
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 10 May 2019
Sec. Rheumatology
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00102
This article is part of the Research Topic Synovial Tissue: Turning the Page to Precision Medicine in Arthritis? View all 16 articles
The synovial tissue is a primary target of multiple diseases characterized by different pathogenic mechanisms, including infective, deposition, neoplastic, and chronic immune-inflammatory pathologies. Synovial biopsy can have a relevant role in differential diagnosis of specific conditions in clinical practice, although its exploitation remains relatively limited. In particular, no validated synovial-tissue-derived biomarkers are currently available in the clinic to aid in the diagnosis and management in most frequent forms of chronic inflammatory arthropathies, namely rheumatoid arthritis (RA) and the spondyloarthritides (SpA). In this brief review, we will discuss the current spectrum of clinical applications of synovial biopsy in routine rheumatologic care and will provide an analysis of the perspectives for its potential exploitation in patients with chronic inflammatory arthritides.
The assessment of the pathologic process at peripheral sites has been proved as a source of clinically relevant information in different human pathologies including cancers and systemic autoimmune diseases. Examples of the latter group are the assessment of the salivary glands in sialo adenitis, the muscle in idiopathic inflammatory myopathies or the kidney in systemic lupus erythematosus, in which the qualitative and/or quantitative analysis of local inflammatory processes can be exploited to corroborate diagnosis/classification, facilitate discrimination among disease entities, evaluate prognosis and guide the choice of appropriate treatments (1–3). Similarly, the synovial membrane, being the target of different rheumatologic conditions, holds an intrinsic potential for wide clinical applications, although its exploitation remains, at present, relatively limited. If, on the one hand, the synovial biopsy may offer unique information aiding the diagnosis of infectious and other rare diseases, on the other, no validated synovial tissue-derived biomarkers are currently available in the clinic to support early diagnosis/classification or to guide individual patients' management in most frequent forms of chronic inflammatory arthropathies.
Based on the existence of major unmet needs and on data derived from previous proof-of-concept studies (see next paragraphs), there is now growing attention in expanding the translational applicability of synovial tissue analysis also in this direction (4). One of the most compelling working hypothesis is that the cellular/molecular patho-biology of the inflamed synovial membrane might delineate specific discriminative traits able to improve early diagnosis of undifferentiated forms and patients' stratification into treatment-specific response groups. The introduction of mini-invasive approaches allowing targeted tissue sampling of large and small joints under direct vision of a standard ultrasound (US) machine (US-guided biopsies) is now contributing to make this perspective more realistic, favoring synovial biopsy widespread applicability and allowing the development of multi-center research and clinical trials (5–10). For a detailed update on currently available synovial biopsy techniques, including their advantages, limitations and validation requirements the reader can refer to a recently published review (11).
In the following paragraphs, we will provide a summary of current applications of synovial biopsy in clinical practice and of the background data that are allowing to conceive their extension into the field of stratified medicine.
For the majority of rheumatologic diseases, patients' interview, clinical examination, imaging and serological tests are usually sufficient to establish a diagnosis and monitor treatment response. The analysis of the synovial tissue can be, however, of assistance for diagnostic purposes in course of arthritis of undetermined origin, allowing the identification of specific traits of a restricted, though defined, spectrum of pathologies, including infective, neoplastic and some deposition diseases (Table 1). Whilst specific markers (cellular and/or molecular) related to several of these conditions can be readily identified also through less-invasive approaches, such as the analysis of synovial fluid, the biopsy can be a relevant implementation tool in different situations. Firstly, the collection of synovial tissue can be essential to ensure sampling of joint environments characterized by lack of or limited effusion, as a primary approach or as a “failsafe” mechanism (12, 13). Under certain circumstances, the synovial biopsy can be important also as a complementary or second level approach in the case of fluid availability. Indeed, despite comparative data on fluid vs. tissue diagnostic accuracy for most conventional approaches (microbiological cultures, PCR for infective agents, detection of crystals) remain limited (14–17), results derived from the two compartments, even if focused on the same downstream procedure, have been shown to lack systematic redundancy and either may contemplate false negative results (13–21).
Beyond expanding the analytical substrate, the availability of biopsy specimens may also offer specific information by allowing the integration of microbiological and molecular screenings with the analysis of characteristic histopathologic traits of some infections and rare diseases (see next paragraphs for details).
US-guided dry needle synovial tissue aspiration (22) or synovial biopsy (23) can be considered as diagnostic options when crystal-associated arthropathies are suspected, in particular in patients without synovial effusion or in the case of negative results from synovial fluid. Both monosodium urate (after tissue fixation in absolute alcohol) and calcium pyrophosphate crystals (24) can be detected within tissue specimens as focal deposits of amorphous material or as birefringent structures by polarized light microscopy. As a general notion, inferred from a recent retrospective study of biopsy reports involving synovial tissue between 1998 and 2015, a confirmatory diagnosis of crystal associated arthritis can be established in ~40% of the cases in which the procedure is performed for a primary clinical suspicion (23).
Both synovial fluid or synovial tissue analyses can also contribute to the differential diagnosis of other rarer deposition diseases including amyloid arthropathy, through Congo-red stain and the identification of typical apple-green birefringent deposits of immunoglobulin free light chains, as well as ochronotic arthritis, typically associated to local accumulation of homogentisic acid polymers and characteristic yellow-brown cartilage debris (25–28).
In keeping with deposition diseases, the diagnosis of suspected infectious arthropathies can be approached either through the analysis of synovial fluid or synovial tissue. Both type of samples have been successfully exploited for the identification of pathogenic organisms through microbiological cultures and molecular analyses. Unfortunately, due to the scarcity of systematic studies, no definite guidelines are currently available to assist clinicians in the selection of the most appropriate strategy when both sites are accessible. Notwithstanding this gap, the availability of synovial specimens can represent, however, a benefit in certain circumstances by offering a wider spectrum of analytical perspectives. These perspectives, which may be of particular relevance in the case of negative results from cultural examinations, include the direct analysis of bacterial and fungal localization in situ through conventional stainings (Gram, Ziehl, Dieterle, periodic acid-Schiff), as well as the evaluation of indirect signs, such as the presence/pathologic aspect of local granulomatous reactions or the degree of perivascular neutrophilic infiltration (29, 30). The latter parameter, though not specific per se, has been reproducibly shown to be a valid discriminative marker of septic arthritis if quantitatively addressed, either through conventional haematoxylin and eosin (H&E) stain or CD15 immunohistochemistry (31–34).
Broad-range 16S rRNA bacterial PCR has not proved to offer major advantages over bacterial culture in the standard diagnostic setting (35) and is considered prone to non-specific results (36). It has been however proposed as a candidate method to monitor the presence of bacterial DNA in synovial samples from patients with septic arthritis during antibiotic treatment (19). Targeted-PCR testing for mycobacteria and difficult-to-culture atypical germs (Borrelia, Tropheryma whipplei) has been similarly applied to both synovial fluid and synovial membrane and can be considered in the case of suspicion of mycobacterial- (37, 38), Lyme- (20, 39) and Whipple's arthritis (20, 40) when a sensitive approach is required.
