- 1Division of Gastroenterology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Integrated Chinese and Western Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3College of Informatics, Huazhong Agricultural University, Wuhan, China
- 4Liver and Infectious Diseases Department, Wuhan Jinyintan Hospital, Wuhan, China
- 5Tuberculosis and Respiratory Department, Wuhan Jinyintan Hospital, Wuhan, China
- 6Department of Medicine, University of California, San Diego, La Jolla, CA, United States
Background and Aim: Liver test abnormalities are common in COVID-19 patients. The aim of our study was to determine risk factors for different liver injury patterns and to evaluate the relationship between liver injury patterns and prognosis in patients with COVID-19.
Methods: We retrospectively analyzed patients admitted between January 1st to March 10th, with laboratory-confirmed COVID-19 and followed them up to April 20th, 2020. Information of clinical features of patients was collected for analysis.
Results: As a result, a total of 838 hospitalized patients with confirmed COVID-19, including 48.8% (409/838) patients with normal liver function and 51.2% (429/838) patients with liver injury were analyzed. Abnormal liver function tests are associated with organ injuries, hypoxia, inflammation, and the use of antiviral drugs. Hepatocellular injury pattern was associated with hypoxia. The mortality of the hepatocellular injury pattern, cholestatic pattern and mixed pattern were 25, 28.2, and 22.3%, respectively, while the death rate was only 6.1% in the patients without liver injury. Multivariate analyses showed that liver injury with cholestatic pattern and mixed pattern were associated with increased mortality risk.
Conclusions: Our study confirmed that hepatocellular injury pattern that may be induced by hypoxia was not risk factor for mortality in SARS-COV-2 infection, while liver injury with mixed pattern and cholestatic pattern that might be induced by SARS-CoV-2 directly might be potential risk factors for increased mortality in COVID-19 patients.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) disease, which is also called Corona Virus Disease 2019 (COVID-19), has been considered as a public health emergency of international concern by World Health Organization (WHO). Currently, SARS-CoV-2 has spread to over 200 countries and areas with 11, 327, 790 confirmed cases, including 532, 340 deaths globally until July 6 (1). Although COVID-19 mainly affects the lower respiratory tract and manifests as pneumonia in humans, a subset of COVID-19 patients present with different degrees of liver injury (2–4).
On the basis of previous reports from China, 15–26% of COVID-19 patients develop severe pneumonia with increased mortality (5). Organ dysfunction including acute kidney injury and liver injury is common in patients with severe pneumonia (5–8). 16–53% of COVID-19 patients had liver injury with abnormal levels of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) accompanied by slightly elevated bilirubin levels during disease progression (2–4). The incidence of liver injury in COVID-19 patients with severe disease (62%) was significantly higher than that in patients with mild disease (25%) (4, 9). In addition, hepatic steatosis and liver injury were also confirmed by liver biopsy from a deceased patient infected with COVID-19 (10), and 78% of deceased COVID-19 patients had liver injury (11). However, whether liver injury is related with the progression of COVID-19 remains controversial (12–14). Besides, liver injury with hepatocyte pattern or mixed pattern had significantly higher risks of developing severe pneumonia in a single-center study of 148 patients (13), while the risk factors for different liver injury patterns remain unclear and a large-scale, multicenter study on the detailed relationships between different liver injury patterns and progression of COVID-19 was absent. In this study, we analyzed data from 838 hospitalized COVID-19 patients in multiple centers, explored the risk factors of liver injury among COVID-19 patients and evaluated the relationship between different patterns of liver injury and the progression of COVID-19. We found that liver injury was closely related with organ injuries, hypoxia, inflammation and the utilization of antiviral drugs, hepatocellular injury pattern that was associated with hypoxia was not risk factor for increased mortality, while liver injury with cholestatic pattern and mixed pattern that may be induced by SARS-CoV-2 directly increased mortality risk for COVID-19 patients.
Methods
Study Design and Participants
This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Ethics Board of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Jinyintan Hospital in China. We obtained the clinical and laboratory data at admission of confirmed COVID-19 patients from two centers including Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Jinyintan Hospital that were designated hospitals in Wuhan to manage patients with COVID-19. We retrospectively analyzed patients admitted between January 1st to March 10th, 2020, and followed them up to April 20th, 2020, with laboratory-confirmed COVID-19 based on real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) assay for nasal and pharyngeal swab specimens (9). The inclusion criteria were: 1. Patients confirmed with COVID-19; 2. Adult patients aged >18 years; 3. Patients had definite clinical outcome (cured or died) before April 20th. Exclusion criteria were: 1. Patients had pre-existing liver disease; 2. Patients aged <18 years. All patients with COVID-19 enrolled in this study were diagnosed according to The World Health Organization Interim Guidance.
