositivity, along with disease activity and pro-inflammatory cytokine levels, is predictive of autonomic dysfunction (150). Moreover, in patients with RA and ankylosing spondylitis, HRV was shown to predict the clinical response to anti-TNF therapy; specifically, patients with more vagal activity responded better to this treatment (142). Biologic, and to a lesser extent synthetic, DMARDs significantly improved autonomic neuropathy, including all of its parasympathetic, sympathetic, and sudomotor components, in patients with RA and ankylosing spondylitis (148).
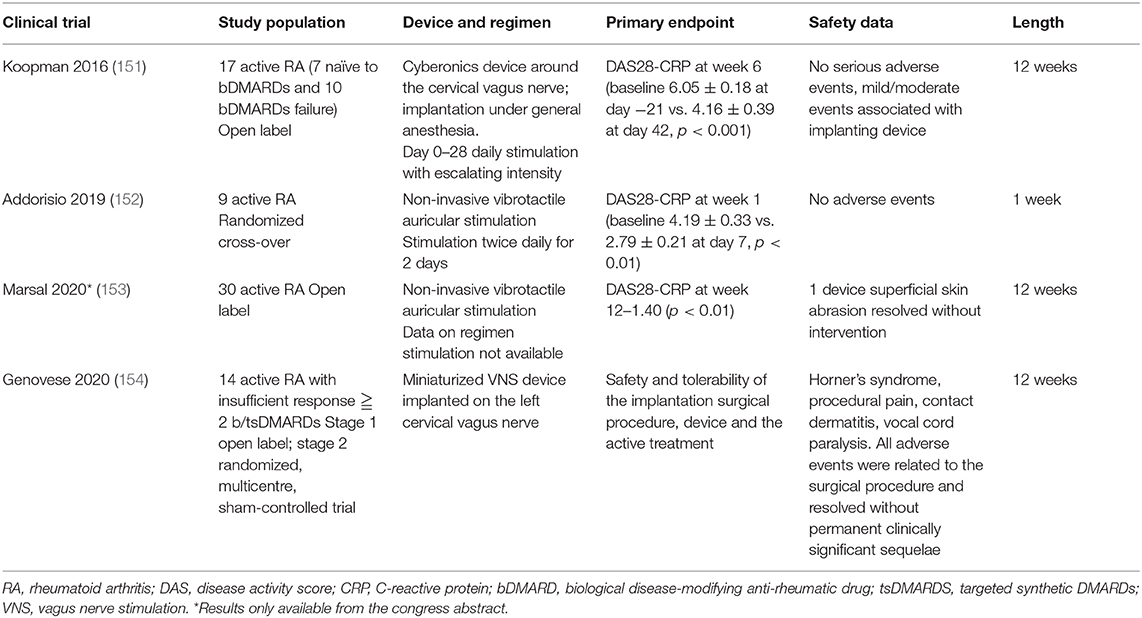
Multiple strategies can be used to achieve these goals. VNS can dampen inflammation early in a discrete and localized manner. Administration of the specific agonist of splenic α7nAChR acts on downstream of the pathway. Antagonization of the SNS is a possible alternative that should be explored. As shown in Table 1, four clinical trials examining VNS have been published (151–154), and one of these has been presented at the last EULAR e-congress (153). As they involved the use of different devices, modalities, timing, and stimulation sites, comparison of their results is (152) not feasible, but the researchers observed significant and rapid declines in the production of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1b after VNS in patients with epilepsy, those with RA, and healthy subjects (151, 152). In the first trial, VNS was performed directly via an electronic device (Cyberonics) implanted under general anesthesia. Three helical coiled cuffs around the vagus nerve and a lead were then tunneled subcutaneously from the neck and connected to a pulse generator placed in a subcutaneous pocket on the chest wall (151). The only adverse events reported were mild/moderate and related to the surgical implanting approach. In the most recent trial, a MicroRegulator device was implanted on the left cervical vagus nerve. The device was well tolerated without adverse events. The sample size was small, but VNS reduced disease activity in 50% of the highly drug refractory RA patients (154). In the other two trials, a non-invasive device consisting of a hand-held probe with a tip producing radial displacement in a circular pattern at the probe was used. Transcutaneous auricular VNS consisted of the application of electrical signals to the cutaneous territory supplied by the auricular branch of the vagus nerve at the cymba concha (152, 153). In all trials, disease activity was rapidly attenuated after VNS in patients with active RA (biologic-naïve subjects and those for whom multiple treatments had failed).
Considering the consistent percentage of patients who do not respond at all to the available drugs or have unsatisfactory responses, have gradual loss of responsiveness over time, or have drug-related adverse events, a non-pharmacological approach such as bioelectronics might be a useful further tool in the successful expansion of the therapeutic armamentarium of RA.
Bioelectronic medicine, based on neuromodulation of the nervous system restoring organ and immune system function, is a new potential tantalizing and promising field. In particular, the use of non-invasive devices has lesser adverse effects than drugs and has greater treatment adherence. Naturally, the therapeutic potential of immuno-autonomics has been further demonstrated, and the optimal neurostimulation parameters to achieve and maintain significant clinical changes are still unknown.
