- 1Department of Rheumatology and Immunology, Shanghai Changzheng Hospital, The Second Military Medical University, Shanghai, China
- 2Department of Radiology, Shanghai Changzheng Hospital, The Second Military Medical University, Shanghai, China
- 3Beijing Tsinghua Chang Gung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
- 4Peking-Tsinghua Center for Life Sciences, Tsinghua University, Beijing, China
Background: Ankylosing spondylitis (AS) is a rheumatic inflammatory disease with unknown etiology, and fatigue is one of the main systemic symptoms of AS. The aim of the current study was to explore the mechanism of AS-associated fatigue (ASF) from multiple aspects, including neuropsychological changes.
Method: A total of 120 AS patients and 78 age- and sex-matched healthy individuals were recruited into the study. Fatigue was assessed by the fatigue item of the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and the Multidimensional Assessment of Fatigue (MAF) scale. Anxiety and depression were assessed by the Hospital Anxiety and Depression Scale (HADS). The cortical thickness and subcortical gray matter volume were assessed using a Philips Achieva 3.0 T TX MRI scanner.
Result: Of the 120 AS patients, 103 (85.8%) reported varying degrees of fatigue. Among these fatigue cases, 33 (32.0%) were in the severe fatigue group (BASDAI-Fatigue ≥ 5), and 70 patients (68.0%) were considered to be in the mild fatigue group (BASDAI-Fatigue > 0 but <5). The BASDAI, ASDAS-CRP, HAD-A, and HAD-D scores of AS patients in the severe fatigue group were all significantly higher than those of patients in the mild fatigue and non-fatigue groups (all, P < 0.05). The structural equation model suggested that AS activity triggered the occurrence of fatigue by inducing psychological change. Finally, head MRI imaging found that the left thalamus volume in AS patients with severe fatigue was significantly larger than that in non-fatigue AS patients and healthy controls (both, P < 0.05).
Conclusion: The study revealed neuropsychological factors involved in fatigue in AS.
Introduction
Ankylosing spondylitis (AS) is a rheumatic inflammatory disease with unknown etiology. Pathologically, AS mainly involves the middle axial bone, as represented by sacroiliitis and enthesitis (1). Clinically, pain, morning stiffness, and fatigue are considered the most common symptoms in AS patients, of which fatigue is a main systemic symptom, with an incidence of 50–70% (2, 3). Fatigue is one important factor contributing to the unsatisfactory treatment outcome and poor quality of life, or even disability, in AS patients. At present, there is no effective treatment for AS-associated fatigue (ASF). Furthermore, it was reported that only 60% of pain and 35% of fatigue can be relieved in AS patients who received biological treatment. Therefore, clinically, how to improve the symptom of fatigue in AS patients remains a challenge.
The etiology of ASF is complex and remains elusive. Previous studies suggested that several factors, including physiology, psychology and behavior, may contribute to its pathogenesis (4). One study revealed that anti-depressants could improve symptoms in AS patients with chronic fatigue syndrome, indicating that psychological factors were associated with ASF (5). Other studies claimed inflammation and autoimmunity as the main causes to ASF (6, 7). Hence, the aim of the present study was to explore the potential mechanism of ASF by analyzing multiple types of data, including clinical indexes and head MRIs, from recruited AS patients.
Methods
Subjects
A total of 120 AS patients were recruited from the Department of Rheumatology and Immunology of Shanghai Changzheng Hospital (Shanghai, China) from July to December 2017. The AS diagnosis was made according to the 1984 modified New York Criteria (8). All patients with any other diseases that may cause fatigue, such as fibromyalgia, malignant tumors or other chronic diseases were excluded from this study. In addition, 78 age- and sex-matched healthy individuals were recruited as controls. Moreover, 10 AS patients with severe fatigue [Bath Ankylosing Spondylitis Disease Activity Index-Fatigue (BASDAI-Fatigue) ≥ 5], 10 AS patients without fatigue (BASDAI-Fatigue = 0), and 6 age- and sex-matched healthy individuals were selected for magnetic resonance imaging (MRI) scanning and analysis. All patients participating in the study were older than 18 years old and provided written informed consent. The research protocol was approved by Shanghai Changzheng Hospital Ethics Committee (approval no 2017SL046).