Data Collection
The medical records of patients were collected and analyzed by the search team from Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Jinyintan Hospital. The clinical symptoms on admission, vital signs, and the pre-existing comorbidities of the patients were obtained from the electronic medical records. The results of the laboratory and imaging examinations on admission were obtained from the electronic system of laboratory and imaging examination. Diagnoses of acute kidney injuries and cardiac injuries were defined according to the Berlin Definition, improving global outcome definition and elevated cardiac biomarker, respectively (10, 15, 16). The degree of severity of COVID-19 (severe vs. non-severe) was defined using the American Thoracic Society guidelines for community-acquired pneumonia (17). For the diagnosis of three patterns of liver injury, initially ALT activity [patients ALT/upper limit of normal (ULN) of ALT] and alkaline phosphatase (ALP) activity (patients ALP/ULN of ALP) is calculated. Then ALT/ALP ratio (R) is determined. Hepatocellular pattern: If ALT alone is elevated ≥5-fold above ULN or R ≥5. Cholestatic pattern: ALP alone is elevated ≥2-fold above ULN or R ≤ 2. Mixed pattern: R >2 to <5 (18, 19).
Statistical Analysis
Categorical variables were presented as numbers and percentages. Chi-square tests with Bonferoni corrections for intragroup comparisons and Fisher's exact tests were used for categorical variables. Continuous values were expressed as means (standard deviations), and were calculated using the Student's t-test or one-way ANOVA for parametric data. Continuous values were expressed as median [interquartile range (IQR)], and were calculated using