
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 13 August 2020
Sec. Geriatric Medicine
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00447
Background: Cognitive impairment is a common condition in older people, and age-related cognitive symptoms may progress to Mild Cognitive Impairment and Dementia. Physical exercise and cognitive training may be useful in maintaining cognitive function, and those developing impaired cognitive function should be advised to plan for the future. The MoCA test is a useful cognitive screening instrument, but the Russian version of this test has not yet been validated. The aim of the present study was to validate the Russian version of the MoCA test.
Methods: The study population included 160 residents of Israel aged 65 years and older with Russian as their mother tongue, 80 of whom were cognitively asymptomatic (AC) and 80 with a clinical diagnosis of MCI. All participants underwent cognitive screening using the Russian version of the MoCA test (MoCA-Ru) as well as evaluation by means of a validated computerized cognitive assessment battery (Neurotrax).
Results: The mean age of the study population was 78 ± 6.6 years and 123 (76.9%) were women. The MoCA-Ru score was higher in the AC group than in those with MCI (24.3 ± 3.74 vs. 20.2 ± 3.07, P < 0.0001). At a cutoff value of ≥25, sensitivity was 0.99 and specificity 0.54, with area under the curve (AUC) of 0.81.
Conclusions: We found the Russian language version of the MoCA test to be a useful cognitive screening instrument for older people with mild cognitive impairment.
As the population ages, the number of older people with cognitive decline is increasing. The transition from normal cognitive aging to mild cognitive impairment (MCI) and subsequent dementia has been well-described (1).
MCI (also termed mild neurocognitive disorder according to DSM 5) (2) is a syndrome in which a person experiencing cognitive symptoms is found to have objective cognitive impairment in one or more domains. While there may be minimal difficulties in instrumental activities of daily living, basic activities of daily living are preserved (3, 4). Early diagnosis of MCI allows for providing advice regarding the possible benefits of interventions, such as cognitive training (5), aerobic exercise (6), and planning for the future.
The prevalence of MCI increases with advancing age. The diagnosis of MCI is clinical and is based on Consensus Criteria (7). The determination of cognitive impairment is based on neuropsychological testing designed to evaluate the main cognitive areas affected by age-related cognitive decline (4). There is a clear need to develop valid screening tests to ascertain whether cognitive symptoms require further evaluation by neuropsychological testing.
The Montreal Cognitive Assessment (MoCA) Test developed by Nasreddine et al. (8) has been validated as a reliable cognitive screening test in the older population. This test, which takes only about 10 min to complete, was designed to be effective as a screening instrument for MCI. It has been translated into many languages, and the official website of MoCA includes over 50 translated versions. Some of these translations have been validated, for example those translated to Chinese (9–18), Korean (19), Japanese (20), Dutch (21), Spanish (22), Italian (23) Portuguese (24, 25), Turkish (26), Polish (27), Georgian (28), Arabic (29, 30) and Hebrew (31). A comparison of the findings of these validation studies will be presented later in the Discussion section.
The Russian language is the 7th most common spoken language in the world, with about 260 million people speaking the language globally, and about 150 million native Russian speakers (https://en.wikipedia.org/wiki/Russian_language). Over one million of the 9 million residents of Israel speak Russian. About 20.9% of citizens aged 65 years and older in Israel define Russian as their mother tongue, and Russian is the most common native language in Israel in this age group (32, 33).
To the best of our knowledge the MoCA questionnaire has not yet been validated in Russian. The aim of the present study was to validate the Russian version of MoCA-Ru in a population of Israelis aged 65 years of age and older who define Russian as their native tongue.
The study population included 160 participants aged 65 years and older with Russian as their mother tongue, 80 with a diagnosis of MCI according to Consensus Criteria (7) and 80 cognitively asymptomatic controls (AC) based on self-report. According to the Consensus Criteria (7), MCI was diagnosed when “(a) the person is neither normal nor demented; (b) there is evidence of cognitive deterioration shown by either objectively measured decline over time and/or subjective report of decline by self and/or informant in conjunction with objective cognitive deficits; and (3) activities of daily living are preserved and complex instrumental functions are either intact or minimally impaired.”