Conclusion
The innovative view of the ANS and RA axis described here allows for the development of new strategies for RA management. The ANS influences key aspects of joint pathophysiology and may be a target of novel therapeutic approaches. The emergence of immuno-autonomics and encouraging results of clinical trials could have extraordinary implications for RA monitoring and treatment. Further research in this field will be useful to understand the full potential of therapeutic models based on the brain–joint axis, with the integration of different findings.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This research was supported by the grant Bando Straordinario per Progetti Interdipartimentali (project code 1079) from the Università degli Studibreak di Milano.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. (2019) 78:1463–71. doi: 10.1136/annrheumdis-2019-215920
2. Cao X, Aballay A. Neural inhibition of dopaminergic signaling enhances immunity in a cell-non-autonomous manner. Curr Biol. (2016) 26:2398. doi: 10.1016/j.cub.2016.08.046
3. Aydemir M, Yazisiz V, Basarici I, Avci AB, Erbasan F, Belgi A, et al. Cardiac autonomic profile in rheumatoid arthritis and systemic lupus erythematosus. Lupus. (2010) 19:255–61. doi: 10.1177/0961203309351540
4. Stojanovich L, Milovanovich B, de Luka SR, Popovich-Kuzmanovich D, Bisenich V, Djukanovich B, et al. Cardiovascular autonomic dysfunction in systemic lupus, rheumatoid arthritis, primary Sjogren syndrome and other autoimmune diseases. Lupus. (2007) 16:181–5. doi: 10.1177/0961203306076223
5. Taylor PC, Holman AJ. Rheumatoid arthritis and the emergence of immuno-autonomics. Rheumatology. (2019) 58:2079–80. doi: 10.1093/rheumatology/key225
6. Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. (2011) 31:1409–17. doi: 10.1007/s00296-011-1999-3
7. Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. (2011) 334:98–101. doi: 10.1126/science.1209985
8. Kawashima K, Fujii T, Moriwaki Y, Misawa H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. (2012) 91:1027–32. doi: 10.1016/j.lfs.2012.05.006
9. Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. (2011) 334:101–5. doi: 10.1126/science.1210301
10. Li M, Shi J, Tang JR, Chen D, Ai B, Chen J, et al. Effects of complete Freund's adjuvant on immunohistochemical distribution of IL-1beta and IL-1R I in neurons and glia cells of dorsal root ganglion. Acta Pharmacol Sin. (2005) 26:192–8. doi: 10.1111/j.1745-7254.2005.00522.x
12. Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. (2003) 9:125–34. doi: 10.1007/BF03402177
13. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. (2000) 85:1–17. doi: 10.1016/S1566-0702(00)00215-0
14. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. (2000) 405:458–62. doi: 10.1038/35013070
15. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. (2003) 421:384–8. doi: 10.1038/nature01339
16. Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. (2004) 10:1216–21. doi: 10.1038/nm1124
17. Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, et al. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. (2009) 15:195–202. doi: 10.2119/molmed.2009.00039
18. Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. (2002) 195:781–8. doi: 10.1084/jem.20011714
19. van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. (2006) 130:1822–30. doi: 10.1053/j.gastro.2006.02.022
20. Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. Am J Physiol Gastrointest Liver Physiol. (2007) 293:G560–7. doi: 10.1152/ajpgi.00098.2007
21. Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. (2006) 131:1122–30. doi: 10.1053/j.gastro.2006.08.016
22. Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J Exp Med. (2014) 211:2583–98. doi: 10.1084/jem.20141132
23. Mina-Osorio P, Rosas-Ballina M, Valdes-Ferrer SI, Al-Abed Y, Tracey KJ, Diamond B. Neural signaling in the spleen controls B-cell responses to blood-borne antigen. Mol Med. (2012) 18:618–27. doi: 10.2119/molmed.2012.00027
24. Koopman FA, Tang MW, Vermeij J, de Hair MJ, Choi IY, Vervoordeldonk MJ, et al. Autonomic dysfunction precedes development of rheumatoid arthritis: a prospective cohort study. EBioMedicine. (2016) 6:231–7. doi: 10.1016/j.ebiom.2016.02.029
25. Adlan AM, Lip GY, Paton JF, Kitas GD, Fisher JP. Autonomic function and rheumatoid arthritis: a systematic review. Semin Arthrit Rheum. (2014) 44:283–304. doi: 10.1016/j.semarthrit.2014.06.003
26. Koopman FA, Stoof SP, Straub RH, Van Maanen MA, Vervoordeldonk MJ, Tak PP. Restoring the balance of the autonomic nervous system as an innovative approach to the treatment of rheumatoid arthritis. Mol Med. (2011) 17:937–48. doi: 10.2119/molmed.2011.00065
27. Koopman FA, van Maanen MA, Vervoordeldonk MJ, Tak PP. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J Intern Med. (2017) 282:64–75. doi: 10.1111/joim.12626
28. Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. (2004) 25:363–70. doi: 10.1016/j.ehj.2003.12.003
29. Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. (2007) 13:178–84. doi: 10.2119/2006-00112.Sloan
30. Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J Intern Med. (2009) 265:439–47. doi: 10.1111/j.1365-2796.2008.02023.x
31. Malave HA, Taylor AA, Nattama J, Deswal A, Mann DL. Circulating levels of tumor necrosis factor correlate with indexes of depressed heart rate variability: a study in patients with mild-to-moderate heart failure. Chest. (2003) 123:716–24. doi: 10.1378/chest.123.3.716