the Mann–Whitney U or Kruskal-Wallis H test with Bonferoni corrections for intragroup comparisons for non-parametric data. The correlation of hypoxia/inflammation and abnormal liver function test was examined using Spearman Rho's (ρ) correlation. To identify the risk factors for poor outcomes, univariate and multivariate Cox regression analyses were performed and reported as hazard ratios (HRs) and 95% confidence intervals (95% CIs). All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) (version 13.0, IBM Corp, Armonk, NY, USA). A significance level of P ≤ 0.05 was used for all models (two-sided).
Results
Clinical Characteristics of Patients
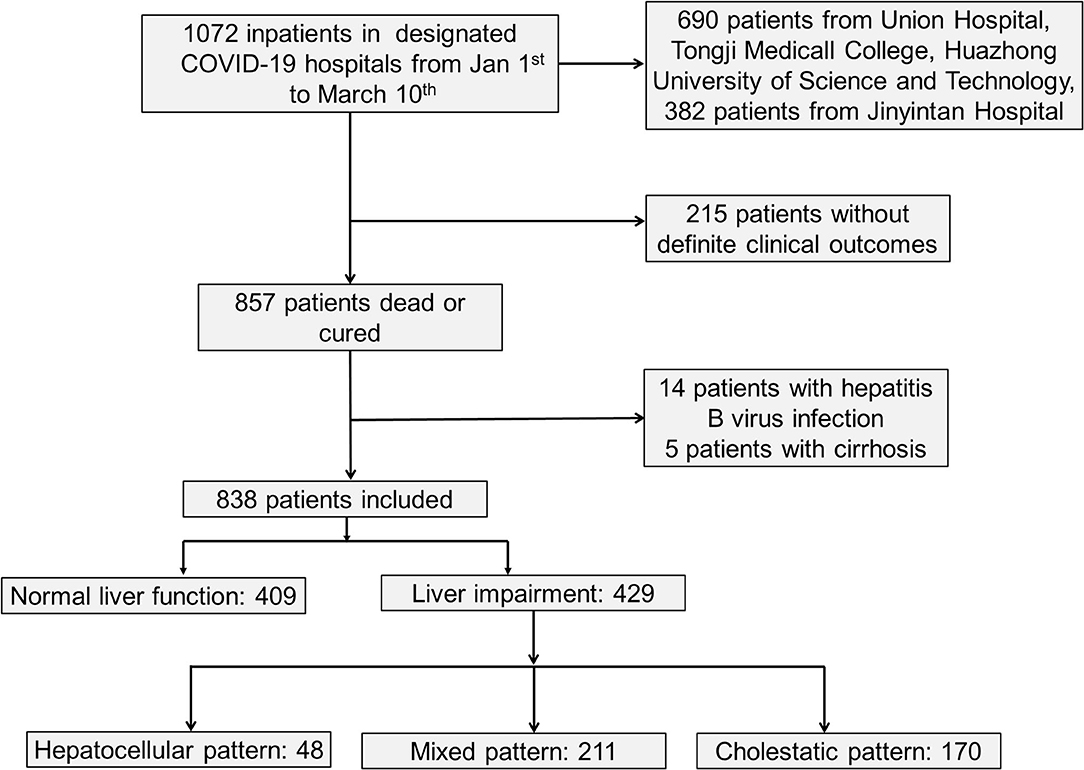
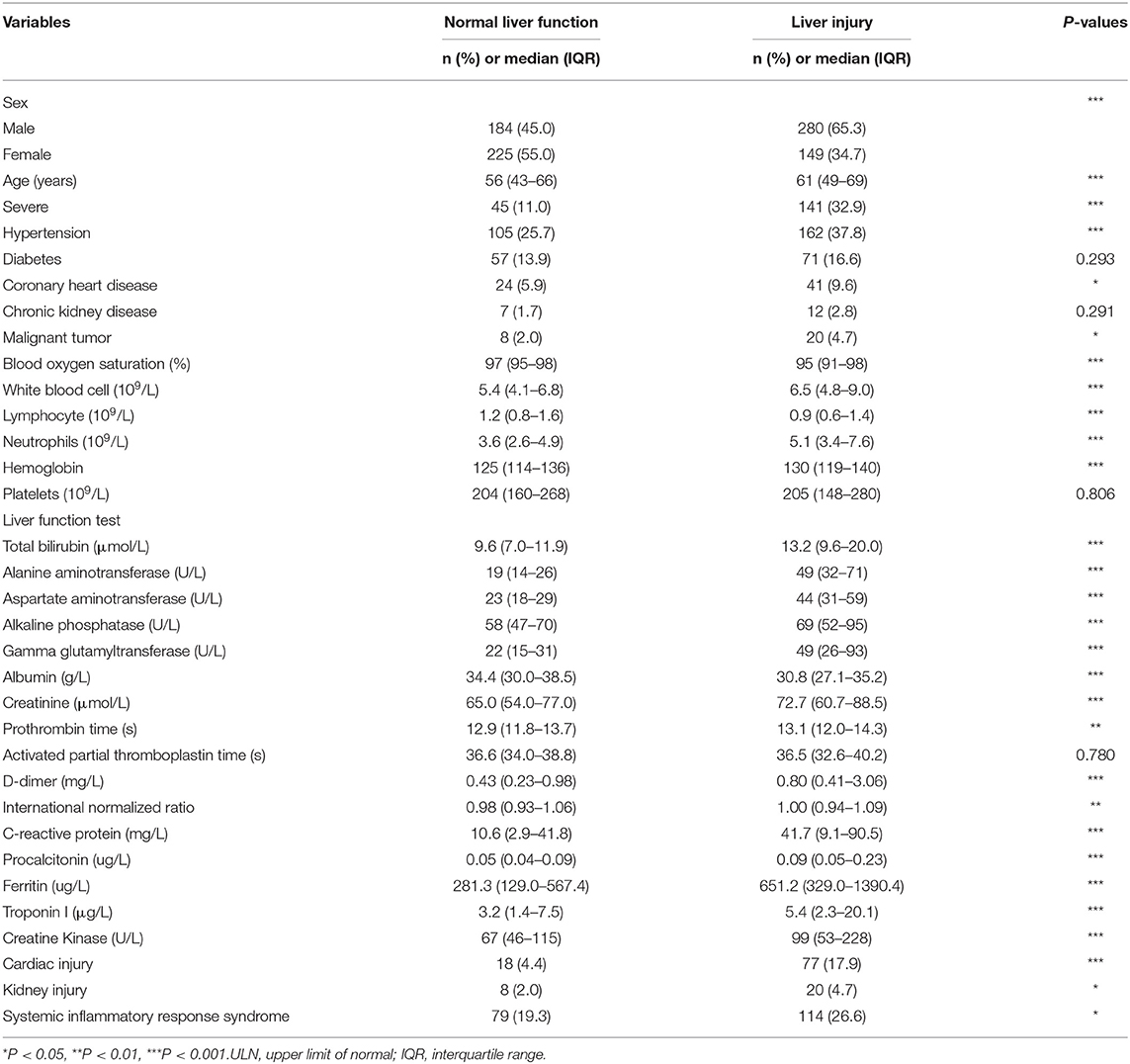
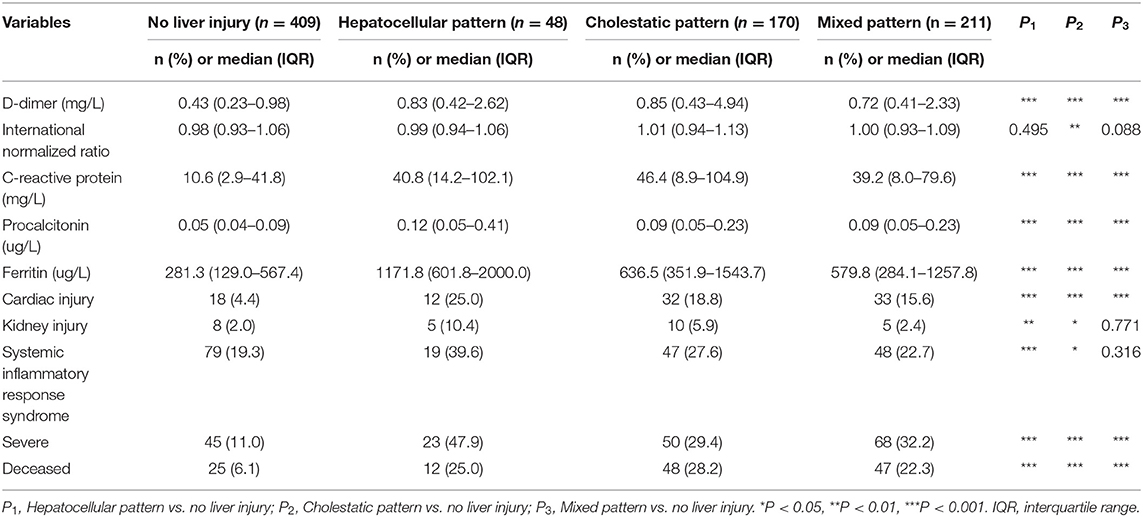
As shown in Figure 1 and Table 1, a total of 838 hospitalized patients with confirmed COVID-19, including 48.8% (409/838) patients with normal liver function and 51.2% (429/838) patients with liver injury were analyzed. A total of 65.3% (280/429) male patients had liver injury, while in female patients, this proportion was significantly lower. The average age for patients with liver injury was 61, which is older than that in patients with normal liver function. In addition, more patients with liver injury had severe COVID-19. These results indicated that male and older patients and patients with more severe disease were more susceptible to liver injury.
Among them, 31.8% (267/838) had hypertension, 15.3% (128/838) had diabetes, and 7.8% (65/838) had coronary heart diseases (Table 1). A higher proportion of COVID-19 patients with liver injury had pre-existing hypertension, coronary heart diseases and malignant tumor, while there was no difference in pre-existing diabetes or chronic renal diseases between the subgroups with normal liver function and liver injury (Table 1).
