
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Med. , 09 May 2019
Sec. Gastroenterology
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00103
Today, daylight saving time is observed in nearly 80 countries around the world, including the European Union, the USA, Canada, and Russia. The benefits of daylight saving time in energy management have been questioned since it was first introduced during World War I and the latest research has led to varying results. Meanwhile, adverse effects of seasonal time shifts on human biology have been postulated and the European Union is planning to abandon the biannual clock change completely. Medical studies have revealed a correlation of seasonal time shifts with increased incidences of several diseases including stroke, myocardial infarction, and unipolar depressive episodes. Moreover, studies in mice have provided convincing evidence, that circadian rhythm disruption may be involved in the pathogenesis of inflammatory bowel diseases, mainly by disturbing the intestinal barrier integrity. Here, we present previously unpublished data from a large German cohort indicating a correlation of seasonal clock changes and medical leaves due to ulcerative colitis and Crohn's disease. Furthermore, we discuss the health risks of clock changes and the current attempts on reforming daylight saving time from a medical perspective.
Biannual clock changes due to summer time/daylight saving time (DST) are common procedures in most European and North American countries, as well as in parts of South America, Australia, and in New Zealand (1). Historically, George Vernon Hudson, a British/New Zealand entomologist and astronomer, was supposedly the first scientist publicly proposing advantages of a seasonal clock change in 1898 (2). In 1784 however, Benjamin Franklin already discussed a waste of candles due to an extended nightlife in Paris in a curious letter to the Journal du Paris suggesting that Parisians need to rise earlier. Although characterized by satiric elements his letter seems to follow the serious purpose of saving candles and decreasing energy expenditure (3). Mainly in an attempt to save resources during World War I, the German Empire and Austria-Hungary were the first countries to introduce DST on 30 April of 1916. Until the end of the war most European countries, Russia, and the US joined them in an unusual case of mutual agreement (4).
In recent years the proposed benefits of DST are being critically discussed. Scientific approaches to evaluate the advantages or disadvantages of DST in terms of energy management have come to inconsistent results. Indeed, there are studies showing minor energy savings due to reduced use of electrical lighting (5–7), whereas others find increased electricity demands for cooling and heating to surpass these savings (8, 9). A 2017 meta-analysis of 44 studies comprehensively states that DST saves ~0.34% of energy in respective countries. As could be expected, there are differences depending on the latitudes of the countries and a larger distance to the equator leads to a higher efficacy of DST (10). Regarding energy management, it may therefore be reasonable to keep seasonal clock changes in countries with high variation of daylight hours, but to abandon them in other regions.
The European Union (EU) has recently conducted a poll asking their citizens whether to maintain or abandon biannual clock changes (11). Reportedly, 4.6 million participants gave their vote setting a record high for public consultations by the EU (12). It is no surprise, that there is public interest in this subject, because seasonal time shifts affect every inhabitant of concerned countries and force them to adjust their biorhythm twice a year. A study from 2015 attempted to measure the welfare effects of time shifts in Germany and Great Britain and reported reduced life satisfaction after the shift to summer time (13). On 31 August of 2018 the European Commission issued a press release stating that 76% of respondents considered the time shifts due to DST a negative or very negative experience corresponding to the aforementioned study. Moreover, 84% of respondents voted for abolishing biannual clock change (14). Although no other public consultation by the European Union generated as many responses, it may still be biased by an overall low participation rate ranging from 0.02% of the population in the United Kingdom (likely reduced by the upcoming Brexit) to 3.79% in Germany. Nevertheless, the European Commission proposed the abandonment of biannual clock changes in favor of permanent summer time to the European Parliament and the Council (14). Very recently, the European Parliament has voted for discontinuing seasonal clock changes. However, before this decision will be executed, negotiations with the responsible EU ministers represented in the Council of the European Union need to be conducted (15).
