
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 24 April 2020
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00127
This article is part of the Research Topic Antimicrobial Resistance As a Global Public Health Problem: How Can We Address It? View all 50 articles
 Xiaolan Ai1†
Xiaolan Ai1† Fei Gao1†
Fei Gao1† Shuwen Yao2
Shuwen Yao2 Bingshao Liang1
Bingshao Liang1 Jialiang Mai1
Jialiang Mai1 Zhile Xiong1
Zhile Xiong1 Xiantang Chen3
Xiantang Chen3 Zhuwei Liang1
Zhuwei Liang1 Hongling Yang1
Hongling Yang1 Zhiying Ou1
Zhiying Ou1 Sitang Gong1
Sitang Gong1 Yan Long1*
Yan Long1* Zhenwen Zhou1*
Zhenwen Zhou1*Background: Staphylococcus aureus (S. aureus) is a major pathogen of human infections. Its fecal carriage serves as a risk factor for nosocomial transmission and disease development. However, the rate of S. aureus fecal carriage among Chinese children has not yet been reported. Therefore, we sought to investigate the prevalence, characterization, and drug resistance of S. aureus isolated from pediatric patients' feces in Southern China.
Methods: Fecal samples (2059) from pediatric patients in three centers in Guangzhou were cultured. From which, 412 S. aureus isolates were identified via selective mediums and automated VITEK Mass Spectrometer analysis. Antibiotic susceptibility was determined and DNA sequencing of seven housekeeping genes were used for multilocus sequence typing analysis.
Results: The fecal carriage rates were 20.0% for S. aureus and 4.5% for methicillin-resistant S. aureus (MRSA). Moreover, S. aureus fecal carriage was positively correlated with outpatient status and gastroenteritis diagnosis. Moreover, age-related patterns were observed with respect to prevalence of S. aureus. Besides, a total of 76 sequence types (STs) were identified, including 25 newly assigned STs and 28 clonal complexes (CCs). ST188, ST6, and ST15 were the most prevalent methicillin-sensitive S. aureus (MSSA) clones, while ST59 and ST45 were the major MRSA clones. S. aureus isolates also exhibited high rates of penicillin (84.2%), erythromycin (38.8%), and clindamycin (35.9%) resistance. Specifically, all ST30 and ST338 isolates were resistant to erythromycin and clindamycin, 61% of ST7 were resistant to tetracycline, and 84% of ST45 exhibited resistance and intermediate resistance to rifampicin. Also, CC59 (ST338 and ST59) and CC45 exhibited different antibiotic resistance patterns.
Conclusion: These results demonstrate the colonization dynamics and molecular epidemiology of S. aureus in child feces in Southern China. Further, they suggest an urgency for strengthening the surveillance programs in China and provide important information for the prevention and treatment of S. aureus infection.
Staphylococcus aureus (S. aureus) is a major pathogen of human infection that causes diseases ranging from minor skin infections to severe bacteremia, necrotizing pneumonia, and life-threatening sepsis (1–3), and thus is a major global threat to human health. S. aureus can colonize multifarious body regions, including the anterior nares (4), skin (5), intestinal tract (6), oropharynx (7), and so on. Colonization is a crucial risk factor for the subsequent development of infections (8). Specifically, the importance of S. aureus fecal colonization was described as early as 1960 (9), in a study that demonstrated rectal carriage of S. aureus earlier than from the nose or throat. Subsequently, several studies have confirmed the clinical importance of S. aureus fecal carriage (10, 11). Additionally, S. aureus fecal carriage may contribute to environmental contamination (12), which can lead to nosocomial transmission and infection. Previous studies have reported fecal carriage of S. aureus in adults from Nigeria (13) and India (14), and a recent study investigated intestinal colonization by S. aureus and Clostridium difficile in healthy adult fecal samples from China (15), and a few studies have reported on S. aureus isolated from pediatric patients' feces samples in China.
Multilocus sequence typing (MLST) has become one of the most popular methods for evaluating S. aureus strains; however, only limited MLST studies of S. aureus from stool samples are available. Methicillin-sensitive (MSSA) strains ST30, ST398, and ST133 were detected from 100 healthy human fecal samples in Spain (16), while ST15, ST188, and ST59 were identified in six S. aureus isolates from stool specimens of diarrheal infants (17). Unfortunately, the diversity of molecular S. aureus types in these studies was limited due to the relatively small population size, which may have led to misinterpretation or inaccurate conclusions to be drawn regarding S. aureus colonization in fecal samples.
In addition to molecular characterization, antibiotic resistance, notably regarding the emergence and evolution of multi-drug-resistant (MDR) S. aureus, has become a major focal point in research across the world. Methicillin-resistant S. aureus (MRSA), which begins with resistance to methicillin or most β-lactam antibiotics and gradually develops co-resistance to vancomycin (18, 19), limits the use of alternative anti-infective drugs and threatens patient's health. Hence, drug resistance should be closely monitored to provide the basis for clinical antibacterial infection treatment, including exploring antibiotic resistance of S. aureus isolated from fecal samples.
Thus, the aims of this investigation were to evaluate the prevalence, molecular genotyping and antibiotic resistance of S. aureus isolated from pediatric patients' fecal samples in Southern China.
All patients were recruited voluntarily and provided informed consent from the participants or the guardians. The study was approved by the research ethics committee of the Guangzhou women and children's medical center (registration no. 2016081029).
