- 1Department of Infectious Diseases, First Hospital of China Medical University, Shenyang, China
- 2Department of Pulmonary and Critical Care Medicine, First Hospital of China Medical University, Shenyang, China
Cryptococcal disease is an opportunistic infection that occurs primarily among people with advanced HIV disease and is an important cause of morbidity and mortality. Spontaneous pneumothorax (SP) is rare in acquired immune deficiency syndrome (AIDS) patients with pulmonary cryptococcosis (PC), but when it occurs, rapid and effective treatment is crucial to the prognosis, with mortality rates varying from 30 to 60%. SP is related to pneumonia mainly due to bacterial infections and pneumocystic jirovecii pneumonia (PJP). However, SP caused by PC is rare. When it occurs, it is often fatal and refractory, which is a challenge both for patients and clinicians. Here, we report a case of SP during the treatment of cryptococcal disease in a patient with AIDS. The pneumothorax remained despite chest tube drainage and evolved into a bronchopleural fistula that was confirmed by the Chartis system. The pneumothorax was significantly resolved following the placement of 2 endobronchial valves (EBVs). The patient tolerated the procedure very well and the pneumothorax gradually resolved. When immunocompromised patients suffer from refractory pneumothorax or prolonged air leaks, EBV implantation may be a feasible and minimally invasive procedure for this vulnerable population.
Background
Cryptococcal disease is an opportunistic infection that occurs primarily among people with advanced human immunodeficiency virus (HIV) and is an important cause of morbidity and mortality (1, 2). Spontaneous pneumothorax (SP) is a well-recognized complication of acquired immune deficiency syndrome (AIDS) and is also a major factor that affects patient prognosis. AIDS-related SP is often recurrent and refractory to chest tube drainage. Interventional bronchoscopy can help rapidly confirm the presence of a fistula and administer precise treatment. Here, we report a case of AIDS-related SP in a patient infected with cryptococcus, resulting in a bronchopleural fistula that was successfully treated by the placement of endobronchial valves (EBVs) through a bronchoscope.
Case Presentation
A 25-year-old man was admitted to our hospital for intermittent fever, non-productive cough, headache and vomiting for 2 weeks. On admission, his vital signs were as follows: temperature, 36.5°C; respiratory rate, 16 bpm; pulse, 80 bpm; and blood pressure, 102/57 mmHg. the laboratory data revealed a white blood cell (WBC) count of 4.29 × 109/L (3.50–9.50*109/L), a neutrophil ratio (NE%) of 84.4% (40.0–75.0%), a C-reactive protein (CRP) level of 71.7 mg/L (0–5.00 mg/L) and a procalcitonin (PCT) level of 0.06 ng/mL (0–0.05 ng/mL). The HIV antibody confirmation test was positive, with a T-cell count of 25 cells/μL. The cerebrospinal fluid (CSF) smear and blood culture were positive for Cryptococcus neoformans. The other routine biochemistry results were normal. Chest computed tomography (CT) revealed an irregular thick-walled cavitation in the left lower lobe and cystic lesions in the right lower lobe (Figure 1A). The diagnosis was AIDS with cryptococcal meningitis and pulmonary cryptococcosis (PC). Antifungal treatment was started with intravenous amphotericin B (1 mg/ kg/day) and oral flucytosine (100 mg/kg/day).
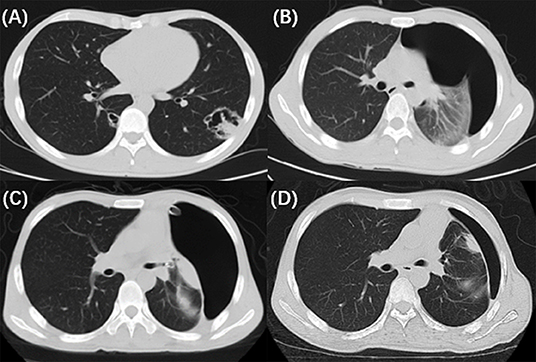
Figure 1. The radiological and bronchoscopic imaging manifestation. (A) Chest CT revealed an irregular thick-walled cavitation in the left lower lobe and cystic lesions in the right lower lobe. (B) Repeat chest CT on the 18th day showed pneumothorax in the left lung. (C) A follow-up chest CT (2 days after EBV implantation) showed partial regression of the pneumothorax. (D) Chest CT acquired after the valves removed revealed that the pneumothorax improved gradually.
