- 1Department of Rheumatology, Peking Union Medical College Hospital, Chinese Academy of Medical Science & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Key Laboratory of Ministry of Health, Beijing, China
- 2Department of Cardiology, Peking Union Medical College Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, China
- 3Department of Nephrology, Children's Hospital Affiliated to Capital Institute of Pediatrics, Beijing, China
Introduction: Cardiac involvement in eosinophilic granulomatosis with polyangiitis (EGPA) is associated with a poor prognosis and high mortality; however, few studies about cardiac involvement in EGPA in the Chinese population are available. We conducted this study to determine the clinical characteristics and overall outcomes of Chinese EGPA patients with cardiac involvement.
Materials and Methods: We retrospectively collected the clinical data of 83 patients diagnosed with EGPA and analyzed the differences between the patients with and without cardiac involvement.
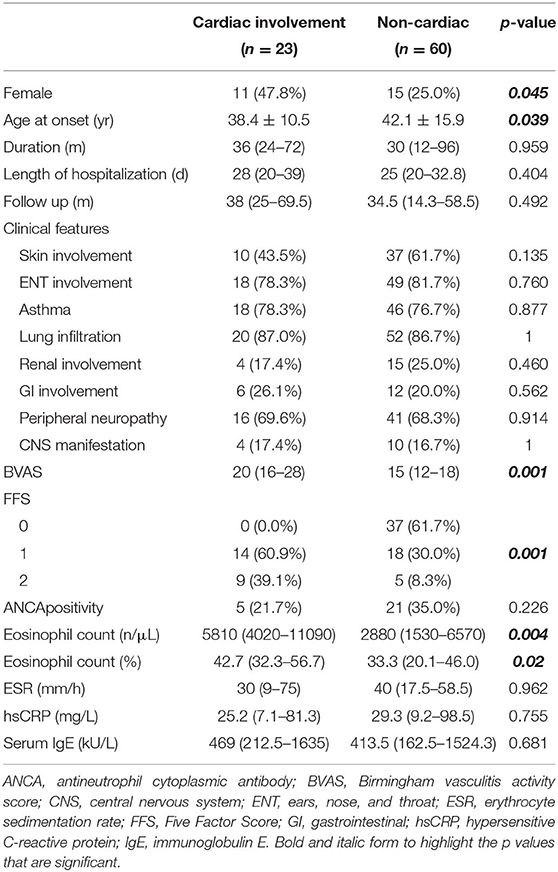
Results: The prevalence of cardiac involvement in EGPA in this cohort was 27.7%. Compared with those without cardiac involvement, EGPA patients with cardiac involvement tended to have a younger age at onset (mean ± SD: 38.4 ± 10.5 vs. 42.1 ± 15.9 years, respectively, p = 0.039), higher eosinophil count (median [IQR]: 5810 [4020–11090] vs. 2880 [1530–6570] n/μL, respectively, p = 0.004), higher disease activity assessed using the Birmingham vasculitis activity score (BVAS) (median [IQR]: 20 [16–28] vs. 15 [12–18], respectively, p = 0.001), and poorer prognosis (Five Factor Score [FFS] ≥ 1: 100% vs. 38.3%, respectively, p = 0.001). In the cardiac involvement group, 43.5% of patients were asymptomatic, but cardiac abnormalities could be detected by cardiac examinations. With appropriate treatment, the overall outcomes of EGPA patients with cardiac involvement in our cohort were good, with only 3 (13.0%) patients dying in the acute phase and no patients dying during follow-up.
Conclusions: Cardiac involvement in EGPA was associated with a younger age at onset, higher eosinophil count, higher disease activity, and a poorer prognosis. Comprehensive cardiac examinations and appropriate treatment are essential to improve the prognosis of those with cardiac involvement.
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome, was first described by Churg and Strauss as a rare autoimmune disease characterized by severe asthma, hypereosinophilia, and symptoms of necrotizing vasculitis in various organs (1). It is classified as antineutrophil cytoplasmic antibody (ANCA)—associated systemic vasculitis affecting small- to medium-sized vessels (2), with ANCA positivity ranging from 26.2 to 85% (3–9).
As a common target organ, cardiac involvement has been reported in 16.1–92% of patients with EGPA according to previous studies (1, 4, 9–16). The cardiac manifestations of EGPA are diverse, ranging from myocarditis, arrhythmia, valvular defects, heart failure, myocardial infarction, and pericardial effusion to cardiac tamponade (11, 13, 16–18). Cardiac involvement in EGPA strongly predicts a worse prognosis (5, 19) and higher mortality (5, 14, 20–22). To date, there have been few data available on cardiac involvement in EGPA patients in the Chinese population. We retrospectively evaluated the data of 83 Chinese patients with EGPA to analyze and describe the prevalence, clinical characteristics, prognostic factors, and outcomes of EGPA patients with cardiac involvement to increase general understanding and provide meaningful information for physicians to use during clinical practice.