Data on the 80 MCI patients were collected from the medical records of the Comprehensive Geriatric Assessment Unit of the Clalit Health Services in Be'er Sheva. In this Unit, frail community-dwelling older individuals who reside in the Be'er Sheva region undergo clinical geriatric assessment. The multi-disciplinary staff of the Unit include geriatricians, a nurse, an occupational therapist, a social worker and a dietician. The comprehensive geriatric assessment routinely includes cognitive and functional assessments.
The AC group was a convenience sample recruited by one of the investigators (AV) from a community clinic in Be'er Sheva. Based on self-reporting of symptoms, inclusion criteria for this group were: (a) no cognitive symptoms suggesting cognitive decline, and (b) no complaint relating to cognition-related difficulties in instrumental activities of daily living (IADL). In both groups, individuals with acute medical conditions, as well as those with bipolar disorder, depression, or schizophrenia, were excluded from the study. All participants provided their informed consent to take part in the study. The study was approved by the Helsinki Committee of the Meir Hospital (Approval #104/2014C).
All participants underwent cognitive screening using the Russian version of the MoCA test (8), as well as cognitive assessment by means of the Russian version of the Neurotrax computerized battery, which has been validated for MCI in other languages (34).
The MoCA test includes eight parts with a maximum score of 30 (a score of 30 represents the best cognitive state). A score of 26 or above is considered normal in the English language version (8) and in some of the studies in other languages (12, 17, 20, 21, 29, 31). In the present study we used the original version of MoCA in Russian as translated by Posochin O.B. and Smirnov A.J. (https://www.mocatest.org/wp-content/uploads/2015/tests-instructions/MoCA-Test-Russian_2010.pdf).
The Neurotrax computerized cognitive assessment battery does not require that the subject has previous computer experience and uses standard neuropsychological tests that were adapted for computer use. The results are grouped into cognitive domains, including memory, attention, visuospatial and executive functions, and a composite score for global cognitive function is calculated. Results are corrected for age and education level according to database norms, with a mean score for each domain being 100, and normal being within one standard deviation (15 points). The test takes about 45 min to administer and requires the use of a mouse and number keyboard keys. It has been translated into various languages, including Russian (http://www.neurotrax.com).
The sample size of 80 in each group was calculated with α = 0.05 and a power of 80% between MoCA and Neurotrax. It was calculated using the Epinfo program 6 (Statcal). We aimed to recruit ninety individuals to each group, anticipating a dropout rate of ~15%.
The data were analyzed using the Receiving Operating Characteristic (ROC) method to test the capacity of each research instrument (MoCA compared to Neurotrax as the gold standard) to distinguish between patients with MCI and cognitively asymptomatic older participants. Sensitivity, specificity, positive and negative predictive values and the Youden Index (Sensitivity + Specificity −1) values were computed for various MOCA-Ru cutoff points (≥22, 23, 24, 25, 26). In addition, an ANOVA test was performed to compare continuous variables, and chi square or Fisher exact tests were performed for categorical variables. Statistical significance was set at P < 0.05.
The mean age of the study population was 78 ± 6.6 years and 123 (76.9%) were women. On average the participants had immigrated to Israel 20.1 ± 8.8 years earlier. Only 15 participants (9.4%) had <10 years of education, 52 (32.5%) had 10 years of education and 93 (58.1%) had more than 10 years of education. The socio-demographic data of the two study groups are presented in Table 1. The AC group were older and had lived in Israel for a longer period.
The overall score for MoCA-Ru was higher in the AC group (24.3 ± 3.74 vs. 20.2 ± 3.07, P ≥ 0.0001). This difference was true for all the test components except for Memory and Language 2 (Table 2). The internal consistency of the MoCA-Ru was limited, with a Cronbach's α = 0.65.
The global score of the Neurotrax battery was significantly higher in the AC group than in the MCI group (84.3 ± 10.7 vs. 79.9 ± 8.9, respectively, P = 0.005), but the only domain with a significant difference was Memory [86.7 ± 14.5 (AC) vs. 84.3 ± 10.7 (MCI), P < 0.0001]. Only six of the 80 participants in the AC group had a Neurotrax global score ≥100. For that reason, in a post hoc analysis, a sub-group of 93 participants was created including 52 participants from the MCI group with a global Neurotrax score ≤ 85 (more than one standard deviation below normal) and 41 from the AC group with a global score above 85 (more than one standard deviation below normal). The calculation of power for this sample was 98%. In this sub-group analysis the MoCA-Ru score was 25.66 ± 3.5 in the AC group and 19.4 ± 3.0 in the MCI group (P < 0.0001).