Data Collection
The clinical data of all participants were collected and summarized in Supplement Table 1. Likewise, the clinicopathological parameters of AS patients, including extra-articular manifestations, C-reactive protein (CRP) levels, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI) score, CRP-based Ankylosing Spondylitis Disease Activity Score (ASDAS-CRP), were also recorded in Supplement Table 2.
Measures of Clinical Variables
To assess the fatigue status of AS patients objectively and effectively, BASDAI scoring was used, which is the most commonly used method and has been verified by many studies (3, 9, 10). Briefly, a BASDAI-Fatigue score ≥ 5 was defined as severe fatigue, a BASDAI-Fatigue score > 0 but <5 was defined as mild fatigue, and a BASDAI-Fatigue score = 0 was defined as non-fatigue. In addition, considering that BASDAI-fatigue scoring could only assess fatigue from a single dimension, the Multidimensional Assessment of Fatigue (MAF), which can evaluate fatigue in AS patients from multiple dimensions, including the degree, severity, distress, the impact on life, and timing, was also used in this study (11, 12). Fatigue in healthy controls was also assessed by the MAF. Moreover, Cronbach's α coefficient was analyzed to determine the internal consistency of the MAF in assessing fatigue in AS patients. A value > 0.6 indicates good reliability, a value > 0.8 indicates very good reliability, and values > 0.9 represent excellent reliability.
Anxiety and depression were assessed by the Hospital Anxiety and Depression Scale (HADS) (13), which consists of 14 items and two dimensions to indicate anxiety or depression. Briefly, a score of 0–7 indicates no anxiety or depression; a score of 8–10 indicates the possibility of anxiety or depression; and a score of 11–21 indicates a high possibility of anxiety or depression.
Neuropathic pain was assessed by a pain detection questionnaire (PDQ) (14), where a score <12 indicates the absence of neuropathic pain; 19 > PDQ ≥ 12 indicates the possibility of neuropathic pain; and a PDQ score ≥ 19 indicates a high possibility of neuropathic pain.
MRI
Head MRI data were acquired using a Philips Achieva 3.0T TX MRI scanner equipped with an 8-channel coil. A whole-brain 3D high-resolution anatomic scan was performed with a T1-weighted 3D Turbo field echo sequence (repetition time 8.1 ms, echo time 3.7 ms, 160 sagittal slices, voxel size 1 × 1 mm, matrix 256 × 256 pixels, field of view 240 × 240 mm, and flip angle 8). Cortical thickness and sub-cortical gray matter volume were calculated by FreeSurfer software (15, 16).
Statistical Analysis
Statistical analysis was performed by SPSS16.0, and the normally distributed data are presented as the mean ± standard deviation (x ± SD). Normally distributed continuous variables were verified by two-sample t-tests, and analysis of variance was used for multi-group differences, and unordered categorical variables were verified by chi-squared tests. The structural equation model was analyzed by Amos 23.0.0, and the relationship between latent variables was analyzed by a structural equation model (SEM).
Results
General Condition of the Patients
According to Supplement Table 1, among the 120 AS patients, 104 were male, and 16 were female. The patients' ages ranged from 18 to 66 years old, with a mean age of 36.4 ± 10.5 years. Overall, the MAF-degree, MAF-severity, and MAF-timing fatigue scores in AS patients in the previous week were significantly higher than those in healthy controls (all, P < 0.01).
Fatigue-Related Factors
After the assessment of fatigue, of the 120 AS patients, 103 (85.8%) reported varying degrees of fatigue. Among these fatigue patients, 33 (32.0%) were in the severe fatigue group (BASDAI-Fatigue ≥ 5), and 70 patients (68.0%) were considered to be in the mild fatigue group (BASDAI-Fatigue>0 but <5). As illustrated in Supplement Table 2, the demographic, social and disease-related data of the three groups were then analyzed. The BASDAI, ASDAS-CRP, anxiety (HAD-A), and depression (HAD-D) scores in the severe fatigue group were significantly higher than those in the mild fatigue and non-fatigue groups (all, P < 0.01).
