
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 23 October 2019
Sec. Rheumatology
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00238
This article is part of the Research TopicInterstitial Lung Disease in the Context of Systemic Disease: Pathophysiology, Treatment and OutcomesView all 13 articles
Rheumatoid arthritis (RA) is a type of inflammatory arthritis that affects ~1% of the general population. Although arthritis is the cardinal symptom, many extra-articular manifestations can occur. Lung involvement and particularly interstitial lung disease (ILD) is among the most common. Although ILD can occur as part of the natural history of RA (RA-ILD), pulmonary fibrosis has been also linked with methotrexate (MTX); a condition also known as MTX-pneumonitis (M-pneu). This review aims to discuss epidemiological, diagnostic, imaging and histopathological features, risk factors, and treatment options in RA-ILD and M-pneu. M-pneu, usually has an acute/subacute course characterized by cough, dyspnea and fever. Several risk factors, including genetic and environmental factors have been suggested, but none have been validated. The diagnosis is based on clinical and radiologic findings which are mostly consistent with non-specific interstitial pneumonia (NSIP), more so than bronchiolitis obliterans organizing pneumonia (BOOP). Histological findings include interstitial infiltrates by lymphocytes, histiocytes, and eosinophils with or without non-caseating granulomas. Treatment requires immediate cessation of MTX and commencement of glucocorticoids. RA-ILD shares the same symptomatology with M-pneu. However, it usually has a more chronic course. RA-ILD occurs in about 3–5% of RA patients, although this percentage is significantly increased when radiologic criteria are used. Usual interstitial pneumonia (UIP) and NSIP are the most common radiologic patterns. Several risk factors have been identified for RA-ILD including smoking, male gender, and positivity for anti-citrullinated peptide antibodies and rheumatoid factor. Diagnosis is based on clinical and radiologic findings while pulmonary function tests may demonstrate a restrictive pattern. Although no clear guidelines exist for RA-ILD treatment, glucocorticoids and conventional disease modifying antirheumatic drugs (DMARDs) like MTX or leflunomide, as well as treatment with biologic DMARDs can be effective. There is limited evidence that rituximab, abatacept, and tocilizumab are better options compared to TNF-inhibitors.
Rheumatoid arthritis (RA) is the most common inflammatory arthritis with a worldwide prevalence of about 1% and a female predominance of about 3:1 (1). While there are numerous synthetic and biologic disease-modifying antirheumatic drugs (DMARDs) that can halt progression of the articular manifestations of the disease, data on extraarticular manifestations are less conclusive. Over the past few years, the lung has become a major focus in terms of pathophysiology and overall prognosis (2). In clinical practice, there are perceived discrepancies regarding pulmonary toxicity between pulmonologists and rheumatologists, especially regarding methotrexate (MTX) and the potential risks of long-term pulmonary fibrosis. Over the past few years, more evidence has evolved adding to the controversy. To make matters more complex, the pulmonary toxicity of biological therapies is less clear. Therefore, rheumatologists are frequently faced with the situation of how to treat joint manifestations effectively in the presence of interstitial lung disease (ILD) since evidence regarding pulmonary safety is sparse. In this review article, we aim to summarize the available evidence regarding MTX-associated pneumonitis (M-pneu), RA-ILD, and discuss treatment options based on available evidence.
A focused literature review including the keywords “methotrexate,” “pneumonitis,” “interstitial lung disease,” and “rheumatoid arthritis” was performed. In addition, articles from the personal archives of the authors or references from key papers were included if deemed relevant by the authors.
The frequency of M-pneu has been reported to range between 0.3 and 11.6% (3–6), depending on the methodology used and the criteria applied for M-pneu diagnosis. Interestingly, since 2001, no cases of M-pneu have been reported in randomized clinical trials of MTX in RA (7). M-pneu generally has an acute or subacute course and is usually observed within the first year of treatment (8). However, cases of late-onset M-pneu have been also described (9, 10).
Symptomatology mainly pertains to dry cough and dyspnea observed in more than 80% of the patients. Fever also occurs in more than 60% of them (3, 11, 12). Some authors have suggested that mild peripheral blood eosinophilia is present in about 25–40% of patients with sub-acute M-pneu (4, 9–11). Also, in case-series from patients with M-pneu it was demonstrated that peripheral blood lymphocytes dropped at the time of M-pneu and went back to normal after recovery (13). These findings, although very useful in everyday clinical practice, remain to be confirmed in larger studies.
Pathogenic mechanisms underlying M-pneu are unclear. It is considered by many investigators to be a hypersensitivity reaction, while interleukin-8 has been implicated in the pathogenesis (14). It should also be noted that patients receiving MTX are also at an increased risk for developing MTX-related lymphoproliferative disorder (LPD) (15). Interestingly, LPD regresses in many cases after the withdrawal of MTX (15, 16). Recent studies investigating the clinical and histopathologic characteristics of these patients have shown that in half of these cases this is linked to Epstein-Barr virus infection (15, 17) with p38 MAP kinase, PI3 kinase, and MEK pathways being implicated (18). The lung can also be involved in the context of MTX-related LPD (15, 16, 19, 20): Cases of lung lymphomatoid granulomatosis, a rare entity characterized histologically by multiple nodular lesions and vessel wall infiltration by lymphoid cells, have been described (16, 19, 20).
Several risk factors have been identified (Table 1), but it is remains uncertain to what extent they contribute to the occurrence of M-pneu. These factors include: age more than 60 years, diabetes mellitus, hypoalbuminemia, previous use of DMARDs), renal dysfunction, male gender, increased Health Assessment Questionnaire (HAQ) score, decreased pain Visual Analog Scale (VAS) score and pre-existing lung disease (6, 12, 21–23). However, these have not been replicated in other studies (24). Genetic factors might also play a role. In a Japanese population, an association between M-pneu and the HLA-A31:01 haplotype has been described (25). However, in a Genome Wide Association Study in a United Kingdom population, these results were not reproduced, but three Single Nucleotide Polymorphisms (SNPs) have been found to be associated with M-pneu occurrence with borderline significance (26). Environmental factors also possibly contribute. It has been suggested that increased latitude is related to an increased risk for M-pneu development. In fact, Jordan et al. using data from the New Zealand ministry of health showed that the incidence rate ratio for M-pneu was increased by 16% per one degree of increasing latitude (27).
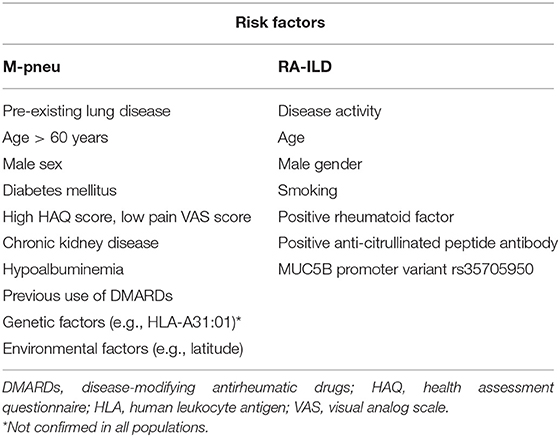
Table 1. Proposed risk factors for the development of methotrexate-associated pneumonitis (M-pneu) and rheumatoid-arthritis-interstitial lung disease (RA-ILD).
