- 1Wenzhou Key Laboratory of Sanitary Microbiology, Key Laboratory of Laboratory Medicine, Ministry of Education, School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, China
- 2Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN, United States
- 3State Key Laboratory of Rice Biology and Ministry of Agriculture Key Laboratory of Agricultural Entomology, Institute of Insect Sciences, Zhejiang University, Hangzhou, China
- 4Optometry and Eye Hospital and School of Ophthalmology, School of Biomedical Engineering, Wenzhou Medical University, Wenzhou, China
The two-component response regulator Rrp2 is a key activator controlling the production of numerous virulence factors of Borrelia burgdorferi, the Lyme disease pathogen. Previously it was shown that the cognate histidine kinase HK2 is not required for Rrp2 activation in vitro, nor for mammalian infection upon needle inoculation, raising the question whether HK2 has any role in the enzootic cycle of B. burgdorferi. In this study, we demonstrated that HK2 is not required for spirochetal survival in the tick vector. When fed on naive mice, the hk2 mutant had reduced infectivity through the route of tick bite, suggesting that the spirochetes lacking HK2 had a disadvantage in the enzootic cycle. Furthermore, overexpression of hk2 reduced the level of Rrp2 phosphorylation, suggesting that HK2 can function as a phosphatase to dephosphorylate Rrp2. Strains overexpressing hk2 impaired the expression of RpoN regulon whose activation is dependent on Rrp2 phosphorylation and activation, and had reduced infectivity in mice. Taken together, these results demonstrate that although HK2 does not play an essential role in Rrp2 activation, it is important for the optimal fitness of B. burgdorferi in the enzootic cycle.
Introduction
The Lyme disease pathogen, Borrelia burgdorferi, B. afzelii, and B. garinii, is maintained in nature in two drastic different hosts, an Ixodes tick and a mammalian host. As an obligate parasite with a small genome, B. burgdorferi has evolved using its limited signaling and regulatory repertoire to adapt to both host environments. Comparing to free living bacteria such as Escherichia coli, which has more than 30 two-component signal transduction systems (TCSs), B. burgdorferi has reduced to two sets of TCS, HK1/Rrp1 and HK2/Rrp2 (in addition to the chemotactic CheA-CheY system) and has evolved to employ these two TCSs to survive in each of the two hosts encountered in the enzootic cycle. HK1/Rrp1, a c-di-GMP producing system, controls spirochete's adaptation to the tick vector (1–7), whereas HK2/Rrp2 is essential for B. burgdorferi to establish infection in the mammalian host (8–11).
The function of HK2/Rrp2 is largely based on the study of the response regulator Rrp2. A typical TCS consists of a histidine kinase as a sensor and a corresponding response regulator that mediates the cellular response (12). Rrp2 is a member of NtrC family transcriptional activator. It contains three putative functional domains: an N-terminal response regulator receiver domain, a central activation domain, and a C-terminal helix-turn-helix (HTH) DNA-binding domain (38). The central activation domain of Rrp2 is highly conserved among a group of transcriptional activators (bacterial enhancer-binding proteins) that specifically activate genes from alternative sigma factor 54 (RpoN or σ54)-type promoter. The only gene with a σ54-type promoter in B. burgdorferi identified to date is rpoS, which encodes the second alternative sigma factor RpoS (σS). Thus, upon activation by phosphorylation at its N-terminal receiver domain, Rrp2 activates its central domain, leading to activation of an alternative sigma factor cascade, σ54-σS cascade (also called RpoN-RpoS pathway or Rrp2-RpoN-RpoS pathway) in B. burgdorferi [for review, see (13–15)]. Rrp2, along with a transcriptional activator BosR and a repressor BadR, regulates σ54-σS sigma factor cascade, which in turn controls ospC, dbpB/A, bbk32, and many other mammalian infection-associated genes (8–11, 16–26).
Relative to the downstream targets controlled by Rrp2, the upstream event that activates Rrp2 is poorly understood. The gene hk2 (BB0763) is adjacent to rrp2 (BB0764) in the genome and purified HK2 is capable of phosphorylating Rrp2 in vitro, making HK2 qualified as the cognate histidine kinase for Rrp2 (8, 27). However, we and others previously showed that disruption of hk2 does not affect ospC expression and activation of σ54-σS cascade, suggesting that HK2 is not essential for Rrp2 phosphorylation (27, 28). The hk2 mutant is also capable of infecting mice upon needle infection. This raises question whether HK2 plays any role in the enzootic cycle of B. burgdorferi. In this study, we further investigated the hk2 mutant phenotype in the tick phase of the enzootic cycle, showing that the hk2 mutant had a reduced infection via tick infestation. We also took another approach to study HK2 functions by overexpressing hk2. The results showed that HK2 is functional and important for maximum fitness in the enzootic cycle of B. burgdorferi.
Results
HK2 Is Not Required for Spirochete Survival During Tick Feeding
Previously we showed that the hk2 mutant had normal infectivity in mice upon needle inoculation (28). To examine whether HK2 plays a role in tick phase of the enzootic cycle, naive Ixodes scapularis larvae were fed on immunocompetent C3H/HeN mice that were needle-infected with wild-type or the hk2 mutant spirochetes. During and after depletion, engorged larvae were then subjected to immunofluorescent assay (IFA) (Figure 1A) and qPCR analyses (Figure 1B) to assess the spirochetal numbers. The result showed that there was no significant difference in spirochetal numbers in the tick midguts at 48 h during feeding or after feeding on mice infected with wild-type and the hk2 mutant B. burgdorferi, suggesting that HK2 is not required for spirochetal survival during tick feeding (Figures 1A,B).
