- 1Department of General Surgery and Central Laboratory, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 2School of Biotechnology and Biomolecular Sciences, University of New South Wales, Sydney, NSW, Australia
- 3Gastrointestinal and Liver Unit, Prince of Wales Hospital, University of New South Wales, Sydney, NSW, Australia
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract mainly comprising two forms including Crohn's disease (CD) and ulcerative colitis (UC). IBD is a lifelong relapsing remitting disease and relapses occur at random patterns which are unpredictable. Fecal biomarkers have been increasingly used to assess disease activity in IBD due to their positive correlations with intestinal inflammation. Recent studies have also assessed the use of fecal biomarkers in predicting relapse and post-operative recurrence. This review provides information from global studies of using fecal calprotectin, lactoferrin and S100A12 to predict relapse in IBD. Strategies for further studies and the use of these fecal biomarkers for personalized management in IBD are also discussed.
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract comprising of two major subsets, Crohn's disease (CD) and ulcerative colitis (UC) (1). Inflammatory bowel disease is a lifelong disease and patients often experience multiple episodes of relapse and remission. Relapses in IBD occur at a random pattern, which are unpredictable. Endoscopy is not used routinely for disease monitoring due to its invasiveness and cost. Current monitoring of disease relapses in patients with IBD is symptom based (1). In order to improve patient management, various studies have assessed the use of fecal biomarkers in predicting disease relapse (2).
Fecal biomarkers have attracted a great attention owning to their non-invasiveness and cost effectiveness. Fecal biomarkers used in IBD are bioproducts resulted from inflammatory responses in the intestinal mucosa. Calprotectin is the most studied fecal biomarker. Lactoferrin, S100A12 and other fecal biomarkers have also been examined in recent years. Most of the studies have reported that these biomarkers correlate well with the endoscopic score and histological inflammation in patients with IBD (3–12).
Recent studies have also assessed the use of fecal biomarkers in predicting relapse and post-operative recurrence. In this review article, we provide comprehensive and updated information from global studies on the use of fecal calprotectin, lactoferrin and S100A12 to predict relapse in IBD. We have also discussed strategies for further studies and the use of these fecal biomarkers for personalized management in IBD.
Biology of Calprotectin, Lactoferrin, and S100A12
Fecal biomarkers used in IBD are either actively secreted by or released from necrotic immune cells during inflammatory responses at the intestinal mucosa. They have a wide variety of biological functions including antimicrobial activity, proinflammatory activity, degradation of extracellular matrix and intracellular pathogens, as well as cellular and metabolic activities.
Calprotectin
Calprotectin is a cytoplasmic protein prominently found in neutrophils that accounts for more than 40% of the cytosolic proteins in neutrophils, and to a lesser extent in monocytes and macrophages. Calprotectin is released to extracellular environment during inflammatory responses upon neutrophil activation or necrosis and induces neutrophil chemotaxis and adhesion. Calprotectin is stable for up to 1 year when stored at −20°C, and stable for 7 days when stored at 4°C and room temperature (13–15).
The physiologically active conformation of calprotectin is a heterodimer complex consisting of S100A8 and S100A9 and both proteins belong to the S100 family. The S100A8 and S100A9 subunits consist of 93 and 113 amino acids with molecular weight of 10.8 and 13.2 kDa, respectively (16, 17). Each subunit is able to bind two calcium ions. In addition to the calcium binding site, each heterodimer displays two transition metal binding sites at the interface of S100A8/S100A9, the first site binds manganese and zinc, while the second site binds zinc only (18–21).
As a metal chelating agent, calprotectin binds transition metals with high affinity and efficiently sequester them away from invading microbial pathogens, thereby starves invading pathogens, limiting their growth and resulting in a process called “nutritional immunity” (22–25). At the site of infection, calprotectin is not only abundantly released by neutrophils, but also epithelial cells and other immune cells, thereby playing a critical role in host defense against various bacterial species such as Listeria monocytogenes, Salmonella Typhimurium, Borrelia burgdorferi, Helicobacter pylori, Staphylococcus aureus, as well as fungal pathogens including Candida albicans (26–33). Interestingly, some bacterial pathogens harbor mechanisms allowing them to evade the harmful environment created by calprotectin. For examples, H. pylori is able to alter its outer membrane via lipid A modification, thus evading the antimicrobial activity of calprotectin. The growth of S. Typhimurium was actually elevated over competing commensal microbes in the presence of calprotectin due to the presence of ZnuABC zinc transporter, which enables the bacterium to acquire zinc under zinc-limiting conditions (34, 35).
Lactoferrin
Lactoferrin is present in most exocrine secretions such as milk, saliva, tears, mucosal secretions, and plasma (36). Secretory epithelia and neutrophils are the main sources of lactoferrin. Lactoferrin is stable for up to 7 days when stored at 4°C or room temperature (37–39).
Human lactoferrin is an 80 kDa glycoprotein containing ~700 amino acids. The single polypeptide chain forms two homologous globular domains, namely N-terminal lobe and C-terminal lobe, respectively, depending on their localization, and each terminal lobe contains two domains (N1, N2, C1, and C2), resulting in a deep cleft conformation for iron-binding (40).
Lactoferrin has antimicrobial activity. Lactoferrin binds free iron, which inhibits the growth of iron-dependent bacterial species and reduces bacterial biofilm formation (41). Lactoferrin can also bind to receptors on bacterial surface, which induces death of Gram-negative bacteria due to a disruption in the cell wall and inhibits the formation of bacterial biofilms. Under inflammatory conditions, the levels of lactoferrin are increased.
S100A12
S100A12 is also a protein of the S100 family that is predominately expressed and secreted by neutrophils. Human S100A12 contains 91 amino acids with a molecular weight of 10.4 kDa and the protein is stable for 7–10 days when stored at room temperature (42–44). Similar to calprotectin, S100A12 is able to bind calcium, iron and zinc. As a metal chelating agent, S100A12 also has antimicrobial activity (45–47). Furthermore, S100A12 has chemotactic characteristic that recruits mast cells and monocytes to the site of inflammation (48–50). S100A12 is able to bind a number of cellular receptors. Recent evidence suggest that S100A12 stimulate proinflammatory responses in monocytes via Toll-like receptor 4, leading to upregulated monocyte expression of proinflammatory cytokines including interleukin (IL)-1β, IL-6, and IL-8 (51). S100A12 is overexpressed in inflammatory conditions.