This study enrolled children from three medical centers in Southern China between August and November 2018, including Guangzhou Women and Children's Medical Center (Tianhe District, central Guangzhou), Guangzhou Children's Hospital (Yuexiu District, western Guangzhou), and Zengcheng Maternity and Children's Health Care Center (Zengcheng District, northern Guangzhou). A total of 2059 non-duplicate pediatric stool samples (1308 outpatients and 751 inpatients) were collected. Approximately 20 mg of stool sample was streaked onto a selective mannitol salt agar medium (Hope Bio-technology, Qingdao, China) within 4 h of sample collection, and incubated in a humidified atmosphere at 37°C with 5% CO2 for 24 h. Suspected S. aureus colonies from each sample were evaluated based on morphology and sub-cultured on Columbia Blood Agar Medium (Detgerm Microbiology Technology, Guangzhou, China) (20). All isolates were further identified for their species assignment by the automated VITEK MS (bioMérieux, Marcy-l'Étoile, France). Identified S. aureus was further confirmed by detecting femB (21). We also collected a range of clinical information from the laboratory information system, including gender, age, types, diagnosis, and fecal occult blood test (FOBT). Accordingly, the patients were classified into six age groups: 0–28 days, newborn; 28 days–3 months, young infant; 3 months–1 year, older infant; 1–3 years, child; 3–6 years, pre-school age; 6–18 years, school age and puberty (22, 23). Among these patients, the oldest was 17 years old, the youngest was 1 day, and the median age was 10 months and 23 days.
Antibiotic susceptibility for 15 antibiotics (penicillin, oxacillin, gentamicin, rifampicin, levofloxacin, ciprofloxacin, trimethoprim/sulfamethoxazole, clindamycin, erythromycin, macrodantin, linezolid, vancomycin, quinoputin/dafutin, tetracycline, and tigecycline) was detected by VITEK 2 AST-GP67 cards (bioMérieux) using the automated VITEK2 compact system (bioMérieux). Antibiotic minimum inhibitory concentration (MIC) was determined according to the published guidelines (24). The quality control strain used in antibiotic susceptibility analysis was S. aureus ATCC 29213. MRSA was defined as an oxacillin-resistant isolate, and multidrug-resistant (MDR) isolates were identified as isolates with resistance to three or more non-β-lactam antibiotics (25).
Total DNA was extracted from 1.0 ml of nutrient broth medium culture grown overnight. After centrifugation, the supernatant was discarded and the S. aureus isolates were resuspended in 200 μl of enzymatic lysis buffer (Sangon Biotech, Shanghai, China). Subsequently, S. aureus solution was incubated at 37°C for 30 min with 3 μl of lysostaphin (Sigma-Aldrich, Shanghai, China), mixed with 200 μl of Buffer BD (Sangon Biotech), and 200 μl of 100% ethanol (Guangzhou Chemical Reagent Factory, Guangzhou, China), and then transferred to an absorbing column (Sangon Biotech). Next, the Ezup Column Bacterial Genomic DNA Extraction Kit (Sangon Biotech) was used in accordance with the manufacturer's instructions.
The femB gene plays an important role in formatting the pentaglycine bridges that stabilize peptidoglycan chains in S. aureus, while the mecA gene is the most important cause of S. aureus resistance to oxacillin (26). Thus, to further confirm the presence S. aureus and MRSA, we detected the expression of femB and mecA by PCR. The primers used for femB and mecA genes were described previously (27), and extracted DNA was amplified using TaqTM (Takara, Tokyo, Japan) following the manufacturer's instructions. Following amplification and extension, the PCR amplicons were separated on 1% agarose gels stained with ethidium bromide and visualized under UV illumination (TEX-20 M, Life Technologies, Carlsbad, USA).
All isolates were analyzed by multilocus sequence typing (MLST) according to a previously published procedure (28). The PCR products were purified and sequenced by a commercial sequencing company (Beijing Genomics Institute, Shenzhen, China). DNA sequencing of seven housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL) was used for MLST analysis. Sequence types (STs) were determined by searching the S. aureus MLST database (https://pubmlst.org/saureus/), which included new emerging MLST alleles and MLST types. Clonal complex (CC) analysis was conducted using the eBURST v.3 programme (https://www.mlst.net/eburst/) according to our previously described protocol (27). Based on STs, a UPGMA dendrogram was constructed with START2.
Statistical analyses were carried out with SPSS software 20 (SPSS Inc., Chicago, USA). The chi-square (χ2) test or Fisher's exact test were applied to dichotomous or categorical variables, which were described as frequencies and proportions. P < 0.05 was considered statistically significant.
A total of 2059 fecal samples were collected from pediatric patients in three hospitals, from which 412 S. aureus and 93 MRSA isolates were identified. The overall colonization prevalence of S. aureus and MRSA were 20.0 and 4.5%, respectively. Accordingly, we classified the 2059 patients into two groups consisting of those that were S. aureus positive (n = 412) and negative (n = 1,647), to analyze the correlation of S. aureus fecal carriage with different clinical features. As shown in Table 1, the fecal carriage of S. aureus was not associated with patient gender (P = 0.149); however, it was positively correlated with outpatient status and gastroenteritis diagnosis (P < 0.01). We also observed a positive relationship between S. aureus and different age groups. The minimum S. aureus carriage rate was in newborn patients (7.3%), while the maximum rate was in infant patients (young infants, 26.6% and older infants 26.3%), after which the carriage rate gradually descended with increasing age of the patients (Table 1). In addition, we observed a positive correlation between FOBT results and S. aureus carriage (P < 0.01, Table 1).
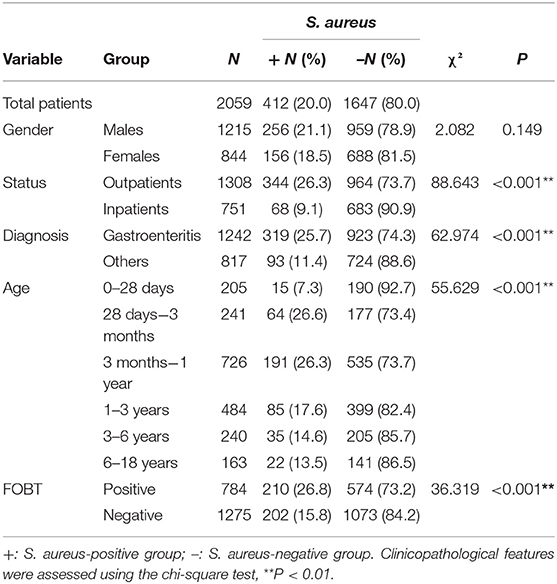
Table 1. Correlation of fecal carriage of S. aureus in pediatric patients with different clinical features.
