
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 23 July 2020
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00265
This article is part of the Research Topic Pathogenesis, Diagnosis and Treatment of Lyme and other Tick-borne Diseases View all 17 articles
Clinical evaluation of Lyme Borreliosis (LB) is the starting point for its diagnosis. The patient's medical history and clinical symptoms are fundamental for disease recognition. The heterogeneity in clinical manifestations of LB can be related to different causes, including the different strains of Borrelia, possible co-infection with other tick transmitted pathogens, and its interactions with the human host. This review aims at describing the heterogeneous symptoms of Lyme Borreliosis, as well as offering a practical approach for recognition of the disease, both in terms of clinical features and diagnostic/research tools.
The genus Borrelia includes three Groups: Lyme Borreliosis (LB), Reptil Associated (REP), and Relapsing Fever (RF) Group (1).
Lyme disease or Lyme borreliosis (LB) is an anthropozoonosis, caused by different genospecies of the Borrelia burgdorferi sensu lato complex. The main tick vector for Borrelia species in Europe is the Ixodes ricinus (2), in America the Ixodes scapularis and Ixodes pacificus (3–5), while in Asia (6) and Russia (7) it is the Ixodes persulcatus. These ticks are possible vectors of Lyme Borreliosis (LB) as well as other pathogens, including viruses, intracellular bacteria, and Protozoa which can co-infect humans (LB co-infections) (8, 9). There are several B. burgdorferi sensu lato genospecies, directly associated with human LB. However, only three genospecies, namely Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii, have been systemically related to LB (4, 10). In addition, four other genospecies have been occasionally detected in humans: B. bissettiae (4, 5), B. lusitaniae (6, 7), B. spielmanii (8), and B. valaisiana (9), especially in Europe (11). Specificity in terms of dominating hosts has been reported both across and within continents (12, 13). The spatial distribution of the different genospecies allocates Borrelia burgdorferi sensu stricto in North America [and possibly B. mayonii, although this causes a disease somewhat distinct from typical LB (14)] and five species in Europe and Asia, B. afzelii, B. garinii, B. burgdorferi, B. spielmanii, and B. bavariensis (15). The heterogeneity in terms of genospecies can mirror different clinical manifestations of LB due to host specialization and tissue tropism. Although overlapping, distinct spectra of clinical manifestations have been recognized for the three main genospecies. In detail, B. burgdorferi sensu stricto is mostly associated with arthritis and neuroborreliosis, B. garinii with neuroborreliosis, and B. afzelii with chronic skin conditions such as acrodermatitis chronica atrophicans (10).
Spirochetes circulate in small amounts in the blood even in acute LB patients (16), with the exception of Borrelia mayonii which has been reported to cause high spirochetemia (14, 17). Depending on the case and genospecies, they can grow in several tissues (18), including skin, nervous and joint system, although less frequently LB can also affect eyes, heart, spleen, and other tissues.
Based on the spatial variability of Borrelia, for an accurate diagnosis, it could be useful to know if the patient has visited other countries or continents.
Some clinical aspects that can be helpful for a correct diagnosis of LB will be described hereafter. Figure 1, instead, shows an overview of possible overlapping scenarios defining LB. Furthermore, a brief description of laboratory investigation tools is included at the end of the review.
Patients sometimes seek medical assistance after a tick bite. In this case, the first step is to remove the tick with small tweezers or an ad hoc tool at the level of the rostrum. Afterwards, it is important to inform the patient of the symptoms, which, in the case of Borrelia infection, may develop in days/weeks. It is also possible to submit the tick for identification and testing for different pathogens. The identification of pathogens within the tick defines a possibility, not the certainty of developing LB (19).
Recognition of an EM rash is very important in LB as it is a hallmark symptom of LB, even when the patient does not recall the tick bite. However, as it has been observed, in rare cases the tick can still be attached to the center of the EM (20, 21). The geographical area where the patient was bitten as well as the date are important elements that should be gathered from the patient. Other variables to establish are: the time elapsed between the tick bite and the appearance of the erythema (usually 5–30 days) and its diameter, especially if larger than 5 cm (22). The most important diagnostic criterion is the EM centrifugal evolution. Erythema migrans (Figure 2) is pathognomonic for LB, therefore it should be treated immediately as serology testing to confirm infection is not necessary. Nevertheless, the clinical presentation of an EM can vary considerably (23). Several clinical variations have been observed, such as smaller-sized-EM of about the size of a coin, oval shaped EM with no darker outline, red-violet EM (erysipeloid), EM with vesicles which mimics herpes simplex or herpes zoster (24), painful EM (burning), itchy EM, hidden EM (scalp), and EM with atrophic evolution (25). It has been shown that in some cases of EM, Borrelia infection can already be disseminated (26).
Differential diagnoses include: mycosis fungoides, granuloma annulare, and interstitial granulomatous dermatitis (IGD), tinea corporis (mini EM), and erythema necroticans migrans.
Serological testing is not recommended because of their poor sensitivity in the early stages of LB. In order to achieve the best outcome for patients, antibiotic treatment should be started without delay.
Secondary EM is characterized by multiple erythematous lesions, which do not develop round the site of the tick bite. It can consist of a few or several plaques that can be located throughout the body (27). The lesions are multiple and can vary from a few cm to more than 20 cm, and are more frequently observed in children (22). The presence of multiple annular erythemas may precede the onset of neurological manifestations, especially in adults.
Borrelia lymphocytoma is defined as a B-cell pseudo-lymphoma that occurs in response to the presence of Borrelia antigens in the skin. Borrelial lymphocytoma can develop when EM is present and mimics a tick-bite reactive nodule. It is relatively frequent in Europe, while it is seldom observed in the US, because in most cases it is caused by Borrelia afzelii and more rarely by B. garinii and B. bissettii (28). Clinically, it appears as a solitary (rarely multiple) soft and non-tender bluish-red nodule or plaque with a size between 1 and 5 cm, sharply demarcated. It is typically found on the ear lobe (Figure 3), the mammary areola, and less frequently on the scrotum or the axillary fold. Extra-cutaneous signs and symptoms are very infrequent. The presence of Borrelia biofilm in human infected skin tissues has been demonstrated (29).
In the presence of this clinical manifestation the following exams should be performed: serology for Borrelia burgdorferi (ELISA and Western-Blot), β2-Microglobulin, and serological tests for Ehrlichia (Anaplasma) (30). Histological examination of skin biopsy and immunohistochemistry to define immunophenotype are also suggested (usually CD20 positive, Bcl-2 negative, κ and λ light chain expressed in an equivalent manner and Borrelia-PCR on DNA from skin slides).
Differential diagnosis includes cutaneous marginal zone lymphoma (PCMZL, Figure 4), which clinically and histologically may present similarities at the immunophenotype. PCMZL is generally CD20, CD22, CD79a, and BCL-2 positive, whereas it is CD5, CD10, Bcl-6, and CD23 negative, and the κ/λ light chain ratio in the histological tissue is very high (31). Borrelia's detection in PCMZL is included in the EORTC guidelines (32, 33).
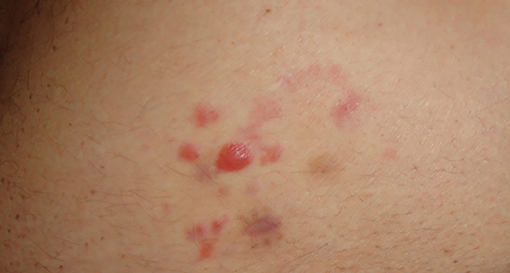
Figure 4. Primary cutaneous B cell marginal zone lymphoma of the trunk. Of note the image that has been already published refers to the same patient but it is slightly different from this one.
PCR for Borrelia on tissue's DNA (frozen or formalin-fixed and paraffin-embedded) can target OspA as reported by Cerroni (34), but also p41 (flagellin) and p66 (35). Skin biopsy specimens from the site of the lesion can also be submitted for culture and isolation of Borrelia.
ACA is the pathognomonic symptom of late LB. Patients, at presentation, should be asked whether they remember being bitten by a tick several months or even years before and whether they ever had an EM. Since the clinical appearance of ACA is not distinctive, it is of key importance to be generally alerted of the possibility of ACA in patients with bluish-red discoloration of a limb with or without swelling and/or atrophy, especially where LB is endemic (36, 37).
Unilateral acrocyanosis is present in the initial phases. This feature is followed by atrophy of the upper and/or lower limbs in an asymmetric manner, which, due to thinning and consequent greater transparency of the skin, allows the vessels of the dermis to be more visible. This condition leads over time to thinning of the most involved limb (22). ACA (Figure 5) is usually localized on the limbs, however, the face is also an acral site, and in some cases, it is difficult to distinguish the ACA of the face from Parry-Romberg syndrome, which may be a variant (38).
In addition to ACA, in some cases, other atrophic-sclerodermic manifestations may be related to LB (39, 40).
Serology by chemiluminescence is usually very high in VlsE IgG; in Western-Blot, p93 (p83/100) and DbpA are generally observed.
Skin biopsy for histological examination and PCR for Borrelia are also possible for research purposes. Isolation of Borrelia in BSK medium from skin lesion can result in the growth of Borrelia afzelii (or more rarely valaisania, lusitaniae, or yangtze).
Other possible skin manifestations that have been associated with LB are: urticaria (41), purpura (42), and erythema nodosum (Baggio-Yoshinari syndrome) (43).
Important information to be obtained from patients includes: the geographical area where the patient lives (if endemic or not for LB), if, in the previous weeks or months the patient has been in wooded areas, if he/she has traveled or has been camping, or has spent time in public parks and gardens or if he/she owns any pets. Requested information should also include the date of the onset of symptoms, recollection of a tick bite and/or of a circular erythema as well as the location and the duration of the skin lesion. In the case of a positive, response, the patient should be asked if he/she was previously treated with antibiotics, what type of antibiotics, and what the duration of treatment was. Other clinical manifestations can be fever, lymphadenopathy, balance disorders, dizziness, and photophobia (44).
Arthritis occurs after 4 days to 2 years (average, 6 months) from EM (45–49). In a European group of patients, the period between the tick bite or EM to the onset of arthritis ranged from 10 days to 16 months, with an average of 3 months (50). A summary of the articular involvement of LB is reported in Table 1.
In the early phase, the patient presents mono- or oligoarticular migrant arthralgia at the level of the large joints. The first affected joint is often near the site of the EM or the tick bite. However, sometimes other large or small joints, such as the temporomandibular joint (TMA), are also affected (51). Over time, the duration of joint arthralgia tends to lengthen, while painless intervals become shorter.