Tissue-directed analyses give also the unique opportunity to broaden the diagnostic spectrum in patients with unclassified arthritis, allowing the identification of specific (non-infective) conditions characterized by typical synovial histopathologic features and less traceable changes in the synovial fluid. Examples of these conditions, in which the synovial biopsy may have a primary diagnostic role, include primary synovial malignancies (lymphomas, sarcomas), metastatic tumors and some benign proliferative lesions like pigmented villonodular synovitis and synovial chondromatosis (the latter characterized by a minor risk of malignant transformation). In all these conditions, the in situ evaluation by conventional histopathologic analyses can be required to integrate and corroborate imaging findings for a defined differential diagnosis (20, 41–43).
The standard histologic analysis of the synovium can be instrumental also in the diagnosis of arthritis in patients affected by some non-Langerhans cell histiocytic disorders, uncommon conditions characterized by multi-system involvement due to dysregulated accumulation of mononuclear phagocytes. In some forms with adult-onset (multicentric reticulohistiocytosis, Erdheim-Chester disease), patients can display severe joint involvement, typically associated to abnormal sub-lining infiltration of CD68-positive (CD1a- and S100-negative) histiocytes and multinucleated giant cells with a lipid-laden or PAS-positive ground-glass cytoplasm (44–46).
Altogether, these data, generated over the last decades, demonstrated the utility of synovial tissue collection for differential diagnosis, but left partially unclear the actual output of the procedure in the real-life setting of a rheumatology clinic, in particular for what concerns most recent approaches, such as US-guided biopsy. This issue has now been addressed by independent groups demonstrating, quite consistently, a success rate of around 82–96% in obtaining samples suitable for analysis with the potential to achieve a diagnosis in between 16 and 20% of the cases (13, 20, 47), depending on the inclusion criteria and study design (13, 20, 47).
Beyond its possible application for differential diagnosis of unclassified arthritis, synovial tissue examination has been also proved to be a valuable source of surrogate biomarkers of response-to-treatment. Evidence supporting this concept derives from pioneering studies performed in the last two decades demonstrating, through the evaluation of serial arthroscopic biopsies, the sensitivity-to-change and external responsiveness of sub-lining CD68+ macrophages in relationship to variations of clinical composite indices in rheumatoid arthritis (RA). The reduction of CD68+ sub-lining macrophages has been shown to be associated to effective treatment, less influenced by placebo compared to clinical parameters, and to be a valid measure of response to treatments characterized by different mechanisms of action (48–51). Collectively, these observations have corroborated the value of synovial tissue analysis for the development of markers of early patho-biologic effect, thus potentially exploitable to accelerate decisions (including dose selection) in early phase I/II clinical trials. Strengthening the general applicability of these data, the correlation between modulation of the number of CD68+ sublining macrophages with clinical response to treatment has been recently confirmed also through the assessment of US-guided biopsies restricted to tissue collection from small joints (8).
If, on the one hand, the studies presented in the previous sections delineated the conditions that can be diagnosed through a synovial biopsy, on the other, they also shed further emphasis on what synovial tissue sampling cannot currently offer in routine clinical practice. The possibility to identify specific traits for most common forms of systemic chronic inflammatory arthropathies, namely RA and spondyloarthritis (SpA) remains, indeed, impracticable. This issue is relevant both in patho-biologic and clinical terms and has been the object of intense investigation in the past. Indeed, since early diagnosis and treatment in these conditions are linked to improved long-term outcomes (52, 53), the identification of disease-specific pathologic changes would contribute not only to improve comprehension of disease pathogenesis but, potentially, also to improve current models for early outcome prediction in undifferentiated forms (54–56).
RA and SpA synovitis (evaluated at a group level) do display measurable differences compared with post-traumatic and degenerative conditions, in terms of gene expression (57), histopathologic score (Krenn's, IMSYC) (58, 59), and cell proliferation rate (60). None of the analyzed parameters, however, has so far proved a sufficient degree of diagnostic accuracy due to intra-disease variability and overlapping features. The same concept applies for what concerns the overall level of micro-anatomic organization of inflammatory infiltrate that has been shown, as expected from studies in different pathologic contexts (61), to present similar qualitative characteristics (62, 63).
Despite these data and the observed gross analogies, there is now growing evidence from independent studies that a detailed comparative analysis of specific components of the inflammatory process may actually allow to detect multiple and congruent biological differences among diseases, in particular if patients' characteristics and the overall degree of joint inflammation are appropriately matched. One of the most compelling aspects that has been reproducibly confirmed relates to the characteristics of the vascular system. Synovial vascularity has been shown to display macroscopic and microscopic differences between RA and SpA, with the latter associated to an increased distribution of tortuous blood vessels in the sub-lining both in early and established disease (64–67). Accordingly, the level of synovial production of angiogenic factors (VEGF and Ang2 mRNA and protein) is significantly increased in psoriatic arthritis (PsA) compared to RA, with a prominent differential expression in perivascular regions. Since Ang2 expression in the presence of VEGF is functionally implicated in angiogenesis and vessel destabilization, it has been proposed that the observed high levels of Ang2/VEGF in PsA joint could inhibit stabilization of the new vessels, resulting in the formation of more “plastic” vessels (68).
The existence of peculiar biological traits characteristic of SpA synovial stroma has been confirmed by gene expression analyses. In this context, of particular interest is the work performed by Yeremenko et al. (69) who, by pan-genomic microarrays of synovial samples from patients with SpA and RA matched for the local degree of histological inflammation, demonstrated a robust disease-specific, inflammation-independent myogene expression signature in SpA synovitis. Synovial tissue staining identified the myogene expressing cells as α-SMA positive, vimentin-positive, prolyl 4-hydroxylase-positive, CD90+ and CD146+ mesenchymal cells, confirming their significant over-representation in the lining and sub-lining of the inflamed SpA synovium.
No differential characteristics, instead, have been reproducibly recognized in the distribution of major lymphocyte populations (conventional CD3+ T cells, CD8+ T cells, B cells, plasma cells) and of lining/sub-lining CD68+ macrophages (65–67, 70), although an increased prevalence of alternatively activated CD163+ macrophages (67, 71) and IL17 producing mast cells (72) has been reported in SpA.
In conclusion, data derived from several independent studies demonstrate that, despite a shared inflammatory background, the inflamed synovium of different forms of chronic inflammatory arthritides can associate to differential cellular and molecular traits. Further research and novel multi-center observational studies (56, 73) are needed to improve our mechanistic comprehension of these traits and delineate their predictive value in real-life clinical practice.
If differences in the synovial characteristics can be captured between different clinical entities, a cutting-edge question is whether clinically relevant differences can be reliably distinguished also within the same disease, a fundamental premise to conceive the possible integration of synovial biopsy into a precision medicine algorithm.