Subsequently we divided the 412 positive S. aureus patients into two groups, MRSA (n = 93) and MSSA (n = 319), to explore the relationship of MRSA and clinical features. However, we found that MRSA was significantly correlated with inpatient status (P = 0.002), and not gender, age, or FOBT results (Table 2).
The antibiotic susceptibility results for the 412 S. aureus isolates according to MLST are presented in Table 3. All 93 MRSA strains were resistant to cefoxitin screening and carried the mecA gene. The S. aureus strains exhibited highest rate of resistance to penicillin (PEN, 84.2%), followed by erythromycin (ERY, 38.8%), clindamycin (CLI, 35.9%), tetracycline (TE, 14.6%), and sulfamethoxazole-trimethoprim (SXT, 6.1%). The resistance rates of antibiotics were lower for gentamicin (GEN, 2.7%), levofloxacin (LEV, 1.9%), ciprofloxacin (CIP, 1.9%), and rifampicin (RIF, 0.7%); however, all isolates were susceptible to macrodantin, linezolid, vancomycin, dalfopristin/quinupristin (QDA), and tigecycline. Compared to the MSSA group, the MRSA group had significantly higher rates of resistance to PEN (P < 0.01), ERY (P < 0.01), CLI (P < 0.01), and TE (P = 0.03) and intermediate resistance to RIF (P < 0.01). Although 22.8% of all strains exhibited MDR, the MRSA group had a significantly higher rate (74.2%) compared to that of the MSSA group (7.8%) (P < 0.01).
According to the results of the MLST method, 76 unique STs were identified among 412 S. aureus isolates, including 25 novel STs. Based on eBURST analysis, the 76 STs were classified into 28 CCs, including 14 groups and 14 singletons (Figures 1, 2). The three most abundant STs among all S. aureus isolates were ST188 (12.9%), ST45 (12.1%), and ST59 (10.0%), comprising 35% of all isolates. Among the MRSA group, the three most abundant STs were ST59 (37.6%), ST45 (35.5%), and ST1 (5.4%), comprising 78.5% of all strains. Among MSSA group, ST188 (16.0%), ST6 (9.7%), and ST15 (8.5%) were the three prevalent STs. Further, the most common CCs among all strains were CC188, CC45, and CC59, representing 39.6% of all clones. Specifically, within the MRSA group, CC59 (41.9%), CC45 (35.5%), and CC1 (6.5%) were the three most abundant CCs, while in the MSSA group, the most common clone was CC188 (17.9%), followed by CC5 (11.6%) and CC6 (11.0%) (Table 4).
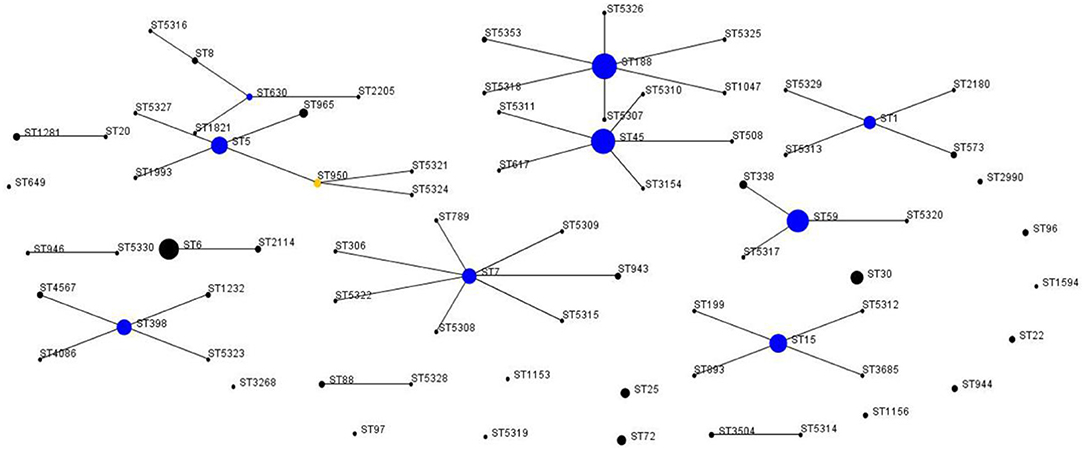
Figure 1. Auto-edited eBURST diagram of 412 S. aureus isolates based on the MLST data. The auto-edited eBURST diagram produced using 6/7 group definition shows 76 STs, including 14 groups and 14 singletons. Each dot implies an MLST ST and the dot area indicates the prevalence of the ST in the MLST data of this study. The linked clusters within the population snapshot should represent clonal complexes, and the primary founders and subgroup founders of these linked clusters are colored blue and yellow.