32. Vlcek M, Rovensky J, Blazicek P, Radikova Z, Penesova A, Kerlik J, et al. Sympathetic nervous system response to orthostatic stress in female patients with rheumatoid arthritis. Ann N Y Acad Sci. (2008) 1148:556–61. doi: 10.1196/annals.1410.026
33. Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, et al. Evidence for brain glial activation in chronic pain patients. Brain. (2015) 138(Pt 3):604–15. doi: 10.1093/brain/awu377
34. Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. (2002) 7:468–73. doi: 10.1038/sj.mp.4000995
35. Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. (2004) 56:819–24. doi: 10.1016/j.biopsych.2004.02.009
36. Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. (2006) 86:1009–31. doi: 10.1152/physrev.00049.2005
37. Yang X, Yang HB, Xie QJ, Liu XH, Hu XD. Peripheral inflammation increased the synaptic expression of NMDA receptors in spinal dorsal horn. Pain. (2009) 144:162–9. doi: 10.1016/j.pain.2009.04.005
38. Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. (2005) 135:659–78. doi: 10.1016/j.neuroscience.2005.03.051
39. Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. (2004) 155:119–26. doi: 10.1016/j.jneuroim.2004.06.009
40. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. (2006) 59:1116–27. doi: 10.1016/j.biopsych.2006.02.013
41. Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. (2008) 64:484–90. doi: 10.1016/j.biopsych.2008.04.016
42. Zimmerman G, Shaltiel G, Barbash S, Cohen J, Gasho CJ, Shenhar-Tsarfaty S, et al. Post-traumatic anxiety associates with failure of the innate immune receptor TLR9 to evade the pro-inflammatory NFkappaB pathway. Transl Psychiatry. (2012) 2:e78. doi: 10.1038/tp.2012.4
43. Wartolowska K, Hough MG, Jenkinson M, Andersson J, Wordsworth BP, Tracey I. Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum. (2012) 64:371–9. doi: 10.1002/art.33326
44. Chang L, Shoptaw S, Normand J. Brain abnormalities in HIV and stimulant users: interventions and prevention. J Food Drug Anal. (2013) 21:S7–9. doi: 10.1016/j.jfda.2013.09.021
45. Li Q, Cheung C, Wei R, Cheung V, Hui ES, You Y, et al. Voxel-based analysis of postnatal white matter microstructure in mice exposed to immune challenge in early or late pregnancy. Neuroimage. (2010) 52:1–8. doi: 10.1016/j.neuroimage.2010.04.015
46. Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. (2008) 28:1398–403. doi: 10.1523/JNEUROSCI.4123-07.2008
47. Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. (2012) 64:2398–403. doi: 10.1002/art.34412
48. Bruchfeld A, Goldstein RS, Chavan S, Patel NB, Rosas-Ballina M, Kohn N, et al. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J Intern Med. (2010) 268:94–101. doi: 10.1111/j.1365-2796.2010.02226.x
49. Grimsholm O, Rantapaa-Dahlqvist S, Dalen T, Forsgren S. Unexpected finding of a marked non-neuronal cholinergic system in human knee joint synovial tissue. Neurosci Lett. (2008) 442:128–33. doi: 10.1016/j.neulet.2008.06.082
50. Bennett FC, Molofsky AV. The immune system and psychiatric disease: a basic science perspective. Clin Exp Immunol. (2019) 197:294–307. doi: 10.1111/cei.13334
51. Jeppesen R, Benros ME. Autoimmune diseases and psychotic disorders. Front Psychiatry. (2019) 10:131. doi: 10.3389/fpsyt.2019.00131
52. Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci. (2019) 1437:57–67. doi: 10.1111/nyas.13712
53. el-Miedany YM, el-Rasheed AH. Is anxiety a more common disorder than depression in rheumatoid arthritis? Joint Bone Spine. (2002) 69:300–6. doi: 10.1016/S1297-319X(02)00368-8
54. VanDyke MM, Parker JC, Smarr KL, Hewett JE, Johnson GE, Slaughter JR, et al. Anxiety in rheumatoid arthritis. Arthritis Rheum. (2004) 51:408–12. doi: 10.1002/art.20474
55. Covic T, Cumming SR, Pallant JF, Manolios N, Emery P, Conaghan PG, et al. Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS). BMC Psychiatry. (2012) 12:6. doi: 10.1186/1471-244X-12-6
56. Sato E, Nishimura K, Nakajima A, Okamoto H, Shinozaki M, Inoue E, et al. Major depressive disorder in patients with rheumatoid arthritis. Mod Rheumatol. (2013) 23:237–44. doi: 10.3109/s10165-012-0643-8
57. Uguz F, Kucuk A, Aydogan S, Arslan S, Kurt HG, Toker A, et al. Is major depression associated with serum levels of tumor necrosis factor-alpha in patients with rheumatoid arthritis? J Psychosom Res. (2015) 79:530–2. doi: 10.1016/j.jpsychores.2015.09.011
58. Katz P, Margaretten M, Trupin L, Schmajuk G, Yazdany J, Yelin E. Role of sleep disturbance, depression, obesity, and physical inactivity in fatigue in rheumatoid arthritis. Arthritis Care Res. (2016) 68:81–90. doi: 10.1002/acr.22577
59. Cheon YH, Lee SG, Kim M, Kim HO, Sun Suh Y, Park KS, et al. The association of disease activity, pro-inflammatory cytokines, and neurotrophic factors with depression in patients with rheumatoid arthritis. Brain Behav Immun. (2018) 73:274–81. doi: 10.1016/j.bbi.2018.05.012
60. Kwiatkowska B, Klak A, Raciborski F, Maslinska M. The prevalence of depression and insomnia symptoms among patients with rheumatoid arthritis and osteoarthritis in Poland: a case control study. Psychol Health Med. (2019) 24:333–43. doi: 10.1080/13548506.2018.1529325
