
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 31 January 2020
Sec. Rheumatology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00012
This article is part of the Research Topic Interstitial Lung Disease in the Context of Systemic Disease: Pathophysiology, Treatment and Outcomes View all 13 articles
Objective: This study aimed at clarifying the prevalence, risk factors, outcome, and outcome-related factors of acute exacerbation of interstitial lung disease (AE-ILD) in patients with idiopathic inflammatory myopathy (IIM).
Methods: Data of IIM patients who were admitted to the First Affiliated Hospital of Zhejiang University (FAHZJU) from September 2007 to September 2019 were retrospectively collected. And the IIM patients with AE-ILD formed the case group. In addition, age and sex matched IIM patients without AE-ILD were randomly selected to constitute the control group. A 1:2 case-control study and intragroup analysis were performed to identify risk factors for development of AE-ILD in IIM patients and unfavorable short-term outcome in AE-ILD patients through comparison, univariate and multivariate logistic regression analysis.
Results: AE-ILD occurred in 64 out of 665 IIM patients (9.6%) with a short-term mortality rate of 39.1%. And the 64 IIM patients with AE-ILD formed the case group. Besides, 128 age and sex matched IIM patients without AE-ILD were randomly selected to constitute the control group. The retrospective case-control study revealed that elevated on-admission disease activity (P < 0.001), lower percent-predicted diffusing capacity of the lung for carbon monoxide (DLCO%, P = 0.013) and diagnosis of clinically amyopathic dermatomyositis (CADM, P = 0.007) were risk factors for development of AE-ILD in IIM patients. The following intragroup analysis indicated that elevated on-admission disease activity (P = 0.008) and bacterial infection (P = 0.003) were significantly correlated with the unfavorable short-term outcome of patients complicated with AE-ILD. In addition, combined use of steroid and disease modifying antirheumatic drugs (DMARDs, P = 0.006) was found to significantly reduce the short-term mortality in IIM patients with AE-ILD.
Conclusion: AE-ILD is a less frequent but fatal complication in IIM patients with elevated on-admission disease activity, lower DLCO% and diagnosis of CADM working as risk factors, indicating the potential roles of autoimmune abnormality and hypoxia in development of AE-ILD. Elevated on-admission disease activity and bacterial infection could predict unfavorable short-term outcome of IIM patients with AE-ILD. A therapeutic regimen of steroid and DMARDs was found to reduce short-term death in these patients.
Idiopathic inflammatory myopathies (IIM) are a group of autoimmune diseases that primarily target the skeleton muscles (1, 2). Dermatomyositis (DM) and polymyositis (PM) are two conventional subtypes of IIM, while clinically amyopathic dermatomyositis (CADM) is a newly recognized subset of DM with typical skin rash of DM and slight muscular damage. Although the incidence of DM, PM, and CADM was considerably low in common people, the high mortality rate, the various clinical manifestations, and multiple complications have drawn much attention from clinicians and researchers. In published studies, the 10-year survival rate for patients with DM, PM, or CADM ranged from 51 to 91% (3). An ~4.5% in-hospital mortality rate was seen in two retrospective studies (3, 4).
Multiple organs apart from muscle are often affected as well, leading to critical worsening of the life quality and outcome of these patients (5). Among the multiple extramuscular complications of IIM, interstitial lung disease (ILD) was identified as both the most frequent and severe involvement, leading to a significant elevation in mortality rate (6). Moreover, acute exacerbation of ILD (AE-ILD), which used to be mainly studied in patients with idiopathic pulmonary fibrosis, has also been noticed in patients with connective tissue disease (CTD). In CTD patients, AE-ILD was reported to occur at a 1-year frequency of 1.25–3.3%, at a lifetime incidence of 7.2% in CTD patients, and contributed to a high mortality rate within these patients (7, 8). In the past few years, there existed a few reports and small-sample studies of AE-ILD, or rapid progression of ILD, in IIM patients. However, systemic understandings including the incidence of AE-ILD, its risk factors and outcome in IIM patients remained unclear. It is thus necessary to uncover the enigma by figuring out factors correlated with AE-ILD in patients with DM, PM, or CADM, and factors associated with outcome of patients with AE-ILD.
In this study, we retrospectively reviewed the medical records of 424 patients with DM, PM, and CADM who were admitted to our center from February 2011 to February 2019, and performed a case-control analysis to identify potential related risk factors for AE-ILD among these patients. Besides, factors affecting the short-term outcome of patients with AE-ILD were as well-probed into via subgroup analysis.
Medical records of adult patients who were admitted to the inpatient department of the Qingchun division of the First Affiliated Hospital of Zhejiang University (FAHZJU) with the diagnosis of DM, PM, or CADM from September 2007 to September 2019 was reviewed and collected. The approval (Reference Number: 2019-646) of the Institutional Review Board (IRB) of the FAHZJU was acquired before the initiation of the study, and written informed consent from each patient involved was acquired as well. The inclusion criteria of this study were: (1) age over 18 years old; (2) the diagnosis of DM or PM fulfilled the diagnostic criteria of Bohan and Peter (9), and the diagnosis of CADM met the criteria developed by Sontheimer (10). Exclusion criteria were: (1) overlap syndromes with other connective tissue diseases; (2) hospitalization for causes unrelated to myositis and its complications, such as fracture, pregnancy, cataract, and appendicitis etc.; (3) myopathies that might be related to thyroid dysfunction, excessive exercises, inherited, or metabolic disorders, recent use of muscle-impairment drugs including statins, chloroquine, colchicine, entecavir, traditional Chinese medicine, etc.; (4) loss to follow-up within 2 weeks after discharge.