On the 18th day of hospitalization, the patient suddenly felt severe chest pain and dyspnea. A repeated chest CT showed pneumothorax (Figure 1B). A chest tube was placed on his left side and yielded symptomatic improvement. Four weeks later, a follow-up CT showed that his left lung did not re-expand. The diagnosis of persistent air leaks (PALs) was confirmed. The patient presented intermittent spasms secondary to cryptococcal meningitis and respiratory failure [Partial pressure of oxygen/Fractional inspired oxygen concentration (PaO2/FiO2): 227.4, FiO2: 33%]. Because the patient was weak and in poor clinical condition (respiratory rate, 25 bpm; pulse, 106 bpm; and blood pressure, 95/58 mmHg.), had respiratory failure and intermittent spasms, and was not a surgical candidate, we inserted EBVs to block the air leak and facilitate healing. The source of the air leaks was in LB3 and LB4+5, which was identified by the Chartis system (Pulmonx SARL, CHARTIS CONSOLE, Switzerland). The Chartis system consists of a balloon catheter and a console that houses flow and pressure sensors (Figure 2A). Airflow is measured through the sensors in the console. When assessing an airway that is exposed to a leak, due to the strong negative pressure in the pleural cavity, the Chartis system visually displays an abnormal block of constant negative pressure (Figure 3). Two EBVs (EBV-TS-4.0 and EBV-TS-5.5) were implanted into the desired airway (Figure 2B).
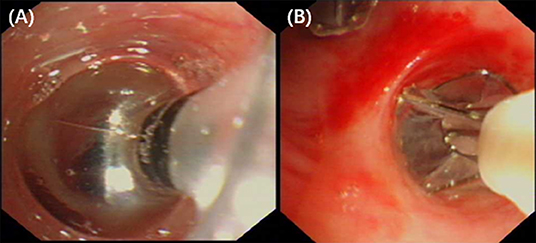
Figure 2. (A) Bronchoscopy revealed that the balloon of the Chartis system occluded the lobar bronchus to identify the lung segments with air leaks. (B) Bronchoscopy revealed two EBVs were implanted into the airway of LB3 and LB4+5.
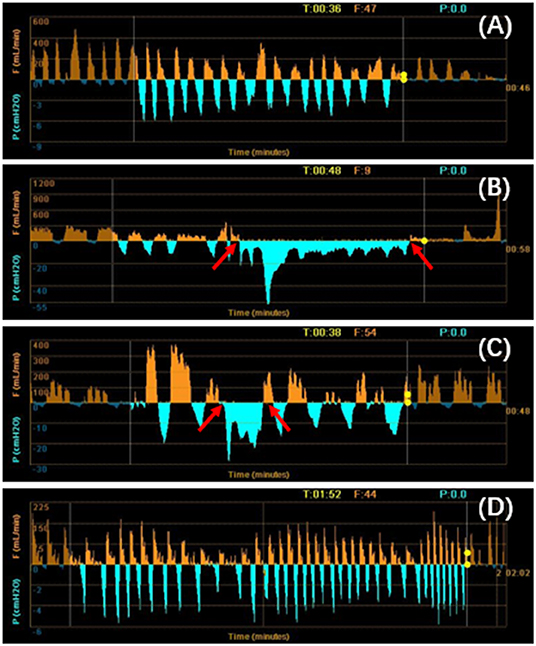
Figure 3. (A–D) Using the Chartis system to identify the lung segments with air leaks. When the balloon occluded the LB4+5 (B) and LB3 (C), a negative pressure was exerted to the exhaust port of the thoracic drainage bottle. The patient's inspiratory pressure was overlapped with the negative pressure created by the vacuum. The negative pressure generated by the vacuum was displayed as a low-level continuous negative pressure at both the inspiratory and expiratory phases (between arrows). It was the sign of the presence of air leaks. There were no air leaks in the left lower lobe (A) and LB1+2 (D). T, time; P, pressure; F, flow.
The patient tolerated the procedure very well with an immediate reduction in air leakage and subjective alleviation of dyspnea. A follow-up chest CT 1 week later showed partial regression of the pneumothorax (Figure 1C). Two months later, the valves were removed. The pneumothorax improved gradually (Figure 1D), and no adverse events related to the EBVs were observed.