Materials and Methods
Patients
We retrospectively collected the data of patients diagnosed with EGPA and hospitalized in Peking Union Medical College Hospital from December 2001 to June 2018 by retrieving medical records. All patients fulfilled the American College of Rheumatology criteria for the classification of EGPA (23). Briefly, patients who met four or more of the following diagnostic criteria were diagnosed with EGPA: (1) asthma, (2) eosinophilia > 10%, (3) mononeuropathy or polyneuropathy, (4) non-fixed pulmonary infiltrates, (5) paranasal sinus abnormality, and (6) extravascular eosinophils. The Birmingham vasculitis activity score (BVAS) (24) was used to assess disease activity and the revised Five Factor Score (FFS) (20) to evaluate the prognosis of all EGPA patients included in this study. According to previous studies (14, 17, 25), cardiac manifestations are attributable to EGPA when patients present with significant arrhythmia, pericardial effusion, acute heart failure, regional or global wall motion abnormalities, diastolic dysfunction, cardiomyopathy, valvular dysfunction, and myocardial enzyme abnormalities that cannot be explained by other etiologies. The study was approved by the Ethics Committee of Peking Union Medical College Hospital and was conducted in accordance with the Helsinki Declaration. Written informed consent could not be obtained due to the retrospective nature of this study.
Clinical Data
All clinical data including age at disease onset, disease duration, initial symptoms, organ involvement, length of hospitalization, clinical manifestations, laboratory findings, imaging, treatment, and follow-up were recorded. Laboratory findings, including complete blood count, serum immunoglobulin E (IgE) levels, hypersensitive C-reactive protein (hsCRP), erythrocyte sedimentation rate (ESR), ANCA test, myocardial enzyme levels, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels, liver function tests, and serum creatinine taken at diagnosis and after treatment were recorded retrospectively. Imaging studies included electrocardiogram (ECG), 24 hours of dynamic electrocardiogram (DCG), ultrasound, magnetic resonance imaging (MRI), computed tomography (CT), and neuroelectrophysiological examination. In addition, for patients who underwent a lung, skin, renal, or heart biopsy when indicated, the pathology results were also recorded.
Statistical Analysis
Continuous variables are shown as the mean ± standard deviation (SD) or median (interquartile range [IQR]), while categorical variables are shown as the number (percentage). Continuous variables were analyzed using the Student's t-test or Mann-Whitney U-test, and categorical variables were analyzed using the chi-square test or Fisher's exact test, as necessary. The Type-1 error was determined to be 5%. Statistical analyses were performed using IBM SPSS statistics (Version 23.0, IBM, Armonk, NY, USA).
Results
Patient Data and Clinical Manifestations
Of the 83 patients with EGPA included in this study, 57 (68.7%) were men and 26 (31.3%) were women, and the mean age (± SD) was 47.3 ± 14.3 years. Twenty-three (27.7%) patients were diagnosed with cardiac involvement. The median duration before diagnosis was 36 months (IQR, 12–84 months). Sixty-four (77.1%) patients had asthma, 67 (80.7%) had paranasal sinus abnormalities, and non-fixed pulmonary infiltrates were observed in 72 (86.7%) patients. Additionally, 19 (22.9%) and 18 (21.7%) patients were diagnosed with renal and gastrointestinal involvement, respectively. Fourteen (16.9%) patients had central nervous system involvement, while 57 (68.7%) patients had manifestations of peripheral neuropathy.
For laboratory findings, all but 1 patient underwent an ANCA test. The results were positive in 26 (31.7%) patients, among which 4 (15.4%) were cANCA-positive and 22 (84.6%) were pANCA-positive. Additionally, the median absolute eosinophil count was 4160/μL (IQR, 1830–8780) for the EGPA patients in this study.
Cardiac Involvement
In this study, 23 (27.7%) patients were diagnosed with cardiac involvement, among which 13 (56.5%) presented with chest pain and dyspnea, and 10 (43.5%) were asymptomatic. A diagnosis of cardiac involvement was made using a combination of clinical symptoms, myocardial enzyme levels, ECG findings, and cardiac imaging examinations. Three (13.0%) patients were admitted to the hospital due to cardiac involvement, while the other patients were found to have cardiac involvement during hospitalization. EGPA patients with cardiac involvement had a younger age at onset than those without cardiac involvement (mean ± SD: 38.4 ± 10.5 vs. 42.1 ± 15.9 years, respectively, p = 0.039) (Figure 1A). Furthermore, women were more likely than men to have cardiac involvement in this cohort, since 47.8% (n = 11) and 25.0% (n = 15) in the cardiac and non-cardiac involvement group were women, respectively (p = 0.045). Patients with cardiac involvement tended to have higher eosinophil counts than those without cardiac involvement (median [IQR]: 5810 [4020–11090] vs. 2880 [1530–6570] /μL, respectively, p = 0.004) (Figure 1B). Although not significant, the percentage of ANCA positivity was lower in those with cardiac involvement than in those without (21.7 vs. 35.0%, respectively, p = 0.226). There were no differences between the two groups in terms of organ involvement or hsCRP, ESR, or serum IgE levels. These data are shown in detail in Table 1.