61. Milic V, Grujic M, Barisic J, Marinkovic-Eric J, Duisin D, Cirkovic A, et al. Personality, depression and anxiety in primary Sjogren's syndrome - Association with sociodemographic factors and comorbidity. PLoS ONE. (2019) 14:e0210466. doi: 10.1371/journal.pone.0210466
62. Nichter B, Norman S, Haller M, Pietrzak RH. Physical health burden of PTSD, depression, and their comorbidity in the U.S. veteran population: morbidity, functioning, and disability. J Psychosom Res. (2019) 124:109744. doi: 10.1016/j.jpsychores.2019.109744
63. Aurrecoechea E, Llorca Diaz J, Diez Lizuain ML, McGwin G Jr, Calvo-Alen J. Gender-associated comorbidities in rheumatoid arthritis and their impact on outcome: data from GENIRA. Rheumatol Int. (2017) 37:479–85. doi: 10.1007/s00296-016-3628-7
64. Hsieh MC, Hsu CW, Lu MC, Koo M. Increased risks of psychiatric disorders in patients with primary Sjogren's syndrome-a secondary cohort analysis of nationwide, population-based health claim data. Clin Rheumatol. (2019) 38:3195–203. doi: 10.1007/s10067-019-04705-z
65. Lok EY, Mok CC, Cheng CW, Cheung EF. Prevalence and determinants of psychiatric disorders in patients with rheumatoid arthritis. Psychosomatics. (2010) 51:338–e8. doi: 10.1176/appi.psy.51.4.338
66. Khongsaengdao B, Louthrenoo W, Srisurapanont M. Depression in Thai patients with rheumatoid arthritis. J Med Assoc Thai. (2000) 83:743–7.
67. Katon WJ, Buchwald DS, Simon GE, Russo JE, Mease PJ. Psychiatric illness in patients with chronic fatigue and those with rheumatoid arthritis. J Gen Int Med. (1991) 6:277–85. doi: 10.1007/BF02597420
68. Timonen M, Viilo K, Hakko H, Sarkioja T, Ylikulju M, Meyer-Rochow VB, et al. Suicides in persons suffering from rheumatoid arthritis. Rheumatology. (2003) 42:287–91. doi: 10.1093/rheumatology/keg082
69. Evers AW, Kraaimaat FW, Geenen R, Jacobs JW, Bijlsma JW. Longterm predictors of anxiety and depressed mood in early rheumatoid arthritis: a 3 and 5 year followup. J Rheumatol. (2002) 29:2327–36.
70. Covic T, Adamson B, Spencer D, Howe G. A biopsychosocial model of pain and depression in rheumatoid arthritis: a 12-month longitudinal study. Rheumatology. (2003) 42:1287–94. doi: 10.1093/rheumatology/keg369
71. Wang SL, Chang CH, Hu LY, Tsai SJ, Yang AC, You ZH. Risk of developing depressive disorders following rheumatoid arthritis: a nationwide population-based study. PLoS ONE. (2014) 9:e107791. doi: 10.1371/journal.pone.0107791
72. Ryu E, Chamberlain AM, Pendegraft RS, Petterson TM, Bobo WV, Pathak J. Quantifying the impact of chronic conditions on a diagnosis of major depressive disorder in adults: a cohort study using linked electronic medical records. BMC Psychiatry. (2016) 16:114. doi: 10.1186/s12888-016-0821-x
73. Kojima M, Kojima T, Suzuki S, Oguchi T, Oba M, Tsuchiya H, et al. Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Rheum. (2009) 61:1018–24. doi: 10.1002/art.24647
74. Kuriya B, Joshi R, Movahedi M, Rampakakis E, Sampalis JS, Bombardier C, et al. High disease activity is associated with self-reported depression and predicts persistent depression in early rheumatoid arthritis: results from the ontario best practices research initiative. J Rheumatol. (2018) 45:1101–8. doi: 10.3899/jrheum.171195
75. Hamed SA, Selim ZI, Elattar AM, Elserogy YM, Ahmed EA, Mohamed HO. Assessment of biocorrelates for brain involvement in female patients with rheumatoid arthritis. Clin Rheumatol. (2012) 31:123–32. doi: 10.1007/s10067-011-1795-1
76. Tylee DS, Sun J, Hess JL, Tahir MA, Sharma E, Malik R, et al. Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. Am J Med Genet Part B Neuropsychiatr Genet. (2018) 177:641–57. doi: 10.1002/ajmg.b.32652
77. Doeglas DM, Suurmeijer TP, van den Heuvel WJ, Krol B, van Rijswijk MH, van Leeuwen MA, et al. Functional ability, social support, and depression in rheumatoid arthritis. Qual Life Res. (2004) 13:1053–65. doi: 10.1023/B:QURE.0000031339.04589.63
78. Zyrianova Y, Kelly BD, Gallagher C, McCarthy C, Molloy MG, Sheehan J, et al. Depression and anxiety in rheumatoid arthritis: the role of perceived social support. Ir J Med Sci. (2006) 175:32–6. doi: 10.1007/BF03167946
79. Zagar I, Delimar V, Pap M, Peric D, Laktasic Zerjavic N, Peric P. Prevalence and correlation of depressive symptoms with functional scores, therapy and disease activity among croatian patients with rheumatoid arthritis: a preliminary study. Psychiatr Danub. (2018) 30:452–8. doi: 10.24869/psyd.2018.452
80. Cakirbay H, Bilici M, Kavakci O, Cebi A, Guler M, Tan U. Sleep quality and immune functions in rheumatoid arthritis patients with and without major depression. Int J Neurosci. (2004) 114:245–56. doi: 10.1080/00207450490269471
81. Melikoglu MA, Melikoglu M. The relationship between disease activity and depression in patients with Behcet disease and rheumatoid arthritis. Rheumatol Int. (2010) 30:941–6. doi: 10.1007/s00296-009-1080-7
82. Ryan S, McGuire B. Psychological predictors of pain severity, pain interference, depression, and anxiety in rheumatoid arthritis patients with chronic pain. Br J Health Psychol. (2016) 21:336–50. doi: 10.1111/bjhp.12171
83. Santos EF, Duarte CM, Ferreira RO, Pinto AM, Geenen R, da Silva JP. Multifactorial explanatory model of depression in patients with rheumatoid arthritis: a structural equation approach. Clin Exp Rheumatol. (2019) 37:641–8.