Calprotectin, Lactoferrin, and S100A12 In Predicting Relapse In IBD
The gold standard of defining clinical remission or relapse relies on endoscopic mucosal healing and histological scoring of inflammation. Majority of the quiescent IBD patients have residual inflammation in the colonic mucosa, and when the degree of inflammation reaches a critical level, symptomatic relapse occurs (52). Various research groups have examined the use of fecal biomarkers as predictive markers for relapse and they are summarized in Table 1. Most of these studies assessed calprotectin and few examined lactoferrin and S100A12. Of the 31 studies listed in Table 1, 29 studies examined calprotectin, three studies examined lactoferrin and one study examined S100A12. Some of these studies have examined multiple fecal biomarkers.
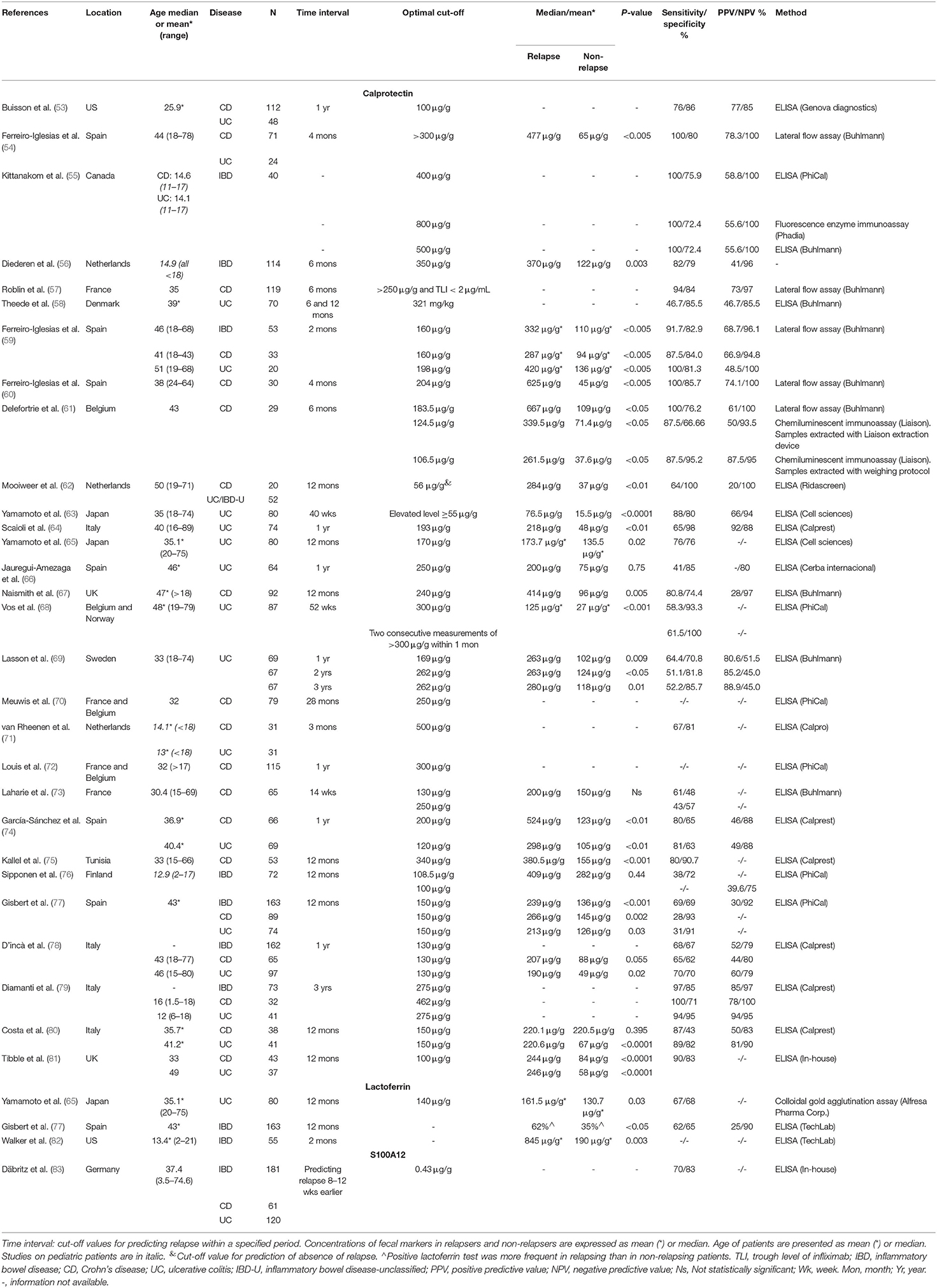
Table 1. Summary of studies investigating fecal biomarkers for the prediction of relapses in inflammatory bowel disease.
The reported sensitivities, specificities and the cut-off values in different studies assessing fecal calprotectin as a biomarker in predicting relapse varied greatly. Of the 29 studies of calprotectin listed in Table 1, the sensitivities for predicting CD, UC, and IBD ranged from 28 to 100%, 31 to 100%, and 38 to 100%, respectively. The specificities for predicting CD, UC, and IBD ranged from 43 to 52%, 63 to 100%, and 69 to 100%, respectively. The cut-off values for CD, UC, and IBD varied from 106.5 to 462 μg/g, 120 to 321 μg/g, and 100 to 800 μg/g, respectively (Table 1). Twenty-one studies compared the levels of calprotectin of relapsed and non-relapsed patients, of which 18 studies (85.7%) found that the levels of fecal calprotectin in relapsed patients were significantly higher, indicating that the levels of fecal calprotectin reflect the levels of inflammation in the intestinal mucosal tissues. A meta-analysis by Mao et al. analyzed combined data from six studies in Table 1, comprising a total of 672 adult IBD patients (318 UC and 354 CD) (84). They reported that the pooled sensitivity and specificity of fecal calprotectin in predicting relapse in quiescent IBD to be 78 and 73%, respectively (84). However, this meta-analysis did not state the cut-off values of the pooled data, the cut-off values in the six original studies varied from 100 to 340 μg (74, 75, 77, 78, 80, 81).