The sensitivity, specificity, positive, and negative values and the Youden Index for MoCA-Ru were calculated for the entire study population, as well as for the 93 participants in the selected sub-group (Table 3).
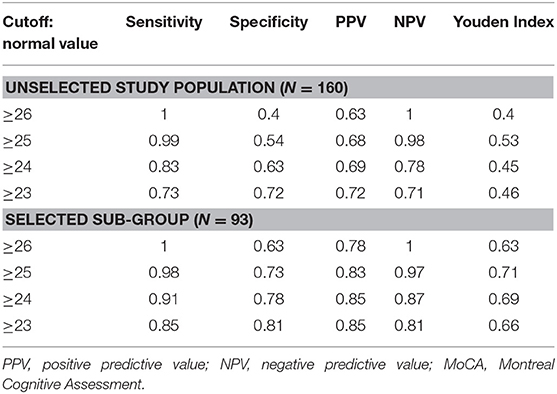
Table 3. Sensitivity, specificity, positive, and negative predictive values and Youden Index for the MoCA-Ru test, with different cutoffs.
In the unselected population of 160 participants the normal optimal cut-off for the MoCA-Ru with the highest Youden Index was ≥25, with a sensitivity of 0.99, but with a low specificity of 0.54. In the selected group of 93 participants the optimal cut-off for MoCA-Ru was similarly ≥25, with higher specificity (0.73) and similar sensitivity (0.98).
In the ROC curve analysis of the MoCA-Ru score, the area under the curve (AUC) was 0.81 for all subjects and 0.91 for the selected population, confirming that the test has a good discriminating capacity for differentiating participants with MCI from asymptomatic controls (Figures 1, 2).
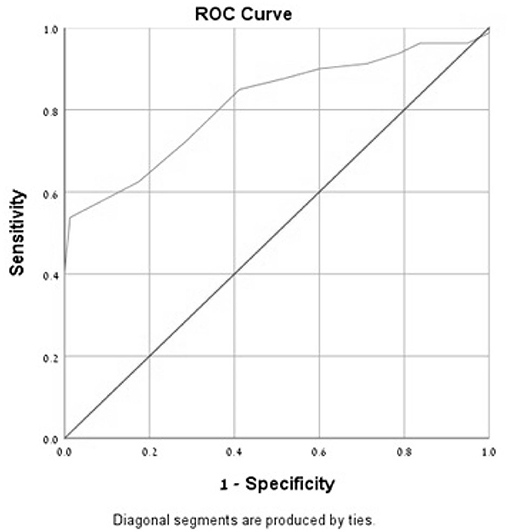
Figure 1. Receiver-operator characteristics curves (ROC) of the MoCA-Ru test in an unselected study population (cut-off 25).
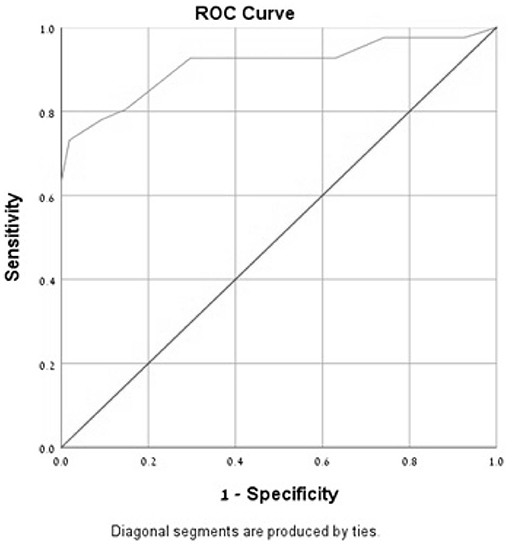
Figure 2. Receiver-operator characteristics curves (ROC) of the MoCA-Ru test in a selected study population (cut-off 25).