84. Dickens C, Jackson J, Tomenson B, Hay E, Creed F. Association of depression and rheumatoid arthritis. Psychosomatics. (2003) 44:209–15. doi: 10.1176/appi.psy.44.3.209
85. Mok CC, Lok EY, Cheung EF. Concurrent psychiatric disorders are associated with significantly poorer quality of life in patients with rheumatoid arthritis. Scand J Rheumatol. (2012) 41:253–9. doi: 10.3109/03009742.2012.664648
86. Soosova MS, Macejova Z, Zamboriova M, Dimunova L. Anxiety and depression in Slovak patients with rheumatoid arthritis. J Ment Health. (2017) 26:21–7. doi: 10.1080/09638237.2016.1244719
87. Garip Y, Eser F, Bodur H. Comorbidities in Turkish patients with rheumatoid arthritis: association with the health-related quality of life in terms of disease activity, functional and radiological status, severity of pain, and social and emotional functioning. Acta Reumatol Port. (2016) 41:344–9.
88. Zautra AJ, Parrish BP, Van Puymbroeck CM, Tennen H, Davis MC, Reich JW, et al. Depression history, stress, and pain in rheumatoid arthritis patients. J Behav Med. (2007) 30:187–97. doi: 10.1007/s10865-007-9097-4
89. Rogers HL, Brotherton HT, de Luis A, Olivera-Plaza SL, Cordoba-Patino AF, Pena-Altamar ML. Depressive symptoms are independently associated with pain perception in Colombians with rheumatoid arthritis. Acta Reumatol Port. (2015) 40:40–9.
90. Piccinni A, Maser JD, Bazzichi L, Rucci P, Vivarelli L, Del Debbio A, et al. Clinical significance of lifetime mood and panic-agoraphobic spectrum symptoms on quality of life of patients with rheumatoid arthritis. Compr Psychiatry. (2006) 47:201–8. doi: 10.1016/j.comppsych.2005.08.002
91. Jobski K, Kollhorst B, Garbe E, Schink T. The risk of ischemic cardio- and cerebrovascular events associated with oxycodone-naloxone and other extended-release high-potency opioids: a nested case-control study. Drug Saf. (2017) 40:505–15. doi: 10.1007/s40264-017-0511-8
92. Lee YC, Kremer J, Guan H, Greenberg J, Solomon DH. Chronic opioid use in rheumatoid arthritis: prevalence and predictors. Arthritis Rheumatol. (2019) 71:670–7. doi: 10.1002/art.40789
93. Dunne FJ, Dunne CA. Fibromyalgia syndrome and depression: common pathways. Br J Hosp Med. (2012) 73:211–7. doi: 10.12968/hmed.2012.73.4.211
94. Ingegnoli F, Schioppo T, Ubiali T, Ostuzzi S, Bollati V, Buoli M, et al. Patient perception of depressive symptoms in rheumatic diseases: a cross-sectional survey. J Clin Rheumatol. (2020). doi: 10.1097/RHU.0000000000001564. [Epub ahead of print].
95. Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Fitzgerald JD, Ranganath VK, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. (2012) 35:537–43. doi: 10.5665/sleep.1742
96. Cabrera-Marroquin R, Contreras-Yanez I, Alcocer-Castillejos N, Pascual-Ramos V. Major depressive episodes are associated with poor concordance with therapy in rheumatoid arthritis patients: the impact on disease outcomes. Clin Exp Rheumatol. (2014) 32:904–13.