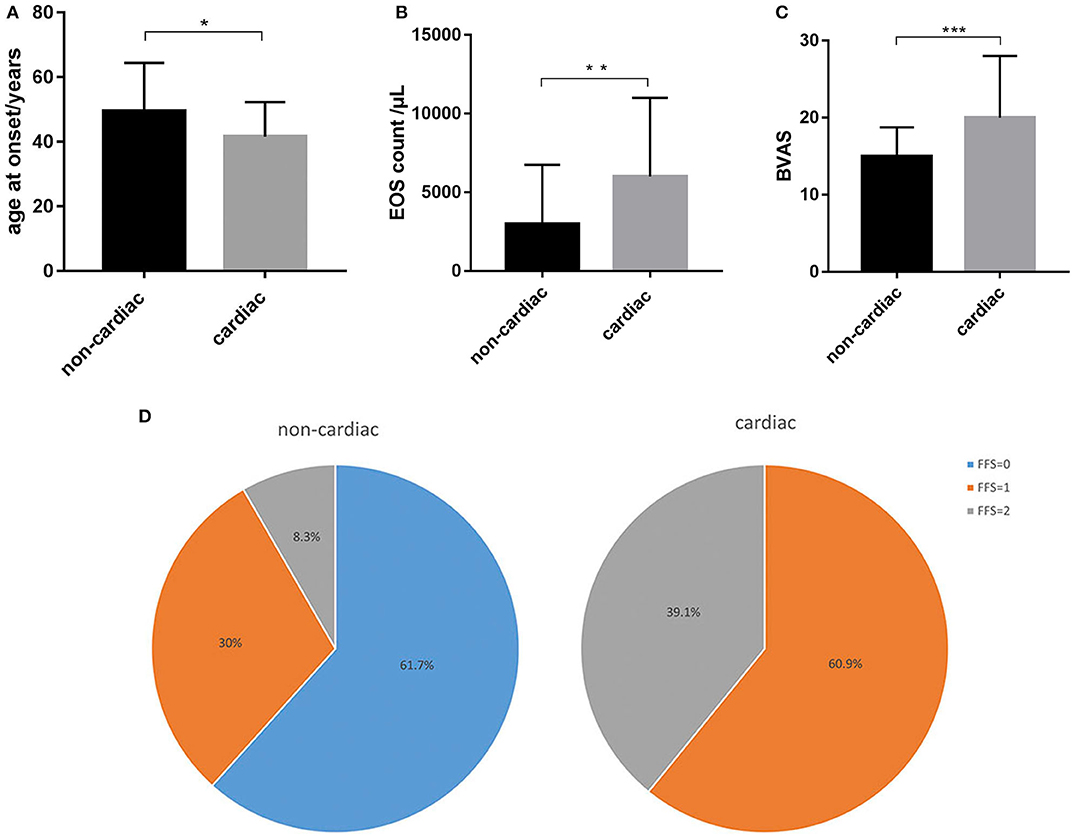
Figure 1. Distinct characteristics between EGPA patients with and without cardiac involvement. (A) EGPA patients with cardiac involvement had a younger age at onset than those without cardiac involvement. (B) Patients with cardiac involvement had higher eosinophil counts than those without cardiac involvement. (C) Patients with cardiac involvement had higher BVAS than those without cardiac involvement. (D) Patients with cardiac involvement had a poorer prognosis (FFS≥1) than those without. *p < 0.05, **p < 0.01, ***p < 0.001.