Using BASDAI and ASDAS-CRP as the disease activity index, HAD-A and HAD-D as the neuropsychological index, and the 5 MAF dimensions as the fatigue index, the SEMs of the indexes were finally analyzed. The model was successfully fitted (probability level = 0.211; CMIN/DF = 1.238; CFI = 0.992; NFI = 0.962; and RMSEA = 0.045). According to the SEM (Figure 1), disease hyperactivity could alter the psychological status (β = 0.61, P = 0.026), and the psychological status could induce the occurrence of fatigue (β = 0.64, P = 0.001), but disease activity did not directly induce the occurrence of fatigue (β = 0.05, P = 0.8). Collectively, these results demonstrated that psychological factors played a critical role in the occurrence of ASF.
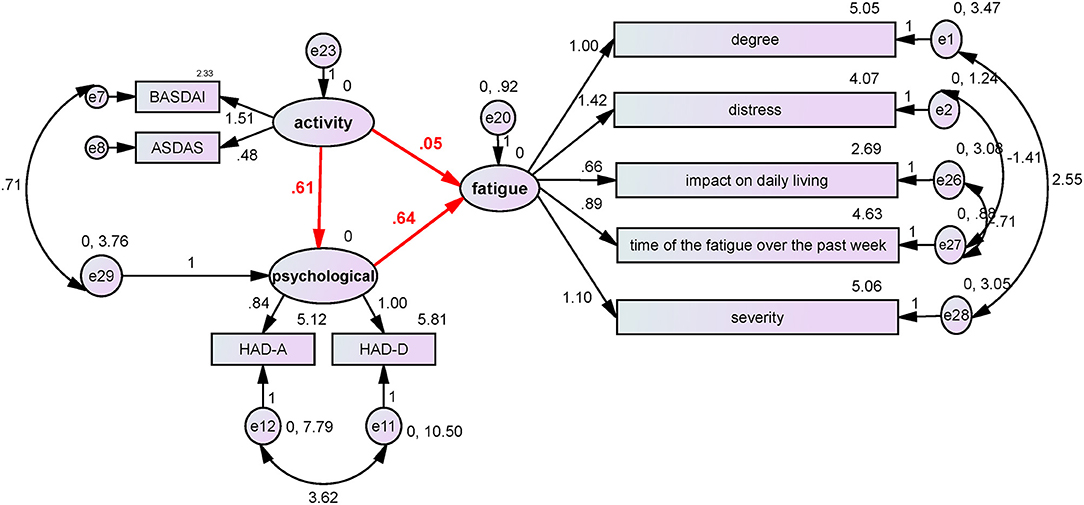
Figure 1. The influence of disease activity and neuropsychological factors on fatigue. BASDAI and ASDAS-CRP were used as the disease activity index, HAD-A and HAD-D were used as the neuropsychological index, and the five MAF items were used as the fatigue index. The structure equation model was successfully established (probability level = 0.211; CMIN/DF = 1.238; CFI = 0.992; NFI = 0.962; and RMSEA = 0.045). According to the SEM, disease hyperactivity could alter the psychological status (β = 0.61, P = 0.026), and the psychological status could induce the occurrence of fatigue (β = 0.64, P = 0.001), but disease activity did not directly induce the occurrence of fatigue (β = 0.05, P = 0.8). The results demonstrated that psychological factors played a critical role in the occurrence of ASF.
Next, the Cronbach's α coefficient of the MAF was analyzed to determine the internal consistency of the MAF in assessing fatigue in AS patients. The results showed that the Cronbach's α coefficient of the MAF in AS was 0.90, suggesting that the MAF had a relatively high internal consistency and reliability.
