- 1Center for Psychosocial Medicine, Institute of Medical Psychology, University Hospital Heidelberg, Heidelberg, Germany
- 2Practice for Psychoendocrinology and Psychotherapy, Heilbronn, Germany
- 3Department of Psychology, University of Regina, Regina, SK, Canada
- 4Women's Mental Health Research Program, Department of Psychiatry, University of Illinois at Chicago, Chicago, IL, United States
- 5Department of Psychiatry, University of North Carolina, Chapel Hill, NC, United States
- 6Department of Gynecological Endocrinology, Poznan University of Medical Sciences, Poznan, Poland
- 7Department of Psychology, University of Zurich, Zurich, Switzerland
Women worldwide are two to three times more likely to suffer from depression in their lifetime than are men. Female risk for depressive symptoms is particularly high during the reproductive years between menarche and menopause. The term “Reproductive Mood Disorders” refers to depressive disorders triggered by hormonal fluctuations during reproductive transitions including the perimenarchal phase, the pre-menstrual phase, pregnancy, the peripartum period and the perimenopausal transition.
Here we focus on reproductive mood disorders manifesting in adult life. We propose a research agenda that draws together several reproductive mood disorders and investigates which genetic, endocrinological, neural, and psychosocial factors can explain depressive symptoms during phases of hormonal transitions in women. Based on current research it is assumed that some women experience an increased sensitivity to not only fluctuations in reproductive steroids (estrogen and progesterone), but also stress-related steroids. We integrate both dynamics into the concept of “steroid hormone sensitivity,” expanding on the concept of “reproductive hormone sensitivity.” We suggest that a differential response of the stress steroid system including corticosteroids, neurosteroids, like allopregnanolone and the GABA-A Receptor complex, as well as a differential (epi)genetic risk in serotonergic and GABAergic signaling, are moderators or mediators between changes in the reproductive steroid system and the physiological, affective, and cognitive outcomes manifesting in reproductive mood disorders. We point to the lack of research on the role of psychosocial factors in increasing a woman's stress level and at some point also the sensitivity of her stress steroid system within the etiology of Reproductive Mood Disorders.
Drawing together the evidence on various reproductive mood disorders we seek to present a basis for the development of more effective pharmacological, social, and psychological treatment interventions and prevention strategies for women susceptible to these disorders. This could pave the way for new research as well as medical and psychological teaching and practice- such as a new type of Practice for Gynecological Psychoneuroendocrinology- with the aim of working on and ultimately offering more integrative forms of support not yet available to women suffering from depression during hormonal transitions. In medical history women have been left alone with this integrative challenge.
Introduction
Women all over the world are two to three times more likely to suffer from depression in their lifetime compared to men (1). This risk is particularly high during the reproductive years between menarche and menopause. This suggests that mood disorders triggered by changes in reproductive steroid hormones during reproductive transitions- including the perimenarchal phase with first-onset depression (2–4), the menstrual cycle, pregnancy, the peripartum period and the menopause transition- may account for an important proportion of this increased risk. Indeed, an estimated 13–19% of reproductive-aged women experience clinically significant premenstrual mood disturbance each month (5, 6); 25% experience significant mood symptoms during or following pregnancy (7) and approximately 45–68% of women experience clinically significant mood symptoms during the menopausal transition (8). While each disorder is unique, it is also believed that increased sensitivity to fluctuations in reproductive steroid hormone levels represent an underlying etiologic process common to all three reproductive phases (9). For this reason, this cluster of mood disorders occurring in the context of reproductive transitions are frequently referred to as “Reproductive Mood Disorders” (RMDs) in academic circles and will be the focus of this review.
We will begin by briefly introducing the key neuroendocrine factors featured in all RMDs and will then proceed in the following sections with a more in depth look at three RMDs occurring in adult life: premenstrual dysphoric disorder (PMDD), peripartum depression (PPD), and perimenopausal depression (PMD). We will also describe reproductive steroid hormone environments that characterize each reproductive transition, and the evidence for the involvement of increased sensitivity to reproductive steroid hormones in the etiology of each disorder, as well as the role of stress-relevant mechanisms like the hypothalamic pituitary adrenal (HPA) axis, neurosteroids and GABARAR receptors. Furthermore, we end each section with genetic considerations specific to each RMD as well as neuroimaging findings. As a final step, we seek to draw together the evidence from the various RMDs to outline a common etiology, while also discussing potential differences between them. Building on earlier initiatives to discuss a shared etiology between the RMDs [e.g., (10–12)], we expand the previously established concept of reproductive steroid hormone sensitivity toward the concept of “steroid hormone sensitivity,” in order to also integrate the contributions of stress-related steroid hormones and the neurosteroid allopregnanolone (ALLO) at the GABARAR receptor, a central switch between the reproductive hormonal system and the HPA Axis. We argue that women suffering from RMDs not only have an abnormal reaction to normal reproductive steroid hormone changes, but also an abnormal reaction to normal stress steroid hormone mechanisms including ALLO signaling. Psychosocial factors will briefly be discussed as a pivotal compound in the role of stress sensitivity in these disorders. Taken together, our perspective on RMDs is conceptualized with the ultimate goal of informing their improved diagnosis and treatment as well as improving the early identification of women who are at the highest risk of RMDs to enable prevention.
Key Neuroendocrine Factors In Reproductive Mood Disorders
Sex differentiation in the prevalence of mood disorders begins with puberty (13). Before adolescence, the rates of depression are similar in girls and boys or even slightly higher in boys (12). However, after menarche, a sharp rise in the prevalence of mood disorders and suicidal behaviors in women can be observed (14, 15). Although perimenarchal mood disorders could clearly be considered a RMD, only limited research is available on the underlying psychoneuroendocrinological mechanisms [see for instance Smith (16)]. However, as will be presented in the Neuroimaging Section The Role of Serotonergic Function in RMDs: Possible Direct and Interactive Pathways below, the findings from this strand of research clearly distinguish pubertal depression from the other adult RMDs. Therefore, we here focus on RMDs manifesting in adult life.
Sensitivity to Changes in Reproductive Steroids
Reproductive steroid hormones including estrogens, progesterone and androgens are regulated by the hypothalamic-pituitary-gonadal (HPG) axis. Briefly, as the initial part of the HPG axis, the hypothalamus releases gonadotropin-releasing hormone (GnRH), which stimulates the anterior pituitary to produce and secrete the gonadotropins- luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH passes the bloodstream and stimulates the release of reproductive steroid hormones from the gonads followed by a negative feedback loop, as illustrated in Figure 1. The HPG axis not only regulates reproductive function, but also other physiological functioning, and influences emotion and cognition [as e.g., reviewed in Gurvich et al. (17)].
The marked increase in mood symptoms during times of reproductive transitions suggests that common underlying reproductive steroid hormone-based mechanisms influence mood symptoms in women. The role of reproductive steroid hormones in regulating the switch into dysfunctional affective states in a susceptible group of women has been well-established by studies showing abnormal symptomatic responses to experimental steroid hormone manipulations in those with PMDD (18), peripartum depression (19), and perimenopausal depression (20), however, the source of this susceptibility remains unknown. After nearly two decades of research devoted to clarifying the etiology of RMDs, there is compelling evidence that these disorders are not characterized by absolute levels in reproductive steroid hormones but result from an increased sensitivity to changes in reproductive steroid hormones instead [see for instance (10, 11, 19–22)]. Therefore, research on RMDs moves more and more toward a focus on understanding affective responses to changes in reproductive steroid hormone levels and the underlying neuroendocrinological and genetic mechanisms underlying these responses (23). Also, the ratio between the reproductive steroid hormones estrogen (E2) and progesterone (P4) has been shown to be crucial in the development of psychiatric disorders (24).
Sensitivity to Changes in Stress-Related Steroid Hormones, Neurosteroids, and the GABAA Receptor Complex
Psychosocial stress is positively associated with the development and severity of mood disturbances during all phases of reproductive transition (25–29). In line with this, there is consistent research showing dysregulations of the hypothalamic-pituitary-adrenal (HPA) axis in depressive disorders. The effector hormones of the HPA axis (most prominently cortisol) act on glucocorticoid- and mineralocorticoid receptors in the central nervous system and provide negative feedback to further regulate HPA axis activation [(30); also see Figure 1]. Overall, a hyperexcitable HPA axis (for instance as a result of significant early life stress or trauma) has been found to be a common feature of depression (31, 32).
The γ-aminobutyric acid (GABA) system plays a critical role in inhibiting the HPA axis at the level of the paraventricular nucleus (PVN) of the hypothalamus, with GABA as the dominant inhibitory neurotransmitter (33–35). Neuroactive steroids (NAS) are metabolites of cholesterol or steroidal precursors that constitute potent and rapid allosteric modulators of the GABARA Rreceptor (36). Among them, a neuroactive P4 metabolite, the neurosteroid 3α-OH-5αβ-pregnan-20-one, or allopregnanolone (ALLO) is of particular interest in this context (37). ALLO acts as a positive allosteric modulator of the GABARA Rreceptor with potent anxiolytic and tranquilizing effects. Animal research demonstrates an HPA axis-dampening effect of ALLO: ALLO administration in rats attenuated stress-induced release of adrenocorticotropic hormone (ACTH) and cortisol; ALLO also attenuates the stimulated release of the corticotropin-releasing hormone (CRH) and prevents hypothalamic CRH gene expression in adrenalectomized rodents [reviewed in Girdler and Klatzkin (38)].
During the fetal and infant period, important changes in gene expression related to intracellular chloride concentration resulting in altered GABAergic tone have been observed. Change in gene expression affecting these mechanisms seems to occur for some women, but not all and it can be argued that this can influence a woman's risk for RMDs (37). During early development, GABA is depolarizing and mostly excitatory due to high [Cl−]. Here it plays a key role by regulating a number of processes including the migration, morphological maturation, and differentiation of neurons (39). GABA mediates Cl(-)dependent inhibitory postsynaptic potentials and alterations in these mechanisms e.g., due to epigenetic changes due to excessive stress as described below are related to GABA's potential implication in the pathogenesis of disorders, RMDs (37).
Apart from such alterations in GABA-activated CL- channels, another aspect that is relevant to a better understanding of a potential mechanism sensitizing some women for RMDs is that certain stem cells continue to be excitatory in adulthood. In relation to this aspect of stem cells it can be said that cognitive functions in adults are modulated by hippocampal neurogenesis which in adult humans occurs exclusively on the level of the dentate gyrus (40). The only product of hippocampal neurogenesis are granule cells as the principal excitatory neurons of the dentate gyrus (41). They provide excitatory input to the pyramidal cells of CA3 (42). The precursor cells from which adult neurogenesis originates receive synaptic input from various other brain regions including dopaminergic fibers from the ventral tegmental area, serotonergic projections from the raphe nuclei, acetylcholinergic input from the septum, and GABAergic connections from local interneurons (43, 44).
It has been argued that genetic or epigenetic modifications of the GABARA Rreceptor may contribute to a woman's sensitivity to ALLO and, in turn, her risk for mood disturbances in response to reproductive steroid hormone fluctuations. For instance, stress in childhood or puberty has been argued to modify the expression of the αR4Rβδ GABARA Rreceptor and plasticity on mood and cognition in the face of stress (45). This particular receptor subunit combination is sensitive for ALLO. Further, the biosynthesis of ALLO has been argued to be affected by excessive stress, which in turn compromises cognitive and affective functioning (46). As Locci and Pinna (46) emphasize, stress-induced down-regulation of ALLO biosynthesis and changes in the GABARA Rreceptor have been related to disorders like posttraumatic stress disorder (PTSD) and depression. On another note, lower serum ALLO levels were found to be associated with higher serotonergic binding in the prefrontal cortex regulating higher cognitive functions and top-down regulation of emotions, while higher ALLO levels were associated with lower alertness (47). Sundstrom Poromaa et al. suggest that this could explain the higher well-being in the follicular phase of the menstrual cycle. Further, sensitivity (and resulting affective vulnerability) might be related to epigenetic changes in genes determining serotonergic functioning (48). However, there is no scientific evidence yet, that abnormalities in serotonergic signaling in depressive responses (49) apply to women suffering from RMDs.
In sum, it is theoretically possible that increased mood sensitivity to ALLO fluctuation (caused by fluctuations in reproductive steroid hormones), manifesting as altered GABAergic tone, would have important consequences for mood outcomes in the face of stress. As such, dysregulation in the stress steroid system is considered in the current manuscript as a potential player in the RMDs, perhaps mediating the relationship between reproductive steroid hormone fluctuation as well as subsequent ALLO fluctuation and mood disturbance in a subset of women. Very recently, a comprehensive review on the mechanisms between GABAergic dysfunction and the HPA axis in the context of depression has been provided (37). However, as it is noted in this review, most of the presented studies have employed male subjects only, and the findings may not be translatable to the female sex. Given the sex differences in stress reactivity (50), behavior (51), and a potential differential in the GABARARRsR Rexpression linked to reproductive steroid hormones (52), our review focuses on findings from female subjects.
Concerning the role of ALLO in these mechanisms, at this point, it could be helpful to clearly separate out two issues: First, paradoxical effects of (normal levels of) ALLO due to a epigenetic modification of αR4Rβδ GABAR and, and second, compromised ability to synthesize ALLO (i.e., low ALLO levels) due to epigenetic modification concerning 3a-HSD and other enzymes needed to synthesize ALLO from P4. They are likely to represent two separate issues, since the first is about sensitivity of the system to normal ALLO changes and the latter is about deficits in levels of ALLO. As will be outlined below, the RMDs described in this manuscript differ in the availability of studies demonstrating the former vs. the latter. In sum, the role of the epigenetically modified GABARAR receptor composition for the activation of the receptor by neurosteroids needs further elucidation in order to better understand RMDs.
