
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 28 October 2020
Sec. Hematology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.588526
This article is part of the Research TopicThrombotic Microangiopathies, Diagnostic and Therapeutic AdvancesView all 11 articles
 Mouhamed Yazan Abou-Ismail1,2*
Mouhamed Yazan Abou-Ismail1,2* Yasmin Arafah1,2
Yasmin Arafah1,2 Pingfu Fu1
Pingfu Fu1 Shufen Cao1
Shufen Cao1 Alvin H. Schmaier1,2
Alvin H. Schmaier1,2 Lalitha Nayak1,2
Lalitha Nayak1,2Background: Immune thrombotic thrombocytopenic purpura (iTTP) is a rare, life-threatening disorder managed with plasma exchange (PLEX) and steroids. Addition of rituximab (RTX) to initial disease treatment has been shown to lower future relapse rates. Information as to whether upfront cyclophosphamide (CTX) treatment is helpful in reducing relapse is not known.
Methods: In a retrospective cohort study, we identified all patients at our institution diagnosed with iTTP between 2010 and 2019. We analyzed outcomes of cumulative incidence of relapse (CIR) and duration of remission.
Results: Thirty Nine patients were studied. Group A (n = 10) included patients who received upfront PLEX and steroids alone, and Group B (n = 28) included those who received either upfront RTX (n = 23) or CTX (n = 5) in addition to PLEX and steroids. The 2-year CIR was 50% in Group A and 27.7% in Group B, with a median duration of remission of 43.6 months vs. 108.3 months, respectively (p = 0.04). Group A was associated with a HR=8.7 (95% CI: 1.27, 59.45), p = 0.027 for duration of remission. There was no significant difference between CTX and RTX in both outcomes of CIR and duration of remission. We observed a potential impact on remission duration based on the presenting absolute neutrophil count (HR = 0.74, 95% CI: 0.58, 0.96) and serum creatinine (HR = 1.42, 95% CI: 1.03, 1.94).
Conclusion: There was no significant difference in iTTP relapse outcomes between upfront RTX and CTX. Absolute neutrophil count and serum creatinine may have a role in predicting relapse. Larger, prospective studies are needed to evaluate these findings.
Auto-immune thrombotic thrombocytopenic purpura (iTTP) is a rare, life-threatening disorder caused by auto-antibodies against ADAMTS13. It is characterized by a severe thrombotic microangiopathy (TMA) that leads to organ failure and is associated with high morbidity and mortality. The mortality rate of the untreated disease is around 90%, and treatment with corticosteroids and therapeutic plasma exchange (PLEX) reduces that rate to around 10%. (1, 2) Although this treatment induces remission, disease relapse remains a common problem. Relapse is estimated to occur in around 30–50% of patients after achieving initial remission (3, 4). Since iTTP is mediated by an antibody against ADAMTS13, additional immunosuppressive therapy given upfront has been shown to lower relapse rates (5–9).
Rituximab (RTX) is a monoclonal antibody that targets B-cells, which produce the antibody responsible for causing iTTP. It was first used in treating this disease in the relapsed or refractory setting in the early 2000s, and was successful in inducing remission (10, 11). The Phase II trials by Scully et al. and Chen et al. demonstrated safety and efficacy of using upfront RTX (8, 12). In the former trial, RTX was associated with 10% relapse rate compared to 57% in historical controls, which was a statistically significant reduction (8). It has since been used in the upfront setting in various studies and shown to lower relapse rates (5–9). While recent common practice has shifted toward adding RTX to steroids and PLEX as front-line treatment for acute initial iTTP, this practice has not been rigorously examined.