The time intervals observed in studies examining fecal calprotectin in Table 1 were from 2 months to 3 years. More than 50% of these studies observed patients for a time interval of 1 year or above. The remaining studies observed patients for shorter terms such as 2, 4, or 6 months. There were no specific traits associated with observation term intervals in respect of cut-off values, sensitivities and specificities.
Most of the studies on fecal calprotectin in predicting IBD relapse were from Europe. Of the 29 studies examining calprotectin in Table 1, 23 were from Europe, two from North America, two from UK, one from Africa, and there were only two studies from Asian populations, both of which were from the same research group in Japan (63, 65).
Enzyme-linked immunosorbent assay (ELISA) was used in quantifying the levels of calprotectin in stools in 23 out of the 29 studies in Table 1. The remaining studies used other methods such as Lateral Flow Assay, chemiluminescent immunoassay, colloidal gold agglutination assay, and fluorescence enzyme immunoassay. The ELISA kits used by these studies were from eight different manufacturers and one study used in-house ELISA. The studies by Kittanakom et al. and Delefortrie et al. have compared different methods in quantifying fecal calprotectin for predicting relapse of IBD and CD, respectively (55, 61). Kittanakom et al. (55) reported the cut-off values of 400 and 500 μg/g when using ELISA kits supplied by two different manufacturers, however the cut-off was of a much higher value (800 μg/g) when fluorescence enzyme immunoassay was used. Delefortrie et al. showed cut-off values of 124.5 and 106.5 μg/g when the same chemiluminescent immunoassay was performed with different sample extraction methods, but the cut-off was much higher (183.5 μg/g) when Lateral Flow Assay was used (61). These results showed that variations can be introduced due to different detection methods used in various studies.
To date, only three studies have investigated the use of fecal lactoferrin in predicting relapse in IBD, of which only the study from Japan was able to identify an optimal cut-off value (65). However, this study did not find a statistically significant difference of fecal lactoferrin levels between relapsed and non-relapsed patients. The remaining two studies from Spain and US, although have found a significant difference of fecal lactoferrin levels between relapsed and non-relapsed patients, but they did not report optimal cut-off values for prediction of relapse (77, 82). Only one study had examined the use of S100A12 for predicting relapse in IBD. By using an in-house ELISA, Däbritz et al. showed that a cut-off value of 0.43 μg/g was able to predict relapse 8–12 weeks earlier with sensitivity and specificity being 70 and 83% respectively.
Calprotectin, Lactoferrin, and S100A12 In Predicting Post-Operative Recurrence In CD
A non-invasive biomarker with predictive potential to identify patients without recurrence would be desirable to avoid post-operative endoscopies. In recent years, the use of fecal calprotectin in predicting post-operative recurrence in CD has been evaluated by various studies. Limited studies have also examined lactoferrin and S100A12. These studies are listed in Table 2.
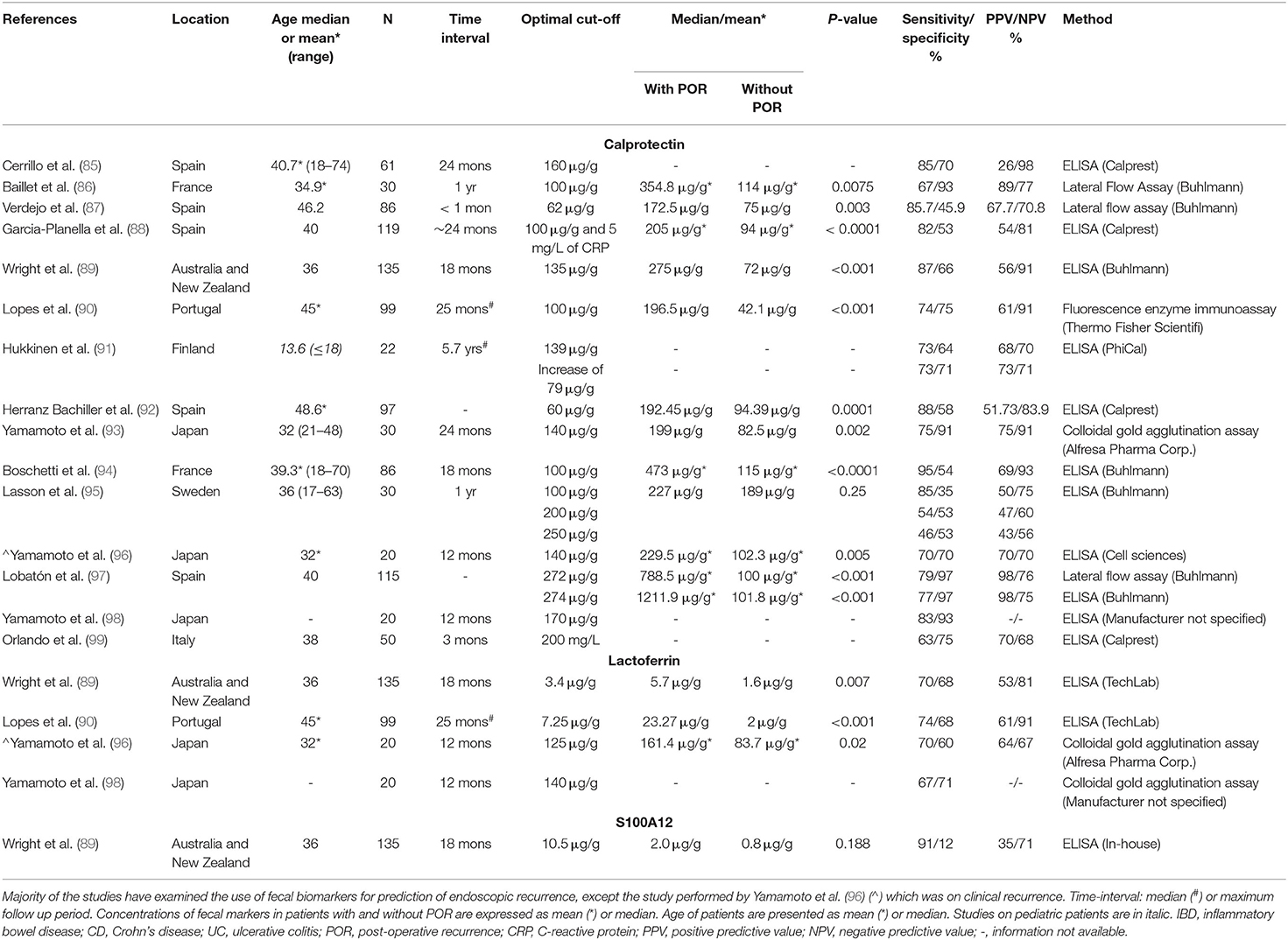
Table 2. Summary of studies investigating fecal biomarkers for the prediction of post-operative recurrence in patients with Crohn's disease.
