
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 03 November 2020
Sec. Obstetrics and Gynecological Surgery
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.581982
Mixed serous-endometrioid endometrial carcinoma is a type of endometrial cancer with relatively low incidence. The genetic factors contributing to the tumorigenesis of mixed carcinoma remains to be explored. Here, we report the first identification of two germline mutations in BRCA1 and MSH2 in a woman with mixed serous papillary adenocarcinoma and endometrioid carcinoma. Immunohistochemistry analysis showed loss of MSH2 and MSH6 protein expression in the endometrioid component. The patient showed partial response to tislelizumab treatment following progression on chemotherapy. Two germline mutations in BRCA1 and MSH2 may collectively promote the tumorigenesis of uterine endometrium with two distinct histological components.
Endometrial carcinoma (EC) is the second most common gynecologic malignancy in China, with an estimated 63,400 new cases and 21,800 deaths in 2015 (1). The vast majority of ECs are sporadic, and hereditary tumor syndrome [most commonly Lynch syndrome (LS)] accounts for ~5% of cases (2). LS is characterized by the identification of germline pathogenetic mutations in mismatch repair (MMR) genes (mainly including MLH1, MSH2, MSH6, PMS2), microsatellite instability and loss of MMR protein expression, which are usually related to endometrioid histology. Besides, Shu et al. reported that BRCA1 germline mutations may increase the risk for serous or serous-like ECs (3). Concurrent pathogenic variants in different genes in one individual is extremely rare. In this study, we identified two germline pathogenic mutations in the BRCA1 and MSH2 genes in a patient with mixed endometrioid and serous EC (EEC-SC).
A 52-year-old woman underwent laparoscopic total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and dissection of pelvic and para-aortic lymph nodes in June, 2018. Pathological findings confirmed the diagnosis of stage IIIA serous papillary adenocarcinoma mixed with endometrioid carcinoma with squamous differentiation (~70% for serous carcinoma component and 30% for endometroid carcinoma component, respectively) (Figure 1A), with tumor metastasis to both fallopian tubes. Immunohistochemistry analysis showed loss of MSH2 (Figure 1B) and MSH6 (Figure 1C) protein expression in the EEC component. She received 6 cycles of paclitaxel and carboplatin as adjuvant therapy with complete response. Postoperative routine follow-up examination in May, 2019 showed that serum CA125 was elevated (71.66 U/ml), but CT examination did not reveal any abnormalities. The level of serum CA125 increased to 100.90 U/ml after 1 month, and ultrasound examination also showed enlarged paraaortic lymph node of 2.5 cm in diameter, suggesting tumor recurrence. Rechallenge of paclitaxel and carboplatin for one cycle failed with continued increase of CA125 to 120.1 U/ml. Subsequently, the patient switched to oxaliplatin combined with pegylated liposomal doxorubicin for one cycle with primary progression. Evaluation after the chemotherapy on August 1, 2019 showed serum CA125 level increased to 231.6 U/ml. CT scan suggested an enlarged left para-aortic lymph node (37*30 mm) (Supplementary Figures 1A,E).