Medical records of all patients enrolled were retrospectively collected by reviewing the electronic medical record (EMR) system. Data including demographic information, course of disease, duration of diagnosis delay, clinical manifestations, or complications, on-admission disease activity, results of pulmonary function test, preceding comorbidities, harmful hobbies, imaging reports, laboratory findings, medications, as well as short-term outcome were acquired and analyzed. ILD, subtype of ILD and AE-ILD were evaluated by radiologists using high-resolution computed tomography (HRCT). In absence of diagnostic criteria dedicated to AE-ILD in patients with CTD, an updated criteria of acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) was adopted based on the experience of published studies on AE-ILD in CTD patients. The updated criteria included previous or concurrent diagnosis of ILD, acute worsening or development of dyspnea typically <1 month duration, computed tomography with new bilateral ground-glass opacity and/or consolidation superimposed on a background pattern consistent with usual interstitial pneumonia (UIP) pattern, and deterioration not fully explained by cardiac failure or fluid overload (11). Compared with the previous diagnostic criteria for AE-ILD proposed in 2007 (12), the new criteria does not demand thorough exclusion of infection. And infection has been found to participate in the pathogenesis and progression of idiopathic pulmonary fibrosis (IPF) (13). As previously suggested, the occurrence of this clinical and radiological manifestation in a background of possible or inconsistent with UIP pattern was also considered diagnostic for AE in CTD patients (14, 15). Cases manifested as UIP pattern were identified based on their radiologic appearance on HRCT: the presence of basal-dominant reticular opacities and predominantly basal and subpleural distribution of honeycomb lesions, with multiple equal-sized cystic lesions of 2–10 mm diameter with a thick wall (16). Diagnosis of bacterial, fungal, or tuberculosis infection was a comprehensive decision based on the essential positive result of etiological detection, HRCT manifestation, clinical symptoms, infection-related laboratory abnormalities, treatment of intravenous antibiotics, and antifungal drugs, positive response after treatment, etc. The etiological detection was defined as the culture of bronchoalveolar lavage fluid (BALF) and sputum. Sputum culture result counted only if >25 squamous epithelial cells per low-power field were observed (17). In bacterial infections, the thresholds for positivity of quantitative cultures were applied: 105 cfu/ml for sputum culture (17), 104 cfu/mL for bronchoalveolar lavage (18). For patients with infection of Candida albican or Candida glabrata, the BALF or sputum culture should show a visually medium to large amount of C. albicans or C. glabrata in the sample. The repeated cultures of BALF or sputum were routinely initiated before intravenous use of antibiotics or anti-fungal medications. Meanwhile diagnosis of virus infection, to be specific, Epstein-Barr virus (EBV) or Cytomegalo virus (CMV) infection, relied on the screening of serum antibody and DNA of these two viruses. Identification of gastrointestinal hemorrhage was based on repeated positive results of fecal occult blood test. To minimize omission of lymphadenectasis, hepatomegaly, and splenomegaly, the identification was based on records of physical examination together with reports of ultrasound examination, computed tomography and positron emission tomography. On-admission disease activity was routinely assessed by the Myositis Disease Activity Assessment Visual Analog Scales (MYOACT) within the first week of admission (19). Immunosuppressive regimens used during hospitalization were categorized into four groups: (1) steroid monotherapy; (2) steroid + disease-modifying antirheumatic drugs (DMARDs); (3) steroid + intravenous immunoglobulin (IVIG); (4) steroid + DMARDs +IVIG. In this study, usage of DMARDs included usage of mycophenolate mofetil (MMF), thalidomide, hydroxychloroquine, cyclosporine, azathioprine, methotrexate, cyclophosphamide, etc. Short-term mortality, or unfavorable short-term outcome, referred to in-hospital mortality or death within 2 weeks of hospital discharge.
To probe into factors exerting significant influence on development of AE-ILD within patients with DM, PM, or CADM, a case-control study was performed. Patients diagnosed with AE-ILD constituted the case group. And ILD patients without AE-ILD were selected using a systematic sampling method by matching age and sex with cases with AE-ILD at a proportion of 1:2. Comparisons, univariate and multivariate logistic regression analysis were performed between the case group and the control group. To clarify the time axis of risk factors and results, only clinical manifestations or complications that happened before the diagnosis of AE-ILD would be taken into account for patients with AE-ILD. In order to identity potential factors affecting the short-term outcome of the AE-ILD patients involved, the AE-ILD patients were further divided into two groups: patients who died in hospital or within 2 weeks of hospital discharge were defined as the mortality group, and those who survived after 2 weeks of hospital discharge were categorized as the survival group. Comparisons and logistic regression analysis were made between the two groups of patients regarding age, sex, clinical features, disease activity, laboratory findings, etc.
Statistical analysis was performed using SPSS 22.0 (Chicago, IL, USA) and R 3.6.1. The normality of continuous variables was tested by the Kolmogorov-Smirnov goodness-of-fit model. Continuous variables were expressed as mean ± SD if normally distributed and median (quartiles) if skewed. Ordinal categorical variables were as well shown as median (quartiles). Unordered categorical variables were presented as numbers and percentages. Independent sample t-test was used to compare normally distributed continuous variables. And Mann-Whitney U-test was applied to compare skewed continuous variables or ordinal categorical variables. Chi-square test and Fisher's exact test were used to compare unordered categorical variables. All tests were two-sided and a P < 0.05 was considered statistically significant. Univariate and multivariate logistic regression analyses were subsequently adopted to identify risk factors for AE-ILD in patients with PM, DM or CADM as well as risk factors for unfavorable short-term outcome in AE-ILD. In the study of risk factors for AE-ILD, explanatory factors with P < 0.1 in the univariate logistic regression analysis were entered into the multivariate logistic regression analysis. In the process of figuring out risk factors for unfavorable short-term outcome, however, factors with P < 0.05 in univariate analysis were enrolled into the multivariate logistic regression analysis owing to the limited number of AE-ILD patients. For normally distributed continuous variables with missing values, inputation using expectation maximization (EM) algorithm was performed for those that passed univariate screening. Multivariate logistic regression analysis with a stepwise forward likelihood ratio (LR) method was used to determine the statistically significant factors. Results from the multivariate logistic regression were presented as an odds ratio (OR) with 95% confidence interval (CI). A two-sided P < 0.05 was considered to be statistically significant. If there existed any positive result in serum biomarkers or disease activity in multivariate logistic regression analysis, a receiver operating characteristic (ROC) curve analysis would be performed to evaluate its predictive value for development and outcome of AE-ILD.