A diagnosis of M-pneu is based on the clinical and radiologic findings. Other diagnostic modalities like pulmonary function tests (PFTs) and bronchoalveolar lavage (BAL) might prove to be helpful as well. However, the differential diagnosis, which includes infections, like Pneumocystis jirovecii pneumonia (PJP), viral and atypical pneumonias, and ILD due to RA (RA-ILD), is difficult to be made (11).
Performance of PFTs routinely for diagnostic or prognostic purposes is still under debate (12). Although some studies have demonstrated only a minor effect of MTX on PFTs (28), two prospective studies have found that there are some alterations: Khadadah et al. (29), describe that after 2 years of treatment of low-dose MTX, patients may develop a restrictive pattern with significant decline in total lung capacity (TLC), functional residual capacity (FRC), forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and an increase in the FEV1/FVC ratio. Similarly, Cottin et al. (30), examining 124 patients treated with MTX, described a reduction of FVC, FEV1, and diffusing capacity of the lung for carbon monoxide (DLCO)/alveolar volume (VA). However, these changes could not predict the 3.2% of patients who developed M-pneu in their study (30). On the other hand, Saravanan et al. (8), have suggested that PFT abnormalities [low FEV1, vital capacity (VC) and diffusing transfer of the lung for carbon monoxide (TLCO)] might have a prognostic role, carrying a higher risk for M-pneu development in RA patients.
Of note, in published guidelines for MTX treatment in RA, based on literature review and expert opinion it is stated that PFTs with DLCO should be performed in patients with pre-existing lung disease or current symptoms (low strength of recommendation [D]) (6). In pediatric populations, some studies do not describe any abnormalities in children with juvenile idiopathic arthritis (JIA) treated with MTX (31, 32), while others conclude that there are some alterations in PFTs, like decrease of the mid-mean expiratory flow (MMEF) and DLCO (33, 34) or an increase in the TLC, FRC and residual volume (RV) (35). However, these are not affected by MTX and they were rather attributed to JIA per se. Besides, none of these patients developed clinically significant lung disease in these studies (33).
BAL examination is often performed in these patients. Most investigators agree that a lymphocytic pattern is observed (36), although cases of with BAL neutrophilia have been also reported (10, 37). Lymphocytosis in BAL is not specific for M-pneu as it is also seen in interstitial pneumonitis due to RA (36, 38) and in RA patients treated with MTX without respiratory symptoms (39). A recent systematic literature review examining characteristics of BAL in M-pneu has shown that lymphocytosis was present in the majority (89%) of BAL samples, while high levels of neutrophils were present in only 17% (40). In fact, six cytological patterns were identified (four with predominant lymphocytosis and two in which neutrophilia was the principal finding (40). It has been also suggested that predominance of CD4+ T cells in BAL is suggestive of M-pneu (36) but there is some evidence that an increased CD4/CD8 ratio can also be found in other RA patients, usually those with pulmonary involvement (40). Also, the CD4/CD8 ratio can be found low or normal in about half of the M-pneu patients. Chikura et al. suggested that neutrophils are increased in the BAL of patients with M-pneu having received treatment for <6 months and with a cumulative dose of <300 mg, while the opposite was the case for lymphocyte numbers (41). These results were independent of the indication for which MTX was given (i.e., RA, Primary biliary cholangitis, Psoriatic arthritis, and others). Finally, serum levels of KL-6, a glycoprotein antigen, and surfactant protein D, both expressed mainly by type II pneumocytes, have been proposed as biomarkers for diagnosing and monitoring M-pneu (42). However, they are found to be increased in other lung diseases as well (43), therefore their utility, if any, in the setting of M-pneu remains to be defined.
Transbronchial lung biopsy (TBLB) might also be a useful diagnostic adjunct. In a study evaluating 44 patients with drug-induced lung injury, 75% underwent TBLB (44). TBLB was diagnostically helpful in 75%. Although histopathology alone cannot diagnose M-pneu, it may provide useful supplemental information that can be incorporated with clinical, radiologic, laboratory, and other features in the final diagnosis (44).
Radiological findings reflect the underlying histopathologic process and include mostly non-specific interstitial pneumonia (NSIP), more so than bronchiolitis obliterans organizing pneumonia (BOOP) (45): on chest radiography, M-pneu gives rise to diffuse heterogeneous opacities in NSIP or bilateral scattered heterogeneous or homogeneous opacities with a peripheral distribution in the upper and lower lobes in BOOP. On CT scanning, scattered or diffuse ground-glass opacities are seen in early NSIP and basal fibrosis in the later stages of the disease. In BOOP, poorly defined nodular consolidations, centrilobular nodules, bronchiolitic (tree-in-bud) changes and bronchial dilatation are the dominant features (Figures 1A–C) (6, 46). In a study examining CT findings in M-pneu, it was found that in the majority of the patients, these lesions subsided during a mean follow-up period of 31 days (46).
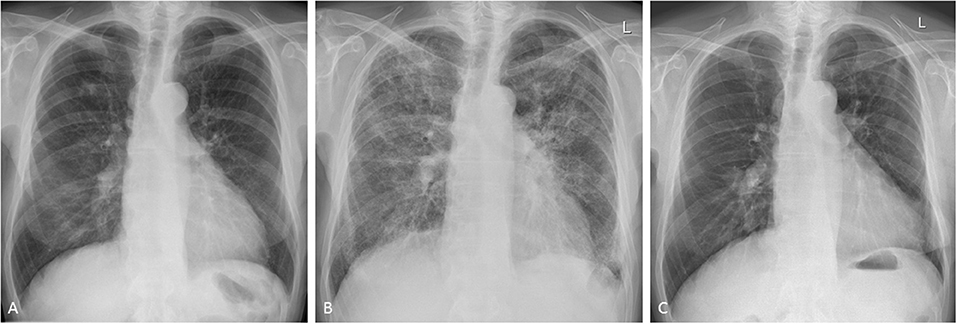
Figure 1. Methotrexate-induced pneumonitis in a 77-year-old man with rheumatoid arthritis. (A) Posterior-anterior chest radiograph immediately before the initiation of treatment. Following 10 days of methotrexate, the patient experienced progressive dyspnea and fever. Follow-up chest radiography showed bilateral heterogeneous opacities in all lung zones. (B) The patient was transferred to the intensive care unit for supportive treatment. High-dose glucocorticoids were administered and gradually withdrawn following clinical and radiological improvement. Initial high-resolution CT scanning showed diffuse infiltrates and bilateral patchy consolidations with only very limited ground-glass opacities (images not shown). (C) Seven months after stopping methotrexate, the changes of pulmonary toxicity had fully resolved.