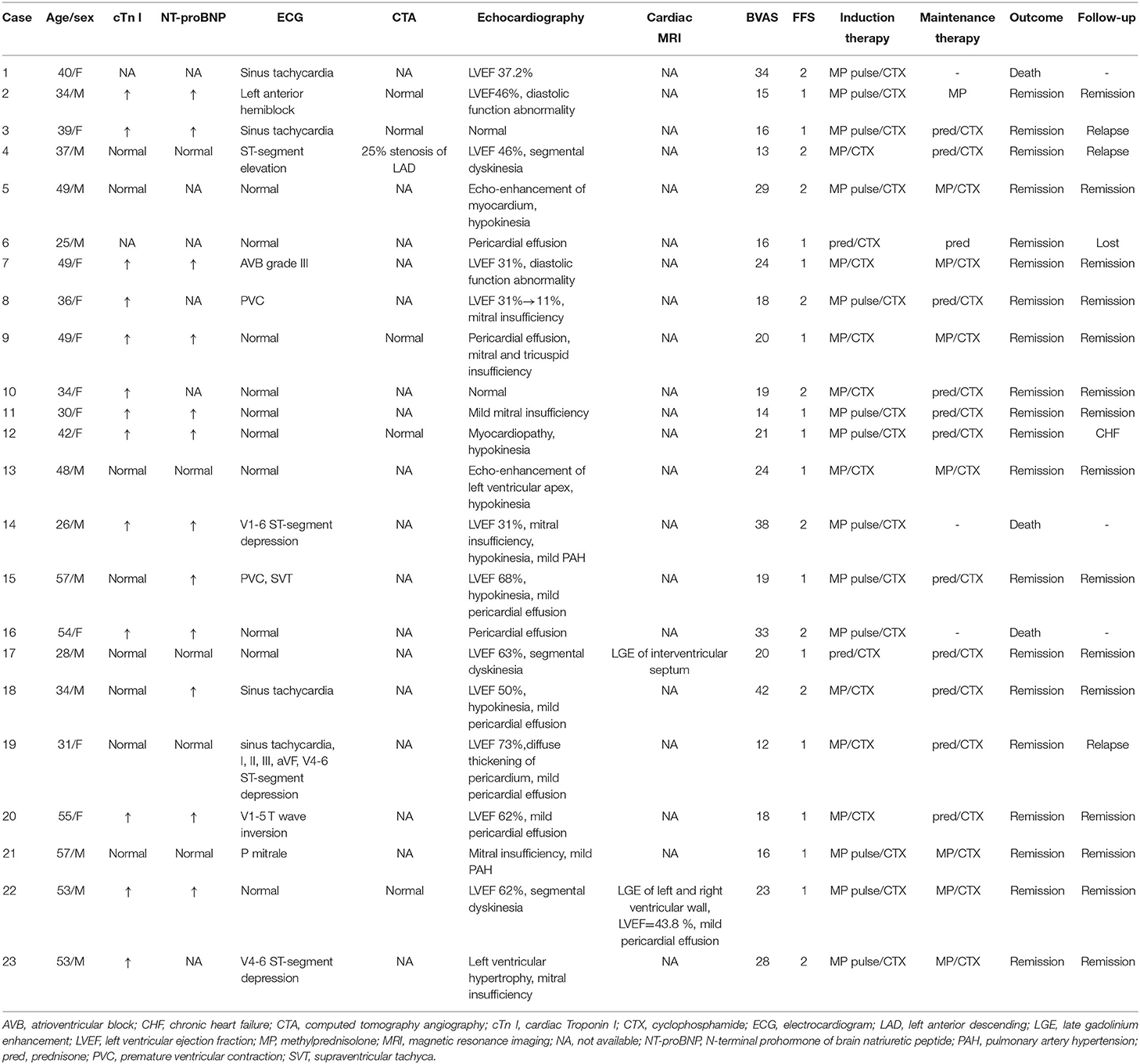
All patients with cardiac involvement underwent an ECG test. Four (17.4%) were found to have sinus tachycardia and 5 (21.7%) had ST-segment or T wave abnormalities. One (4.3%) patient with ST-segment elevation progressed to cardiac arrest but survived due to timely cardio-pulmonary resuscitation. One (4.3%) patient was found to have a third-degree atrioventricular block requiring support from a temporary pacemaker. Ten (43.5%) patients did not present with ECG abnormalities but had elevated myocardial enzymes or echocardiographic changes. Thirteen patients (56.5%) had elevated cardiac Troponin I (cTn I) levels, and elevated NT-proBNP levels were found in 12 patients (52.2%).
An echocardiography was performed on all patients with cardiac involvement, and 21 (95.5%) patients were found to have cardiac disorders. Six (26.1%) patients had decreased left ventricular ejection fraction (LVEF; median [IQR]: 34.1% [31%−46%]), among which one patient had extremely low LVEF (11%), leading to cardiogenic shock and the need for extracorporeal membrane oxygenation support. Eleven (47.8%) patients had diastolic function abnormalities or segmental dyskinesia, and 6 (26.1%) patients had mitral or tricuspid insufficiency. Mild to moderate pericardial effusion was observed in 7 (30.4%) patients. Only 2 (8.7%) patients underwent a cardiac MRI. Both of these patients were found to have late gadolinium enhancement of the myocardium and one had decreased LVEF (43.8%) which was not identified on echocardiography. None of the 6 patients who underwent a coronary artery CT angiography (CTA) had major abnormalities that could be characterized as cardiac involvement. These findings are shown in Table 2.