A total of 665 patients treated at FAHZJU with a diagnosis of DM, PM, or CADM between September 2007 and September 2019 were enrolled into this study, including 334 with DM, 264 with PM, and 67 with CADM. Four hundred and eighty-three patients (72.6%) were identified to be complicated with ILD. Sixty-four out of 665 patients were diagnosed with AE-ILD during their stay in hospital (Figure 1). The incidence of AE-ILD was 9.6% in patients with DM, PM, or CADM, and 13.3% in patients who were complicated with ILD at the same time. To be specific, the incidence of AE-ILD in patient with DM, PM, and CADM were 10.8, 5.7, and 19.4%, respectively. In the 665 patients, the average age for AE-ILD patients was 57.7 ± 11.9 years, which was significantly higher than that of the patients without AE-ILD (53.1 ± 13.7 years, P = 0.011). Among the 64 AE-ILD patients, 25 were males and 39 were females. The proportion of males in AE-ILD patients was not significantly different from that in non-AE-ILD patients (39.1 vs. 32.3%, P = 0.272). Short-term mortality rate for AE-ILD and non-AE-ILD patients were 39.1 vs. 5.7% (P < 0.001).
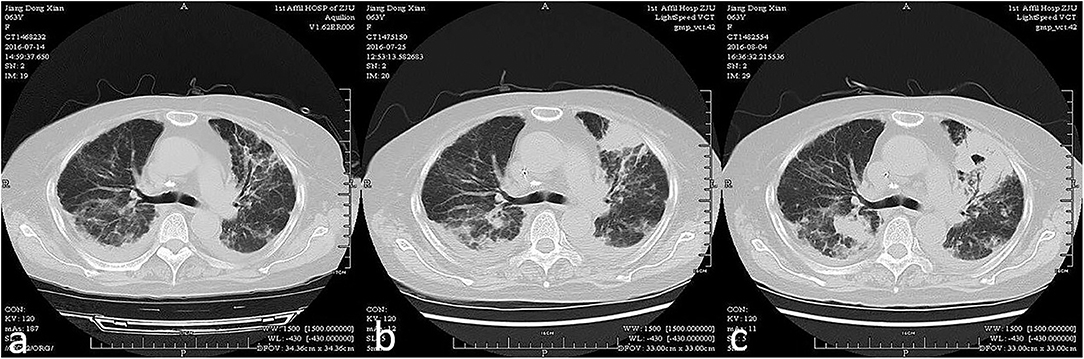
Figure 1. Acute exacerbation of interstitial lung disease of a patient within 3 weeks (from a–c chronologically).
In total, 64 AE-ILD patients and 128 ILD patients without occurrence of AE-ILD were included in the case-control analysis to identify risk factors for AE-ILD in patients with DM, PM, or CADM. Due to the retrospective nature of this study, only 137 patients (54 of AE-ILD patients and 83 of patients without AE-ILD) received pulmonary function test within the first week of hospitalization. The case group presented more frequently with treatment of steroid + IVIG (P = 0.034), diagnosis of CADM (P = 0.034) and less frequently with allergic history (P = 0.049). Higher levels of serum ferritin (P = 0.027) and C reactive protein (CRP, P = 0.004) were seen in patients with AE-ILD. On-admission disease activity, which was evaluated by MYOACT score, was as well-significantly higher for patients in the case group (P < 0.001). In addition, AE-ILD patients were found to present with lower level of percent-predicted diffusing capacity of the lung for carbon monoxide (DLCO%, P = 0.009; Table 1, Supplementary Data 1).
Univariate analysis showed that there were eight factors associated with AE-ILD at the level of P < 0.1. These factors included elevated on-admission disease activity (P < 0.001), lower DLCO% (P = 0.010), serum ferritin (P = 0.058), CRP (P = 0.037), hypertension (P = 0.065), allergic history (P = 0.058), treatment of steroid + IVIG (P = 0.038), and diagnosis of CADM (P = 0.038) (Supplementary Table 1). Inputation was performed for DLCO% before multivariate logistic regression analysis. Using Kolmogorov-Smirnov test, DLCO% was found a continuous variable that was subject to normal distribution. EM inputation was hereby performed to handle the impact of missing values more appropriately. Afterwards, all variables with P < 0.1 were entered into the multivariate logistic regression analysis, and elevated on-admission disease activity (P < 0.001), lower DLCO% (P = 0.013), and diagnosis of CADM (P = 0.007) were found to be significantly different between the case group and the control group. The results were found similar to those without EM imputation (Table 2). As presented in Figure 2, the optimal cut-off value of the on-admission disease activity for AE-ILD was >7.5, with a sensitivity of 76.6% and a specificity of 57.0%. The area under the curve (AUC) was 0.705.
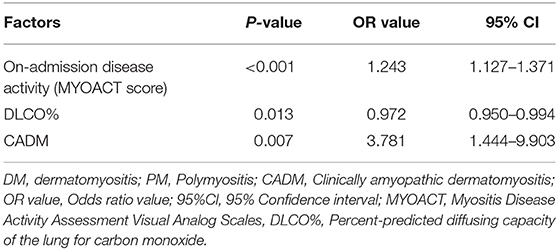
Table 2. Multivariate logistic regression analysis of risk factors for AE-ILD in patients with DM, PM, or CADM.
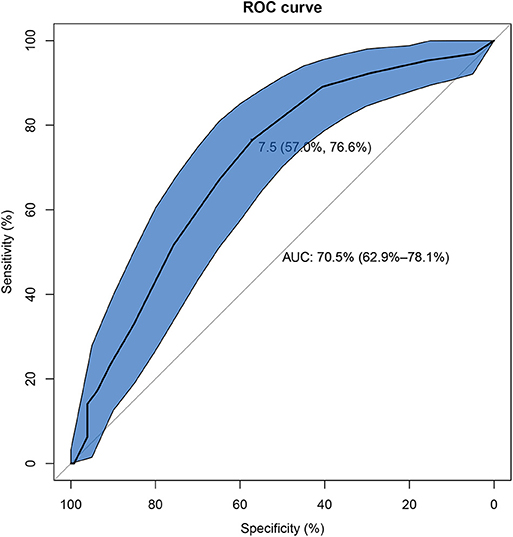
Figure 2. The receiver operating characteristic curve of on-admission disease activity for development of AE-ILD in IIM patients. AE-ILD, Acute exacerbation of interstitial lung disease; IIM, Idiopathic inflammatory myopathies.