Precision medicine is an approach to disease treatment and prevention that takes into account individual patho-biologic variability, thus allowing to predict more accurately which treatment or prevention strategy for a particular disease will be more suitable in specific groups of patients. This perspective, which differs substantially from conventional approaches based on the “average person,” represents a major objective of modern healthcare systems due to both clinical and socio-economic needs. Chronic inflammatory arthritides have several characteristics that make them ideally suitable for stratification. These include the high degree of clinical heterogeneity that characterizes both RA and SpA, the degree of variability of response to specific treatments within each disease, and the similar degree of efficacy of specific treatments across individuals affected by different diseases (74). Whilst a rudimental level of stratification is already applied to RA, through the distinction of autoantibody-positive and -negative sub-groups (75), it is quite clear that these categories, per se, are not sufficient to entirely explain the heterogeneity of the disease and that a finer profiling is required (76). Since the synovial membrane represents one of the primary targets of these conditions, it is expected that dissecting its pathologic traits could be a privileged window on disease pathogenic spectrum (4).
Several studies performed in recent years have set the technical, pathological and clinical bases to support the scientific rationale of exploiting synovial biopsy for a precision medicine approach to arthritis.
In technical terms, the collection of a limited amount of tissue from a single procedure has been proved sufficient to obtain a reliable assessment of different histopathologic markers (6, 8, 77–79) and gene expression (80) in one joint. Despite differences among studies, depending on the adopted technique and measurement unit (number of specimens, mm2), all reported data were falling within the feasibility range of a routinely applicable procedure. As a complementary observation, the assessment of different characteristics (selected histopathologic markers and of T cell clonal expansions) in one joint has been shown to be representative of the same parameters in other joints in RA (81, 82). Notwithstanding the possible existence of variability in transcriptome signatures and epigenetic traits among different sites (83), current results collectively suggest that the analysis of few synovial specimens from a single accessible site can be informative on the systemic process.
In pathological terms, there is now extensive evidence indicating that the cross-sectional evaluation of the synovium from a single joint does actually allow the identification of defined inter-individual differences in RA. This concept has been supported by independent studies focused on different analytical perspectives: immuno-histology (84, 85), gene expression profiling of whole tissue (86–92), and RNA-seq data from isolated synovial cells (93).
A critical issue remains the interpretation of the observed heterogeneity and two main models are currently emerging. In particular, whilst some studies have described the variability of synovial characteristics primarily as a function of the overall degree of inflammation intensity (92, 94), other analyses have proposed the existence of a more qualitative spectrum, with the identification of distinct synovitis categories, each characterized by congruent histological, molecular and cytological correlates (95, 96). Based on the relative enrichment of specific gene sets, these categories have been defined by Dennis et al. (95) as: (i) the lymphoid phenotype, enriched in genes related to B-T lymphocyte activation-differentiation, immunoglobulin production and antigen presentation; (ii) the myeloid phenotype, also characterized by processes associated with TNFα and IL-1β production, TLR and NOD-like receptor signaling, Fcγ-receptor-meditated phagocytosis; (iii) the fibroid phenotype, enriched for genes associated with TGFβ and BMP signaling, together with SMAD binding, but lacking enrichment of any immune system processes; (iv) the low inflammatory phenotype, showing only enrichment for inflammatory and wound response processes. These phenotypes, or similar patterns according to a recently revised classification (97), have been shown to present measurable associations with specific biomarkers in peripheral blood (CXCL13 and soluble ICAM-1 for the lymphoid and myeloid phenotype, respectively) and to be detectable in early-untreated RA, strengthening their differential biologic impact also at systemic level and in the absence of treatment biases (95, 97).
In clinical terms, despite it remains unclear whether the heterogeneity of synovial features does reflect fixed characteristics of specific disease subsets or dynamic phases conditioned by fluctuations of the inflammatory process, we have now proof-of-concept evidence that the assessment of synovial inter-individual differences does actually have the potential to predict clinically-relevant outcomes. Data supporting this idea derive from independent observational studies based on patients' stratification through either histological parameters or molecular signatures. Associations between synovial pathologic traits and clinical response to specific treatments has been obtained in studies focusing on agent targeting different molecular pathways, including anti-TNF (95, 98–104), IL-6 inhibitors (105), or B cell depleting agents (106–108), pointing at a wide spectrum of applicability. The assessment of synovial patho-biology in single joints has been shown also to hold an intrinsic potential for the development of prognostic biomarkers, as it can be inferred, for example, by the association between B cell-rich/lymphoid synovitis (109) and radiographic progression, recently confirmed in independent RA cohorts (85, 97, 104).
Despite current advancements, the development of a valid personalized approach to RA or SpA, based on synovial biopsy and applicable at community level still remains a very ambitious target. It should be indeed emphasized that data derived from available prediction studies, though promising, did not always led to univocal conclusions. Although differences might be obviously related to the limited sample size, differences in the definition of exposure variables and pre-set confounders, we might also consider that both RA and SpA are likely to be determined, as the majority of immune-inflammatory diseases, by a complex series of events controlled by polygenic, environmental and endocrine factors (110, 111). Some of these events might express themselves also at systemic level and in different anatomic compartments (112–115), providing a source of variability that may be missed by restricting the analysis to downstream inflammatory reactions. Response to treatment can be also influenced by patient-related subjective factors not directly reconcilable to measurable peripheral events (116). Thus, unlike oncology, in which the conception of a precision approach can be primarily based on genetic drivers, the approach to systemic immune-inflammatory diseases might require considering additional levels of complexity through the integration of different systems and clinical parameters (117) (Figure 1). A direct example supporting this hypothesis derives from the work performed by Lauwerys et al. demonstrating that the diagnostic accuracy of synovial analyses based on gene expression data increases from 56.8 to 98.6% by the addition of specific clinical symptoms in the prediction algorithm (57). Large size prospective multi-center clinical trials testing the relevance of biopsy-based patient stratification are currently in progress and are expected to offer direct insights into the actual predictive weight of synovial biopsy.