The articular involvement in the late phase has different clinical features compared to the typical migrant myo-arthralgia of early LB. The clinical symptomatology is not easy to distinguish from arthritis due to other causes. The disorder can become chronic or intermittent, with attacks lasting from a couple of weeks to a few months, which can be followed by resolution of symptoms. The intensity of the attacks decreases over time. Hyperpyrexia is not usually present, but a general sense of fatigue is common.
Swelling of the joints with marked functional impotence is often present. Affected knees, for instance, may have very large effusions (synovial fluid) (52). If those injuries are not diagnosed and treated, the patient will possibly experience erosion of the cartilage and bone which can lead to permanent damage of the joint.
Muscular system involvement includes myalgia, muscle weakness, and myositis (53) with difficulty in raising the arms above the head, carrying weights, and climbing stairs; and dysphagia, with difficulty breathing due to the involvement of intercostal muscles (inter-costal diaphragm). In some cases, these symptoms can simulate a dermatomyositis (41).
To confirm diagnosis, it is useful to perform a serological ELISA test followed by a Western Blot. In case the patient reports having headaches and/or a fever, tests for TBE, Ehrlichia (Anaplasma), Rickettsia, and Bartonella coinfections are suggested. A rheumatologic examination can be also requested.
Serum IgG antibodies for B. burgdorferi s.l. are present in high titers in patients with Lyme arthritis, while a negative IgG serology rules out the diagnosis (54, 55). Serological investigation of synovial fluid is not helpful because of the absence of a blood–synovial barrier; IgG antibody concentration in serum and synovial fluid will be equivalent.
In some cases, it can be useful to perform a PCR for Borrelia using DNA from synovial fluid or from a biopsy fragment of the synovium (56).
If the clinical picture is suggestive of LB, but the serology is negative, the clinical symptoms should over-rule a negative test, as pointed out by Burgdorfer. Commercial test kits are often inaccurate and can give negative results even in advanced LB. A negative test does not demonstrate the absence of LB and further investigations are needed to rule out differential diagnoses, such as that for an autoimmune disease (57).
Involvement of the nervous system occurs in up to 15% of patients with untreated LB (58). A summary of the possible neurological manifestations in LB is reported in Table 2.
Headache is the most frequent symptom. Cranial nerve involvement may occur, particularly that of the facial nerve (80%). Facial paralysis is bilateral in 25% (59, 60). Paralysis of the III, IV, VI cranial nerve, and optic neuritis can be observed.
Among children in Europe, the most common manifestations are facial nerve palsy (about 55%) and lymphocytic meningitis (about 30%) (61).
Meningopolyneuritis (Garin-Bujadoux-Bannwarth) with radicular pain and sometimes paresis of extremities or the abdominal wall (62, 63), neurologic bladder (64), and paresthesia can be observed. Myelitis is a rare manifestation of LB; although monofocal or multifocal lesions of the cervical spinal cord (65) have been described, as well as lombosacral myelitis (66) and acute transverse myelitis.
Pseudo tumor cerebri associated with LB was first described in 1985 (67). Subsequently, other cases have been described mainly in children (68) and rarely in adults (69).
Infection of the central nervous system is observed in 2–4% of Lyme neuroborreliosis, typically in the late or chronic stage of the disease (70). Encephalitis presents non-specific MRI findings of diffuse involvement of the brain parenchyma. Cerebral, cerebellar parenchyma, and thalami can be involved (71).
Neuroborreliosis can be associated with speech disorders, recent cognitive, and affective disorders (72), psychiatric disorders, states of anxiety, depression (73), and states of panic, and restless syndrome can be related to LB (74).
Cerebral vasculitis in patients with LB is observed in about 0.3% of cases (75). In some cases, the possibility of infection or co-infection (76) with Borrelia miyamotoi, which can be transmitted by the same tick as LB, should be considered (77, 78).
Neurological examination is suggested in order to rule out a differential diagnosis. In addition to the serological tests for anti-Borrelia antibodies by ELISA and Western Blot, it is also possible to perform a PCR for the detection of Borrelia DNA in cerebrospinal fluid (79) as well as an ELISA for Chemokine 13 (80).
Peripheral neuropathy can be detected in about 5–10% of Lyme neuroborreliosis cases. It can present as a chronic asymmetric neuropathy, usually without intrathecal antibodies (81).
For late neuroborreliosis, a careful examination is suggested for possible acrodermatitis chronica atrophicans (acral acrocyanotic appearance, and to verify any differences in limbs diameter) (82), and possibly a biopsy (for example on the ankle presenting neuropathic alterations) for histological examination of the small nervous fibers. Small fiber neuropathy (SFN) can be observed after antibiotic treatment (Post-treatment Lyme disease syndrome—PTLDS) and may be responsible for sensory symptoms (83).
In most patients, examination of the cerebrospinal fluid (CSF) reveals lymphocytic pleocytosis, damage to the blood-CSF-barrier, and an intrathecal synthesis of immunoglobulin IgM, IgG, and sometimes IgA (84); the protidorrachia is normal or slightly increased; the glycorrachia is normal or only slightly diminished.
During paralysis of the facial nerve, the CSF often presents lymphocytic pleocytosis even in the absence of signs and symptoms of meningitis (85).
After the onset of neurological symptoms, for a short time, intrathecal synthesis may not be detectable and CSF pleocytosis may be absent especially in children with isolated paralysis of the seventh cranial nerve (86). The production of intrathecal antibodies can continue even after recovery. On the other hand, intrathecal synthesis of specific antibodies is lacking in many patients with neuroborreliosis.
The use of chemokine (C–X–C motif) ligand 13 (CXCL13), a B-cell attracting chemokine, was debated for the laboratory diagnosis of acute Lyme neuroborreliosis in CSF (87). CXCL13 can be detected in CSF early in the disease and it has been reported to decrease with treatment (88). However, CXCL13 is not specific for Lyme neuroborreliosis and can also be found in some other inflammatory diseases of the CNS (88).
The different genospecies are often related to different clinical manifestations. Borrelia garinii is mainly related to typical early Lyme Neuroborreliosis (i.e., pain, meningoradiculoneuritis, or Bannwarth syndrome) while Borrelia valaisiana causes neurologic Lyme manifestations less frequently (89); Borrelia afzelii is less specific for neurologic manifestations as radicular pain and meningeal symptoms are rarely present (79). It is observed more often in late Neuroborreliosis by diffusion from the skin to small nerve fibers, often deriving from Acrodermatitis chronica atrophicans (82). It is able to cross the blood-brain barrier, but has a limited ability to produce inflammation in the CSF. The role of this genospecies has yet to be fully clarified.
The involvement of the heart is observed in 4–10% of patients with LB, of whom 90% have Lyme carditis (90, 91). The most frequent manifestations are:
• Atrioventricular Conduction disorder or other rhythm disorders,
• Pericarditis (94),
• Postural Orthostatic Tachycardia Syndrome (POTS) (95).
In addition to dyspnea, chest pain, or irregular heartbeat, typical symptoms include syncope episodes (93). On physical examination, 35% of patients had bradycardia and about 15% tachycardia.
If heart involvement in LB is suspected, a cardiological examination is suggested. The following investigations should be addressed: 12-channel ECG and 24-h ECG Holter (query: rhythm analysis, PQ interval, QRS width, ectopic beats), chest X-ray (question: heart size, congestion); echocardiography (diameter, ejection fraction, abnormal wall movement, pericardial effusion); cardiac MRI, and in selected cases myocardial biopsy for histological examination and cultural isolation of Borrelia (96). Electrophysiological examination can be done only in selected cases to confirm the diagnosis and establish a prognosis, as it is a highly invasive procedure and can cause arrhythmia. Patients should be clearly informed about the procedure and its associated risk.
Ocular manifestations can be linked to a direct involvement of the eye or can be secondary to Neuroborreliosis. Ocular involvement, is possible at every stage of LB and they can be summarized as follows:
• Follicular conjunctivitis often self-limited, and,
• Photophobia.
They can appear in the first stages of LB.
In the early disseminated phase, these manifestations are possible:
• Macular edema,
• Uveitis and Iridocyclitis,
• Optic Neuritis and Neuroretinitis,
• Retinal Vasculitis and Choroiditis,
• Branch Retinal Vein Occlusion (BRVO) (97),
• White Dot Syndrome (98),
• Stromal Keratitis and Episcleritis.
Intermediate uveitis is the most common uveitis in LB. Posterior uveitis is mostly associated with chorioretinal involvement (99).
Keratitis is characteristic of the second and third stages of LB and may either be interstitial or ulcerative. Episcleritis and scleritis are rare and can be observed mainly in the late phase of LB (100).
Regarding ocular manifestations due to Neuroborreliosis, they include:
• Myositis of Extraocular Muscles,
• Facial Palsy and other Cranial Nerve Palsies (101),
• Horner's syndrome (102).
Coinfections should be suspected in the following cases (103, 104):
✓ in the presence of fever and headache,
✓ in patients diagnosed with LB, who do not clinically improve or,
✓ whose symptoms have changed (e.g., appearance of febrile episodes) after adequate antibiotic treatment,
✓ when patients have leukopenia and neutropenia, persistent after treatment, or high ESR,
✓ when patients present purple, persistent skin lesions, even the same purpuric Erythema migrans (in our experience).
In these cases tests for Rickettsia, Anaplasma (105), Bartonella, Babesia (106), and TBE (FSME Frühsommer-Meningoenzephalitis) (105, 107) and Powassan virus (108) are suggested.
The spirochetes may persist in affected organs even months to years after the initial infection, causing a chronic form of illness. Therefore, antimicrobial agents have been found to have a role in all stages of the disease (109).
When patients come to the Lyme Disease Center, because they have been found to be positive for anti-Borrelia antibodies, it is necessary to request an accurate medical history including the geographical area where the patient lives, recollection of a tick bite, and if applicable, the recollection of a circular rash, its possible location, and its duration. This collection of information should be followed by an accurate examination for the presence of LB related symptoms. Medical history should also include any previous antibiotic treatment.
In the absence of any reported tick bite or EM and related clinical manifestations, if the serological test results are positive in IgG antibodies it is recommended to perform a WB, whereas positive IgM may not be specific, and serology should be repeated after 6 months.
When the skin, the myo-articular system, and/or the nervous, cardiac or ocular systems are involved, specific investigations must be carried out, as indicated in the two previous paragraphs.
These patients should also be subjected to immunological testing, as Borrelia antigens can induce autoimmune diseases in predisposed subjects (Trigger Factor).