Liver Biochemical Abnormalities Were Related With Organ Injuries
We evaluated the relationship between liver biochemical abnormalities and other laboratory results in COVID-19 patients. Patients with liver biochemical abnormalities showed more severe lymphocytopenia with lower counts of lymphocytes, more inflammation as indicated by elevated level of serum white blood cells, C-reactive protein (CRP) and procalcitonin (PCT), higher level of D-dimers, kidney injury as indicated by elevated level of serum creatinine, and higher level of serum ferritin. Importantly, patients with liver biochemical abnormalities had a higher incidence of cardiac injury, kidney injury, and systemic inflammatory response syndrome (SIRS) (Table 1). These results indicated that liver biochemical abnormalities were closely associated with cardiac injury, kidney injury and systemic inflammatory response that play important roles in COVID-19 patients.
Liver Biochemical Abnormalities Were Closely Associated With Hypoxia/Inflammation
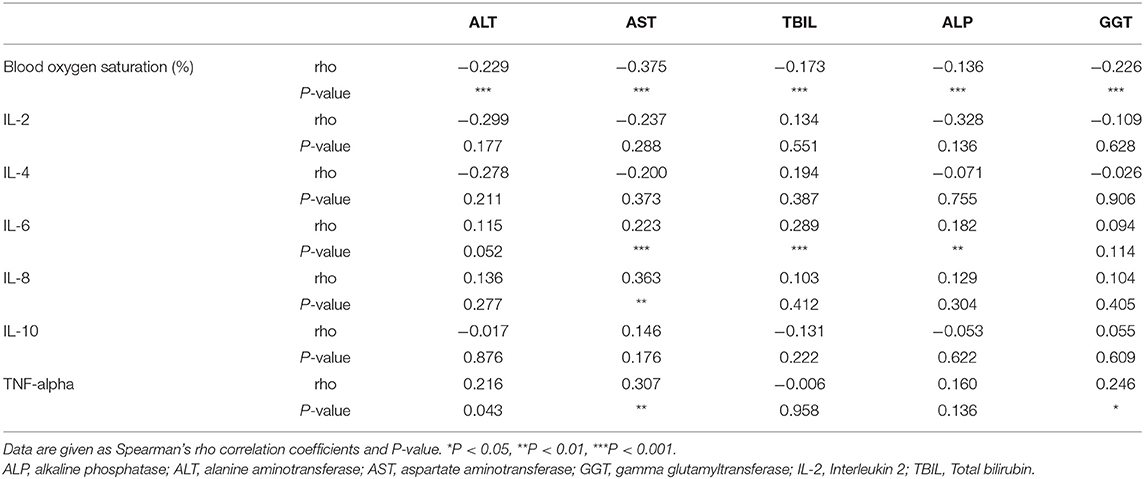
Hypoxia and inflammatory cytokine storm are considered as risk factor for liver biochemical abnormalities in patients with COVID-19 (20). Therefore, we analyzed the relationship between liver biochemical abnormalities and hypoxia or inflammatory cytokines. As shown in Table 1, patients with liver injury had much lower blood oxygen saturation. Correlation analysis showed that blood oxygen saturation was negatively related with ALT, AST, TBIL, ALP, and GGT indicating that hypoxia might contribute to liver injury (Table 2).