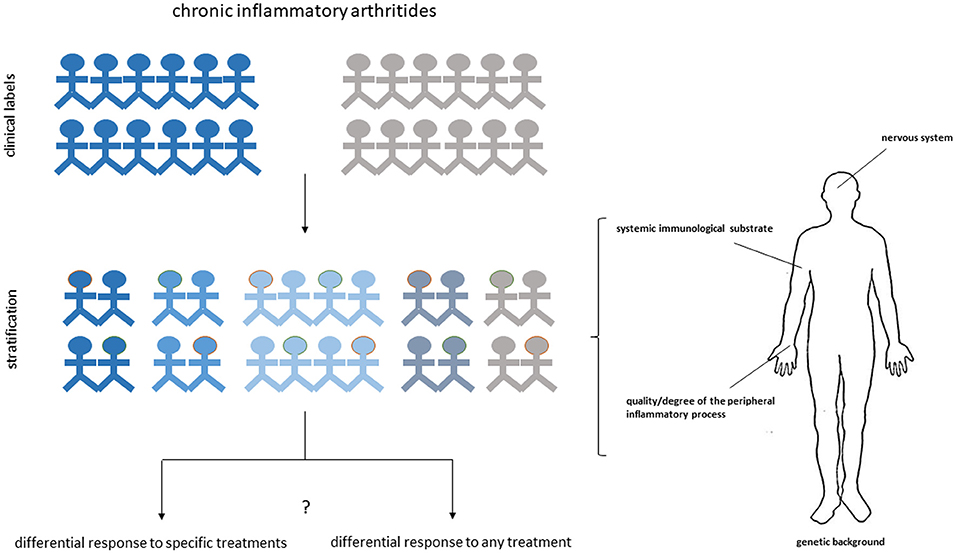
Figure 1. Multidimensional approach for personalized medicine in arthritis. Different anatomic and functional dimensions can cooperate to delineate the heterogeneous phenotype of chronic inflammatory arthritides and the predisposition of subgroup of patients to respond to specific treatments or to respond to any treatment. Dissecting the synovial histological and molecular characteristics of each individual patient may provide a fundamental contribution to the stratification process by offering a privileged window on the differential expression of the disease at target sites.
Taken together, the studies discussed in this review highlight the important, though circumstantial role that synovial biopsy can have in current clinical practice. Depending on the clinical context, it may complement and in some cases substitute less invasive procedures, offering the possibility to integrate microbiologic and histopathologic data. The combination of these approaches in certain circumstances can be essential to achieve a definite diagnosis in patients with arthritis of undetermined origin. The spectrum of applicability of synovial biopsy remains, however, relatively limited mostly due to the lack of validated markers for the diagnosis and management of major forms of chronic inflammatory arthritis.
The next challenge is thus to define the exploitability of the heterogeneous molecular and cellular patterns that characterize the synovial tissue in RA and SpA for the development of novel diagnostic markers and multi-dimensional precision medicine algorithms. Based on recent data from observational studies and the technological advancements in synovial tissue sampling and analysis this perspective seems now more realistic. Of considerable relevance in this direction is the recent introduction of novel cutting-edge tools allowing transcriptional profiling and single-cell RNA sequencing of infiltrating cells isolated from synovial samples (118, 119). This technology, which has already contributed to the achievement of important goals in the characterization of novel cell subsets in RA (93, 120, 121), is expected in the near future to play a key role also in the field of biomarker discovery and in clinical translation. It is indeed likely that, compared to whole-tissue gene expression analyses, the assessment of synovial characteristics at single cell level might dramatically expand our possibilities to screen specific aspects of the pathogenic process and to unravel intrinsic characteristics of the disease. The fine deconstruction of the histopathological, molecular and cellular heterogeneity of the synovial inflammatory process by means of integrated high-throughput approaches might also lead in the near future to a novel taxonomic classification of chronic inflammatory arthritides, firmly rooted in basic pathogenic processes and, possibly, spanning across the boundaries of conventional clinical labels.
AM, SB, and SR contributed to literature review and preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AN declared a past co-authorship with one of the authors AM to the handling editor.
1. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjogren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. (2017) 76:9–16. doi: 10.1136/annrheumdis-2016-210571
2. Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, Visser M, et al. 2017 European league against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. (2017) 76:1955–64. doi: 10.1136/annrheumdis-2017-211468corr1
3. Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. (2012) 64:797–808. doi: 10.1002/acr.21664
4. Orr C, Vieira-Sousa E, Boyle DL, Buch MH, Buckley CD, Canete JD, et al. Synovial tissue research: a state-of-the-art review. Nat Rev Rheumatol. (2017) 13:463–75. doi: 10.1038/nrrheum.2017.161
5. Koski JM, Helle M. Ultrasound guided synovial biopsy using portal and forceps. Ann Rheum Dis. (2005) 64:926–9. doi: 10.1136/ard.2004.027409
6. Scire CA, Epis O, Codullo V, Humby F, Morbini P, Manzo A, et al. Immunohistological assessment of the synovial tissue in small joints in rheumatoid arthritis: validation of a minimally invasive ultrasound-guided synovial biopsy procedure. Arthritis Res Ther. (2007) 9:R101. doi: 10.1186/ar2302
7. Kelly S, Humby F, Filer A, Ng N, Di Cicco M, Hands RE, et al. Ultrasound-guided synovial biopsy: a safe, well-tolerated and reliable technique for obtaining high-quality synovial tissue from both large and small joints in early arthritis patients. Ann Rheum Dis. (2015) 74:611–7. doi: 10.1136/annrheumdis-2013-204603
8. Humby F, Kelly S, Hands R, Rocher V, DiCicco M, Ng N, et al. Use of ultrasound-guided small joint biopsy to evaluate the histopathologic response to rheumatoid arthritis therapy: recommendations for application to clinical trials. Arthritis Rheumatol. (2015) 67:2601–10. doi: 10.1002/art.39235
9. Humby F, Kelly S, Bugatti S, Manzo A, Filer A, Mahto A, et al. Evaluation of minimally invasive, ultrasound-guided synovial biopsy techniques by the OMERACT filter–determining validation requirements. J Rheumatol. (2016) 43:208–13. doi: 10.3899/jrheum.141199
10. Humby F, Romao VC, Manzo A, Filer A, Bugatti S, Vieira-Sousa E, et al. A multicenter retrospective analysis evaluating performance of synovial biopsy techniques in patients with inflammatory arthritis: arthroscopic versus ultrasound-guided versus blind needle biopsy. Arthritis Rheumatol. (2018) 70:702–10. doi: 10.1002/art.40433
11. Humby FC. Synovial tissue sampling in rheumatological practice-past developments and future perspectives. Front Med. (2019) 6:4. doi: 10.3389/fmed.2019.00004