Circadian rhythm disruption (CRD), as frequently present in our modern 24-h societies, has been suggested to contribute to various diseases. Among them are metabolic (16, 17), cardiovascular (18) and neuropsychiatric disorders (19), as well as different types of cancer (16, 20, 21). More surprisingly, clock changes due to DST are associated with exacerbation of some medical conditions, even though the time shift is only 1 h. Incidences of myocardial infarction were significantly increased on the first 3 days after clock change to summer time in a large Swedish cohort taking into account a time span of 15 years (22). Importantly, this result was later reproduced in five independent studies from Scandinavia, Croatia, Germany, and the USA (23) with a maximum increase of acute infarctions of 24% on Mondays following time shift in spring (24).
Circadian variation in the onset of strokes has been established since the early 1990s by several studies showing increased incidences in the morning (25). More recently, a Finnish study showed elevated hospitalizations due to ischemic stroke in the first 2 days after DST transitions from 2004 to 2013 further supporting time transition-related effects on cardiovascular diseases (26).
Finally, data from the Danish Psychiatric Central Registry show that the transition from summer time to standard time is associated with increased rates of unipolar depressive episodes in the course of 10 weeks (27), implicating that negative effects—at least for depression—might be present for a longer period of time.
As a side note, there is also an ongoing controversy about a possible impact of seasonal time shifts on the rate of traffic accidents. In 1996 Stanley Coren published a Correspondence article in the New England Journal of Medicine reporting increased numbers of traffic accidents on Mondays following the spring time shift in Canada (28). Coren's results are supported by a 2001 study, which reported slightly but significantly increased fatal accidents in the USA following spring time shifts in a data set of 21 years (29). Coren speculated that the loss of 1 h of sleep was the underlying cause, because the autumn time shift conversely showed reduced traffic incidents. However, numerous other studies do not support Coren's data. Overall, these studies even suggest the seasonal time shift to reduce traffic incidents in the long run, possibly by providing an extra hour of daylight in busier evening traffic hours (30–34).
The two main mechanisms by which circadian regulation of the intestine takes place are vagal afferents from the suprachiasmatic nucleus and intestinal expression of clock genes (35–37). These include, among others, CLOCK (Circadian Locomotor Output Cycles Kaput), BMAL1 (= ARNTL, Aryl hydrocarbon receptor nuclear translocator-like protein 1), CRY1/2 (cryptochrome 1/2), PER1-3 (period circadian protein homolog 1–3) and DBP (D-site binding protein), which are key factors in self-sustaining transcriptional-translational feedback loops (TTFL) that constitute the foundation of circadian regulation on a molecular level within mammalian cells (38–41). In brief, CLOCK and BMAL1 proteins form a heterodimeric transcription factor inducing the expression of their own negative regulators PER1-3 and CRY1-2 in a negative TTFL. PER and CRY proteins accumulate in the cytoplasm and are phosphorylated by casein kinase Iε (CKIε) and glycogen synthase kinase-3 (GSK3). Subsequently, they translocate back to the nucleus, where they repress transcriptional activation by their own activators CLOCK and BMAL1. Gradually, PER and CRY proteins are degraded closing the loop and allowing the cycle to start anew (42). An accessory feedback loop depends on REV-ERBα/ß (= NR1D1/2, nuclear receptor subfamily 1, group D, member 1/2) and RORα/ß (= NR1F1/2, nuclear receptor subfamily 1, group F, member 1/2), which are expressed in a circadian rhythm in intestinal tissue as well, regulating transcription of BMAL1 (38).
These circadian clock gene/protein oscillations need to be relayed to the expression of effector genes in order to exert functional effects in peripheral organs. DBP is considered a prototypic local transducer of these signals, which was shown in liver tissue, where it affects the circadian expression of cytochrome P450 enzymes (43, 44). In recent years, it has become evident that post-translational modification of the aforementioned gene products is an additional key factor in regulating cellular clock rhythms (42). In the intestinal tract, several clock genes are preferentially expressed in epithelial cells and in the enteric nervous system implying a relevant role in the coordination of intestinal functions (39, 45). Microarray analysis show that ~4% of all distal colonic genes are expressed with circadian rhythms many of which are involved in cell signaling, proliferation, inflammation, intestinal motility, and secretion (46). Indeed, gastrointestinal motility (45), gastric acid secretion (47), intestinal regeneration (48), activity of mucosal enzymes and carbohydrate as well as peptide absorption have been shown to be regulated in a circadian manner (37, 49–52).