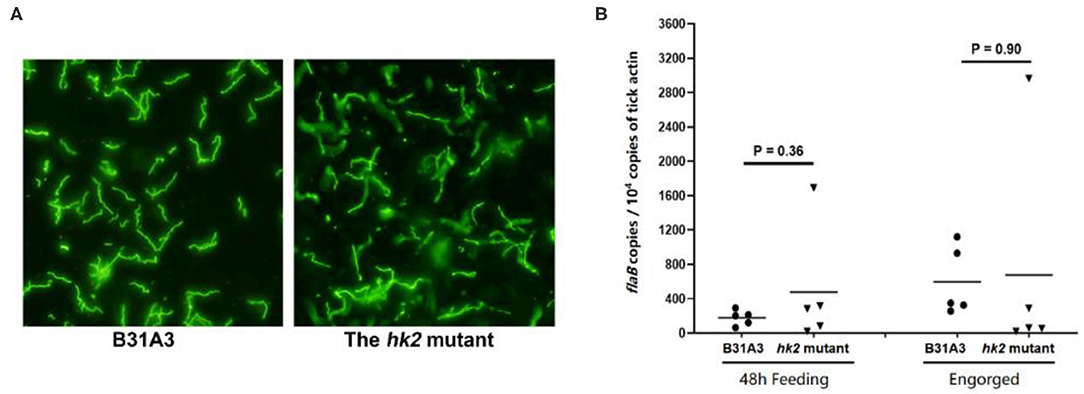
Figure 1. IFA and qPCR analyses of spirochetes in fed larvae. (A) Unfed I. scapularis larvae harboring either the wild type B31A3 or the hk2 mutant were fed on naive C3H/HeN mice, and engorged larvae were subjected to IFA analysis using fluorescein isothiocyanate-labeled anti-B. burgdorferi antibody. Five ticks were examined for each group, and a representative image for each group of ticks is shown. (B) 48 h feeding larvae or fully fed larvea were collected and subjected to qPCR analyses. Each data points were generated from the DNA sample extracted from three larvae. The copy numbers of the flaB gene of B. burgdorferi was used for caculating spirochetal numbers, which is nurmalized by 104 copies of the tick actin gene.
The hk2 Mutant Has Normal Level of Activation of σ54-σS Sigma Factor Cascade During Tick Feeding
We and others showed that HK2 is not required for Rrp2 activation and σ54-σS cascade activation under the in vitro cultivation conditions (27, 28). In the enzootic cycle of B. burgdorferi, spirochetes colonizing in the midgut of unfed ticks begin to replicate and activate σ54-σS cascade when ticks feed (14). Whether HK2 is required for σ54-σS cascade activation during tick feeding has not been examined. Thus, infected fed larvae from above experiments were allowed to molt to nymphs. Flat nymphs were fed on naive mice. Engorged nymphs were collected for quantitative RT-PCR analysis to determine the levels of ospC expression, a surrogate for σ54-σS cascade activation. As shown in Figure 2, there was no significant difference in levels of ospC expression between wild-type spirochetes and the hk2 mutant during tick feeding, suggesting that HK2 is not required for σ54-σS cascade activation in vitro as well as during tick feeding.
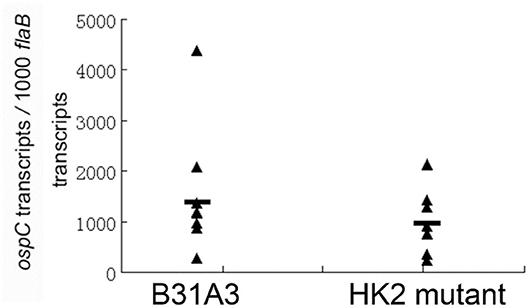
Figure 2. qRT-PCR analyses of spirochetes during tick feeding. Flat I. scapularis nymphs infected with either the wild-type strain B31A3 or the hk2 mutant were fed on naive C3H/HeN mice, and engorged nymphs were subjected to qPCR analyses. Seven ticks were examined for each group, and each data point was from one nymph. The level of ospC expression were nornalized with the level of flaB transcripts of B. burgdorferi. No significant difference was observed between the two groups.
The hk2 Mutant Has Reduced Infectivity via Tick Infestation
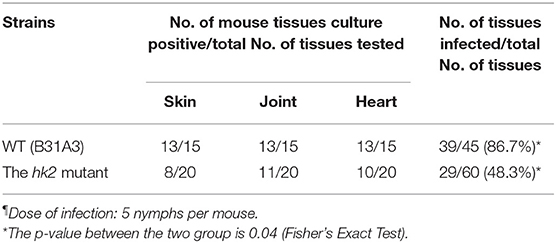
Although HK2 is not required for mammalian infection via needle inoculation using in vitro cultured spirochetes, it remains to be determined whether HK2 plays a role via tick infestation. Accordingly, flat nymphs harboring wild-type or the hk2 mutant spirochetes were allowed to feed on groups of naive C3H/HeN mice. Three weeks after tick bites, mice were sacrificed, and tissue biopsies including skin, joint, and heart were collected for culturing the presence of spirochetes. While 86.7% of mouse tissues infected with ticks harboring wild-type B. burgdorferi were culture positive, 48.3% mouse tissues infected with ticks harboring the hk2 mutant were culture positive (Table 1), suggesting that the hk2 mutant has reduced infectivity via the route of tick infection.
Overexpression of hk2 Impairs Activation of σ54-σS Sigma Factor Cascade
To further investigate the function of HK2, we took another approach by overexpressing hk2 by transforming B. burgdorferi with a shuttle vector carrying a hk2 gene driven by a constitutive flaB promoter. The shuttle vectors were transformed into wild-type strain B31A3. As expected, transformed clones carrying a flaB promoter-driven hk2 had a much higher level of Hk2 than that of wild-type strain (Figure 3). Overexpression of hk2 dramatically reduced production of RpoS and RpoS-dependent surface lipoproteins such as OspC, DbpA, and BBK32 (Figure 3). Further qRT-PCR analyses showed that transcripts of rpoS and several RpoS-dependent genes (ospC, bb0680, bb0844, bba07, bba73) were significantly reduced upon HK2 overexpression (Figure 4). These results indicate that overexpression of hk2 impaired the activation of σ54-σS cascade.