These studies again reported varied sensitivities, specificities and cut-off values. Studies examining calprotectin reported sensitivities between 46 and 95% and specificities between 45.9 and 97%. The cut-off values also ranged from 60 to 274 μg/g. In the study by Lasson et al. (95) three different cut-off values (100, 200, and 250 μg/g) were assessed, and the corresponding sensitivities were 85, 54, and 46%, respectively. Nevertheless, this study did not detect a significantly different levels of fecal calprotectin in patients with and without post-operative recurrence while the other studies did (Table 2). A meta-analysis performed by Tham et al. on examining the use of fecal calprotectin for detection of post-operative endoscopic recurrence in CD showed that a significant threshold effect was observed for fecal calprotectin values of 50, 100, 150, and 200 μg/g; while the optimal diagnostic accuracy was obtained for fecal calprotectin value of 150 μg/g, with a pooled sensitivity and specificity being 70 and 69%, respectively (100).
Four studies have examined lactoferrin, which all showed significantly different fecal lactoferrin levels in patients with and without post-operative recurrence. However, the cut-off values ranged from 3.4 to 140 μg/g (Table 2). Only one study has examined S100A12 in pediatric patients using an in-house ELISA, which reported a sensitivity of 90% and specificity of 12%, and no significant difference in fecal S100A12 levels was observed in patients with and without post-operative recurrence (Table 2).
Discussion and Suggestions
Studies from diverse geographical regions of the world, mainly from Europe, have examined the use of fecal biomarkers in predicting disease relapse and post-operative recurrence in patients with IBD. Calprotectin is the most studied marker, and several studies also examined lactoferrin and few have investigated S100A12. The consistent information from these studies is that the level of calprotectin increases along with the intestinal mucosal inflammation, which is consistent with the biological functions of this protein. However, whether it can be used to predict disease relapse and post-operative recurrence is inconclusive from the current studies.
Several factors from these studies have contributed to the uncertainty of using fecal biomarkers in predicting disease relapse and post-operative recurrence. Firstly, the cut-off values used in these studies varied remarkably, making it difficult to draw reliable conclusion. Secondly, different detection methods were used, which may produce inconsistent results. Thirdly, the time intervals observed in different studies were random, which again makes it difficult to compare the results between studies. Further studies therefore are warranted to determine whether these fecal biomarkers are reliable predicative markers in the management of IBD. We suggest the following strategies.
Use Fecal Biomarkers as Markers for Personalized Management in IBD
The degree of mucosal inflammation, the level of inflammation that can cause clinical symptoms and the response to different therapeutic agents in individual patients with IBD vary greatly. Given this, fecal biomarkers are perhaps best used in personalized management. Fecal samples can be collected at different stages of IBD in individual patients and the levels of fecal biomarkers can then be measured. Changes in levels of fecal biomarkers can be used to monitor and predict disease progress in individual patients, which may lead to an enhanced patient management.
Coordinated Multi-Center Analysis
Coordinated multi-center studies from different geographic regions are needed in order to determine whether fecal biomarkers can be used as reliable predictive markers for patients with IBD globally. Samples in different centers should be collected at multiple but consistently defined timepoints. Given that ELISA was the most commonly used quantification method in previous studies, perhaps this method should still be used. However, ELISA kits provided by different manufacturers should be compared. Consistently defined cut-off values should be used for data analysis. This approach is more likely to produce conclusive data regarding whether fecal biomarkers can be used as cohort markers to predict disease relapse in patients with IBD.