An alternative therapy, cyclophosphamide (CTX), has been used in relapsed iTTP. CTX is effectively used in the management of acquired hemophilia that is caused by auto-antibodies to factor VIII (13, 14). In the European Acquired Hemophilia Registry, steroids combined with cyclophosphamide resulted in more stable complete remission (70%) than rituximab-based regimens (59%) (14). Several small studies have utilized cyclophosphamide in the treatment of relapsed or refractory iTTP where it has been shown to be effective (15–19). However, studies evaluating CTX use in the upfront setting as a first-line treatment along with PLEX and corticosteroids are lacking. There are several advantages to the use of CTX. First, many patients develop infusion reactions to RTX or have other contraindicating comorbidities that may preclude its use upfront. Furthermore, if given concurrently with PLEX, ~65% of RTX may be removed making the optimal dose and frequency of this drug difficult to determine (20). Alternatively, CTX is unlikely to be removed by PLEX due to its low protein-binding rate of 23%, and higher volume of distribution of 0.8 L/kg (21). Furthermore, CTX is far less costly than RTX. As such, having an alternative agent for use in the upfront setting may be beneficial. In this retrospective clinical review, we hypothesized that CTX is as effective as RTX in lowering relapse rate when used in the upfront setting for initial acute iTTP. We compared outcomes of cumulative incidence of relapse (CIR) as well as duration of remission between patients who received upfront CTX and those who received RTX, in addition to PLEX and steroids. Subsequently, in order to evaluate whether there was benefit in the use of any additional immunosuppression besides PLEX and steroids, we compared the cohort of patients who received either additional CTX or RTX with those who received PLEX and steroids alone without any additional immunosuppression. This retrospective investigation also examined several baseline characteristics on initial disease presentation and assessed whether they correlated with a higher risk of relapse.
In a retrospective chart review, we identified all patients diagnosed with iTTP at University Hospitals—Cleveland Medical Center between 2010 and 2019. The study was approved by the Institutional Review Board (IRB). A billing diagnosis code search for “thrombotic microangiopathy” (ICD9: 446.6, ICD10: M31.1) was conducted. We included all patients diagnosed with iTTP, defined as having an ADAMTS13 level of <10% and a positive ADAMTS13 inhibitor or antibody, with clinical evidence of TMA per provider documentation. We excluded patients with a follow-up duration of <30 days, or with TMA due to other causes such as congenital TTP, hemolytic-uremic syndrome, anti-phospholipid syndrome, HELLP syndrome, systemic lupus erythematosus, preeclampsia, accelerated hypertension, and diffuse intravascular coagulopathy. The ADAMTS13 assays were performed at the Blood Center of Wisconsin, measured by Fluorescence Resonance Energy Transfer with a synthetic substrate. Inhibitor assays were performed on those patients with low ADAMTS13 levels.
We divided our cohort into four groups (Figure 1). Group A included patients who received steroids and PLEX alone, without any additional upfront therapy. Group B included patients who received steroids, PLEX, in addition to either RTX or CTX. The latter group was further divided into RTX group and CTX group to compare the effects of these two agents. For our secondary objective of predictors of relapse, we utilized the collective data from the entire cohort (all groups combined).
We defined the “onset of remission” as the date after which the platelet count was greater than or equal to 150,000 /μl and lactate dehydrogenase (LDH) ≤ 246 U/L for a minimum of 48 h. Clinical relapse was defined as recurrence of TMA in addition to ADAMTS13 level of <10%. The “duration of remission” was measured from the date of onset of remission, until the date of first clinical relapse of TTP. For patients who are alive and did not relapse, the end point was the date of last-follow-up.
The cumulative incidence of relapse (CIR) was estimated using Kaplan-Meier method (22) and its difference among treatment groups was examined by log-rank test. The effect of continuous and categorical covariates on thrombotic thrombocytopenic purpura relapse rate was estimated by univariate Cox model (23). The association of categorical variables and continuous variables was examined using chi-square test and Pearson correlation coefficient, respectively, and the difference of continuous measurements among groups was tested using T-test (two groups) or ANOVA (more than 2 groups). The effect of continuous and categorical covariates on time to eradication of inhibitor (from diagnosis time) was estimated by univariate and multivariable linear regression. The effect of continuous and categorical covariates on days of hospitalization duration was also estimated by univariate and multivariable linear regression. All tests are two-sided and p ≤ 0.05 was considered statistically significant.