Of the 64 AE-ILD patients identified in the study, 25 (39.1%) died in hospital or within 2 weeks of hospital discharge. In addition to 36 AE-ILD patients with DM, we also found 15 PM patients and 13 CADM patients who as well-suffered from AE-ILD. And 15 of them (23.4%) manifested as UIP pattern in HRCT. Infection happened to 30 out of 64 adult AE-ILD patients. Ten had bacterial infection, 12 had fungal infection, three were diagnosed with tuberculosis, one was found to have EBV infection. Three suffered from both bacterial and fungal infection, and one had both bacterial and EBV infection. Bacterial (21.9%) and fungal (23.4%) infections were hereby recognized as the two most common infections in AE-ILD patients. Only eight patients with infections (five in bacterial infection, two in fungal infection, and one in tuberculosis infection) were identified based on positive result of BALF smear or culture. To be specific, bacterial infection included four cases of Acinetobacter baumannii, four cases with Stenotrophomonas maltophilia, three case with Klebsiella pneumonia, onc case with Pseudomonas aeruginosa, one case with Staphylococcus haemolyticus, and one case with Staphylococcus aureus. And fungal infection included 10 cases with medium to large amount of C. albicans, three cases with Aspergillus fumigatus, one case with Pneumocystis carinii and one case with C. glabrata. Therefore, infections in patients with AE-ILD were mostly opportunistic infections. Details on infections in the matched control group was provided in Supplementary Data 2. In addition, the most commonly used therapy was a combined application of steroid and DMARDs (45.3%). And MMF (48.3%) was the most frequently used DMARD in this regimen. Patients with unfavorable short-term outcome presented more frequently with dysphagia (P = 0.030), bacterial infection (P = 0.001), hypertension (P = 0.017), treatment of steroid + IVIG (P = 0.013), and less frequently with treatment of steroid + DMARDs (P = 0.001). Higher on-admission disease activity (P = 0.014) was as well-seen in patients with unfavorable outcome (Table 3).
Univariate analysis showed that there were six factors associated with unfavorable short-term outcome in AE-ILD patients at the level of P < 0.05. These factors included dysphagia (P = 0.019) bacterial infection (P = 0.002), on-admission disease activity (P = 0.012), hypertension (P = 0.020), treatment of steroid + DMARDs (P = 0.002) and steroid + IVIG (P = 0.018) (Supplementary Table 2). The following multivariate logistic regression analysis revealed that higher on-admission disease activity (P = 0.008), bacterial infection (P = 0.003), and treatment of steroid+DMARDs (P = 0.006) were significantly correlated with unfavorable short-term outcome in AE-ILD patients (Table 4). As presented in Figure 3, the best cut-off value of the on-admission disease activity for unfavorable short-term outcome in patients with AE-ILD was >8.5, with a sensitivity of 84.0% and a specificity of 43.6%. The AUC was 0.682.
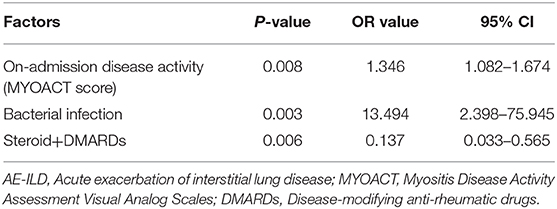
Table 4. Multivariate logistic regression analysis of risk factors for unfavorable short-term outcome in patients complicated with AE-ILD.
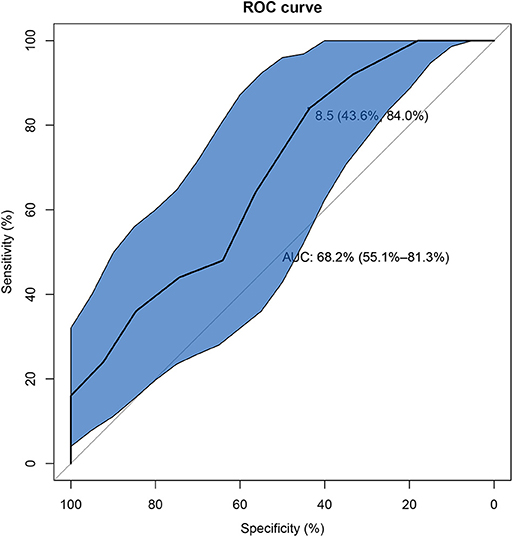
Figure 3. The receiver operating characteristic curve of on-admission disease activity for unfavorable short-term outcome in IIM patients with AE-ILD. IIM, Idiopathic inflammatory myopathies; AE-ILD, Acute exacerbation of interstitial lung disease.
To date, this is the first study to systematically probe into the risk factors for development of AE-ILD in patients with DM, PM, or CADM, and potential factors affecting the short-term outcome of the AE-ILD patients. Preceding studies on acute exacerbation mainly focused on AE-IPF. And the annual incidence of AE-IPF ranged from 7 to 19.1% in different clinical trials and retrospective studies (20–25). Knowledge on AE-ILD in non-IPF patients, namely connective-tissue-disease-related ILD (CTD-ILD), was limited. The reported incidence of AE-ILD in rheumatoid arthritis (RA) patients with ILD was 7.7–22% (26, 27). Tomiyama et al. revealed an AE-ILD incidence of 9.4% in systemic sclerosis (28). In this study, the incidence of AE-ILD was 9.6% in patients with DM, PM, or CADM, and 13.3% in patients complicated with ILD. And the mortality rate of AE-ILD was significantly higher than that in non-AE-ILD patients (39.1 vs. 5.7% P < 0.001). Besides, the average age for AE-ILD patients was as well-higher than that of the patients without AE-ILD (57.7 ± 11.9 vs. 53.1 ± 13.7 years, P = 0.011). Elevated on-admission disease activity, lower DLCO% and diagnosis of CADM were found to be risk factors for development of AE-ILD in patients with DM, PM, or CADM. Moreover, bacterial infection, elevated on-admission disease activity and treatment of steroid + DMARDs were significantly correlated with short-term outcome in AE-ILD patients.