The results of the present study show that the MoCA-Ru test has good discriminating capacity for differentiating participants with MCI from cognitively asymptomatic controls. In contrast to the original English version of the MoCA (8) in which the normal cut-off value was set at ≥26, the suggested cut-off value in our study is ≥25.
Table 4 summarizes findings from previous studies that have evaluated the validity of MoCA among populations from various countries in different languages. It can be seen that the cut-off value varies between studies. A meta-analysis that included nine studies (37) showed that a cut-off of ≥23 had the best diagnostic accuracy.
A clear advantage of our study is that it is the first study to evaluate the validity of MoCA in a Russian speaking population. However, our study has clear limitations, which raise the question as to the generalizability of the study results. An important limitation is that the methodology of validation of the MoCA that we used in our study differed from that used for validation in a number of other languages. The majority of studies (9–12, 14–20, 22, 24–28, 30) included subjects with either MCI or dementia as well as controls. In contrast, as is the case with some of the other reported studies (13, 23, 29, 31), our study compared only subjects with MCI to a control group. However, it is important to note that in the original validation study of the MoCA, the investigators found similar results for both the MCI and dementia groups (8).
Another major limitation of our study is the choice of a group of controls who were cognitively asymptomatic based on self-report alone. While the subjects were diagnosed with MCI based on clinical evaluation, the asymptomatic control group did not undergo formal cognitive evaluation prior to inclusion in the study. This is in contrast to many of the studies where the control group included those with “normal cognition” based on an absence of cognitive symptoms as well as preserved function on cognitive testing (9–20, 31). Nevertheless, some studies did include a group of asymptomatic controls with normal cognitive screening (23, 28–30) but no formal cognitive evaluation.
It is important to note that the Neurotrax global score (84.3 ± 10.7) of the control group raises the possibility that some of the participants indeed had cognitive impairment beyond the level anticipated for their age and education level. We thus performed a sub-group analysis to include only cognitively normal controls based on Neurotrax findings compared to those with MCI. In this analysis the normal cut-off for the MoCA-Ru tests remained at ≤ 25, with improved specificity, supporting the validity of our findings. The internal consistency of the MoCA-Ru in our study was lower than in most of the studies listed in Table 4.
This convenience sample of asymptomatic controls was also older than our subjects with MCI. However, it is important to emphasize that Neurotrax scores are corrected for age and education level. Also, since the prevalence of MCI increases with age, the older age of the controls actually strengthens our findings.
Many of the studies evaluating different language versions of the MoCA utilized neuropsychological batteries (9, 11, 14, 16, 19, 22, 24, 26) while others used the MMSE with the Clinical Dementia Rating Scale (CDR) Scale (12, 15, 17, 27, 30) or even the MMSE alone (23, 28). In our study we used the Neurotrax computerized cognitive assessment battery, which was also used to validate the Hebrew version of the MoCA (31), and which has been shown to be reliable for determining the presence of MCI (34).
Our optimal normal cut-off level was ≥25, which is slightly lower than that of ≥26 found by Nasreddine et al. (8). A possible explanation for this finding may be related to the age of the participants. In our study, the age of the participants was relatively high compared to other studies in this field (Table 4). There is no consensus as to the effect of age on the results of the MoCA test. In some studies, an association with age was found (9, 11–14, 26), while others did not find such an association (10, 22, 35). The association between MoCA and education level has been described (9–14, 16, 22, 26, 35), with a positive association found between education levels and the MoCA score (25). In the present study <10% of the participants had an education level lower than 10 years (which is equivalent to high school education in the former Soviet Union). On comparing our study to those in which the study population was well-educated, the optimal normal cut-off was ≥26, as found in the study by Nasreddine et al. (8) Thus, for example, in the study conducted in Spanish in Colombia by Gil et al. (22) where the normal cut-off was ≥26, the sensitivity of MoCA was 0.99 and the specificity was 0.52, but when the cut-off was set at ≥23 the sensitivity dropped to 0.89 while the specificity increased to 0.79. In the study by Goldstein et al. (36) among African Americans the mean education level was 13.4 years in the MCI group and 10.9 in the control group. At a normal cut-off of ≥26 the sensitivity of MoCA was 1.0 with a specificity of 0.4, while at ≥25 the sensitivity was 0.95 and the specificity 0.63.