97. Euesden J, Matcham F, Hotopf M, Steer S, Cope AP, Lewis CM, et al. The relationship between mental health, disease severity, and genetic risk for depression in early rheumatoid arthritis. Psychosom Med. (2017) 79:638–45. doi: 10.1097/PSY.0000000000000462
98. Liu YL, Szklo M, Davidson KW, Bathon JM, Giles JT. Differential association of psychosocial comorbidities with subclinical atherosclerosis in rheumatoid arthritis. Arthritis Care Res. (2015) 67:1335–44. doi: 10.1002/acr.22635
99. Scherrer JF, Virgo KS, Zeringue A, Bucholz KK, Jacob T, Johnson RG, et al. Depression increases risk of incident myocardial infarction among Veterans Administration patients with rheumatoid arthritis. Gen Hosp Psychiatry. (2009) 31:353–9. doi: 10.1016/j.genhosppsych.2009.04.001
100. Marrie RA, Walld R, Bolton JM, Sareen J, Patten SB, Singer A, et al. Psychiatric comorbidity increases mortality in immune-mediated inflammatory diseases. Gen Hosp Psychiatry. (2018) 53:65–72. doi: 10.1016/j.genhosppsych.2018.06.001
101. Nikiphorou E, de Lusignan S, Mallen C, Roberts J, Khavandi K, Bedarida G, et al. Prognostic value of comorbidity indices and lung diseases in early rheumatoid arthritis: a UK population-based study. Rheumatology. (2020) 59:1296–305. doi: 10.1093/rheumatology/kez409
102. Brandstetter S, Riedelbeck G, Steinmann M, Loss J, Ehrenstein B, Apfelbacher C. Depression moderates the associations between beliefs about medicines and medication adherence in patients with rheumatoid arthritis: cross-sectional study. J Health Psychol. (2018) 23:1185–95. doi: 10.1177/1359105316646440
103. Savka S. Improved quality of life of patients with rheumatoid arthritis and nonpsychotic mental disorders. Wiad Lek. (2019) 72:47–51. doi: 10.36740/WLek201901109
104. Smarr KL, Parker JC, Kosciulek JF, Buchholz JL, Multon KD, Hewett JE, et al. Implications of depression in rheumatoid arthritis: do subtypes really matter? Arthritis Care Res. (2000) 13:23–32. doi: 10.1002/1529-0131(200002)13:1<23::AID-ART5>3.0.CO;2-W
105. Hider SL, Tanveer W, Brownfield A, Mattey DL, Packham JC. Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatology. (2009) 48:1152–4. doi: 10.1093/rheumatology/kep170
106. Hewlett S, Ambler N, Almeida C, Cliss A, Hammond A, Kitchen K, et al. Self-management of fatigue in rheumatoid arthritis: a randomised controlled trial of group cognitive-behavioural therapy. Ann. Rheum Dis. (2011) 70:1060–7. doi: 10.1136/ard.2010.144691
107. Parker JC, Smarr KL, Slaughter JR, Johnston SK, Priesmeyer ML, Hanson KD, et al. Management of depression in rheumatoid arthritis: a combined pharmacologic and cognitive-behavioral approach. Arthritis Rheum. (2003) 49:766–77. doi: 10.1002/art.11459
108. Dhavale HS, Gawande S, Bhagat V, Durge V, Londhe V, Kini S, et al. Evaluation of efficacy and tolerability of dothiepin hydrochloride in the management of major depression in patients suffering from rheumatoid arthritis. J Indian Med Assoc. (2005) 103:291–4.
109. Slaughter JR, Parker JC, Martens MP, Smarr KL, Hewett JE. Clinical outcomes following a trial of sertraline in rheumatoid arthritis. Psychosomatics. (2002) 43:36–41. doi: 10.1176/appi.psy.43.1.36
110. Zautra AJ, Davis MC, Reich JW, Nicassario P, Tennen H, Finan P, et al. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J Consult Clin Psychol. (2008) 76:408–21. doi: 10.1037/0022-006X.76.3.408
111. Gautam S, Tolahunase M, Kumar U, Dada R. Impact of yoga based mind-body intervention on systemic inflammatory markers and co-morbid depression in active Rheumatoid arthritis patients: a randomized controlled trial. Restor Neurol Neurosci. (2019) 37:41–59. doi: 10.3233/RNN-180875
112. Uguz F, Akman C, Kucuksarac S, Tufekci O. Anti-tumor necrosis factor-alpha therapy is associated with less frequent mood and anxiety disorders in patients with rheumatoid arthritis. Psychiatry Clin Neurosci. (2009) 63:50–5. doi: 10.1111/j.1440-1819.2008.01905.x
113. Sun Y, Wang D, Salvadore G, Hsu B, Curran M, Casper C, et al. The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric Castleman's disease. Brain Behav Immun. (2017) 66:156–64. doi: 10.1016/j.bbi.2017.06.014
114. Genty M, Combe B, Kostine M, Ardouin E, Morel J, Lukas C. Improvement of fatigue in patients with rheumatoid arthritis treated with biologics: relationship with sleep disorders, depression and clinical efficacy. A prospective, multicentre study. Clin Exp Rheumatol. (2017) 35:85–92.