Additionally, the host's circadian rhythm regulates diurnal variations of gut microbiota (53). Microbial cues, on the other hand, are transformed into rhythmic downstream signals by oscillating expression of Toll-like receptors in intestinal epithelial cells. These signals result in a tightly regulated circadian expression profile of various genes with crucial functions for metabolic and immunologic homeostasis in the intestine (41, 54), representing a novel facet in bidirectional communication of host and gut microbiota. Disruption of the host's circadian rhythm by varying mechanisms can lead to intestinal dysbiosis, symptoms of irritable bowel syndrome, metabolic dysregulation, increased glucose tolerance, and obesity among others (45, 53–58).
Notably, circadian rhythm disruption (CRD) also induces increased intestinal permeability—a major culprit in metabolic liver and inflammatory bowel diseases (IBD) (59). An early study by Preuss et al. providing limited mechanistic insight showed that shifting the light-dark cycle exacerbates experimental colitis dramatically (60). Accordingly, genetic ablation of one of the key factors of the accessory TTFL, REV-ERBα, leads to increased susceptibility to experimental colitis in mice, whereas mice with increased REV-ERBα activation are protected (61). Moreover, we have recently shown that both genetically (Per1/Per2-mutant mice) and externally induced CRD exacerbates mucosal inflammation via inhibition of intestinal epithelial cell proliferation and induction of necroptosis in a well-established murine model of IBD (62). By demonstrating that sleep deprivation, which is often linked to CRD, worsens colonic inflammation, additional evidence for an important role of the circadian rhythm in intestinal homeostasis was provided (63).
In human IBD circadian genes are downregulated in inflamed and non-inflamed mucosal biopsies and systemically in mononuclear blood cells, correlating with increased markers of inflammation and disease activity (64). Another study from 2015 shows a deregulation of key circadian genes in the mucosa of IBD patients including CRY1, PER1, and PER3 using genome-wide cDNA microarray analysis (65). Accordingly, CRD is suggested as a possible environmental trigger of IBD activation (66, 67). This is intriguing, considering that various environmental factors including psychosomatic disorders, antibiotics, gastrointestinal and upper respiratory infections and stress have been proposed as major triggers of relapse, but lack consistent correlations in prospective studies (68–70). To date, the most reliable clinical predictors for acute flares remain high relapse rates in the past, a short time since the last relapse and disease severity in general, providing only limited mechanistic insight (68, 71).
We analyzed medical leave frequencies due to ulcerative colitis (UC) and Crohn's disease (CD) 30 days before and after seasonal time shifts throughout the years 2010 to 2013 in a large cohort of more than 10 million insurance holders of a German health insurance company. Strikingly, after the autumn time shift significantly more UC patients reported sick leaves (Figure 1A, 12.88/1,000 insurance holders vs. 15.18/1,000, P = 0.0135). Similarly, medical leave frequency for CD patients was significantly increased in the same period of time (Figure 1B, 15.88/1,000 vs. 18.6/1,000, P = 0.0077). The spring time shift led to increased medical leave rates for both disease entities as well, but the differences were not statistically significant (Figures 1A,B). Although the absolute changes are modest and medical leave frequencies are only a surrogate parameter for acute IBD flares, these results indicate a relevant effect of seasonal clock change on symptom severity of IBD patients forcing them to take a medical leave.
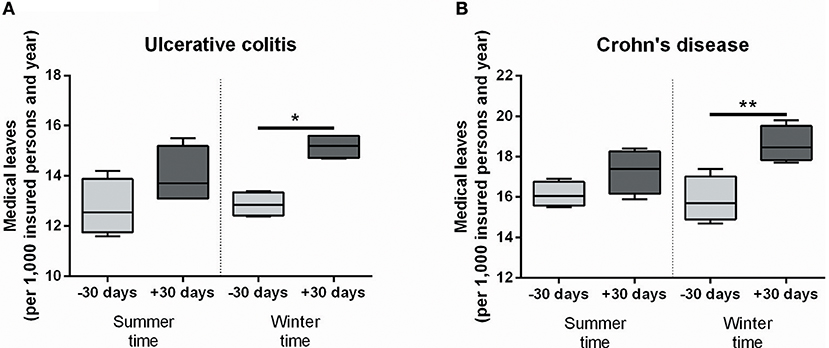
Figure 1. Medical leave frequencies due to IBD increase after seasonal clock changes. Medical leaves are shown per 1,000 insurance holders in the 30 days before and after seasonal clock changes for ulcerative colitis (A) and Crohn's disease (B) as defined by the International Classification of Diseases (ICD-10). Statistical testing was performed using One-Way ANOVA followed by Tukey's honest significant difference test in Prism 6 (GraphPad Software). *P < 0.05; **P < 0.01.