In some cases, Borrelia induces the production of antibodies against certain surface antigens, which cross-react with specific sequences of organism structures (antigenic camouflage). It is known, in fact, that there can be cross-reactivity between OspA and the human leukocyte function antigen (LFA) (110, 111), as well as between Osp and acetylcholine receptors, enolase gamma, and Borrelia Enolase (112).
A thorough diagnostic examination should be based on the clinical picture, the organs involved, the serological pattern, and the tests that have been already performed.
The persistence of symptoms related to LB can be observed in untreated patients as well as in patients who have undergone treatment but continue to present symptoms. Untreated patients can develop persistent signs and symptoms, which usually involve the joints and less commonly the nervous system (113). Patients who instead have been treated mainly report a worsening of subjective symptoms. After 6 months, 36% of patients experienced an increase in fatigue, 20% complained of widespread pain, and 45% of neurocognitive impairment (114). Long-term persistent illness following antibiotic treatment is not uncommon, especially when treatment is delayed. About 10–20% of patients treated for early or late LB experience persistent symptoms, which may last for months or years (115). Symptoms consist of fatigue, joint and muscle pains, recent cognitive disorders, root pain, paresthesia, or dysesthesia. If we analyze the group of patients treated for Neuroborreliosis, this percentage increases significantly. Eikeland found that in Europe only 56% of patients treated with antibiotics for neuroborreliosis were symptom-free 30 months after treatment (116, 117).
Some published authors of medical research recognize mainly two clinical scenarios: the first characterized by typical symptoms of post-Lyme disease when symptoms persist for <6 months, and post-treatment Lyme disease syndrome or chronic Lyme disease if symptoms are debilitating and persist after treatment (118).
In the International Lyme and associated diseases society (ILADS) guidelines, “chronic Lyme disease” is described as a multisystem illness with persistent symptoms (119, 120), including fatigue, cognitive dysfunction, headaches, sleep disturbances, and other neurologic features, such as demyelinating disease, peripheral neuropathy, and sometimes motor neuron disease, neuropsychiatric presentations, cardiac presentations (including electrical conduction delays and dilated cardiomyopathy), and musculoskeletal problems (121–123). The cause may consist in residual damage to tissues and the immune system and cytokine production (122, 123), which occurs as a consequence of the infection causing possible modification of protein antigens located on the cell membrane. According to certain controlled studies, post-treatment Lyme disease syndrome (PTLDS) has often been shown to be non-responsive to antibiotic therapy. Several hypotheses have been suggested in order to explain PTLDS, among them, the presence of bacterial debris, autoimmunity, and co-infections, (120, 124, 125). In several studies, persistent Borrelia was isolated by culture or PCR (126–139).
The effectiveness of Ceftriaxone in several cases supports the hypothesis of bacterial persisters which survive in spite of previous antibiotic treatment (140). Delong et al. (140) have reported that retreatment can be effective, but further studies are needed to assess the role of antibiotics for persistent infection. It has been demonstrated that the persistence of Borrelia burgdorferi is likely due to the development of biologically less active permanent forms (Spheroblasts and round shapes) and of biofilm (141, 142). Biofilm analysis (Clinical Biofilm Ring Test—cBRT) (143) and treatment can produce an improvement in test results (144). In some cases, Borrelia can induce the production of antibodies against certain surface antigens, which cross-react with specific sequences of organism structures (antigenic camouflage). OspA is known, in fact, to cross-react with LFA, as well as Osp with Acetylcholine receptors. Treatment of B. burgdorferi in the stationary phase can result in a higher probability of regrowth once antibiotic treatment is interrupted (119).
Post-Treatment Lyme Disease Symptoms (PTLDS) and Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) have several clinical features in common, including fatigue, musculoskeletal pain, and cognitive difficulties. The Canadian Clinical Criteria for CFS/ME diagnosis include the following symptoms: Fatigue > 6 months, limited physical activity, unrefreshing sleep, impaired thinking and speech, vertigo, post-exertional fatigue, stress induced by exertion, reduced concentration, orthostatic intolerance, food intolerance (145).
Immunologic mechanisms have been suspected to play a role in both PTLDS and CFS/ME.
In CFS/ME patients, serum Activin B levels were significantly elevated compared with control subjects. Elevated Activin B levels together with normal Activin A levels identified patients with the diagnostic symptoms of CFS/ME (146, 147).
It has also been hypothesized that there is an immunosignature specific to CFS/ME and that this could aid the diagnosis. Scientists were in fact able to identify a 256-peptide signature that separates CFS/ME samples from healthy controls (148).
An increase in levels and frequency of IgG anti-neural antibody reactivity has been found in PTLDS. The anti-neural antibody response was independent from serologic positivity for antibodies to Borrelia burgdorferi; however there was no significant difference in the prevalence of anti-neural antibody reactivity between CFS/ME patients and healthy controls (149).
It is documented that trans-placental transmission of the spirochetes from the mother to the fetus is possible, and Borrelia starts crossing the placenta (150, 151) during the first month, unlike Treponema, which passes through the placenta barrier starting from the 5th month. A case of congenital Lyme with multiple annular erythema at birth has been reported in a child whose mother reported having an erythema migrans during pregnancy. Culture of skin biopsy from the child‘s skin lesion was positive for Borrelia garinii and rapid recovery was achieved after antibiotic therapy (152). A study on seven pregnant European women with EM and Borrelia isolated from blood indicated that the course and outcome of early LB was uneventful when pregnant women were treated with intravenous ceftriaxone, and that the outcome of their pregnancies was good (153). Therefore, in case of pregnancy, antibiotic prophylaxis treatment may be appropriate in the case of tick bites in endemic areas.
Below is a description of the symptoms of LB in children with potential exposure to tick bites, who have been diagnosed with EM or positive serological results or clinical manifestations compatible with LB.
Clinical suspicion of Lyme disease is based on the following clinical manifestations: for early localized LB, the presence of erythema migrans, often on the face, possibly associated with conjunctivitis and/or photophobia; for early disseminated LB the presence of multiple annular erythemas, Borrelial lymphocytoma, cranial neuritis, headache and/or pain and stiffness in the neck, migrant myo-arthralgia with possible involvement of the temporomandibular joint, alterations of electrocardiogram suggestive of carditis; for late BL the presence of arthritis. Acrodermatitis chronica atrophicans can also occur in children, but it is rare (154).
Patients with non-specific symptoms (e.g., fever or fatigue without specific manifestations of early, disseminated or late Lyme disease) are classified as probably not affected by Lyme disease. These patients should be considered positive only if, after 1 month, serology tests demonstrate serum conversion.
In some cases a rapid test response is required, ELISA or CLIA (155). Clinical evaluation plays a fundamental role when having to make initial decisions regarding children who visit the pediatric emergency room.
Several commercial products are available for detecting IgG and/or IgM antibodies against Borrelia burgdorferi s.l. complex. Test systems comprise different techniques including the Enzyme-linked immunosorbent assay (ELISA), the Enzyme-Immunoassay (EIA), the Enzyme-Linked Fluorescence Assay (ELFA), the Chemoluminescence Immunoassay (CLIA), Luminex, Fluoro-Immunoassay (FIA), and Western Blots/Immunoblots. Some tests use antigens obtained from native Borrelia bacteria, whilst others use manufacturing methods to prepare recombinant antigens. In some assays a mixture of both are used.
The European and North American guidelines indicate that the diagnosis of LB is currently based on a two-tier serology at all stages of the infection, except when erythema migrans is present (156). The two-tier testing procedure includes ELISA or EIA or VlsE/C6 as the first test and a Western Blot/Immunoblot assay as a confirmatory test. The VlsE Complex (variable major protein-like sequence Expressed—Vmp 35 kDa) is a surface protein formed by three defined domains: two invariable constant regions at the COOH and NH2 terminals, and one internal variable region. The invariable, internal areas are masked and protected by the “in vivo” external variable regions. Due to the continuous modifications of its external antigenically variable component, Borrelia is able to escape the immune system.
After the death of the spirochetes, the VlsE protein is presented in its entirety to the immune system, which can thus induce the production of antibodies against the preserved and invariable regions of VlsE. The dosage of the VlsE protein and its sixth invariant region (IR6) peptide of Borrelia burgdorferi has been reported to quantitatively vary after antibiotic treatment (157–159), although VlsE and C6 are detected both in convalescent and healthy people, and thus they do not differentiate between active and past infection. OspC is used for detection of specific IgM antibodies in the first stage of the serologic test, either as a single antigen or as a mixture with other antigens.
Immunoblot (western blot) is generally used to confirm positivity and can characterize the immune responses to specific proteins of Borrelia burgdorferi s.l. complex. The test kit manufacturers clearly define the interpretation for positive, negative, and equivocal samples.
The European Union Concerted Action on Lyme Borreliosis/EUCALB has conducted a multicenter study for the standardization of the interpretative criteria of immunoblot results in Europe. Although a set of eight bands were identified as significant in each participant laboratory, no single rule was formulated for use across Europe (160). The sensitivity of serological tests for diagnosis of LB is highly heterogeneous, varying with clinical manifestations (161). Average sensitivity estimates of 50% for erythema migrans, 77% for neuroborreliosis, 97% for acrodermatitis chronica atrophicans, and 73% for unspecified LB have been reported (162). Overall, the mean sensitivity of the serologic test was reported in a meta-analysis to be 59.5% (range: 30.6–86.2%) (163). Most European and North American guidelines recommend searching for intrathecal antibody production for the diagnosis of early Lyme neuroborreliosis (156).
In recent years, other commercially available serological tests have been developed for Borrelia detection. Among them, the TickPlex assay is an ELISA-based test, which also contains a new antigen for round bodies/persister forms of Borrelia. This assay has been reported to be useful in different stages of LB and the upgraded test also allows to simultaneously determine IgM and IgG antibodies of several tick-transmitted bacterial and viral pathogens (https://www.arminlabs.com/en/tests/tickplex).
Direct detection of B. burgdorferi sensu lato can be achieved by culture of the infectious agent, by microscopy, and by the use of molecular methods for the detection of Borrelia nucleic acids. These methods vary in sensitivity and procedure complexity. They can provide evidence for the presence of intact spirochetes or spirochete components, such as DNA or protein, in tick vectors, reservoir hosts, or patients.
Although in vitro cultivation of Borrelia from clinical samples represents the golden standard for proving an active infection, this method cannot be routinely used for diagnosis as it is time consuming and has low clinical sensitivity (54, 164). Borrelia burgdorferi sensu lato culture can be obtained from various tissues and body fluids with variable yield using dedicated media, such as the modified Kelly-Pettenkofer medium (MKP), the Barbour-Stoenner-Kelly II (BSK-II) medium, and the commercially available BSK-H medium (165, 166). Borrelia cultivation from clinical samples is mostly successful from skin biopsy when compared to blood and CSF cultures (165, 167).