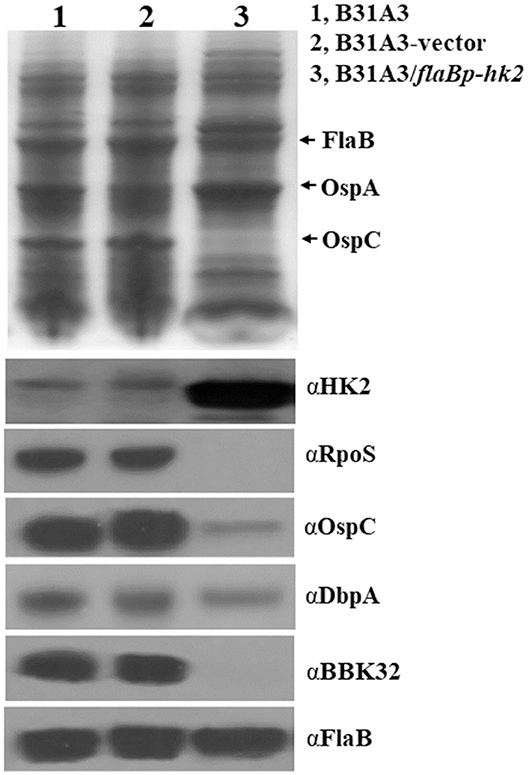
Figure 3. Overexpression of HK2 impaired production of RpoS and RpoS-controlled lipoproteins. Spirochetes were cultivated in BSK-II medium at 37°C and were harvested at the stationary phase, and whole-cell lysates were subjected to SDS-PAGE (top) and Western blot analyses (bottom). The positions of proteins and antibodies used are indicated on the right. B31A3, wild-type B. burgdorferi; B31A3-vector, B31A3 carrying an empty shuttle vector; B31A3/flaBp-HK2, B31A3 carrying a vector harboring a hk2 gene driven by a constitutive flaB promoter.
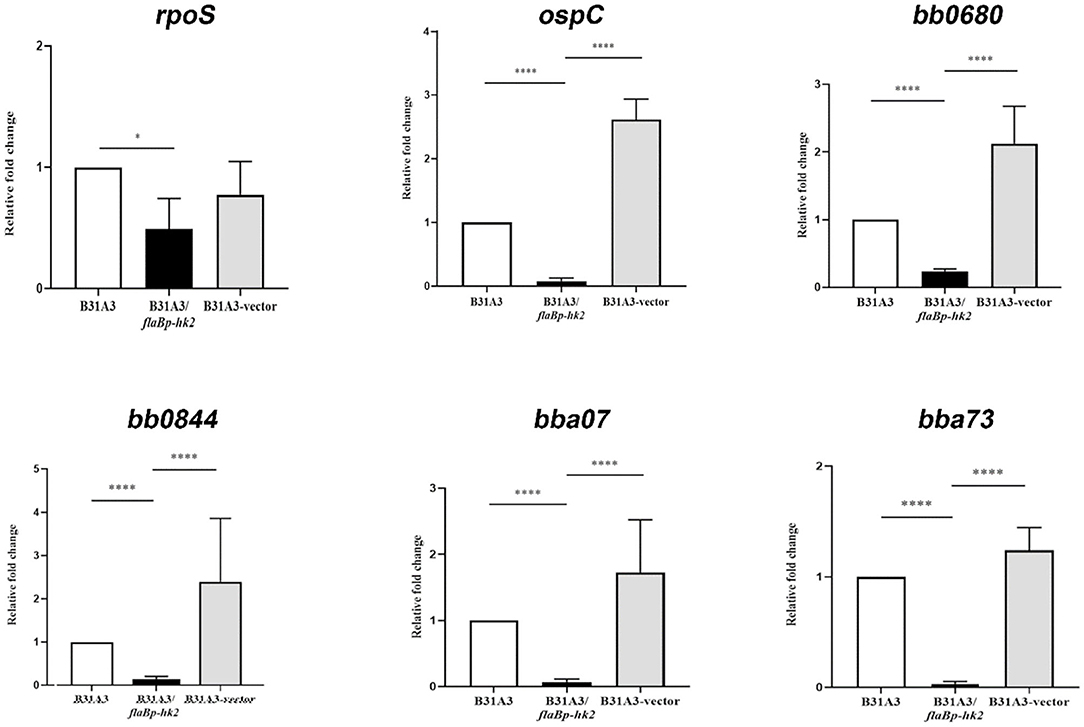
Figure 4. qRT-PCR analyses of several RpoS-controlled genes. Spirochetes were cultured at 37°C in BSK-II medium and harvested in the late-log phase. RNAs were extracted and subjected to qRT-PCR analyses. Samples were first normalized with the flaB level, and then levels of gene expression were reported relative to that of wild-type B31A3 (with the level of expression of each gene in B31A3 as 1.0). *p < 0.01; ****p < 0.0001.