12. Johnson JS, Freemont AJ. A 10 year retrospective comparison of the diagnostic usefulness of synovial fluid and synovial biopsy examination. J Clin Pathol. (2001) 54:605–7. doi: 10.1136/jcp.54.8.605
13. Coiffier G, Ferreyra M, Albert JD, Stock N, Jolivet-Gougeon A, Perdriger A, et al. Ultrasound-guided synovial biopsy improves diagnosis of septic arthritis in acute arthritis without enough analyzable synovial fluid: a retrospective analysis of 176 arthritis from a French rheumatology department. Clin Rheumatol. (2018) 37:2241–9. doi: 10.1007/s10067-018-4160-9
14. Fink B, Makowiak C, Fuerst M, Berger I, Schafer P, Frommelt L. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J Bone Joint Surg Br. (2008) 90:874–8. doi: 10.1302/0301-620X.90B7.20417
15. Fink B, Gebhard A, Fuerst M, Berger I, Schafer P. High diagnostic value of synovial biopsy in periprosthetic joint infection of the hip. Clin Orthop Relat Res. (2013) 471:956–64. doi: 10.1007/s11999-012-2474-5
16. Williams JL, Norman P, Stockley I. The value of hip aspiration versus tissue biopsy in diagnosing infection before exchange hip arthroplasty surgery. J Arthroplasty. (2004) 19:582–6. doi: 10.1016/j.arth.2003.11.011
17. Cross MC, Kransdorf MJ, Chivers FS, Lorans R, Roberts CC, Schwartz AJ, et al. Utility of percutaneous joint aspiration and synovial biopsy in identifying culture-positive infected hip arthroplasty. Skeletal Radiol. (2014) 43:165–8. doi: 10.1007/s00256-013-1757-6
18. Graf SW, Buchbinder R, Zochling J, Whittle SL. The accuracy of methods for urate crystal detection in synovial fluid and the effect of sample handling: a systematic review. Clin Rheumatol. (2013) 32:225–32. doi: 10.1007/s10067-012-2107-0
19. van der Heijden IM, Wilbrink B, Vije AE, Schouls LM, Breedveld FC, Tak PP. Detection of bacterial DNA in serial synovial samples obtained during antibiotic treatment from patients with septic arthritis. Arthritis Rheum. (1999) 42:2198–203. doi: 10.1002/1529-0131(199910)42:10<2198::AID-ANR23>3.0.CO;2-N
20. Najm A, Orr C, Heymann MF, Bart G, Veale DJ, Le Goff B. Success rate and utility of ultrasound-guided synovial biopsies in clinical practice. J Rheumatol. (2016) 43:2113–9. doi: 10.3899/jrheum.151441
21. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. (2014) 42:385–91. doi: 10.1007/s15010-013-0567-z
22. Slot O, Terslev L. Ultrasound-guided dry-needle synovial tissue aspiration for diagnostic microscopy in gout patients presenting without synovial effusion or clinically detectable tophi. J Clin Rheumatol. (2015) 21:167–8. doi: 10.1097/RHU.0000000000000228
23. Moses V, Asirvatham JR, McHugh J, Ike R. Synovial biopsy in the diagnosis of crystal-associated arthropathies. J Clin Rheumatol. (2019). doi: 10.1097/RHU.0000000000000993. [Epub ahead of print].
24. Filippou G, Tacchini D, Adinolfi A, Bertoldi I, Picerno V, Toscano C, et al. Histology of the synovial membrane of patients affected by osteoarthritis and calcium pyrophosphate dihydrate crystal deposition disease vs. osteoarthritis alone: a pilot study. Scand J Rheumatol. (2016) 45:538–9. doi: 10.3109/03009742.2016.1150508
25. Munoz-Gomez J, Bergada-Barado E, Gomez-Perez R, Llopart-Buisan E, Subias-Sobrevia E, Rotes-Querol J, et al. Amyloid arthropathy in patients undergoing periodical haemodialysis for chronic renal failure: a new complication. Ann Rheum Dis. (1985) 44:729–33. doi: 10.1136/ard.44.11.729
26. Lakhanpal S, Li CY, Gertz MA, Kyle RA, Hunder GG. Synovial fluid analysis for diagnosis of amyloid arthropathy. Arthritis Rheum. (1987) 30:419–23. doi: 10.1002/art.1780300409
27. Kruithof E, Baeten D, Veys EM, De Keyser F, Suykens S, De Wilde L, et al. Case Number 29: ochronosis: synovial histopathological characteristics. Ann Rheum Dis. (2004) 63:130. doi: 10.1136/ard.2003.013912
28. Bhangle S, Panush RS, Berman EL, Schumacher HR. Clinical images: synovial fluid clues to ochronosis. Arthritis Rheum. (2012) 64:473. doi: 10.1002/art.33409
29. Gerlag DM, Tak PP. How to perform and analyse synovial biopsies. Best Pract Res Clin Rheumatol. (2013) 27:195–207. doi: 10.1016/j.berh.2013.03.006
30. Bresnihan B. Are synovial biopsies of diagnostic value? Arthritis Res Ther. (2003) 5:271–8. doi: 10.1186/ar1003
31. Pandey R, Drakoulakis E, Athanasou NA. An assessment of the histological criteria used to diagnose infection in hip revision arthroplasty tissues. J Clin Pathol. (1999) 52:118–23. doi: 10.1136/jcp.52.2.118
32. Morawietz L, Tiddens O, Mueller M, Tohtz S, Gansukh T, Schroeder JH, et al. Twenty-three neutrophil granulocytes in 10 high-power fields is the best histopathological threshold to differentiate between aseptic and septic endoprosthesis loosening. Histopathology. (2009) 54:847–53. doi: 10.1111/j.1365-2559.2009.03313.x
33. Della Beffa C, Slansky E, Pommerenke C, Klawonn F, Li J, Dai L, et al. The relative composition of the inflammatory infiltrate as an additional tool for synovial tissue classification. PLoS ONE. (2013) 8:e72494. doi: 10.1371/journal.pone.0072494
34. Pessler F, Dai L, Diaz-Torne C, Ogdie A, Gomez-Vaquero C, Paessler ME, et al. Increased angiogenesis and cellular proliferation as hallmarks of the synovium in chronic septic arthritis. Arthritis Rheum. (2008) 59:1137–46. doi: 10.1002/art.23915
35. Jalava J, Skurnik M, Toivanen A, Toivanen P, Eerola E. Bacterial PCR in the diagnosis of joint infection. Ann Rheum Dis. (2001) 60:287–9. doi: 10.1136/ard.60.3.287
36. van der Heijden IM, Wilbrink B, Tchetverikov I, Schrijver IA, Schouls LM, Hazenberg MP, et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. (2000) 43:593–8. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1
37. Fernandes S, Vieira-Sousa E, Furtado C, Costa A, Barros R, Fonseca JE. A diagnosis of disseminated tuberculosis based on knee arthroscopic guided synovial biopsy in the context of monoarthritis. Acta Reumatol Port. (2016) 41:256–9.