Previously published data on increases of IBD flares in the autumn/winter months compared to spring are partially reproduced by our data, although the surrogate parameters differ from study to study (68, 71, 72). Climatic differences as well as sunlight exposure and associated vitamin D activation are possible causes for these seasonal variations and may partially explain the here observed increase after the autumn time shift, but certainly not after the clock change in spring, when sunlight exposition increases and temperatures rise (73, 74). Notably, the variations in relapse frequency observed in these studies are predominantly registered months before the dates of clock changes and do not coincide with them (68, 72). It is therefore unlikely that increased medical leave rates after seasonal clock changes in our data are merely caused by the change of seasons. Furthermore, it should be noted that several other studies did not report seasonality or even conversely registered a rise of IBD flares in spring (75–77). Possible causes for the varying results include the definition of flares, different geographical and genetic backgrounds of study populations and environmental factors such as infections, climate, and food (78).
Many intestinal diseases among them IBD may be heavily influenced by psychological factors. Therefore, it is important to consider negative psychosomatic effects of seasonal time shifts as effectors in our data, as reflected by increased depressive episodes after autumn time shift (27). Nevertheless, our data are coherent with abundant experimental evidence of a crucial role of circadian rhythm in the maintenance of intestinal homeostasis and barrier function. Although we are not able to distinguish between immunologic and psychosomatic mechanisms underlying our results, these data may ultimately imply socioeconomic costs of biannual clock changes as increased medical leaves entail fewer working hours. Future prospective studies measuring hospitalization rates and disease activity scores of IBD patients are, however, needed to conclusively support an effect of seasonal clock changes on IBD relapse rates.
DST is a highly polarizing issue, concerning most people in western civilizations. Data on energy savings due to DST are inconsistent, but DST appears more favorable in countries located remote from the equator. Epidemiological data suggest a link of seasonal time changes to increased incidences of acute myocardial infarction, ischemic stroke, and depression. We here provide additional data indicating a correlation of seasonal clock changes with a surrogate parameter of acute IBD flares, corroborated by existing experimental evidence on circadian regulation of the intestinal homeostasis in mice and humans. Further prospective studies will be needed to further support these results. From a medical perspective—and setting aside small energy savings and highly questionable effects on traffic incidents—the abolishment of biannual clock changes should be seriously considered. Following a public consultation, the European Union is currently evaluating a permanent switch to summer time and it would not be surprising if other regions were following this example in the years to come.
BF analyzed and interpreted the data and drafted the manuscript. TS was a major contributor in writing the manuscript. HO provided important intellectual content. SD analyzed and interpreted the data. CS was a major contributor in writing the script and provided important intellectual content. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Raw data were kindly provided by the Scientific Institute of the Allgemeine Ortskrankenkasse (WIdO, AOK Berlin). We acknowledge financial support for Open Access publication by Land Schleswig-Holstein within the funding programme Open Access Publikationsfonds.
ARNTL/BMAL1, Aryl hydrocarbon receptor nuclear translocator-like protein 1; CKIε, casein kinase Iε; CLOCK, Circadian Locomotor Output Cycles Kaput; CD, Crohn's disease; CRD, circadian rhythm disruption; CRY1, cryptochrome 1; DBP, D-site binding protein; DST, daylight saving time/summer time; GSK3, glycogen synthase kinase-3; IBD, inflammatory bowel diseases; NR1D1/2 (REV-ERBα/β), nuclear receptor subfamily 1, group D, member 1/2; NR1F1/2 (RORα), nuclear receptor subfamily 1, group F, member 1/2 (RAR-related orphan receptor alpha/beta); PER1-3, period circadian protein homolog 1; TTFL, transcriptional-translational feedback loop; UC, ulcerative colitis.