Discussion/Conclusion
Spontaneous pneumothorax is a rare but serious complication of opportunistic infections in AIDS patients, with mortality rates varying from 30 to 60% (3), and is related to pneumonia mainly due to bacterial infections and pneumocystic jirovecii pneumonia (PJP) (4). However, SP caused by PC is rare. When it occurs, it is often fatal and refractory, which is a challenge both for patients and clinicians.
Generally, chest tube evacuation of intrathoracic air using a water seal or surgery with a thoracoscopic poudrage/pleurectomy remains the standard treatment for SP (5). Interventional bronchoscopy is helpful for the treatment of SP. The EBV system (Zephyr EBV), works as a one-way valve and is placed in the targeted airway proximal to the area of the air leak, preventing the entrance of air during inspiration and for the drainage of bronchial secretions, thereby enabling the lung to re-expand and heal (6). Early bronchoscopic intervention in patients with AIDS-related SP may shorten the duration of chest tube drainage and help improve the disease prognosis.
There are other minimally invasive and bronchoscopic therapeutic options for prolonged air leakage, such as endobronchial Watanabe spigot (EWS), a type of silicone bronchial blocker. Previous studies revealed that EWS insertion was an effective modality for managing prolonged air leaks caused secondary to pneumothorax, empyema, and postoperative complications (7–9). Infection control at the target bronchus is important for a prolonged duration of EWS indwelling. EWSs should be placed after achieving infection control with antibiotic therapy (10). Chest tube and underwater seal drainage should also be placed to drain the empyema and gain control of the infection.
In contrast to other blocking devices, EBVs allow for expiration and the clearance of bronchial secretions, thereby reducing the risk of post obstructive pneumonia (11). Furthermore, EBVs can be easily removed when the air leaks are resolved to allow adequate time for tissue healing (11). We summarized the published cases using EBVs for PAL(s) in Table 1 (12–27). Only three cases were of patients with AIDS, and our case was the only patient whose SP was secondary to PC. EBVs are well-tolerated and effective for the treatment of PALs. Complications are rare and include pneumonia (13), migration of valves (17), and bacterial colonization. It is important to identify the source of the air leak. We can use a chest drainage system to assess the air leaks. A balloon occlusion test was performed to identify the affected region. The balloon was inflated to achieve complete occlusion in the lobar, segmental, and subsegmental bronchi. The affected airway was identified by the reduction or elimination of the air leak through the chest tube 15–20 s after the occlusion. The Chartis system (Pulmonx) is also a good choice for detecting air leaks, as previously mentioned (28, 29). When the source of the leakage is confirmed, the Chartis system is helpful for confirming the absence of collateral ventilation in the target bronchus which is a key factor for a favorable clinical response. Collateral ventilation from the adjacent lobes through collateral channels could prevent target lobe atelectasis, which potentially limits the clinical response after endoscopic bronchial occlusion (29).