Disease Activity, Treatment, Outcomes, and Follow-Up
The patients with cardiac involvement had higher BVASs than those without cardiac involvement (median [IQR]: 20 [16–28] vs. 15 [12–18], respectively, p = 0.001), which indicated greater disease activity in the cardiac involvement group (Figure 1C). Moreover, patients with cardiac involvement tended to have a poorer prognosis than those without (FFS ≥1: 100 vs. 38.3%, respectively, p = 0.001) (Figure 1D).
All patients with cardiac involvement received a combination of glucocorticoids and cyclophosphamide (CTX) as induction treatment. The methylprednisolone pulse therapy involved intravenous methylprednisolone 1g per day for 3 days followed by high dose glucocorticoids. High-dose glucocorticoid treatment was defined as oral or intravenous glucocorticoids equivalent to prednisone 1–2 mg/(kg·d). Fifteen (65.2%) patients received methylprednisolone pulse in addition to CTX therapy, and 8 (34.8%) received high-dose glucocorticoids in addition to CTX. Maintenance treatment included glucocorticoids and CTX (n = 18) or glucocorticoids alone (n = 2). In terms of the outcomes for the EGPA patients with cardiac involvement, one patient died of infectious shock due to gut perforation, one patient died of multi-organ failure, and one died from a cerebral hernia. Twenty (87.0%) patients achieved remission after treatment, which included an improvement in LVEF, a decrease in myocardial enzymes, and/or a normal ECG.
For the patients with cardiac involvement, the mean follow-up period was 38 months (IQR, 25–69.5), and one patient was lost to follow-up. Three (15.8%) patients relapsed and were readmitted to the hospital during the follow-up period. The relapsed organs for these patients were the heart, ear, and lung, and all patients achieved remission after treatment. No relationship was observed between relapsing and disease activity, which was measured using the BVAS or FFS at baseline. One patient (5.3%) progressed to heart failure, 15 (78.9%) patients achieved long-term remission, and no patients died during follow-up. These findings are shown in Table 2.
Discussion
EGPA is believed to have a better prognosis than other types of ANCA-associated vasculitis (5, 26), though cardiac involvement is an independent risk factor of mortality (5, 20). Previous studies have demonstrated a prevalence of cardiac involvement in EGPA ranging from 16.1 to 92%, while the prevalence in our cohort was 27.7%, which was relatively low. One reason may be that only two patients underwent a cardiac MRI, which is more sensitive than other imaging studies and can identify cardiac abnormalities in EGPA patients even after a normal ECG and echocardiography as previous studies have suggested (9, 14, 27–29). Patients with cardiac involvement had characteristics that were distinct from those without cardiac involvement in this cohort. According to previous studies (11, 12, 17, 30), EGPA is more common in males than in females, which was also observed in our cohort (68.7 vs. 31.3%, respectively). However, there were more female patients (47.8%) in the cardiac involvement group than in the non-cardiac involvement group (25.0%), indicating that female patients had a higher incidence of cardiac involvement in this population. Furthermore, patients with cardiac involvement in our study tended to have a younger age at onset compared with those without cardiac involvement. This finding was not identified in other studies. Many studies have demonstrated that ANCA negativity is correlated with heart and lung involvement (4, 6, 19, 25), while ANCA positivity is correlated with renal involvement and pulmonary hemorrhage (4, 6, 31). Thus, it has been suggested that cardiac involvement in EGPA is the result of eosinophilic infiltration in the tissue (1, 32), and that eosinophilia plays a more significant role than ANCA in EGPA with cardiac involvement (15). In our study, we also noted a higher eosinophil count in the group with cardiac involvement, which was consistent with the findings of previous studies (15, 25). The percentage of ANCA positivity was lower in the group with cardiac involvement in this study, although this finding was not statistically significant. Many of the patients had taken glucocorticoids before diagnosis, which may explain this phenomenon.