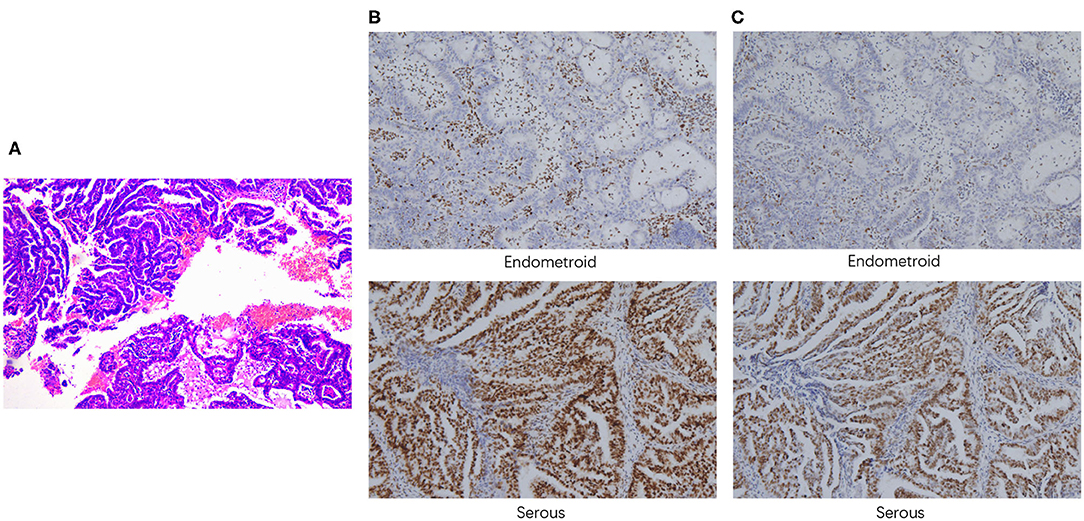
Figure 1. Pathological findings. (A) H&E staining demonstrated the presence of both serous and endometrioid components. Immunohistochemistry staining of MSH2 (B) and MSH6 (C) (100x magnification).
The patient came to our hospital for second opinion. To seek for potential targeted therapies and immunotherapies, paired tumor-normal next-generation sequencing of 1,021 cancer-related genes was performed using tumor tissue and peripheral blood. Of great interest, two heterozygous germline mutations in BRCA1 (NM_007294.3 c.3348_3351delAGTT p.V1117Rfs*11) and MSH2 (large deletion of exons 4-16) were identified (Figures 2B–D). Besides, a total of 72 somatic mutations were detected, including putative or known functional mutations in PTEN, ARID1A, TP53, FBXW7, and KRAS (Supplementary Table 1). In addition, genetic testing results showed that microsatellites were highly unstable, and tumor mutation burden was extremely high (51.84 muts/Mb).
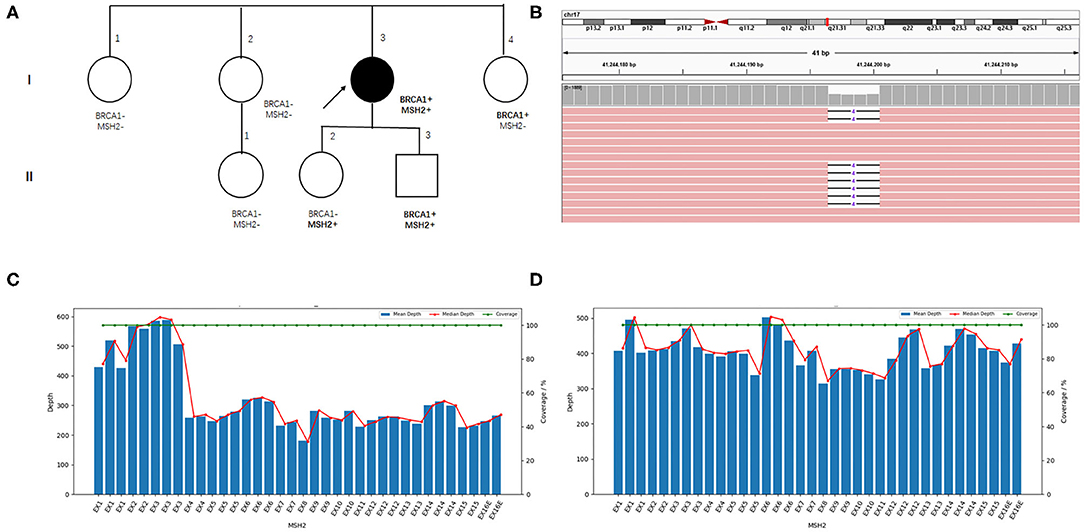
Figure 2. Family pedigree and germline mutations identified in the index patient. (A) Family pedigree. The index patient is indicated with an arrow, and the circle filled with black color denotes endometrial cancer. (B) Visualized sequencing data shows the BRCA1 variant. MSH2 exons coverage depth analysis in the index patient (C) and another sample with the same batch (D).