Interactions Between the HPA and the HPG Axes: The Central Role of GABAergic Mechanisms
A link between HPA axis activation and HPG axis suppression has long been established via a suppression of GnRH signaling to the pituitary and corresponding decrease in FSH and LH dampening gonadal activity (53). In depressed females (as well as males) HPA axis activity is inversely related to HPG axis activity (31). Based on this, a bi-directional relationship between the experience of stress and activation of the HPG axis has been suggested: Variability in sex steroids has been related to stress vulnerability and perimenstrual psychiatric risk (24, 54), impaired affective adjustment during the peripartum period (55, 56) and perimenopausal depressive symptoms (20).
At the level of the central nervous system, we consider GABAergic mechanisms as mediators between the HPA and HPG axes in order to advance our understanding of RMDs. The GABAergic Deficit Hypothesis of Major Depression posits that defects in GABAergic neural inhibition can contribute to the etiology of depressive symptoms and that future antidepressant therapies could be improved by focusing on the restoration of GABAergic neurotransmission (57, 58). For comprehensive reviews of the role of the GABARA RReceptor, the relevant subunits in mood disorders in the aftermath of stress as well as the role of ALLO and allotetrahydrodeoxycorticosterone (THDOC) in this please see the work of Maguire [e.g., (37)] as well as Locci and Pinna (46).
Both, the HPA and the HPG axes are regulated by GABAergic transmission at the level of CRH and gonadotropin-releasing hormone (GnRH) neurons. Higher neurosteroid sensitivity has been found in GABARA RRs incorporating the δ subunit. Therefore, stress-derived neurosteroids at GABARA Rreceptors containing δ subunits have been hypothesized to regulate the cross-talk between the HPA and the HPG (59). As suggested from the research above, fluctuations in P4, and subsequent fluctuations in ALLO could be involved in both HPA and the HPG axes functioning. However, evidence on the role of ALLO at central GABARAR receptors, their interplay between the HPA and the HPG axes and the genesis of RMDs is very limited, because ALLO mechanisms cannot be measured in the living human brain. Based on the available data, it can be hypothesized that women susceptible to RMDs show higher sensitivity to stress during phases of reproductive steroid hormone variability. This sensitivity corresponds to altered ALLO-dependent functioning at the GABARAR receptor in the PVN (see Figure 1). In fact, there is evidence of a shift in somatic GABA currents (ERGABAR) in parvocellular neurons in the PVN after chronic early life stress (60). In sum, chronic stress seems to lead to compromised GABAergic control of the HPA axis.
Neuroimaging Findings of Reproductive Mood Disorders
Although much of the literature on the neurobiology of MDD, which did not make a distinction between men and women, may still provide valuable insight for RMDs, there are very few functional brain imaging in fact comparing dysfunctional neuronal networks in depressed men and women [e.g., (61)]. Pubertal depression that may manifest as first-onset depression in the perimenarchal phase was found to produce neuroimaging results that significantly differ from the adult Reproductive Mood disorders (PMDD, PPD, PMD) and are more akin to activation patterns observed in Major Depressive Disorder (MDD) (62). The identification of a female depression biotype, as well as differences between female mood disorders across the reproductive cycle can provide essential insights concerning their potential respective treatment. The sub-sections of this article that portray each of these disorders also include a section reviewing the neuroimaging findings for the specific reproductive mood disorder manifesting in adult life.
The Role of Serotonergic Function in RMDs: Possible Direct and Interactive Pathways
Altered serotonergic function has also been observed among women with RMDs, and therefore these mechanisms should be considered when building theoretical and empirical models of RMD. Deficits in serotonergic function are observed across RMDs [as reviewed in Schiller et al. (63)], and pharmacologic fMRI studies have demonstrated that estrogen and progestin administration (vs. placebo) cause increased 5-HT2A binding in brain areas critically involved in emotion regulation, including the anterior cingulate cortex, dorsolateral prefrontal cortex, and lateral orbitofrontal cortex (64). The interactions between sex hormones and the serotonergic system in both healthy women and those with RMDs are extensively reviewed elsewhere [e.g., (63, 65)]. In later sections on various adult reproductive mood disorders, we will discuss both serotonergic abnormalities and potential interactions with the GABAergic system as they relate to specific RMDs.
It could also be the case that the serotonergic and GABAergic systems interact to cause RMD symptoms (66). As reviewed by Birzniece et al. (66), a clear interaction between the GABAergic and the serotonergic systems has been described in the hippocampus. In this particular area of the brain serotonin neurons have been found to frequently end at inhibitory GABAergic interneurons (67). In the context of the relationship between the GABAergic and the serotonergic system in reproductive mood disorders (discussed in more detail below) there have been findings that in vivo administration of low doses of a 5HT1A receptor agonist with anxiolytic effects enhances GABA stimulated ClP− Puptake in cortico-hippocampal synaptoneurosomes (68). More experimental studies are needed to determine the relevance of these interactive mechanisms in the regulation of behavior and RMD symptoms. In this paper, we retain a primary focus on the GABA/ALLO system while acknowledging the importance of the serotonin system in RMDs, as there is a possibility that there are important interactions between the GABAergic and serotonin systems in the etiology of RMDs.
Premenstrual Mood Disorders
Premenstrual mood disorders include premenstrual syndrome (PMS), PMDD, and premenstrual exacerbation (PME) of ongoing mood disorder (69). It is estimated that 13–19% of naturally cycling women experience clinically significant emotional PMS symptoms (5, 6) and that about 5% meet diagnostic criteria for PMDD (70), a severe form of PMS recently formalized as a new diagnosis in the DSM-5 (71). PMDD is characterized by the recurrent luteal phase emergence of clinically significant affective symptoms that become minimal or absent after the onset of menstruation (72–74). This “on-off” pattern in which symptoms are fully confined to the luteal phase has been emphasized as a critical diagnostic feature for selecting “pure” PMS/PMDD (i.e., without comorbidities) in research studies; however, there may be important variability in the exact timing of the onset and resolution of symptoms in PMDD and related syndromes (75). With respect to PME prevalence, prospective data from epidemiologic studies indicate that roughly 60% of women with depressive disorders demonstrate clinically significant PME of at least one depressive symptoms (76). Therefore, premenstrual mood disorders affect a large portion of women. While PMDD was not coded for in the ICD-10, PMDD is new in ICD-11 where it is put under gynecologic disorders, but is cross-listed as a depressive disorder. This might cause confusion, since there is no evidence for a gynecologic pathology in PMDD. ICD-11 will not come into effect until the year 2022, which means that it is currently not used in most places. At the moment there is an ICD-11 Browser made available by the World Health Organization (WHO), where these new categories can be found (World Health Organization, ICD-11 Browser, Version 2019).
Sensitivity to Changes in Reproductive Steroids in PMS/PMDD
The reproductive steroid hormones E2 and P4 change in a predictable fashion across the prototypical 4-week menstrual cycle. In the first week, which starts with menstrual bleeding, both E2 and P4 are low and stable, followed by a large abrupt peak in E2 at the end of the second week, just prior to ovulation. In the third week, formation of the corpus luteum after ovulation leads to a two-week elevation of P4 and secondary elevation of E2. In the final week, both E2 and P4 plateau and then fall precipitously in the final few days prior to menses onset. In the paragraphs that follow, we outline what is known about the pathophysiology of PMS/PMDD. Since the pathophysiology of PME of depression has been found to be unique from PMDD but has yet to be clarified in its own right (i.e., PME of depression is resistant to various effective treatments for PMDD; Bixo et al. (77), Freeman et al. (78), Peters et al. (79)], we will focus our review below on the role of reproductive steroid hormones in PMS/PMDD (i.e., those with luteal phase confinement of symptoms).
As yet, studies have failed to demonstrate consistent abnormalities in E2, P4, or ALLO hormone levels among women with PMS/PMDD. Instead, premenstrual mood disorders are thought to be caused by an aberrant response to normal fluctuations in reproductive steroid hormones. A series of clinical trials demonstrate that GnRH agonists effectively treat the symptoms of PMDD by creating a medical menopause characterized by low, stable levels of reproductive steroid hormones [reviewed in Wyatt et al. (80)]. However, experimental studies demonstrate that addback of luteal phase levels of E2, P4, or their combination causes a resurgence of PMDD-like symptoms not observed in controls and not precipitated by placebo (18). Recently, Schmidt et al. (22) have also demonstrated that this symptomatic response to addback of reproductive steroid hormones is time-limited, remitting after 1 month of stable addback. This series of experiments suggests that it is the postovulatory changes in reproductive steroid hormones—and not the elevated luteal hormone levels themselves—that precipitate symptoms in PMS/PMDD.
Further, experimental work demonstrates that PMS/PMDD are caused by an abnormal sensitivity to normal postovulatory surges in reproductive steroid hormones rather than perimenstrual reproductive steroid hormone withdrawal. Schmidt et al. (81) tested the effects of reproductive steroid hormone withdrawal on symptoms of PMDD in three groups of women; one third received placebo in the midluteal phase, and the other two thirds received mifepristone, a competitive P4 receptor antagonist that causes menses (breakdown of endometrium) and luteolysis (involution of the corpus luteum and associated E2 and P4 withdrawal). Of those receiving mifepristone, half also received human chorionic gonadotropin (HcG), which rescued the corpus luteum, preventing reproductive steroid hormone withdrawal (while still allowing mifepristone-induced menses for blinding purposes); the other half of those on mifepristone received a placebo, which resulted in both mifepristone-induced menses and mifepristone-induced luteolysis with attendant early reproductive steroid hormone withdrawal. The authors reported no significant mood differences between the three groups (midluteal induction of early menses and hormone withdrawal with mifepristone + placebo injection, midluteal induction of early menses but normal midluteal hormone levels with mifepristone + HcG injection, and a natural midluteal phase without menses or hormone withdrawal on dual placebos). This indicates that neither initiation of menses nor induction of hormone (and attendant ALLO) withdrawal alone underlie symptoms of PMDD. In combination with the results of studies described above, we conclude that an abnormal post-ovulatory sensitivity to surges in reproductive steroid hormones (and not a perimenstrual sensitivity to reproductive steroid hormone withdrawal) likely precipitates the symptoms of PMS/PMDD.
Sensitivity to Changes in ALLO and the HPA Axis in PMS/PMDD
Although general psychiatric populations have been found to show reduced biosynthesis of ALLO (46), a recent experimental metabolomics study demonstrated normal P4 metabolism to GABAergic neurosteroids (e.g., ALLO) in PMS/PMDD relative to controls (82). Instead of being caused by a general reduction in ALLO levels (e.g., similar to that observed in depression or PTSD), evidence is accumulating to support the notion that PMS/PMDD are caused by an abnormal neural sensitivity to normal ALLO changes. A recent randomized controlled trial demonstrated preliminary evidence for efficacy of dutasteride in PMDD, a 5α-reductase inhibitor that prevents the metabolism of P4 to its GABAergic neurosteroid metabolites such as ALLO (83). This indicates that it is an altered sensitivity to postovulatory ALLO surges, and not solely E2 or P4 surges, that triggers symptoms of PMS/PMDD.
This altered response to ALLO surges in PMS/PMDD might be caused by abnormal or insufficient plasticity of the GABARAR receptor in response to the postovulatory rise in ALLO [e.g., an upregulation of the α4βδ GABARAR receptor; Shen et al. (84)]. This argument is supported by preliminary evidence for the efficacy of UC1010 (Sepranolone), which acts as an antagonist at the neurosteroid binding site of the GABARAR receptor at which ALLO is active, thereby preventing the adverse effects of ALLO surges in PMDD (77). Notably, these combined results would appear to rule out insufficient ALLO biosynthesis (either peripherally or in the CNS) as the cause of PMS/PMDD, since blockade of the neurosteroid binding site is therapeutic rather than further triggering PMS/PMDD symptoms. In sum, it appears that symptoms of PMS/PMDD are probably related to an abnormal regulation of the GABARAR receptor in response to postovulatory surges in ALLO.
Perimenstrual studies demonstrate that GnRH-agonist suppression of reproductive steroid hormones blunts the normative HPA axis response to stress in healthy women, and that addback administration of luteal levels of P4—and not E2—recapitulate the luteal phase potentiation of the HPA axis response to stress (85, 86). Since ALLO is known to limit the extent and duration of the HPA axis response to stress, it is likely that luteal phase increases in P4 potentiate the HPA axis response through other (e.g., genomic) mechanisms (87).
While the HPA axis and related cortisol output has been extensively studied in observational studies of PMS/PMDD, there is no consistent evidence for abnormal cortisol levels or responses to stress in PMS/PMDD [reviewed in Kiesner and Granger (88)]. Rigorous experimental studies (in which women with and without PMDD are tested during ovarian suppression with GnRH agonist, E2 addback, and P4 addback) demonstrate no differences in reproductive steroid hormone regulation of the HPA axis among women with PMS/PMDD compared to controls, including normal effects of administration of reproductive steroid hormones on output of CRH by the hypothalamus, ACTH by the pituitary, and glucocorticoids by the adrenals, as well as normal effects of administration of reproductive steroid hormones on glucocorticoid receptor feedback (85). Therefore, this may be an area of pathophysiology in which PMS/PMDD differs from other mood disorders such as unipolar depression and may also differ from the pathophysiology of peripartum or perimenopausal mood episodes (compare e.g., also sections HPA Axis Dysregulation and the Neurosteroid Hypothesis of Peripartum Depression and HPA Axis and GABA-ergic Mechanisms in Perimenopausal Depression).