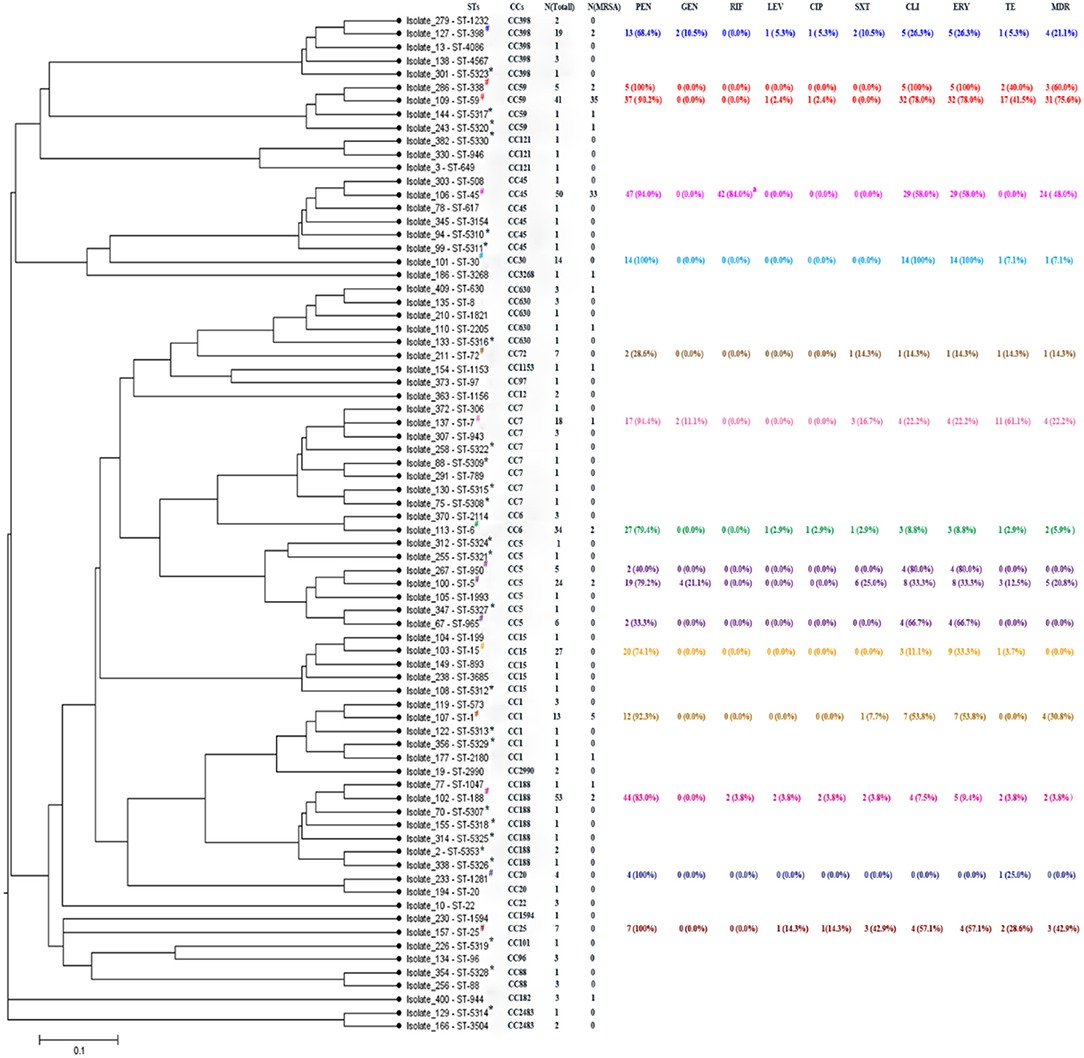
Figure 2. START2 analysis, genotypes, and drug resistances of Staphylococcus aureus isolates. STs, sequence types; CCs, clonal complexes; Each ST randomly selected one isolate as shown above. PEN, penicillin; GEN, gentamicin; RIF, rifampicin; LEV, levofloxacin; CIP, ciprofloxacin; SXT, sulfamethoxazole-trimethoprim; CLI, clindamycin; ERY, erythrocin; TE, tetracycline; MDR, multidrug resistance, representing antibiotic resistance of non β-lactamase. *Representing newly assigned STs. #Representing 16 STs that each had more than three strains. aResistance and intermediary resistance of RIF. Different colors represent different clonal complexes.
As shown in Figure 2, there were 25 newly assigned STs (ST5307 to ST5330, and ST5353) in this study, of which many were single-locus variants (SLVs). Among the 25 novel STs, 26 strains were identified, including 2 MRSA and 24 MSSA isolates; both MRSA isolates belonged to CC59, and the most abundant CC in the MSSA groups was CC188. Finally, we identified 18 novel SLVs in seven housekeeping genes, which were subsequently assigned as new alleles (Table S1).
When analyzing the correlation between antibiotic resistance profiles and unique sequence types (STs) in genotypes of the S. aureus isolates, 16 STs each had more than three strains that were selected in this study. Some specific STs were determined to be closely associated with certain antibiotic resistance patterns, while some exhibited high sensitivity. As shown in Figure 2 and Figure S1, all ST338, ST25, ST30, and ST1281 isolates were resistant to PEN, while ST72, ST950, and ST965 showed high sensitivity to PEN. However, ST950 and ST965 showed high resistance to CLI and ERY, yet were sensitive to all other antibiotics, with an MDR rate of 0%, similar to the MDR rate of ST1281 and ST15. Similarly, ST188, with the largest number of strain types and largest antibiotic resistance coverage, had a very low MDR rate (3.8%). All ST30 and ST338 isolates were resistant to ERY and CLI. ST59, which belongs to CC59 with ST338, also showed a high resistance rate (78%) to ERY and CLI. Additionally, ST59 and ST338 had a higher rate of resistance to TE, with the two highest MDR rates of 75.6 and 60.0%, respectively. In addition to the STs mentioned above, ST7 exhibited the highest resistance rate (61.1%) to TE, while ST45, another predominant ST in the MRSA isolate group, had the highest resistance and intermediate resistance rate (84.0%) to RIF (Figure 2 and Figure S1).
S. aureus fecal carriage may contribute to environmental contamination, facilitate nosocomial transmission, and promote human disease development. Moreover, fecal carriage of S. aureus among children is more likely to cause disease infection due to their immature and underdeveloped immune system (29). Since S. aureus fecal colonization has been identified as a risk factor for infection disease development (30), our findings may serve to advance the current understanding regarding S. aureus fecal colonization dynamics and prevention of S. aureus infection.
In this study, the S. aureus fecal carriage rate was determined to be 20.0% in pediatric patients from Guangzhou, while the MRSA carriage rate was 4.5%. These results agree with the reported prevalence of pooled estimates for S. aureus and MRSA fecal carriage rates (16.8–36.3%, 0.7–27.0%, respectively) (30). However, the prevalence of S. aureus in this study was higher than that reported from participants with nosocomial diarrhea in Germany (7%) (31) compared to healthy adults in China (3.51%) (15), but was lower than that reported in a previous Nigerian study (31.7%) (13). Moreover, in China, the prevalence of MRSA nasal colonization in children between 2005 and 2015 was 4.4% (32), similar to the 4.5% carriage rate detected in our study, but slightly higher than a previous study in American children with cancer (2.9%) (33). Although the prevalence of S. aureus and MRSA is dynamic due to differences in geographical regions, age, gender, and health status, future studies continue to be warranted to better characterize the cause of these differences.