Overexpression of hk2 Reduces Infectivity
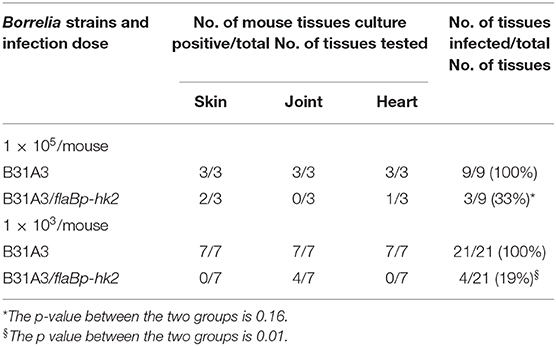
Given the importance of σ54-σS cascade in mammalian infection, we examined the ability of the hk2 overexpression strain to infect mice. Groups of immunocompetent C3H/HeN mice were inoculated with either a high dose (1 × 105 spirochetes per mouse) or a low dose (1 × 103 spirochetes per mouse) of wild-type B. burgdorferi B31A3 or the hk2 overexpression strain B31A3/flaBp-hk2. Four weeks post-inoculation, mice were sacrificed, and various mouse tissues (skin, heart, and joint) were collected and cultured for spirochete growth. All mouse tissues from mice inoculated with wild-type B. burgdorferi B31A3 were culture positive, whereas mouse tissues from mice inoculated with B31A3/flaBp-hk2 were 33% (p = 0.16) and 19% (p = 0.01) culture positive for high dose group and low dose group, respectively (Table 2), indicating that overexpressing hk2 reduced the infectivity of B. burgdorferi in mice.
HK2 Functions as a Phosphatase of Rrp2
We further investigated possible mechanisms underlying the phenotypes of HK2 overexpression. Some two-component histidine kinases can function as phosphatase (29, 30). Given that activation of σ54-σS cascade requires Rrp2 phosphorylation, we postulate that Hk2 may function as a phosphatase for Rrp2. Because aspartate phosphorylation of response regulators has short half-life and is very unstable, and antibodies that recognize phospho-Asp are not available, we performed Phos-tag™ acrylamide gel electrophoresis that uses dinuclear metal complex as a specific phosphate-binding agent to chelate phosphate (31, 32), a method that has shown to successfully separate phosphorylated and unphosphorylated forms of other response regulator proteins (33). Accordingly, B. burgdorferi lysates were subjected to Phos-tag SDS-PAGE followed by immunoblotting using anti-Rrp2 monoclonal antibody. Two distinct bands were observed in B31A3 (Figure 5, lane 1): the lower band corresponded to unphosphorylated Rrp2 and the top band corresponded to phosphorylated Rrp2 (p-Rrp2). When the cell lysate was treated by heat (boiling) prior to Phos-tag SDS-PAGE, the top band disappeared (Figure 5, lane 2), consistent with the fact that Asp-phosphorylation is unstable and heat sensitive to heat. The strain with HK2 overexpression dramatically reduced the intensity of the top band corresponding to phosphorylated Rrp2 (Figure 5, last lane), whereas the strain carrying the same shuttle vector that overexpressed an unrelated protein HD-GYP did not affect the level of Rrp2 phosphorylation (Figure 5, lane 4). This result suggests that although HK2 is not required for Rrp2 phosphorylation, it can function as a phosphatase to dephosphorylate Rrp2, and the impaired activation of σ54-σS cascade by HK2 overexpression is, at least in part, due to the reduced level of Rrp2 phosphorylation.
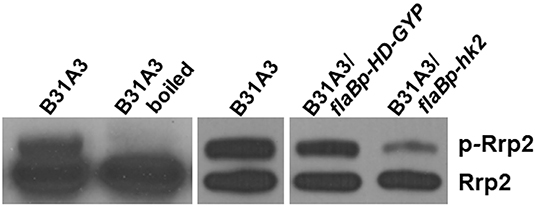
Figure 5. Overexpressing HK2 reduces the level of phosphorylated Rrp2 in B. burgdorferi. Phos-tag SDS-PAGE and immunoblotting was used to detect both phosphorylated and dephosphorylated Rrp2 in the cell. Wild-type B. burgdorferi B31A3, B31A3 carrying a shuttle vector harboring a unrelated protein HD-GYP (B31A3/flaBp-HD-GYP), or B31A3 carrying a shuttle vector harboring a hk2 gene driven by a flaB promoter GYP (B31A3/flaBp-hk2), were harvested at mid-log phase and cell lysates were prepared and separated on 7.5% SDS-PAGE containing 0, 5, 10, and 25 uM Phos-tag followed by immunoblotting using anti-Rrp2 antibody. p-Rrp2, the band corresponds to phosphorylated Rrp2. As a unphosphorylated Rrp2 control, B31A3 was also treated by boiling (lane 2) prior to Phos-tag SDS-PAGE (Rrp2 phosphorylation is unstable and sensitive to heat).
We and others showed that Rrp2 phosphorylation is required for cell growth, in addition to activation of σ54-σS cascade (34, 35). If HK2 overexpression reduces the level of Rrp2 phosphorylation, one would expect that it will affect spirochetal growth. Indeed, B31A3/flaBp-hk2 displayed a distinct show growth rate than B31A3 and B31A3 carrying an empty shuttle vector (Figure 6). This observation further supports the hypothesis that HK2 functions as a phosphatase of Rrp2.
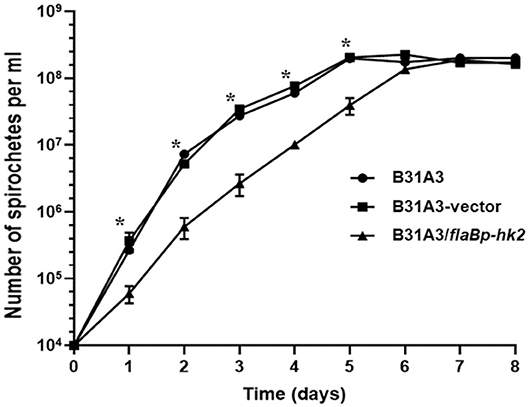
Figure 6. HK2 overexpression resuls in reduced growth rate. Wild-type B. burgdorferi strain B31A3, B31A3 carrying an empty shuttle vector (B31A3-vector), or B31A3 carrying a shuttle vector harboring a hk2 gene driven by a flaB promoter (B31A3/flaBp-hk2) were cultivated in standard BSK-II medium at 37°C with a initial cell density of 1 × 104 spirochetes/ml. Numbers of spirochetes were enumerated under a dark-field microscope. Each data point is the average of data from three independent cultures. *p < 0.01 (paired student test).