The most common histopathological pattern observed includes interstitial infiltrates by lymphocytes, histiocytes, and eosinophils with or without granulomas (36). Granulomas, usually non-caseating, are also identified in some patients, while hyperplastic type II pneumocytes and perivascular inflammation are also commonly seen (47). Other patterns have also been described and often coexist with interstitial pneumonitis, such as diffuse and organized alveolar damage (3, 12, 47). The latter seems to be more frequent in acute cases of M-pneu (47).
In suspected M-pneu MTX should be discontinued immediately. Often, treatment with steroids is required (8). Other immunosuppressive drugs, such as cyclophosphamide (CYC), have also been administered successfully (48). Tocilizumab (TCZ), given its efficacy as monotherapy in RA, is also an attractive therapeutic option, since its use has been reported to be beneficial (38).
The prognosis of M-pneu is generally good and most patients recover fully (8), however, mortality is reported to be relatively high reaching 17.6% (6, 11). Other smaller studies have reported even higher figures up to 30% (49). Besides, in a review assessing patients (including individuals with RA) who developed M-pneu, the percentage was 13% (47). Furthermore, a study by Chikura et al. examining 56 RA patients with M-pneu suggested that mortality was more increased in patients who developed pneumonitis after treated with MTX for <6 months compared to those treated for a longer time period (41). It is suggested that this difference in mortality is accompanied by specific histopathologic features and characteristics in the BAL examination (41). Re-introduction of MTX in patients who have developed M-pneu has led to recurrence of lung injury and in many cases to death (11, 49). There are single cases, however, in which the drug has been re-introduced successfully (50).
RA is not merely a disease of the joints. It is a true systemic inflammatory disease with effects on many organs and organ systems. A variety of pulmonary manifestations can be seen in RA including pulmonary nodules, pleural effusions, bronchiectasis, and, most importantly, ILD (2).
ILD is a frequently under-recognized complication of RA. The estimated prevalence is heavily dependent on the ascertainment method used. Bongartz et al. reported a lifetime risk of 7.7%, a 9-fold increase over the general population (51). Studies using the ERAS and ERAN early arthritis cohorts as well as the ILD specific BRILL study in the UK reported a prevalence of RA-ILD of 3–5% (52, 53) (Table 2). All of these studies identified clinical RA-ILD; if screening of asymptomatic individuals with RA is utilized, the prevalence of ILD increases depending on the performance characteristics of the screening methodology used. High resolution CT scanning identifies ILD in 19–67% of RA patients depending on the thresholds for diagnosis employed (54, 55). A study performing unselected histological assessment of pulmonary tissue in RA patients revealed evidence of ILD in 80% of patients (69). For these studies in which ILD was diagnosed based on radiologic and histological data, it should be noted that they probably overestimate clinically relevant RA-ILD. Patients were included irrespective of pulmonary symptoms and many of them had normal PFTs.
Increasing evidence supports a primary role for the lung in initiating RA pathogenesis and RA-ILD may occur prior to the onset of the joint disease (70–72). Known predictors of RA-ILD include RA severity, age, male sex, smoking, and seropositivity for rheumatoid factor or anti-citrullinated peptide antibodies anti-citrullinated peptide antibodies (51, 71) (Table 1). In the past several years, biomarkers for RA-ILD have been suggested: Citrullinated isoforms of heat shock protein 90 (hsp90) have been shown to be potentially useful as a biomarker of RA-ILD (73). Hsp90 could also be identified in BAL specimens (74). Recently, the gain-of-function MUC5B promoter variant rs35705950, has been found to be associated with the development of ILD in RA patients with an Odds Ratio of 3.1 (75). This is especially interesting given that the same MUC5B variant is the strongest known risk factor for idiopathic pulmonary fibrosis (IPF), which shares many similarities with RA-ILD (76).
The clinical findings in RA-ILD are similar to those previously described for M-pneu with dyspnoea and non-productive cough with or without fever predominating (7). Most typically, RA-ILD develops insidiously over time and may be present and asymptomatic for a significant period. This diagnostic delay may be further exacerbated by the fact that patient's rheumatoid joint disease may limit their ability to exercise sufficiently to precipitate exertional dyspnea. Clinical examination findings may be absent in early disease but ultimately the majority of patients will have fine bibasal crepitations (77). The majority of those with an usual interstitial pneumonia (UIP) pattern RA-ILD will also develop clubbing, similar to IPF patients (77). Radiologic findings are of little help in distinguishing the two disorders with a significant degree of overlapping features (46). However, a key distinguishing feature can be chronicity. MTX-pneu is typically a fulminant acute process (11) (Table 2). A more indolent subacute or chronic development of radiologic findings strongly favors RA-ILD. In this scenario, historic radiologic imaging demonstrating evidence of similar but early ILD changes argues against MTX-pneu. However, RA-ILD may present as a fulminant and potentially fatal process, including early in the disease process (2, 78, 79).
The diagnosis of RA-ILD can generally be made by a combination of clinical features as described above and congruent findings on chest imaging. It is important to remember that RA patients are, at least equally, and in often cases more likely, to develop other causes of dyspnea and cough than the general population. For example, the risk of infection, including atypical infections, pulmonary emboli, and lung cancer, are all increased in RA patients (80–82). PFTs may provide evidence of restrictive lung disease with a reduced TLCO/DCLO generally being the first manifestation.
Bronchoscopy and BAL may be performed to rule out other diagnoses. BAL is frequently abnormal in RA-ILD, but the findings are non-specific and rarely diagnostically useful. In rare cases open lung biopsy may be needed to confirm a diagnosis, in general when an alternative diagnosis is suspected.
Apart from treatment-related complications, the thoracic manifestations of RA are plentiful (56) and include pleural changes, large airway involvement and, more so than with other collagen vascular diseases, a usual interstitial pneumonia (UIP) pattern of interstitial lung disease as distinct from a non-specific interstitial pneumonia (NSIP) or other patterns (71, 83) (Table 2). Clinically relevant ILD is less common, comprising basal cystic changes (honey combing), peripheral reticular opacities and bronchioloectasis, best seen on CT scanning, and lower lobe volume loss which may advance in the chronic stage (Figure 2). Bronchiolitis obliterans has been described in RA, while follicular bronchiolitis is more common, showing small nodular changes on CT. Rheumatoid nodules as large as 5 cm are more likely in men, typically occur in smokers and may be seen prior to the articular manifestation of the disease. Nodules may cavitate, occasionally calcify and rarely rupture.