A total of 39 patients met the inclusion and exclusion criteria. One patient received mycophenolate mofetil, and was not included in the primary analysis of CIR, but was included in the secondary objective of the study on laboratory predictors of relapse. Ten patients received steroids and PLEX alone (Group A), and 28 patients received steroids, PLEX, and either RTX or CTX (Group B). Of those, 23 received RTX (375 mg/m2 weekly for four doses), and five received CTX (400 mg/m2 every 3 weeks for six doses). The average age of diagnosis was 44 years. The average duration of follow-up was 76 months. There were no significant differences in baseline characteristics between any of the groups (Table 1). Upfront administration of CTX was not associated with any significant side effects in the five treated patients.
The median duration of remission in Group A vs. Group B was 43.6 months (95% CI: 0.5, 114.3) and 108.3 months (95% CI: 36.8, N/A) respectively (p = 0.04). The CIR at 48 months in Group A was 50 vs. 27.7% (CTX = 33.3%, RTX = 27.2%) in Group B (Table 2) In the multivariable analysis, Group A was associated with a statistically significant hazard ratio of 8.7 (95% CI: 1.27, 59.45), p = 0.027 for time to clinical relapse, compared to Group B. There was no statistically significant difference in CIR between RTX and CTX in the Kaplan–Meier analysis (Figure 2, Table 2).
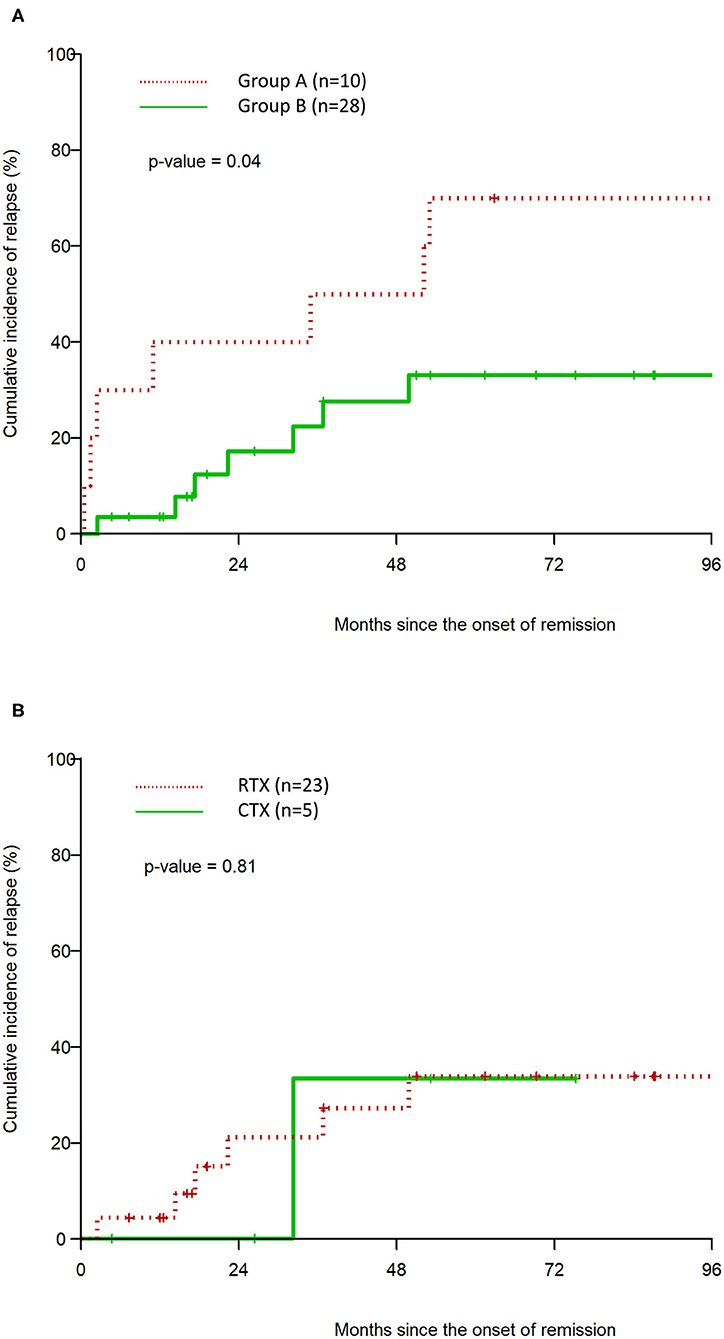
Figure 2. Kaplan–Meier estimation of cumulative incidence of clinical relapse after remission for Group A vs. Group B (A), and CTX vs. RTX (B).