Previous studies revealed that declined forced vital capacity (FVC), low diffusing capacity of the lung for carbon monoxide (DLCO), pulmonary hypertension, comorbid coronary artery disease, surgical resection of lung cancers and various infections etc. were found to be risk factors for AE-ILD (29–31). However, the results were not homogeneous in different studies. In this study, decreased DLCO%, which reflected lower diffusing capacity, was found to be a risk factor for AE-ILD in patients with DM, PM, or CADM. The role of lower DLCO% in AE-ILD was not clear. On the one hand, lower DLCO% reflected decreased gas-exchanging function of lung. With no significant alteration in pulmonary ventilation function etc., decreased gas-exchanging function would lead to hypoxia, which could subsequently contribute to progress of ILD. Hypoxia have been recognized to induce progress of interstitial lung disease through augmenting oxidative and inflammatory pathways, increasing the total lung collagen content and heterogeneous structural alterations (32–34). On the other hand, decreased DLCO% could be an early-stage manifestation of AE-ILD since ILD and its progression could result in impaired diffuse capacity via alveolar structural alteration, thickening of alveolar capillary wall, etc. Lower DLCO% seemed to be both initiating factor and consequence of AE-ILD.
MYOACT score works as a systemic evaluation of disease activity of IIM (19, 35). After adjusting for other factors, elevated on-admission MYOACT score was found to be related to development of AE-ILD in IIM patients. The role of CTD disease activity in AE-ILD was disputable in published studies. In a retrospective study concerning RA patients receiving tocilizumab treatment, AE-ILD was found to be positively related to disease activity of RA (36). However, no similar association was seen in RA patients treated by corticosteroids and immunosuppressants. The predictive role of MYOACT score in this study might lie in the partially overlapped pathological mechanism between AE-ILD and IIM. Elevated levels of several cytokines and chemokines, namely IL-6, IL-8, IL-17, IL-23, etc., were seen in peripheral blood, muscle or skin of IIM patients, and were consistent with disease activity (37). Meanwhile several studies also observed significant elevation of cytokines and chemokines including IL-6, IL-8 in patients with ILD exacerbation, and the elevation was found to be related to worse outcome (38, 39). The partially overlapped pathological mechanism made baseline disease activity a valuable predictor of AE-ILD. Besides, after adjusting for factors including infections, medication, pulmonary function, etc., the significance of on-admission disease activity could, to some extent, demonstrated the role of autoimmune abnormality in development of AE-ILD. In 2011, Shu etc. found that initial disease activity, which was evaluated by MYOACT score, was not significantly correlated with long-term outcome of IIM patients (40). And no linkage between initial disease activity and short-term outcome of hospitalized IIM patients was reported previously. By narrowing down to DM, PM, or CADM patients complicated with AE-ILD, on-admission disease activity, which was evaluated by MYOACT score, was found to herald unfavorable short-term outcome in this study.
However, the evaluation of disease activity demands ability for communication, which would be difficult in patients with mental retardation or disturbed behavior. It would thus be of great significance to identify serum biomarkers for development and outcome of AE-ILD in IIM patients. Researchers in Hamamatsu University found that higher levels of ferritin predicted development of AE-IPF and unfavorable outcome (41). However, in this study, serum ferritin was not found to be significantly related to development of AE-ILD after adjusting for other clinical features. Nor was it identified to predict short-term outcome of IIM patients with AE-ILD. Preceding study also revealed that CRP could be used to predict development of AE-ILD in patients receiving non-pulmonary surgery (42). Nevertheless, no statistical significance for CRP was seen in IIM patients with regard to development and outcome of AE-ILD. Further studies would be demanded to identify serum biomarkers for development and outcome of AE-ILD in CTD patients.
In addition to the high prevalence of ILD in CADM patients, preceding studies proposed that rapidly progressive pattern of ILD was more frequently seen in CADM patients compared with patients with DM or PM (43, 44). After multivariate logistic regression analysis, diagnosis of CADM was found to be a risk factor for AE-ILD in patients with DM, PM or CADM, which was consistent with the past clinical findings. Although CD8+ T cells were found to play a key role in development of IIM-related ILD, high proportion of CD4+ T cells seemed to play a greater role in acute exacerbation of ILD. Suda and his colleagues focused on CADM patients and found that the CD4/CD8 ratio in bronchoalveolar lavage fluid (BALF) was higher in patients with rapidly progressive ILD in comparison to that in chronic ILD patients (45). Ito et al. demonstrated similar results in BALF and peripheral blood of patients with DM (46). Moreover, Mukae et al. uncovered a higher CD4/CD8 ratio in BALF of CADM-related ILD patients compared with that in ILD patients with classic DM (43). Taken together, the higher proportion of CD4+ T cells in BALF seem to link diagnosis of CADM with higher incidence of AE-ILD. Confirmation of the role of higher proportion of CD4+ T cells and exploration of its detailed mechanism in immune abnormality of AE-ILD in IIM patients demands further exploration.
In-hospital IIM patients regularly received immunosuppressive therapy, which greatly increased their vulnerability to bacterial, fungal, or viral infection. More infections, opportunistic bacterial and fungal infections in particular, were hereby identified in this study. Although infectious triggers were found in 10–30% of patients with AE in preceding study (47), no significant association was found between infections and development of AE-ILD after adjusting for disease activity, pulmonary function, medication, etc. In the following intragroup analysis, bacterial infection was found to be associated with unfavorable short-term outcome in DM, PM, or CADM patients complicated with AE-ILD. Similar linkage between infection and short-term outcome was seen in IIM patients (3, 4). And opportunistic infection was as well-recognized as a major cause of mortality in patients with IIM-related ILD (48). However, this is the first study identifying infection as risk factor for unfavorable short-term outcome in patients complicated with AE-ILD.