Despite normal regulation of the HPA axis by reproductive steroid hormones (85), there is some evidence to indicate that trauma and recent life stressors increase the severity of hormone-related symptom expression in PMS/PMDD. Cross-sectional studies have observed a correlation between traumatic experiences and retrospectively-self-reported PMDD symptoms [a method known to have a very high false positive rate; Pilver et al. (89)]; however more rigorous studies in which PMDD was prospectively-diagnosed did not find increased exposure to trauma in individuals with PMS/PMDD (90). PMDD patients with high ALLO levels have been found to show blunted nocturnal cortisol levels in comparison to healthy controls who had low ALLO serum concentrations. It also has been argued that diurnal secretion of cortisol may be influenced by ALLO levels during the luteal phase (91). However, these findings do not replicate in another experimental study on PMDD, where affected women did not show altered ALLO metabolism following ovarian suppression and E2 or P4 addback (82). Nonetheless, a longitudinal study of patients with prospectively-diagnosed PMDD found that the strength of the daily link between P4 levels and symptoms was stronger in patients with histories of trauma, which may indicate that trauma increases the severity (rather than the occurrence) of hormone sensitive mood symptoms in PMS/PMDD (92). In addition to historical trauma exposure, current life stressors may exacerbate or prolong the mood effects of hormone sensitivity in women with PMS/PMDD. In one observational study, cycles preceded by higher-than-usual perceived stress showed greater premenstrual increases in mood symptoms (93). Another prospective study of medical students who were (or were not) beginning a stressful night shift assignment found greater increases in premenstrual mood changes among students starting the stressful rotation (94). Since the HPA axis has been found to be normal in PMS/PMDD, more work is needed to understand how internal and environmental stressors may interact with hormone sensitivity to increase cyclical mood symptoms.
Brain Imaging Findings on PMDD
Neuroimaging studies of PMDD remain rare and of mixed quality [for a thorough review and critique of this literature, see Comasco and Sundström-Poromaa (95)]. However, a few patterns of interest have emerged and have begun to shed light on some possible neurobiological underpinnings of PMDD. A few studies comparing gray matter volumes between women with PMDD and healthy controls have observed larger posterior cerebellum and increased gray matter density in the hippocampal cortex, as well as lower gray matter density in the parahippocampal cortex (96, 97). Some studies have noted differences in brain function between those with and without PMDD regardless of cycle phase, including altered activation in the dorsolateral prefrontal cortex (98) and increased amygdala response to negative stimuli (99). Other studies show differences in how the cycle impacts brain function in PMDD. In a proton magnetic resonance spectroscopy study comparing PMDD and controls, cortical GABA decreased from the follicular to the luteal phases in controls, but increased from the follicular to the luteal phase in PMDD (100). Amygdala function may also respond abnormally to progesterone in PMDD; one study found that GABAergic progesterone metabolites predicted lower amygdala reactivity to social negative pictures in healthy controls, whereas the opposite relationship was found in those with PMDD (99). Finally, one study found that, relative to healthy controls, women with PMDD showed greater late luteal phase increases in activation of the left insula during a cognitive processing task (101). In sum, while there are some promising findings that may point to the underlying neurobiology of hormone sensitivity in PMDD, more systematic, and experimental imaging work is required to move this area forward.
Interactions Between the Serotonergic and the GABAergic system in PMDD
As discussed by Birzniece et al. (66) SSRIs may increase inhibitory processes in the limbic structures of the brain involved in the emotional as well as cognitive regulation by hyperpolarization of neuronal membranes enhancing GABA-stimulated Cl− uptake. Women diagnosed with PMDD have been shown to have a decreased sensitivity toward GABAA receptor active substances, especially during the luteal phase (102), when altered serotonergic activity is also observed [reviewed in Hantsoo and Epperson (103)]. Serotonin reuptake inhibitors represent an effective treatment, especially by means of intermittent administration in the luteal phase (104). One possible mechanism is that the SSRI treatment increases metabolism of progesterone to allopregnanolone and normalizes the tolerance to neurosteroids observed during the luteal phase in PMDD (105). However, there is also some evidence that the benefit of SSRIs in PMDD is dependent on their serotonergic mechanisms (106), since coadministration of the serotonin receptor antagonist metergoline (vs. SSRI alone) is able to undermine the benefit of SSRIs in PMDD. Therefore, more work is needed to determine whether alterations of serotonin and GABA systems represent additive or interactive risk factors for PMDD.
Genetic Factors in PMS/PMDD
Although a few studies have identified single-nucleotide polymorphisms (SNP) that differentiate PMS/PMDD cases from controls (107, 108), caution should be exercised when interpreting such studies (109). However, with a focus on epigenetic alterations a recent study of lymphoblastoid cell line cultures from women with PMS/PMDD and controls has demonstrated notable abnormalities in the cellular epigenetic processing of reproductive steroid hormones in PMDD, including altered mRNA expression of several ESC/E(Z) complex genes, both in control samples and in samples treated with E2 and P4 (110). Epigenetic changes across the menstrual cycle and in response to hormonal manipulations may represent a critical area of research to move forward our understanding of the pathophysiology of PMS/PMDD.
Peripartum Mood Disorders
Diagnostic codes for peripartum mood disorders in the ICD and DSM diagnostic manuals differ and there is not a consistent definition. The ICD-10 (111) restricts to either pregnancy or postpartum onset, while in the DSM-5 (71), there is a “peripartum onset” specifier that includes both pregnancy and postpartum in the affective disorders section. ICD-11 distinguishes between mild, moderate, and severe episodes of depression and also between single and recurrent episodes, as well as with and without psychotic symptoms that are supplemented with “current episode perinatal” in the codes ICD 11 6A70-6A71 (World Health Organization ICD-11 Browser, Version 2019).
Up to 25% of women experience significant depressive symptoms following pregnancy (7, 112), however, it is estimated that about 80% of postpartum depression (PPD) cases are not recognized and officially diagnosed, which means that only 20% of affected women receive the treatment they need. Notably, affective symptoms are often already present during pregnancy and the presence of such symptoms in pregnancy has been shown to be among the strongest predictors of PPD (113).
Reproductive Hormonal Changes During the Peripartum Period
For a comprehensive overview concerning normal reproductive steroid hormone fluctuations see Schock et al. (114). It has long been hypothesized that withdrawal of reproductive steroid hormones occurring in the postpartum period plays an important role in the etiology of PPD. One of the first studies providing strong evidence of this used a hormone manipulation paradigm simulating the reproductive steroid hormone withdrawal after parturition among women with a history of PPD and among women without. It was shown that the same hormone manipulation procedure produced a significant increase in depressive symptoms among the women with a history of PPD but not among women without. This suggests that PPD may result from an increased sensitivity to the changes in reproductive steroid hormones that characterize the postpartum period (19). Also, there are several small RCTs finding hormone therapy to be an effective treatment for postpartum depressive symptoms which also supports the role of hormonal withdrawal in the etiology of PPD (115).
While the mechanisms by which withdrawal from E2 and P4 triggers depressive mood in a subset of postpartum women are not fully understood, there is a growing body of research suggesting that increased sensitivity to psychosocial stress may play a role. For example, a history of stressful life events is a strong predictor of the development of PPD (116). Furthermore, psychological (e.g., adjustment to the role as a mother) and physiological stressors (e.g., sleep deprivation) markedly affect subjective well-being, affective symptoms, and physiological stress responses after birth (117). In addition, standard laboratory experiments have shown that stress-susceptibility prior to birth can predict postpartum mood symptoms (118).
HPA Axis Dysregulation and the Neurosteroid Hypothesis of Peripartum Depression
Stress increasing factors for depression and anxiety for instance during pregnancy have been reviewed as risk factors for these disorders, e.g., lack of partner or of social support, a history of abuse or of domestic violence; personal history of mental illness; unplanned or unwanted pregnancy; adverse events in life and high perceived stress; present and past pregnancy complications and pregnancy loss (119). In light of the important role that psychosocial stressors and increased sensitivity to such stressors likely play in the development of peripartum depression, it is not surprising that there is more and more evidence implicating alterations in HPA axis functioning in the etiology of peripartum depression (119). The cortisol response of pregnant women (and new mothers, respectively), with a vulnerability for depression during reproductive transitions may vary as a function of the social support they receive, as well as other stress reducing interventions they may have access to (120). Pregnant women suffering from depression show a significantly higher cortisol response to stressors compared to their controls (118). Pregnant women with prepartum depression and/or anxiety disorder measured in the third trimester have also been found to show a higher cortisol response to stress, but only in the case of comorbidity with e.g., anxiety disorders (121). Also, higher mid-term CRH levels were associated with PPD (122). Further, a significant correlation between the cortisol awakening response and major depression was found in pregnant women (123). Setting pre-partum and postpartum mood problems into context in peripartum research can contribute to our understanding of RMDs, as we see a continued rise in E2 in the third trimester accompanied by an increase in mood symptoms and ultimately also the peak in depressive symptoms in the second and third postpartum week where accumulated pre-partum psychiatric burden adds up with postpartum hormone withdrawal (124).
In recent years, research on peripartum depression has evolved toward examining the role of neurosteroids (e.g., ALLO and DHEA) in the genesis of the disorder and a “hormone-sensitive” phenotype of postpartum depression has been proposed [e.g., (125)]. Dehydroepiandrosterone (DHEA) plays a particular role in affective dysregulation and abnormal DHEA secretion has been found in major depression [e.g., (126)]. Also, DHEA has been found to have anti-depressive effects on both, men and women (127). However, the neurosteroid ALLO has increasingly moved into the center of attention. Regardless of PPD or a healthy state, postpartum women show reduced cortical GABA and ALLO, comparable to healthy women in their follicular phase (128), which also suggests that normal absolute hormone levels are not the underlying cause, but the fluctuation of hormones. There appears to be a vulnerability or sensitivity in a subpopulation of women to the development of peripartum depression (125). It has been shown that the increasing levels of neuroactive steroids (NAS) during pregnancy are crucially related to modifications in the expression of specific GABARA Rreceptor subunits (129). During pregnancy and after parturition, due to major changes in reproductive hormones, the expression of the GABARAR receptor δ subunit is altered (52, 130, 131). ALLO levels increase during pregnancy and the GABARA Rreceptor δ subunit is downregulated. After parturition ALLO levels decrease rapidly, the GABARA R receptor δ subunit is recovered. Based on research with rodents it has been hypothesized that i0n women at risk for PPD, this regulatory mechanism seems to be compromised. For a comprehensive review on these mechanisms, also with respect to the stress axis we recommend the most recent work by Walton and Maguire (132). Altered NAS and GABA profiles have been found in women at risk for peripartum depression. Peripartum GABA levels in women at risk for PPD manifested at a significantly lower level compared to healthy controls (133). In pregnant women whose stress regulatory mechanisms are compromised with respect to this GABARAR receptor complex (for genetic or epigenetic reasons), there can be extremely high stress levels. So, the vulnerability to stress-related psychological disorders like anxiety and depression increases significantly, depending also on the potency of exogenous stressors such as social stressors (119). Neurosteroids play a key role in endogenous stress modulation (134). In sum, as it is hypothesized that a flexible plasticity of GABARAR receptor subunits is compromised in PPD women, suggesting a dysfunction in adapting to peripartal hormonal changes. This would also correspond with the key notion proposed by Rubinow and Schmidt (23) that RMDs represent a problem of switching between affective states.
Brain Imaging Findings on Peripartum Depression
Women with PPD have been found to show altered functional connectivity and activity in brain areas key for executive functions as well as emotion and reward processing. For a comprehensive review of structural and functional connectivity and molecular imaging research please see Duan et al. (135). Neuroimaging findings on PPD center around a decreased resting state functional connectivity between the anterior cingulate cortex, amygdala, hippocampus, and the dorsolateral prefrontal cortex against the background of falling progesterone and ALLO levels after giving birth (136). First fMRI and spectroscopy studies have started to bring GABA and ALLO mechanisms on screen in peripartum depressed women (137). GABA levels were found to be correlated with marked differences in the connectivity of the dorsomedial prefrontal cortex within the default mode network, which also correlated with depression cores in PPD women (137). Also, compromised connectivity has been reported between the amygdala and prefrontal cortex, which implies dysfunctions in emotion processing (138). Concerning an overlap between MDD and PPD functional abnormalities, findings are conflicting. While some authors argue that the network dysfunctions are identical between the disorders (138, 139), others argue that only perimenarchal depressive disorders share the same neuroimaging findings with MDD as mentioned earlier (62). Here, it has to be considered that the majority of neuroimaging studies had included cases suffering from PPD and MDD as well. Future research will have to make clear distinctions in this respect. In particular the hypothalamus, the amygdala, the anterior cingulate, the orbitofrontal and dorsolateral prefrontal cortices, the insula and the striatum seem to be key areas of interest to the etiology of PPD (140). In sum, fMRI studies demonstrate hypoactivation of brain regions studied in women with postpartum depression compared to those without PPD (62).
Interactions Between the Serotonergic and the GABAergic system in Peripartum Depression
The link between the serotonergic system and the GABAergic system in the peripartum period is a research area of increasing interest. Antidepressant treatment with SSRI's has been shown to restore plasma and CSF ALLO levels in association with improvements in depressive symptoms (141, 142). Therefore, increasing levels of ALLO may play an important role in the antidepressant and anti-anxiety pharmacological effects of SSRIs (46). In addition, the recent FDA approval of brexanolone (Zulresso) for the treatment of postpartum depression provides further evidence that a positive allosteric modulator of GABA-A improves depression in the postpartum period (143, 144). However, the relation between brexanolone treatment in women with PPD and associated serotonin levels has not yet been shown.
Genetic Factors in Peripartum Depression
There is a growing interest in understanding the genetic signature of peripartum depression. The literature consists largely of smaller genetic epidemiological and linkage studies (145–149). More recently, a study of the heritability of PPD using the Swedish national twin register estimated the heritability of PPD to be 54 and 44% using twin and sibling designs, respectively. In this same population, the heritability of depression occurring outside of the peripartum period was estimated to be 32% (150). These findings suggest that one-third of the genetic contribution to peripartum depression was unique and did not overlap with the genetic component of depression outside of the peripartum period. Therefore, the increased heritability, as well as increased genetic overlap with other mood disorders, makes peripartum depression an interesting candidate for future genetic investigations. Genetic aspects represent an important person-level variable in a potential etiological model of reproductive disorders, as will be shown in the graphical abstract introduced below as a precursor to such an etiological model (section Discussion: From “Reproductive Steroid Hormone Sensitivity” to “Steroid Hormone Sensitivity”).