In a study from Georgia (28), the mean education level was 11.5 years in the MCI group and 11.6 years in the control group. At the recommended cut-off value of ≥26 the sensitivity of MoCA was 1.0 and the sensitivity was 0.44, but when the cut-off was set at ≥22 the specificity increased to 0.69 without any change in sensitivity (1.0). It is important to note that it is appropriate to compare the results of the Georgian study with those of the present study not only since the populations in both studies were well-educated, but also because the two populations were composed of former residents of the USSR who received an education in schools with similar curricula.
A clear advantage of our study is that it is the first study to evaluate the validity of MoCA in a Russian speaking population. However, this study has clear limitations. The major limitation of the study, which was already discussed in detail above, was that the participants in the control group did not undergo a comprehensive cognitive, affective, and functional assessment, and that their eligibility was based on self-reported normal cognitive and instrumental function. We can thus not exclude the possibility that subjects in the control group may indeed have suffered cognitive impairment. As well, the participants in the control group were recruited from community clinics and not at random. All these factors raise the question as to the generalizability of the study results. Several variables were not collected in the control group. For example, we do not have data on chronic co-morbidity or medical treatment for these participants, so we could not compare the two study groups beyond basic socio-demographic details.
Another limitation of the present study is related to the optimal cut-off, which was determined on the basis of the data analyses. This way of determining the optimal cut-off can increase the risk for bias (38) and could lead to an overestimation of sensitivity and specificity, especially in small studies (39).
In conclusion, we found the Russian language version of the MoCA test to be useful as a screening tool for MCI. Further studies should aim to further validate this instrument and to determine the optimal normal cut-off value for MoCA-Ru in the older population with impaired cognitive function.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by Helsinki Committee of the Meir Hospital (Approval #104/2014C). The patients/participants provided their written informed consent to participate in this study.
TF designed the study, was responsible for the statistical design of the study, and for carrying out the statistical analysis. AV designed the study, collected the data, and assisted with writing the article. TD and BP designed the study and assisted with writing the article. YP designed the study and wrote the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Campbell NL, Unverzagt F, LaMantia MA, Khan BA, Boustani MA. Risk factors for the progression of mild cognitive impairment to dementia. Clin Geriatr Med. (2013) 29:873–93. doi: 10.1016/j.cger.2013.07.009
2. Association. AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC (2013).
3. Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. (2006) 367:1262–70. doi: 10.1016/S0140-6736(06)68542-5
4. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Int Med. (2014) 275:214–28 doi: 10.1111/joim.12190
5. Butler M, McCreedy E, Nelson VA, Desai P, Ratner E, Fink HA, et al. Does cognitive training prevent cognitive decline?: a systematic review. Anna Int Med. (2018) 168:63–8. doi: 10.7326/M17-1531
6. Song D, Yu DSF, Li PWC, Lei Y. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: a systematic review and meta-analysis. Int J Nursing Stud. (2018) 79:155–64. doi: 10.1016/j.ijnurstu.2018.01.002
7. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Int Med. (2004) 256:240–6. doi: 10.1111/j.1365-2796.2004.01380.x
8. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