Author Contributions
FL played a major role in writing the manuscript. LZhu and LZha conceived the project. LZhu, LZha, SL, and SR provided critical feedback and helped in editing the manuscript. All authors have approved the final version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China under Grant Number 51672003.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chang S, Malter L, Hudesman D. Disease monitoring in inflammatory bowel disease. World J Gastroenterol. (2015) 21:11246. doi: 10.3748/wjg.v21.i40.11246
2. Lopez RN, Leach ST, Lemberg DA, Duvoisin G, Gearry RB, Day AS. Fecal biomarkers in inflammatory bowel disease. J Gastroenterol Hepatol. (2017) 32:577–82. doi: 10.1111/jgh.13611
3. Røseth A, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. (2004) 39:1017–20. doi: 10.1080/00365520410007971
4. Sipponen T, Savilahti E, Kärkkäinen P, Kolho K-L, Nuutinen H, Turunen U, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn's disease. Inflamm Bowel Dis. (2008) 14:1392–8. doi: 10.1002/ibd.20490
5. Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. (2008) 103:162. doi: 10.1111/j.1572-0241.2007.01556.x
6. Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. (1997) 58:176–80. doi: 10.1159/000201441
7. Bunn SK, Bisset WM, Main MJ, Golden BE. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2001) 32:171–7. doi: 10.1097/00005176-200102000-00015
8. Schoepfer AM, Trummler M, Seeholzer P, Seibold-Schmid B, Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. (2008) 14:32–9. doi: 10.1002/ibd.20275
9. Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. (2010) 105:162–9. doi: 10.1038/ajg.2009.545
10. Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment Pharmacol Ther. (2008) 28:1221–9. doi: 10.1111/j.1365-2036.2008.03835.x
11. Garnero P, Préaudat C, Vermeire S. Fecal S100A12 levels measured by a new ELISA are increased in ulcerative colitis (UC) and Crohn's disease (CD) and correlates with intestinal damage. J Transl Med. (2010) 8:1. doi: 10.1186/1479-5876-8-S1-P40
12. Rogler G, Biedermann L. Clinical utility of biomarkers in IBD. Curr Gastroenterol Rep. (2015) 17:26. doi: 10.1007/s11894-015-0449-x
13. Tøn H, Brandsnes Ø, Dale S, Holtlund J, Skuibina E, Schjønsby H, et al. Improved assay for fecal calprotectin. Clin Chim Acta. (2000) 292:41–54. doi: 10.1016/S0009-8981(99)00206-5
14. Haisma S-M, van Rheenen PF, Wagenmakers L, Kobold AM. Calprotectin instability may lead to undertreatment in children with IBD. Arch Dis Child. (2020) 105:996–8. doi: 10.1136/archdischild-2018-316584
15. Lasson A, Stotzer P-O, Öhman L, Isaksson S, Sapnara M, Strid H. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis. (2015) 9:26–32. doi: 10.1016/j.crohns.2014.06.002
16. Siegenthaler G, Roulin K, Chatellard-Gruaz D, Hotz R, Saurat JH, Hellman U, et al. A heterocomplex formed by the calcium-binding proteins MRP8 (S100A8) and MRP14 (S100A9) binds unsaturated fatty acids with high affinity. J Biol Chem. (1997) 272:9371–7. doi: 10.1074/jbc.272.14.9371
17. Shirley SH, von Maltzan K, Robbins PO, Kusewitt DF. Melanocyte and melanoma cell activation by calprotectin. J Skin Cancer. (2014) 2014:846249. doi: 10.1155/2014/846249
18. Korndörfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9) 2 heterotetramer, calprotectin, illustrates how conformational changes of interacting α-helices can determine specific association of two EF-hand proteins. J Mol Biol. (2007) 370:887–98. doi: 10.1016/j.jmb.2007.04.065
19. Wei L, Liu M, Xiong H. Role of Calprotectin as a Biomarker in Periodontal Disease. Mediators Inflamm 2019;2019. doi: 10.1155/2019/3515026
20. Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. PNAS. (2013) 110:3841–6. doi: 10.1073/pnas.1220341110
21. Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. (2011) 10:158–64. doi: 10.1016/j.chom.2011.07.004
22. Brophy MB, Hayden JA, Nolan EM. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J Am Chem Soc. (2012) 134:18089–100. doi: 10.1021/ja307974e
23. Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, et al. Role of calprotectin in withholding zinc and copper from Candida albicans. Infect Immun. (2018) 86:e00779–17. doi: 10.1128/IAI.00779-17
24. Nakashige TG, Zygiel EM, Drennan CL, Nolan EM. Nickel sequestration by the host-defense protein human calprotectin. J Am Chem Soc. (2017) 139:8828–36. doi: 10.1021/jacs.7b01212
25. Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol. (2015) 11:765. doi: 10.1038/nchembio.1891
26. Champaiboon C, Sappington KJ, Guenther BD, Ross KF, Herzberg MC. Calprotectin S100A9 calcium-binding loops I and II are essential for keratinocyte resistance to bacterial invasion. J Biol Chem. (2009) 284:7078–90. doi: 10.1074/jbc.M806605200
27. Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression inhibits bacterial binding to mucosal epithelial cells. Infect Immun. (2001) 69:3692–6. doi: 10.1128/IAI.69.6.3692-3696.2001
28. Zaia A, Sappington K, Nisapakultorn K, Chazin W, Dietrich E, Ross KF, et al. Subversion of antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm of TR146 epithelial cells after invasion by Listeria monocytogenes. Mucosal Immunol. (2009) 2:43–53. doi: 10.1038/mi.2008.63
29. Lusitani D, Malawista SE, Montgomery RR. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect Immun. (2003) 71:4711–6. doi: 10.1128/IAI.71.8.4711-4716.2003
30. Gaddy JA, Radin JN, Loh JT, Piazuelo MB, Kehl-Fie TE, Delgado AG, et al. The host protein calprotectin modulates the Helicobacter pylori cag type IV secretion system via zinc sequestration. PLoS Pathog. (2014) 10. doi: 10.1371/journal.ppat.1004450
31. Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14). J Infect Dis. (2000) 182:1272–5. doi: 10.1086/315810
32. Loomans HJ, Hahn BL, Li Q-Q, Phadnis SH, Sohnle PG. Histidine-based zinc-binding sequences and the antimicrobial activity of calprotectin. J Infect Dis. (1998) 177:812–4. doi: 10.1086/517816
33. Sohnle PG, Collins-Lech C, Wiessner JH. The zinc-reversible antimicrobial activity of neutrophil lysates and abscess fluid supernatants. J Infect Dis. (1991) 164:137–42. doi: 10.1093/infdis/164.1.137
34. Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe. (2012) 11:227–39. doi: 10.1016/j.chom.2012.01.017
35. Hantke K. Bacterial zinc transporters and regulators. In: Maret W, editor. Zinc Biochemistry, Physiology, and Homeostasis. Dordrecht: Springer (2001). p. 53–63.
36. Legrand D. Overview of lactoferrin as a natural immune modulator. J Pediatr. (2016) 173:S10–5. doi: 10.1016/j.jpeds.2016.02.071
37. Guerrant R, Araujo V, Soares E, Kotloff K, Lima A, Cooper W, et al. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J Clin Microbiol. (1992) 30:1238–42. doi: 10.1128/JCM.30.5.1238-1242.1992
38. Uchida K, Matsuse R, Tomita S, Sugi K, Saitoh O, Ohshiba S. Immunochemical detection of human lactoferrin in feces as a new marker for inflammatory gastrointestinal disorders and colon cancer. Clin Biochem. (1994) 27:259–64. doi: 10.1016/0009-9120(94)90027-2
39. Saitoh O, Kojima K, Kayazawa M, Sugi K, Tanaka S, Nakagawa K, et al. Comparison of tests for fecal lactoferrin and fecal occult blood for colorectal diseases: a prospective pilot study. Intern Med. (2000) 39:778–82. doi: 10.2169/internalmedicine.39.778
40. Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, Mazurier J. Lactoferrin structure and functions. In: Bösze Z, editor. Bioactive Components of Milk. New York, NY: Springer (2008). p. 163–94.