Underlying pathophysiology can be used to probe the heterogeneity of peripartum depression. We hypothesize there may be different phenotypes of peripartum depression. Evidence from the international PACT Consortium (Postpartum Depression: Action Toward Causes and Treatment), has worked to help define the heterogeneity of peripartum depression and has described different phenotypes based on severity of symptoms, timing of onset of depressive symptoms, and presence of suicidal thoughts, race, and ethnicity among others (151–153). The PACT Consortium is also interested in the underlying genetic signature of PPD and is currently working on a large-scale Genome-wide association study of PPD (154). These approaches will increase our understanding of phenotypic differences but there remains an important gap between defining phenotypes and understanding the underlying mechanistic differences.
Consequently, the next step is to investigate the underlying pathophysiology that may be unique to the observed heterogeneity in PPD. For example, it could also be hypothesized that women with a susceptibility to fluctuations in reproductive steroid hormones who already show signs of depression during pregnancy may not develop a natural tolerance toward ALLO changes during pregnancy and postpartum, which could imply that they show an increased sensitivity in their key receptors for ALLO. The recent approval of brexanolone, a formulation of ALLO, in the US for PPD is highly relevant to this hypothesis. The women studied in the clinical trials had severe PPD with onset of symptoms in the third trimester or within the first month postpartum (143, 144).
Perimenopausal Mood Disorders
The menopausal transition describes the reproductive phase transitioning from regular menstrual cycles to the complete cessation of menses, which marks the onset of menopause. Between ages 42 and 55, nearly all women experience the menopause transition, which, on average, extends 5–6 years leading up to the last menstrual period [e.g., Avis and McKinlay (155), Oldenhave et al. (156), Treloar (157)]. A recent review article identified 12 cross-sectional studies comparing rates of elevated depressive symptoms in pre- and peri-menopausal women and concluded that 45–68% of perimenopausal women, vs. only 28–31% of premenopausal women, report clinically significant elevations in depressive symptoms (8). Currently, perimenopausal depression does not have a diagnostic code that is distinct from major depression in either the ICD-10 (111) or the DSM-5 (71). ICD-11 still does not code for perimenopausal depression- this RMD would have to be coded by “Other specified menopausal and perimenopausal disorders, GA20Y” (World Health Organization, ICD-11 Browser, Version 2019).While the Center for Epidemiologic Studies Depression Scale (CES-D) is the questionnaire that is most commonly used to assess depressive symptoms in the menopause transition, there has been a call for a menopause-specific scale that would better take both physical and affective symptoms into consideration (158). In this review, we focus on reproductive hormonal fluctuations as a critical factor in perimenopausal problems. However, for the sake of completion- and as will also be referred to in the concluding research outlook- other stress responsive systems also need to be taken into consideration. For instance, the immune system, as well as the thyroid hormonal system can be at the root of climacteric symptoms (159). So it is of utmost importance to rule out other causes like thyroid disorder or autoimmune disorder (160) in women presenting with perimenopausal depression. Also, the links between the reproductive hormonal and the immune system have to be kept in mind, in particular in the context of Hormone Therapy in the Perimenopausal Transition (161). Fluctuations in Progesterone levels have also been shown to alter immune responses and susceptibility to infections at diverse mucosal sites including the genital, gastrointestinal, and respiratory tracts. So the immunomodulatory effects of Progesterone-based compounds need to be given thorough consideration in the treatment of perimenopausal symptoms (162). Reproductive hormonal changes seem to be at the core of those interactions with other hormonal system disorders as well as immunological disturbances, so for this review, we focus on fluctuations in reproductive hormones.
Reproductive Steroid Hormone Changes of the Menopause Transition
The menopausal transition is characterized by several hormonal changes, triggered by a diminishing number of ovarian follicles and fluctuating levels of FSH (163). Beginning in the early menopause transition and continuing into the late transition, is the appearance of menstrual cycles that are characterized by elevated luteal phase E2 levels, which can reach levels that are as high as double those generally seen in the late follicular phase (164, 165). E2 levels in the early follicular phase, on the other hand, have been shown, at times, to reach lower levels than typically observed in reproductive-aged women (166). Furthermore, the low-E2 early follicular phase lengthens due to a delayed ovarian response to FSH, resulting in a longer cycle (167). While P4 levels remain intact throughout the early menopause transition, luteal P4 is lower, on average, throughout the late menopause transition (168). Anovulatory cycles, characterized by low P4 but variable E2 levels, also become increasingly common, with 60–70% of cycles being anovulatory in the late transition (169).
Evidence that an Abnormal Sensitivity to Normal Perimenopausal Reproductive Hormonal Changes Contributes to Perimenopausal Depression
Several studies implicate extreme fluctuation in E2 in the development of perimenopausal depression. Perhaps the strongest evidence comes from a placebo-controlled study by Schmidt et al. (20), which experimentally induced E2 withdrawal using an E2 patch and observed a marked increase in depressive symptoms in the following weeks among women with a history of perimenopausal depression that had been responsive to E2, but not among those without. These findings are in line with three studies observing a relationship between greater E2 fluctuation and elevated risk for depressive symptoms (170–172), as well as a recent placebo-controlled RCT examining the efficacy of transdermal E2 in the prevention of depressive symptoms among perimenopausal and early postmenopausal women (173). In this 12-month study, the rate of clinically significant depressive symptoms was found to be lower among women assigned to transdermal E2 (100 ug/day) vs. placebo (17 vs. 32%). Interestingly, women in the early menopause transition were found to experience the greatest mood benefit, perhaps suggesting that its effects were due to its ability to stabilize the hormonal environment rather than increase low E2 levels.
HPA Axis and GABA-ergic Mechanisms in Perimenopausal Depression
In light of the importance of both hormonal and psychosocial contributions to the development of perimenopausal depression, it has been hypothesized that the hormonal environment of the menopause transition, particularly increased E2 fluctuation, may interact with the stress axis to confer an increased sensitivity to psychosocial stress and increased vulnerability to mood disturbance (163). However, when compared to PMDD and peripartum depression, research directly examining this potential interaction is greatly lacking. In one study cited above (163), a significant interaction was found between E2 fluctuation and the presence of major stressful life events such that E2 fluctuation was predictive of depressive mood among women experiencing one or more events but not among those without baseline stress. Furthermore, the 12-month RCT of transdermal E2 cited above found that the mood benefits of E2 were enhanced among women with greater baseline stressful life events (173). While two studies to date suggest that basal cortisol levels are unrelated to depressive mood in the menopause transition (126, 174), there is some evidence that altered diurnal cortisol patterns may be associated with perimenopausal depressive symptoms. For example, an ancillary of the Study of Women Across the Nation (SWAN) including 408 midlife women, found that depressive symptom score was associated with a flatter diurnal cortisol slope (175). A second study has also observed a relationship between greater weekly changes in E2 and elevated morning cortisol among women with current perimenopausal depression but not among euthymic controls (171, 172). This latter finding would be consistent with the notion that increased HPA axis activation following E2 (and, in turn, ALLO) fluctuations may play a role in perimenopausal depression.
The potential involvement of the GABAergic system and GABAergic neurosteroids, such as ALLO, in the etiology of perimenopausal depression remains largely theoretical as little clinical research directly testing its contribution has been conducted. In a rat model, ovariectomy has been shown to greatly reduce brain ALLO concentrations while administration of 17β estradiol (0.1 or 1 ug per day for 14 days) has been shown to restore ALLO to pre-ovariectomy levels in the hippocampus and hypothalamus (176), an effect that is likely explained by E2's modulation of the enzymes involved in the conversion of P4 to ALLO, 5α-reductase, and 3α-hydroxysteroid dehydrogenase (177). It is therefore possible that the shift between the more extreme E2 levels that characterize the menopause transition, either alone or in conjunction with P4, may result in a more dramatic change in ALLO concentrations than would be seen in a typical menstrual cycle, thus triggering depressive mood in a subset of women for whom the GABARAR receptor fails to adjust to the rapid change in neurosteroid levels. These women may also be more prone to depressive mood when first experiencing a hypogonadal state in the late perimenopausal and early postmenopausal phases: in line with this possibility, a recent study examined the correlation between serum ALLO levels and mood in 140 late perimenopausal and early postmenopausal women and found that ALLO was negatively correlated with feelings of guilt among the early postmenopausal women (29).
Brain Imaging Studies in Perimenopausal Depression
To date, there has been little research examining the neural correlates of perimenopausal depression. One study by Berent-Spillson et al. (178) compared brain activation patterns in euthymic pre-, peri-, and postmenopausal women in response to an emotion identification task. Perimenopausal women were found to have a negative bias in identifying the emotions exhibited by the neutral faces; furthermore, both peri- and postmenopausal women exhibited less limbic activation and greater activation of the tempo-parietal-occipital junction (178), a pattern considered to indicate a more cognitively-mediated decision-making process than an emotionally-mediated one. The cognitively-mediated decision-making process was associated with greater depressive symptoms across all three groups. Laboratory sessions occurred in the early follicular (low E2) phase among cycling women, though E2 levels did not correlate with any of the outcomes assessed, suggesting that these differences are not related to differences in hormone levels, but that perhaps greater hormonal instability and/ or hormonal withdrawal is the hormonal driver behind these observed differences. Other imaging studies, while not conducted in perimenopausal women per se, have used experimental hormonal manipulations in other populations to inform our understanding of the menopause transition. These studies have primarily focused on the involvement of the serotonergic system and are therefore described in the next section.
Interactions Between the Serotonergic and the GABAergic system in Perimenopausal Depression
As with PMDD and PPD, the potential involvement of other neurotransmitter systems apart from GABA have been investigated in perimenopausal depression, with the serotonergic system receiving the most attention. Neocortical serotonin transporter binding (resulting in greater serotonin reuptake and therefore lower serotoninergic tone in this region) has been found to increase in response to an experimentally induced hypogonadal state mimicking that observed in the late menopause transition (49). Furthermore, cortical serotonin transporter binding has been found to decrease (179) while 5-HTR2AR receptor binding has been found to increase (180) following the administration of estrogen therapy in postmenopausal women. One final study examining serotoninergic influences in perimenopausal depression examined the interaction between tryptophan depletion and estradiol treatment on brain activation during an emotion identification task among early postmenopausal women (181). Specifically, it was found that tryptophan depletion reduced activation of the dorsolateral prefrontal cortex and medial frontal/cingulate gyrus compared to sham depletion but that this effect was eliminated with the administration of estradiol. Also relevant to the potential involvement of the serotonergic system in perimenopausal depression is one study comparing the volume of monoamine oxidase A, an enzyme involved in the degradation of serotonin, in the prefrontal cortex of pre-, peri-, and postmenopausal women (182). This study found that on average, women in the perimenopausal age range (41–51 years) had 34% greater monoamine oxidase A volume compared to young reproductive-aged women and 16% greater than older women.
The above studies implicating the serotonergic system in the etiology of perimenopausal depression are consistent with research finding that selective serotonin reuptake inhibitors (SSRIs) are an effective treatment for perimenopausal depression [see Maki et al. (183) for review]. Taken together, these research findings suggest that the serotonergic system has a role to play in the etiology of perimenopausal depression; however, in light of the complex interactions that are well-documented between the GABAergic and serotonergic system, this certainly does not preclude the simultaneous involvement of the GABAergic system as more thoroughly provided above. Interactions between the two systems remain to be explored in depth in the perimenopausal context.
Genetic Factors in Perimenopausal Depression
Current research examining potential genetic contributions to the development of perimenopausal depression is limited. The largest study to date involved 1,538 pre- and perimenopausal women ages 42–55 participating in the Study of Women's Health Across the Nation (SWAN). In this study, specific polymorphisms of three genes involved in the metabolism of E2 and estrone (E1)–the CYP1A1 gene among Caucasian and African American women, the 17HSD gene among Chinese women, and the CYP 19 gene among Japanese women – were found to be associated with an increased risk of elevated depressive symptoms (184). However, neither the ESR1 nor the ESR2 gene, respectively, encoding estrogen receptors α and β, were found to be relevant for risk of depressive symptoms. A second study including 488 women ages 42–68, of which 156 were perimenopausal (54 depressed and 102 controls), observed depression-related differences in the frequency of polymorphisms for the MAO-A and MTHFR genes, which are relevant for monoamine oxidation and methylation, respectively. However, several other genes, including the ESR1 gene, several genes involved in serotonergic transmission (5HTR2A, 5HTR1B, and 5HTR2C), and the GABRB1 gene, which codes for a subunit of the GABARAR receptor, were not found to be relevant for depression in the perimenopausal subgroup (185). However, statistical power to detect such effects may have been limited. A third study including 391 Chinese women (191 with major depressive disorder, 200 controls) found that, among women ages 40–60, a gene-by-environment interaction between negative life events and allelic variations of the ESR2 gene could be seen in relation to major depressive disorder. However, no such interaction was observed among younger women, raising the possibility that the hormonal environment of the menopause transition, when combined with negative life events, may trigger depressive mood in women with a genetic predisposition involving the ESR2 gene. However, there is clearly much work to be done in clarifying the role that genetics play in the etiology of perimenopausal depression. Future research that more carefully defines the menopause transition via menstrual bleeding patterns using the Stages of Reproductive Aging Workshop +10 (STRAW+10) criteria (186), excluding postmenopausal women, is needed. The STRAW+10 guidelines define the early menopause transition based on the appearance of a menstrual cycle length 7+ days shorter or longer than usual and the late menopause transition based on the occurrence of two skipped cycles, but <1 year since the last menstrual period.
Discussion: From “Reproductive Steroid Hormone Sensitivity” to “Steroid Hormone Sensitivity”
Based on the research findings presented in this review, the following conclusions can be made with a fair degree of confidence. First, rigorous hormonal manipulation studies have demonstrated strong evidence that increased mood sensitivity to changes in reproductive steroid hormones plays a key role in the etiology of all three RMDs discussed—PMS/PMDD (18), PPD (19), and PMD (20). The evidence that increased sensitivity to ALLO fluctuation mediates the link between reproductive steroid hormone changes and mood disturbance is quite strong in the context of PMDD (83) and PPD (143). However, further work is needed to examine the role that sensitivity to ALLO fluctuation triggered by E2 or P4 fluctuation may play in the development of perimenopausal depression. Although it seems likely that genetic and epigenetic contributions play an important role in conferring an increased sensitivity to ALLO fluctuation, we are far from having a clear and consistent picture about the specific genes involved.