The Cox regression analysis of the effect of initial inhibitor level, absolute neutrophil count (ANC), nadir platelet count, and serum creatinine on the outcome of duration of remission is summarized below, and highlighted in Table 3. The analysis on all other variables, including age, absolute lymphocyte count, neutrophil/lymphocyte ratio, peak LDH on admission, fibrinogen, D-Dimer, presence of autoimmune disease, and presence of malignancy did not show a statistically significant impact on duration of remission. Similarly, the time to initiation of CTX or RTX from the time of disease diagnosis did not demonstrate an effect on the duration of remission.
Although the initial inhibitor level showed a trend toward an increased risk of clinical relapse per unit increase the results did not meet statistical significance in the univariate analysis (HR: 1.16, 95% CI: 0.95, 1.44), p = 0.17. However, this effect was amplified after controlling for type of upfront therapy in the multivariable analysis (HR: 1.23, 95% CI: 0.97, 1.56), p = 0.084.
A higher ANC on presentation was associated with a statistically significantly longer duration of remission per unit increase. This effect maintained statistical significance in the multivariable analysis, with an HR of 0.74 (95% CI: 0.58, 0.96). Also, a Kaplan-Meier graph of a dichotomized separation of high or low ANC (above or below the median of 7.6 × 109/L) shows a statistically significant decrease in the risk of CIR in the high ANC group (Figure 3A), with p = 0.015. Although the average ANC between the groups was not different, within the entire cohort there is a gradient of ANC values. Those ANC values >7.6 × 109/L demonstrate the improved CIR finding.
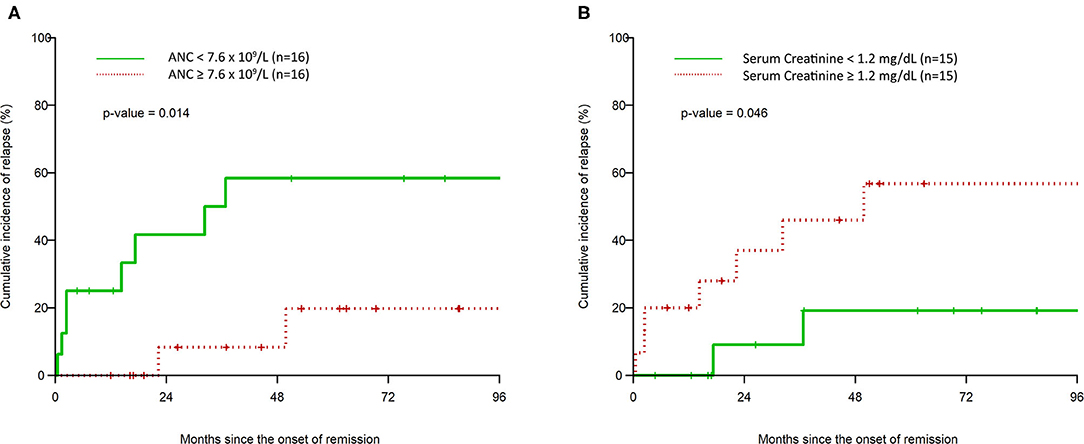
Figure 3. Kaplan–Meier estimation of cumulative incidence of clinical relapse after remission based on high vs. low initial absolute neutrophil count (A) and high vs. low initial serum creatinine (B).
The nadir platelet count on presentation was not associated with a statistically significant change in time to clinical relapse, with HR of 0.98 (0.90, 1.06), p-value: 0.60.
The serum creatinine level on presentation was associated with a decreased time to clinical relapse per unit increase, which was statistically significant in the multivariable analysis (HR: 1.42, 95% CI: 1.03, 1.94). A Kaplan–Meier graph of a dichotomized separation of high or low serum creatinine (above or below the median of 1.2 mg/dL) shows a statistically significant increase in the risk of CIR in the high serum creatinine group (Figure 3B), with p = 0.046.