41. Kruzel ML, Zimecki M, Actor JK. Lactoferrin in a Context of Inflammation-Induced Pathology. Front Immunol. (2017) 8:1438. doi: 10.3389/fimmu.2017.01438
42. Vrabie R, Kane S. Noninvasive markers of disease activity in inflammatory bowel disease. Gastroenterol Hepatol. (2014) 10:576.
43. de Jong NS, Leach ST, Day AS. Fecal S100A12: a novel noninvasive marker in children with Crohn's disease. Inflamm Bowel Dis. (2006) 12:566–72. doi: 10.1097/01.ibd.0000227626.72271.91
44. Ilg EC, Troxler H, Bürgisser DM, Kuster T, Markert M, Guignard F, et al. Amino acid sequence determination of human S100A12 (P6, calgranulin C, CGRP, CAAF1) by tandem mass spectrometry. Biochem Biophys Res Commun. (1996) 225:146–50. doi: 10.1006/bbrc.1996.1144
45. Haley KP, Delgado AG, Piazuelo MB, Mortensen BL, Correa P, Damo SM, et al. The human antimicrobial protein calgranulin C participates in control of Helicobacter pylori growth and regulation of virulence. Infect Immun. (2015) 83:2944–56. doi: 10.1128/IAI.00544-15
46. Jackson E, Little S, Franklin DS, Gaddy JA, Damo SM. Expression, purification, and antimicrobial activity of S100A12. JoVE. (2017) e55557. doi: 10.3791/55557
47. Cunden LS, Gaillard A, Nolan EM. Calcium ions tune the zinc-sequestering properties and antimicrobial activity of human S100A12. Chem Sci. (2016) 7:1338–48. doi: 10.1039/C5SC03655K
48. Miranda LP, Tao T, Jones A, Chernushevich I, Standing KG, Geczy CL, et al. Total chemical synthesis and chemotactic activity of human S100A12 (EN-RAGE). FEBS Lett. (2001) 488:85–90. doi: 10.1016/S0014-5793(00)02392-9
49. Yan WX, Armishaw C, Goyette J, Yang Z, Cai H, Alewood P, et al. Mast cell and monocyte recruitment by S100A12 and its hinge domain. J Biol Chem. (2008) 283:13035–43. doi: 10.1074/jbc.M710388200
50. Foell D, Wittkowski H, Ren Z, Turton J, Pang G, Daebritz J, et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. (2008) 216:183–92. doi: 10.1002/path.2394
51. Foell D, Wittkowski H, Kessel C, Lüken A, Weinhage T, Varga G, et al. Proinflammatory S100A12 can activate human monocytes via Toll-like receptor 4. Am J Respir Crit Care Med. (2013) 187:1324–34. doi: 10.1164/rccm.201209-1602OC
52. Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis. (2009) 15:1746–54. doi: 10.1002/ibd.20920
53. Buisson A, Mak WY, Andersen MJ Jr, Lei D, Kahn SA, Pekow J, et al. Faecal calprotectin is a very reliable tool to predict and monitor the risk of relapse after therapeutic de-escalation in patients with inflammatory bowel diseases. J Crohns Colitis. (2019) 13:1012–24. doi: 10.1093/ecco-jcc/jjz023
54. Ferreiro-Iglesias R, Barreiro-de Acosta M, Lorenzo-Gonzalez A, Dominguez-Muñoz JE. Accuracy of consecutive fecal calprotectin measurements to predict relapse in inflammatory bowel disease patients under maintenance with anti-TNF therapy. J Clin Gastroenterol. (2018) 52:229–34. doi: 10.1097/MCG.0000000000000774
55. Kittanakom S, Shajib M, Garvie K, Turner J, Brooks D, Odeh S, et al. Comparison of fecal calprotectin methods for predicting relapse of pediatric inflammatory bowel disease. Can J Gastroenterol Hepatol. (2017) 2017:1450970. doi: 10.1155/2017/1450970
56. Diederen K, Hoekman D, Leek A, Wolters V, Hummel T, de Meij T, et al. Raised faecal calprotectin is associated with subsequent symptomatic relapse, in children and adolescents with inflammatory bowel disease in clinical remission. Aliment Pharmacol Ther. (2017) 45:951–60. doi: 10.1111/apt.13950
57. Roblin X, Duru G, Williet N, Del Tedesco E, Cuilleron M, Jarlot C, et al. Development and internal validation of a model using fecal calprotectin in combination with infliximab trough levels to predict clinical relapse in Crohn's disease. Inflamm Bowel Dis. (2017) 23:126–32. doi: 10.1097/MIB.0000000000000986
58. Theede K, Holck S, Ibsen P, Kallemose T, Nordgaard-Lassen I, Nielsen AM. Fecal calprotectin predicts relapse and histological mucosal healing in ulcerative colitis. Inflamm Bowel Dis. (2016) 22:1042–8. doi: 10.1097/MIB.0000000000000736
59. Ferreiro-Iglesias R, Barreiro-de Acosta M, Otero Santiago M, Lorenzo Gonzalez A, Alonso de la Pena C, Benitez Estevez AJ, et al. Fecal calprotectin as predictor of relapse in patients with inflammatory bowel disease under maintenance infliximab therapy. J Clin Gastroenterol. (2016) 50:147–51. doi: 10.1097/MCG.0000000000000312
60. Ferreiro-Iglesias R, Barreiro-de Acosta M, Lorenzo-Gonzalez A, Dominguez-Muñoz JE. Usefulness of a rapid faecal calprotectin test to predict relapse in Crohn's disease patients on maintenance treatment with adalimumab. Scand J Gastroenterol. (2016) 51:442–7. doi: 10.3109/00365521.2015.1115546
61. Delefortrie Q, Schatt P, Grimmelprez A, Gohy P, Deltour D, Collard G, et al. Comparison of the Liaison® calprotectin kit with a well established point of care test (Quantum Blue-Bühlmann-Alere®) in terms of analytical performances and ability to detect relapses amongst a Crohn population in follow-up. Clin Biochem. (2016) 49:268–73. doi: 10.1016/j.clinbiochem.2015.10.010