1. Daylight Saving Time Around the World 2018. Available online at: https://www.timeanddate.com/time/dst/2018.html (accessed April 30, 2019).
3. Franklin B. Essay on daylight saving. In: Goodman NG, editor. The Ingenious Dr. Franklin; Selected Scientific Letters of Benjamin Franklin. University of Pennsylvania Press (1931). p. 17–22. Available online at: http://www.webexhibits.org/daylightsaving/franklin3.html (accessed April 30, 2019).
4. Daylight Saving Time Statistics. Available online at: https://www.timeanddate.com/time/dst/statistics.html (accessed April 30, 2019).
5. Verdejo H, Becker C, Echiburu D, Escudero W, Fucks E. Impact of daylight saving time on the chilean residential consumption. Energy Pol. (2016) 88:456–64. doi: 10.1016/j.enpol.2015.10.051
6. Awad Momani M, Yatim B, Ali MAM. The impact of the daylight saving time on electricity consumption—a case study from Jordan. Energy Pol. (2009) 37:2042–51. doi: 10.1016/j.enpol.2009.02.009
7. Mirza FM, Bergland O. The impact of daylight saving time on electricity consumption: Evidence from southern Norway and Sweden. Energy Pol. (2011) 39:3558–71. doi: 10.1016/j.enpol.2011.03.057
8. Kotchen MJ, Grant LE. Does daylight saving time save energy? evidence from a natural experiment in Indiana. Rev Econ Stat. (2011) 93:1172–85. doi: 10.1162/REST_a_00131
9. Aries MBC, Newsham GR. Effect of daylight saving time on lighting energy use: a literature review. Energy Pol. (2008) 36:1858–66. doi: 10.1016/j.enpol.2007.05.021
10. Havranek T, Herman D, Irsova Z. Does Daylight Saving Save Energy? A Meta-Analysis. (2016). Available online at: https://mpra.ub.uni-muenchen.de/74518/ (accessed April 30, 2019).
11. European Commission. Have Your Say: Commission Launches Public Consultation on Daylight Saving Time. European Commission Dly News (2018). Available online at: http://europa.eu/rapid/press-release_MEX-18-4370_en.htm#2 (accessed April 30, 2019).
12. European Commission. Have Your Say: More Than 4.6 Million Responses Received in Public Consultation on Summertime Arrangements. European Commission Dly News (2018). Available online at: http://europa.eu/rapid/press-release_MEX-18-5043_en.htm (accessed April 30, 2019).
13. Kuehnle D, Wunder C. Using the life satisfaction approach to value daylight savings time transitions: evidence from Britain and Germany. J Happiness Stud. (2016) 17:2293–323. doi: 10.1007/s10902-015-9695-8
14. European Commission. Summertime Consultation: 84% Want Europe to Stop Changing the Clock. European Commission - Press release (2018). Available online at: http://europa.eu/rapid/press-release_IP-18-5302_en.htm (accessed April 30, 2019).
15. European Parliament. European Parliament Legislative Resolution of 26 March 2019 on the Proposal for a Directive of the European Parliament and of the Council Discontinuing Seasonal Changes of Time and Repealing Directive 2000/84/EC. European Parliament-Press Release (2019). Available online at: http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//NONSGML+TA+P8-TA-2019-0225+0+DOC+PDF+V0//EN (accessed April 30, 2019).
16. Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell. (2016) 30:909–24. doi: 10.1016/j.ccell.2016.10.007
17. Maury E, Hong HK, Bass J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab. (2014) 40:338–46. doi: 10.1016/j.diabet.2013.12.005
18. Takeda N, Maemura K. Circadian clock and cardiovascular disease. J Cardiol. (2011) 57:249–56. doi: 10.1016/j.jjcc.2011.02.006
19. Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. (2010) 11:589–99. doi: 10.1038/nrn2868
20. Papagiannakopoulos T, Bauer MR, Davidson SM, Heimann M, Subbaraj L, Bhutkar A, et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. (2016) 24:324–31. doi: 10.1016/j.cmet.2016.07.001
21. Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med. (2012) 18:1249–60. doi: 10.2119/molmed.2012.00077
22. Janszky I, Ljung R. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. (2008) 359:1966–8. doi: 10.1056/NEJMc0807104
23. Manfredini R, Fabbian F, De Giorgi A, Zucchi B, Cappadona R, Signani F, et al. Daylight saving time and myocardial infarction: should we be worried? a review of the evidence. Eur Rev Med Pharmacol Sci. (2018) 22:750–5. doi: 10.3390/jcm8030404
24. Sandhu A, Seth M, Gurm HS. Daylight savings time and myocardial infarction. Open Hear. (2014) 1:e000019. doi: 10.1136/openhrt-2013-000019
25. Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. (1998) 29:992–6. doi: 10.1161/01.STR.29.5.992
26. Sipilä JOT, Ruuskanen JO, Rautava P, Kytö V. Changes in ischemic stroke occurrence following daylight saving time transitions. Sleep Med. (2016) 27–28:20–4. doi: 10.1016/j.sleep.2016.10.009
27. Hansen BT, Sønderskhov KM, Hageman I, Dinesen PT, Østergaarde SD. Daylight savings time transitions and the incidence rate of unipolar depressive episodes. Epidemiology. (2017) 28:346–53. doi: 10.1097/EDE.0000000000000580
28. Coren S. Daylight savings time and traffic accidents. N Engl J Med. (1996) 334:924–5. doi: 10.1056/NEJM199604043341416
29. Varughese J, Allen RP. Fatal accidents following changes in daylight savings time: the American experience. Sleep Med. (2001) 2:31–36. doi: 10.1016/S1389-9457(00)00032-0
30. Ferguson S. Traffic accidents and daylight saving time. N Engl J Med. (1996) 335:355–7. doi: 10.1056/NEJM199608013350517
31. Ferguson SA, Preusser DF, Lund AK, Zador PL, Ulmer RG. Daylight saving time and motor vehicle crashes: the reduction in pedestrian and vehicle occupant fatalities. Am J Public Health. (1995) 85:92–5. doi: 10.2105/AJPH.85.1.92
32. Vincent A. Effects of daylight savings time on collision rates. N Engl J Med. (1998) 339:1167–8. doi: 10.1056/NEJM199810153391617
33. Sood N, Ghosh A. The short and long run effects of daylight saving time on fatal automobile crashes. B E J Econom Anal Pol. (2007) 7:1–22. doi: 10.2202/1935-1682.1618
34. Broughton J, Sedman R. The Potential Effects on Road Casualties of Double British Summertime. (1989) Available online at: https://trl.co.uk/sites/default/files/RR228.pdf (accessed April 30, 2019).
35. Scheving LA. Biological clocks and the digestive system. Gastroenterology. (2000) 119:536–49. doi: 10.1053/gast.2000.9305
36. Pardini L, Kaeffer B, Trubuil A, Bourreille A, Galmiche JP. Human intestinal circadian clock: expression of clock genes in colonocytes lining the crypt. Chronobiol Int. (2005) 22:951–61. doi: 10.1080/07420520500395011
37. Tavakkolizadeh A, Ramsanahie A, Levitsky LL, Zinner MJ, Whang EE, Ashley SW, et al. Differential role of vagus nerve in maintaining diurnal gene expression rhythms in the proximal small intestine. J Surg Res. (2005) 129:73–8. doi: 10.1016/j.jss.2005.05.023
38. Mazzoccoli G, Francavilla M, Pazienza V, Benegiamo G, Piepoli A, Vinciguerra M, et al. Differential patterns in the periodicity and dynamics of clock gene expression in mouse liver and stomach. Chronobiol Int. (2012) 29:1300–11. doi: 10.3109/07420528.2012.728662