A diagnosis of cardiac involvement was made using a combination of clinical manifestations; myocardial enzyme levels; and ECG, coronary artery CTA, echocardiography, and cardiac MRI findings. Chest pain was a common symptom in patients with cardiac involvement, which is in accordance with some previous studies (15, 33). However, almost half of the patients were asymptomatic or had normal ECGs, and cardiac abnormalities were only detected by echocardiography or cardiac MRI. Therefore, to exclude cardiac involvement even after a normal ECG and the absence of cardiac manifestations, an overall evaluation of the patient's heart condition should be performed, as recommended by previous studies (14, 17), especially in patients with an early age of onset and a high eosinophil count according to the results of our study. EGPA patients with cardiac involvement have been reported to have higher FFSs (9, 25), which was also noted in our cohort, suggesting that patients with cardiac involvement have a poorer prognosis. Additionally in our study, patients with cardiac involvement had higher BVASs, which predicts greater disease activity and severe organ damage. However, the application of glucocorticoids and the combination of immunosuppressive agents such as CTX in severe cases significantly improved the outcomes for EGPA (5, 19). All patients with cardiac involvement in our study received the induction treatment, which included methylprednisolone pulse therapy or high-dose glucocorticoids combined with CTX, and most patients achieved remission during the acute phase. Due to this intensive therapy, the overall outcomes of patients with cardiac involvement in our cohort were good, similar to the results of some previous studies (25, 34). Therefore, early diagnosis and appropriate treatment are essential to prevent the acceleration of cardiac involvement in patients with EGPA (17, 34, 35). Recently, mepolizumab, a fully humanized monoclonal antibody that blocks IL-5 (36), was approved by the US Food and Drug Administration for use in EGPA. Mepolizumab, which could lead to a significant decrease in peripheral absolute eosinophil counts, has been effective in EGPA treatment (37–40), leading to longer periods of remission and reduced doses of glucocorticoids. Since cardiac involvement in EGPA has been correlated with a high peripheral eosinophil count, mepolizumab could be an effective treatment for EGPA with cardiac involvement.
This is the first study to our knowledge with a relatively large sample size addressing EGPA patients in China with cardiac involvement. We identified that the clinical characteristics of patients with cardiac involvement are distinct from those of patients without cardiac involvement in this cohort, which may provide meaningful information for physicians to use during clinical practice. Moreover, our study suggests that cardiac involvement predicts a poorer prognosis and higher disease activity in EGPA patients. Comprehensive cardiac examinations and appropriate treatments are essential to diagnose cardiac involvement early, thus improving outcomes for this patient population.
However, our study has several limitations. First, this is a cross-sectional and retrospective study, so biases may have influenced the results. Additionally, the fact that endomyocardial biopsies were not performed to confirm the diagnosis of cardiac involvement is another limitation of our study.
In conclusion, EGPA patients with cardiac involvement tended to have a younger age at onset, higher eosinophil count, higher disease activity, and a poorer prognosis than those without cardiac involvement. Comprehensive cardiac examinations are therefore necessary to diagnose cardiac involvement early. High-dose glucocorticoids combined with CTX was an effective treatment used to prevent the acceleration of cardiac involvement.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Peking Union Medical College Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YC collected and analyzed the data and wrote the manuscript. JZ, JL, QW, and HY collected and analyzed the data and revised the manuscript. XG, WZ, YZ, FZ, and XZ designed the study, re-confirmed the diagnoses of all the patients, and revised the manuscript. SZ and YF designed the study, analyzed the data, and wrote the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81671620 and 81971545) and the CAMS Innovation Fund for Medical Sciences (CIFMS, 2017-I2M-3-015).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a pre-print at Research Square (41).
References
1. Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. (1951) 27:277–301.
2. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. (2013) 65:1–11. doi: 10.1002/art.37715
3. Eustace JA, Nadasdy T, Choi M. Disease of the month. The Churg Strauss Syndrome. J Am Soc Nephrol. (1999) 10:2048–55.
4. Sinico RA, Di Toma L, Maggiore U, Bottero P, Radice A, Tosoni C, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. (2005) 52:2926–35. doi: 10.1002/art.21250
5. Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine. (1999) 78:26–37. doi: 10.1097/00005792-199901000-00003
6. Sable-Fourtassou R, Cohen P, Mahr A, Pagnoux C, Mouthon L, Jayne D, et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med. (2005) 143:632–8. doi: 10.7326/0003-4819-143-9-200511010-00006
7. Tervaert JWC, Elema JD, Kallenberg CGM. Clinical and histopathological association of 29kD-ANCA and MPO-ANCA. APMIS. (1990) 98(Suppl 19):35. doi: 10.1111/j.1600-0463.1990.tb05722.x
8. Reid AJ, Harrison BD, Watts RA, Watkin SW, McCann BG, Scott DG. Churg-Strauss syndrome in a district hospital. QJM. (1998) 91:219–229. doi: 10.1093/qjmed/91.3.219
9. Dunogue B, Terrier B, Cohen P, Marmursztejn J, Legmann P, Mouthon L, et al. Impact of cardiac magnetic resonance imaging on eosinophilic granulomatosis with polyangiitis outcomes: a long-term retrospective study on 42 patients. Autoimmun Rev. (2015) 14:774–80. doi: 10.1016/j.autrev.2015.04.013
10. Abu-Shakra M, Smythe H, Lewtas J, Badley E, Weber D, Keystone E. Outcome of polyarteritis nodosa and Churg-Strauss syndrome: an analysis of twenty-five patients. Arthritis Rheum. (1994) 37:1798–803. doi: 10.1002/art.1780371214
11. Chumbley LC, Harrison EG Jr, DeRemee RA. Allergic granulomatosis and angiitis (Churg-Strauss syndrome): Report and analysis of 30 cases. Mayo Clin Proc. (1977) 52:477–84.