To determine which inflammatory cytokine is associated with liver injury, we measured serum level of Interleukin (IL)-2, IL-4, IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α) in patients with normal liver function and with liver injury. As shown in Supplementary Table 1, serum IL-6 and TNF-α were significantly increased in the liver injury group. IL-6 was positively correlated with the increased AST, TBIL, and ALP. IL-8 was positively correlated with increased AST, and TNF-α was positively correlated with increased ALT, AST, and GGT (Table 2). These results indicate that inflammatory cytokines might contribute to liver injury in patients with COVID-19.
Lopinavir/Litonavir and Ribavirin Increased the Risk of Liver Biochemical Abnormalities
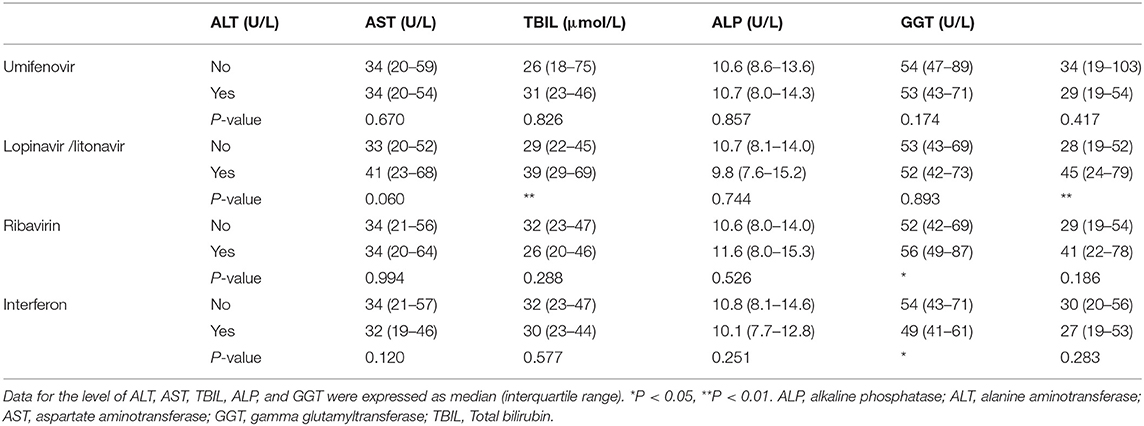
Antiviral drugs recommended to treat COVID-19 include umifenovir (arbidol hydrochloride), lopinavir/litonavir, ribavirin and interferon, which are metabolized in liver and can induce hepatotoxicity. We tested an association of antiviral drug use and liver injury in COVID-19 patients. As shown in Table 3, the level of ALT, AST, ALP, GGT, and TBil showed no significant difference between patients with and without umifenovir treatment. Patients treated with lopinavir/litonavir had higher levels of AST and GGT (Table 3). In addition, use of ribavirin slightly increased the level of ALP (Table 3). These results indicate that there is an association between the use of lopinavir /litonavir and ribavirin as the antiviral drugs and increased liver injury in COVID-19 patients.
Hepatocellular Injury Pattern Was Closely Related With Hypoxia
To further understand the characteristic of liver injury in patients with COVID-19, we classified the liver biochemical abnormalities as hepatocellular pattern, cholestatic pattern and mixed pattern according to the ALT/ALP ratio. Most patients (49.2%) manifested with a mixed liver injury pattern (211/429), and 39.6% of patients presented with cholestatic pattern (170/429). Hepatocellular pattern only accounted for 11.2% of patients (48/429). There was no difference between these three liver injury patterns in terms of gender, age, severity of COVID-19 and pre-existing diseases (Supplementary Table 2).