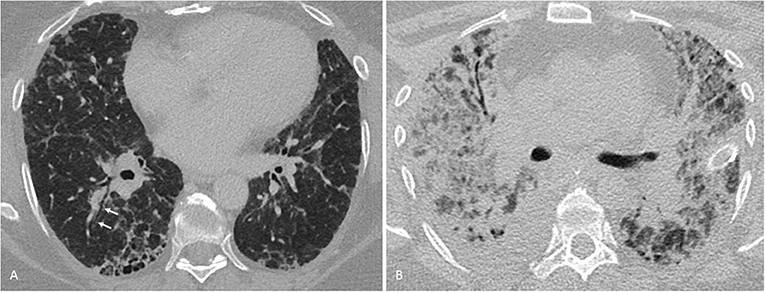
Figure 2. Interstitial lung disease in a 56-year-old woman with rheumatoid arthritis. (A) One millimeter transverse axial CT-section through the lung bases show subpleural honeycombing and early traction bronchiectasis (arrows), consistent with a usual interstitial pneumonia pattern. (B) Nine months later, the patient developed severe dyspnea at rest and required mechanical ventilation. On bronchoalveolar lavage, influenza A virus was found to be present. A follow-up CT now showed a small right-sided pleural effusion and multifocally confluent consolidation, partially obscuring equally patchy bilateral ground-glass opacification. A few thickened septae (crazy-paving pattern) could be delineated (not shown). These findings were consistent with a viral pneumonia. Despite extracorporeal membrane oxygenation therapy, the patient deceased.
Findings on BAL are generally abnormal but non-specific in RA-ILD. Common findings include some form of neutrophil or lymphocytic predominant leucocytosis, or alterations in T-lymphocyte ratios (36, 41, 59, 84–86). Histologic findings are congruent with those seen with the underlying ILD phenotype, including neutrophilic or lymphocytic infiltrates, and fibrotic changes. A number of histopathological findings have been suggested to aid in the differentiation of MTX-pneu from RA-ILD including type II pneumocyte hyperplasia and fibroblast proliferation (11). However, these features have also been reported in RA-ILD.
Glucocorticoids remain an important part of the acute management of RA-ILD. The optimum longer-term management of RA-ILD is uncertain, however, given the known factors predictive of RA-ILD described above it is logical that good RA disease control should be the cornerstone of any strategy (61). This is supported by the significant decline in the reported frequency of RA-ILD as RA treatment options have advanced (87). Given its proven efficacy in RA joint disease there is good reason to expect that MTX may be a justified part of any treatment strategy in an RA patient with ILD; evidence to support this strategy is beginning to emerge (60, 88). Despite previous concerns over potential pulmonary toxicity with leflunomide, this agent also appears to be potentially beneficial for RA-ILD (62). In the setting of RA-ILD, the choice of biological therapy is not clear: A recent review of the literature identified seven studies and 28 case reports, which showed an increased mortality with the use of tumor necrosis factor-inhibitors (TNF-i) (63). In this analysis, female sex and longer disease duration were associated with ILD onset or worsening (63). The heterogeneity in the reported outcome measures was too large to draw any firm conclusions. Other agents, such as Abatacept (ATC) have been investigated in few studies: In a Japanese study, deterioration of RA-ILD was described in 11 of 131 patients (8.4%) and was associated with concomitant MTX use (Odds Ratio of 12.75) (89). By contrast, a multicentric analysis from Spain concluded that ATC was associated with stable ILD in about two thirds of the patients (64). The role of TCZ in RA-ILD is less clear. A retrospective study in Japan showed worsening of ILD with TCZ in only six of 78 patients (7.7%) (65) or even improvement (38). These findings are in line with data from clinical trials in Systemic sclerosis (90), where it has been shown to preserve lung function, although this was not the primary endpoint.
Preliminary evidence of a particular role for Rituximab (RTX) is beginning to emerge (66, 67, 91, 92). An observational study of 56 patients with RA-ILD treated with RTX showed that 16% improved and 52% remained stable; a particularly impressive response given the aggressive natural history of RA-ILD (11, 66). This is logical given the association of RA-ILD with other known predictors of Rituximab response, in particular seropositivity (68).
Other agents are currently under investigation in the treatment of RA-ILD: The anti-fibrotic tyrosine kinase inhibitor nintedanib has been shown to be effective in an animal model of RA-ILD; the same agent has demonstrable efficacy in RCTs in IPF and, recently, also in systemic sclerosis (93–95). Another anti-fibrotic agent, pirfenidone, has been shown to downregulate profibrotic pathways in a bleomycin-induced mouse model and lung biopsy specimens from RA-ILD patients (96). Figure 3 depicts our proposed treatment approach to the treatment of pulmonary manifestations in RA.
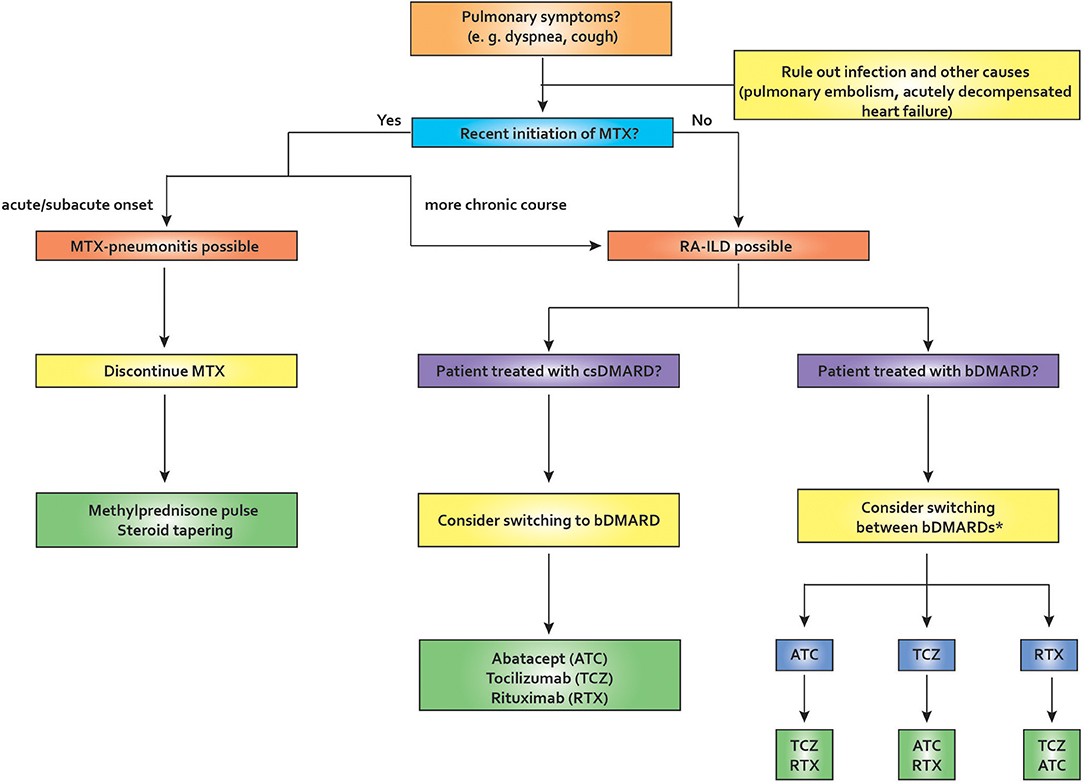
Figure 3. Proposed algorithm for pulmonary symptoms in rheumatoid arthritis. In the setting of recent MTX initiation, MTX-pneu is always a concern, especially if the onset of symptoms is acute or sub-acute. In this case, MTX needs to be stopped and usually glucocorticoid therapy and supportive care in an intensive care unit is required. If the onset is more insidious, RA-ILD is a possibility. After ruling out other causes of pulmonary symptoms, management should depend on various factors, including comorbities, age, disease activity, and others. If a patient is diagnosed as having RA-ILD and receives a csDMARD, switching to a bDMARD may be appropriate. If a patient is already on bDMARD therapy, switching therapies may be required. Many authors tend to avoid TNF-inhibitors in this situation, but the evidence is weak. ATC, abatacept; bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; ILD, interstitial lung disease; MTX, methotrexate; MTX-pneu, MTX-pneumonitis; RA, rheumatoid arthritis; RTX, rituximab; TCZ, tocilizumab. *TNF-inhibitors have been reported to be associated with worsening lung function in RA-ILD (weak evidence level).