62. Mooiweer E, Severs M, Schipper ME, Fidder HH, Siersema PD, Laheij RJ, et al. Low fecal calprotectin predicts sustained clinical remission in inflammatory bowel disease patients: a plea for deep remission. J Crohns Colitis. (2015) 9:50–5. doi: 10.1093/ecco-jcc/jju003
63. Yamamoto T, Shimoyama T, Matsumoto K. Consecutive monitoring of faecal calprotectin during mesalazine suppository therapy for active rectal inflammation in ulcerative colitis. Aliment Pharmacol Ther. (2015) 42:549–58. doi: 10.1111/apt.13308
64. Scaioli E, Scagliarini M, Cardamone C, Liverani E, Ugolini G, Festi D, et al. Clinical application of faecal calprotectin in ulcerative colitis patients. Eur J Gastroenterol Hepatol. (2015) 27:1418–24. doi: 10.1097/MEG.0000000000000461
65. Yamamoto T, Shiraki M, Bamba T, Umegae S, Matsumoto K. Fecal calprotectin and lactoferrin as predictors of relapse in patients with quiescent ulcerative colitis during maintenance therapy. Int J Colorectal Dis. (2014) 29:485–91. doi: 10.1007/s00384-013-1817-3
66. Jauregui-Amezaga A, López-Cerón M, Aceituno M, Jimeno M, Rodríguez de Miguel C, Pinó-Donnay S, et al. Accuracy of advanced endoscopy and fecal calprotectin for prediction of relapse in ulcerative colitis: a prospective study. Inflamm Bowel Dis. (2014) 20:1187–93. doi: 10.1097/MIB.0000000000000069
67. Naismith GD, Smith LA, Barry SJ, Munro JI, Laird S, Rankin K, et al. A prospective evaluation of the predictive value of faecal calprotectin in quiescent Crohn's disease. J Crohns Colitis. (2014) 8:1022–9. doi: 10.1016/j.crohns.2014.01.029
68. Vos MD, Louis EJ, Jahnsen J, Vandervoort JG, Noman M, Dewit O, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. (2013) 19:2111–7. doi: 10.1097/MIB.0b013e31829b2a37
69. Lasson A, Simrén M, Stotzer P-O, Isaksson S, Öhman L, Strid H. Fecal calprotectin levels predict the clinical course in patients with new onset of ulcerative colitis. Inflamm Bowel Dis. (2013) 19:576–81. doi: 10.1097/MIB.0b013e31827e78be
70. Meuwis M-A, Vernier-Massouille G, Grimaud J, Bouhnik Y, Laharie D, Piver E, et al. Serum calprotectin as a biomarker for Crohn's disease. J Crohns Colitis. (2013) 7:e678–83. doi: 10.1016/j.crohns.2013.06.008
71. van Rheenen PF. Role of fecal calprotectin testing to predict relapse in teenagers with inflammatory bowel disease who report full disease control. Inflamm Bowel Dis. (2012) 18:2018–25. doi: 10.1002/ibd.22896
72. Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, et al. Maintenance of remission among patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. (2012) 142:63–70.e5. doi: 10.1053/j.gastro.2011.09.034
73. Laharie D, Mesli S, El Hajbi F, Chabrun E, Chanteloup E, Capdepont M, et al. Prediction of Crohn's disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther. (2011) 34:462–9. doi: 10.1111/j.1365-2036.2011.04743.x
74. García-Sánchez V, Iglesias-Flores E, González R, Gisbert JP, Gallardo-Valverde JM, González-Galilea Á, et al. Does fecal calprotectin predict relapse in patients with Crohn's disease and ulcerative colitis? J Crohns Colitis. (2010) 4:144–52. doi: 10.1016/j.crohns.2009.09.008
75. Kallel L, Ayadi I, Matri S, Fekih M, Mahmoud NB, Feki M, et al. Fecal calprotectin is a predictive marker of relapse in Crohn's disease involving the colon: a prospective study. Eur J Gastroenterol Hepatol. (2010) 22:340–5. doi: 10.1097/MEG.0b013e32832bab49
76. Sipponen T, Kolho K-L. Faecal calprotectin in children with clinically quiescent inflammatory bowel disease. Scand J Gastroenterol. (2010) 45:872–7. doi: 10.3109/00365521003782389
77. Gisbert JP, Bermejo F, Pérez-Calle J-L, Taxonera C, Vera I, McNicholl AG, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. (2009) 15:1190–8. doi: 10.1002/ibd.20933
78. D'incà R, Dal Pont E, Di Leo V, Benazzato L, Martinato M, Lamboglia F, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. (2008) 103:2007–14. doi: 10.1111/j.1572-0241.2008.01870.x
79. Diamanti A, Colistro F, Basso M, Papadatou B, Francalanci P, Bracci F, et al. Clinical role of calprotectin assay in determining histological relapses in children affected by inflammatory bowel diseases. Inflamm Bowel Dis. (2008) 14:1229–35. doi: 10.1002/ibd.20472
80. Costa F, Mumolo M, Ceccarelli L, Bellini M, Romano M, Sterpi C, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut. (2005) 54:364–8. doi: 10.1136/gut.2004.043406
81. Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. (2000) 119:15–22. doi: 10.1053/gast.2000.8523
82. Walker TR, Land ML, Kartashov A, Saslowsky TM, Lyerly DM, Boone JH, et al. Fecal lactoferrin is a sensitive and specific marker of disease activity in children and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2007) 44:414–22. doi: 10.1097/MPG.0b013e3180308d8e
83. Däbritz J, Langhorst J, Lügering A, Heidemann J, Mohr M, Wittkowski H, et al. Improving relapse prediction in inflammatory bowel disease by neutrophil-derived S100A12. Inflamm Bowel Dis. (2013) 19:1130–8. doi: 10.1097/MIB.0b013e318280b1cd