The mortality rate of patients with AE-ILD was relatively high. For patients with IPF, 46% of deaths are secondary to AE and median survival period after AE is 3-4 months (49). And a high mortality rate (55.6%) was as well-seen in CTD patients with AE-ILD (14). In this study, the short-term mortality rate of AE-ILD group was 39.1%. The relatively high mortality rate of AE-ILD patients indicated much room for improvement in therapeutic regimens. In IIM patients with AE-ILD, a combined use of steroid and DMARDs was found to reduce the short-term mortality rate of these patients. Meanwhile no significant effect was identified in the application of intravenous immunoglobulin. Preceding study revealed a favorable response of exacerbation of ILD in RA patients after receiving a combined therapy of steroid and DMARDs (50). And cyclosporine, tacrolimus, and cyclophosphamide were the major DMARDs used in this study. However, the mostly commonly used DMARD in our study was MMF, the use of which has been proved effective in myositis-related ILD (51). The combined use of steroid and MMF in CTD patients with AE-ILD deserved further exploration in the future. Intravenous immunoglobulin, which was as well-frequently used in patients with ILD or AE-ILD, still played a disputable role in treatment of AE-ILD, especially CTD-related AE-ILD (29, 52). Biologics could be viewed as a two-edge sword in AE-ILD. On the one hand, rituximab, etc. have shown optimistic result in therapy of several AE-ILD cases (52, 53). On the other hand, biologics have also been reported to induce AE-ILD (54, 55). Apart from immunosuppressant treatment, empirical antibiotic therapy is also considered for all patients (56). Application of azithromycin and prophylactic use of co-trimoxazole were found effective in several clinical trials (57–59). Besides, antifibrotic medication, anti-acid therapy, plasma exchange, Polymyxin-B-immobilized fiber column (PMX) and fluid management were as well-found to have potential, yet disputable effect on outcome of AE-ILD patients (29–31).
The most significant limitations of this study are the retrospective and observational nature of the study and the small sample size. Furthermore, absence of records of pulmonary hypertension and several myositis-associated antibodies in over half of the patients also restrained us from figuring out their roles in development of AE-ILD among IIM patients. A large prospective cohort study is essential to confirm our findings and fill in the gaps. In spite of all the limitations, we intended to shed some light on the future study of AE-ILD in patients with DM, PM, or CADM.
AE-ILD is a fatal complication in IIM patients. Elevated on-admission disease activity, lower DLCO% and diagnosis of CADM were found to be risk factor for development of AE-ILD in patients with DM, PM, or CADM. Speculations on the roles of autoimmune abnormality and hypoxia in development of AE-ILD were hereby brought up. In addition, elevated on-admission disease activity, bacterial infection could be used to predict unfavorable short-term outcome in AE-ILD patients. A therapeutic regimen of steroid and DMARDs was found to reduce short-term death in IIM patients with AE-ILD.
All datasets generated for this study are included in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of Zhejiang University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors met the criteria for authorship established by the International Committee of Medical Journal Editors. Specifically, JLia and HC were responsible for substantial contributions to the conception, design, analysis, drafting the work, revising the work, and reviewing of the manuscript. YK, CS, WC, and JLin assisted with the data gathering, revising the work, and reviewing of the manuscript. All the authors listed have approved for publication of the content and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work.
This study was supported in part by the grants from National Natural Science Foundation of China (81701602).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors appreciate the assistance of Bei Xu and Yuli Wang in verification of ILD and AE-ILD.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00012/full#supplementary-material
1. Dalakas MC. Pathogenesis and therapies of immune-mediated myopathies. Autoimmun Rev. (2012) 11:203–6. doi: 10.1016/j.autrev.2011.05.013
2. Marasco E, Cioffi E, Cometi L, Valentini V, Zanframundo G, Neri R, et al. One year in review 2018: idiopathic inflammatory myopathies. Clin Exp Rheumatol. (2018) 36:937–47.
3. Murray SG, Schmajuk G, Trupin L, Lawson E, Cascino M, Barton J, et al. A population-based study of infection-related hospital mortality in patients with dermatomyositis/polymyositis. Arthritis Care Res. (2015) 67:673–80. doi: 10.1002/acr.22501
4. Wu C, Wang Q, He L, Yang E, Zeng X. Hospitalization mortality and associated risk factors in patients with polymyositis and dermatomyositis: a retrospective case-control study. PLoS ONE. (2018) 13:e0192491. doi: 10.1371/journal.pone.0192491
5. Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. (2018) 5:109–29. doi: 10.3233/JND-180308
6. Barba T, Fort R, Cottin V, Provencher S, Durieu I, Jardel S, et al. Treatment of idiopathic inflammatory myositis associated interstitial lung disease: a systematic review and meta-analysis. Autoimmun Rev. (2019) 18:113–22. doi: 10.1016/j.autrev.2018.07.013