A second assertion that can be made at this stage of research on RMDs is that proximal psychosocial stress increases susceptibility for mood disturbance in the context of reproductive transitions. In the case of PPD and perimenopausal depression, there is some evidence that HPA axis dysregulation may either be a correlate of depressive symptoms or may play a partial role in the development of mood disturbance. However, the evidence for its role as a primary mechanism underlying the etiology of these RMDs is relatively weak. More work is needed, in particular also on PMDD, to identify the mechanisms by which psychosocial stress may increase risk for and/or exacerbate RMDs. Overall, the GABARAR receptor in specific subunit combinations has been found to be a clear center piece in these mechanisms between the stress hormonal axis and the reproductive hormonal axis (37).
Third, the serotonergic system has also been implicated in all three RMDs. However, we do not consider this body of research as being contradictory to the simultaneous involvement of GABAergic neurosteroids. Indeed, there is considerable evidence suggesting that the serotonergic system is directly modulated by these neurosteroids, including ALLO (46, 66). In sum, clear linkages between the GABAergic and the serotonergic systems have been found in the hippocampus, where serotonin neurons frequently end at inhibitory GABAergic interneurons (67). Also in vivo administration of low doses of a 5HT1A receptor agonist that comes with anxiolytic effects enhances GABA stimulated ClP− Puptake in cortico-hippocampal synaptoneurosomes (68).
Recent literature presented earlier in this manuscript and also in the following closes the circle to chronic stress or trauma in childhood and youth, that has been established as a clear risk factor for RMDs. Life stress that causes developmental insults alters exactly those GABA-stimulated chloride mechanisms in CRH neurons that constitute a key mechanism in the GABAergic control of the HPA axis. These insights link to and build forth on another research tradition that might benefit research on RMDs: research on the psychobiology and molecular genetics of resilience (187). The stress resilience of women with RMDs seems to be severely compromised, which relates to the very few existent studies on chronic stress and early life stress reported earlier in this article in this context. In a recent and comprehensive review on this topic, Hodes and Epperson (188) elaborate on the existing findings concerning the difference between men and women in response to significant life stress. The findings presented in this review confirm that life stress leading to developmental insults, in particular during childhood and puberty, is unmasked in women during hormonal fluctuations in reproductive transitions. In women, this then manifests in stress-related disorders such as depression, anxiety and posttraumatic stress disorder. In contrast, prenatal and early postnatal stress in men tends to manifest in other symptoms, such as those pertaining to the autism spectrum disorders as well-attention-deficit/ hyperactivity disorder. Further research in this particular context of reproductive transitions and life stress in women could hold valuable keys for improving the mental health of women affected by RMDs.
Also, the brain imaging evidence presented in this article shows that there is far more research needed to get a clear picture of shared activation patterns in RMDs, as the studies for each RMD are to heterogenous. In sum, a couple of aspects have to be kept in mind when drafting a common etiological model for RMDs: a clear difference in brain activation patterns has been found between adult RMDs and premenarchal mood problems, in that the latter was more akin to MDD. So it seems sensible to draft a common etiological model for adult RMDs only. In brain imaging studies GABA also played a central role, in that GABAergic fluctuations were clearly tied to fluctuations in the reproductive hormonal system. Also, decreasing ALLO levels were found to be linked to changes in brain activation pattern. So, brain imaging research also warrants the inclusion of the GABAergic system and its activating neurosteroids into an etiological model of RMDs. Not only the GABAergic mechanism at the level of the PVN, activation patterns in the hippocampus, but also compromised connectivity between the amygdala and the prefrontal cortex played a major role in RMDs. Some researchers found an identical network dysfunction and blunted brain activation patterns for all RMDs. Concerning serotonergic functioning hypogonadal states were found to come with greater serotonergic uptake and decreased serotonin transporter binding blunting serotonergic functioning in brain imaging studies. Both, the serotonergic as well as the GABAergic system need to find a place in a joint etiological model.
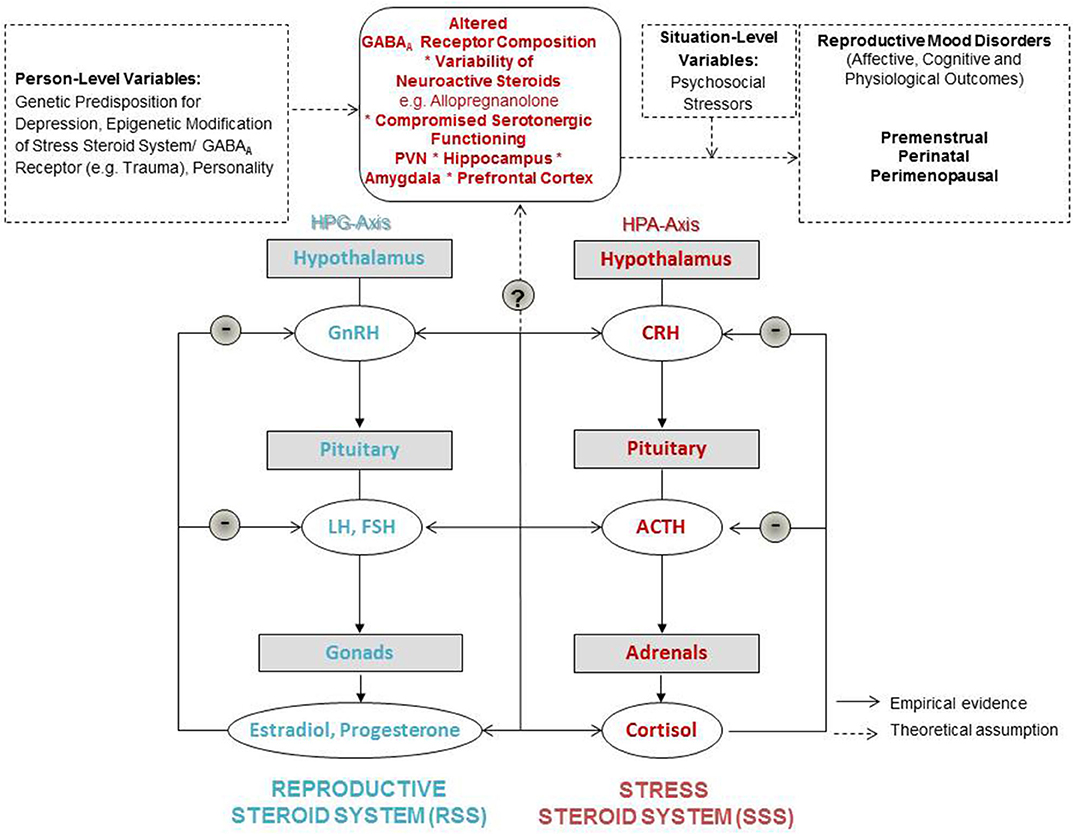
In light of the above, in Figure 1 we present a graphical abstract that could serve as a stepping stone for etiological models for RMDs. In this graphic we integrate the supposed shared mediators and moderators of RMDs. The model expands on reproductive steroid hormone sensitivity as a concept suggesting that rather than absolute steroid hormone levels, it is the sensitivity toward relative changes in reproductive steroid hormones (indicated by plus and minus symbols) which drives affective, cognitive, and physiological outcomes (11). These outcomes can also be expected to be closely related and depend on situation-level variables of women such as psychosocial stressors, as well as person-level variables suches the genetic predisposition and epigenetic modifications of the systems introduced and labeled in the following.
Therefore, we wish to add a focus on a sensitivity to stress via altered GABARA Rreceptor and ALLO functioning on a central nervous system level. Thus, we find it helpful to propose to expand on the concept of reproductive steroid hormone sensitivity toward introducing a more integrative concept of “steroid hormone sensitivity”: We include both, a sensitivity in the reproductive steroid system (RSS) as well as what we label “the stress steroid system” (SSS). The dynamics between those two steroid systems needs in-depth investigation. There are to date no integrative data on these neuroendocrine dynamics and their influence on women's everyday life. Shared sensitivity to normal reproductive steroid and neurosteroid concentrations seems the focal point in the Stress steroid system for all RMDs. Therefore, we position GABARA Rreceptor composition as the central switch between the RSS and the SSS in our graphical abstract, as this receptor regulates both systems as reviewed earlier in this manuscript. The composition and plasticity of this receptor seems key in whether the cross-talk between the two steroid systems is flexible and adaptable during hormonal fluctuations. Whether a woman with a vulnerable genetic or epigenetic set-up including compromised GABAergic modulation develops RMDs then depends on the social context, including its stressors and supportive factors.
Conclusion
For all three RMDs manifesting in adult women's life—PMDD, PPD and perimenopausal depression—there is strong evidence that biological factors (e.g., hormonal, genetic) and psychosocial factors (e.g., daily-life burden/stressors) interact to predict depressive symptoms. However, so far, much of the research has tended to examine these mood disorders from one perspective or the other, failing to consider how an individual's biological vulnerability may interact with her social environment to predict the risk for depression. As a result, clinicians frequently have a limited view of RMDs: obstetricians/gynecologists may tend to view these disorders as being purely hormonal, responsive only to pharmacological intervention, while counselors and social workers may fail to appreciate the degree to which biological influences play a role.
Furthermore, despite increasing evidence that the RMDs may have much in common, they are still being investigated in isolation, with research teams specializing in one disorder or the other. This is also reflected in the diagnostic manuals ICD [e.g., (111)] and DSM-5 (71), where these disorders are spread across different diagnostic categories of codes. RMDs still have to find their adequate place in diagnostic manuals and it is continued research on RMDs that is paving the way for this.
Expanding our perspective toward the more comprehensive concept of “Steroid Hormone Sensitivity” that integrates interactions between both, the reproductive steroid system (RSS) and the stress steroid system (SSS) might serve as a neuroendocrine basis for a better understanding of the underlying differential stress sensitivity experienced by affected women in daily life. Psychosocial stress effects manifesting in affective as well as cognitive symptoms may be of particular importance in these mechanisms. Compromised in their cognitive functioning and in turn stressed by these disabling symptoms, these women may spiral down in a vicious circle between affective, physiological and cognitive symptoms ultimately resulting in what we diagnose as RMDs. Researchers in both research traditions on depresssion- those on reproductive steroids as well as those on stress steroids- seem to be more and more turning toward abnormalities in receptor plasticity. The GABARA RReceptor could be the key switch between the reproductive and the stress steroid system with mounting evidence at a subunit level within this receptor complex: a compromised plasticity required during hormonal fluctuations. Concluding from our review, the role of the δ subunit within the αR4Rβδ GABA Receptor seems to be key in a successful endocrine modulation of reproductive transitions in an environment marked by social stressors.
Working with a common etiological model may not only help us all to proceed in our joint understanding of RMDs, but it may also facilitate the development of new therapeutic approaches combining psychosocial and neuroendocrine aspects. Detecting psychosocial stressors such as the lack of support and finding solutions for them is one of those aspects. From a neuroendocrine point of view, elevating ALLO levels has been discussed as a therapeutic approach to stress-related disorders (46). The FDA approval of a formulation of ALLO (Brexanolone) in the treatment of postpartum depression has, therefore, broad implications for our field. Others have also focused on the translocator protein (18kDa) (TSPO) which is key for neurosteroidogenesis (189) or a novel, synthetic, neuroactive steroid SGE-516 to improve postpartal depression-like symptoms in mice (190). Overall, the role of GABARA RReceptor composition for the activation of the receptor by neurosteroids (37, 46) should be at the center of pharmacological endeavors. In this context there is still far more in-depth evidence needed for the epigenetic regulation of GABAergic transmission. In depression research addressing GABAergic deficits means moving from the mere treatment of the symptoms of depression toward correcting causal neurochemical imbalances.
From a methodological point of view, we encourage the use of parallel methodologies across the three RMDs and suggest testing for common neuroendocrinological, genetic and psychosocial contributors to these disorders. Several methodological challenges will have to be carefully taken into account: the adequate measurement points in all disorders; interaction effects between reproductive steroid hormones and sex hormone binding globulin (SHBG), dehydroepiandrosterone DHEA and androgen measurement; inflammation markers; the choice of biomarkers for genetic phenotyping (such as serotonin, genotyping receptors, but also transporters); other neurotransmitters, targeting GABA receptors and ALLO in humans. With respect to psychosocial factors, confounding factors such as aging and self-image in the case of menopause or anxiety in younger women having to deal with family and workload have to be considered too. The growing high-risk demographic of single working mothers would be a good example, as the prevalence of depression in this group is significantly higher than in married controls due to chronically high stress levels (191). Sub-clusters of patients within the respective RMDs will have to be carefully distinguished as may have become clear in the course of this review. Current research that is exploring the crosstalk between Glucocorticoids, Reproductive Hormones and Immunity (192) may also benefit from picking up this thread of research on RMDs.
Ultimately, our review is meant to further establish RMDs as a phase-sensitive distinct clinical entity warranting the development of a new diagnostic category on RMDs in future editions of ICD and DSM. In particular, in the face of the compelling evidence available on perimenopausal depression, it is urgently necessary to insert a respective diagnostic category, as even the upcoming ICD-11 does not make any reference to this particular RMD. Integrating our knowledge on these health issues of women in a bigger picture allows us to take a psychoneuroendocrinologically informed stance on fundamental societal questions that have long been of pressing relevance. These include the differential social support and stress profiles in the context of different depression prevalence between men and women (120) and involves factors like the increase in stress-related diseases world-wide.