Imaging Findings
Ten severe AS patients with fatigue (F+), 10 non-fatigue AS (F–) patients and six healthy controls were chosen to participate in the MRI study. As shown in Supplement Table 3, there were no significant differences in age, sex, body mass index (BMI), disease duration, CRP, and PDQ between the groups (all, P > 0.05).
To compare the cortical volume (including that of sense-associated regions, motor-associated regions and the limbic system) and subcortical gray matter volume (including that of the thalamus, putamen, and caudate) segmentation between the three groups, a head MRI scan was performed in these patients. The left thalamus volume of AS patients with severe fatigue (8,407 ± 739.4 mm3) was significantly larger than that in non-fatigue AS patients (7,708 ± 308.1 mm3) and healthy participants (7,481 ± 295.8 mm3) (both, P < 0.05; Figure 2). However, the differences in other indicators were not significant among the three groups.
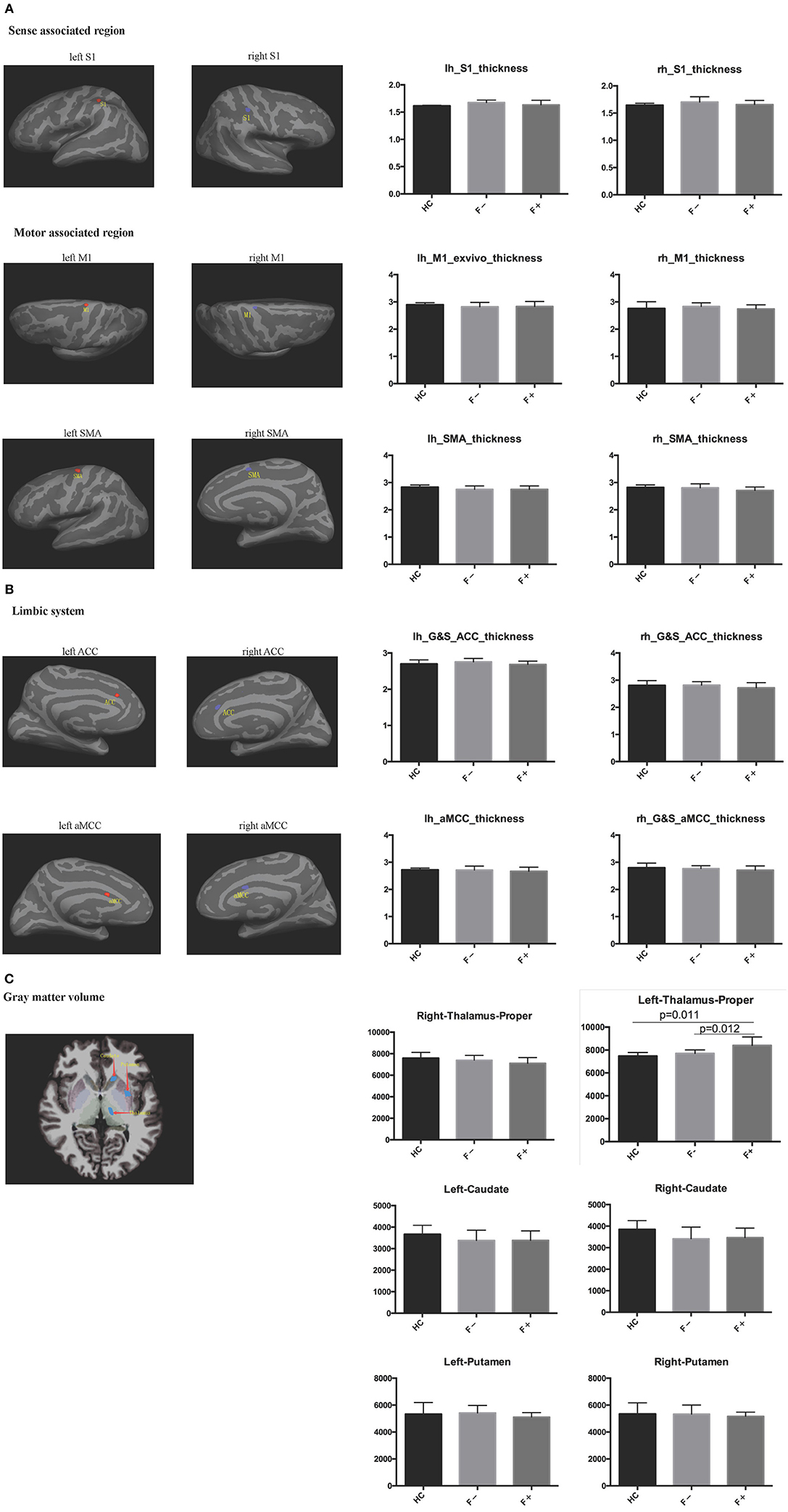
Figure 2. Cortical and subcortical gray matter segmentation was performed in head MRI. (A) Sense-associated regions and motor-associated regions; (B) Limbic system; (C) Gray matter volume. The left thalamus volume in AS patients with severe fatigue was significantly larger than that of non-fatigue AS patients and healthy controls.