39. Hoogerwerf WA, Hellmich HL, Cornélissen G, Halberg F, Shahinian VB, Bostwick J, et al. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. (2007) 133:1250–60. doi: 10.1053/j.gastro.2007.07.009
40. Polidarová L, Sládek M, Soták M, Sumová A. Hepatic duodenal and colonic circadian clocks differ in their persistence under conditions of constant light and in their entrainment by restricted feeding. Chron. (2011) 28:204–15. doi: 10.3109/07420528.2010.548615
41. Froy O, Chapnik N. Circadian oscillation of innate immunity components in mouse small intestine. Mol Immunol. (2007) 44:1954–60. doi: 10.1016/j.molimm.2006.09.026
42. Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. (2007) 8:139–48. doi: 10.1038/nrm2106
43. Lavery DJ, Lopez-Molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, et al. Circadian expression of the steroid 15 alpha-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Mol Cell Biol. (1999) 19:6488–99. doi: 10.1128/MCB.19.10.6488
44. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. (2002) 418:935–41. doi: 10.1038/nature00965
45. Hoogerwerf WA. Role of clock genes in gastrointestinal motility. Am J Physiol Liver Physiol. (2010) 299:549–55. doi: 10.1152/ajpgi.00147.2010
46. Hoogerwerf WA, Sinha M, Conesa A, Luxon BA, Shahinian VB, Cornélissen G, et al. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology. (2008) 135:2019–29. doi: 10.1053/j.gastro.2008.08.048
47. Hoogerwerf WA. Biologic clocks and the gut. Curr Gastroenterol Rep. (2006) 8:353–9. doi: 10.1007/s11894-006-0019-3
48. Stokes K, Cooke A, Chang H, Weaver DR, Breault DT, Karpowicz P. The circadian clock gene BMAL1 coordinates intestinal regeneration. Cell Mol Gastroenterol Hepatol. (2017) 4:95–114. doi: 10.1016/j.jcmgh.2017.03.011
49. Saito H, Terada T, Shimakura J, Katsura T, Inui K. Regulatory mechanism governing the diurnal rhythm of intestinal H + /peptide cotransporter 1 (PEPT1). Am J Physiol Liver Physiol. (2008) 295:G395–402. doi: 10.1152/ajpgi.90317.2008
50. Pan X, Terada T, Irie M, Saito H, Inui K-I. Diurnal rhythm of H + -peptide cotransporter in rat small intestine. Am J Physiol Liver Physiol. (2002) 283:G57–64. doi: 10.1152/ajpgi.00545.2001
51. Pan X, Terada T, Okuda M, Inui K-I. The diurnal rhythm of the intestinal transporters SGLT1 and PEPT1 Is regulated by the feeding conditions in rats. J Nutr. (2004) 134:2211–5. doi: 10.1093/jn/134.9.2211
52. Pan X, Hussain MM. Clock is important for food and circadian regulation of macronutrient absorption in mice. J Lipid Res. (2009) 50:1800–13. doi: 10.1194/jlr.M900085-JLR200
53. Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. (2014) 159:514–29. doi: 10.1016/j.cell.2014.09.048
54. Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. (2013) 153:812–27. doi: 10.1016/j.cell.2013.04.020
55. Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev. (2010) 23:155–68. doi: 10.1017/S0954422410000016
56. Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, et al. Circadian disorganization alters intestinal microbiota. PLoS ONE. (2014) 9:e97500. doi: 10.1371/journal.pone.0097500
57. Voigt RM, Summa KC, Forsyth CB, Green SJ, Engen P, Naqib A, et al. The circadian clock mutation promotes intestinal dysbiosis. Alcohol Clin Exp Res. (2016) 40:335–47. doi: 10.1111/acer.12943
58. Rosselot AE, Hong CI, Moore SR. Rhythm and bugs: circadian clocks, gut microbiota, and enteric infections. Curr Opin Gastroenterol. (2016) 32:7–11. doi: 10.1097/MOG.0000000000000227
59. Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, et al. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS ONE. (2013) 8:e67102. doi: 10.1371/journal.pone.0067102
60. Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek FW. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am J Physiol Regul Integr Comp Physiol. (2008) 295:R2034–40. doi: 10.1152/ajpregu.00118.2008