Borrelia burgdorferi sensu lato detection by light microscopy is not feasible in clinical practice. The low Borrelia load does not allow a direct recognition of the spirochetes in tissue slides for routine diagnostic procedures. However, for specific purposes, the Warthin-Starry's silver stain (168, 169) and more recently the focus floating microscopy (FFM) (170–173), which are light microscopy-based techniques, can be used to detect Borrelia in clinical tissues. In addition, Borrelia species were also detected by electron microscopy in human samples from myocardial tissues (174) and crystalline keratopathy (175).
Among molecular methods of detecting Borrelia's nucleic acids, PCR-based methods are the most widely used for confirmation of Borrelia infection (167). However, Borrelia diagnosis continues to be very difficult, even by PCR (176). PCR sensitivity for Borrelia diagnosis is, indeed, highly variable, because of the multiple factors involved in its detectability by PCR. The type of starting material (blood, skin biopsies, cerebrospinal fluid, synovial fluid), the DNA extraction protocols, the possible use of systems for enrichment of microbial DNA, the PCR targets and PCR approach (nested PCR, real time PCR, digital PCR, PCR followed by hybridization, etc.) influence PCR sensitivity (167, 177, 178). The variability in specimens mentioned above and target amplification have also been found in the CE-IVD PCR assays developed for Borrelia detection (177). Low bacterial concentration is the main concern, and a further hypothesis regarding the possibility that during infection Borrelia invades the intracellular niche has been suggested (176). Moreover, different non-motile atypical morphologies of B. burgdorferi (s.l.) spirochetes have been reported. These include looped or ring-shaped forms, blebs, round bodies, and cell wall deficient forms; spirochete colonies or biofilm aggregates have also been described. The above-mentioned morphologies can impact Borrelia detectability by PCR. Biofilm busters to increase Borrelia load have been suggested for more accurate PCR tests (144). Borrelia PCR from skin biopsy from patients with ECM and ACA usually has a higher rate of positivity, but with large variation among studies (167). However, as the lesions are per se pathognomonic of LB, PCR is now only used for research purposes for those lesions. The diagnostic sensitivity of PCR in body fluids is highly variable, depending on the sample type, on the volume of the sample and on the contamination from PCR inhibitors (179). In synovial fluid, PCR for Borrelia detection is more sensitive than in blood and CSF (167). Borrelia targets for PCR must be genetically stable and should enable the detection of all pathogen of Borrelia species. They can be located on the chromosome or on plasmid DNA. The most frequent chromosomal targets that have been reported in clinical studies are flagellin (26, 164, 180–182), 16S rRNA gene (180, 183–185), the gene codifying for the 66 kDa protein (26, 56, 184, 185), while the most used plasmid target is OspA (56, 180, 183, 186–188), which has been also reported to be more stable after degradation of spirochetes (178). At present the major concern in Borrelia diagnosis by PCR is the lack of standardization of the protocols and analyzed targets (167, 177, 178). This heterogeneity in terms of PCR protocols and samples makes it difficult to diagnose LB unequivocally by PCR in settings in which the pre-test probability of LB is very low, including for instance patients suspected of late LB, with negative serology (178).
Because of the limits of serology in detecting the Borrelia sensu lato complex in clinical samples, other commercially available tests have been developed. Among them, the T cell response tests, including the lymphocyte transformation test (LTT and MELISA) and the enzyme linked immuno-spot (EliSpot) test have been commercialized. They are based on the detection in patients' blood of Borrelia-specific T-lymphocyte, notably the T helper lymphocytes, which are reported to circulate in the blood in detectable numbers only during an active immune response against Borrelia and to persist in a non-florid infection in lymphoid organs (189).
Alternative tests to the traditional serology and PCR for Borrelia detection have also been proposed. Among them, Luminex-based approaches for Borrelia detection have been reported. This multiplex- high-throughput technique was used for the simultaneous detection of the plasmid contents of different B. burgdorferi strains (10 Ag-Luminex technology) (190), but also to diagnose Borrelia miyamotoi in the serum of European patients (191) as well as for the simultaneous detection of 10 insect-borne pathogens, including Borrelia (192). An immuno-PCR (iPCR) assay, which takes advantage of the PCR properties to increase the sensitivity of standard ELISA (193), was also developed and evaluated for the detection of antibodies to the B. burgdorferi C6 peptide (194). Other approaches refer to the metabolic profiling for early Lyme disease (195) and the measurement of IFN-γ after incubating blood with Borrelia antigens. The latter method was reported to be potentially useful in the laboratory diagnosis of early Lyme disease, even after antibiotic treatment (196).
Written informed consent was obtained as part of the hospital procedures from the individuals and/or minor' legal guardian/next of kin for the publication of any potentially identifiable images included in this article.
GT managed the clinical aspect of the review, MR the section dedicated to serology, SB the section related to direct diagnosis go LB. All authors drafted and revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Erica Falkingham for the language revision of the manuscript and the Associazione Lyme Italia e Conifezioni for supporting Lyme Borreliosis studies and dissemination.
1. Margos G, Gofton A, Wibberg D, Dangel A, Marosevic D, Loh SM, et al. The genus Borrelia reloaded. PLoS One. (2018) 13:e0208432. doi: 10.1371/journal.pone.0208432
2. Ackermann R, Kabatzki J, Boisten HP, Steere AC, Grodzicki RL, Hartung S, et al. Ixodes ricinus spirochete and European erythema chronicum migrans disease. Yale J Biol Med. (1984) 57:573–80.
3. Barton WE, Gray EW, Shipes D. An initial investigation of the status of Borrellia burgodorferi and its suspected primary vector, Ixodes scapularis, in South Carolina. J S C Med Assoc. (1992) 88:5–8.
4. Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo-Candiani J, Welsh O. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. (2019) 38:201–8. doi: 10.1007/s10096-018-3417-1
5. Bissett ML, Hill W. Characterization of Borrelia burgdorferi strains isolated from Ixodes pacificus ticks in California. J Clin Microbiol. (1987) 25:2296–301. doi: 10.1128/JCM.25.12.2296-2301.1987
6. Zhang ZF. [Investigation of Lyme disease in northeast of China]. Zhonghua Liu Xing Bing Xue Za Zhi. (1989) 10:261–4.
7. Kriuchechnikov VN, Korenberg EI, Shcherbakov SV, Kovalevskii Iu V, Levin ML. [Identification of Borrelia isolated in the USSR from Ixodes persulcatus Schulze ticks]. Zh Mikrobiol Epidemiol Immunobiol. (1988) 12:41–4.
8. Kwon HY, Im JH, Park YK, Durey A, Lee JS, Baek JH. Two imported cases of Babesiosis with complication or co-infection with Lyme disease in Republic of Korea. Korean J Parasitol. (2018) 56:609–13. doi: 10.3347/kjp.2018.56.6.609
9. Mancini F, Vescio MF, Toma L, Di Luca M, Severini F, Caccio SM, et al. Detection of tick-borne pathogens in ticks collected in the suburban area of Monte Romano, Lazio Region, Central Italy. Ann Ist Super Sanita. (2019) 55:143–50. doi: 10.4415/ANN_19_02_06
10. Strnad M, Honig V, Ruzek D, Grubhoffer L, Rego ROM. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl Environ Microbiol. (2017) 83:e00609–17. doi: 10.1128/AEM.00609-17
11. Stanek G, Reiter M. The expanding Lyme Borrelia complex–clinical significance of genomic species? Clin Microbiol Infect. (2011) 17:487–93. doi: 10.1111/j.1469-0691.2011.03492.x
12. Rimoldi SG, Merli S, Bestetti G, Giacomet V, Cislaghi G, Grande R, Sanzani S, et al. Occurrence of Lyme disease infection in a non endemic area in Northern Italy. G Ital Dermatol Venereol. (2020) 155:320–24. doi: 10.23736/S0392-0488.18.05941-2
13. Kilpatrick AM, Dobson ADM, Levi T, Salkeld DJ, Swei A, Ginsberg HS, et al. Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Philos Trans R Soc Lond B Biol Sci. (2017) 372:20160117. doi: 10.1098/rstb.2016.0117
14. Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, Davis JP, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. (2016) 16:556–64. doi: 10.1016/S1473-3099(15)00464-8
15. Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. (2012) 379:461–73. doi: 10.1016/S0140-6736(11)60103-7
16. Wallach FR, Forni AL, Hariprashad J, Stoeckle MY, Steinberg CR, Fisher L, et al. Circulating Borrelia burgdorferi in patients with acute Lyme disease: results of blood cultures and serum DNA analysis. J Infect Dis. (1993) 168:1541–3. doi: 10.1093/infdis/168.6.1541
17. Wilhelmsson P, Lindgren PE. Detection of a novel Lyme borreliosis pathogen. Lancet Infect Dis. (2016) 16:511–2. doi: 10.1016/S1473-3099(15)00483-1
18. Trevisan G, Cinco M. Lyme disease. A general survey. Int J Dermatol. (1990) 29:1–8. doi: 10.1111/j.1365-4362.1990.tb03745.x
19. Faulde MK, Rutenfranz M, Hepke J, Rogge M, Gorner A, Keth A. Human tick infestation pattern, tick-bite rate, and associated Borrelia burgdorferi s.l. infection risk during occupational tick exposure at the Seedorf military training area, northwestern Germany. Ticks Tick Borne Dis. (2014) 5:594–9. doi: 10.1016/j.ttbdis.2014.04.009
20. Afzelius A. Verhandlungen der dermatologischen Gesellschaft zu Stockholm. Arch Dermatol Syph. (1910) 101:404. doi: 10.1007/BF01832773
21. Lipschutz B. Über eine seltene Erythem Form (Erythema chronicum migrans). Arch Dermatol Syphilis. (1914) 118:349. doi: 10.1007/BF02076105
22. Mullegger RR, Glatz M. Skin manifestations of lyme borreliosis: diagnosis and management. Am J Clin Dermatol. (2008) 9:355–68. doi: 10.2165/0128071-200809060-00002
23. Sharma A, Guleria S, Sharma R, Sharma A. Lyme disease: a case report with typical and atypical lesions. Indian Dermatol Online J. (2017) 8:124–7. doi: 10.4103/2229-5178.202271
24. Trevisan G, Stinco G, Cattonar P, Nobile C. Unusual onset of Lyme borreliosis simulating herpes zoster. J Eur Acad Dermatol Venereol. (1995) 4:299–300. doi: 10.1111/j.1468-3083.1995.tb00357.x
25. Trevisan G, Agolzer A, Grizzo A. Erythema chronicum migrans et atrophicans of the breast in a baby girl. Eur J Pediatr Dermatol. (1992) 2:25–8.