Discussion
Fatigue is a self-perception of persistent exhaustion and a decrease in physical and mental abilities, of which the characteristics include tiredness, lack of energy and motivation, and difficulty in concentrating attention on work or decision making (17). In AS patients, fatigue is one of the main systemic symptoms, occurring in 50–70% of cases (2, 3, 12, 18). In the present study, we found that 85.8% of AS patients exhibited varying degrees of fatigue symptoms, including 32.0% patients with severe fatigue. To date, the BASDAI-Fatigue score is the main indicator of ASF (19). However, this scoring system can only reflect a single dimension related to the degree of fatigue. Therefore, the MAF system, which can measure multiple other dimensions such as distress, impact on daily life activities, and timing of fatigue in the previous week, is also used to assess ASF (12) and, therefore, reflects the overall status of ASF in a more comprehensive way. In this study, the results revealed a correlation between the MAF dimensions of degree and severity and the BASDAI-Fatigue score. In addition, the Cronbach's α coefficient of the MAF was 0.90 in AS patients, suggesting that the MAF had a relatively high internal consistency and reliability.
Usually, the occurrence of fatigue in AS patients is considered to be associated with pathological factors, including the decreased ability of skeletomuscular activity due to inflammation, disease activity, and osteoporosis. However, some studies have discovered that complete relief of fatigue was achieved in only 35% of AS patients who received biomedical drugs (20). In our study, there was no significant difference in CRP levels between the severe fatigue, mild fatigue and non-fatigue groups, suggesting that there might be extra-inflammatory factors contributing to the occurrence of ASF, including psychological, social and demographic factors (21). Further study of the structural equation model showed that psychological changes played a key role in the occurrence of ASF. As such, we speculated that psychological changes might directly trigger the occurrence of fatigue. Alternatively, the impact of disease activity on ASF was through psychological changes. To some extent, this view was partly in line with the finding of Wu et al. (20) that residual fatigue was prominent after treatment with tumor necrosis factor inhibitors, suggesting that part of fatigue pathophysiology is unrelated to disease activity. Fatigue is the subjective perception of patients, which can be affected by pain, pressure, limited spinal mobility, and other factors in the assessment of fatigue in AS patients. Other studies have demonstrated that anti-depressants, including tricyclics and selective 5-HT reuptake inhibitors, could markedly improve the symptoms of patients with chronic fatigue syndrome (5, 22). Therefore, the exploration of whether mood regulatory drugs can be used to relieve the symptoms of ASF is warranted.