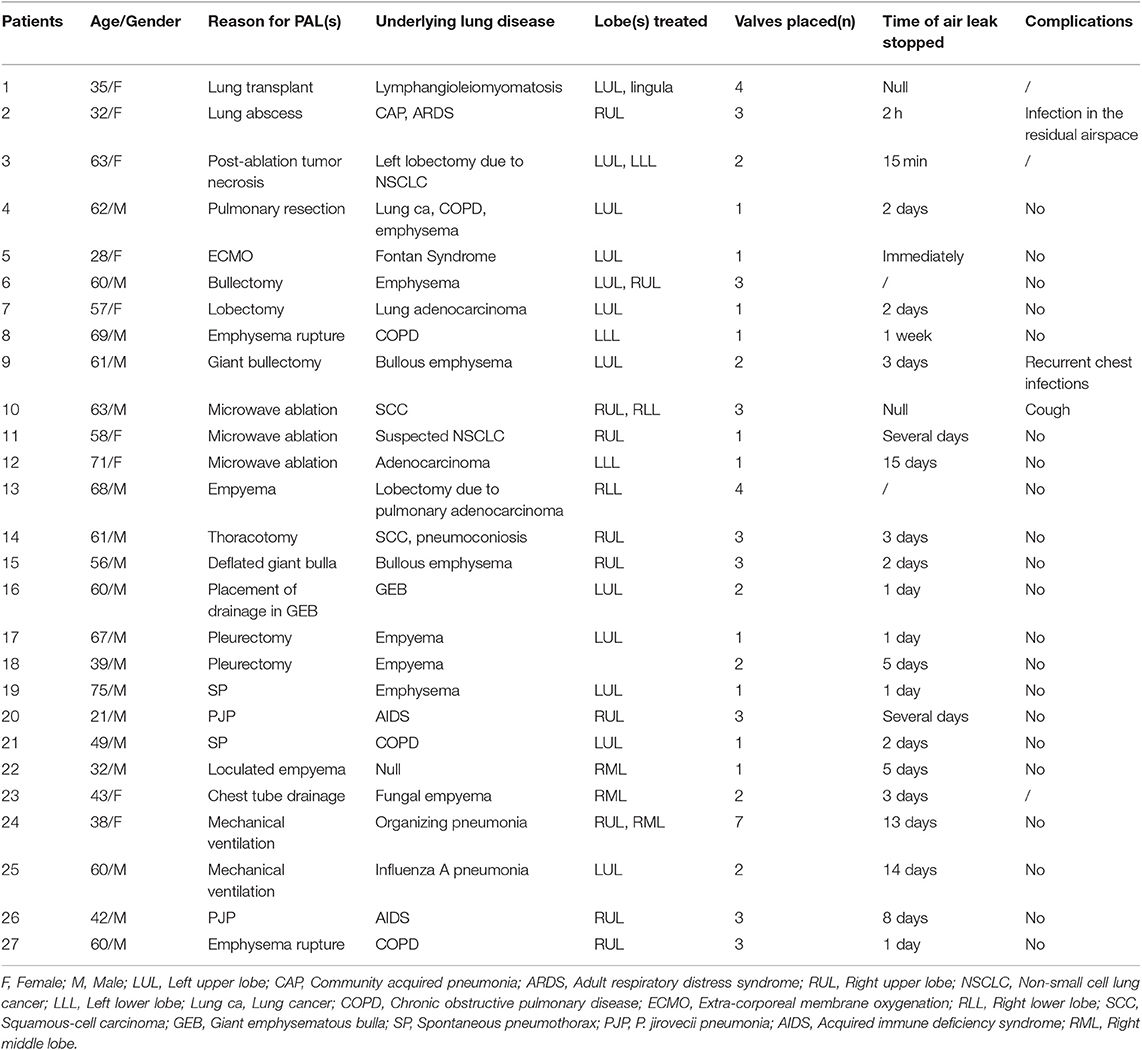
Table 1. Summary of patients' demographics and characteristics for the published cases using EBV for PAL(s).
In conclusion, SP caused by PC is rare but life threatening, and EBV placement may be a good choice for patients with AIDS who are non-surgical candidates due to their extremely poor condition.
Data Availability Statement
All datasets for this study are included in the article.
Ethics Statement
Written informed consent was obtained from the participant for the publication of this case report and any potentially identifying images/information.
Author Contributions
GH made substantial contributions to the conception and design of the work. GH, YW, and YZ helped to collect the data from the cases. GH, YW, and CL wrote the manuscript. GH and YW interpreted the data for the work. GH and HM performed the bronchoscopy. All authors revised the paper critically for important intellectual content, provided final approval of the version to be published, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated, and resolved and contributed toward the acquisition of data for the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AIDS, Acquired immune deficiency syndrome; SP, Spontaneous pneumothorax; PC, Pulmonary cryptococcosis; EBV, Endobronchial valve; WBC, White blood cell; NE%, Neutrophil ratio; CRP, C-reactive protein; PCT, Procalcitonin; CSF, cerebrospinal fluid; CT, Computed tomography; PALs, Persistent air leaks.
References
1. Guidelines for The Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. WHO Guidelines Approved by the Guidelines Review Committee. Geneva (2018).