ILD in general has a poor prognosis, however, this is even more true of RA-ILD, which has an ominous prognosis with a Hazard Ratio (HR) for death of 2.86 (51). Overall, respiratory causes are the second most common cause of death in patients with RA; symptomatic RA-ILD contributes 13% of the excess mortality associated with RA (51, 53, 97). Median survival following a diagnosis of RA-ILD is <3 years (2, 97). Acute fulminant RA-ILD occurring rapidly following disease onset is well-documented and frequently fatal (2, 78, 79). RA-ILD patients with a UIP pattern on imaging have increased mortality compared to other patterns, with a relative risk of 2.39 for UIP compared to NSIP (98). As well as the inherent mortality associated with RA-ILD itself, these patients are also at significantly increased risk of pulmonary infection (71, Figure 2).
Methotrexate pneumonitis usually presents acutely but its incidence has been decreasing over time. Suspension of MTX and administration of glucocorticoid pulse therapy are usually required. In the long term, MTX therapy may associate with a lower incidence of RA-ILD, thus questioning the fear of progressive pulmonary fibrosis associated with this agent. Regarding bDMARDs, ATC, TCZ, or RTX appear more promising than TNF-i in patients requiring more intense immunosuppression although the evidence base for this remains weak.
Future studies should aim at determining the exact prevalence of RA-ILD in early stage RA patients and will certainly rely on PFTs and imaging with CT at baseline and during the disease course to help identify patients at high risk for progression.
GF, EN, and RC wrote the first draft of the manuscript. PK edited and revised the manuscript and drafted the figures. JL edited the manuscript and contributed figures. All authors revised the manuscript critically and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. (2018) 4:18001. doi: 10.1038/nrdp.2018.1
2. Shaw M, Collins BF, Ho LA, Raghu G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev. (2015) 24:1–16. doi: 10.1183/09059180.00008014
3. Atzeni F, Boiardi L, Sallì S, Benucci M, Sarzi-Puttini P. Lung involvement and drug-induced lung disease in patients with rheumatoid arthritis. Expert Rev Clin Immunol. (2013) 9:649–57. doi: 10.1586/1744666X.2013.811173
4. Barrera P, Laan RF, van Riel PL, Dekhuijzen PN, Boerbooms AM, van de Putte LB. Methotrexate-related pulmonary complications in rheumatoid arthritis. Ann Rheum Dis. (1994) 53:434–9. doi: 10.1136/ard.53.7.434
5. Dawson JK, Graham DR, Desmond J, Fewins HE, Lynch MP. Investigation of the chronic pulmonary effects of low-dose oral methotrexate in patients with rheumatoid arthritis: a prospective study incorporating HRCT scanning and pulmonary function tests. Rheumatology. (2002) 41:262–7. doi: 10.1093/rheumatology/41.3.262
6. Pavy S, Constantin A, Pham T, Gossec L, Maillefert J-F, Cantagrel A, et al. Methotrexate therapy for rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion. Joint Bone Spine. (2006) 73:388–95. doi: 10.1016/j.jbspin.2006.01.007
7. Conway R, Low C, Coughlan RJ, O'Donnell MJ, Carey JJ. Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol. (2014) 66:803–12. doi: 10.1002/art.38322
8. Saravanan V, Kelly CA. Reducing the risk of methotrexate pneumonitis in rheumatoid arthritis. Rheumatology. (2004) 43:143–7. doi: 10.1093/rheumatology/keg466
9. Salehi M, Miller R, Khaing M. Methotrexate-induced Hypersensitivity Pneumonitis appearing after 30 years of use: a case report. J Med Case Rep. (2017) 11:174. doi: 10.1186/s13256-017-1333-0
10. Yamakawa H, Yoshida M, Takagi M, Kuwano K. Late-onset methotrexate-induced pneumonitis with neutrophilia in bronchoalveolar lavage fluid. BMJ Case Rep. (2014) 2014:bcr2014206123. doi: 10.1136/bcr-2014-206123
11. Kremer JM, Alarcón GS, Weinblatt ME, Kaymakcian MV, Macaluso M, Cannon GW, et al. Clinical, laboratory, radiographic, and histopathologic features of methotrexate-associated lung injury in patients with rheumatoid arthritis: a multicenter study with literature review. Arthritis Rheum. (1997) 40:1829–37. doi: 10.1002/art.1780401016
12. Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum. (2014) 43:613–26. doi: 10.1016/j.semarthrit.2013.09.005
13. Inokuma S, Kono H, Kohno Y, Hiramatsu K, Ito K, Shiratori K, et al. Methotrexate-induced lung injury in patients with rheumatoid arthritis occurs with peripheral blood lymphocyte count decrease. Ann Rheum Dis. (2006) 65:1113–4. doi: 10.1136/ard.2005.045211
14. Fujimori Y, Kataoka M, Tada S, Takehara H, Matsuo K, Miyake T, et al. The role of interleukin-8 in interstitial pneumonia. Respirology. (2003) 8:33–40. doi: 10.1046/j.1440-1843.2003.00420.x
15. Yamakawa H, Yoshida M, Katagi H, Hirooka S, Okuda K, Ishikawa T, et al. Pulmonary and retroperitoneal lesions induced by methotrexate-associated lymphoproliferative disorder in a patient with rheumatoid arthritis. Mod Rheumatol. (2016) 26:441–4. doi: 10.3109/14397595.2014.898559
16. Kameda H, Okuyama A, Tamaru J-I, Itoyama S, Iizuka A, Takeuchi T. Lymphomatoid granulomatosis and diffuse alveolar damage associated with methotrexate therapy in a patient with rheumatoid arthritis. Clin Rheumatol. (2007) 26:1585–9. doi: 10.1007/s10067-006-0480-2
17. Kurita D, Miyoshi H, Ichikawa A, Kato K, Imaizumi Y, Seki R, et al. Methotrexate-associated lymphoproliferative disorders in patients with rheumatoid arthritis: clinicopathologic features and prognostic factors. Am J Surg Pathol. (2019) 43:869–84. doi: 10.1097/PAS.0000000000001271
18. Feng W, Cohen JI, Fischer S, Li L, Sneller M, Goldbach-Mansky R, et al. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J Natl Cancer Inst. (2004) 96:1691–702. doi: 10.1093/jnci/djh313
19. Barakat A, Grover K, Peshin R. Rituximab for pulmonary lymphomatoid granulomatosis which developed as a complication of methotrexate and azathioprine therapy for rheumatoid arthritis. Springerplus. (2014) 3:751. doi: 10.1186/2193-1801-3-751
20. Shimada K, Matsui T, Kawakami M, Nakayama H, Ozawa Y, Mitomi H, et al. Methotrexate-related lymphomatoid granulomatosis: a case report of spontaneous regression of large tumours in multiple organs after cessation of methotrexate therapy in rheumatoid arthritis. Scand J Rheumatol. (2007) 36:64–7. doi: 10.1080/03009740600902403
21. Alarcón GS, Kremer JM, Macaluso M, Weinblatt ME, Cannon GW, Palmer WR, et al. Risk factors for methotrexate-induced lung injury in patients with rheumatoid arthritis. A multicenter, case-control study. Methotrexate-Lung Study Group. Ann Intern Med. (1997) 127:356–64. doi: 10.7326/0003-4819-127-5-199709010-00003
22. Ohosone Y, Okano Y, Kameda H, Fujii T, Hama N, Hirakata M, et al. Clinical characteristics of patients with rheumatoid arthritis and methotrexate induced pneumonitis. J Rheumatol. (1997) 24:2299–303.