Fatigue can be classified as either peripheral or central, with peripheral fatigue mainly being related to decreased muscular tone or metabolic abnormalities and central fatigue mainly being related to structural or functional abnormalities of the spinal cord, cerebral cortex or subcortical gray matter; usually, these two forms of fatigue may co-exist in chromic disease-associated fatigue such as ASF (21). Using MRI, some researchers discovered that the cerebral gray matter, specifically areas related to attention and the somatosensory cortex, were thinner in AS patients with severe fatigue; meanwhile, the integrity of the cerebral white matter connecting the abovementioned related areas was reduced (23). Moreover, compared with healthy controls, the cortex in the primary somatosensory area, insular lobe, anterior cingulate and anterior cingulate cortex was thinner, while the volume of the gray matter of the thalamus and putamen was increased (24). In accordance with the previous studies of Wu et al. (23) and Andreasen et al. (25), our results in this study showed that the left thalamus volume in AS patients with severe fatigue was significantly larger than that in AS patients without fatigue and healthy controls. No significant changes were observed in the right thalamus volume or the thickness of the cerebral cortex, which was inconsistent with the findings in the study of Wu et al. (23). One possible reason for this inconsistency is the small sample size in our study. In addition, it has been reported that significantly higher activity in the left thalamus (26) and higher blood flow blood flow in the left thalamus (27) were observed in patients with chronic fatigue syndrome. Therefore, we suggest that the increased left thalamus volume in AS patients is of great significance. In addition, we did not find any significant change in the thickness of the cortex in the primary somatosensory area in AS patients, and we speculated that there was no significant difference in the pain detection score between AS patients with severe fatigue and those without fatigue. Previous studies also demonstrated that inflammation, chronic pain and enterobacteria could induce structural changes in the nervous system and neurotransmitters (28–33). However, few studies have reported structural and functional changes in the nervous system in AS, and the conclusions that they drew are not all consistent. In the present study, we demonstrated that clinical changes in the nervous system played an important role in the subjective symptoms of fatigue in AS patients. However, larger-sample studies or magnetic resonance spectroscopy (MRS) are necessary to verify structural and functional changes in the central nervous system of AS patients.
In conclusion, we analyzed multiple types of data, including clinical indexes and head MRI scans, from recruited AS patients and revealed neuropsychological factors contributing to fatigue in AS, which sheds new light on the pathogenesis of AS fatigue and could provide novel therapeutic strategies for ASF. However, despite these findings, there are still some limitations to our study. For instance, decreased muscle strength and limited spinal joint mobility are very common symptoms in AS patients that may affect the assessment of fatigue in these patients. However, methods for eliminating those effects are currently lacking. In addition, considering economic cost, operation difficulty and the large amount of data, a small number of AS patients were included in the head MRI study. Further studies with more samples should be carried out to verify and expand the research results in the future.
Conclusion
In the current study, we found that the BASDAI, ASDAS-CRP, anxiety (HAD-A), and depression (HAD-D) scores of the severe fatigue group were significantly higher than those of the mild fatigue and non-fatigue groups. In MRI, we further found that the left thalamus volume in severe fatigue AS patients was significantly larger than that in non-fatigue AS patients and healthy participants. Taken together, our study suggests that neuropsychological factors play an important role in the pathogenesis of fatigue in AS.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics committee of Changzheng Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors were involved in drafting the manuscript, revising it critically for important intellectual content, and in the final approval of the publication.
Funding
This work was supported by National Science Foundation of China (Grant 81430031) and China Ministry of Science and Technology (973 Program of China 2014CB541800).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the patients and the investigators who took part in this study. The authors also acknowledge Dong Wei (SAS Company, Shanghai) for data analysis and Zheng Jun and Li Shiqi (East China Normal University) for data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2019.00271/full#supplementary-material
Abbreviations
AS, ankylosing spondylitis; ASF, ankylosing spondylitis-associated fatigue; MRI, magnetic resonance imaging; CRP, C-reactive protein; BASDAI, bath ankylosing spondylitis disease activity index; BASFI, bath ankylosing spondylitis functional index; ASDAS-CRP, CRP-based ankylosing spondylitis disease activity score; MAF, multidimensional assessment of fatigue; HADS, hospital anxiety and depression scale; PDQ (neuropathic pain assessment), pain detection questionnaire; SEM, structural equation model.