115. Bottomley JM, LeReun C, Diamantopoulos A, Mitchell S, Gaynes BN. Vagus nerve stimulation (VNS) therapy in patients with treatment resistant depression: a systematic review and meta-analysis. Compr Psychiatry. (2019) 98:152156. doi: 10.1016/j.comppsych.2019.152156
116. Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. (2018) 11:203–13. doi: 10.2147/JIR.S163248
117. Isik A, Koca SS, Ozturk A, Mermi O. Anxiety and depression in patients with rheumatoid arthritis. Clin Rheumatol. (2007) 26:872–8. doi: 10.1007/s10067-006-0407-y
118. Lisitsyna TA, Veltishchev DY, Seravina OF, Kovalevskaya OB, Starovoytova MN, Desinova OV. Comparative analysis of anxiety-depressive spectrum disorders in patients with rheumatic diseases. Ter Arkh. (2018) 90:30–7. doi: 10.26442/terarkh201890530-37
119. Hassan AA, Nasr MH, Mohamed AL, Kamal AM, Elmoghazy AD. Psychological affection in rheumatoid arthritis patients in relation to disease activity. Medicine. (2019) 98:e15373. doi: 10.1097/MD.0000000000015373
120. Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, et al. Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol Psychiatr Sci. (2019) 28:333–42. doi: 10.1017/S2045796017000579
121. Watad A, Bragazzi NL, Adawi M, Aljadeff G, Amital H, Comaneshter D, et al. Anxiety disorder among rheumatoid arthritis patients: insights from real-life data. J Affect Disord. (2017) 213:30–4. doi: 10.1016/j.jad.2017.02.007
122. Reinhorn IM, Bernstein CN, Graff LA, Patten SB, Sareen J, Fisk JD, et al. Social phobia in immune-mediated inflammatory diseases. J Psychosom Res. (2020) 128:109890. doi: 10.1016/j.jpsychores.2019.109890
123. Stebbings S, Herbison P, Doyle TC, Treharne GJ, Highton J. A comparison of fatigue correlates in rheumatoid arthritis and osteoarthritis: disparity in associations with disability, anxiety and sleep disturbance. Rheumatology. (2010) 49:361–7. doi: 10.1093/rheumatology/kep367
124. Mangelli L, Gribbin N, Buchi S, Allard S, Sensky T. Psychological well-being in rheumatoid arthritis: relationship to 'disease' variables and affective disturbance. Psychother Psychosom. (2002) 71:112–6. doi: 10.1159/000049354
125. Karimi S, Yarmohammadian MH, Shokri A, Mottaghi P, Qolipour K, Kordi A, et al. Predictors and effective factors on quality of life among Iranian patients with rheumatoid arthritis. Mater Sociomed. (2013) 25:158–62. doi: 10.5455/msm.2013.25.158-162
126. Esen SA, Karabulut Y, Esen I, Atmis V. Effects of the Disease Characteristics and the treatment on psychological status in patients with rheumatoid arthritis and ankylosing spondylitis. Curr Rheumatol Rev. (2018) 14:271–8. doi: 10.2174/1573397113666170728123518
127. Yilmaz V, Umay E, Gundogdu I, Karaahmet ZO, Ozturk AE. Rheumatoid arthritis: are psychological factors effective in disease flare? Eur J Rheumatol. (2017) 4:127–32. doi: 10.5152/eurjrheum.2017.16100
128. Liu Y, Ho RC, Mak A. The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis. (2012) 15:183–7. doi: 10.1111/j.1756-185X.2011.01673.x
129. Murphy H, Dickens C, Creed F, Bernstein R. Depression, illness perception and coping in rheumatoid arthritis. J Psychosom Res. (1999) 46:155–64. doi: 10.1016/S0022-3999(98)00073-7
130. Whitehouse CE, Fisk JD, Bernstein CN, Berrigan LI, Bolton JM, Graff LA, et al. Comorbid anxiety, depression, and cognition in MS and other immune-mediated disorders. Neurology. (2019) 93:1081–2. doi: 10.1212/WNL.0000000000006854
131. Adina TS, Mihaela-Simona S, Lucia CP, Stefan Cristian D, Maria B, Lili BA, et al. The influence of socio-demographic factors, lifestyle and psychiatric indicators on adherence to treatment of patients with rheumatoid arthritis: a cross-sectional study. Medicina. (2020) 56:178–192. doi: 10.3390/medicina56040178
132. Mattey DL, Dawes PT, Hassell AB, Brownfield A, Packham JC. Effect of psychological distress on continuation of anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. J Rheumatol. (2010) 37:2021–4. doi: 10.3899/jrheum.100050
133. Urman A, Taklalsingh N, Sorrento C, McFarlane IM. Inflammation beyond the joints: rheumatoid arthritis and cardiovascular disease. Scifed J Cardiol. (2018) 2:1000019.
134. Mavrogeni S, Dimitroulas T, Sfikakis PP, Kitas GD. Heart involvement in rheumatoid arthritis: multimodality imaging and the emerging role of cardiac magnetic resonance. Semin Arthritis Rheum. (2013) 43:314–24. doi: 10.1016/j.semarthrit.2013.05.001
135. Markousis-Mavrogenis G, Koutsogeorgopoulou L, Dimitroulas T, Katsifis G, Vartela V, Mitsikostas D, et al. Is there a brain/heart interaction in rheumatoid arthritis and seronegative spondyloartropathies? A combined brain/heart magnetic resonance imaging reveals the answer. Curr Rheumatol Rep. (2020) 22:39. doi: 10.1007/s11926-020-00922-7
136. Adlan AM, Paton JF, Lip GY, Kitas GD, Fisher JP. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J Physiol. (2017) 595:967–81. doi: 10.1113/JP272944
137. Fisher JP, Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens. (2012) 26:463–75. doi: 10.1038/jhh.2011.66
138. Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. (2009) 135:302–7. doi: 10.1016/j.ijcard.2008.03.056