To access the cancer risk for the family members of the patient, Sanger sequencing and RT-PCR were performed to confirm the presence of germline BRCA1 and MSH2 mutations in her family members, although she has no family history of cancer. Her younger sister (I-4) and daughter (II-2) carry the BRCA1 and MSH2 mutations, respectively. Unfortunately, her son harbors both the BRCA1 and MSH2 mutations (Figures 2A, Supplementary Figures 2, 3).
The patient was then treated with 200 mg intravenous tislelizumab every 3 weeks from September 4, 2019. Partial remission was achieved at 9 weeks after treatment with the shrinkage of an enlarged left para-aortic lymph node (Supplementary Figures 1B,F). In addition, the normalization of serum CA125 (12.28 U/ml) was observed after 2 months of treatment. CT scan at 15 weeks after treatment demonstrated continuous shrinkage of the enlarged lymph node (Supplementary Figures 1C,G). She has been on treatment for 4 months, and discontinued the treatment for 2 months due to the impact of COVID-19 outbreak. Fortunately, the CT scan did not reveal progression as a result of drug interruption (Supplementary Figures 1D,H). She continued the treatment and is still in follow-up. During the treatment, she experienced grade 1 treatment-related elevation of alanine transaminase (ALT) and aspartate transaminase (AST), which was relieved after symptomatic treatment.
The case illustrates that the genetic factors of ECs are complex and may result in different histologic presentations. MSH2 large deletions were identified in 6.4% (28/439) families with LS (4). In this case, a novel heterozygous MSH2 large deletion was identified in the index patient using a well-designed panel-based NGS test. This reminds us that professionals for genetic variants interpretation should be aware of this rare type of mutation in practice. The relationship between mutations in the BRCA1 gene and EC is controversial. Multiple studies found that BRCA mutation carriers may have an elevated risk of EC, while others suggested that the increased risk may be associated with tamoxifen treatment (3, 5–7). In our case, the index patient did not have history of breast cancer and tamoxifen treatment. A large retrospective study showed that the incidence of serous/serous-like ECs in BRCA1 mutation carriers is significantly higher than expected. Biron-Shental et al. also found that high rate of BRCA germline mutation in SC patients accompanied by strong familial cancer history may indicate that SC is a part of HBOC (8). Therefore, we speculated that the SC component in the patient may be associated with the BRCA1 germline mutation. Two germline mutations in BRCA1 and MSH2 may collectively promote the tumorigenesis of a single lesion with two distinct pathological components.
EC can be routinely classified into two distinct histological subtypes. Type I (~80–90%, mainly endometrioid adenocarcinoma) and type II (relatively uncommon, primarily serous and clear cell adenocarcinoma) tumors are distinct at the molecular level. High frequency of POLE, PTEN, CTNNB1, PIK3R1, ARID1A, KRAS mutations and microsatellite instability are found in type I tumors, whereas mutations in TP53 and FBXW7, and somatic copy number alterations are more frequently found in type II carcinomas (9, 10). Coenegrachts et al. found that in majority of the cases, SC and EEC components in mixed EEC-SC exhibit distinct molecular characteristics, but have similar mutation profiles compared to SC and EEC cancers, respectively (11, 12). In the present case with mixed histological components, frequently mutated genes in endometrioid tumors (PTEN, ARID1A, KRAS) and serous tumors (TP53, FBXW7) are all mutated. Our results were consistent with their findings and supported the divergent clonal evolution in mixed ECs.