References
1. Durmus D, Sarisoy G, Alayli G, Kesmen H, Çetin E, Bilgici A, et al. Psychiatric symptoms in ankylosing spondylitis: their relationship with disease activity, functional capacity, pain and fatigue. Compr Psychiatry. (2015) 62:170–7. doi: 10.1016/j.comppsych.2015.07.016
2. Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A. Ankylosing spondylitis: an overview. Ann Rheum Dis. (2002) 61(Suppl 3):iii8–18. doi: 10.1136/ard.61.suppl_3.iii8
3. Van Tubergen A, Coenen J, Landewé R, Spoorenberg A, Chorus A, Boonen A, et al. Assessment of fatigue in patients with ankylosing spondylitis: a psychometric analysis. Arthritis Care Res. 47:8–16. doi: 10.1002/art1.10179
4. Sparks JA, Costenbader KH. Genetics, environment, and gene-environment interactions in the development of systemic rheumatic diseases. Rheum Dis Clin North Am. (2014) 40:637–57. doi: 10.1016/j.rdc.2014.07.005
5. Chi-Un P, Marks DM, Patkar AA, Masand PS, Patrick L, Alessandro S. Pharmacological treatment of chronic fatigue syndrome: focusing on the role of antidepressants. Expert Opin Pharmacother. (2009) 10:1561. doi: 10.1517/14656560902988510
6. Han R, Yang X, Chen M, Zhang X, Yuan Y, Hu X, et al. Changes and clinical significance of CD8+CD122+ T cells in the peripheral blood of patients with ankylosing spondylitis. Clin Rheumatol. (2017) 37:1–8. doi: 10.1007/s10067-017-3887-z
7. Rezaiemanesh A, Abdolmaleki M, Abdolmohammadi K, Aghaei H, Pakdel FD, Fatahi Y, et al. Immune cells involved in the pathogenesis of ankylosing spondylitis. Biomed Pharmacother. (2018) 100:198. doi: 10.1016/j.biopha.2018.01.108
8. Calin A. Comment on article by van der Linden et al. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. (1985) 28:357. doi: 10.1002/art.1780280321
9. Dernis-Labous E, Messow M, Dougados M. Assessment of fatigue in the management of patients with ankylosing spondylitis. Rheumatology. (2003) 42:1523–8. doi: 10.1093/rheumatology/keg421
10. Yesim A, Altinay Goksel K, Servet A, Yesim K, Nurullah A. A Turkish version of the bath ankylosing spondylitis disease activity index: reliability and validity. Rheumatol Int. (2005) 25:280–4. doi: 10.1007/s00296-003-0432-y
11. Piper BF, Lindsey AM, Dodd MJ, Ferketich S, Paul SM, Weller S. The development of an instrument to measure the subjective dimension of fatigue. Medicine. (1989) 1:199–208. doi: 10.1007/978-1-349-13397-0_25
12. Aissaoui N, Rostom S, Hakkou J, Ghziouel K, Berrada BR, Abouqal R, et al. Fatigue in patients with ankylosing spondylitis: prevalence and relationships with disease-specific variables, psychological status, and sleep disturbance. Rheumatol Int. (2012) 32:2117–24. doi: 10.1007/s00296-011-1928-5
13. Stern AF. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361. doi: 10.1111/j.1600-0447.1983.tb09716.x
14. Rainer F, Ralf B, Ulrich G, TöLle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. (2006) 22:1911–20. doi: 10.1185/030079906X132488
15. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. (1999) 9:179–94. doi: 10.1006/nimg.1998.0395
16. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. (2000) 97:11050–5. doi: 10.1073/pnas.200033797
17. Lewis G, Wessely S. The epidemiology of fatigue: more questions than answers. J Epidemiol Commun Health. (1992) 46:92–7. doi: 10.1136/jech.46.2.92
18. Emilce Edith S, María Florencia M, Fernando DP, José Antonio MC, Gustavo C. Fatigue assessment and its impact in the quality of life of patients with ankylosing spondylitis. Clin Rheumatol. (2015) 34:497–501. doi: 10.1007/s10067-014-2682-3
19. Yacoub YI, Amine B, Laatiris A, Abouqal R, Hajjaj-Hassouni N. Assessment of fatigue in Moroccan patients with ankylosing spondylitis. Clin Rheumatol. (2010) 29:1295–9. doi: 10.1007/s10067-010-1558-4
20. Wu Q, Inman RD, Davis KD. Tumor necrosis factor inhibitor therapy in ankylosing spondylitis: differential effects on pain and fatigue and brain correlates. Pain. (2015) 156:297–304. doi: 10.1097/01.j.pain.0000460310.71572.16
22. Chi-Un P, Prakash M. Duloxetine: a new psychopharmacologic treatment option for fibromyalgia? Curr Psychiatry Rep. (2008) 10:237.