In our study, the fecal carriage of S. aureus was positively correlated with outpatient status and gastroenteritis diagnosis. Pediatric gastroenteritis primarily manifests as abdominal pain, diarrhea, and vomiting, and these patients comprise the majority of outpatients. Moreover, S. aureus has been described as the most common global causative pathogen of food-borne illness, while studies have reported it to be associated with infantile diarrhea (17), corresponding with our observed positive correlation between S. aureus fecal carriage and gastroenteritis in children. In addition, our study found that fecal S. aureus colonization was lowest during the first 4 weeks of life, after which it increased rapidly during the first year, followed by a gradual decline until 17 years of age. This may be explained by the underdeveloped intestinal function and microbiota composition in newborn patients. With improved intestinal function and increased diversity of intestinal microbes, S. aureus colonization increased within the following year. Further, human milk oligosaccharides have been suggested to be a strong contributor to bacterial reproduction in the infant gut and to stimulate S. aureus growth (34), providing an important function for breastfeeding in early life. Subsequently, with the consumption of a comprehensive diet and enhanced immune function, the fecal carriage of S. aureus decreases with increasing age. Additionally, within this study, S. aureus fecal carriage was higher in patients that tested FOBT positive, which may be explained by virulence factors, especially staphylococcal enterotoxins, produced by S. aureus, causing intestinal damage that leads to intestinal bleeding. However, this hypothesis requires further validation.
We also determined that fecal carriage of MRSA was positively associated with inpatients status as hospitalized patients are more likely to be infected with MRSA (35). Interestingly, 7 of the 68 hospitalized patients with S. aureus in their stool also had it within in their sputum or alveolar lavage fluid, demonstrating similar antibiotic susceptibility patterns, including 2 MRSA and 5 MSSA isolates (data not shown). This may suggest that the S. aureus isolated from stool sample was consistent with the source in the sputum or alveolar lavage fluid, and fecal carriage of S. aureus may be associated with infection in other parts of the body.
Based on the MLST results, 412 S. aureus isolates were divided into 76 STs, with fewer MRSA isolates (14 STs) than MSSA isolates (69 STs), indicating that MRSA isolates were more genetically stable than MSSA isolates. The most commonly reported S. aureus isolates in China are diverse and include ST1 (36), ST6 (20), ST5 (37), and ST188 (38) according to different regions, ages, and resources. Similarly, many different MSSA isolates have been identified throughout China, including ST7 and ST188 (39). In our study, ST188, ST6, and ST15 were the most frequently observed STs in the MSSA group. ST188 was reported as a major cause of childhood infections in China, due to its high adhesion and biofilm formation ability (38). In addition, ST15 and ST188 were the two most prevalent clones isolated from infantile diarrhea fecal samples (17). Although the types of MSSA strains are diverse, the most abundant ones have not changed significantly among children in China.
MRSA strains demonstrated strong homology with the prevalent clones in this study, which were determined to be ST59 (37.6%) and ST45 (35.5%). This result was consistent with a previous study reported by Ding et al. in Chinese children (40). ST59, a predominant MRSA clone causing CA-MRSA infections among children (41), was not only predominant in Chinese cities, including Shanghai (39), Sichuan (42), and Taiwan (43), but also is considered to be a prevalent isolate throughout the Asia-Pacific region (44). It is worth noting that ST45, the second most prevalent MRSA strain, has an increased carriage rate in children, compared to 18.8% for the MRSA isolated in our previous study (27). ST45, known as a Berlin clone, was also a common isolate throughout European countries, and has now spread to Australia (45), Singapore (46), and China (41). Further, previous studies from Shanghai have shown that ST239 was the most frequent MRSA clone between 2005 and 2010 (47), which was replaced by the increasingly abundant ST5, ST59, and ST398 clones between 2008 and 2017 (48). However, ST239 was not identified in our study, and ST5 and ST398 only accounted for 2.2% of MRSA isolates. Alternatively, ST45 and ST59 were not only the two dominant clones in the MRSA group, but also the second and the third most abundant clones in all S. aureus isolates. ST45-MRSA is attributed to the acquisition of mecA by a MSSA clone in the community (49). Therefore, our results suggest that ST45 would alter the MRSA clone structure in this region of China, allowing for the development of additionally prominent clones in Chinese children, such as ST59.
The drug resistance of S. aureus has attracted great attention worldwide, especially in China, due to the abuse of antibiotics and the recent emergence of MDR bacteria. Consistent with a previous report (50), we show that S. aureus isolates exhibited a high rate of resistance to PEN, ERY, and CLI, which may be the result of the excessive use of PEN and macrolides (51). The current study demonstrated that S. aureus was more susceptible to vancomycin and linezolid, and displayed 100% sensitivity to vancomycin, linezolid, macrodantin, and QDA. Similarly, relatively low rates of resistance to CIP and LEV were observed in S. aureus isolates, which may be a result from the infrequent use of fluoroquinolones in pediatric patients due to their reported cartilage toxicity. In addition, S. aureus strains can differ in their antibiotic resistance patterns with specific STs. CC59 (ST59 and ST338) exhibited the highest MDR rate and a specific antibiotic resistance pattern (ERY-CLI-TE), while another common clone, CC45 (ST45), exhibited a different antibiotic resistance pattern (ERY-CLI-RIF). ST45 resistance or intermediate resistance to RIF is caused by rpoB mutations (52). However, strains assigned to the same cluster seemed to have similar resistance patterns, suggesting that further genotyping of the S. aureus strains may assist in designing more effective clinical treatment regimens.