Immune checkpoint inhibitors provide an optional treatment strategy for patients with LS-related EC. In 2017, pembrolizumab, a mono-clonal antibody targeting programmed death receptor-1 (PD-1), was approved for microsatellite instability–high (MSI-H)/mismatch-repair–deficient (dMMR) solid tumors that have progressed after prior therapy and have no satisfactory alternative treatment options. A phase II study of pembrolizumab monotherapy in patients with MSI-H/dMMR endometrial cancer (n = 49) demonstrated an objective response rate (ORR) of 57.1% (95% CI, 42.2–71.2%), with a median progression-free survival (PFS) of 25.7 months (95% CI, 4.9 months to not reached) (13, 14). Tislelizumab, another anti PD-1 antibody, has been approved by NMPA for the treatment of recurrent and refractory classical Hodgkin lymphoma, as well as previously treated locally advanced or metastatic urothelial carcinoma with PD-L1 high expression. Multiple clinical studies demonstrated that tislelizumab monotherapy was well tolerated and effective in patients with advanced solid tumors, including urothelial, lung and gastric carcinoma, with the objective response rate ranging from 13 to 25% (15–17). To date, no clinical trial has been conducted to investigate the clinical activity of tislelizumab in patients with endometrial cancer. In our index patient, microsatellites were highly unstable. She has a germline mutation in MSH2, while IHC showed MSH2 and MSH6 expression were lost in the EEC component. She received tislelizumab with a good response after progression on multiple lines of chemotherapy. Due to the identification of the BRCA1 germline mutation, poly ADP-ribose polymerase inhibitors monotherapy or combined with immunotherapy may be used in the subsequent lines of treatment. Tumors with BRCA1/2 pathogenic mutations have higher level of genomic instability, and this may generate more neoantigens, which may be associated with better efficacy when receiving treatment with immune checkpoint inhibitors (18). Based on this rational, combined treatment with PARP inhibitors and immunotherapies has shown promising efficacy in multiple clinical trials (19, 20). The combination of tislelizumab with a novel PARP inhibitor—pamiparib was evaluated in solid tumors in a phase Ia/b clinical trial. Ten (20%) of 49 patients achieved an objective response, including two complete responses and eight partial responses (21).
In conclusion, this is the first report of two germline mutations in BRCA1 and MSH2 identified in a woman with mixed EEC-SC. Tumor heterogeneity at the level of germline and somatic aberrations may collectively promote the histological divergence in mixed EEC-SC.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by ethics committee of Peking University Cancer Hospital & Institute. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YG: conception and design and study supervision. HW and RC: acquisition of data. HZ and MY: analysis and interpretation of data and writing, review, and/or revision of the manuscript. All authors contributed to the article and approved the submitted version.
MY and RC were employed by the company Geneplus-Beijing.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.581982/full#supplementary-material
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
2. Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. (2019) 19:510–21. doi: 10.1038/s41568-019-0177-x
3. Shu CA, Pike MC, Jotwani AR, Friebel TM, Soslow RA, Levine DA, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. (2016) 2:1434–40. doi: 10.1001/jamaoncol.2016.1820
4. van der Klift H, Wijnen J, Wagner A, Verkuilen P, Tops C, Otway R, et al. Molecular characterization of the spectrum of genomic deletions in the mismatch repair genes MSH2, MLH1, MSH6, and PMS2 responsible for hereditary nonpolyposis colorectal cancer (HNPCC). Genes Chromosomes Cancer. (2005) 44:123–38. doi: 10.1002/gcc.20219
5. Thompson D, Easton DF, Breast Cancer Linkage Consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. (2002) 94:1358–65. doi: 10.1093/jnci/94.18.1358
6. Beiner ME, Finch A, Rosen B, Lubinski J, Moller P, Ghadirian P, et al. The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations. A prospective study. Gynecol Oncol. (2007) 104:7–10. doi: 10.1016/j.ygyno.2006.08.004