As we tried to show in this review, the hormonal as well as the psychosocial environment of the menopause transition is clearly implicated in the development of for instance perimenopausal depression. There is mounting evidence that the presence of psychosocial stress may interact with this hormonal environment to confer an increased mood vulnerability. Taking such a psychoneuroendocrinologically informed stance also calls for new teaching contents in medical and psychological faculties as well as a new type of practices for Gynecological Psychoneuroendocrinology (193). In this new type of practice treatment options will be offered that integrate neuroendocrinological knowledge with psychiatric, gynecological, urological, and social evidence as well as the latest insights of sexual medicine. This new type of practice supports women in finding adequate solutions to their problems, an extraordinarily demanding integrative effort of various medical, social and psychological services that women were left alone with for most of this world's medical history. In research, teaching and practice that takes such a multidisciplinary stance, a whole array of aspects can be taken into account in finding health solutions for women. For instance, also the aspects of pain and inflammation that are linked to depression (194) has never been elucidated in its link to altered GABAergic mechanisms found in RMDs. Neuro-orthopedic, gynecological as well as urological inflammations such as cystitis often represent masked depressive processes. These are aspects which would greatly benefit progress on RMDs in research and practice if addressed in such a multidisciplinary way. On the basis of a longitudinal, multi-disciplinary perspective on RMDs that takes psychosocial and neuroendocrine factors into account, far more effective prevention and intervention strategies including pharmacological, psychotherapeutic, and psychosocial approaches may be developed, with the aim to improve mental health of the many women in this world affected by reproductive mood disorders.
Author Contributions
SS-S and BD: conception and design of the work. SS-S, JG, TE-M, SM-B, KS, RS, A-LZ, UE, and BD: drafting the article. SS-S, BD, and UE: critical revision of the article. SS-S, JG, TE-M, SM-B, KS, RS, A-LZ, UE, and BD: final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the reviewers for their immensely helpful input. In addition, on a personal note, SS-S wishes to thank her beloved Schweizer-Schubert Family for their love and support during the making of this manuscript.
References
1. Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. (1996) 276:293–9. doi: 10.1001/jama.1996.03540040037030
2. Lewis G, Ioannidis K, van Harmelen AL, Neufeld S, Stochl J, Lewis G, et al. The association between pubertal status and depressive symptoms and diagnoses in adolescent females: a population-based cohort study. PLoS ONE. (2018) 13:e0198804. doi: 10.1371/journal.pone.0198804
3. Sequeira ME, Lewis SJ, Bonilla C, Smith GD, Joinson C. Association of timing of menarche with depressive symptoms and depression in adolescence: mendelian randomisation study. Br J Psychiatry. (2017) 210:39–46. doi: 10.1192/bjp.bp.115.168617
4. Tondo L, Pinna M, Serra G, De Chiara L, Baldessarini RJ. Age at menarche predicts age at onset of major affective and anxiety disorders. Eur Psychiatry. (2017) 39:80–85. doi: 10.1016/j.eurpsy.2016.08.001
5. Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. (2003) 28:1–23. doi: 10.1016/S0306-4530(03)00098-2
6. Wittchen HU, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. (2002) 32:119–32. doi: 10.1017/S0033291701004925
7. Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. (2005) 106 (5 Pt. 1):1071–83. doi: 10.1097/01.AOG.0000183597.31630.db
8. Maki PM, Kornstein SG, Joffe H, Bromberger JT, Freeman EW, Athappilly G, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. (2018) 25:1069–85. doi: 10.1097/GME.0000000000001174
9. Payne JL, Palmer JT, Joffe H. A reproductive subtype of depression: conceptualizing models and moving toward etiology. Harv Rev Psychiatry. (2009) 17:72–86. doi: 10.1080/10673220902899706
10. Deecher D, Andree TH, Sloan D, Schechter LE. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology. (2008) 33:3–17. doi: 10.1016/j.psyneuen.2007.10.006
11. Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci. (2008) 33:331–43.
12. Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. (2003) 74:67–83. doi: 10.1016/S0165-0327(02)00432-9
13. Beesdo K, Hofler M, Leibenluft E, Lieb R, Bauer M, Pfennig A. Mood episodes and mood disorders: patterns of incidence and conversion in the first three decades of life. Bipolar Disord. (2009) 11:637–49. doi: 10.1111/j.1399-5618.2009.00738.x
14. Lewinsohn PM, Rohde P, Seeley JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clin Psychol Rev. (1998) 18:765–94. doi: 10.1016/S0272-7358(98)00010-5
15. Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the national comorbidity survey replication adolescent supplement. JAMA Psychiatry. (2013) 70:300–10. doi: 10.1001/2013.jamapsychiatry.55
16. Smith SS. The influence of stress at puberty on mood and learning: role of the α4βδ GABAA receptor. Neuroscience. (2013) 249:192–213. doi: 10.1016/j.neuroscience.2012.09.065
17. Gurvich C, Hoy K, Thomas N, Kulkarni J. Sex differences and the influence of sex hormones on cognition through adulthood and the aging process. Brain Sci. (2018) 8:163. doi: 10.3390/brainsci8090163
18. Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. (1998) 338:209–16. doi: 10.1056/NEJM199801223380401
19. Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. (2000) 157:924–30. doi: 10.1176/appi.ajp.157.6.924
20. Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. (2015) 72:714–26. doi: 10.1001/jamapsychiatry.2015.0111
21. Daly RC, Danaceau MA, Rubinow DR, Schmidt PJ. Concordant restoration of ovarian function and mood in perimenopausal depression. Am J Psychiatry. (2003) 160:1842–6. doi: 10.1176/appi.ajp.160.10.1842
22. Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. (2017) 174:980–9. doi: 10.1176/appi.ajp.2017.16101113
23. Rubinow DR, Schmidt PJ. Is there a role for reproductive steroids in the etiology and treatment of affective disorders? Dialogues Clin Neurosci. (2018) 20:187–96. doi: 10.31887/DCNS.2018.20.3/drubinow
24. Eisenlohr-Moul TA, DeWall CN, Girdler SS, Segerstrom SC. Ovarian hormones and borderline personality disorder features: preliminary evidence for interactive effects of estradiol and progesterone. Biol Psychol. (2015) 109:37–52. doi: 10.1016/j.biopsycho.2015.03.016
25. Eser D, Schule C, Baghai TC, Romeo E, Rupprecht R. Neuroactive steroids in depression and anxiety disorders: clinical studies. Neuroendocrinology. (2006) 84:244–54. doi: 10.1159/000097879
26. Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. (2004) 61:62–70. doi: 10.1001/archpsyc.61.1.62
27. Holsboer F, Ising M. Central CRH system in depression and anxiety–evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. (2008) 583:350–7. doi: 10.1016/j.ejphar.2007.12.032
28. Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol. (2001) 97:77–80. doi: 10.1097/00006250-200101000-00016
29. Slopien R, Pluchino N, Warenik-Szymankiewicz A, Sajdak S, Luisi M, Drakopoulos P, et al. Correlation between allopregnanolone levels and depressive symptoms during late menopausal transition and early postmenopause. Gynecol Endocrinol. (2018) 34:144–7. doi: 10.1080/09513590.2017.1371129
30. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Rev. (2000) 21:55–89. doi: 10.1210/er.21.1.55
31. Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. (2005) 4:141–94. doi: 10.1016/j.arr.2005.03.003
32. Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. (2008) 31:464–8. doi: 10.1016/j.tins.2008.06.006
33. Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. (2008) 213:63–72. doi: 10.1007/s00429-008-0192-2
34. Patchev VK Hassan AH Holsboer DF Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. (1996) 15:533–40. doi: 10.1016/S0893-133X(96)00096-6
35. Patchev VK Shoaib M Holsboer F Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. (1994) 62:265–71. doi: 10.1016/0306-4522(94)90330-1
36. Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. (1986) 232:1004–7. doi: 10.1126/science.2422758
37. Maguire J. Neuroactive steroids and GABAergic involvement in the neuroendocrine dysfunction associated with major depressive disorder and postpartum depression. Front Cell Neurosci. (2019) 13:83. doi: 10.3389/fncel.2019.00083
38. Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. (2007) 116:125–39. doi: 10.1016/j.pharmthera.2007.05.006
39. Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. (2002) 3:728–39. doi: 10.1038/nrn920
40. Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. (2013) 153:1219–27. doi: 10.1016/j.cell.2013.05.002
41. Bergmann O, Spalding KL, Frisen J. Adult neurogenesis in humans. Cold Spring Harb Perspect Biol. (2015) 7:a018994. doi: 10.1101/cshperspect.a018994
42. Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci USA. (1997) 94:10432–7. doi: 10.1073/pnas.94.19.10432
43. Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res. (2004) 77:155–65. doi: 10.1002/jnr.20116
44. Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, et al. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci. (2006) 24:586–94. doi: 10.1111/j.1460-9568.2006.04924.x
45. Smith SS. α4βδ GABAA receptors and tonic inhibitory current during adolescence: effects on mood and synaptic plasticity. Front Neural Circuits. (2013) 7:135. doi: 10.3389/fncir.2013.00135
46. Locci A, Pinna G. Neurosteroid biosynthesis down-regulation and changes in GABAA receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br J Pharmacol. (2017) 174:3226–41. doi: 10.1111/bph.13843
47. Sundstrom Poromaa I, Comasco E, Backstrom T, Bixo M, Jensen P, Frokjaer VG. Negative association between allopregnanolone and cerebral serotonin transporter binding in healthy women of fertile age. Front Psychol. (2018) 9:2767. doi: 10.3389/fpsyg.2018.02767
48. Schneider I, Kugel H, Redlich R, Grotegerd D, Burger C, Burkner PC, et al. Association of serotonin transporter gene AluJb methylation with major depression, amygdala responsiveness, 5-HTTLPR/rs25531 polymorphism, and stress. Neuropsychopharmacology. (2018) 43:1308–16. doi: 10.1038/npp.2017.273
49. Frokjaer VG, Pinborg A, Holst KK, Overgaard A, Henningsson S, Heede M, et al. Role of serotonin transporter changes in depressive responses to sex-steroid hormone manipulation: a positron emission tomography study. Biol Psychiatry. (2015) 78:534–43. doi: 10.1016/j.biopsych.2015.04.015
50. Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. (2015) 18:1413–20. doi: 10.1038/nn.4112
51. Shansky RM. Sex differences in behavioral strategies: avoiding interpretational pitfalls. Curr Opin Neurobiol. (2018) 49:95–8. doi: 10.1016/j.conb.2018.01.007
52. Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. (2009) 34 Suppl 1:S84–90. doi: 10.1016/j.psyneuen.2009.06.019
53. Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. (1998) 129:229–40. doi: 10.7326/0003-4819-129-3-199808010-00012
54. Kleinstauber M, Schmelzer K, Ditzen B, Andersson G, Hiller W, Weise C. Psychosocial profile of women with premenstrual syndrome and healthy controls: a comparative study. Int J Behav Med. (2016) 23:752–63. doi: 10.1007/s12529-016-9564-9
55. La Marca-Ghaemmaghami P, Dainese SM, Stalla G, Haller M, Zimmermann R, Ehlert U. Second-trimester amniotic fluid corticotropin-releasing hormone and urocortin in relation to maternal stress and fetal growth in human pregnancy. Stress. (2017) 20:231–40. doi: 10.1080/10253890.2017.1312336
56. La Marca-Ghaemmaghami P, Ehlert U. Stress during pregnancy: experienced stress, stress hormones, and protective factors. Eur Psychol. (2015) 20:102–19. doi: 10.1027/1016-9040/a000195
57. Luscher B, Fuchs T. GABAergic control of depression-related brain states. Adv Pharmacol. (2015) 73:97–144. doi: 10.1016/bs.apha.2014.11.003
58. Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. (2011) 16:383. doi: 10.1038/mp.2010.120
59. Camille Melon L, Maguire J. GABAergic regulation of the HPA and HPG axes and the impact of stress on reproductive function. J Steroid Biochem Mol Biol. (2016) 160:196–203. doi: 10.1016/j.jsbmb.2015.11.019
60. Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, et al. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: relevance to neurosteroids and programming of the stress response. J Neurosci. (2013) 33:19534–54. doi: 10.1523/JNEUROSCI.1337-13.2013
61. Jenkins LM, Kendall AD, Kassel MT, Patrón VG, Gowins JR, Dion C, et al. Considering sex differences clarifies the effects of depression on facial emotion processing during fMRI. J Affect Disord. (2018) 225:129–36. doi: 10.1016/j.jad.2017.08.027
62. Stickel S, Wagels L, Wudarczyk O, Jaffee S, Habel U, Schneider F, et al. Neural correlates of depression in women across the reproductive lifespan–an fMRI review. J Affect Disord. (2018) 246:556–70. doi: 10.1016/j.jad.2018.12.133
63. Schiller CE, Johnson SL, Abate AC, Schmidt PJ, Rubinow DR. Reproductive steroid regulation of mood and behavior. Compr Physiol. (2016) 6:1135. doi: 10.1002/cphy.c150014
64. Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, et al. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biol Psychiatry. (2000) 48:854–60. doi: 10.1016/S0006-3223(00)00967-7
65. Rubinow DR, Schmidt PJ, Roca CA. Estrogen–serotonin interactions: implications for affective regulation. Biol Psychiatry. (1998) 44:839–50. doi: 10.1016/S0006-3223(98)00162-0
66. Birzniece V, Bäckström T, Johansson IM, Lindblad C, Lundgren P, Löfgren M, et al. Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Res Rev. (2006) 51:212–39. doi: 10.1016/j.brainresrev.2005.11.001
67. Gulyás AI, Acsády L, Freund TF. Structural basis of the cholinergic and serotonergic modulation of GABAergic neurons in the hippocampus. Neurochem Int. (1999) 34:359–72. doi: 10.1016/S0197-0186(99)00041-8
68. Soderpalm B, Andersson G, Enerback C, Engel JA. In vivo administration of the 5-HT1A receptor agonist 8-OH-DPAT interferes with brain GABA (A)/benzodiazepine receptor complexes. Neuropharmacology. (1997) 36:1071–7. doi: 10.1016/S0028-3908(97)00105-6
69. Ismaili E, Walsh S, O'Brien PMS, Backstrom T, Brown C, Dennerstein L, et al. Fourth consensus of the international society for premenstrual disorders (ISPMD): auditable standards for diagnosis and management of premenstrual disorder. Arch Womens Ment Health. (2016) 19:953–8. doi: 10.1007/s00737-016-0631-7
70. Gehlert S, Song IH, Chang CH, Hartlage SA. The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychol Med. (2009) 39:129–36. doi: 10.1017/S003329170800322X
71. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, TX: American Psychiatric Association (2013).