Discussion
The Rrp2-RpoN-RpoS pathway, or the σ54-σS alternative sigma factor cascade, is the most studied regulatory pathway in B. burgdorferi (13, 14). It plays a major role in controlling differential gene expression during the process of the spirochetal transmission from ticks to mammals and has thus been called “Gatekeeper” (11). Therefore, understanding how this pathway is activated is important for our understanding of how B. burgdorferi migrates between ticks and mammals. It has been perplexing that HK2, being the cognate histidine kinase of Rrp2, showed no effect on activation of σ54-σS cascade in vitro and is dispensable for mammalian infection via the route of needle inoculation (27, 28). Given that B. burgdorferi has a compact genome and that the hk2 gene is highly conserved among all B. burgdorferi strains including B. garinii and B. afzelii, it is unlikely that hk2 is no longer needed for B. burgdorferi and is in the process of gene loss through genome reduction. In this study, we showed that strain lacking HK2 reduced infectivity via tick bites, the nature route of infection. Tightly controlled hk2 expression is also important for mammalian infection, as HK2 overexpression led to reduced infectivity in mice. We also successfully employed the Phos-tag method, which not only allowed us to detect phosphorylated form Rrp2 but also showed that although HK2 is not required for Rrp2 phosphorylation in vitro, it can function as a phosphatase that dephosphorylates Rrp2. Together, this study demonstrates that HK2 is not what was previously perceived dispensable for the pathogenesis of B. burgdorferi; rather, it plays an important role in the enzootic cycle of B. burgdorferi.
The observation that the hk2 mutant showed different infection outcomes between needle inoculation vs. tick bite underlines the importance of using nature route of tick infestation for assessment of infectivity of a B. burgdorferi mutant. It has been reported that different route of infection by B. burgdorferi can have different infectivity and tissue tropism (36, 37). For example, the dbpBA mutant lacking decorin-binding proteins A and B showed avirulent phenotype by needle-inoculation, but later was demonstrated to be fully infectious via tick infestation (36). One of the obvious reasons for such different outcomes is that spirochetes cultivated in vitro used for needle inoculation have different gene expression profile from that in ticks. In this regard, we examined the ospC expression of the hk2 mutant during tick feeding, as ospC expression is the surrogate for activation of σ54-σS cascade (Figure 2). This is important because the previous conclusion that HK2 is not required for Rrp2-RpoS-RpoS activation is based on spirochetes grown in vitro. Whether HK2 plays a role in Rrp2-RpoS-RpoS activation in vivo has not been examined. Based on the result from the current study, we now can conclude that HK2 is not essential for Rrp2-RpoS-RpoS activation in vivo, i.e., during tick feeding. This data also indicates that the reduced infectivity of the hk2 mutant via tick bite was not due to a defect in activation of σ54-σS cascade, suggesting HK2 may influence other pathways or genes. For instance, HK2 may regulate expression of genes important for spirochetal migration from the tick midgut to salivary gland, whereas needle inoculation bypasses such requirement.
Our results show that Hk2 can function as a phosphatase to dephosphorylate Rrp2, which could explain why Hk2 overexpression resulted in an impaired activation of σ54-σS cascade and reduced infectivity in mice. However, this observation does not exclude other effects of HK2 overexpression that might also contribute to the phenotype. For example, HK2 overexpression might sequester potential HK2 binding ligand or interfere the interacting partner of Rrp2 such as RpoN and possibly BosR. One caveat of this study is that the phenotype of HK2 overexpression in ticks was not examined. B31A3/flab-hk2 is defective in mice, which hampered us feeding ticks on infected mice. Further study using artificial feeding to infect ticks is warranted to confirm the HK2 overexpression phenotype in ticks. Nevertheless, although a lot needs to be learned about HK2 function, this work demonstrates, for the first time, that the hk2 mutant is defective in the enzootic cycle of B. burgdorferi, and HK2 can function as a phosphatase for Rrp2. That is, despite the fact that HK2 is not required for Rrp2 phosphorylation in vitro, HK2 is important to the enzootic cycle of B. burgdorferi and further studies are warranted to elucidate the function of HK2 including the role of the putative PAS domain, the signal HK2 may senses, and the nature of the defect of the hk2 mutant in the enzootic cycle.
Materials and Methods
B. burgdorferi Strains and Culture Conditions
Low-passage, virulent B. burgdorferi strain B31A3 (a gift from Dr. Patricia Rosa, Rocky Mountain Laboratories, NIH) was used in this study. A B31A3 derived hk2 mutant used in this study was constructed previously by our laboratory (28). Spirochetes were cultivated in Barbour-Stoenner-Kelly (BSK-II) medium supplemented with 6% normal rabbit serum (Pel-Freez Biologicals, Rogers, AR) (38) at 37°C with 5% CO2. For the HK2 overexpression B. burgdorferi strain, 300 μg/ml of kanamycin antibiotics was added to the cultures. The constructed shuttle vector (pHX55-HK2) was maintained in Escherichia coli strain DH5α.
Construction of the Strain With Overexpression of hk2
For hk2 overexpression, the PCR fragments of the wild-type hk2 gene and the flaB promoter were fused at the ATG site, and the combined fragment was then cloned into the BamH1 and PstI sites of the shuttle vector pJD55 (36), resulting in pHX55-HK2. The constructed shuttle vector was then transformed into B31A3, and kanamycin-resistant Borrelia transformants were confirmed by PCR for the presence of pHX55-HK2 and by Western blot for HK2 overproduction. Plasmid profiles of the confirmed transformants were determined by multiple PCR analyses for each of the endogenous plasmids as described previously (39, 40). One of the HK2 overexpression clones that had plasmid profiles identical to parental B31A3 was chosen for further study.