Our study results demonstrate that the upfront use of immunosuppressive therapy, in addition to steroids and PLEX is associated with a reduction in the cumulative incidence of relapse and prolongs the duration of remission. The median duration of remission was significantly higher in Group B (108.3 months) compared to steroids and PLEX alone in Group A (43.6 months) with a HR of 8.7 (95% CI: 1.27, 59.45) after controlling for baseline characteristics in the multivariable model. Consistent with previously reported data, these findings demonstrate that the addition of immunosuppressive therapy is associated with a longer initial remission in iTTP. Our data is derived from a combined cohort of patients who received either CTX or RTX and demonstrates the benefit of any additional upfront immunosuppression besides PLEX and steroids in this disease. The sample size in the RTX and CTX groups individually was too small to demonstrate a statistically significant difference in outcomes in either group.
We then compared the outcomes between those who received RTX or CTX upfront. The use of upfront RTX has been shown in several studies to lower relapse rates (6–9). CTX, on the other hand, has not been studied in the upfront setting, as opposed to relapsed or refractory disease. While our sample size is small, the Kaplan–Meier curves showed clear overlap and no statistically significant changes in relapse outcomes in patients who received upfront CTX as compared to RTX. However, in order to achieve 80% power to detect a difference, the required total sample size is 308 patients. Nevertheless, our data demonstrate that it would be beneficial to evaluate the use of upfront CTX in larger, prospective studies. Low-dose pulse intravenous CTX used for iTTP is associated with far less adverse effects compared to higher doses of CTX, which can lead to infectious complications, bone marrow suppression, and long-term risk for malignancy (24). The risks with low-dose CTX are outweighed in the context of preventing relapse of life-threatening iTTP.
The second goal of our study was to investigate baseline characteristics that may help predict the risk of relapse. Identification of risk factors predictive of relapse would make a stronger argument for adding an upfront immunosuppressive agent, or using more aggressive therapy at diagnosis. A previous study by Tuncer et al. demonstrated that male sex, severe thrombocytopenia, and higher LDH pre-/post-treatment ratio were associated with higher risk of relapse (3). However, from all the different baseline characteristics we analyzed, we identified three different variables that may impact relapse outcomes: inhibitor level, ANC, and serum creatinine.
The inhibitor level on presentation did not show a statistically significant effect on duration of remission in the univariate analysis. However, when we controlled for the type of therapy used (Group A vs. Group B), its effect was amplified and trended closer toward statistical significance. This suggests that with larger study samples, this effect may be more pronounced. Of note, there are limitations in the inhibitor assay that we use since it does not report levels above 8 arbitrary units. Unlike previously reported data, our study did not demonstrate a statistically significant impact of nadir platelet count on relapse risk (3). Further, we found that a higher serum creatinine was associated with a shorter duration of remission and a statistically significant separation in the Kaplan–Meier analysis for CIR. We suspect that this may reflect a more aggressive nature of the disease and its microvascular complications, which may also translate into a higher tendency for clinical relapse.
We proposed that an elevated ANC may correlate with higher risk of relapse. However, contrary to our hypothesis, we found that a higher ANC was actually associated with a longer duration of remission. The protective effect of a higher ANC maintains statistical significance in the multivariable analysis after controlling for all other characteristics and is statistically significant in the Kaplan-Meier separation. At present, the mechanistic basis of this observation is not known. There is emerging evidence in the literature that neutrophils may inhibit B-cell responses, especially in the context of autoimmune diseases (25). In murine models, data suggest a role for neutrophils in suppressing immunoglobulin and antibody production in B lymphocytes, as well as slowing disease progression of murine lupus (26–28). There is also evidence that such an effect may occur in humans as well. A study by Lelis et al. has shown that myeloid derived suppressor cells, which may function as pathologically activated neutrophils, have a role in modulating B cell responses by suppressing B-cell proliferation and antibody production (29). Since iTTP is also an autoimmune disease caused by auto-antibodies against ADAMTS13, a modulatory effect on the B cell production of antibodies probably would correlate with a more durable remission.