72. Ditzen B, Nussbeck F, Drobnjak S, Spörri C, Wüest DU, Ehlert U, et al. Validierung eines deutschsprachigen DSM-IV-TR basierten fragebogens zum prämenstruellen syndrom. Z Kl Psych Psychoth. (2011) 40:149–59. doi: 10.1026/1616-3443/a000095
73. La Marca-Ghaemmaghami P, Ehlert U. Gynecological health: psychosocial aspects. In: James WD, editors. International Encyclopedia of the Social & Behavioral Sciences, 2 ed (Oxford: Elsevier) (2015). p. 462–8.
74. Stute P, Bodmer C, Ehlert U, Eltbogen R, Ging A, Streuli I, et al. Interdisciplinary consensus on management of premenstrual disorders in Switzerland. Gynecol Endocrinol. (2017) 33:342–8. doi: 10.1080/09513590.2017.1284788
75. Eisenlohr-Moul TA, Kaiser G, Weise C, Schmalenberger KM, Kiesner J, Ditzen B, et al. Are there temporal subtypes of premenstrual dysphoric disorder?: using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. (2019) 50:964–72. doi: 10.1017/S0033291719000849
76. Hartlage SA, Brandenburg DL, Kravitz HM. Premenstrual exacerbation of depressive disorders in a community-based sample in the United States. Psychosom Med. (2004) 66:698–706. doi: 10.1097/01.psy.0000138131.92408.b9
77. Bixo M Ekberg K Poromaa IS Hirschberg AL Jonasson AF Andreen L . Treatment of premenstrual dysphoric disorder with the GABAA receptor modulating steroid antagonist sepranolone (UC1010)-A randomized controlled trial. Psychoneuroendocrinology. (2017) 80:46–55. doi: 10.1016/j.psyneuen.2017.02.031
78. Freeman EW, Sondheimer SJ, Rickels K. Gonadotropin-releasing hormone agonist in the treatment of premenstrual symptoms with and without ongoing dysphoria: a controlled study. Psychopharmacol Bull. (1997) 33:303–9.
79. Peters W, Freeman MP, Kim S, Cohen LS, Joffe H. Treatment of premenstrual breakthrough of depression with adjunctive oral contraceptive pills compared with placebo. J Clin Psychopharmacol. (2017) 37:609–14. doi: 10.1097/JCP.0000000000000761
80. Wyatt KM, Dimmock PW, Ismail KM, Jones PW, O'Brien PM. The effectiveness of GnRHa with and without 'add-back' therapy in treating premenstrual syndrome: a meta analysis. BJOG. (2004) 111:585–93. doi: 10.1111/j.1471-0528.2004.00135.x
81. Schmidt PJ, Nieman LK, Grover GN, Muller KL, Merriam GR, Rubinow DR. Lack of effect of induced menses on symptoms in women with premenstrual syndrome. N Engl J Med. (1991) 324:1174–9. doi: 10.1056/NEJM199104253241705
82. Nguyen TV, Reuter JM, Gaikwad NW, Rotroff DM, Kucera HR, Motsinger-Reif A, et al. The steroid metabolome in women with premenstrual dysphoric disorder during GnRH agonist-induced ovarian suppression: effects of estradiol and progesterone addback. Transl Psychiatry. (2017) 7:e1193. doi: 10.1038/tp.2017.146
83. Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, et al. 5alpha-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. (2016) 41:1093–102. doi: 10.1038/npp.2015.246
84. Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, et al. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci. (2007) 10:469–77. doi: 10.1038/nn1868
85. Lee EE, Nieman LK, Martinez PE, Harsh VL, Rubinow DR, Schmidt PJ. ACTH and cortisol response to Dex/CRH testing in women with and without premenstrual dysphoria during GnRH agonist-induced hypogonadism and ovarian steroid replacement. J Clin Endocrinol Metab. (2012) 97:1887–96. doi: 10.1210/jc.2011-3451
86. Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, et al. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab. (2003) 88:3057–63. doi: 10.1210/jc.2002-021570
87. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology. (2014) 231:3619–34. doi: 10.1007/s00213-014-3572-8
88. Kiesner J, Granger DA. A lack of consistent evidence for cortisol dysregulation in premenstrual syndrome/premenstrual dysphoric disorder. Psychoneuroendocrinology. (2016) 65:149–64. doi: 10.1016/j.psyneuen.2015.12.009
89. Pilver CE, Levy BR, Libby DJ, Desai RA. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Women's Mental Health. (2011) 14:383-93. doi: 10.1007/s00737-011-0232-4
90. Segebladh B, Bannbers E, Kask K, Nyberg S, Bixo M, Heimer G, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. (2011) 90:746–52. doi: 10.1111/j.1600-0412.2011.01151.x
91. Segebladh B, Bannbers E, Moby L, Nyberg S, Bixo M, Backstrom T, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. (2013) 16:131–7. doi: 10.1007/s00737-013-0327-1
92. Eisenlohr-Moul TA, Rubinow DR, Schiller CE, Johnson JL, Leserman J, Girdler SS. Histories of abuse predict stronger within-person covariation of ovarian steroids and mood symptoms in women with menstrually related mood disorder. Psychoneuroendocrinology. (2016) 67:142–52. doi: 10.1016/j.psyneuen.2016.01.026
93. Gollenberg AL, Hediger ML, Mumford SL, Whitcomb BW, Hovey KM, Wactawski-Wende J, et al. Perceived stress and severity of perimenstrual symptoms: the BioCycle Study. J Women's Health. (2010) 19:959–67. doi: 10.1089/jwh.2009.1717
94. Namavar Jahromi B, Pakmehr S, Hagh-Shenas H. Work stress, premenstrual syndrome and dysphoric disorder: are there any associations? Iran Red Cresc Med J. (2011) 13:199–202.
95. Comasco E, Sundström-Poromaa I. Neuroimaging the menstrual cycle and premenstrual dysphoric disorder. Curr. Psychiatry Rep. (2015) 17:77. doi: 10.1007/s11920-015-0619-4
96. Berman SM, London ED, Morgan M, Rapkin AJ. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. (2013) 146:266–71. doi: 10.1016/j.jad.2012.06.038
97. Jeong HG, Ham BJ, Yeo HB, Jung IK, Joe SH. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. (2012) 140:260–7. doi: 10.1016/j.jad.2012.02.010
98. Baller EB, Wei SM, Kohn PD, Rubinow DR, Alarcón G, Schmidt PJ, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. (2013) 170:305–14. doi: 10.1176/appi.ajp.2012.12030385
99. Gingnell M, Ahlstedt V, Bannbers E, Wikström J, Sundström-Poromaa I, Fredrikson M. Social stimulation and corticolimbic reactivity in premenstrual dysphoric disorder: a preliminary study. Biol Mood Anxiety Disord. (2014) 4:3. doi: 10.1186/2045-5380-4-3
100. Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W. Cortical gamma-aminobutyric acid levels across the menstural cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. (2002) 59:851–8. doi: 10.1001/archpsyc.59.9.851
101. Bannbers E, Gingnell M, Engman J, Morell A, Comasco E, Kask K, et al. The effect of premenstrual dysphoric disorder and menstrual cycle phase on brain activity during response inhibition. J Affect Disord. (2012) 142:347–50. doi: 10.1016/j.jad.2012.04.006
102. Sundstrom I, Andersson A, Nyberg S, Ashbrook D, Purdy RH, Bäckström T. Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology. (1998) 67:126–38. doi: 10.1159/000054307
103. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. (2015) 17:87. doi: 10.1007/s11920-015-0628-3
104. Halbreich U, Kahn LS. Treatment of premenstrual dysphoric disorder with luteal phase dosing of sertraline. Expert Opin Pharmacother. (2003) 4:2065–78. doi: 10.1517/14656566.4.11.2065
105. Sundstrom I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. (1998) 23:73–88. doi: 10.1016/S0306-4530(97)00064-4
106. Roca CA, Schmidt PJ, Smith MJ, Danaceau MA, Murphy DL, Rubinow DR. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. (2002) 159:1876–81. doi: 10.1176/appi.ajp.159.11.1876
107. Huo L, Straub RE, Roca C, Schmidt PJ, Shi K, Vakkalanka R, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. (2007) 62:925–33. doi: 10.1016/j.biopsych.2006.12.019
108. Pakharenko L. Effect of estrogen receptor gene ESR1 polymorphism on development of premenstrual syndrome. Georgian Med News. (2014) 1:37–41. doi: 10.30841/2708-8731.1.2020.471239
109. Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF, et al. No support for historical candidate gene or candidate gene-by-interaction hypotheses for major depression across multiple large samples. Am J Psychiatry. (2019) 176:376–87. doi: 10.1176/appi.ajp.2018.18070881
110. Dubey N, Hoffman JF, Schuebel K, Yuan Q, Martinez PE, Nieman LK, et al. The ESC/E(Z) complex, an effector of response to ovarian steroids, manifests an intrinsic difference in cells from women with premenstrual dysphoric disorder. Mol Psychiatry. (2017) 22:1172–84. doi: 10.1038/mp.2016.229
111. World Health Organization. ICD-10: International Statistical Classificiation of Diseases and Related Health Problems: Tenth Revision, 2nd ed. (2004) Geneva: WHO.
112. Meltzer-Brody S, Boschloo L, Jones I, Sullivan PF, Penninx BW. The EPDS-Lifetime: assessment of lifetime prevalence and risk factors for perinatal depression in a large cohort of depressed women. Arch Womens Ment Health. (2013) 16:465–73. doi: 10.1007/s00737-013-0372-9
113. Martini J, Petzoldt J, Einsle F, Beesdo-Baum K, Hofler M, Wittchen HU. Risk factors and course patterns of anxiety and depressive disorders during pregnancy and after delivery: a prospective-longitudinal study. J Affect Disord. (2015) 175:385–95. doi: 10.1016/j.jad.2015.01.012
114. Schock H, Zeleniuch-Jacquotte A, Lundin E, Grankvist K, Lakso HÅ, Idahl A, et al. Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study. BMC Pregnancy Childbirth. (2016) 16:146. doi: 10.1186/s12884-016-0937-5
115. Gregoire A, Kumar R, Everitt B, Studd J. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. (1996) 347:930–3. doi: 10.1016/S0140-6736(96)91414-2
116. Gelaye B, Rondon MB, Araya R, Williams MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. (2016) 3:973–82. doi: 10.1016/S2215-0366(16)30284-X
117. Norhayati MN, Hazlina NH, Asrenee AR, Emilin WM. Magnitude and risk factors for postpartum symptoms: a literature review. J Affect Disord. (2015) 175:34–52. doi: 10.1016/j.jad.2014.12.041
118. Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med. (2006) 68:931–7. doi: 10.1097/01.psy.0000244385.93141.3b
119. Biaggi A, Conroy S, Pawlby S, Pariante CM. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. (2016) 191:62–77. doi: 10.1016/j.jad.2015.11.014
120. Schweizer S, Müller M, Reck C, Wallwiener M, Wallwiener S. Peripartale Depression bei instrumentellen und emotionellen Defiziten in der sozialen Unterstützung: Erforschung psychoneuroendokrinologisch-protektiver Effekte einer Achtsamkeitsintervention. Geburtshilfe und Frauenheilkunde Heft. (2019) 79:209. doi: 10.1055/s-0039-1678372
121. Evans LM, Myers MM, Monk C. Pregnant women's cortisol is elevated with anxiety and depression - but only when comorbid. Arch Womens Ment Health. (2008) 11:239–48. doi: 10.1007/s00737-008-0019-4
122. Iliadis SI, Sylven S, Hellgren C, Olivier JD, Schijven D, Comasco E, et al. Mid-pregnancy corticotropin-releasing hormone levels in association with postpartum depressive symptoms. Depress Anxiety. (2016) 33:1023–30. doi: 10.1002/da.22529
123. Szpunar MJ, Parry BL. A systematic review of cortisol, thyroid-stimulating hormone, and prolactin in peripartum women with major depression. Arch Womens Ment Health. (2017) 21:149–161. doi: 10.1007/s00737-017-0787-9
124. Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: a population-based register study. JAMA. (2006) 296:2582–9. doi: 10.1001/jama.296.21.2582
125. Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectr. (2015) 20:48–59. doi: 10.1017/S1092852914000480
126. Schmidt PJ, Murphy JH, Haq N, Danaceau MA, Clair LSS. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology. (2002) 27:907–20. doi: 10.1016/S0306-4530(02)00004-5
127. Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, et al. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. (2005) 62:154–62. doi: 10.1001/archpsyc.62.2.154
128. Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology. (2006) 186:425–33. doi: 10.1007/s00213-006-0313-7
129. Mostallino MC, Sanna E, Concas A, Biggio G, Follesa P. Plasticity and function of extrasynaptic GABAA receptors during pregnancy and after delivery. Psychoneuroendocrinology. (2009) 34:S74–83. doi: 10.1016/j.psyneuen.2009.06.013
130. Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci. (2009) 29:9592–601. doi: 10.1523/JNEUROSCI.2162-09.2009
131. Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle–linked changes in GABA A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. (2005) 8:797. doi: 10.1038/nn1469
132. Walton N, Maguire J. Allopregnanolone-based treatments for postpartum depression: why/how do they work? Neurobiol Stress. (2019) 11:100198. doi: 10.1016/j.ynstr.2019.100198
133. Deligiannidis KM, Kroll-Desrosiers AR, Mo S, Nguyen HP, Svenson A, Jaitly N, et al. Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology. (2016) 70:98–107. doi: 10.1016/j.psyneuen.2016.05.010
134. Zorumski CF, Paul SM, Covey DF, Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-a receptors and beyond. Neurobiol Stress. (2019) 11:100196. doi: 10.1016/j.ynstr.2019.100196
135. Duan C, Cosgrove J, Deligiannidis KM. Understanding peripartum depression through neuroimaging: a review of structural and functional connectivity and molecular imaging research. Curr Psychiatry Rep. (2017) 19:70. doi: 10.1007/s11920-017-0824-4
136. Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, et al. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res. (2013) 47:816–28. doi: 10.1016/j.jpsychires.2013.02.010
137. Deligiannidis K, Fales C, Kroll-Desrosiers A, Shaffer S, Tan Y, Hall J, et al. Resting-state functional connectivity, cortical gaba and allopregnanolone in postpartum depression: a functional magnetic imaging and spectroscopy study. Biol Psychiatry. (2019) 85:S114. doi: 10.1016/j.biopsych.2019.03.286
138. Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. (2010) 167:1373–80. doi: 10.1176/appi.ajp.2010.09081235
139. Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, et al. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry. (2011) 70:395–9. doi: 10.1016/j.biopsych.2011.02.021
140. Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World J Biol Psychiatry. (2010) 11:165–80. doi: 10.3109/15622970903131571
141. Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. (1998) 155:910–3. doi: 10.1176/ajp.155.7.910
142. Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. (1998) 95:3239–44. doi: 10.1073/pnas.95.6.3239
143. Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. (2017) 390:480–9. doi: 10.1016/S0140-6736(17)31264-3
144. Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. (2018) 392:1058–70. doi: 10.1016/S0140-6736(18)31551-4
145. Costas J, Gratacos M, Escaramis G, Martin-Santos R, de Diego Y, Baca-Garcia E, et al. Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. J Psychiatr Res. (2010) 44:717–24. doi: 10.1016/j.jpsychires.2009.12.012
146. Forty L, Jones L, Macgregor S, Caesar S, Cooper C, Hough A, et al. Familiality of postpartum depression in unipolar disorder: results of a family study. Am J Psychiatry. (2006) 163:1549–53. doi: 10.1176/ajp.2006.163.9.1549
147. Mahon PB, Payne JL, MacKinnon DF, Mondimore FM, Goes FS, Schweizer B, et al. Genome-wide linkage and follow-up association study of postpartum mood symptoms. American Journal of Psychiatry. (2009) 166:1229–37. doi: 10.1176/appi.ajp.2009.09030417
148. Murphy-Eberenz K, Zandi PP, March D, Crowe RR, Scheftner WA, Alexander M, et al. Is perinatal depression familial? J Affect Disord. (2006) 90:49–55. doi: 10.1016/j.jad.2005.10.006
149. Treloar SA, Martin NG, Bucholz KK, Madden PA, Heath AC. Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychol Med. (1999) 29:645–54. doi: 10.1017/S0033291799008387
150. Viktorin A, Meltzer-Brody S, Kuja-Halkola R, Sullivan PF, Landen M, Lichtenstein P, et al. Heritability of perinatal depression and genetic overlap with nonperinatal depression. Am J Psychiatry. (2016) 173:158–65. doi: 10.1176/appi.ajp.2015.15010085
151. Di Florio A, Putnam K, Altemus M, Apter G, Bergink V, Bilszta J, et al. The impact of education, country, race and ethnicity on the self-report of postpartum depression using the edinburgh postnatal depression scale. Psychol Med. (2017) 47:787–99. doi: 10.1017/S0033291716002087
152. PACT. Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry. (2015) 2:59–67. doi: 10.1016/S2215-0366(14)00055-8
153. Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, et al. Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. Lancet Psychiatry. (2017) 4:477–85. doi: 10.1016/S2215-0366(17)30136-0
154. Guintivano J, Krohn H, Lewis C, Byrne EM, Henders AK, Ploner A, et al. PPD ACT: an app-based genetic study of postpartum depression. Transl Psychiatry. (2018) 8:260. doi: 10.1038/s41398-018-0305-5
155. Avis NE, McKinlay SM. The massachusetts women's health study: an epidemiologic investigation of the menopause. J Am Med Women's Assoc. (1995) 50:45–9, 63.