Mouse Infection With B. burgdorferi via Needle Inoculation
All mouse experiments were approved by the IACUC committee of Indiana University School of Medicine (IUSM) under the protocol number #11339. Four-week-old C3H/HeN mice (Harlan, Indianapolis, IN) were subcutaneously inoculated with doses of spirochetes as indicated. Mice were euthanized at the end of the experiments, and multiple tissues (joint, heart, skin) were harvested. All tissues were cultivated in 2 ml of the BSK-II medium (Sigma-Aldrich, St. Louis, MO) containing an antibiotic mixture of phosphomycin (2 mg/ml), rifampin (5 mg/ml), and amphotericin B (250 mg/ml) (Sigma-Aldrich) to inhibit bacterial and fungal contamination. All cultures were maintained at 37°C and examined for the presence of spirochetes by dark-field microscopy beginning from 5 days after inoculation. A single growth-positive culture was used as the criterion to determine positive mouse infection.
Tick-Mouse Cycle of B. burgdorferi
Ixodes scapularis egg masses were purchased from Oklahoma State University. The tick-mouse experiments were conducted in IUSM and approved were approved by the IACUC committee of IUSM under protocol number #11339. Unfed larvae were fed on groups of mice (C3H/HeN, three mice per group, 150–200 larvae per mouse) that were needle infected with spirochetes. Ticks were allowed to feed to repletion (3–4 days) and then collected within 24 h. A portion of fed larvae were subjected to analyses. The remaining fed larvae were maintained in the tick incubator and allowed to molt to the nymphal stage (about 5 weeks). One month after molting, unfed nymphs were then allowed to feed on naive C3H/HeN mice. Fully engorged nymphal ticks were collected within 24 h of repletion and subjected to analyses. Mice infected with tick bites were subjected to infection analyses as described above.
Immunofluorescence Assay (IFA)
IFA was performed as reported previously (3). Briefly, the entire contents of a fed tick were smeared and fixed on silylated microscope slides (CEL Associates, Pearland, TX). The slides were incubated with BacTrace fluorescein isothiocyanate-conjugated goat anti-B. burgdorferi antibody (Kirkegaard and Perry Laboratories Gaithersburg, MD) at 37°C. Samples were observed using an Olympus BX50 fluorescence microscope. Twenty ticks from each group were examined by IFA.
qPCR and qRT-PCR
For qPCR analyses of B. burgdorferi DNA in ticks, DNA samples were extracted from ticks using a DNeasy tissue kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. qPCR was performed with primer pairs of qflaB-F/R and qTactin-F/R as described previously (41). Calculations of relative DNA copy number (represented by flaB) were normalized with the copy number of the tick actin gene.
For quantification of ospC transcripts of B. burgdorferi in ticks, RNA samples were extracted from ticks using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocols. To reduce trace amounts of DNA contamination, samples were further digested with RNase-free DNaseI (Qiagen), purified using the RNeasy mini kit (Qiagen), and analyzed with NanoDrop OneC Spectrophotometer (Thermo Fisher Scientific). DNA-free RNA was confirmed by PCR amplification for the B. burgdorferi flaB gene. cDNA was synthesized using the PrimeScript 1st strand cDNA Synthesis Kit (TaKaRa). Given the low levels of bacterial RNA in ticks, the specific primers for each gene target were used for cDNA synthesis instead of random primers previously (41). To quantify the transcript levels of genes of interest, an absolute quantitation method was used to create a standard curve for the qPCR assay according to the manufacturer's protocol (Strategene, La Jolla, CA). Briefly, the PCR product of the flaB gene served as a standard template. A series of 10-fold dilutions (102-107 copies/ml) of the standard template was prepared, and qPCR was performed to generate a standard curve by plotting the initial template quantity against the Ct values for the standards. The quantity of the targeted genes in the cDNA samples was calculated using their Ct values and the standard curve. The samples were assayed in triplicate using the ABI 7000 Sequence Detection System and PowerUp SYBR Green Master Mix (Applied Biosystems). The levels of the target gene transcript were reported as per 1000 copies of flaB.
For qRT-PCR analyses of gene expression in cultured B. burgdorferi, spirochetes were cultured at 37°C in BSK-II medium and harvested in the late-log phase. RNAs were extracted and subjected to qRT-PCR analyses as described above. Primers used for rpoS, ospC, bb0680, bb0844, bba07, bba73 were described previously (42). Samples were first normalized with the flaB level, and then levels of gene expression were reported relative to that of wild-type B31A3 (with the level of expression of each gene in B31A3 as 1.0).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Immunoblotting
Spirochetes from mid-log cultures were harvested by centrifugation at 8,000 × g for 10 min and washed three times with PBS (pH 7.4) at 4°C. Pellets were suspended in SDS buffer containing 50 mM Tris-HCl (pH 8.0), 0.3% sodium dodecyl sulfate (SDS), and 10 mM dithiothreitol (DTT). Cell lysates (108 cells per lane) were separated by 12% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (GE-Healthcare, Milwaukee, WI). Membranes were blotted with either single or a mixed monoclonal/polyclonal antibodies against HK2 (28), RpoS, OspC, DbpA, BBK32 (43), followed with goat anti-mouse or anti-rat lgG-HRP secondary antibody (1:1,000; Santa Cruz Biotechnology). Detection of horseradish peroxidase activity was determined by the enhanced chemiluminescence method (Thermo Pierce ECL Western Blotting Substrate) with subsequent exposure to X-ray film.