2. He Q, Ding Y, Zhou W, Li H, Zhang M, Shi Y, et al. Clinical features of pulmonary cryptococcosis among patients with different levels of peripheral blood CD4(+) T lymphocyte counts. BMC Infect Dis. (2017) 17:768. doi: 10.1186/s12879-017-2865-z
3. Terzi E, Zarogoulidis K, Kougioumtzi I, Dryllis G, Kioumis I, Pitsiou G, et al. Human immunodeficiency virus infection and pneumothorax. J Thorac Dis. (2014) 6(Suppl. 4):S377–82. doi: 10.3978/j.issn.2072-1439.2014.08.03
4. Rivero A, Perez-Camacho I, Lozano F, Santos J, Camacho A, Serrano A, et al. Etiology of spontaneous pneumothorax in 105 HIV-infected patients without highly active antiretroviral therapy. Eur J Radiol. (2009) 71:264–8. doi: 10.1016/j.ejrad.2008.05.003
5. Tschopp JM, Bintcliffe O, Astoul P, Canalis E, Driesen P, Janssen J, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J. (2015) 46:321–35. doi: 10.1183/09031936.00219214
6. Travaline JM, McKenna RJ Jr, De Giacomo T, Venuta F, Hazelrigg SR, Boomer M, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest. (2009) 136:355–60. doi: 10.1378/chest.08-2389
7. Kaneda H, Minami K, Nakano T, Taniguchi Y, Saito T, Konobu T, et al. Efficacy and long-term clinical outcome of bronchial occlusion with endobronchial Watanabe spigots for persistent air leaks. Respir Investig. (2015) 53:30–6. doi: 10.1016/j.resinv.2014.09.002
8. Morikawa S, Okamura T, Minezawa T, Goto Y, Hayashi M, Yamaguchi T, et al. A simple method of bronchial occlusion with silicone spigots (Endobronchial Watanabe Spigot; EWS(R)) using a curette. Ther Adv Respir Dis. (2016) 10:518–24. doi: 10.1177/1753465816664862
9. Sasada S, Tamura K, Chang YS, Okamoto N, Matsuura Y, Tamiya M, et al. Clinical evaluation of endoscopic bronchial occlusion with silicone spigots for the management of persistent pulmonary air leaks. Intern Med. (2011) 50:1169–73. doi: 10.2169/internalmedicine.50.5016
10. Maki Y, Fujikura Y, Tagami Y, Hamakawa Y, Sasaki H, Misawa K, et al. Empyema with multiple bronchopleural fistulae improved by bronchial occlusion using an endobronchial watanabe spigot with the push and slide method. Int Med. (2019) 58:1315–9. doi: 10.2169/internalmedicine.1877-18
11. Huang X, Ding L, Xu H. Bronchoscopic valve placement for the treatment of persistent air leaks. Medicine. (2018) 97:e0183. doi: 10.1097/MD.0000000000010183
12. Toma TP, Kon OM, Oldfield W, Sanefuji R, Griffiths M, Wells F, et al. Reduction of persistent air leak with endoscopic valve implants. Thorax. (2007) 62:830–3. doi: 10.1136/thx.2005.044537
13. Abu-Hijleh M, Blundin M. Emergency use of an endobronchial one-way valve in the management of severe air leak and massive subcutaneous emphysema. Lung. (2010) 188:253–7. doi: 10.1007/s00408-009-9204-0
14. Gillespie CT, Sterman DH, Cerfolio RJ, Nader D, Mulligan MS, Mularski RA, et al. Endobronchial valve treatment for prolonged air leaks of the lung: a case series. Ann Thorac Surg. (2011) 91:270–3. doi: 10.1016/j.athoracsur.2010.07.093
15. Conforti S, Torre M, Fieschi S, Lomonaco A, Ravini M. Successful treatment of persistent postoperative air leaks following the placement of an endobronchial one-way valve. Monaldi Arch Chest Dis. (2010) 73:88–91. doi: 10.4081/monaldi.2010.304
16. Schiavon M, Marulli G, Zuin A, Nicotra S, Di Chiara F, De Filippis F, et al. Endobronchial valve for secondary pneumothorax in a severe emphysema patient. Thorac Cardiovasc Surg. (2011) 59:509–10. doi: 10.1055/s-0030-1270758
17. Jenkins M, Vaughan P, Place D, Kornaszewska M. Endobronchial valve migration. Eur J Cardiothorac Surg. (2011) 40:1258–60. doi: 10.1016/j.ejcts.2011.02.027