23. Shidara K, Hoshi D, Inoue E, Yamada T, Nakajima A, Taniguchi A, et al. Incidence of and risk factors for interstitial pneumonia in patients with rheumatoid arthritis in a large Japanese observational cohort, IORRA. Mod Rheumatol. (2010) 20:280–6. doi: 10.1007/s10165-010-0280-z
24. Carroll GJ, Thomas R, Phatouros CC, Atchison MH, Leslie AL, Cook NJ, et al. Incidence, prevalence and possible risk factors for pneumonitis in patients with rheumatoid arthritis receiving methotrexate. J Rheumatol. (1994) 21:51–4.
25. Furukawa H, Oka S, Shimada K, Rheumatoid Arthritis-Interstitial Lung Disease Study Consortium, Tsuchiya N, Tohma S. HLA-A*31:01 and methotrexate-induced interstitial lung disease in Japanese rheumatoid arthritis patients: a multidrug hypersensitivity marker? Ann Rheum Dis. (2013) 72:153–5. doi: 10.1136/annrheumdis-2012-201944
26. Bluett J, Owen S-A, Massey J, Alfirevic A, Pirmohamed M, Plant D, et al. HLA-A 31:01 is not associated with the development of methotrexate pneumonitis in the UK population: results from a genome-wide association study. Ann Rheum Dis. (2017) 76:e51. doi: 10.1136/annrheumdis-2017-211512
27. Jordan SR, Stevanovic VR, Herbison P, Dockerty J, Highton J. Methotrexate pneumonitis in rheumatoid arthritis: increased prevalence with increasing latitude: an epidemiological study of trends in new zealand. J Clin Rheumatol. (2011) 17:356–7. doi: 10.1097/RHU.0b013e3182314e34
28. Beyeler C, Jordi B, Gerber NJ, Im Hof V. Pulmonary function in rheumatoid arthritis treated with low-dose methotrexate: a longitudinal study. Br J Rheumatol. (1996) 35:446–52. doi: 10.1093/rheumatology/35.5.446
29. Khadadah ME, Jayakrishnan B, Al-Gorair S, Al-Mutairi M, Al-Maradni N, Onadeko B, et al. Effect of methotrexate on pulmonary function in patients with rheumatoid arthritis–a prospective study. Rheumatol Int. (2002) 22:204–7. doi: 10.1007/s00296-002-0227-6
30. Cottin V, Tébib J, Massonnet B, Souquet PJ, Bernard JP. Pulmonary function in patients receiving long-term low-dose methotrexate. Chest. (1996) 109:933–8. doi: 10.1378/chest.109.4.933
31. Graham LD, Myones BL, Rivas-Chacon RF, Pachman LM. Morbidity associated with long-term methotrexate therapy in juvenile rheumatoid arthritis. J Pediatric. (1992) 120:468–73. doi: 10.1016/S0022-3476(05)80923-0
32. Wallace CA, Bleyer WA, Sherry DD, Salmonson KL, Wedgwood RJ. Toxicity and serum levels of methotrexate in children with juvenile rheumatoid arthritis. Arthritis Rheum. (1989) 32:677–81. doi: 10.1002/anr.1780320604
33. Leiskau C, Thon A, Gappa M, Dressler F. Lung function in children and adolescents with juvenile idiopathic arthritis during long-term treatment with methotrexate: a retrospective study. Clin Exp Rheumatol. (2012) 30:302–7.
34. Schmeling H, Stephan V, Burdach S, Horneff G. Pulmonary function in children with juvenile idiopathic arthritis and effects of methotrexate therapy. Z Rheumatol. (2002) 61:168–72. doi: 10.1007/s003930200025
35. Camiciottoli G, Trapani S, Castellani W, Ginanni R, Ermini M, Falcini F. Effect on lung function of methotrexate and non-steroid anti-inflammatory drugs in children with juvenile rheumatoid arthritis. Rheumatol Int. (1998) 18:11–6. doi: 10.1007/s002960050047
36. Schnabel A, Richter C, Bauerfeind S, Gross WL. Bronchoalveolar lavage cell profile in methotrexate induced pneumonitis. Thorax. (1997) 52:377–9. doi: 10.1136/thx.52.4.377
37. Leduc D, De Vuyst P, Lheureux P, Gevenois PA, Jacobovitz D, Yernault JC. Pneumonitis complicating low-dose methotrexate therapy for rheumatoid arthritis. Discrepancies between lung biopsy and bronchoalveolar lavage findings. Chest. (1993) 104:1620–3. doi: 10.1378/chest.104.5.1620
38. Picchianti Diamanti A, Markovic M, Argento G, Giovagnoli S, Ricci A, Laganà B, et al. Therapeutic management of patients with rheumatoid arthritis and associated interstitial lung disease: case report and literature review. Ther Adv Respir Dis. (2017) 11:64–72. doi: 10.1177/1753465816668780
39. Scherak O, Popp W, Kolarz G, Wottawa A, Ritschka L, Braun O. Bronchoalveolar lavage and lung biopsy in rheumatoid arthritis. In vivo effects of disease modifying antirheumatic drugs. J Rheumatol. (1993) 20:944–9.