Certain limitations were noted within this study. Firstly, more detailed clinical information was difficult to obtain, especially for outpatients, which account for the majority of patients; therefore, the study was limited in its ability to analyze the effect of additional risk factors for S. aureus carriage or MRSA colonization, including premature birth, duration of hospital stay, mother carriage status, history of antibiotic intake, etc. Secondly, inpatient status is strongly correlated with the carriage of MRSA; however, due to the diversity of the hospital environment, we were unable to differentiate MRSA strains based on the various areas in which the inpatients were admitted to; the source of MRSA is worth exploring further. Lastly, we only applied a single method for S. aureus typing, which limited access to more specific and detailed prevalent molecular characterization of S. aureus.
In summary, the S. aureus carriage rate in pediatric feces in Southern China was as high as 20%. MSSA and MRSA exhibited significant differences in genotyping and antimicrobial susceptibility, with ST59 and ST45 emerging as two major MRSA clones and ST188 as the most prevalent MSSA clone. Antibiotic resistance patterns of S. aureus were also found to be closely associated with specific STs. These findings clarify the colonization dynamics and molecular epidemiology of S. aureus from child feces in Southern China and suggest an urgent need to strengthen the surveillance programs in this region, while also providing important information regarding the prevention and treatment of S. aureus infection.
The datasets generated for this study can be found in the https://pubmlst.org/bigsdb?db=pubmlst_saureus_seqdef&page=downloadProfiles&scheme_id=1.
The studies involving human participants were reviewed and approved by the research ethics committee of the Guangzhou women and children's medical center (registration no. 2016081029). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
ZZ and YL initiated and designed the study. XA, FG, SY, BL, JM, ZX, XC, and ZL performed the experiments and/or analyzed the data. XA wrote the draft. HY, ZO, SG, ZZ, and YL revised the manuscript. All authors have approved the final version.
This work was supported by grants from the Natural Science Foundation of Guangdong (Nos. 8451012001001570 and 9151012001000009), Guangdong Science and Technology Department (Nos. 2014A020212013 and 2016A020215013), Medical Health Science and Technology Foundation of Guangzhou (Nos. 20171A010267, and 20181A011039), Paediatric Institute Foundation of Guangzhou Women and Children's Medical Center (Nos. Pre-NSFC-2019-014 and IP-2019-022), and Guangzhou Science Technology and Innovation Commission (No. 201707010010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all the patients who provided their specimens and clinical data for this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00127/full#supplementary-material
Figure S1. Antibiotic resistance of S. aureus isolates from pediatric feces linked to sequence types, as illustrated by the tri-color scale.
1. Lowy FD. Staphylococcus aureus infections. N Engl J Med. (1998) 339:520–32. doi: 10.1056/NEJM199808203390806
2. Abrahamian FM, Moran GJ. Methicillin-resistant Staphylococcus aureus infections. N Engl J Med. (2007) 357:2090. doi: 10.1056/NEJMc072407
3. Yasmin M, El Hage H, Obeid R, El Haddad H, Zaarour M, Khalil A. Epidemiology of bloodstream infections caused by methicillin-resistant Staphylococcus aureus at a tertiary care hospital in New York. Am J Infect Control. (2016) 44:41–6. doi: 10.1016/j.ajic.2015.08.005
4. Millar EV, Chen WJ, Schlett CD, Cui T, Crawford KB, Lanier JB, et al. Frequent use of chlorhexidine-based body wash associated with a reduction in methicillin-resistant Staphylococcus aureus nasal colonization among military trainees. Antimicrob Agents Chemother. (2015) 59:943–9. doi: 10.1128/AAC.03993-14
5. Paharik AE, Parlet CP, Chung N, Todd DA, Rodriguez EI, Van Dyke MJ, et al. Coagulase-negative Staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking Quorum sensing. Cell Host Microbe. (2017) 22:746–56.e5. doi: 10.1016/j.chom.2017.11.001
6. Gagnaire J, Verhoeven PO, Grattard F, Rigaill J, Lucht F, Pozzetto B, et al. Epidemiology and clinical relevance of Staphylococcus aureus intestinal carriage: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. (2017) 15:767–85. doi: 10.1080/14787210.2017.1358611
7. Petersen IS, Larsen PL, Brandelev BL, Hald J, Praetorius C, Welinder R, et al. Close association between oropharyngeal and rhinopharyngeal colonization with Staphylococcus aureus - clues to new insight of MRSA colonization of the oropharynx. J Hosp Infect. (2013) 84:259–62. doi: 10.1016/j.jhin.2013.04.007
8. Bradley SF, Terpenning MS, Ramsey MA, Zarins LT, Jorgensen KA, Sottile WS, et al. Methicillin-resistant Staphylococcus aureus: colonization and infection in a long-term care facility. Ann Intern Med. (1991) 115:417–22. doi: 10.7326/0003-4819-115-6-417
9. Hurst V. Transmission of hospital staphylococci among newborn infants. II. Colonization of the skin and mucous membranes of the infants. Pediatrics. (1960) 25:204–14.