We further evaluated the relationship between different liver injury patterns and laboratory parameters (Supplementary Table 2). Patients with a hepatocellular injury pattern had lower blood oxygen saturation, higher ferritin level and increased kidney injury (Supplementary Table 2). These results indicated that hypoxia might play a critical role in hepatocytes death in COVID-19 patients.
The Association Between Liver Injury and COVID-19 Severity
We finally evaluated the relationship between different patterns of liver injury and COVID-19 outcome (Table 4). It was worth noting that 47.9, 29.4, and 32.2% of COVID-19 patients with hepatocellular pattern, cholestatic pattern and mixed pattern, respectively, were severe cases compared with 11% of patients with normal liver function (Table 4). The death rates of the COVID-19 patients with hepatocellular pattern, cholestatic pattern, and mixed pattern were 25, 28.2, and 22.3%, respectively, compared with 6.1% of patients with normal liver function (Table 4).
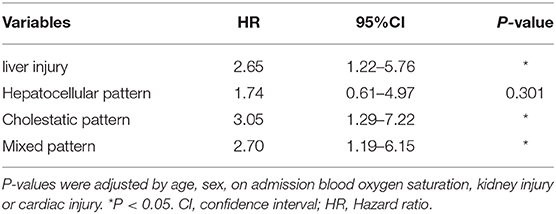
Multivariate analyses showed that the liver injury was associated with increased mortality risk in patients with COVID-19, with an adjusted hazard ratio of 2.65 (1.22–5.76) compared with normal liver function (Table 5). In addition, other factors such as platelet count and coagulation factors that represent the state of liver's ability to function were also associated with increased mortality risk in patients with COVID-19 (Supplementary Tables 3, 4). The levels of platelet count decreased in deceased patients with COVID-19, while prothrombin time, D-dimer and international normalized ratio increased in deceased patients with COVID-19 (Supplementary Table 3). Further analysis showed that liver injury with cholestatic pattern and mixed pattern were associated with increased mortality risks in patients with COVID-19 (Table 5). These results indicate that liver injury with cholestatic pattern and mixed pattern are associated with worse prognosis in patients with COVID-19.
Discussion
Severe respiratory failure is more likely to be the main cause of mortality in COVID-19 patients (21). Other potential factors such as older age, high Sequential Organ Failure Assessment (SOFA) score, and d-dimer > 1 μg/mL were also associated with poor prognosis (22). On the basis of previous reports from China, some COVID-19 patients present with liver injury (7, 8, 23). Since subtypes of liver injury include hepatocellular pattern, cholestatic pattern, and mixed pattern, we mainly evaluated the incidence of three subtypes in COVID-19 patients and assessed their relationship with prognosis of COVID-19. In this study, we found that liver injury with cholestatic and mixed pattern were associated with worse prognosis of patients with COVID-19.
Our data indicate that SARS-CoV-2 associated liver injury correlates with hypoxia/ inflammation. The mechanism by which COVID-19 patients present with liver injury is still unclear. It was reported that SARS-CoV-2 uses angiotensin converting enzyme II (ACE2) for cell entry (24). Apart from the lung alveolar epithelial cells, liver cholangiocytes also express of ACE2 (24, 25). Besides, coronavirus particles were also identified in the liver of two deceased SARS-CoV-2 patients (26). These suggest that SARS-CoV-2 may damage cholangiocytes directly and lead to cholestatic and mixed pattern liver injury in COVID-19 patients. Interestingly, viral inclusions were not observed in the liver biopsy specimens of some COVID-19 patient with liver injury (10), indicating that SARS-CoV-2 may induce liver injury through other mechanisms. Inflammation may also contribute to liver injury as patients with liver injury had higher level of inflammatory markers, such as CRP and PCT (9, 27). Wang et al. (28) confirmed that the inflammatory storm is closely related to multiple organ damage or death in COVID-19 patients. Our data also confirmed that liver injury correlates with inflammatory cytokines. In addition, hypoxia presenting as one of the common symptoms in COVID-19 patients, may be also related with liver injury (29). Our data show that liver injury is negatively correlated with blood oxygen saturation. Adverse effect of some drugs may be another factor to cause liver injury (30, 31). The use of lopinavir/ritonavir before admission was significantly higher in patients with emerging liver injury than that in patients with normal liver functions, suggesting that application of antiviral drugs may lead to liver injury during the process of COVID-19 treatment (27). Our data confirmed that liver injury for COVID-19 patients was associated with the use of certain antiviral drugs, such as lopinavir /litonavir and ribavirin.