40. D'Elia T. Methotrexate-induced pneumonitis: heterogeneity of bronchoalveolar lavage and differences between cancer and rheumatoid arthritis. Inflamm Allergy Drug Targets. (2014) 13:25–33. doi: 10.2174/1871528112666131230013059
41. Chikura B, Sathi N, Lane S, Dawson JK. Variation of immunological response in methotrexate-induced pneumonitis. Rheumatology. (2008) 47:1647–50. doi: 10.1093/rheumatology/ken356
42. Taniguchi K, Usui Y, Matsuda T, Suzuki S, Fujiki K, Yakusiji F, et al. Methotrexate-induced acute lung injury in a patient with rheumatoid arthritis. Int J Clin Pharmacol Res. (2005) 25:101–5.
43. Miyata M, Sakuma F, Fukaya E, Kobayashi H, Rai T, Saito H, et al. Detection and monitoring of methotrexate-associated lung injury using serum markers KL-6 and SP-D in rheumatoid arthritis. Intern Med. (2002) 41:467–73. doi: 10.2169/internalmedicine.41.467
44. Romagnoli M, Bigliazzi C, Casoni G, Chilosi M, Carloni A, Dubini A, et al. The role of transbronchial lung biopsy for the diagnosis of diffuse drug-induced lung disease: a case series of 44 patients. Sarcoidosis Vasc Diffuse Lung Dis. (2008) 25:36–45.
45. Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics. (2000) 20:1245–59. doi: 10.1148/radiographics.20.5.g00se081245
46. Arakawa H, Yamasaki M, Kurihara Y, Yamada H, Nakajima Y. Methotrexate-induced pulmonary injury: serial CT findings. J Thorac Imaging. (2003) 18:231–6. doi: 10.1097/00005382-200310000-00004
47. Imokawa S, Colby TV, Leslie KO, Helmers RA. Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. Eur Respir J. (2000) 15:373–81. doi: 10.1034/j.1399-3003.2000.15b25.x
48. Suwa A, Hirakata M, Satoh S, Mimori T, Utsumi K, Inada S. Rheumatoid arthritis associated with methotrexate-induced pneumonitis: improvement with i.v. cyclophosphamide therapy. Clin Exp Rheumatol. (1999) 17:355–8.
49. Bartram SA. Experience with methotrexate-associated pneumonitis in northeastern England: comment on the article by Kremer et al. Arthritis Rheum. (1998) 41:1327–8.
50. Cook NJ, Carroll GJ. Successful reintroduction of methotrexate after pneumonitis in two patients with rheumatoid arthritis. Ann Rheum Dis. (1992) 51:272–4. doi: 10.1136/ard.51.2.272
51. Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. (2010) 62:1583–91. doi: 10.1002/art.27405
52. Kiely P, Busby AD, Nikiphorou E, Sullivan K, Walsh DA, Creamer P, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open. (2019) 9:e028466. doi: 10.1136/bmjopen-2018-028466
53. Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatology. (2014) 53:1676–82. doi: 10.1093/rheumatology/keu165
54. Bilgici A, Ulusoy H, Kuru O, Celenk C, Unsal M, Danaci M. Pulmonary involvement in rheumatoid arthritis. Rheumatol Int. (2005) 25:429–35. doi: 10.1007/s00296-004-0472-y
55. Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. (2001) 56:622–7. doi: 10.1136/thorax.56.8.622
56. Capobianco J, Grimberg A, Thompson BM, Antunes VB, Jasinowodolinski D, Meirelles GSP. Thoracic manifestations of collagen vascular diseases. Radiographics. (2012) 32:33–50. doi: 10.1148/rg.321105058
57. Searles G, McKendry RJ. Methotrexate pneumonitis in rheumatoid arthritis: potential risk factors. Four case reports and a review of the literature. J Rheumatol. (1987) 14:1164–71.
58. Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. (2007) 4:443–8. doi: 10.1513/pats.200703-045MS
59. Garcia JG, James HL, Zinkgraf S, Perlman MB, Keogh BA. Lower respiratory tract abnormalities in rheumatoid interstitial lung disease. Potential role of neutrophils in lung injury. Am Rev Respir Dis. (1987) 136:811–7. doi: 10.1164/ajrccm/136.4.811
60. Rojas-Serrano J, Herrera-Bringas D, Pérez-Román DI, Pérez-Dorame R, Mateos-Toledo H, Mejía M. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): methotrexate and the severity of lung disease are associated to prognosis. Clin Rheumatol. (2017) 36:1493–500. doi: 10.1007/s10067-017-3707-5
61. Sparks JA, He X, Huang J, Fletcher EA, Zaccardelli A, Friedlander HM, et al. Rheumatoid arthritis disease activity predicting incident clinically-apparent RA-associated interstitial lung disease: A prospective cohort study. Arthritis Rheumatol. (2019) 71:1472–82. doi: 10.1002/art.40904
62. Conway R, Low C, Coughlan RJ, O'Donnell MJ, Carey JJ. Leflunomide use and risk of lung disease in rheumatoid arthritis: a systematic literature review and metaanalysis of randomized controlled trials. J Rheumatol. (2016) 43:855–60. doi: 10.3899/jrheum.150674
63. Huang Y, Lin W, Chen Z, Wang Y, Huang Y, Tu S. Effect of tumor necrosis factor inhibitors on interstitial lung disease in rheumatoid arthritis: angel or demon? Drug Des Devel Ther. (2019) 13:2111–25. doi: 10.2147/DDDT.S204730
64. Fernández-Díaz C, Loricera J, Castañeda S, López-Mejías R, Ojeda-García C, Olivé A, et al. Abatacept in patients with rheumatoid arthritis and interstitial lung disease: a national multicenter study of 63 patients. Semin Arthritis Rheum. (2018) 48:22–7. doi: 10.1016/j.semarthrit.2017.12.012
65. Akiyama M, Kaneko Y, Yamaoka K, Kondo H, Takeuchi T. Association of disease activity with acute exacerbation of interstitial lung disease during tocilizumab treatment in patients with rheumatoid arthritis: a retrospective, case-control study. Rheumatol Int. (2016) 36:881–9. doi: 10.1007/s00296-016-3478-3
66. Md Yusof MY, Kabia A, Darby M, Lettieri G, Beirne P, Vital EM, et al. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years' experience at a single centre. Rheumatology. (2017) 56:1348–57. doi: 10.1093/rheumatology/kex072
67. Fui A, Bergantini L, Selvi E, Mazzei MA, Bennett D, Pieroni MG, et al. Rituximab therapy in interstitial lung disease associated with rheumatoid arthritis. Intern Med J. (2019). doi: 10.1111/imj.14306. [Epub ahead of print].