139. Panoulas VF, Toms TE, Douglas KM, Sandoo A, Metsios GS, Stavropoulos-Kalinoglou A, et al. Prolonged QTc interval predicts all-cause mortality in patients with rheumatoid arthritis: an association driven by high inflammatory burden. Rheumatology. (2014) 53:131–7. doi: 10.1093/rheumatology/ket338
140. Hanvivadhanakul P, Buakhamsri A. Disease activity is associated with LV dysfunction in rheumatoid arthritis patients without clinical cardiovascular disease. Adv Rheumatol. (2019) 59:56. doi: 10.1186/s42358-019-0100-x
141. Sandoo A, Protogerou AD, Hodson J, Smith JP, Zampeli E, Sfikakis PP, et al. The role of inflammation, the autonomic nervous system and classical cardiovascular disease risk factors on subendocardial viability ratio in patients with RA: a cross-sectional and longitudinal study. Arthritis Res Ther. (2012) 14:R258. doi: 10.1186/ar4103
142. Holman AJ, Ng E. Heart rate variability predicts anti-tumor necrosis factor therapy response for inflammatory arthritis. Auton Neurosci. (2008) 143:58–67. doi: 10.1016/j.autneu.2008.05.005
143. Zimmermann M, Vodicka E, Holman AJ, Garrison LP Jr. Heart rate variability testing: could it change spending for rheumatoid arthritis patients in the United States? An exploratory economic analysis. J Med Econ. (2018) 21:712–20. doi: 10.1080/13696998.2018.1470519
144. Hu MX, Milaneschi Y, Lamers F, Nolte IM, Snieder H, Dolan CV, et al. The association of depression and anxiety with cardiac autonomic activity: the role of confounding effects of antidepressants. Depress Anxiety. (2019) 36:1163–72. doi: 10.1002/da.22966
145. Pecanha T, Lima AH. Inflammation and cardiovascular autonomic dysfunction in rheumatoid arthritis: a bidirectional pathway leading to cardiovascular disease. J Physiol. (2017) 595:1025–6. doi: 10.1113/JP273649
146. Fazalbhoy A, Birznieks I, Macefield VG. Individual differences in the cardiovascular responses to tonic muscle pain: parallel increases or decreases in muscle sympathetic nerve activity, blood pressure and heart rate. Exp Physiol. (2012) 97:1084–92. doi: 10.1113/expphysiol.2012.066191
147. Lazzerini PE, Acampa M, Capecchi PL, Hammoud M, Maffei S, Bisogno S, et al. Association between high sensitivity C-reactive protein, heart rate variability and corrected QT interval in patients with chronic inflammatory arthritis. Eur J Intern Med. (2013) 24:368–74. doi: 10.1016/j.ejim.2013.02.009
148. Syngle A, Verma I, Krishan P, Garg N, Syngle V. Disease-modifying anti-rheumatic drugs improve autonomic neuropathy in arthritis: DIANA study. Clin Rheumatol. (2015) 34:1233–41. doi: 10.1007/s10067-014-2716-x
149. Sandhu V, Allen SC. The effects of age, seropositivity and disease duration on autonomic cardiovascular reflexes in patients with rheumatoid arthritis. Int J Clin Pract. (2004) 58:740–5. doi: 10.1111/j.1368-5031.2004.00210.x
150. Syngle V, Syngle A, Garg N, Krishan P, Verma I. Predictors of autonomic neuropathy in rheumatoid arthritis. Auton Neurosci. (2016) 201:54–9. doi: 10.1016/j.autneu.2016.07.008
151. Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. (2016) 113:8284–9. doi: 10.1073/pnas.1605635113
152. Addorisio ME, Imperato GH, de Vos AF, Forti S, Goldstein RS, Pavlov VA, et al. Investigational treatment of rheumatoid arthritis with a vibrotactile device applied to the external ear. Bioelectron Med. (2019) 5:4. doi: 10.1186/s42234-019-0020-4
153. Marsal S, Corominas H, Lopez Lasanta M, Reina-Sanz D, Perez-Garcia C, Borrell Paños H, et al. Pilot clinical study of a non-invasive auricular vagus nerve stimulation device in patients with rheumatoid arthritis. Ann Rheum Dis. (2020) 79:999. doi: 10.1136/annrheumdis-2020-eular.3315
154. Genovese MC, Gaylis NB, Sikes D, Kivitz A, Horowitz DL, Peterfy C, et al. Safety and efficacy of neurostimulation with a miniaturised vagus nerve stimulation device in patients with multidrug-refractory rheumatoid arthritis: a two-stage multicentre, randomised pilot study. Lancet Rheumatol. (2020) 2:e527–38. doi: 10.1016/S2665-9913(20)30172-7
Keywords: vagus nerve, central nervous system, autonomic nervous system, mood disorder, rheumatoid arthritis, depression, therapy
Citation: Ingegnoli F, Buoli M, Antonucci F, Coletto LA, Esposito CM and Caporali R (2020) The Link Between Autonomic Nervous System and Rheumatoid Arthritis: From Bench to Bedside. Front. Med. 7:589079. doi: 10.3389/fmed.2020.589079
Received: 30 July 2020; Accepted: 30 October 2020;
Published: 07 December 2020.
Edited by:
Helena Canhao, New University of Lisbon, PortugalReviewed by:
Theodoros Dimitroulas, Aristotle University of Thessaloniki, GreeceJose Inciarte-Mundo, Hospital Clínic de Barcelona, Spain
Copyright © 2020 Ingegnoli, Buoli, Antonucci, Coletto, Esposito and Caporali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Ingegnoli, ZnJhbmNlc2NhLmluZ2Vnbm9saUB1bmltaS5pdA== orcid.org/0000-0002-6727-1273
†These authors have contributed equally to this work
 Francesca Ingegnoli
Francesca Ingegnoli Massimiliano Buoli
Massimiliano Buoli Flavia Antonucci
Flavia Antonucci Lavinia Agra Coletto
Lavinia Agra Coletto Cecilia Maria Esposito
Cecilia Maria Esposito Roberto Caporali
Roberto Caporali