23. Wu Q, Inman RD, Davis KD. Fatigue in ankylosing spondylitis is associated with the brain networks of sensory salience and attention. Arthr Rheumatol. (2014) 66:295–303. doi: 10.1002/art.38244
24. Qi W, Inman RD, Davis KD. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthr Rheumatol. (2013) 65:1494–503. doi: 10.1002/art.37920
25. Andreasen AK, Jakobsen J, Soerensen L, Andersen H, Petersen T, Bjarkam CR, et al. Regional brain atrophy in primary fatigued patients with multiple sclerosis. Neuroimage. (2010) 50:608–15. doi: 10.1016/j.neuroimage.2009.12.118
26. Cook DB, O'Connor PJ, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. NeuroImage. (2007) 36:108–22. doi: 10.1016/j.neuroimage.2007.02.033
27. Tomoda A, Miike T, Yamada E, Honda H, Moroi T, Ogawa M, et al. Chronic fatigue syndrome in childhood–brain and development. Brain Dev. (2000) 22:60–4. doi: 10.1016/S0387-7604(99)00111-4
28. Schmidt-Wilcke T, Leinisch E, Gänßbauer S, Draganski B, Bogdahn U, Altmeppen J, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. (2006) 125:89–97. doi: 10.1016/j.pain.2006.05.004
29. Marco P, Josef P, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. (2011) 14:1227–35. doi: 10.1038/nn.2923
30. Seminowicz DA, Wideman TH, Lina N, Zeinab HK, Summaya F, Ware MA, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. (2011) 31:7540. doi: 10.1523/JNEUROSCI.5280-10.2011
31. Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. γ-aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. (2012) 113:411–7. doi: 10.1111/j.1365-2672.2012.05344.x
32. Michal S, Jonathan K, Serge R, Alexandre P. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci. (2013) 33:17587–96. doi: 10.1523/JNEUROSCI.3241-13.2013
Keywords: ankylosing spondylitis, fatigue, neuropsychological factors, gray matter, MRI
Citation: Li T, Zhou L, Zhao H, Song J, Wang X, Liu S and Xu H (2019) Fatigue in Ankylosing Spondylitis Is Associated With Psychological Factors and Brain Gray Matter. Front. Med. 6:271. doi: 10.3389/fmed.2019.00271
Received: 26 June 2019; Accepted: 05 November 2019;
Published: 21 November 2019.
Edited by:
Fernando Manuel Pimentel-Santos, New University of Lisbon, PortugalReviewed by:
Mauro Waldemar Keiserman, Hospital São Lucas da PUCRS, BrazilAnabela Barcelos, Centro Hospitalar Baixo Vouga, Portugal
Copyright © 2019 Li, Zhou, Zhao, Song, Wang, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huji Xu, eHVodWppQHNtbXUuZWR1LmNu
†These authors share first authorship
‡These authors share senior authorship
 Ting Li
Ting Li Ling Zhou
Ling Zhou Hongbo Zhao
Hongbo Zhao Jing Song
Jing Song Xiuwen Wang
Xiuwen Wang Shiyuan Liu
Shiyuan Liu Huji Xu
Huji Xu