68. Conway R, Carey JJ. Methotrexate and lung disease in rheumatoid arthritis. Panminerva Med. (2017) 59:33–46. doi: 10.23736/S0031-0808.16.03260-2
69. Cervantes-Perez P, Toro-Perez AH, Rodriguez-Jurado P. Pulmonary involvement in rheumatoid arthritis. JAMA. (1980) 243:1715–9. doi: 10.1001/jama.243.17.1715
70. Catrina AI, Svensson CI, Malmström V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat Rev Rheumatol. (2017) 13:79–86. doi: 10.1038/nrrheum.2016.200
71. Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology. (2017) 56:344–50. doi: 10.1093/rheumatology/kex299
72. Nurmi HM, Purokivi MK, Kärkkäinen MS, Kettunen H-P, Selander TA, Kaarteenaho RL. Variable course of disease of rheumatoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm Med. (2016) 16:107. doi: 10.1186/s12890-016-0269-2
73. Harlow L, Rosas IO, Gochuico BR, Mikuls TR, Dellaripa PF, Oddis CV, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum. (2013) 65:869–79. doi: 10.1002/art.37881
74. Harlow L, Gochuico BR, Rosas IO, Doyle TJ, Osorio JC, Travers TS, et al. Anti-citrullinated heat shock protein 90 antibodies identified in bronchoalveolar lavage fluid are a marker of lung-specific immune responses. Clin Immunol. (2014) 155:60–70. doi: 10.1016/j.clim.2014.08.004
75. Juge P-A, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. (2018) 379:2209–19. doi: 10.1056/NEJMoa1801562
76. Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. (2011) 364:1503–12. doi: 10.1056/NEJMoa1013660
77. Rajasekaran BA, Shovlin D, Lord P, Kelly CA. Interstitial lung disease in patients with rheumatoid arthritis: a comparison with cryptogenic fibrosing alveolitis. Rheumatology. (2001) 40:1022–5. doi: 10.1093/rheumatology/40.9.1022
78. Ellman P, Ball RE. Rheumatoid disease with joint and pulmonary manifestations. Br Med J. (1948) 2:816–20. doi: 10.1136/bmj.2.4583.816
79. Bély M, Apáthy A. Changes of the lung in rheumatoid arthritis–rheumatoid pneumonia. A clinicopathological study. Acta Morphol Hung. (1991) 39:117–56.
80. Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. (2002) 46:2287–93. doi: 10.1002/art.10524
81. Chung W-S, Peng C-L, Lin C-L, Chang Y-J, Chen Y-F, Chiang JY, et al. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis. (2014) 73:1774–80. doi: 10.1136/annrheumdis-2013-203380
82. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. (2015) 17:212. doi: 10.1186/s13075-015-0728-9
83. Lee H-K, Kim DS, Yoo B, Seo JB, Rho J-Y, Colby TV, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. (2005) 127:2019–27. doi: 10.1378/chest.127.6.2019
84. Mornex JF, Cordier G, Pages J, Vergnon JM, Lefebvre R, Brune J, et al. Activated lung lymphocytes in hypersensitivity pneumonitis. J Allergy Clin Immunol. (1984) 74:719–27. doi: 10.1016/0091-6749(84)90236-7
85. Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. (1997) 156:528–35. doi: 10.1164/ajrccm.156.2.9609016
86. Turesson C, Matteson EL, Colby TV, Vuk-Pavlovic Z, Vassallo R, Weyand CM, et al. Increased CD4+ T cell infiltrates in rheumatoid arthritis-associated interstitial pneumonitis compared with idiopathic interstitial pneumonitis. Arthritis Rheum. (2005) 52:73–9. doi: 10.1002/art.20765
88. Rojas-Serrano J, González-Velásquez E, Mejía M, Sánchez-Rodríguez A, Carrillo G. Interstitial lung disease related to rheumatoid arthritis: evolution after treatment. Reumatol Clin. (2012) 8:68–71. doi: 10.1016/j.reumae.2011.12.001
89. Mochizuki T, Ikari K, Yano K, Sato M, Okazaki K. Long-term deterioration of interstitial lung disease in patients with rheumatoid arthritis treated with abatacept. Mod Rheumatol. (2019) 29:413–7. doi: 10.1080/14397595.2018.1481566
90. Khanna D, Denton CP, Lin CJF, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann Rheum Dis. (2018) 77:212–20. doi: 10.1136/annrheumdis-2017-211682
91. Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients-an overview of different types of involvement and treatment. Rheumatology. (2019) kez177. doi: 10.1093/rheumatology/kez177. [Epub ahead of print].
92. Druce KL, Iqbal K, Watson KD, Symmons DPM, Hyrich KL, Kelly C. Mortality in patients with interstitial lung disease treated with rituximab or TNFi as a first biologic. RMD Open. (2017) 3:e000473. doi: 10.1136/rmdopen-2017-000473
93. Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. (2019) 380:2518–28. doi: 10.1056/NEJMoa1903076
94. Redente EF, Aguilar MA, Black BP, Edelman BL, Bahadur AN, Humphries SM, et al. Nintedanib reduces pulmonary fibrosis in a model of rheumatoid arthritis-associated interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. (2018) 314:L998–1009. doi: 10.1152/ajplung.00304.2017
95. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. (2014) 370:2071–82. doi: 10.1056/NEJMoa1402584
96. Wu C, Lin H, Zhang X. Inhibitory effects of pirfenidone on fibroblast to myofibroblast transition in rheumatoid arthritis-associated interstitial lung disease via the downregulation of activating transcription factor 3 (ATF3). Int Immunopharmacol. (2019) 74:105700. doi: 10.1016/j.intimp.2019.105700
97. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. (2008) 26(5 Suppl. 51):S35–61.
98. Singh N, Varghese J, England BR, Solomon JJ, Michaud K, Mikuls TR, et al. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: a systematic literature review and meta-analysis. Semin Arthritis Rheum. (2019). doi: 10.1016/j.semarthrit.2019.04.005. [Epub ahead of print].
Keywords: rheumatoid arthritis, interstitial lung disease, methotrexate, biologics, immunosuppressive therapies
Citation: Fragoulis GE, Nikiphorou E, Larsen J, Korsten P and Conway R (2019) Methotrexate-Associated Pneumonitis and Rheumatoid Arthritis-Interstitial Lung Disease: Current Concepts for the Diagnosis and Treatment. Front. Med. 6:238. doi: 10.3389/fmed.2019.00238
Received: 30 August 2019; Accepted: 10 October 2019;
Published: 23 October 2019.
Edited by:
Burkhard Franz Leeb, Karl Landsteiner Institute for Clinical Rheumatology, Austria; Karl Landsteiner University for Health Sciences, AustriaReviewed by:
Venerino Poletti, Aarhus University Hospital, DenmarkCopyright © 2019 Fragoulis, Nikiphorou, Larsen, Korsten and Conway. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard Conway, ZHJyaWNoYXJkY29ud2F5QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.