9. Dong Y, Yean Lee W, Hilal S, Saini M, Wong TY, Chen CL, et al. Comparison of the montreal cognitive assessment and the mini-mental state examination in detecting multi-domain mild cognitive impairment in a Chinese sub-sample drawn from a population-based study. Int Psychogeriatr. (2013) 25:1831–8. doi: 10.1017/S1041610213001129
10. Hu JB, Zhou WH, Hu SH, Huang ML, Wei N, Qi HL, et al. Cross-cultural difference and validation of the Chinese version of montreal cognitive assessment in older adults residing in Eastern China: preliminary findings. Arch Gerontol Geriatr. (2013) 56:38–43. doi: 10.1016/j.archger.2012.05.008
11. Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatr Neurol. (2011) 24:184–90. doi: 10.1177/0891988711422528
12. Ng A, Chew I, Narasimhalu K, Kandiah N. Effectiveness of montreal cognitive assessment for the diagnosis of mild cognitive impairment and mild Alzheimer's disease in Singapore. Singapore Med J. (2013) 54:616–9. doi: 10.11622/smedj.2013220
13. Ng TP, Feng L, Lim WS, Chong MS, Lee TS, Yap KB, et al. Montreal Cognitive Assessment for screening mild cognitive impairment: variations in test performance and scores by education in Singapore. Dementia Geriatr Cogn Disord. (2015) 39:176–85. doi: 10.1159/000368827
14. Tan JP, Li N, Gao J, Wang LN, Zhao YM, Yu BC, et al. Optimal cutoff scores for dementia and mild cognitive impairment of the montreal cognitive assessment among elderly and oldest-old Chinese population. J Alzheimer's Dis. (2015) 43:1403–12. doi: 10.3233/JAD-141278
15. Tsai JC, Chen CW, Chu H, Yang HL, Chung MH, Liao YM, et al. Comparing the sensitivity, specificity, and predictive values of the montreal cognitive assessment and mini-mental state examination when screening people for mild cognitive impairment and dementia in Chinese population. Arch Psychiatr Nurs. (2016) 30:486–91. doi: 10.1016/j.apnu.2016.01.015
16. Yeung PY, Wong LL, Chan CC, Leung JL, Yung CY. A validation study of the Hong Kong version of Montreal Cognitive Assessment. (HK-MoCA) in Chinese older adults in Hong Kong. Hong Kong Med J. (2014) 20:504–10. doi: 10.12809/hkmj144219
17. Yu J, Li J, Huang X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatr. (2012) 12:156. doi: 10.1186/1471-244X-12-156
18. Zhao S, Guo C, Wang M, Chen W, Wu Y, Tang W, et al. A clinical memory battery for screening for amnestic mild cognitive impairment in an elderly chinese population. J Clin Neurosci. (2011) 18:774–9. doi: 10.1016/j.jocn.2010.07.149
19. Lee JY, Dong Woo L, Cho SJ, Na DL, Hong Jin J, Kim SK, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatr Neurol. (2008) 21:104–10. doi: 10.1177/0891988708316855
20. Fujiwara Y, Suzuki H, Yasunaga M, Sugiyama M, Ijuin M, Sakuma N, et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr Gerontol Int. (2010) 10:225–32. doi: 10.1111/j.1447-0594.2010.00585.x
21. Thissen AJ, van Bergen F, de Jonghe JF, Kessels RP, Dautzenberg PL. [Applicability and validity of the dutch version of the montreal cognitive assessment. (moCA-d) in diagnosing MCI]. Tijdschrift Voor Gerontologie En Geriatrie. (2010) 41:231–40. doi: 10.1007/s12439-010-0218-0
22. Gil L, Ruiz de Sanchez C, Gil F, Romero SJ, Pretelt Burgos F. Validation of the montreal cognitive assessment. (MoCA) in Spanish as a screening tool for mild cognitive impairment and mild dementia in patients over 65 years old in Bogota, Colombia. Int J Geriatr Psychiatr. (2015) 30:655–62. doi: 10.1002/gps.4199
23. Bosco A, Spano G, Caffo AO, Lopez A, Grattagliano I, Saracino G, et al. Italians do it worse. Montreal Cognitive Assessment. (MoCA) optimal cut-off scores for people with probable Alzheimer's disease and with probable cognitive impairment. Aging Clin Exp Res. (2017) 29:1113–20. doi: 10.1007/s40520-017-0727-6
24. Freitas S, Simoes MR, Alves L, Santana I. Montreal cognitive assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. (2013) 27:37–43. doi: 10.1097/WAD.0b013e3182420bfe