38. van der Heijden IM, Wilbrink B, Schouls LM, van Embden JD, Breedveld FC, Tak PP. Detection of mycobacteria in joint samples from patients with arthritis using a genus-specific polymerase chain reaction and sequence analysis. Rheumatology. (1999) 38:547–53. doi: 10.1093/rheumatology/38.6.547
39. van der Heijden IM, Wilbrink B, Rijpkema SG, Schouls LM, Heymans PH, van Embden JD, et al. Detection of Borrelia burgdorferi sensu stricto by reverse line blot in the joints of Dutch patients with Lyme arthritis. Arthritis Rheum. (1999) 42:1473–80. doi: 10.1002/1529-0131(199907)42:7<1473::AID-ANR22>3.0.CO;2-I
40. O'Duffy JD, Griffing WL, Li CY, Abdelmalek MF, Persing DH. Whipple's arthritis: direct detection of Tropheryma whippelii in synovial fluid and tissue. Arthritis Rheum. (1999) 42:812–7. doi: 10.1002/1529-0131(199904)42:4<812::AID-ANR27>3.0.CO;2-S
41. Donovan A, Schweitzer ME, Garcia RA, Nomikos G. Chronic lymphocytic leukemia/small lymphocytic lymphoma presenting as septic arthritis of the shoulder. Skeletal Radiol. (2008) 37:1035–9. doi: 10.1007/s00256-008-0512-x
42. Goldenberg DL, Kelley W, Gibbons RB. Metastatic adenocarcinoma of synovium presenting as an acute arthritis. Diagnosis by closed synovial biopsy. Arthritis Rheum. (1975) 18:107–10. doi: 10.1002/art.1780180202
43. O'Connell JX. Pathology of the synovium. Am J Clin Pathol. (2000) 114:773–84. doi: 10.1309/LWW3-5XK0-FKG9-HDRK
44. Gorman JD, Danning C, Schumacher HR, Klippel JH, Davis JC Jr. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. (2000) 43:930–8. doi: 10.1002/1529-0131(200004)43:4<930::AID-ANR27>3.0.CO;2-A
45. Cavalli G, Guglielmi B, Berti A, Campochiaro C, Sabbadini MG, Dagna L. The multifaceted clinical presentations and manifestations of Erdheim-Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis. (2013) 72:1691–5. doi: 10.1136/annrheumdis-2012-202542
46. Kroot EJ, Weel AE, Hazes JM, Zondervan PE, Heijboer MP, van Daele PL, et al. Diagnostic value of blind synovial biopsy in clinical practice. Rheumatology. (2006) 45:192–5. doi: 10.1093/rheumatology/kei117
47. Sitt JC, Griffith JF, Lai FM, Hui M, Chiu KH, Lee RK, et al. Ultrasound-guided synovial Tru-cut biopsy: indications, technique, and outcome in 111 cases. Eur Radiol. (2017) 27:2002–10. doi: 10.1007/s00330-016-4545-6
48. Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJ, Kraan MC, Baeten D, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. (2005) 64:834–8. doi: 10.1136/ard.2004.029751
49. Baeten D, Houbiers J, Kruithof E, Vandooren B, Van den Bosch F, Boots AM, et al. Synovial inflammation does not change in the absence of effective treatment: implications for the use of synovial histopathology as biomarker in early phase clinical trials in rheumatoid arthritis. Ann Rheum Dis. (2006) 65:990–7. doi: 10.1136/ard.2005.047852
50. Wijbrandts CA, Vergunst CE, Haringman JJ, Gerlag DM, Smeets TJ, Tak PP. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. (2007) 56:3869–71. doi: 10.1002/art.22964
51. Bresnihan B, Pontifex E, Thurlings RM, Vinkenoog M, El-Gabalawy H, Fearon U, et al. Synovial tissue sublining CD68 expression is a biomarker of therapeutic response in rheumatoid arthritis clinical trials: consistency across centers. J Rheumatol. (2009) 36:1800–2. doi: 10.3899/jrheum.090348
52. Zink A, Albrecht K. Rheumatoid arthritis: the benefits of early treatment after decades. Nat Rev Rheumatol. (2017) 13:458–9. doi: 10.1038/nrrheum.2017.104
53. Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. (2015) 74:1045–50. doi: 10.1136/annrheumdis-2013-204858
54. van der Helm-vanMil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. (2007) 56:433–40. doi: 10.1002/art.22380
55. Baeten D, Kruithof E, De Rycke L, Vandooren B, Wyns B, Boullart L, et al. Diagnostic classification of spondylarthropathy and rheumatoid arthritis by synovial histopathology: a prospective study in 154 consecutive patients. Arthritis Rheum. (2004) 50:2931–41. doi: 10.1002/art.20476
56. Kraan MC, Haringman JJ, Post WJ, Versendaal J, Breedveld FC, Tak PP. Immunohistological analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology. (1999) 38:1074–80. doi: 10.1093/rheumatology/38.11.1074
57. Lauwerys BR, Hernandez-Lobato D, Gramme P, Ducreux J, Dessy A, Focant I, et al. Heterogeneity of synovial molecular patterns in patients with arthritis. PLoS ONE. (2015) 10:e0122104. doi: 10.1371/journal.pone.0122104
58. Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. (2006) 49:358–64. doi: 10.1111/j.1365-2559.2006.02508.x
59. Najm A, le Goff B, Venet G, Garraud T, Amiaud J, Biha N, et al. IMSYC immunologic synovitis score: a new score for synovial membrane characterization in inflammatory and non-inflammatory arthritis. Joint Bone Spine. (2018) 86:77–81. doi: 10.1136/annrheumdis-2017-eular.2547
60. Pessler F, Ogdie A, Diaz-Torne C, Dai L, Yu X, Einhorn E, et al. Subintimal Ki-67 as a synovial tissue biomarker for inflammatory arthropathies. Ann Rheum Dis. (2008) 67:162–7. doi: 10.1136/ard.2007.071670
61. Manzo A, Bombardieri M, Humby F, Pitzalis C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: from inflammation to autoimmunity and tissue damage/remodeling. Immunol Rev. (2010) 233:267–85. doi: 10.1111/j.0105-2896.2009.00861.x
62. Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. (2005) 35:1347–59. doi: 10.1002/eji.200425830
63. Canete JD, Santiago B, Cantaert T, Sanmarti R, Palacin A, Celis R, et al. Ectopic lymphoid neogenesis in psoriatic arthritis. Ann Rheum Dis. (2007) 66:720–6. doi: 10.1136/ard.2006.062042
64. Espinoza LR, Vasey FB, Espinoza CG, Bocanegra TS, Germain BF. Vascular changes in psoriatic synovium. A light and electron microscopic study. Arthritis Rheum. (1982) 25:677–84. doi: 10.1002/art.1780250611
65. Veale D, Yanni G, Rogers S, Barnes L, Bresnihan B, Fitzgerald O. Reduced synovial membrane macrophage numbers, ELAM-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum. (1993) 36:893–900. doi: 10.1002/art.1780360705
66. Baeten D, Demetter P, Cuvelier C, Van Den Bosch F, Kruithof E, Van Damme N, et al. Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: influence of disease duration and activity. Ann Rheum Dis. (2000) 59:945–53. doi: 10.1136/ard.59.12.945
67. Kruithof E, Baeten D, De Rycke L, Vandooren B, Foell D, Roth J, et al. Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res Ther. (2005) 7:R569–80. doi: 10.1186/ar1698
68. Fearon U, Griosios K, Fraser A, Reece R, Emery P, Jones PF, et al. Angiopoietins, growth factors, and vascular morphology in early arthritis. J Rheumatol. (2003) 30:260–8.