84. Mao R, Xiao Y-l, Gao X, Chen B-l, He Y, Yang L, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. (2012) 18:1894–9. doi: 10.1002/ibd.22861
85. Cerrillo E, Moret I, Iborra M, Pamies J, Hervás D, Tortosa L, et al. A nomogram combining fecal calprotectin levels and plasma cytokine profiles for individual prediction of postoperative crohn's disease recurrence. Inflamm Bowel Dis. (2019) 25:1681–91. doi: 10.1093/ibd/izz053
86. Baillet P, Cadiot G, Goutte M, Goutorbe F, Brixi H, Hoeffel C, et al. Faecal calprotectin and magnetic resonance imaging in detecting Crohn's disease endoscopic postoperative recurrence. World J Gastroenterol. (2018) 24:641. doi: 10.3748/wjg.v24.i5.641
87. Verdejo C, Hervías D, Roncero Ó, Arias Á, Bouhmidi A, Lorente R, et al. Fecal calprotectin is not superior to serum C-reactive protein or the Harvey-Bradshaw index in predicting postoperative endoscopic recurrence in Crohn's disease. Eur J Gastroenterol Hepatol. (2018) 30:1521–7. doi: 10.1097/MEG.0000000000001284
88. Garcia-Planella E, Mañosa M, Cabré E, Marín L, Gordillo J, Zabana Y, et al. Fecal calprotectin levels are closely correlated with the absence of relevant mucosal lesions in postoperative Crohn's disease. Inflamm Bowel Dis. (2016) 22:2879–85. doi: 10.1097/MIB.0000000000000960
89. Wright EK, Kamm MA, De Cruz P, Hamilton AL, Ritchie KJ, Keenan JI, et al. Comparison of fecal inflammatory markers in Crohn's disease. Inflamm Bowel Dis. (2016) 22:1086–94. doi: 10.1097/MIB.0000000000000671
90. Lopes S, Andrade P, Afonso J, Rodrigues-Pinto E, Dias CC, Macedo G, et al. Correlation between calprotectin and modified Rutgeerts score. Inflamm Bowel Dis. (2016) 22:2173–81. doi: 10.1097/MIB.0000000000000850
91. Hukkinen M, Pakarinen MP, Merras-Salmio L, Koivusalo A, Rintala R, Kolho K-L. Fecal calprotectin in the prediction of postoperative recurrence of Crohn's disease in children and adolescents. J Pediatr Surg. (2016) 51:1467–72. doi: 10.1016/j.jpedsurg.2016.01.017
92. Herranz Bachiller MT, Barrio Andres J, Fernandez Salazar L, Ruiz-Zorrilla R, Sancho Del Val L, Atienza Sanchez R. The utility of faecal calprotectin to predict post-operative recurrence in Crohńs disease. Scand J Gastroenterol. (2016) 51:720–6. doi: 10.3109/00365521.2015.1130164
93. Yamamoto T, Shimoyama T, Umegae S, Matsumoto K. Serial monitoring of faecal calprotectin for the assessment of endoscopic recurrence in asymptomatic patients after ileocolonic resection for Crohn's disease: a long-term prospective study. Therap Adv Gastroenterol. (2016) 9:664–70. doi: 10.1177/1756283X16646562
94. Boschetti G, Moussata D, Stefanescu C, Roblin X, Phelip G, Cotte E, et al. Levels of fecal calprotectin are associated with the severity of postoperative endoscopic recurrence in asymptomatic patients with Crohn's disease. Am J Gastroenterol. (2015) 110:865–72. doi: 10.1038/ajg.2015.30
95. Lasson A, Strid H, Öhman L, Isaksson S, Olsson M, Rydström B, et al. Fecal calprotectin one year after ileocaecal resection for Crohn's disease-a comparison with findings at ileocolonoscopy. J Crohns Colitis. (2014) 8:789–95. doi: 10.1016/j.crohns.2013.12.015
96. Yamamoto T, Shiraki M, Bamba T, Umegae S, Matsumoto K. Faecal calprotectin and lactoferrin as markers for monitoring disease activity and predicting clinical recurrence in patients with Crohn's disease after ileocolonic resection: a prospective pilot study. United Eur Gastroenterol J. (2013) 1:368–74. doi: 10.1177/2050640613501818
97. Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn's disease. J Crohns Colitis. (2013) 7:e641–51. doi: 10.1016/j.crohns.2013.05.005
98. Yamamoto T, Kotze PG. Is fecal calprotectin useful for monitoring endoscopic disease activity in patients with postoperative Crohn's disease? J Crohns Colitis. (2013) 7:e712. doi: 10.1016/j.crohns.2013.08.005
99. Orlando A, Modesto I, Castiglione F, Scala L, Scimeca D, Rispo A, et al. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn's disease: a comparison with ultrasound. Eur Rev Med Pharmacol Sci. (2006) 10:17.
Keywords: inflammatory bowel disease, Crohn's disease, ulcerative colitis, fecal biomarkers, prediction, calprotectin, lactoferrin, S100A12
Citation: Liu F, Lee SA, Riordan SM, Zhang L and Zhu L (2020) Global Studies of Using Fecal Biomarkers in Predicting Relapse in Inflammatory Bowel Disease. Front. Med. 7:580803. doi: 10.3389/fmed.2020.580803
Received: 07 July 2020; Accepted: 01 December 2020;
Published: 17 December 2020.
Edited by:
Fernando Gomollón, University of Zaragoza, SpainReviewed by:
Yong Hua Sheng, University of Queensland, AustraliaEdouard Louis, University of Liège, Belgium
Copyright © 2020 Liu, Lee, Riordan, Zhang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixin Zhu, bHgtemh1QDE2My5jb20=; Li Zhang, bC56aGFuZ0B1bnN3LmVkdS5hdQ==
 Fang Liu
Fang Liu Seul A. Lee2
Seul A. Lee2 Li Zhang
Li Zhang