26. Pauluzzi P, Bonin S, Gonzalez Inchaurraga MA, Stanta G, Trevisan G. Detection of spirochaetal DNA simultaneously in skin biopsies, peripheral blood and urine from patients with erythema migrans. Acta Derm Venereol. (2004) 84:106–10. doi: 10.1080/00015550310006815
27. Di Meo N, Retrosi C, Corneli P, Trevisan G. Multiple erythema migrans due to borreliosis. G Ital Dermatol Venereol. (2019) 154:599–600. doi: 10.23736/S0392-0488.18.05888-1
28. Maraspin V, Nahtigal Klevisar M, Ruzic-Sabljic E, Lusa L, Strle F. Borrelial lymphocytoma in adult patients. Clin Infect Dis. (2016) 63:914–21. doi: 10.1093/cid/ciw417
29. Sapi E, Balasubramanian K, Poruri A, Maghsoudlou JS, Socarras KM, Timmaraju AV, et al. Evidence of in vivo existence of borrelia biofilm in borrelial lymphocytomas. Eur J Microbiol Immunol. (2016) 6:9–24. doi: 10.1556/1886.2015.00049
30. Bonin S, Stinco G, Patriarca MM, Trevisini S, di Meo N, Trevisan G. Could co-infection with anaplasma play a role in Borrelia-associated primary cutaneous marginal zone B-cell lymphomas? Indian J Dermatol Venereol Leprol. (2016) 82:81–4. doi: 10.4103/0378-6323.171011
31. Selva R, Violetti SA, Delfino C, Grandi V, Cicchelli S, Tomasini C, et al. A literature revision in primary cutaneous B-cell lymphoma. Indian J Dermatol. (2017) 62:146–57. doi: 10.4103/ijd.IJD_74_17
32. Roggero E, Zucca E, Mainetti C, Bertoni F, Valsangiacomo C, Pedrinis E, et al. Eradication of Borrelia burgdorferi infection in primary marginal zone B-cell lymphoma of the skin. Hum Pathol. (2000) 31:263–8. doi: 10.1016/S0046-8177(00)80233-6
33. Senff NJ, Noordijk EM, Kim YH, Bagot M, Berti E, Cerroni L, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. (2008) 112:1600–9. doi: 10.1182/blood-2008-04-152850
34. Cerroni L, Zochling N, Putz B, Kerl H. Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J Cutan Pathol. (1997) 24:457–61. doi: 10.1111/j.1600-0560.1997.tb01318.x
35. Gatti A, Stinco G, Trevisini S, di Meo N, Signoretto D, Leonardo E, et al. Electrochemotherapy as a novel treatment for primary cutaneous marginal zone B-cell lymphomas. Dermatol Therapy. (2014) 27:244–7. doi: 10.1111/dth.12128
36. Herxheimer K, Hartmann K. Ueber Acrodermatitis chronica atrophicans. Arch Dermatol Syphilis. (1902) 61:57–76. doi: 10.1007/BF01933570
38. Stinco G, Trevisan G, Martina Patriarca M, Ruscio M, Di Meo N, Patrone P. Acrodermatitis chronica atrophicans of the face: a case report and a brief review of the literature. Acta Dermatovenerol Croat. (2014) 22:205–8. Available online at: https://hrcak.srce.hr/index.php?show=clanak&id_clanak_jezik=187065
39. Aberer E, Wutte N. Atrophosclerodermic manifestations of Lyme borreliosis. Open Dermatol J. (2016) 10(Suppl 1:M4):27–43. doi: 10.2174/1874372201610010027
40. Trevisan G, Stinco G, Nobile C, Bonin S, Stanta G. Detection of Borrelia burgdorferi in skin biopsies from patients with morphea by polymerase chain reaction. J Eur Acad Dermatol Venereol. (1996) 6:15–9. doi: 10.1111/j.1468-3083.1996.tb00127.x
41. Novitch M, Wahab A, Kakarala R, Mukerji R. The emergence of a forgotten entity: dermatomyositis-like presentation of lyme disease in rural wisconsin. Cureus. (2018) 10:e2608. doi: 10.7759/cureus.2608
42. Berger BW. Dermatologic manifestations of Lyme disease. Rev Infect Dis. (1989) 11(Suppl 6):S1475–81. doi: 10.1093/clinids/11.Supplement_6.S1475
43. Mantovani E, Costa IP, Gauditano G, Bonoldi VL, Higuchi ML, Yoshinari NH. Description of Lyme disease-like syndrome in Brazil. Is it a new tick borne disease or Lyme disease variation? Braz J Med Biol Res. (2007) 40:443–56. doi: 10.1590/S0100-879X2007000400002
44. Hercogova J, Brzonova I. Lyme disease in central Europe. Curr Opin Infect Dis. (2001) 14:133–7. doi: 10.1097/00001432-200104000-00004
45. Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. (1987) 107:725–31. doi: 10.7326/0003-4819-107-5-725
47. Gerber MA, Shapiro ED, Burke GS, Parcells VJ, Bell GL. Lyme disease in children in southeastern Connecticut. Pediatric Lyme Disease Study Group. N Engl J Med. (1996) 335:1270–4. doi: 10.1056/NEJM199610243351703
48. Sigal LH, Zahradnik JM, Lavin P, Patella SJ, Bryant G, Haselby R, et al. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant outer-surface protein A Lyme disease Vaccine Study Consortium. N Engl J Med. (1998) 339:216–22. doi: 10.1056/NEJM199807233390402
49. Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T, et al. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. (2002) 34:1184–91. doi: 10.1086/339813
50. Herzer P. Joint manifestations of Lyme borreliosis in Europe. Scand J Infect Dis Suppl. (1991) 77:55–63.
52. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. (2015) 29:269–80. doi: 10.1016/j.idc.2015.02.004
53. Bitar I, Lally EV. Musculoskeletal manifestations of Lyme disease. Med Health R I. (2008) 91:213–5.
54. Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev. (2005) 18:484–509. doi: 10.1128/CMR.18.3.484-509.2005
55. Puius YA, Kalish RA. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am. (2008) 22:289–300, vi–vii. doi: 10.1016/j.idc.2007.12.014
56. Priem S, Burmester GR, Kamradt T, Wolbart K, Rittig MG, Krause A. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann Rheum Dis. (1998) 57:118–21. doi: 10.1136/ard.57.2.118
57. Horowitz RI, Lacout A, Marcy PY, Perronne C. To test or not to test? Laboratory support for the diagnosis of Lyme borreliosis. Clin Microbiol Infect. (2018) 24:210. doi: 10.1016/j.cmi.2017.09.015
58. Marques AR. Lyme Neuroborreliosis. Continuum (Minneap Minn). (2015) 21(6 Neuroinfectious Disease): 1729–44. doi: 10.1212/CON.0000000000000252
59. Halperin JJ. Nervous system Lyme disease. Infect Dis Clin North Am. (2015) 29:241–53. doi: 10.1016/j.idc.2015.02.002
60. Strle F, Stanek G. Clinical manifestations and diagnosis of lyme borreliosis. Curr Probl Dermatol. (2009) 37:51–110. doi: 10.1159/000213070
61. Huppertz HI, Sticht-Groh V. Meningitis due to Borrelia burgdorferi in the initial stage of Lyme disease. Eur J Pediatr. (1989) 148:428–30. doi: 10.1007/BF00595904
62. Ackermann R, Horstrup P, Schmidt R. Tick-borne meningopolyneuritis (Garin-Bujadoux, Bannwarth). Yale J Biol Med. (1984) 57:485–90.
63. Garin C, Bujadoux A. Paralysis by ticks 1922. Clin Infect Dis. (1993) 16:168–9. doi: 10.1093/clinids/16.1.168
64. Dumic I, Vitorovic D, Spritzer S, Sviggum E, Patel J, Ramanan P. Acute transverse myelitis - a rare clinical manifestation of Lyme neuroborreliosis. IDCases. (2019) 15:e00479. doi: 10.1016/j.idcr.2018.e00479
65. Akbik F, Matiello M, Piquet A, Cho T, Cohen A, Venna N. Bibrachial plegia due to Lyme radiculopoliomyelitis-myelitis. J Neurol Sci. (2017) 378:1–2. doi: 10.1016/j.jns.2017.04.028
66. Mantienne C, Albucher JF, Catalaa I, Sevely A, Cognard C, Manelfe C. MRI in Lyme disease of the spinal cord. Neuroradiology. (2001) 43:485–8. doi: 10.1007/s002340100583
67. Raucher HS, Kaufman DM, Goldfarb J, Jacobson RI, Roseman B, Wolff RR. Pseudotumor cerebri and Lyme disease: a new association. J Pediatr. (1985) 107:931–3. doi: 10.1016/S0022-3476(85)80193-1
68. Ezequiel M, Teixeira AT, Brito MJ, Luis C. Pseudotumor cerebri as the presentation of Lyme disease in a non-endemic area. BMJ Case Rep. (2018) 2018:bcr2017222976. doi: 10.1136/bcr-2017-222976
69. Norreslet Gimsing L, Lunde Larsen LS. A rare case of pseudotumor cerebri in adult Lyme disease. Clin Case Rep. (2020) 8:116–9. doi: 10.1002/ccr3.2582
70. Oschmann P, Dorndorf W, Hornig C, Schafer C, Wellensiek HJ, Pflughaupt KW. Stages and syndromes of neuroborreliosis. J Neurol. (1998) 245:262–72. doi: 10.1007/s004150050216
71. Massengo SA, Bonnet F, Braun C, Vital A, Beylot J, Bastard J. Severe neuroborreliosis: the benefit of prolonged high-dose combination of antimicrobial agents with steroids–an illustrative case. Diagn Microbiol Infect Dis. (2005) 51:127–30. doi: 10.1016/j.diagmicrobio.2004.04.022
72. Oczko-Grzesik B, Kepa L, Puszcz-Matlinska M, Pudło R, Zurek A, Badura-Głabik T. Estimation of cognitive and affective disorders occurrence in patients with Lyme borreliosis. Ann Agric Environ Med. (2017) 24:33–8. doi: 10.5604/12321966.1229002
73. Keilp JG, Corbera K, Gorlyn M, Oquendo MA, Mann JJ, Fallon BA. Neurocognition in post-treatment Lyme disease and major depressive disorder. Arch Clin Neuropsychol. (2019) 34:466–80. doi: 10.1093/arclin/acy083
74. Hemmer B, Riemann D, Glocker FX, Lucking CH, Deuschl G. Restless legs syndrome after a borrelia-induced myelitis. Mov Disord. (1995) 10:521–2. doi: 10.1002/mds.870100422
75. Back T, Grunig S, Winter Y, Bodechtel U, Guthke K, Khati D, et al. Neuroborreliosis-associated cerebral vasculitis: long-term outcome and health-related quality of life. J Neurol. (2013) 260:1569–75. doi: 10.1007/s00415-013-6831-4
76. Berghoff W. Chronic Lyme disease and co-infections: differential diagnosis. Open Neurol J. (2012) 6:158–78. doi: 10.2174/1874205X01206010158
77. Fiecek B, Chmielewski T, Tylewska-Wierzbanowska S. Borrelia miyamotoi - new etiologic agent of neuroborreliosis? Przegl Epidemiol. (2017) 71:531–8.