10. Bhalla A, Aron DC, Donskey CJ. Staphylococcus aureus intestinal colonization is associated with increased frequency of S. aureus on skin of hospitalized patients. BMC Infect Dis. (2007) 7:105. doi: 10.1186/1471-2334-7-105
11. Efuntoye MO, Adetosoye AI. Enterotoxigenicity and drug sensitivity of staphylococci from children aged five years and below with sporadic diarrhoea. East Afr Med J. (2003) 80:656–9. doi: 10.4314/eamj.v80i12.8784
12. Boyce JM, Havill NL, Otter JA, Adams NM. Widespread environmental contamination associated with patients with diarrhea and methicillin-resistant Staphylococcus aureus colonization of the gastrointestinal tract. Infect Control Hosp Epidemiol. (2007) 28:1142–7. doi: 10.1086/520737
13. Onanuga A, Temedie TC. Multidrug-resistant intestinal Staphylococcus aureus among self-medicated healthy adults in Amassoma, South-South, Nigeria. J Health Popul Nutr. (2011) 29:446–53. doi: 10.3329/jhpn.v29i5.8898
14. Vandana KE, Varghese G, Krishna S, Mukhopadhyay C, Kamath A, Ajith V. Screening at admission for carrier prevalence of multidrug-resistant organisms in resource-constrained settings: a hospital-based observational study. J Hosp Infect. (2010) 76:180–1. doi: 10.1016/j.jhin.2010.04.015
15. Dong D, Ni Q, Wang C, Zhang L, Li Z, Jiang C, et al. Effects of intestinal colonization by Clostridium difficile and Staphylococcus aureus on microbiota diversity in healthy individuals in China. BMC Infect Dis. (2018) 18:207. doi: 10.1186/s12879-018-3111-z
16. Benito D, Lozano C, Gomez-Sanz E, Zarazaga M, Torres C. Detection of methicillin-susceptible Staphylococcus aureus ST398 and ST133 strains in gut microbiota of healthy humans in Spain. Microb Ecol. (2013) 66:105–11. doi: 10.1007/s00248-013-0240-1
17. Chen Z, Pan WG, Xian WY, Cheng H, Zheng JX, Hu QH, et al. Identification of infantile Diarrhea caused by breast milk-transmitted Staphylococcus aureus infection. Curr Microbiol. (2016) 73:498–502. doi: 10.1007/s00284-016-1088-7
18. Kumar M. Multidrug-resistant Staphylococcus aureus, India, 2013-2015. Emerg Infect Dis. (2016) 22:1666-7. doi: 10.3201/eid2209.160044
19. Tran KN, Rybak MJ. β-Lactam combinations with vancomycin show synergistic activity against vancomycin-susceptible Staphylococcus aureus, vancomycin-intermediate S. aureus (VISA), and heterogeneous VISA. Antimicrob Agents Chemother. (2018) 62:e00157-18. doi: 10.1128/AAC.00157-18
20. Chen Z, Han C, Huang X, Liu Y, Guo D, Ye X. A molecular epidemiological study of methicillin-resistant and methicillin-susceptible Staphylococcus aureus contamination in the airport environment. Infect Drug Resist. (2018) 11:2363–75. doi: 10.2147/IDR.S178584
21. Jonas D, Grundmann H, Hartung D, Daschner FD, Towner KJ. Evaluation of the mecA femB duplex polymerase chain reaction for detection of methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. (1999) 18:643–7. doi: 10.1007/s100960050365
22. Zhao XX, Zhang GQ, Li ZY. Clinical features and etiology of abdominal distension in children. Zhongguo Dang Dai Er Ke Za Zhi. (2019) 21:1022–7.
23. Baraff LJ. Management of fever without source in infants and children. Ann Emerg Med. (2000) 36:602-14. doi: 10.1067/mem.2000.110820
24. CLSI. Performance standards for antimicrobial susceptibility testing. In: 28th Edn CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute (2018).
25. Lee GC, Dallas SD, Wang Y, Olsen RJ, Lawson KA, Wilson J, et al. Emerging multidrug resistance in community-associated Staphylococcus aureus involved in skin and soft tissue infections and nasal colonization. J Antimicrob Chemother. (2017) 72:2461–8. doi: 10.1093/jac/dkx200
26. Giannouli S, Labrou M, Kyritsis A, Ikonomidis A, Pournaras S, Stathopoulos C, et al. Detection of mutations in the FemXAB protein family in oxacillin-susceptible mecA-positive Staphylococcus aureus clinical isolates. J Antimicrob Chemother. (2010) 65:626–33. doi: 10.1093/jac/dkq039
27. Liang B, Mai J, Liu Y, Huang Y, Zhong H, Xie Y, et al. Prevalence and characterization of Staphylococcus aureus isolated from women and children in Guangzhou, China. Front Microbiol. (2018) 9:2790. doi: 10.3389/fmicb.2018.02790
28. Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. (2004) 186:1518–30. doi: 10.1128/JB.186.5.1518-1530.2004
29. Pedro Tda C, Morcillo AM, Baracat EC. Etiology and prognostic factors of sepsis among children and adolescents admitted to the intensive care unit. Rev Bras Ter Intensiva. (2015) 27:240–6. doi: 10.5935/0103-507X.20150044
30. Claassen-Weitz S, Shittu AO, Ngwarai MR, Thabane L, Nicol MP, Kaba M. Fecal carriage of Staphylococcus aureus in the hospital and community setting: a systematic review. Front Microbiol. (2016) 7:449. doi: 10.3389/fmicb.2016.00449
31. Flemming K, Ackermann G. Prevalence of enterotoxin producing Staphylococcus aureus in stools of patients with nosocomial diarrhea. Infection. (2007) 35:356–8. doi: 10.1007/s15010-007-6268-8
32. Lin J, Peng Y, Xu P, Zhang T, Bai C, Lin D, et al. Methicillin-resistant Staphylococcus aureus nasal colonization in Chinese children: a prevalence meta-analysis and review of influencing factors. PLoS ONE. (2016) 11:e0159728. doi: 10.1371/journal.pone.0159728
33. Srinivasan A, Seifried SE, Zhu L, Srivastava DK, Perkins R, Shenep JL, et al. Increasing prevalence of nasal and rectal colonization with methicillin-resistant Staphylococcus aureus in children with cancer. Pediatr Blood Cancer. (2010) 55:1317–22. doi: 10.1002/pbc.22815