69. Yeremenko N, Noordenbos T, Cantaert T, van Tok M, van de Sande M, Canete JD, et al. Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum. (2013) 65:174–85. doi: 10.1002/art.37704
70. van Kuijk AW, Reinders-Blankert P, Smeets TJ, Dijkmans BA, Tak PP. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann Rheum Dis. (2006) 65:1551–7. doi: 10.1136/ard.2005.050963
71. Baeten D, Moller HJ, Delanghe J, Veys EM, Moestrup SK, De Keyser F. Association of CD163+ macrophages and local production of soluble CD163 with decreased lymphocyte activation in spondylarthropathy synovitis. Arthritis Rheum. (2004) 50:1611–23. doi: 10.1002/art.20174
72. Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Canete JD, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. (2012) 64:99–109. doi: 10.1002/art.33396
73. van de Sande MG, Thurlings RM, Boumans MJ, Wijbrandts CA, Modesti MG, Gerlag DM, et al. Presence of lymphocyte aggregates in the synovium of patients with early arthritis in relationship to diagnosis and outcome: is it a constant feature over time? Ann Rheum Dis. (2011) 70:700–3. doi: 10.1136/ard.2010.139287
74. Romao VC, Vital EM, Fonseca JE, Buch MH. Right drug, right patient, right time: aspiration or future promise for biologics in rheumatoid arthritis? Arthritis Res Ther. (2017) 19:239. doi: 10.1186/s13075-017-1445-3
75. Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. (2008) 26:651–75. doi: 10.1146/annurev.immunol.26.021607.090244
76. Bugatti S, Manzo A, Montecucco C, Caporali R. The clinical value of autoantibodies in rheumatoid arthritis. Front Med. (2018) 5:339. doi: 10.3389/fmed.2018.00339
77. Dolhain RJ, Ter Haar NT, De Kuiper R, Nieuwenhuis IG, Zwinderman AH, Breedveld FC, et al. Distribution of T cells and signs of T-cell activation in the rheumatoid joint: implications for semiquantitative comparative histology. Br J Rheumatol. (1998) 37:324–30. doi: 10.1093/rheumatology/37.3.324
78. Kennedy TD, Plater-Zyberk C, Partridge TA, Woodrow DF, Maini RN. Representative sample of rheumatoid synovium: a morphometric study. J Clin Pathol. (1988) 41:841–6. doi: 10.1136/jcp.41.8.841
79. Bresnihan B, Cunnane G, Youssef P, Yanni G, Fitzgerald O, Mulherin D. Microscopic measurement of synovial membrane inflammation in rheumatoid arthritis: proposals for the evaluation of tissue samples by quantitative analysis. Br J Rheumatol. (1998) 37:636–42. doi: 10.1093/rheumatology/37.6.636
80. Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. (2003) 5:R352–60. doi: 10.1186/ar1004
81. Kraan MC, Reece RJ, Smeets TJ, Veale DJ, Emery P, Tak PP. Comparison of synovial tissues from the knee joints and the small joints of rheumatoid arthritis patients: implications for pathogenesis and evaluation of treatment. Arthritis Rheum. (2002) 46:2034–8. doi: 10.1002/art.10556
82. Musters A, Klarenbeek PL, Doorenspleet ME, Balzaretti G, Esveldt REE, van Schaik BDC, et al. In rheumatoid arthritis, synovitis at different inflammatory sites is dominated by shared but patient-specific T cell clones. J Immunol. (2018) 201:417–22. doi: 10.4049/jimmunol.1800421
83. Ai R, Hammaker D, Boyle DL, Morgan R, Walsh AM, Fan S, et al. Joint-specific DNA methylation and transcriptome signatures in rheumatoid arthritis identify distinct pathogenic processes. Nat Commun. (2016) 7:11849. doi: 10.1038/ncomms11849
84. Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O'Fallon WM, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. (2001) 167:1072–80. doi: 10.4049/jimmunol.167.2.1072
85. Bugatti S, Manzo A, Vitolo B, Benaglio F, Binda E, Scarabelli M, et al. High expression levels of the B cell chemoattractant CXCL13 in rheumatoid synovium are a marker of severe disease. Rheumatology. (2014) 53:1886–95. doi: 10.1093/rheumatology/keu163
86. van der Pouw Kraan TC, van Gaalen FA, Kasperkovitz PV, Verbeet NL, Smeets TJ, Kraan MC, et al. Rheumatoid arthritis is a heterogeneous disease: evidence for differences in the activation of the STAT-1 pathway between rheumatoid tissues. Arthritis Rheum. (2003) 48:2132–45. doi: 10.1002/art.11096
87. van der Pouw Kraan TC, van Gaalen FA, Huizinga TW, Pieterman E, Breedveld FC, Verweij CL. Discovery of distinctive gene expression profiles in rheumatoid synovium using cDNA microarray technology: evidence for the existence of multiple pathways of tissue destruction and repair. Genes Immun. (2003) 4:187–96. doi: 10.1038/sj.gene.6363975
88. Tsubaki T, Arita N, Kawakami T, Shiratsuchi T, Yamamoto H, Takubo N, et al. Characterization of histopathology and gene-expression profiles of synovitis in early rheumatoid arthritis using targeted biopsy specimens. Arthritis Res Ther. (2005) 7:R825–36. doi: 10.1186/ar1751
89. Lindberg J, af Klint E, Ulfgren AK, Stark A, Andersson T, Nilsson P, et al. Variability in synovial inflammation in rheumatoid arthritis investigated by microarray technology. Arthritis Res Ther. (2006) 8:R47. doi: 10.1186/ar1903
90. Timmer TC, Baltus B, Vondenhoff M, Huizinga TW, Tak PP, Verweij CL, et al. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. (2007) 56:2492–502. doi: 10.1002/art.22748
91. Huber R, Hummert C, Gausmann U, Pohlers D, Koczan D, Guthke R, et al. Identification of intra-group, inter-individual, and gene-specific variances in mRNA expression profiles in the rheumatoid arthritis synovial membrane. Arthritis Res Ther. (2008) 10:R98. doi: 10.1186/ar2485
92. van Baarsen LG, Wijbrandts CA, Timmer TC, van der Pouw Kraan TC, Tak PP, Verweij CL. Synovial tissue heterogeneity in rheumatoid arthritis in relation to disease activity and biomarkers in peripheral blood. Arthritis Rheum. (2010) 62:1602–7. doi: 10.1002/art.27415
93. Mandelin AM II, Homan PJ, Shaffer AM, Cuda CM, Dominguez ST, Bacalao E, et al. Transcriptional profiling of synovial macrophages using minimally invasive ultrasound-guided synovial biopsies in rheumatoid arthritis. Arthritis Rheumatol. (2018) 70:841–54. doi: 10.1002/art.40453
94. Orange DE, Agius P, DiCarlo EF, Robine N, Geiger H, Szymonifka J, et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. (2018) 70:690–701. doi: 10.1002/art.40428
95. Dennis G Jr, Holweg CT, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA, et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther. (2014) 16:R90. doi: 10.1186/ar4555
96. Pitzalis C, Kelly S, Humby F. New learnings on the pathophysiology of RA from synovial biopsies. Curr Opin Rheumatol. (2013) 25:334–44. doi: 10.1097/BOR.0b013e32835fd8eb
97. Humby F, Lewis M, Ramamoorthi N, Hackney JA, Barnes MR, Bombardieri M, et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann Rheum Dis. (2019) doi: 10.1136/annrheumdis-2018-214539. [Epub ahead of print].