7. Park IN, Kim DS, Shim TS, Lim CM, Lee SD, Koh Y, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. (2007) 132:214–20. doi: 10.1378/chest.07-0323
8. Suda T, Kaida Y, Nakamura Y, Enomoto N, Fujisawa T, Imokawa S, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med. (2009) 103:846–53. doi: 10.1016/j.rmed.2008.12.019
9. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. (1975) 292:344–7. doi: 10.1056/NEJM197502132920706
10. Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. (2002) 46:626–36. doi: 10.1067/mjd.2002.120621
11. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. an international working group report. Am J Respir Crit Care Med. (2016) 194:265–75. doi: 10.1164/rccm.201604-0801CI
12. Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. (2007) 176:636–43. doi: 10.1164/rccm.200703-463PP
13. Invernizzi R, Molyneaux PL. The contribution of infection and the respiratory microbiome in acute exacerbations of idiopathic pulmonary fibrosis. Eur Respir Rev. (2019) 28:190045. doi: 10.1183/16000617.0045-2019
14. Manfredi A, Sebastiani M, Cerri S, Vacchi C, Tonelli R, Della Casa G, et al. Acute exacerbation of interstitial lung diseases secondary to systemic rheumatic diseases: a prospective study and review of the literature. J Thorac Dis. (2019) 11:1621–8. doi: 10.21037/jtd.2019.03.28
15. Papanikolaou IC, Drakopanagiotakis F Polychronopoulos vs. Acute exacerbations of interstitial lung diseases. Curr Opin Pulm Med. (2010) 16:480–6. doi: 10.1097/MCP.0b013e32833ae49d
16. Sato T, Teramukai S, Kondo H, Watanabe A, Ebina M, Kishi K, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. (2014) 147:1604–11.e3. doi: 10.1016/j.jtcvs.2013.09.050
17. Joyce SM. Sputum analysis and culture. Ann Emerg Med. (1986) 15:325–8. doi: 10.1016/S0196-0644(86)80576-5
18. Chastre J, Fagon JY, Bornet-Lecso M, Calvat S, Dombret MC, al Khani R, et al. Evaluation of bronchoscopic techniques for the diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. (1995) 152:231–40. doi: 10.1164/ajrccm.152.1.7599829
19. Isenberg DA, Allen E, Farewell V, Ehrenstein MR, Hanna MG, Lundberg IE, et al. International consensus outcome measures for patients with idiopathic inflammatory myopathies. Development and initial validation of myositis activity and damage indices in patients with adult onset disease. Rheumatology. (2004) 43:49–54. doi: 10.1093/rheumatology/keg427
20. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. (2014) 370:2071–82. doi: 10.1056/NEJMoa1402584
21. Ohshimo S, Ishikawa N, Horimasu Y, Hattori N, Hirohashi N, Tanigawa K, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med. (2014) 108:1031–9. doi: 10.1016/j.rmed.2014.04.009
22. Judge EP, Fabre A, Adamali HI, Egan JJ. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J. (2012) 40:93–100. doi: 10.1183/09031936.00115511
23. Kakugawa T, Sakamoto N, Sato S, Yura H, Harada T, Nakashima S, et al. Risk factors for an acute exacerbation of idiopathic pulmonary fibrosis. Respir Res. (2016) 17:79. doi: 10.1186/s12931-016-0400-1
24. Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. (2011) 37:356–63. doi: 10.1183/09031936.00159709
25. Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. (2006) 27:143–50. doi: 10.1183/09031936.06.00114004
26. Toyoda Y, Hanibuchi M, Kishi J, Kawano H, Morizumi S, Sato S, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia associated with connective tissue disease. J Med Invest. (2016) 63:294–9. doi: 10.2152/jmi.63.294
27. Hozumi H, Nakamura Y, Johkoh T, Sumikawa H, Colby TV, Kono M, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open. (2013) 3:e003132. doi: 10.1136/bmjopen-2013-003132
28. Tomiyama F, Watanabe R, Ishii T, Kamogawa Y, Fujita Y, Shirota Y, et al. High prevalence of acute exacerbation of interstitial lung disease in japanese patients with systemic sclerosis. Tohoku J Exp Med. (2016) 239:297–305. doi: 10.1620/tjem.239.297
29. Leuschner G, Behr J. Acute exacerbation in interstitial lung disease. Front Med. (2017) 4:176. doi: 10.3389/fmed.2017.00176
30. Azadeh N, Moua T, Baqir M, Ryu JH. Treatment of acute exacerbations of interstitial lung disease. Expert Rev Respir Med. (2018) 12:309–13. doi: 10.1080/17476348.2018.1446831
31. Azadeh N, Limper AH, Carmona EM, Ryu JH. The role of infection in interstitial lung diseases: a review. Chest. (2017) 152:842–52. doi: 10.1016/j.chest.2017.03.033
32. Barratt SL, Blythe T, Ourradi K, Jarrett C, Welsh GI, Bates DO, et al. Effects of hypoxia and hyperoxia on the differential expression of VEGF-A isoforms and receptors in Idiopathic Pulmonary Fibrosis (IPF). Respir Res. (2018) 19:9. doi: 10.1186/s12931-017-0711-x
33. Kim JS, Podolanczuk AJ, Borker P, Kawut SM, Raghu G, Kaufman JD, et al. Obstructive sleep apnea and subclinical interstitial lung disease in the multi-ethnic study of atherosclerosis (MESA). Ann Am Thorac Soc. (2017) 14:1786–95. doi: 10.1513/AnnalsATS.201701-091OC
34. Braun RK, Broytman O, Braun FM, Brinkman JA, Clithero A, Modi D, et al. Chronic intermittent hypoxia worsens bleomycin-induced lung fibrosis in rats. Respir Physiol Neurobiol. (2018) 256:97–108. doi: 10.1016/j.resp.2017.04.010
35. Alexanderson H, Lundberg IE. Disease-specific quality indicators, outcome measures and guidelines in polymyositis and dermatomyositis. Clin Exp Rheumatol. (2007) 25(6 Suppl 47):153–8.