25. Memoria CM, Yassuda MS, Nakano EY, Forlenza OV. Brief screening for mild cognitive impairment: validation of the Brazilian version of the Montreal cognitive assessment. Int J Geriatr Psychiatr. (2013) 28:34–40. doi: 10.1002/gps.3787
26. Kaya Y, Aki OE, Can UA, Derle E, Kibaroglu S, Barak A. Validation of montreal cognitive assessment and discriminant power of montreal cognitive assessment subtests in patients with mild cognitive impairment and alzheimer dementia in turkish population. J Geriatr Psychiatr Neurol. (2014) 27:103–9. doi: 10.1177/0891988714522701
27. Magierska J, Magierski R, Fendler W, Kloszewska I, Sobow TM. Clinical application of the Polish adaptation of the Montreal Cognitive Assessment. (MoCA) test in screening for cognitive impairment. Neurol Neurochirurg Polska. (2012) 46:130–9. doi: 10.5114/ninp.2012.28255
28. Janelidze M, Mikeladze N, Bochorishvili N, Dzagnidze A, Kapianidze M, Mikava N, et al. Validity of the georgian montreal cognitive assessment for the screening of mild cognitive impairment and Dementia. Am J Alzheimer's Dis Other Dementias. (2017) 32:36–40. doi: 10.1177/1533317516679304
29. Rahman TT, El Gaafary MM. Montreal cognitive assessment Arabic version: reliability and validity prevalence of mild cognitive impairment among elderly attending geriatric clubs in Cairo. Geriatr Gerontol Int. (2009) 9:54–61 doi: 10.1111/j.1447-0594.2008.00509.x
30. Saleh AA, Alkholy R, Khalaf OO, Sabry NA, Amer H, El-Jaafary S, et al. Validation of montreal cognitive assessment-basic in a sample of elderly egyptians with neurocognitive disorders. Aging Mental Health. (2018) 23: 551–7. doi: 10.1080/13607863.2018.1428936
31. Lifshitz M, Dwolatzky T, Press Y. Validation of the Hebrew version of the MoCA test as a screening instrument for the early detection of mild cognitive impairment in elderly individuals. J Geriatr Psychiatr Neurol. (2012) 25:155–61. doi: 10.1177/0891988712457047
32. Dwolatzky T, Brodsky J, Azaiza F, Clarfield AM, Jacobs JM, Litwin H. Coming of age: health-care challenges of an ageing population in Israel. Lancet. (2017) 389:2542–50. doi: 10.1016/S0140-6736(17)30789-4
34. Dwolatzky T, Whitehead V, Doniger GM, Simon ES, Schweiger A, Jaffe D, et al. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatr. (2003) 3:4. doi: 10.1186/1471-2318-3-4
35. Chu LW, Ng KH, Law AC, Lee AM, Kwan F. Validity of the cantonese chinese montreal cognitive assessment in Southern Chinese. Geriatr Gerontol Int. (2015) 15:96–103. doi: 10.1111/ggi.12237
36. Goldstein FC, Ashley AV, Miller E, Alexeeva O, Zanders L, King V. Validity of the montreal cognitive assessment as a screen for mild cognitive impairment and dementia in African Americans. J Geriatr Psychiatr Neurol. (2014) 27:199–203. doi: 10.1177/0891988714524630
37. Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment. (MoCA) cutoff scores. Int J Geriatr Psychiatr. (2018) 33:379–88. doi: 10.1002/gps.4756
38. Davis DH, Creavin ST, Noel-Storr A, Quinn TJ, Smailagic N, Hyde C, et al. Neuropsychological tests for the diagnosis of Alzheimer's disease dementia and other dementias: a generic protocol for cross-sectional and delayed-verification studies. Cochrane Database Syst Rev. (2013) 28:CD010460. doi: 10.1002/14651858.CD010460
Keywords: MoCA, mild cognitive impairment, Russian version, Israel, old age
Citation: Freud T, Vostrikov A, Dwolatzky T, Punchik B and Press Y (2020) Validation of the Russian Version of the MoCA Test as a Cognitive Screening Instrument in Cognitively Asymptomatic Older Individuals and Those With Mild Cognitive Impairment. Front. Med. 7:447. doi: 10.3389/fmed.2020.00447
Received: 21 September 2019; Accepted: 06 July 2020;
Published: 13 August 2020.
Edited by:
Maw Pin Tan, University of Malaya, MalaysiaReviewed by:
Mario Ulises Pérez-Zepeda, Dalhousie University, CanadaCopyright © 2020 Freud, Vostrikov, Dwolatzky, Punchik and Press. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Press, eWFucEBiZ3UuYWMuaWw=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.