78. Johnson TL, Graham CB, Maes SE, Hojgaard A, Fleshman A, Boegler KA, et al. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick Borne Dis. (2018) 9:1499–507. doi: 10.1016/j.ttbdis.2018.07.009
79. Ornstein K, Berglund J, Bergstrom S, Norrby R, Barbour AG. Three major Lyme Borrelia genospecies (Borrelia burgdorferi sensu stricto, B. afzelii and B. garinii) identified by PCR in cerebrospinal fluid from patients with neuroborreliosis in Sweden. Scand J Infect Dis. (2002) 34:341–6. doi: 10.1080/00365540110080313
80. Schmidt C, Plate A, Angele B, Pfister HW, Wick M, Koedel U, et al. A prospective study on the role of CXCL13 in Lyme neuroborreliosis. Neurology. (2011) 76:1051–8. doi: 10.1212/WNL.0b013e318211c39a
81. Mygland A, Ljostad U, Fingerle V, Rupprecht T, Schmutzhard E, Steiner I, et al. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol. (2010) 17:8–16, e1–4. doi: 10.1111/j.1468-1331.2009.02862.x
82. Ogrinc K, Maraspin V. Nervous system involvement in Lyme borreliosis. Open Dermatol J. (2016) 10:44–54. doi: 10.2174/1874372201610010044
83. Novak P, Felsenstein D, Mao C, Octavien NR, Zubcevik N. Association of small fiber neuropathy and post treatment Lyme disease syndrome. PLoS One. (2019) 14:e0212222. doi: 10.1371/journal.pone.0212222
85. Lotric-Furlan S, Cimperman J, Maraspin V, Ruzic-Sabljic E, Logar M, Jurca T, et al. Lyme borreliosis and peripheral facial palsy. Wien Klin Wochenschr. (1999) 111:970–5.
86. Millner M, Schimek MG, Spork D, Schnizer M, Stanek G. Lyme borreliosis in children. A controlled clinical study based on ELISA values. Eur J Pediatr. (1989) 148:527–30. doi: 10.1007/BF00441549
87. Rupprecht TA, Manz KM, Fingerle V, Lechner C, Klein M, Pfirrmann M, et al. Diagnostic value of cerebrospinal fluid CXCL13 for acute Lyme neuroborreliosis. A systematic review and meta-analysis. Clin Microbiol Infect. (2018) 24:1234–40. doi: 10.1016/j.cmi.2018.04.007
88. Markowicz M, Schotta AM, Kundi M, Bogovic P, Ogrinc K, Strle F, et al. CXCL13 concentrations in cerebrospinal fluid of patients with Lyme neuroborreliosis and other neurological disorders determined by Luminex and ELISA. Ticks Tick Borne Dis. (2018) 9:1137–42. doi: 10.1016/j.ttbdis.2018.04.008
89. Ryffel K, Peter O, Rutti B, Suard A, Dayer E. Scored antibody reactivity determined by immunoblotting shows an association between clinical manifestations and presence of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. Valaisiana in humans. J Clin Microbiol. (1999) 37:4086–92. doi: 10.1128/JCM.37.12.4086-4092.1999
90. Besant G, Wan D, Yeung C, Blakely C, Branscombe P, Suarez-Fuster L, et al. Suspicious index in Lyme carditis: systematic review and proposed new risk score. Clin Cardiol. (2018) 41:1611–6. doi: 10.1002/clc.23102
91. Scheffold N, Herkommer B, Kandolf R, May AE. Lyme carditis–diagnosis, treatment and prognosis. Dtsch Arztebl Int. (2015) 112:202–8. doi: 10.3238/arztebl.2015.0202
92. Kashou AH, Braiteh N, Kashou HE. Reversible atrioventricular block and the importance of close follow-up: two cases of Lyme carditis. J Cardiol Cases. (2018) 17:171–4. doi: 10.1016/j.jccase.2018.01.001
93. Kostic T, Momcilovic S, Perisic ZD, Apostolovic SR, Cvetkovic J, Jovanovic A, et al. Manifestations of Lyme carditis. Int J Cardiol. (2017) 232:24–32. doi: 10.1016/j.ijcard.2016.12.169
94. Ocon AJ, Kwiatkowski AV, Peredo-Wende R, Blinkhorn R. Adult-onset still's disease with haemorrhagic pericarditis and tamponade preceded by acute Lyme disease. BMJ Case Rep. (2018) 2018:bcr2018225517. doi: 10.1136/bcr-2018-225517
95. Noyes AM, Kluger J. A tale of two syndromes: Lyme disease preceding postural orthostatic tachycardia syndrome. Ann Noninvasive Electrocardiol. (2015) 20:82–6. doi: 10.1111/anec.12158
96. Lardieri G, Salvi A, Camerini F, Cinco M, Trevisan G. Isolation of Borrelia burgdorferi from myocardium. Lancet. (1993) 342:490. doi: 10.1016/0140-6736(93)91612-P
97. Mikkila HO, Seppala IJ, Viljanen MK, Peltomaa MP, Karma A. The expanding clinical spectrum of ocular lyme borreliosis. Ophthalmology. (2000) 107:581–7. doi: 10.1016/S0161-6420(99)00128-1
98. Kilic Muftuoglu I, Aydin Akova Y, Gur Gungor S. A case of Lyme disease accompanied by uveitis and white dot syndrome. Turk J Ophthalmol. (2016) 46:241–3. doi: 10.4274/tjo.25991
99. John M, Raman M, Ryan K. A tiny tick can cause a big health problem. Indian J Ophthalmol. (2017) 65:1228–32. doi: 10.4103/ijo.IJO_411_17
100. Mora P, Carta A. Ocular manifestations of Lyme borreliosis in Europe. Int J Med Sci. (2009) 6:124–5. doi: 10.7150/ijms.6.124
101. Sauer A, Hansmann Y, Jaulhac B, Bourcier T, Speeg-Schatz C. [Ocular Lyme disease occurring during childhood: five case reports]. J Fr Ophtalmol. (2012) 35:17–22. doi: 10.1016/j.jfo.2011.03.015
102. Budhram A, Le C, Jenkins ME. Lyme disease presenting with raeder syndrome. Headache. (2018) 58:317–8. doi: 10.1111/head.13220
103. Raileanu C, Moutailler S, Pavel I, Porea D, Mihalca AD, Savuta G, et al. Borrelia diversity and co-infection with other tick borne pathogens in ticks. Front Cell Infect Microbiol. (2017) 7:36. doi: 10.3389/fcimb.2017.00036
104. Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R. Polymicrobial nature of tick-borne diseases. mBio. (2019) 10:e02055-19. doi: 10.1128/mBio.02055-19
105. Ben I, Lozynskyi I. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus and Dermacentor reticulatus and Coinfection with Borrelia burgdorferi and tick-borne encephalitis virus in Western Ukraine. Vector Borne Zoonotic Dis. (2019) 19:793–801. doi: 10.1089/vbz.2019.2450
106. Parveen N, Bhanot P. Babesia microti-Borrelia burgdorferi coinfection. Pathogens. (2019) 8:117. doi: 10.3390/pathogens8030117
107. Kulakowska A, Zajkowska JM, Ciccarelli NJ, Mroczko B, Drozdowski W, Bucki R. Depletion of plasma gelsolin in patients with tick-borne encephalitis and Lyme neuroborreliosis. Neurodegener Dis. (2011) 8:375–80. doi: 10.1159/000324373
108. Khan AM, Shahzad SR, Ashraf MF, Naseer U. Powassan virus encephalitis, severe babesiosis and lyme carditis in a single patient. BMJ Case Rep. (2019) 12:e231645. doi: 10.1136/bcr-2019-231645
109. Baveja S, Oberoi B, Vashisht D, Das P. Lyme disease - a report of atypical cutaneous sequelae. Indian Dermatol Online J. (2019) 10:336–7. doi: 10.4103/idoj.IDOJ_294_18
110. Guerau-de-Arellano M, Huber BT. Development of autoimmunity in Lyme arthritis. Curr Opin Rheumatol. (2002) 14:388–93. doi: 10.1097/00002281-200207000-00009
111. Crowley JT, Drouin EE, Pianta A, Strle K, Wang Q, Costello CE, et al. A highly expressed human protein, apolipoprotein B-100, serves as an autoantigen in a subgroup of patients with Lyme disease. J Infect Dis. (2015) 212:1841–50. doi: 10.1093/infdis/jiv310
112. Maccallini P, Bonin S, Trevisan G. Autoimmunity against a glycolytic enzyme as a possible cause for persistent symptoms in Lyme disease. Med Hypotheses. (2018) 110:1–8. doi: 10.1016/j.mehy.2017.10.024
113. Rebman AW, Aucott JN. Post-treatment Lyme disease as a model for persistent symptoms in Lyme disease. Front Med. (2020) 7:57. doi: 10.3389/fmed.2020.00057
114. Aucott JN, Rebman AW, Crowder LA, Kortte KB. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: is there something here? Qual Life Res. (2013) 22:75–84. doi: 10.1007/s11136-012-0126-6
115. Rebman AW, Bechtold KT, Yang T, Mihm EA, Soloski MJ, Novak CB, et al. The clinical, symptom, and quality-of-life characterization of a well-defined group of patients with posttreatment Lyme disease syndrome. Front Med. (2017) 4:224. doi: 10.3389/fmed.2017.00224
116. Eikeland R, Ljostad U, Helgeland G, Sand G, Flemmen HO, Bo MH, et al. Patient-reported outcome after treatment for definite Lyme neuroborreliosis. Brain Behav. (2020) 10:e01595. doi: 10.1002/brb3.1595
117. Eikeland R, Mygland A, Herlofson K, Ljostad U. European neuroborreliosis: quality of life 30 months after treatment. Acta Neurol Scand. (2011) 124:349–54. doi: 10.1111/j.1600-0404.2010.01482.x
118. Lacout A, El Hajjam M, Marcy PY, Perronne C. The persistent Lyme disease: “True Chronic Lyme Disease” rather than “Post-treatment Lyme Disease Syndrome.” J Glob Infect Dis. (2018) 10:170–1. doi: 10.4103/jgid.jgid_152_17
119. Caskey JR, Embers ME. Persister Development by Borrelia burgdorferi populations in vitro. Antimicrob Agents Chemother. (2015) 59:6288–95. doi: 10.1128/AAC.00883-15
120. Feng J, Shi W, Zhang S, Zhang Y. Persister mechanisms in Borrelia burgdorferi: implications for improved intervention. Emerg Microbes Infect. (2015) 4:e51. doi: 10.1038/emi.2015.51
121. Lantos PM. Chronic Lyme disease. Infect Dis Clin North Am. (2015) 29:325–40. doi: 10.1016/j.idc.2015.02.006