98. van der Pouw Kraan TC, Wijbrandts CA, van Baarsen LG, Rustenburg F, Baggen JM, Verweij CL, et al. Responsiveness to anti-tumour necrosis factor alpha therapy is related to pre-treatment tissue inflammation levels in rheumatoid arthritis patients. Ann Rheum Dis. (2008) 67:563–6. doi: 10.1136/ard.2007.081950
99. Wijbrandts CA, Dijkgraaf MG, Kraan MC, Vinkenoog M, Smeets TJ, Dinant H, et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann Rheum Dis. (2008) 67:1139–44. doi: 10.1136/ard.2007.080440
100. Badot V, Galant C, Nzeusseu Toukap A, Theate I, Maudoux AL, Van den Eynde BJ, et al. Gene expression profiling in the synovium identifies a predictive signature of absence of response to adalimumab therapy in rheumatoid arthritis. Arthritis Res Ther. (2009) 11:R57. doi: 10.1186/ar2678
101. Canete JD, Celis R, Moll C, Izquierdo E, Marsal S, Sanmarti R, et al. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. (2009) 68:751–6. doi: 10.1136/ard.2008.089284
102. Klaasen R, Thurlings RM, Wijbrandts CA, van Kuijk AW, Baeten D, Gerlag DM, et al. The relationship between synovial lymphocyte aggregates and the clinical response to infliximab in rheumatoid arthritis: a prospective study. Arthritis Rheum. (2009) 60:3217–24. doi: 10.1002/art.24913
103. Lindberg J, Wijbrandts CA, van Baarsen LG, Nader G, Klareskog L, Catrina A, et al. The gene expression profile in the synovium as a predictor of the clinical response to infliximab treatment in rheumatoid arthritis. PLoS ONE. (2010) 5:e11310. doi: 10.1371/journal.pone.0011310
104. Orr C, Najm A, Biniecka M, McGarry T, Ng CT, Young F, et al. Synovial immunophenotype and anti-citrullinated peptide antibodies in rheumatoid arthritis patients: relationship to treatment response and radiologic prognosis. Arthritis Rheumatol. (2017) 69:2114–23. doi: 10.1002/art.40218
105. Ducreux J, Durez P, Galant C, Nzeusseu Toukap A, Van den Eynde B, Houssiau FA, et al. Global molecular effects of tocilizumab therapy in rheumatoid arthritis synovium. Arthritis Rheumatol. (2014) 66:15–23. doi: 10.1002/art.38202
106. Gutierrez-Roelens I, Galant C, Theate I, Lories RJ, Durez P, Nzeusseu-Toukap A, et al. Rituximab treatment induces the expression of genes involved in healing processes in the rheumatoid arthritis synovium. Arthritis Rheum. (2011) 63:1246–54. doi: 10.1002/art.30292
107. Teng YK, Levarht EW, Hashemi M, Bajema IM, Toes RE, Huizinga TW, et al. Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis Rheum. (2007) 56:3909–18. doi: 10.1002/art.22967
108. Hogan VE, Holweg CT, Choy DF, Kummerfeld SK, Hackney JA, Teng YK, et al. Pretreatment synovial transcriptional profile is associated with early and late clinical response in rheumatoid arthritis patients treated with rituximab. Ann Rheum Dis. (2012) 71:1888–94. doi: 10.1136/annrheumdis-2011-201115
109. Bugatti S, Vitolo B, Caporali R, Montecucco C, Manzo A. B cells in rheumatoid arthritis: from pathogenic players to disease biomarkers. Biomed Res Int. (2014) 2014:681678. doi: 10.1155/2014/681678
110. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. (2016) 388:2023–38. doi: 10.1016/S0140-6736(16)30173-8
111. Dougados M, Baeten D. Spondyloarthritis. Lancet. (2011) 377:2127–37. doi: 10.1016/S0140-6736(11)60071-8
112. Klareskog L, Catrina AI. Autoimmunity: lungs and citrullination. Nat Rev Rheumatol. (2015) 11:261–2. doi: 10.1038/nrrheum.2015.38
113. Bugatti S, Manzo A, Caporali R, Montecucco C. Inflammatory lesions in the bone marrow of rheumatoid arthritis patients: a morphological perspective. Arthritis Res Ther. (2012) 14:229. doi: 10.1186/ar4115
114. Manzo A, Caporali R, Vitolo B, Alessi S, Benaglio F, Todoerti M, et al. Subclinical remodelling of draining lymph node structure in early and established rheumatoid arthritis assessed by power Doppler ultrasonography. Rheumatology. (2011) 50:1395–400. doi: 10.1093/rheumatology/ker076
115. Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. (2015) 21:895–905. doi: 10.1038/nm.3914
116. Rech J, Hess A, Finzel S, Kreitz S, Sergeeva M, Englbrecht M, et al. Association of brain functional magnetic resonance activity with response to tumor necrosis factor inhibition in rheumatoid arthritis. Arthritis Rheum. (2013) 65:325–33. doi: 10.1002/art.37761
117. Chan AC, Behrens TW. Personalizing medicine for autoimmune and inflammatory diseases. Nat Immunol. (2013) 14:106–9. doi: 10.1038/ni.2473
118. Donlin LT, Rao DA, Wei K, Slowikowski K, McGeachy MJ, Turner JD, et al. Methods for high-dimensonal analysis of cells dissociated from cyropreserved synovial tissue. Arthritis Res Ther. (2018) 20:139. doi: 10.1186/s13075-018-1631-y
119. Stephenson W, Donlin LT, Butler A, Rozo C, Bracken B, Rashidfarrokhi A, et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat Commun. (2018) 9:791. doi: 10.1038/s41467-017-02659-x
120. Canavan M, Walsh AM, Bhargava V, Wade SM, McGarry T, Marzaioli V, et al. Enriched Cd141+ DCs in the joint are transcriptionally distinct, activated, and contribute to joint pathogenesis. JCI Insight. (2018) 3:23. doi: 10.1172/jci.insight.95228
Keywords: synovial biopsy, arthritis, synovitis, biomarkers, precision medicine
Citation: Manzo A, Bugatti S and Rossi S (2019) Clinical Applications of Synovial Biopsy. Front. Med. 6:102. doi: 10.3389/fmed.2019.00102
Received: 20 December 2018; Accepted: 25 April 2019;
Published: 10 May 2019.
Edited by:
João Eurico Fonseca, Universidade de Lisboa, PortugalReviewed by:
Caroline Ospelt, University of Zurich, SwitzerlandCopyright © 2019 Manzo, Bugatti and Rossi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Manzo, YW50b25pby5tYW56b0B1bmlwdi5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.