12. Gaskin G, Clutterbuck EJ, Pusey CD. Renal disease in the Churg-Strauss syndrome: Diagnosis, management and outcome. Contrib Nephrol. (1991) 94:58–65. doi: 10.1159/000420611
13. Solans R, Bosch JA, Perez-Bocanegra C, Selva A, Huguet P, Alijotas J, et al. Churg-Strauss syndrome: outcome and long-term follow-up of 32 patients. Rheumatology. (2001) 40:763–71. doi: 10.1093/rheumatology/40.7.763
14. Hazebroek MR, Kemna MJ, Schalla S, Sanders-van Wijk S, Gerretsen SC, Dennert R, et al. Prevalence and prognostic relevance of cardiac involvement in ANCA-associated vasculitis: eosinophilic granulomatosis with polyangiitis and granulomatosis with polyangiitis. Int J Cardiol. (2015) 199:170–9. doi: 10.1016/j.ijcard.2015.06.087
15. Vinit J, Bielefeld P, Muller G, Pfitzenmeyer P, Bonniaud P, Lorcerie B, et al. Heart involvement in Churg-Strauss syndrome: retrospective study in French Burgundy population in past 10 years. Eur J Intern Med. (2010) 21:341–6. doi: 10.1016/j.ejim.2010.05.004
16. Lanham JG, Elkon KB, Pusey CD, Hughes GR. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine. (1984) 63:65–81. doi: 10.1097/00005792-198403000-00001
17. Dennert RM, van Paassen P, Schalla S, Kuznetsova T, Alzand BS, Staessen JA, et al. Cardiac involvement in Churg-Strauss syndrome. Arthritis Rheum. (2010) 62:627–34. doi: 10.1002/art.27263
18. Haas C, Le Jeunne C, Choubrac P, Durand H, Hugues FC. [Churg-Strauss syndrome. Retrospective study of 20 cases]. Bull Acad Natl Med. (2001) 185:1113–30; discussion 1130–1133. doi: 10.1016/S0001-4079(19)34475-9
19. Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine. (1996) 75:17–28. doi: 10.1097/00005792-199601000-00003
20. Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Le Toumelin P, et al. The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine. (2011) 90:19–27. doi: 10.1097/MD.0b013e318205a4c6
21. Mahr A, Katsahian S, Varet H, Guillevin L, Hagen EC, Hoglund P, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. (2013) 72:1003–10. doi: 10.1136/annrheumdis-2012-201750
22. Bourgarit A, Le Toumelin P, Pagnoux C, Cohen P, Mahr A, Le Guern V, et al. Deaths occurring during the first year after treatment onset for polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: a retrospective analysis of causes and factors predictive of mortality based on 595 patients. Medicine. (2005) 84:323–30. doi: 10.1097/01.md.0000180793.80212.17
23. Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. (1990) 33:1094–100. doi: 10.1002/art.1780330806
24. Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. (1994) 87:671–8.