61. Wang S, Lin Y, Yuan X, Li F, Guo L, Wu B. REV-ERBα integrates colon clock with experimental colitis through regulation of NF-κB/NLRP3 axis. Nat Commun. (2018) 9:4246. doi: 10.1038/s41467-018-06568-5
62. Pagel R, Bär F, Schröder T, Sünderhauf A, Künstner A, Ibrahim SM, et al. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. FASEB J. (2017) 31:4707–19. doi: 10.1096/fj.201700141RR
63. Tang Y, Preuss F, Turek FW, Jakate S, Keshavarzian A. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med. (2009) 10:597–603. doi: 10.1016/j.sleep.2008.12.009
64. Liu X, Yu R, Zhu L, Hou X, Zou K. Bidirectional regulation of circadian disturbance and inflammation in inflammatory bowel disease. Inflamm Bowel Dis. (2017) 23:1741–51. doi: 10.1097/MIB.0000000000001265
65. Palmieri O, Mazzoccoli G, Bossa F, Maglietta R, Palumbo O, Ancona N, et al. Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol Int. (2015) 32:903–16. doi: 10.3109/07420528.2015.1050726
66. Swanson GR, Burgess HJ, Keshavarzian A. Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert Rev Clin Immunol. (2011) 7:29–36. doi: 10.1586/eci.10.83
67. Codoñer-Franch P, Gombert M. Circadian rhythms in the pathogenesis of gastrointestinal diseases. World J Gastroenterol. (2018) 24:4297–303. doi: 10.3748/wjg.v24.i38.4297
68. Riley SA, Mani V, Goodman MJ, Lucas S. Why do patients with ulcerative colitis relapse? Gut. (1990) 31:179–83. doi: 10.1136/gut.31.2.179
69. North CS, Alpers DH, Helzer JE, Spitznagel EL, Clouse RE. Do life events or depression exacerbate inflammatory bowel disease? a prospective study. Ann Intern Med. (1991) 114:381–6. doi: 10.7326/0003-4819-114-5-381
70. Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol. (2010) 105:1994–2002. doi: 10.1038/ajg.2010.140
71. Liverani E, Scaioli E, Digby RJ, Bellanova M, Belluzzi A. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol. (2016) 22:1017–33. doi: 10.3748/wjg.v22.i3.1017
72. Zeng L, Anderson FH. Seasonal change in the exacerbations of Crohn's disease. Scand J Gastroenterol. (1996) 31:79–82. doi: 10.3109/00365529609031631
73. Nielsen OH, Rejnmark L, Moss AC. Role of vitamin D in the natural history of inflammatory bowel disease. J Crohn's Colitis. (2018) 12:742–52. doi: 10.1093/ecco-jcc/jjy025
74. Ananthakrishnan AN. Environmental triggers for inflammatory bowel disease. Curr Gastroenterol Rep. (2013) 15:302. doi: 10.1007/s11894-012-0302-4
75. Koido S, Ohkusa T, Saito H, Yokoyama T, Shibuya T, Sakamoto N, et al. Seasonal variations in the onset of ulcerative colitis in Japan. World J Gastroenterol. (2013) 19:9063–8. doi: 10.3748/wjg.v19.i47.9063
76. Lewis JD, Aberra FN, Lichtenstein GR, Bilker WB, Brensinger C, Strom BL. Seasonal variation in flares of inflammatory bowel disease. Gastroenterology. (2004) 126:665–73. doi: 10.1053/j.gastro.2003.12.003
77. Bai A, Guo Y, Shen Y, Xie Y, Zhu X, Lu N. Seasonality in flares and months of births of patients with ulcerative colitis in a Chinese population. Dig Dis Sci. (2009) 54:1094–8. doi: 10.1007/s10620-008-0453-1
Keywords: daylight saving time, seasonal clock changes, circadian rhythm disruption, inflammatory bowel diseases, ulcerative colitis, Crohn's disease, European Union
Citation: Föh B, Schröder T, Oster H, Derer S and Sina C (2019) Seasonal Clock Changes Are Underappreciated Health Risks—Also in IBD? Front. Med. 6:103. doi: 10.3389/fmed.2019.00103
Received: 04 October 2018; Accepted: 26 April 2019;
Published: 09 May 2019.
Edited by:
Murat Toruner, Ankara University, TurkeyReviewed by:
Giuseppe Losurdo, University of Bari Medical School, ItalyCopyright © 2019 Föh, Schröder, Oster, Derer and Sina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bandik Föh, YmFuZGlrLmZvZWhAdWtzaC5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.