34. Hunt KM, Preuss J, Nissan C, Davlin CA, Williams JE, Shafii B, et al. Human milk oligosaccharides promote the growth of staphylococci. Appl Environ Microbiol. (2012) 78:4763–70. doi: 10.1128/AEM.00477-12
35. Milstone AM, Goldner BW, Ross T, Shepard JW, Carroll KC, Perl TM. Methicillin-resistant Staphylococcus aureus colonization and risk of subsequent infection in critically ill children: importance of preventing nosocomial methicillin-resistant Staphylococcus aureus transmission. Clin Infect Dis. (2011) 53:853–9. doi: 10.1093/cid/cir547
36. Wu S, Huang J, Wu Q, Zhang J, Zhang F, Yang X, et al. Staphylococcus aureus isolated from retail meat and meat products in China: incidence, antibiotic resistance and genetic diversity. Front Microbiol. (2018) 9:2767. doi: 10.3389/fmicb.2018.02767
37. Li X, Fang F, Zhao J, Lou N, Li C, Huang T, Li Y. Molecular characteristics and virulence gene profiles of Staphylococcus aureus causing bloodstream infection. Braz J Infect Dis. (2018) 22:487–94. doi: 10.1016/j.bjid.2018.12.001
38. Wang Y, Liu Q. Phylogenetic analysis and virulence determinant of the host-adapted Staphylococcus aureus lineage ST188 in China. Emerg Microbes Infect. (2018) 7:45. doi: 10.1038/s41426-018-0048-7
39. Wang X, Liu Q, Zhang H, Li X, Huang W, Fu Q, et al. Molecular characteristics of community-associated Staphylococcus aureus isolates from pediatric patients with bloodstream infections between 2012 and 2017 in Shanghai, China. Front Microbiol. (2018) 9:1211. doi: 10.3389/fmicb.2018.01211
40. Ding YL, Fu J, Chen J, Mo SF, Xu S, Lin N, et al. Molecular characterization and antimicrobial susceptibility of Staphylococcus aureus isolated from children with acute otitis media in Liuzhou, China. BMC Pediatr. (2018) 18:388. doi: 10.1186/s12887-018-1366-6
41. Wang L, Liu Y, Yang Y, Huang G, Wang C, Deng L, et al. Multidrug-resistant clones of community-associated meticillin-resistant Staphylococcus aureus isolated from Chinese children and the resistance genes to clindamycin and mupirocin. J Med Microbiol. (2012) 61:1240–7. doi: 10.1099/jmm.0.042663-0
42. Tan S, Wan C, Wang H, Zhou W, Shu M. Relationship between nasal carrier isolates and clinical isolates in children with Staphylococcus aureus infections. Microb Pathog. (2019) 127:233–8. doi: 10.1016/j.micpath.2018.11.032
43. Yao K, Wang L, Liu Y, Dong F, Song W, Zhen J, et al. Nasal methicillin-resistant Staphylococcus aureus colonization among otherwise healthy children aged between 2 months and 5 years in northern Taiwan, 2005-2010. BMC Infect Dis. (2018) 51:756–62. doi: 10.1016/j.jmii.2017.07.014
44. Huh K, Chung DR. Changing epidemiology of community-associated methicillin-resistant Staphylococcus aureus in the Asia-Pacific region. Expert Rev Anti Infect Ther. (2016) 14:1007–22. doi: 10.1080/14787210.2016.1236684
45. Dotel R, O'Sullivan MVN, Davis JS, Newton PJ, Gilbert GL. Molecular epidemiology of methicillin-resistant Staphylococcus aureus isolates in New South Wales, Australia, 2012-2017. Infect Dis Health. (2019) 24:134–40. doi: 10.1016/j.idh.2019.04.002
46. Htun HL, Kyaw WM, de Sessions PF, Low L, Hibberd ML, Chow A. Methicillin-resistant Staphylococcus aureus colonisation: epidemiological and molecular characteristics in an acute-care tertiary hospital in Singapore. Epidemiol Infect. (2018) 146:1785–92. doi: 10.1017/S0950268818001966
47. Song Y, Du X, Li T, Zhu Y, Li M. Phenotypic and molecular characterization of Staphylococcus aureus recovered from different clinical specimens of inpatients at a teaching hospital in Shanghai between 2005 and 2010. J Med Microbiol. (2013) 62:274–82. doi: 10.1099/jmm.0.050971-0
48. Dai Y, Liu J, Guo W, Meng H, Huang Q, He L, et al. Decreasing methicillin-resistant Staphylococcus aureus (MRSA) infections is attributable to the disappearance of predominant MRSA ST239 clones, Shanghai, 2008-2017. Emerg Microbes Infect. (2019) 8:471–8. doi: 10.1080/22221751.2019.1595161
49. Witte W. Antibiotic resistance in gram-positive bacteria: epidemiological aspects. J Antimicrob Chemother. (1999) 44(Suppl. A), 1-9. doi: 10.1093/jac/44.suppl_1.1
50. Qin Y, Wen F, Zheng Y, Zhao R, Hu Q, Zhang R. Antimicrobial resistance and molecular characteristics of methicillin-resistant Staphylococcus aureus isolates from child patients of high-risk wards in Shenzhen, China. Jpn J Infect Dis. (2017) 70:479–84. doi: 10.7883/yoken.JJID.2016.328
51. Chen B, Dai X, He B, Pan K, Li H, Liu X, et al. Differences in Staphylococcus aureus nasal carriage and molecular characteristics among community residents and healthcare workers at Sun Yat-Sen University, Guangzhou, Southern China. BMC Infect Dis. (2015) 15:303. doi: 10.1186/s12879-015-1032-7
Keywords: Staphylococcus aureus, prevalence, characterization, drug resistance, child fecal carriage
Citation: Ai X, Gao F, Yao S, Liang B, Mai J, Xiong Z, Chen X, Liang Z, Yang H, Ou Z, Gong S, Long Y and Zhou Z (2020) Prevalence, Characterization, and Drug Resistance of Staphylococcus Aureus in Feces From Pediatric Patients in Guangzhou, China. Front. Med. 7:127. doi: 10.3389/fmed.2020.00127
Received: 10 November 2019; Accepted: 23 March 2020;
Published: 24 April 2020.
Edited by:
Ilana L. B. C. Camargo, University of São Paulo, BrazilReviewed by:
Rima Abdallah Moghnieh, Makassed General Hospital, LebanonCopyright © 2020 Ai, Gao, Yao, Liang, Mai, Xiong, Chen, Liang, Yang, Ou, Gong, Long and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Long, bG9uZ3lhbmdtY0AxNjMuY29t; Zhenwen Zhou, enp3NjI0OEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.