18. Alexander ES, Healey TT, Martin DW, Dupuy DE. Use of endobronchial valves for the treatment of bronchopleural fistulas after thermal ablation of lung neoplasms. J Vasc Interv Radiol. (2012) 23:1236–40. doi: 10.1016/j.jvir.2012.06.009
19. van Zeller M, Bastos P, Fernandes G, Magalhaes A. Clinical challenges of persistent pulmonary air-leaks–case report. Rev Port Pneumol. (2014) 20:162–6. doi: 10.1016/j.rppneu.2013.07.004
20. Fiorelli A, Accardo M, Vicidomini G, Santini M. LigaSure meets endobronchial valve in a case of lung cancer with pneumoconiosis. Transl Lung Cancer Res. (2013) 2:308–10. doi: 10.3978/j.issn.2218-6751.2012.12.09
21. Ambrosino N, Ribechini A, Allidi F, Gabbrielli L. Use of endobronchial valves in persistent air leaks: a case report and review of the literature. Expert Rev Respir Med. (2013) 7:85–90. doi: 10.1586/ers.12.76
22. Fiorelli A, Costanzo S, Carelli E, Di Costanzo E, Santini M. Bronchoscopic treatment of complex persistent air leaks with endobronchial one-way valves. Gen Thorac Cardiovasc Surg. (2016) 64:234–8. doi: 10.1007/s11748-014-0479-6
23. Vicencio AG, Tozzi M, Thompson C, Satchell M, Delbello D, Ting A, et al. Intrabronchial valves for treatment of alveolar-pleural fistula in a patient with Pneumocystis jirovecii pneumonia. J Bronchology Interv Pulmonol. (2014) 21:346–9. doi: 10.1097/LBR.0000000000000104
24. Qi F, Tian Q, Chen L, Li C, Zhang S, Liu X, et al. Use of endobronchial valve insertion to treat relapsing pneumothorax: a case report and literature review. Clin Respir J. (2017) 11:411–8. doi: 10.1111/crj.12355
25. Kalatoudis H, Nikhil M, Zeid F, Shweihat Y. Bronchopleural fistula resolution with endobronchial valve placement and liberation from mechanical ventilation in acute respiratory distress syndrome: a case series. Case Rep Crit Care. (2017) 2017:3092457. doi: 10.1155/2017/3092457
26. Ghiani A, Hansen M, Tsitouras K, Neurohr C. Endobronchial one-way valve therapy facilitates weaning from extracorporeal membrane oxygenation in a patient with ARDS and persistent air leak. Case Rep Crit Care. (2018) 2018:9736217. doi: 10.1155/2018/9736217
27. Bader S, Faul C, Raab S, Schwaiblmair M, Berghaus TM. Successful long-term treatment of persistent pulmonary air leak in Pneumocystis jirovecii pneumonia by unidirectional endobronchial valves. Respir Med Case Rep. (2018) 25:170–3. doi: 10.1016/j.rmcr.2018.08.021
28. Lee SW, Shin SY, Park TS, Choi YY, Park JC, Park J, et al. Clinical utility of quantitative CT analysis for fissure completeness in bronchoscopic lung volume reduction: comparison between CT and chartis. Korean J Radiol. (2019) 20:1216–25. doi: 10.3348/kjr.2018.0724
Keywords: endobronchial valves, bronchopleural fistula, pneumothorax, cryptococcosis, AIDS
Citation: Wen Y, Liang C, Zhou Y, Ma H and Hou G (2020) Endobronchial Valves for the Treatment of Bronchopleural Fistula and Pneumothorax Caused by Pulmonary Cryptococcosis in an AIDS Patient. Front. Med. 7:51. doi: 10.3389/fmed.2020.00051
Received: 07 August 2019; Accepted: 03 February 2020;
Published: 18 February 2020.
Edited by:
Vincent Cottin, Université Claude Bernard Lyon 1, FranceReviewed by:
Levent Dalar, Istanbul Bilim University, TurkeyPeter Korsten, Nephrology and Rheumatology University Medical Center Göttingen, Germany
Copyright © 2020 Wen, Liang, Zhou, Ma and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Hou, aG91Z2FuZ2NtdUAxNjMuY29t
 Ying Wen1
Ying Wen1 Gang Hou
Gang Hou