Our study is the first to examine outcomes in a subset of patients treated with upfront CTX therapy for initial iTTP, and suggests similar time to first relapse and 4-year CIR as compared to RTX. Although our numbers are small, our institutional experience suggests that CTX may be considered as alternative therapy in patients intolerant to RTX. Consistent with the currently published literature, PLEX and steroid therapy alone was associated with a significantly shorter duration of remission compared to additional immunosuppressive therapy. Initial inhibitor level, serum creatinine, and ANC may offer a predictive role in the risk for disease relapse. The results of these investigations indicate that there is room for more diverse approaches to the management of iTTP. Larger prospective studies are warranted to confirm these observations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MA-I and YA collected the data. MA-I, AHS, and LN wrote the manuscript. AHS and LN supervised and mentored the study. All authors contributed to the article and approved the submitted version.
LN was supported by HL142647, BGT670026, and HL143402. AHS was supported in-part by Department of Army BC150596P1, HL126645, NIH grants AI130131, HL144113, HL143402, CA223301 and a Shire Investigator-Initiated Support grant.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian apheresis study group. N Engl J Med. (1991) 325:393–7. doi: 10.1056/NEJM199108083250604
2. Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. (1991) 325:398–403. doi: 10.1056/NEJM199108083250605
3. Tuncer HH, Oster RA, Huang ST, Marques MB. Predictors of response and relapse in a cohort of adults with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: a single-institution experience. Transfusion. (2007) 47:107–14. doi: 10.1111/j.1537-2995.2007.01071.x
4. Vesely SK, George JN, Lammle B, Studt JD, Alberio L, El-Harake MA, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. (2003) 102:60–8. doi: 10.1182/blood-2003-01-0193
5. Tsai HM, Shulman K. Rituximab induces remission of cerebral ischemia caused by thrombotic thrombocytopenic purpura. Eur J Haematol. (2003) 70:183–5. doi: 10.1034/j.1600-0609.2003.00026.x
6. Lim W, Vesely SK, George JN. The role of rituximab in the management of patients with acquired thrombotic thrombocytopenic purpura. Blood. (2015) 125:1526–31. doi: 10.1182/blood-2014-10-559211
7. Westwood JP, Thomas M, Alwan F, McDonald V, Benjamin S, Lester WA, et al. Rituximab prophylaxis to prevent thrombotic thrombocytopenic purpura relapse: outcome and evaluation of dosing regimens. Blood Adv. (2017) 1:1159–66. doi: 10.1182/bloodadvances.2017008268
8. Scully M, McDonald V, Cavenagh J, Hunt BJ, Longair I, Cohen H, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. (2011) 118:1746–53. doi: 10.1182/blood-2011-03-341131
9. Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood. (2016) 127:3092–4. doi: 10.1182/blood-2016-03-703827
10. Chemnitz J, Draube A, Scheid C, Staib P, Schulz A, Diehl V, et al. Successful treatment of severe thrombotic thrombocytopenic purpura with the monoclonal antibody rituximab. Am J Hematol. (2002) 71:105–8. doi: 10.1002/ajh.10204
11. Zheng X, Pallera AM, Goodnough LT, Sadler JE, Blinder MA. Remission of chronic thrombotic thrombocytopenic purpura after treatment with cyclophosphamide and rituximab. Ann Intern Med. (2003) 138:105–8. doi: 10.7326/0003-4819-138-2-200301210-00011
12. Chen H, Fu A, Wang J, Wu T, Li Z, Tang J, et al. Rituximab as first-line treatment for acquired thrombotic thrombocytopenic purpura. J Int Med Res. (2017) 45:1253–60. doi: 10.1177/0300060517695646
13. Lian EC, Larcada AF, Chiu AY. Combination immunosuppressive therapy after factor VIII infusion for acquired factor VIII inhibitor. Ann Intern Med. (1989) 110:774–8. doi: 10.7326/0003-4819-110-10-774
14. Collins P, Baudo F, Knoebl P, Levesque H, Nemes L, Pellegrini F, et al. Immunosuppression for acquired hemophilia a: results from the european acquired haemophilia registry (EACH2). Blood. (2012) 120:47–55. doi: 10.1182/blood-2012-02-409185
15. Beloncle F, Buffet M, Coindre JP, Munoz-Bongrand N, Malot S, Pene F, et al. Splenectomy and/or cyclophosphamide as salvage therapies in thrombotic thrombocytopenic purpura: the French TMA reference center experience. Transfusion. (2012) 52:2436–44. doi: 10.1111/j.1537-2995.2012.03578.x
16. Hertzberg MS, Koutts J. Oral cyclophosphamide for refractory or relapsing thrombotic thrombocytopenic purpura (TTP). Aust N Z J Med. (1997) 27:439. doi: 10.1111/j.1445-5994.1997.tb02204.x
17. Allan DS, Kovacs MJ, Clark WF. Frequently relapsing thrombotic thrombocytopenic purpura treated with cytotoxic immunosuppressive therapy. Haematologica. (2001) 86:844–50.