122. Bonin S, Zanotta N, Sartori A, Bratina A, Manganotti P, Trevisan G, et al. Cerebrospinal fluid cytokine expression profile in multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. Immunol Invest. (2018) 47:135–45. doi: 10.1080/08820139.2017.1405978
123. Hein TM, Sander P, Giryes A, Reinhardt JO, Hoegel J, Schneider EM. Cytokine expression patterns and single nucleotide polymorphisms (SNPs) in patients with chronic borreliosis. Antibiotics (Basel). (2019) 8:107. doi: 10.3390/antibiotics8030107
124. Crossland NA, Alvarez X, Embers ME. Late disseminated Lyme disease: associated pathology and spirochete persistence posttreatment in rhesus macaques. Am J Pathol. (2018) 188:672–82. doi: 10.1016/j.ajpath.2017.11.005
125. Hodzic E, Imai DM, Escobar E. Generality of post-antimicrobial treatment persistence of Borrelia burgdorferi strains N40 and B31 in genetically susceptible and resistant mouse strains. Infect Immun. (2019) 87:e00442-19. doi: 10.1128/IAI.00442-19
126. Haupl T, Hahn G, Rittig M, Krause A, Schoerner C, Schonherr U, et al. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. (1993) 36:1621–6. doi: 10.1002/art.1780361118
127. Lawrence C, Lipton RB, Lowy FD, Coyle PK. Seronegative chronic relapsing neuroborreliosis. Eur Neurol. (1995) 35:113–7. doi: 10.1159/000117104
128. Lee SH, Vigliotti JS, Vigliotti VS, Jones W, Shearer DM. Detection of borreliae in archived sera from patients with clinically suspect Lyme disease. Int J Mol Sci. (2014) 15:4284–98. doi: 10.3390/ijms15034284
129. Masters E, Lynxwiler P, Rawlings J. Spirochetemia after continuous high-dose oral amoxicillin therapy. Infect Dis Clin Pract. (1994) 3:207–8. doi: 10.1097/00019048-199405000-00016
130. Middelveen MJ, Sapi E, Burke J, Filush KR, Franco A, Fesler MC, et al. Persistent Borrelia infection in patients with ongoing symptoms of Lyme disease. Healthcare (Basel). (2018) 6:33. doi: 10.3390/healthcare6020033
131. Murgia R, Cinco M. Induction of cystic forms by different stress conditions in Borrelia burgdorferi. APMIS. (2004) 112:57–62. doi: 10.1111/j.1600-0463.2004.apm1120110.x
132. Mursic VP, Wanner G, Reinhardt S, Wilske B, Busch U, Marget W. Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants. Infection. (1996) 24:218–26. doi: 10.1007/BF01781096
133. Oksi J, Nikoskelainen J, Viljanen MK. Comparison of oral cefixime and intravenous ceftriaxone followed by oral amoxicillin in disseminated Lyme borreliosis. Eur J Clin Microbiol Infect Dis. (1998) 17:715–9. doi: 10.1007/s100960050166
134. Pfister HW, Preac-Mursic V, Wilske B, Schielke E, Sorgel F, Einhaupl KM. Randomized comparison of ceftriaxone and cefotaxime in Lyme neuroborreliosis. J Infect Dis. (1991) 163:311–8. doi: 10.1093/infdis/163.2.311
135. Phillips SE, Mattman LH, Hulinska D, Moayad H. A proposal for the reliable culture of Borrelia burgdorferi from patients with chronic Lyme disease, even from those previously aggressively treated. Infection. (1998) 26:364–7. doi: 10.1007/BF02770837
136. Preac-Mursic V, Pfister HW, Spiegel H, Burk R, Wilske B, Reinhardt S, et al. First isolation of Borrelia burgdorferi from an iris biopsy. J Clin Neuroophthalmol. (1993) 13:155–61; discussion 62.
137. Preac-Mursic V, Weber K, Pfister HW, Wilske B, Gross B, Baumann A, et al. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection. (1989) 17:355–9. doi: 10.1007/BF01645543
138. Schmidli J, Hunziker T, Moesli P, Schaad UB. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J Infect Dis. (1988) 158:905–6. doi: 10.1093/infdis/158.4.905
139. Franck M, Ghozzi R, Pajaud J, Lawson-Hogban NE, Mas M, Lacout A, et al. Borrelia miyamotoi: 43 cases diagnosed in France by real-time PCR in patients with persistent polymorphic signs and symptoms. Front Med. (2020) 7:55. doi: 10.3389/fmed.2020.00055
140. Delong AK, Blossom B, Maloney EL, Phillips SE. Antibiotic retreatment of Lyme disease in patients with persistent symptoms: a biostatistical review of randomized, placebo-controlled, clinical trials. Contemp Clin Trials. (2012) 33:1132–42. doi: 10.1016/j.cct.2012.08.009
141. Sapi E, Kasliwala RS, Ismail H, Torres JP, Oldakowski M, Markland S, et al. The long-term persistence of Borrelia burgdorferi antigens and DNA in the tissues of a patient with Lyme disease. Antibiotics (Basel). (2019) 8:183. doi: 10.3390/antibiotics8040183
142. Feng J, Li T, Yee R, Yuan Y, Bai C, Cai M, et al. Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: implications for understanding persistence, Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discov Med. (2019) 27:125–38.
143. Di Domenico EG, Cavallo I, Guembe M, Prignano G, Gallo MT, Bordignon V, et al. The clinical Biofilm Ring Test: a promising tool for the clinical assessment of biofilm-producing Candida species. FEMS Yeast Res. (2018) 18:foy025. doi: 10.1093/femsyr/foy025
144. Lacout A, Dacher V, El Hajjam M, Marcy PY, Perronne C. Biofilms busters to improve the detection of Borrelia using PCR. Med Hypotheses. (2018) 112:4–6. doi: 10.1016/j.mehy.2018.01.005
145. Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome. J Chronic Fatigue Syndrome. (2003) 11:7–115. doi: 10.1300/J092v11n01_02
146. Lidbury BA, Kita B, Lewis DP, Hayward S, Ludlow H, Hedger MP, et al. Activin B is a novel biomarker for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) diagnosis: a cross sectional study. J Transl Med. (2017) 15:60. doi: 10.1186/s12967-017-1161-4
147. Lidbury BA, Kita B, Richardson AM, Lewis DP, Privitera E, Hayward S, et al. Rethinking ME/CFS diagnostic reference intervals via machine learning, and the utility of activin B for defining symptom severity. Diagnostics (Basel). (2019) 9:79. doi: 10.3390/diagnostics9030079
148. Gunther OP, Gardy JL, Stafford P, Fluge O, Mella O, Tang P, et al. Immunosignature analysis of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Mol Neurobiol. (2019) 56:4249–57. doi: 10.1007/s12035-018-1354-8
149. Ajamian M, Cooperstock M, Wormser GP, Vernon SD, Alaedini A. Anti-neural antibody response in patients with post-treatment Lyme disease symptoms versus those with myalgic encephalomyelitis/chronic fatigue syndrome. Brain Behav Immun. (2015) 48:354–5. doi: 10.1016/j.bbi.2015.04.006
150. MacDonald AB. Gestational Lyme borreliosis. Implications for the fetus. Rheum Dis Clin North Am. (1989) 15:657–77.
151. Waddell LA, Greig J, Lindsay LR, Hinckley AF, Ogden NH. A systematic review on the impact of gestational Lyme disease in humans on the fetus and newborn. PLoS One. (2018) 13:e0207067. doi: 10.1371/journal.pone.0207067
152. Trevisan G, Stinco G, Cinco M. Neonatal skin lesions due to a spirochetal infection: a case of congenital Lyme borreliosis? Int J Dermatol. (1997) 36:677–80.
153. Maraspin V, Ruzic-Sabljic E, Pleterski-Rigler D, Strle F. Pregnant women with erythema migrans and isolation of borreliae from blood: course and outcome after treatment with ceftriaxone. Diagn Microbiol Infect Dis. (2011) 71:446–8. doi: 10.1016/j.diagmicrobio.2011.07.017
154. Menni S, Pistritto G, Piccinno R, Trevisan G. Acrodermatitis chronica atrophicans in an Italian child. Acta Derm Venereol. (1996) 76:243.
155. Nigrovic LE, Bennett JE, Balamuth F, Levas MN, Chenard RL, Maulden AB, et al. Accuracy of clinician suspicion of Lyme disease in the emergency department. Pediatrics. (2017) 140:e20171975. doi: 10.1542/peds.2017-1975
156. Eldin C, Raffetin A, Bouiller K, Hansmann Y, Roblot F, Raoult D, et al. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med Mal Infect. (2019) 49:121–32. doi: 10.1016/j.medmal.2018.11.011
157. Peltomaa M, McHugh G, Steere AC. Persistence of the antibody response to the VlsE sixth invariant region (IR6) peptide of Borrelia burgdorferi after successful antibiotic treatment of Lyme disease. J Infect Dis. (2003) 187:1178–86. doi: 10.1086/374376
158. Levy SA, O'Connor TP, Hanscom JL, Shields P, Lorentzen L, Dimarco AA. Quantitative measurement of C6 antibody following antibiotic treatment of Borrelia burgdorferi antibody-positive nonclinical dogs. Clin Vaccine Immunol. (2008) 15:115–9. doi: 10.1128/CVI.00340-07
159. Philipp MT, Bowers LC, Fawcett PT, Jacobs MB, Liang FT, Marques AR, et al. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J Infect Dis. (2001) 184:870–8. doi: 10.1086/323392
160. Robertson J, Guy E, Andrews N, Wilske B, Anda P, Granstrom M, et al. A European multicenter study of immunoblotting in serodiagnosis of lyme borreliosis. J Clin Microbiol. (2000) 38:2097–102. doi: 10.1128/.38.6.2097-2102.2000
161. Control ECfDPa. A Systematic Literature Review on the Diagnostic Accuracy of Serological Tests for Lyme Borreliosis. Stockholm: ECDC (2016). 95 p.