25. Neumann T, Manger B, Schmid M, Kroegel C, Hansch A, Kaiser WA, et al. Cardiac involvement in Churg-Strauss syndrome: impact of endomyocarditis. Medicine. (2009) 88:236–43. doi: 10.1097/MD.0b013e3181af35a5
26. Keogh KA, Specks U. Churg-Strauss syndrome: clinical presentation, antineutrophil cytoplasmic antibodies, and leukotriene receptor antagonists. Am J Med. (2003) 115:284–90. doi: 10.1016/S0002-9343(03)00359-0
27. Miszalski-Jamka T, Szczeklik W, Sokołowska B, Karwat K, Belzak K, Mazur W, et al. Standard and feature tracking magnetic resonance evidence of myocardial involvement in Churg-Strauss syndrome and granulomatosis with polyangiitis (Wegener's) in patients with normal electrocardiograms and transthoracic echocardiography. Int J Cardiovasc Imaging. (2013) 29:843–53. doi: 10.1007/s10554-012-0158-6
28. Wassmuth R, Gobel U, Natusch A, Schneider W, Kettritz R, Dietz R, et al. Cardiovascular magnetic resonance imaging detects cardiac involvement in Churg-Strauss syndrome. J Card Fail. (2008) 14:856–60. doi: 10.1016/j.cardfail.2008.07.227
29. Cereda AF, Pedrotti P, De Capitani L, Giannattasio C, Roghi A. Comprehensive evaluation of cardiac involvement in eosinophilic granulomatosis with polyangiitis (EGPA) with cardiac magnetic resonance. Eur J Intern Med. (2017) 39:51–6. doi: 10.1016/j.ejim.2016.09.014
30. Liu GY, Ventura IB, Achtar-Zadeh N, Elicker BM, Jones KD, Wolters PJ, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in north american patients with idiopathic pulmonary fibrosis. Chest. (2019) 156:715–23. doi: 10.1016/j.chest.2019.05.014
31. Sinico RA, Di Toma L, Maggiore U, Tosoni C, Bottero P, Sabadini E, et al. Renal involvement in Churg-Strauss syndrome. AJKD. (2006) 47:770–9. doi: 10.1053/j.ajkd.2006.01.026
32. Saito Y, Okada S, Funabashi N, Kobayashi Y. ANCA-negative eosinophilic granulomatosis with polyangitis (EGPA) manifesting as a large intracardiac thrombus and glomerulonephritis with angionecrosis. BMJ Case Reports. (2016) doi: 10.1136/bcr-2016-216520
33. Pakbaz M, Pakbaz M. Cardiac involvement in eosinophilic granulomatosis with polyangiitis: a meta-analysis of 62 case reports. J Tehran Heart Cent. (2020) 15:18–26. doi: 10.18502/jthc.v15i1.3334
34. Frustaci A, Gentiloni N, Chimenti C, Natale L, Gasbarrini G, Maseri A. Necrotizing myocardial vasculitis in Churg-Strauss syndrome: clinicohistologic evaluation of steroids and immunosuppressive therapy. Chest. (1998) 114:1484–9. doi: 10.1378/chest.114.5.1484
35. Cohen P, Pagnoux C, Mahr A, Arene J-P, Mouthon L, Le Guern V, et al. Churg-Strauss syndrome with poor-prognosis factors: a prospective multicenter trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in forty-eight patients. Arthritis Care Res. (2007) 57:686–93. doi: 10.1002/art.22679
36. Smith DA, Minthorn EA, Beerahee M. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti-interleukin-5 monoclonal antibody. Clin Pharmacokinet. (2011) 50:215–27. doi: 10.2165/11584340-000000000-00000
37. Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. (2017) 376:1921–32. doi: 10.1056/NEJMoa1702079
38. Moosig F, Gross WL, Herrmann K, Bremer JP, Hellmich B, Hellmich B. Targeting interleukin-5 in refractory and relapsing Churg-Strauss syndrome. Ann Intern Med. (2011) 155:341–3. doi: 10.7326/0003-4819-155-5-201109060-00026
39. Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. (2010) 125:1336–43. doi: 10.1016/j.jaci.2010.03.028
40. Herrmann K, Gross WL, Moosig F. Extended follow-up after stopping mepolizumab in relapsing/refractory Churg-Strauss syndrome. Clin Exp Rheumatol. (2012) 30:S62–S5. doi: 10.1186/ar3747
Keywords: eosinophilic granulomatosis with polyangitis, cardiac involvement, clinical characteristics, outcome, Chinese population
Citation: Chen Y, Guo X, Zhou J, Li J, Wu Q, Yang H, Zhang S, Fei Y, Zhang W, Zhao Y, Zhang F and Zeng X (2020) Cardiac Involvement in Eosinophilic Granulomatosis With Polyangiitis: A Retrospective Study in the Chinese Population. Front. Med. 7:583944. doi: 10.3389/fmed.2020.583944
Received: 16 July 2020; Accepted: 06 November 2020;
Published: 10 December 2020.
Edited by:
Lorenzo Cavagna, Fondazione Ospedale San Matteo (IRCCS), ItalyReviewed by:
Marlene Plüß, University Medical Center Göttingen, GermanyCong-Qiu Chu, Oregon Health and Science University, United States
Copyright © 2020 Chen, Guo, Zhou, Li, Wu, Yang, Zhang, Fei, Zhang, Zhao, Zhang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shangzhu Zhang, aXJlbmV6aGFuZ3B1bWNoQDE2My5jb20=; Yunyun Fei, ZmVpeXVueXVuQHB1bWNoLmNu
 Yingying Chen
Yingying Chen Xiaoxiao Guo2
Xiaoxiao Guo2 Jing Li
Jing Li Shangzhu Zhang
Shangzhu Zhang Yunyun Fei
Yunyun Fei Wen Zhang
Wen Zhang Xiaofeng Zeng
Xiaofeng Zeng