18. Coppo P, Froissart A. Treatment of thrombotic thrombocytopenic purpura beyond therapeutic plasma exchange. Hematol Am Soc Hematol Educ Program. (2015) 2015:637–43. doi: 10.1182/asheducation-2015.1.637
19. Guzicka-Kazimierczak R, Kazimierczak A, Clark J. Promising results from cyclophosphamide based immunosuppression therapy of thrombotic thrombocytopenic purpura. Pomeranian J Life Sci. (2015) 61:34–40. doi: 10.21164/pomjlifesci.48
20. McDonald V, Manns K, Mackie IJ, Machin SJ, Scully MA. Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost. (2010) 8:1201–8. doi: 10.1111/j.1538-7836.2010.03818.x
21. Samtleben W, Mistry-Burchardi N, Hartmann B, Lennertz A, Bosch T. Therapeutic plasma exchange in the intensive care setting. Ther Apher. (2001) 5:351–7. doi: 10.1046/j.1526-0968.2001.00383.x
22. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. (2012) 53:458–480.
23. Cox DR. Regression models and life-tables. J Roy Stat Soc Ser B. (1972) 34:187–202. doi: 10.1111/j.2517-6161.1972.tb00899.x
24. Martin-Suarez I, D'Cruz D, Mansoor M, Fernandes AP, Khamashta M, Hughes G. Immunosuppressive treatment in severe connective tissue diseases: effects of low dose intravenous cyclophosphamide. Ann Rheum Dis. (1997) 56:481–7. doi: 10.1136/ard.56.8.481
25. Costa S, Bevilacqua D, Cassatella MA, Scapini P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology. (2019) 156:23–32. doi: 10.1111/imm.13005
26. Bird AK, Chang M, Barnard J, Goldman BI, Meednu N, Rangel-Moreno J, et al. Neutrophils slow disease progression in murine lupus via modulation of autoreactive germinal centers. J Immunol. (2017) 199:458–66. doi: 10.4049/jimmunol.1700354
27. Jee J, Bonnegarde-Bernard A, Duverger A, Iwakura Y, Cormet-Boyaka E, Martin TL, et al. Neutrophils negatively regulate induction of mucosal IgA responses after sublingual immunization. Mucosal Immunol. (2015) 8:735–45. doi: 10.1038/mi.2014.105
28. Kamenyeva O, Boularan C, Kabat J, Cheung GY, Cicala C, Yeh AJ, et al. Neutrophil recruitment to lymph nodes limits local humoral response to staphylococcus aureus. PLoS Pathog. (2015) 11:e1004827. doi: 10.1371/journal.ppat.1004827
Keywords: thrombotic thrombocytopenic purpura, rituximab, cyclophosphamide, treatment, relapse
Citation: Abou-Ismail MY, Arafah Y, Fu P, Cao S, Schmaier AH and Nayak L (2020) Outcomes of Immune Thrombotic Thrombocytopenic Purpura (iTTP) With Upfront Cyclophosphamide vs. Rituximab. Front. Med. 7:588526. doi: 10.3389/fmed.2020.588526
Received: 29 July 2020; Accepted: 24 September 2020;
Published: 28 October 2020.
Edited by:
Magali J. Fontaine, University of Maryland, Baltimore, United StatesReviewed by:
Andrew Yee, Baylor College of Medicine, United StatesCopyright © 2020 Abou-Ismail, Arafah, Fu, Cao, Schmaier and Nayak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mouhamed Yazan Abou-Ismail, eWF6YW4uYWJvdS1pc21haWxAaGNpLnV0YWguZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.