156. Oldenhave A, Jaszmann LJ, Haspels AA, Everaerd WTA. Impact of climacteric on well-being: a survey based on 5213 women 39 to 60 years old. Am J Obstet Gynecol. (1993) 168:772–80. doi: 10.1016/S0002-9378(12)90817-0
157. Treloar AE. Menstrual cyclicity and the pre-menopause. Maturitas. (1981) 3:249–64. doi: 10.1016/0378-5122(81)90032-3
158. Willi J, Ehlert U. Assessment of perimenopausal depression: a review. J Affect Disord. (2019) 249:216–22. doi: 10.1016/j.jad.2019.02.029
159. Slopien R, Owecki M, Slopien A, Bala G, Meczekalski B. Climacteric symptoms are related to thyroid status in euthyroid menopausal women. J Endocrinol Invest. (2019) 43:75–80. doi: 10.1007/s40618-019-01078-7
160. Taneja V. Sex hormones determine immune response. Front Immunol. (2018) 9:1931. doi: 10.3389/fimmu.2018.01931
161. Ghosh M, Rodriguez-Garcia M, Wira CR. The immune system in menopause: pros and cons of hormone therapy. J Steroid Biochem Mol Biol. (2014) 142:171–175. doi: 10.1016/j.jsbmb.2013.09.003
162. Hall OJ, Klein S. Progesterone-based compounds affect immune responses and suceptibility to infections at diverse mucosal sites. Immunology. (2017) 10:1097–107. doi: 10.1038/mi.2017.35
163. Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. (2015) 172:227–36. doi: 10.1176/appi.ajp.2014.14070918
164. Hale GE, Burger H. Hormonal changes and biomarkers in late reproductive age, menopausal transition and menopause. Best Prac Res Clin Obstet Gynaecol. (2009) 23:7–23. doi: 10.1016/j.bpobgyn.2008.10.001
165. Hale GE Zhao X Hughes CL Burger HG Robertson DM Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the staging of reproductive aging workshop (STRAW) staging system. J Clin Endocrinol Metab. (2007) 92:3060–7. doi: 10.1210/jc.2007-0066
166. Shideler S, DeVane G, Kalra P, Benirschke K, Lasley B. Ovarian-pituitary hormone interactions during the perimenopause. Maturitas. (1989) 11:331–9. doi: 10.1016/0378-5122(89)90029-7
167. Miro F, Parker S, Aspinall L, Coley J, Perry P, Ellis J. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM study. J Clin Endocrinol Metab. (2004) 89:4910–5. doi: 10.1210/jc.2003-031731
168. Hale GE, Robertson DM, Burger HG. The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol. (2014) 142:121–31. doi: 10.1016/j.jsbmb.2013.08.015
169. O'Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, et al. Progesterone and ovulation across stages of the transition to menopause. Menopause. (2009) 16:1178. doi: 10.1097/gme.0b013e3181aa192d
170. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. (2006) 63:375. doi: 10.1001/archpsyc.63.4.375
171. Gordon JL, Eisenlohr-Moul TA, Rubinow DR, Schrubbe L, Girdler SS. Naturally occurring changes in estradiol concentrations in the menopause transition predict morning cortisol and negative mood in perimenopausal depression. Clin Psychol Sci. (2016) 4:919–35. doi: 10.1177/2167702616647924
172. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, Girdler SS. Estradiol variability, stressful life events, and the emergence of depressive symptomatology during the menopausal transition. Menopause. (2016) 23:257–66. doi: 10.1097/GME.0000000000000528
173. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, Girdler SS. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. (2018) 75:149–57. doi: 10.1001/jamapsychiatry.2017.3998
174. Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES. Increased urinary cortisol levels during the menopause transition. Menopause. (2006) 13:212–21. doi: 10.1097/01.gme.0000198490.57242.2e
175. Knight JM, Avery EF, Janssen I, Powell LH. Cortisol and depressive symptoms in a population-based cohort of midlife women. Psychosom Med. (2010) 72:855–61. doi: 10.1097/PSY.0b013e3181f4ab87
176. Genazzani AR, Bernardi F, Stomati M, Monteleone P, Luisi S, Rubino S, et al. Effects of estradiol and raloxifene analog on brain, adrenal and serum allopregnanolone content in fertile and ovariectomized female rats. Neuroendocrinology. (2000) 72:162–70. doi: 10.1159/000054583
177. Pluchino N, Cubeddu A, Giannini A, Merlini S, Cela V, Angioni S, et al. Progestogens and brain: an update. Maturitas. (2009) 62:349–55. doi: 10.1016/j.maturitas.2008.11.023
178. Berent-Spillson A, Marsh C, Persad C, Randolph J, Zubieta JK, Smith Y. Metabolic and hormone influences on emotion processing during menopause. Psychoneuroendocrinology. (2017) 76:218–25. doi: 10.1016/j.psyneuen.2016.08.026
179. Jovanovic H, Kocoska-Maras L, Rådestad AF, Halldin C, Borg J, Hirschberg AL, et al. Effects of estrogen and testosterone treatment on serotonin transporter binding in the brain of surgically postmenopausal women–a PET study. Neuroimage. (2015) 106:47–54. doi: 10.1016/j.neuroimage.2014.11.003
180. Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, et al. Increase in prefrontal cortex serotonin2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. (2003) 160:1522–4. doi: 10.1176/appi.ajp.160.8.1522
181. Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology. (2012) 37:372–82. doi: 10.1016/j.psyneuen.2011.07.007
182. Rekkas PV, Wilson AA, Lee VWH, Yogalingam P, Sacher J, Rusjan P, et al. Greater monoamine oxidase A binding in perimenopausal age as measured with carbon 11–labeled harmine positron emission tomography. JAMA Psychiatry. (2014) 71:873–9. doi: 10.1001/jamapsychiatry.2014.250
183. Maki PM, Kornstein SG, Joffe H, Bromberger JT, Freeman EW, Athappilly G, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women's Health. (2019) 28:117–34. doi: 10.1089/jwh.2018.27099.mensocrec
184. Kravitz HM, Janssen I, Lotrich FE, Kado DM, Bromberger JT. Sex steroid hormone gene polymorphisms and depressive symptoms in women at midlife. Am J Med. (2006) 119 (9 Suppl. 1), S87–93. doi: 10.1016/j.amjmed.2006.07.010
185. Rozycka A, Slopien R, Slopien A, Dorszewska J, Seremak-Mrozikiewicz A, Lianeri M, et al. The MAOA, COMT, MTHFR and ESR1 gene polymorphisms are associated with the risk of depression in menopausal women. Maturitas. (2016) 84:42–54. doi: 10.1016/j.maturitas.2015.10.011
186. Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the stages of reproductive aging workshoop+10: addressing the unfinished agenda of staging reproductive aging. Climacteric. (2012) 15:105–14. doi: 10.3109/13697137.2011.650656
187. Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. (2009) 10:446–57. doi: 10.1038/nrn2649
188. Hodes GE, Epperson CN. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry. (2019) 86:421–32. doi: 10.1016/j.biopsych.2019.04.028
189. Schule C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol. (2014) 113:79–87. doi: 10.1016/j.pneurobio.2013.09.003
190. Melon L, Hammond R, Lewis M, Maguire J. A novel, synthetic, neuroactive steroid is effective at decreasing depression-like behaviors and improving maternal care in preclinical models of postpartum depression. Front Endocrinol. (2018) 9:703. doi: 10.3389/fendo.2018.00703
191. Kim GE, Choi HY, Kim EJ. Impact of economic problems on depression in single mothers: a comparative study with married women. PLoS ONE. (2018) 13:e0203004. doi: 10.1371/journal.pone.0203004
192. Bereshchenko O, Bruscoli S, Riccardi C. Glucocorticoids, sex hormones, and immunity. Front Immunol. (2018) 9:1332. doi: 10.3389/fimmu.2018.01332
193. Schweizer S. Die Praxis für gynäkologische Psychoneuroendokrinologie- Vermessung eines Zukunftsfelds im Rahmen einer Fallstudie zur Praxengründung. Geburtshilfe und Frauenheilkunde Heft. (2020) 80:8. doi: 10.1055/s-0039-3402967
Keywords: depression, stress, allopregnanolone, GABARA receptor, premenstrual, perinatal, perimenopausal, peripartal
Citation: Schweizer-Schubert S, Gordon JL, Eisenlohr-Moul TA, Meltzer-Brody S, Schmalenberger KM, Slopien R, Zietlow A-L, Ehlert U and Ditzen B (2021) Steroid Hormone Sensitivity in Reproductive Mood Disorders: On the Role of the GABAA Receptor Complex and Stress During Hormonal Transitions. Front. Med. 7:479646. doi: 10.3389/fmed.2020.479646
Received: 20 June 2019; Accepted: 20 August 2020;
Published: 18 January 2021.
Edited by:
Cornelia Weise, University of Marburg, GermanyReviewed by:
Torbjorn Backstrom, Umeå University, SwedenJana Strahler, University of Giessen, Germany
Copyright © 2021 Schweizer-Schubert, Gordon, Eisenlohr-Moul, Meltzer-Brody, Schmalenberger, Slopien, Zietlow, Ehlert and Ditzen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophie Schweizer-Schubert, Praxis@Dr-Schweizer-Schubert.com; Beate Ditzen, Beate.Ditzen@med.uni-heidelberg.de
 Sophie Schweizer-Schubert
Sophie Schweizer-Schubert Jennifer L. Gordon
Jennifer L. Gordon Tory A. Eisenlohr-Moul
Tory A. Eisenlohr-Moul Samantha Meltzer-Brody
Samantha Meltzer-Brody Katja M. Schmalenberger
Katja M. Schmalenberger Radoslaw Slopien6
Radoslaw Slopien6 Anna-Lena Zietlow
Anna-Lena Zietlow Ulrike Ehlert
Ulrike Ehlert Beate Ditzen
Beate Ditzen