Our data show that patients with liver injury had higher level of serum ferritin and more severe hypoxia especially for patients with hepatocellular injury pattern. It was reported that SARS-CoV-2 destroys hemoglobin in red blood cells, dissociating iron and deoxyhemoglobin, causing less hemoglobin that can carry oxygen, and producing symptoms of respiratory distress and hypoxia (32). Besides, iron dissociated from heme will be stored in ferritin, thus the level of ferritin in serum will be increased (32). In addition, hypoxia may also induce liver injury (29), and damage hepatocytes. Therefore, we propose that the elevated level of serum ferritin and hypoxia are related with hepatocyte injury.
Similar to other reports (6, 33), our study showed that liver injury in COVID-19 patients was frequent but mild in nature. The patterns of liver injury were mostly cholestatic and mixed pattern, which maybe induced by SARS-CoV-2 directly. Besides, liver injury with cholestatic and mixed patterns were associated with the worse prognosis of COVID-19. Thus, clinical physicians should pay more attention to COVID-19 patients with cholestatic and mixed pattern liver injury and give effective treatment method.
Our study has some limitations. Liver injury in COVID-19 is associated with hepatotoxic drug intake and tissue hypoxia. Therefore, increased liver injury induced by hypoxia, or hepatotoxic drug intake may not be reflective of risk factors for mortality in SARS-COV-2 infection. Although large enough to conduct valid comparisons among groups, the sample size remains limited. Larger studies should be performed to further evaluate the predictive value of liver injury for prognosis of COVID-19. In addition, this was a retrospective study, and an unknown bias may exist.
In summary, our study demonstrates that liver injury is closely related with organ injuries, hypoxia, inflammation, and the use of antiviral drugs. Hepatocellular injury pattern is associated with hypoxia, and increased hepatocellular injury may not be risk factor for mortality in SARS-COV-2 infection, while liver injury with cholestatic, and mixed patterns that may be induced by SARS-CoV-2 directly may be potential risk factors for increased mortality in patients with COVID-19. COVID-19 patients with liver injury especially for cholestatic and mixed patterns should be monitored and evaluated frequently and performed effective treatment.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Jinyintan Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
HChu was responsible for study concept and design, analysis and interpretation of data, and drafting of the manuscript. TB was responsible for acquisition, analysis of data, and critical revision of the manuscript. LH, LX, LYao, RZ, ZL, LZ, CH, SS, QH, and YZ provided assistance in data acquisition and revision of the manuscript. LC and XN provided assistance in data analysis and revision of the manuscript. QZ, HChe, and BS provided critical revision of the manuscript. LYan and XH was responsible for the study concept and design, critical revision of the manuscript, and study supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Hubei Province Novel Pneumonia Emergency Research (No. 2020FCA014 to XH) and the NIH funded San Diego Digestive Diseases Research Center (P30 DK120515 to BS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.584342/full#supplementary-material
Abbreviations
ACE2, angiotensin converting enzyme II; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cis, confidence intervals; COVID-19, Corona Virus Disease 2019; CRP, C-reactive protein; GGT, gamma glutamyltransferase; HRs, hazard ratios; IL-6, Interleukin 6; IQR, interquartile range; ORs, odds ratios; PCT, procalcitonin; R, ratio; RT-PCR, real-time reverse-transcriptase polymerase-chain-reaction; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; SIRS, systemic inflammatory response syndrome; TNF-α, tumor necrosis factor-α; WHO, World Health Organization; ULN, upper limit of normal.
References
1. World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report-209. Available online at: https:// https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200816-covid-19-sitrep-209.pdf (accessed August 16, 2020).
2. Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. (2020) 368:m606. doi: 10.1136/bmj.m606
3. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. (2020) 20:425–34. doi: 10.1016/S1473-3099(20)30086-4
4. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China medical treatment expert group for, clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
5. Special Expert Group for Control of the Epidemic of Novel Coronavirus Pneumonia of the Chinese Preventive Medicine Association. An update on the epidemiological characteristics of novel coronavirus pneumoniaCOVID-19. Zhonghua Liu Xing Bing Xue Za Zhi. (2020) 41:139–44. doi: 10.3760/cma.j.issn.0254-6450.2020.02.002
6. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–69. doi: 10.1001/jama.2020.1585
7. Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, et al. A 55-day-old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, heart damage. J Infect Dis. (2020) 221:1775–81. doi: 10.1093/infdis/jiaa113
8. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
9. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
10. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–22. doi: 10.1016/S2213-2600(20)30076-X
11. Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE. (2020) 15:e0235458. doi: 10.1371/journal.pone.0235458
12. Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. (2020) 40:2095–103. doi: 10.1111/liv.14455
13. Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: abnormal liver function tests. J Hepatol. (2020) 73:566–74. doi: 10.1016/j.jhep.2020.04.006
14. Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. (2020) 72:389–98. doi: 10.1002/hep.31301
15. Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the berlin definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
16. Kellum JA, Lameire N, K.Group AGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical Care. (2013) 17:204. doi: 10.1186/cc11454
17. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. (2019) 200:e45–67. doi: 10.1164/rccm.201908-1581ST
18. Danan G, Benichou C. Causality assessment of adverse reactions to drugs–I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. (1993) 46:1323–30. doi: 10.1016/0895-4356(93)90101-6
19. Kang Y, Kim SH, Park SY, Park BY, Lee JH, An J, et al. Evaluation of drug-induced liver injury developed during hospitalization using electronic health record (EHR)-based algorithm. Allergy Asthma Immunol Res. (2020) 12:430–42. doi: 10.4168/aair.2020.12.3.430
20. Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, et al. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. (2020) 8:18–24. doi: 10.14218/JCTH.2020.00018
21. Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. (2020) 24:198. doi: 10.1186/s13054-020-02911-9
22. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
23. Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. (2020) 5:428–30. doi: 10.1016/S2468-1253(20)30057-1
24. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579:270–3. doi: 10.1038/s41586-020-2012-7
25. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. (2020) 526:135–40. doi: 10.1016/j.bbrc.2020.03.044
26. Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. (2020) 73:807–16. doi: 10.1016/j.jhep.2020.05.002
27. Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. (2020) 18:1561–6. doi: 10.1016/j.cgh.2020.04.002
28. Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. (2020) 57:102833. doi: 10.1016/j.ebiom.2020.102833
29. Yang L, Wang W, Wang X, Zhao J, Xiao L, Gui W, et al. Creg in hepatocytes ameliorates liver ischemia/reperfusion injury in a TAK1-dependent manner in mice. Hepatology. (2019) 69:294–313. doi: 10.1002/hep.30203
30. Yancheva N, Tzonev R. A case of late presentation of darunavir-related cholestatic hepatitis. Int J STD AIDS. (2019) 30:620–2. doi: 10.1177/0956462419826723
31. Marty FM, Vidal-Puigserver J, Clark C, Gupta SK, Merino E, Garot D, et al. Intravenous zanamivir or oral oseltamivir for hospitalised patients with influenza: an international, randomised, double-blind, double-dummy, phase 3 trial. Lancet Respir Med. (2017) 5:135–46. doi: 10.1016/S2213-2600(16)30435-0
32. Liu W, Li H. COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. Available online at: https://chemrxiv.org/articles/COVID-19_Disease_ORF8_and_SurfaceGlycoprotein_Inhibit_Heme_Metabolism_by_Binding_to_Porphyrin/11938173 (accessed April 25, 2020).
Keywords: liver impairment, hepatocellular pattern, cholestatic pattern, mixed pattern, prognosis
Citation: Chu H, Bai T, Chen L, Hu L, Xiao L, Yao L, Zhu R, Niu X, Li Z, Zhang L, Han C, Song S, He Q, Zhao Y, Zhu Q, Chen H, Schnabl B, Yang L and Hou X (2020) Multicenter Analysis of Liver Injury Patterns and Mortality in COVID-19. Front. Med. 7:584342. doi: 10.3389/fmed.2020.584342
Received: 17 July 2020; Accepted: 11 September 2020;
Published: 20 October 2020.
Edited by:
Liang Wen, Zhejiang University, ChinaReviewed by:
Ming-Hua Zheng, First Affiliated Hospital of Wenzhou Medical University, ChinaHiroshi Nakase, Sapporo Medical University, Japan
Copyright © 2020 Chu, Bai, Chen, Hu, Xiao, Yao, Zhu, Niu, Li, Zhang, Han, Song, He, Zhao, Zhu, Chen, Schnabl, Yang and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Yang, aGVwYXlhbmdAMTYzLmNvbQ==; Xiaohua Hou, aG91eGhAaHVzdC5lZHUuY24=
†These authors have contributed equally to this work
 Huikuan Chu
Huikuan Chu Tao Bai
Tao Bai Liuying Chen1
Liuying Chen1 Lilin Hu
Lilin Hu Li Xiao
Li Xiao Lin Yao
Lin Yao Rui Zhu
Rui Zhu Xiaohui Niu
Xiaohui Niu Zhonglin Li
Zhonglin Li Lei Zhang
Lei Zhang Chaoqun Han
Chaoqun Han Shuangning Song
Shuangning Song Qi He
Qi He Ling Yang
Ling Yang