Phos-Tag SDS-PAGE
Spirochetes were grown at mid-log phase and harvested by centrifugation for 1 min at 4°C. Cell pellets were washed twice with ice-cold PBS and resuspended in 1 x SDS-PAGE sample buffer. To get rid of the cell debris, the samples were centrifuged at 4°C for 3 min. The supernatants were then loaded on 7.5% Phos-tag SDS-PAGE gels with or without Phos-tag (33). Phos-tag acrylamide AAL-107 was purchased from Wako Chemicals USA. To prepare the control sample in which all Rrp2 molecules are dephosphorylated, cell lysates were boiled for 5 min prior to loading into Phos-tag gel. The gels were run in MOPS buffer (100 mM Tris-HCl, 100 mM MOPS, 0.1% SDS, and 5 mM sodium bisulfite) at 100V, 4°C followed by treatment with transfer buffer (25 mM Tris-HCl, 192 mM glycine, 20% methanol) containing 1 mM EDTA at room temperature with gentle shaking for 15 min to remove the zinc from the gel. The gel was further washed in new transfer buffer without EDTA for 15 min at room temperature with gentle shaking. Separated proteins in the gels were transferred onto NC or PVDF membranes for immunoblot.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by the IACUC committee of Indiana University School of Medicine (IUSM) under the protocol number # 11339.
Author Contributions
QL and HX performed the experiment and wrote the paper. YZha and JY performed the experiment. JD performed the experiment and data analysis. YZho analyzed the data and edits the paper. XY and YL designed and wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
Funding for this work was partially provided by NIH grants AI083640 (to XY) and the National Science Foundation of China 81772229 and support of Key Discipline of Zhejiang Province in Medical Technology (First Class, Category A) (to YL). This investigation was partially conducted in a facility with support from research facilities improvement program grant number C06 RR015481-01 from the National Center for Research Resources, NIH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rogers EA, Terekhova D, Zhang H, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol. (2009) 71:1551–73. doi: 10.1111/j.1365-2958.2009.06621.x
2. Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, et al. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect Immun. (2011) 79:3117–30. doi: 10.1128/IAI.05136-11
3. He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, et al. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. (2011) 7:e1002133. doi: 10.1371/journal.ppat.1002133
4. Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol Microbiol. (2011) 81:219–31. doi: 10.1111/j.1365-2958.2011.07687.x
5. Sultan SZ, Pitzer JE, Boquoi T, Hobbs G, Miller MR, Motaleb MA. Analysis of the HD-GYP domain cyclic dimeric GMP phosphodiesterase reveals a role in motility and the enzootic life cycle of Borrelia burgdorferi. Infect Immun. (2011) 79:3273–83. doi: 10.1128/IAI.05153-11
6. Sze CW, Smith A, Choi YH, Yang X, Pal U, Yu A, et al. Study of the response regulator Rrp1 reveals its regulatory role in chitobiose utilization and virulence of Borrelia burgdorferi. Infect Immun. (2013) 81:1775–87. doi: 10.1128/IAI.00050-13
7. Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, Radolf JD. Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission. Infect Immun. (2015) 83:3043–60. doi: 10.1128/IAI.00315-15
8. Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci USA. (2003) 100:11001–6. doi: 10.1073/pnas.1834315100
9. Caimano MJ, Eggers CH, Hazlett KR, Radolf JD. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun. (2004) 72:6433–45. doi: 10.1128/IAI.72.11.6433-6445.2004
10. Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun. (2008) 76:3844–53. doi: 10.1128/IAI.00467-08
11. Caimano MJ, Groshong AM, Belperron A, Mao J, Hawley KL, Luthra A, et al. The RpoS gatekeeper in Borrelia burgdorferi: an invariant regulatory scheme that promotes spirochete persistence in reservoir hosts and niche diversity. Front Microbiol. (2019) 10:1923. doi: 10.3389/fmicb.2019.01923
12. Stock AM, Robinson VL, Goudreau PN. Two component signal transduction. Ann Rev Biochem. (2000) 69:183–215. doi: 10.1146/annurev.biochem.69.1.183
13. Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol. (2011) 65:479–99. doi: 10.1146/annurev.micro.112408.134040
14. Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Micro. (2012) 10:87–99. doi: 10.1038/nrmicro2714
15. Stevenson B, Seshu J. Regulation of gene and protein expression in the lyme disease spirochete. Curr Top Microbiol Immunol. (2017) 415:83–112. doi: 10.1007/82_2017_49
16. Hübner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci USA. (2001) 98:12724–9. doi: 10.1073/pnas.231442498
17. Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, et al. Borrelia burgdorferi s54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci USA. (2005) 102:5162–7. doi: 10.1073/pnas.0408536102
18. Yang XF, Lybecker MC, Pal U, Alani SM, Blevins J, Revel AT, et al. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J Bacteriol. (2005) 187:4822–9. doi: 10.1128/JB.187.14.4822-4829.2005
19. Caimano M, Iyer R, Eggers C, Gonzalez C, Morton E, Gilbert M, et al. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. (2007) 65:1193–217. doi: 10.1111/j.1365-2958.2007.05860.x