162. Leeflang MM, Ang CW, Berkhout J, Bijlmer HA, Van Bortel W, Brandenburg AH, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. (2016) 16:140. doi: 10.1186/s12879-016-1468-4
163. Cook MJ, Puri BK. Commercial test kits for detection of Lyme borreliosis: a meta-analysis of test accuracy. Int J Gen Med. (2016) 9:427–40. doi: 10.2147/IJGM.S122313
164. Cerar T, Ruzic-Sabljic E, Glinsek U, Zore A, Strle F. Comparison of PCR methods and culture for the detection of Borrelia spp. in patients with erythema migrans. Clin Microbiol Infect. (2008) 14:653–8. doi: 10.1111/j.1469-0691.2008.02013.x
165. Veinovic G, Cerar T, Strle F, Ruzic-Sabljic E. Influence of MKP medium stored for prolonged periods on growth and morphology of Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. APMIS. (2014) 122:230–5. doi: 10.1111/apm.12129
166. Wilske B, Fingerle V, Schulte-Spechtel U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol Med Microbiol. (2007) 49:13–21. doi: 10.1111/j.1574-695X.2006.00139.x
167. RuŽić-Sabljić E, Cerar T. Progress in the molecular diagnosis of Lyme disease. Expert Rev Mol Diagn. (2017) 17:19–30. doi: 10.1080/14737159.2016.1246959
168. MacDonald AB. Borrelia burgdorferi tissue morphologies and imaging methodologies. Eur J Clin Microbiol Infect Dis. (2013) 32:1077–82. doi: 10.1007/s10096-013-1853-5
169. Wanyura H, Wagner T, Samolczyk-Wanyura D. Borrelia burgdorferi–a potentially aetiological factor in TMJ disorders? Preliminary report. J Craniomaxillofac Surg. (2008) 36:28–33. doi: 10.1016/j.jcms.2007.05.007
170. Eisendle K, Grabner T, Zelger B. Focus floating microscopy: “gold standard” for cutaneous borreliosis? Am J Clin Pathol. (2007) 127:213–22. doi: 10.1309/3369XXFPEQUNEP5C
171. Eisendle K, Grabner T, Kutzner H, Zelger B. Possible role of Borrelia burgdorferi sensu lato infection in lichen sclerosus. Arch Dermatol. (2008) 144:591–8. doi: 10.1001/archderm.144.5.591
172. Eisendle K, Baltaci M, Kutzner H, Zelger B. Detection of spirochaetal microorganisms by focus floating microscopy in necrobiosis lipoidica in patients from central Europe. Histopathology. (2008) 52:877–84. doi: 10.1111/j.1365-2559.2008.03051.x
173. Derler AM, Eisendle K, Baltaci M, Obermoser G, Zelger B. High prevalence of “Borrelia-like” organisms in skin biopsies of sarcoidosis patients from Western Austria. J Cutan Pathol. (2009) 36:1262–8. doi: 10.1111/j.1600-0560.2009.01271.x
174. Kuchynka P, Palecek T, Grus T, Schramlova J, Krsek D, Vitkova I, et al. A high frequency of viral agents yet absence of Borrelia burgdorferi is seen within the myocardium of subjects with normal left ventricular systolic function: an electron microscopy study. Folia Microbiol. (2016) 61:129–35. doi: 10.1007/s12223-015-0417-8
175. Dietrich T, Geissdorfer W, Schlotzer-Schrehardt U, Holbach L, Schoerner C, Seitz B. Borrelia-associated crystalline keratopathy with intracorneal detection of Borrelia garinii by electron microscopy and polymerase chain reaction. Cornea. (2008) 27:498–500. doi: 10.1097/ICO.0b013e318162a8f5
176. Lacout A, Mone Y, Franck M, Marcy PY, Mas M, Veas F, et al. Blood cell disruption to significantly improve the Borrelia PCR detection sensitivity in borreliosis in humans. Med Hypotheses. (2018) 116:1–3. doi: 10.1016/j.mehy.2018.04.012
177. Bonin S. Diagnostic tools for borrelia assessment in humans. Open Dermatol J. (2016) 10(Suppl 1: M7):62–9. doi: 10.2174/1874372201610010062
178. van Dam AP. Molecular diagnosis of Borrelia bacteria for the diagnosis of Lyme disease. Expert Opin Med Diagn. (2011) 5:135–49. doi: 10.1517/17530059.2011.555396
179. Nolte O. Nucleic acid amplification based diagnostic of Lyme (Neuro-)borreliosis - Lost in the jungle of methods, targets, and assays? Open Neurol J. (2012) 6:129–39. doi: 10.2174/1874205X01206010129
180. de Leeuw BH, Maraha B, Hollemans L, Sprong H, Brandenburg AH, Westenend PJ, et al. Evaluation of Borrelia real time PCR DNA targeting OspA, FlaB and 5S-23S IGS and Borrelia 16S rRNA RT-qPCR. J Microbiol Methods. (2014) 107:41–6. doi: 10.1016/j.mimet.2014.09.001
181. Jenkins A, Hvidsten D, Matussek A, Lindgren PE, Stuen S, Kristiansen BE. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Norway: evaluation of a PCR test targeting the chromosomal flaB gene. Exp Appl Acarol. (2012) 58:431–9. doi: 10.1007/s10493-012-9585-2
182. Mantovani E, Marangoni RG, Gauditano G, Bonoldi VL, Yoshinari NH. Amplification of the flgE gene provides evidence for the existence of a Brazilian borreliosis. Rev Inst Med Trop Sao Paulo. (2012) 54:153–7. doi: 10.1590/S0036-46652012000300007
183. O'Rourke M, Traweger A, Lusa L, Stupica D, Maraspin V, Barrett PN, et al. Quantitative detection of Borrelia burgdorferi sensu lato in erythema migrans skin lesions using internally controlled duplex real time PCR. PLoS One. (2013) 8:e63968. doi: 10.1371/journal.pone.0063968
184. Pintore MD, Ceballos L, Iulini B, Tomassone L, Pautasso A, Corbellini D, et al. Detection of invasive Borrelia burgdorferi strains in North-Eastern Piedmont, Italy. Zoonoses Public Health. (2015) 62:365–74. doi: 10.1111/zph.12156
185. Santino I, Berlutti F, Pantanella F, Sessa R, del Piano M. Detection of Borrelia burgdorferi sensu lato DNA by PCR in serum of patients with clinical symptoms of Lyme borreliosis. FEMS Microbiol Lett. (2008) 283:30–5. doi: 10.1111/j.1574-6968.2008.01134.x
186. Floris R, Menardi G, Bressan R, Trevisan G, Ortenzio S, Rorai E, et al. Evaluation of a genotyping method based on the ospA gene to detect Borrelia burgdorferi sensu lato in multiple samples of lyme borreliosis patients. New Microbiol. (2007) 30:399–410.
187. Gooskens J, Templeton KE, Claas EC, van Dam AP. Evaluation of an internally controlled real-time PCR targeting the ospA gene for detection of Borrelia burgdorferi sensu lato DNA in cerebrospinal fluid. Clin Microbiol Infect. (2006) 12:894–900. doi: 10.1111/j.1469-0691.2006.01509.x
188. Livey I, O'Rourke M, Traweger A, Savidis-Dacho H, Crowe BA, Barrett PN, et al. A new approach to a Lyme disease vaccine. Clin Infect Dis. (2011) 52(Suppl 3):s266–70. doi: 10.1093/cid/ciq118
189. von Baehr V, Doebis C, Volk HD, von Baehr R. The lymphocyte transformation test for borrelia detects active lyme borreliosis and verifies effective antibiotic treatment. Open Neurol J. (2012) 6:104–12. doi: 10.2174/1874205X01206010104
190. Norris SJ, Howell JK, Odeh EA, Lin T, Gao L, Edmondson DG. High-throughput plasmid content analysis of Borrelia burgdorferi B31 by using Luminex multiplex technology. Appl Environ Microbiol. (2011) 77:1483–92. doi: 10.1128/AEM.01877-10
191. Jahfari S, Sarksyan DS, Kolyasnikova NM, Hovius JW, Sprong H, Platonov AE. Evaluation of a serological test for the diagnosis of Borrelia miyamotoi disease in Europe. J Microbiol Methods. (2017) 136:11–6. doi: 10.1016/j.mimet.2017.02.013
192. Wang HY, Wu SQ, Jiang L, Xiao RH, Li T, Mei L, et al. Establishment and optimization of a liquid bead array for the simultaneous detection of ten insect-borne pathogens. Parasit Vectors. (2018) 11:442. doi: 10.1186/s13071-018-2996-0
193. Theel ES. The Past, Present, and (possible) future of serologic testing for Lyme disease. J Clin Microbiol. (2016) 54:1191–6. doi: 10.1128/JCM.03394-15
194. Halpern MD, Jain S, Jewett MW. Enhanced detection of host response antibodies to Borrelia burgdorferi using immuno-PCR. Clin Vaccine Immunol. (2013) 20:350–7. doi: 10.1128/CVI.00630-12
195. Molins CR, Ashton LV, Wormser GP, Hess AM, Delorey MJ, Mahapatra S, et al. Development of a metabolic biosignature for detection of early Lyme disease. Clin Infect Dis. (2015) 60:1767–75. doi: 10.1093/cid/civ185
Keywords: Lyme disease, Borrelia, clinical symptoms, diagnosis, clinical heterogeneity
Citation: Trevisan G, Bonin S and Ruscio M (2020) A Practical Approach to the Diagnosis of Lyme Borreliosis: From Clinical Heterogeneity to Laboratory Methods. Front. Med. 7:265. doi: 10.3389/fmed.2020.00265
Received: 23 January 2020; Accepted: 14 May 2020;
Published: 23 July 2020.
Edited by:
Christian Perronne, Assistance Publique Hopitaux De Paris, FranceReviewed by:
Natasha Rudenko, Institute of Parasitology, CzechiaCopyright © 2020 Trevisan, Bonin and Ruscio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giusto Trevisan, dHJldmlzYW5AdW5pdHMuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.