36. Akiyama M, Kaneko Y, Yamaoka K, Kondo H, Takeuchi T. Association of disease activity with acute exacerbation of interstitial lung disease during tocilizumab treatment in patients with rheumatoid arthritis: a retrospective, case-control study. Rheumatol Int. (2016) 36:881–9. doi: 10.1007/s00296-016-3478-3
37. Rider LG, Aggarwal R, Machado PM, Hogrel JY, Reed AM, Christopher-Stine L, et al. Update on outcome assessment in myositis. Nat Rev Rheumatol. (2018) 14:303–18. doi: 10.1038/nrrheum.2018.33
38. Marchioni A, Tonelli R, Ball L, Fantini R, Castaniere I, Cerri S, et al. Acute exacerbation of idiopathic pulmonary fibrosis: lessons learned from acute respiratory distress syndrome? Crit Care. (2018) 22:80. doi: 10.1186/s13054-018-2002-4
39. Papiris SA, Tomos IP, Karakatsani A, Spathis A, Korbila I, Analitis A, et al. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine. (2018) 102:168–72. doi: 10.1016/j.cyto.2017.08.019
40. Shu XM, Lu X, Xie Y, Wang GC. Clinical characteristics and favorable long-term outcomes for patients with idiopathic inflammatory myopathies: a retrospective single center study in China. BMC Neurol. (2011) 11:143. doi: 10.1186/1471-2377-11-143
41. Enomoto N, Oyama Y, Enomoto Y, Mikamo M, Karayama M, Hozumi H, et al. Prognostic evaluation of serum ferritin in acute exacerbation of idiopathic pulmonary fibrosis. Clin Respir J. (2018) 12:2378–89. doi: 10.1111/crj.12918
42. Takao S, Masuda T, Yamaguchi K, Sakamoto S, Horimasu Y, Nakashima T, et al. High preoperative C-reactive protein level is a risk factor for acute exacerbation of interstitial lung disease after non-pulmonary surgery. Medicine. (2019) 98:14296. doi: 10.1097/MD.0000000000014296
43. Mukae H, Ishimoto H, Sakamoto N, Hara S, Kakugawa T, Nakayama S, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. (2009) 136:1341–7. doi: 10.1378/chest.08-2740
44. Saketkoo LA, Ascherman DP, Cottin V, Christopher-Stine L, Danoff SK, Oddis CV. Interstitial lung disease in idiopathic inflammatory myopathy. Curr Rheumatol Rev. (2010) 6:108–19. doi: 10.2174/157339710791330740
45. Suda T, Fujisawa T, Enomoto N, Nakamura Y, Inui N, Naito T, et al. Interstitial lung diseases associated with amyopathic dermatomyositis. Eur Respir J. (2006) 28:1005–12. doi: 10.1183/09031936.06.00038806
46. Ito M, Kaise S, Suzuki S, Kazuta Y, Sato Y, Miyata M, et al. Clinico-laboratory characteristics of patients with dermatomyositis accompanied by rapidly progressive interstitial lung disease. Clin Rheumatol. (1999) 18:462–7. doi: 10.1007/s100670050139
47. Atochina EN, Beck JM, Preston AM, Haczku A, Tomer Y, Scanlon ST, et al. Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species. Infect Immun. (2004) 72:6002–11. doi: 10.1128/IAI.72.10.6002-6011.2004
48. Marie I, Hachulla E, Cherin P, Hellot MF, Herson S, Levesque H, et al. Opportunistic infections in polymyositis and dermatomyositis. Arthritis Rheum. (2005) 53:155–65. doi: 10.1002/art.21083
49. Suzuki A, Kimura T, Kataoka K, Matsuda T, Yokoyama T, Mori Y, et al. Acute exacerbation of idiopathic pulmonary fibrosis triggered by Aspergillus empyema. Respir Med Case Rep. (2018) 23:103–6. doi: 10.1016/j.rmcr.2018.01.004
50. Ota M, Iwasaki Y, Harada H, Sasaki O, Nagafuchi Y, Nakachi S, et al. Efficacy of intensive immunosuppression in exacerbated rheumatoid arthritis-associated interstitial lung disease. Mod Rheumatol. (2017) 27:22–8. doi: 10.3109/14397595.2016.1173816
51. Huapaya JA, Silhan L, Pinal-Fernandez I, Casal-Dominguez M, Johnson C, Albayda J, et al. Long-term treatment with azathioprine and mycophenolate mofetil for myositis-related interstitial lung disease. Chest. (2019) 156:896–906. doi: 10.1016/j.chest.2019.05.023
52. So H, Wong VTL, Lao VWN, Pang HT, Yip RML. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin Rheumatol. (2018) 37:1983–9. doi: 10.1007/s10067-018-4122-2
53. Tokunaga K, Hagino N. Dermatomyositis with rapidly progressive interstitial lung disease treated with rituximab: a report of 3 cases in Japan. Internal Med. (2017) 56:1399–403. doi: 10.2169/internalmedicine.56.7956
54. Kawashiri SY, Kawakami A, Sakamoto N, Ishimatsu Y, Eguchi K. A fatal case of acute exacerbation of interstitial lung disease in a patient with rheumatoid arthritis during treatment with tocilizumab. Rheumatol Int. (2012) 32:4023–6. doi: 10.1007/s00296-010-1525-z
55. Matsumoto T, Iwano S, Takahashi N, Asai S, Watanabe T, Asai N, et al. Association between chest computed tomography findings and respiratory adverse events in rheumatoid arthritis patients undergoing long-term biological therapy. Int J Rheum Dis. (2019) 22:626–35. doi: 10.1111/1756-185X.13434
56. Maher TM, Whyte MK, Hoyles RK, Parfrey H, Ochiai Y, Mathieson N, et al. Development of a consensus statement for the definition, diagnosis, and treatment of acute exacerbations of idiopathic pulmonary fibrosis using the delphi technique. Adv Ther. (2015) 32:929–43. doi: 10.1007/s12325-015-0249-6
57. Kawamura K, Ichikado K, Suga M, Yoshioka M. Efficacy of azithromycin for treatment of acute exacerbation of chronic fibrosing interstitial pneumonia: a prospective, open-label study with historical controls. Respiration. (2014) 87:478–84. doi: 10.1159/000358443
58. Shulgina L, Cahn AP, Chilvers ER, Parfrey H, Clark AB, Wilson EC, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax. (2013) 68:155–62. doi: 10.1136/thoraxjnl-2012-202403
Keywords: interstitial lung disease, dermatomyositis, polymyositis, complication, outcome
Citation: Liang J, Cao H, Ke Y, Sun C, Chen W and Lin J (2020) Acute Exacerbation of Interstitial Lung Disease in Adult Patients With Idiopathic Inflammatory Myopathies: A Retrospective Case-Control Study. Front. Med. 7:12. doi: 10.3389/fmed.2020.00012
Received: 31 August 2019; Accepted: 13 January 2020;
Published: 31 January 2020.
Edited by:
Maximilian F. Konig, Division of Rheumatology, Johns Hopkins Medicine, United StatesReviewed by:
Cheng-De Yang, Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, ChinaCopyright © 2020 Liang, Cao, Ke, Sun, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Lin, bGluamluemp1QHpqdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.