20. Ouyang Z, Blevins JS, Norgard MV. Transcriptional interplay among the regulators Rrp2, RpoN, and RpoS in Borrelia burgdorferi. Microbiology. (2008) 154:2641–58. doi: 10.1099/mic.0.2008/019992-0
21. Hyde JA, Shaw DK, Smith Iii R, Trzeciakowski JP, Skare JT. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol Microbiol. (2009) 74:1344–55. doi: 10.1111/j.1365-2958.2009.06951.x
22. Ouyang Z, Kumar M, Kariu T, Haq S, Goldberg M, Pal U, et al. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol Microbiol. (2009) 74:1331–43. doi: 10.1111/j.1365-2958.2009.06945.x
23. Ouyang Z, Deka RK, Norgard MV. BosR (BB0647) controls the RpoN-RpoS regulatory pathway and virulence expression in Borrelia burgdorferi by a novel DNA-binding mechanism. PLoS Pathog. (2011) 7:e1001272. doi: 10.1371/journal.ppat.1001272
24. Dunham-Ems SM, Caimano MJ, Eggers CH, Radolf JD. Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog. (2012) 8:e1002532. doi: 10.1371/journal.ppat.1002532
25. Miller CL, Karna SLR, Seshu J. Borrelia host adaptation regulator (BadR) regulates rpoS to modulate host adaptation and virulence factors in Borrelia burgdorferi. Mol Microbiol. (2013) 88:105–24. doi: 10.1111/mmi.12171
26. Ouyang Z, Zhou J. BadR (BB0693) controls growth phase-dependent induction of rpoS and bosR in Borrelia burgdorferi via recognizing TAAAATAT motifs. Mol Microbiol. (2015) 98:1147–67. doi: 10.1111/mmi.13206
27. Burtnick MN, Downey JS, Brett PJ, Boylan JA, Frye JG, Hoover TR, et al. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol Microbiol. (2007) 65:277–93. doi: 10.1111/j.1365-2958.2007.05813.x
28. Xu H, Caimano MJ, Lin T, He M, Radolf JD, Norris SJ, et al. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. (2010) 6:e1001104. doi: 10.1371/journal.ppat.1001104
29. Capra EJ, Laub MT. Evolution of two-component signal transduction systems. Ann Rev Microbiol. (2012) 66:325–347. doi: 10.1146/annurev-micro-092611-150039
30. Buschiazzo A, Trajtenberg F. Two-component sensing and regulation: how do histidine kinases talk with response regulators at the molecular level? Ann Rev Microbiol. (2019) 73:507–528. doi: 10.1146/annurev-micro-091018-054627
31. Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. (2006) 5:749–57. doi: 10.1074/mcp.T500024-MCP200
32. Yamada S, Nakamura H, Kinoshita E, Kinoshita-Kikuta E, Koike T, Shiro Y. Separation of a phosphorylated histidine protein using phosphate affinity polyacrylamide gel electrophoresis. Anal Biochem. (2007) 360:160–2. doi: 10.1016/j.ab.2006.10.005
33. Barbieri CM, Stock AM. Universally applicable methods for monitoring response regulator aspartate phosphorylation both in vitro and in vivo using Phos-tag-based reagents. Anal Biochem. (2008) 376:73–82. doi: 10.1016/j.ab.2008.02.004
34. Groshong AM, Gibbons NE, Yang XF, Blevins JS. Rrp2, a prokaryotic enhancer-like binding protein, is essential for viability of Borrelia burgdorferi. J Bacteriol. (2012) 194:3336–42. doi: 10.1128/JB.00253-12
35. Yin Y, Yang Y, Xiang X, Wang Q, Yang Z-N, Blevins J, et al. Insight into the dual functions of bacterial enhancer-binding protein Rrp2 of Borrelia burgdorferi. J Bacteriol. (2016) 198:1543–52. doi: 10.1128/JB.01010-15
36. Blevins J, Hagman KE, Norgard MV. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. (2008) 8:82. doi: 10.1186/1471-2180-8-82
37. Sertour N, Cotté V, Garnier M, Malandrin L, Ferquel E, Choumet V. Infection kinetics and tropism of Borrelia burgdorferi sensu lato in mouse after natural (via ticks) or artificial (needle) infection depends on the bacterial strain. Front Microbiol. (2018) 9:1722. doi: 10.3389/fmicb.2018.01722
38. Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. (1984) 57:521–5.
39. Bunikis I, Kutschan-Bunikis S, Bonde M, Bergström S. Multiplex PCR as a tool for validating plasmid content of Borrelia burgdorferi. J Microbiol Methods. (2011) 86:243–7. doi: 10.1016/j.mimet.2011.05.004
40. Xiang X, Yang Y, Du J, Lin T, Chen T, Yang XF, et al. Investigation of ospC expression variation among Borrelia burgdorferi strains. Front Cell Infect Microbiol. (2017) 7:1–9. doi: 10.3389/fcimb.2017.00131
41. Xu HJ, He M, He JJ, Yang XF. Role of the surface lipoprotein BBA07 in the enzootic cycle of Borrelia burgdorferi. Infect Immun. (2010) 78:2910–8. doi: 10.1128/IAI.00372-10
42. He M, Zhang J-J, Ye M, Lou Y, Yang XF. The cyclic di-GMP receptor PlzA controls virulence gene expression through RpoS in Borrelia burgdorferi. Infect Immun. (2014) 82:445–52. doi: 10.1128/IAI.01238-13
Keywords: lyme disease, Borrelia (Borreliella) burgdorferi, two-component system (TCS), Rrp2-HK2, OspC
Citation: Liu Q, Xu H, Zhang Y, Yang J, Du J, Zhou Y, Yang XF and Lou Y (2020) Role of HK2 in the Enzootic Cycle of Borrelia burgdorferi. Front. Med. 7:573648. doi: 10.3389/fmed.2020.573648
Received: 17 June 2020; Accepted: 07 September 2020;
Published: 26 October 2020.
Edited by:
Ying Zhang, Zhejiang University, ChinaReviewed by:
Melissa Jo Caimano, University of Connecticut Health Center, United StatesUtpal Pal, University of Maryland, College Park, United States
Copyright © 2020 Liu, Xu, Zhang, Yang, Du, Zhou, Yang and Lou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: X. Frank Yang, eGZ5YW5nQGl1LmVkdQ==; Yongliang Lou, bHlsQHdtdS5lZHUuY24=
 Qiang Liu
Qiang Liu Haijun Xu
Haijun Xu Yan Zhang
Yan Zhang Jing Yang
Jing Yang Jimei Du
Jimei Du Yan Zhou
Yan Zhou X. Frank Yang
X. Frank Yang Yongliang Lou
Yongliang Lou