7. Segev Y, Iqbal J, Lubinski J, Gronwald J, Lynch HT, Moller P, et al. The incidence of endometrial cancer in women with BRCA1 and BRCA2 mutations: an international prospective cohort study. Gynecol Oncol. (2013) 130:127–31. doi: 10.1016/j.ygyno.2013.03.027
8. Biron-Shental T, Drucker L, Altaras M, Bernheim J, Fishman A. High incidence of BRCA1-2 germline mutations, previous breast cancer and familial cancer history in Jewish patients with uterine serous papillary carcinoma. Eur J Surg Oncol. (2006) 32:1097–100. doi: 10.1016/j.ejso.2006.03.032
9. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. (2013) 497:67–73. doi: 10.1038/nature12113
10. Lax SF. Pathology of endometrial carcinoma. Adv Exp Med Biol. (2017) 943:75–96. doi: 10.1007/978-3-319-43139-0_3
11. Coenegrachts L, Garcia-Dios DA, Depreeuw J, Santacana M, Gatius S, Zikan M, et al. Mutation profile and clinical outcome of mixed endometrioid-serous endometrial carcinomas are different from that of pure endometrioid or serous carcinomas. Virchows Arch. (2015) 466:415–22. doi: 10.1007/s00428-015-1728-5
12. Gatius S, Cuevas D, Fernandez C, Roman-Canal B, Adamoli V, Piulats JM, et al. Tumor heterogeneity in endometrial carcinoma: practical consequences. Pathobiology. (2018) 85:35–40. doi: 10.1159/000475529
13. O'Malley D, Marabelle A, De Jesus-Acosta A, Piha-Paul SA, Arkhipov A, Longo F, et al. 1044P - Pembrolizumab in patients with MSI-H advanced endometrial cancer from the KEYNOTE-158 study. Ann Oncol. (2019) 30:v425–6. doi: 10.1093/annonc/mdz250.052
14. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord J-P, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. (2020) 38:1–10. doi: 10.1200/JCO.19.02105
15. Deva S, Lee J, Lin C, Yen C, Millward M, Chao Y, et al. 70O A phase Ia/Ib trial of tislelizumab, an anti-PD-1 antibody (ab), in patients (pts) with advanced solid tumors. Ann Oncol. (2018) 29(Suppl._10):mdy487.042. doi: 10.1093/annonc/mdy487.042
16. BeiGene. BeiGene Announces Clinical Results on Tislelizumab Presented at the 22nd Annual Meeting of the Chinese Society of Clinical Oncology (CSCO). BeiGene (2019).
17. Ye D, Liu J, Zhou A, Zou Q, Li H, Fu C, et al. First report of efficacy and safety from a phase II trial of tislelizumab, an anti-PD-1 antibody, for the treatment of PD-L1+ locally advanced or metastatic urothelial carcinoma (UC) in Asian patients. Ann Oncol. (2019) 30:v367. doi: 10.1093/annonc/mdz249.019
18. Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. (2016) 7:13587–98. doi: 10.18632/oncotarget.7277
19. Konstantinopoulos PA, Waggoner SE, Vidal GA, Mita MM, Fleming GF, Holloway RW, et al. TOPACIO/Keynote-162 (NCT02657889): A phase 1/2 study of niraparib + pembrolizumab in patients (pts) with advanced triple-negative breast cancer or recurrent ovarian cancer (ROC)—Results from ROC cohort. J Clin Oncol. (2018) 36(15_Suppl.):106. doi: 10.1200/JCO.2018.36.15_suppl.106
20. Bang Y-J, Kaufman B, Geva R, Stemmer SM, Hong S-H, Lee J-S, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): results in patients with relapsed gastric cancer. J Clin Oncol. (2019) 37(4_Suppl.):140. doi: 10.1200/JCO.2019.37.4_suppl.140
21. Friedlander M, Meniawy T, Markman B, Mileshkin L, Harnett P, Millward M, et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. (2019) 20:1306–15. doi: 10.1016/S1470-2045(19)30396-1
Keywords: double germline mutations, mixed serous-endometrioid endometrial carcinoma, next-generation sequencing, tislelizumab, tumor heterogeneity
Citation: Zheng H, Yuan M, Wu H, Chen R and Gao Y (2020) Case Report: Double Germline Mutations in BRCA1 and MSH2 in a Patient With Mixed Serous-Endometrioid Endometrial Carcinoma. Front. Med. 7:581982. doi: 10.3389/fmed.2020.581982
Received: 31 July 2020; Accepted: 05 October 2020;
Published: 03 November 2020.
Edited by:
Zaleha Abdullah Mahdy, National University of Malaysia, MalaysiaReviewed by:
Prafull Ghatage, Tom Baker Cancer Centre, CanadaCopyright © 2020 Zheng, Yuan, Wu, Chen and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunong Gao, Z2FveXVub25nQHZpcC5zaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.