
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 19 February 2019
Sec. Geriatric Medicine
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00026
Background/Aim: Current evidence in the literature supports associations between frailty, cognitive impairment, and dementia. The study aim was to describe the risk of cognitive disorders associated with physical frailty in older adults from community-based studies.
Methods: We performed a systematic review and meta-analysis, using MEDLINE, PsycINFO, Scopus, and Web of Science as databases for the search. Cohort and longitudinal studies were included in qualitative analysis and quantitative synthesis. For inclusion, studies had to assess dementia and cognitive impairment as a primary or secondary outcome, and describe the prevalence of frailty among participants at baseline and follow-up.
Results: Of the 2,210 studies retrieved by the systematic review, 6 relevant studies were included in a meta-analysis. Baseline frailty was significantly associated with an increased risk of geriatric cognitive disorders (pooled OR = 1.80, 95% CI = 1.11–2.92; p = 0.02). Heterogeneity across the studies was significant (I2 = 79%).
Conclusions: The analyses confirmed that frail older adults were at higher risk of incident cognitive disorders than non-frail elders. Frailty status seems to be most associated with the risk of incident dementia. Frailty may represent a risk factor for dementia and could constitute a novel modifiable target in early cognitive impairment.
From its definition, frailty can be understood as a state of higher vulnerability to stressors attributed to a lower homeostatic reserve due to an age-related multisystem physiological change (1). Frailty refers to a potentially reversible pathological aging process that occurs at an intermediate stage between aging-related diseases (senility) and relevant adverse outcomes such as disability and death (2). It is a common geriatric condition with a mean prevalence of 10% (3). Gill et al. conducted a study investigating risk factors associated with disability in the last year of life and reported that frailty was the condition most frequently leading to death (4).
Several types of operational definitions have emerged contributing to the diagnosis of frailty, ranging from physical or phenotype criteria [e.g., Fried's phenotype criteria (1)] to multidimensional models [e.g., Frailty Index (5)]. A third frailty model warranting special attention is the biopsychosocial model (another multidimensional model) which combines physical and psychosocial domains (6). This construct is oriented toward the social sciences and emphasizes the importance of an integral conceptual definition of frailty (7). In general, independently of the validated criteria used, the diagnosis of frailty is associated with adverse health outcomes (falls, disability, hospitalization, institutionalization, or death) (2).
Current evidence in the literature from cross-sectional and longitudinal studies has shown relationships between frailty and cognitive disorders (including mild cognitive impairment and dementia) (8–10). Frailty may increase the future risk of mild cognitive impairment (MCI) and all-cause dementia in cognitively unimpaired populations, as well as accelerate cognitive decline of these individuals (11). Furthermore, components of frailty appeared to be related to pathological findings of Alzheimer's disease (AD) and vascular dementia, supporting the notion of a possible common biological pathway between frailty and cognitive disorders (12, 13). Despite this evidence, there is debate over the magnitude of the association between frailty and cognitive impairment. Some longitudinal studies show that frailty is associated with dementia, especially vascular dementia (14–16). Frailty was identified retrospectively, or using non-validated criteria, in many other studies (10, 13, 17, 18). Additionally, in previous systematic reviews and meta-analyses, more recently published studies were not included and the number of incident cognitive impairment cases among frail participants was not clearly reported (19–21).
Interest in this field of research has been growing rapidly in the past 5 years (22, 23). It is thus essential to define the relevant aspects that are useful for the definition of the construct of cognitive frailty for use in both clinical practice and research (22, 23). Therefore, the understanding of the relationship between frailty and geriatric cognitive disorders could contribute to new interventions for the prevention and management of both conditions. Finally, the main objective of this systematic review and meta-analysis was to describe the risk of development of cognitive disorders in previously cognitively unimpaired community-dwelling older adults or those with MCI associated with frailty at baseline from longitudinal and cohort studies.
A systematic literature search of PubMed (MEDLINE), SCOPUS, PsycINFO, and Web of Science from 1st March 2001 through January 2018 was conducted according to the Standards for Systematic Reviews (24) and the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (25). The publication period was decided based on the most widely used definition of frailty, Fried's phenotype criteria (1), published on 1st March, 2001. In addition to the date limit, the following filters were used: English language, humans, aged 65 years or older. The inclusion criteria were: (i) older adults without dementia at baseline; (ii) community-dwelling population; (iii) cohort or longitudinal studies; (iv) frailty defined according to common, validated and recognized criteria, and evaluated prospectively; (v) incidence of geriatric cognitive disorders at the end of a follow-up of at least 2 years; and finally; (vi) if dementia was the main outcome, it had to be diagnosed based on well-known established criteria such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) (26) or National Institute of Neurological and Communicative Disorders and Stroke, Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) (27) or National Institute of Neurological Disorders and Stroke, Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS-AIREN) (28).
The search terms used included the following: [(“cognition”[MeSH] OR “cognition”) OR (“cognitive dysfunction”[MeSH] OR (“cognitive” AND “dysfunction”) OR “cognitive dysfunction” OR (“mild” AND “cognitive” AND “impairment” OR “mild cognitive impairment”) OR (“dementia”[MeSH] OR “dementia”)] AND [(“frailty”[MeSH] OR “frailty”) OR (“frail elderly”[MeSH] OR (“frail” AND “elderly”) OR “frail elderly”)]. The bibliographies of relevant reviews and meta-analyses involving frailty and cognitive impairment were also manually searched and additional references obtained from outside experts.
Two independent authors reviewed each study abstract according to the inclusion criteria, and the full text of all studies retrieved by the literature search for eligibility. Cohort and longitudinal studies that assessed dementia and cognitive impairment as a primary or secondary outcome and described the prevalence of frailty among participants at baseline were included in the quantitative synthesis. Only studies conducted among community-dwelling older adults were included. Studies that were reviews, editorials or letters, clinical, and cross-sectional studies were excluded. Any disagreement over studies selected by any of the authors was resolved by consensus of the authors involved.
Two authors extracted the data according to a predefined format for presentation: author, year; population, exposures, comparators, outcomes, and study design. The authors abstracted study design information, population characteristics at baseline, exposure details, disease prevalence at baseline, and incidence at the end of follow-up, and risk estimates such as OR (Odds ratio) or HR (Hazard ratio) with 95% confidence intervals (95%CI) from all included studies into a standardized table. Two authors assessed the quality and risk of bias for each study included in the qualitative analysis. The Newcastle-Ottawa Quality Assessment Scale (29) was used for this evaluation of quality, where each study was assessed for good standards on four items of selection, one item of comparability, and three items of outcome, yielding a total of 8 stars (points) (comparability can be scored with up to two stars).
All evidence drawn from the studies was described qualitatively and summarized in Table 1. We also analyzed the results from the studies using quantitative estimates of effects by the Mantel-Haenszel method. Thus, a random-effects meta-analysis was conducted to estimate the odds ratio of cognitive decline between frail and non-frail participants using the RevMan software, version 5.3 (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014). Confidence interval was set at 95%, and the level of significance was set at <5%.
The systematic search of the literature yielded 2,200 citations. A further 10 studies had not been identified and were added manually. Of the 2,210 records, 867 studies were removed by search filters: publication period (March, 2001 to January, 2018), English language, humans, aged over 65 years. Of the 1,343 records, 1,258 studies considered not relevant were excluded, giving a total of 85 studies for full-text review. Twenty-one reviews, nine editorials or letters; eight clinical studies; and eight cross-sectional studies were subsequently excluded. Five studies were excluded for not categorizing frailty status or showing an association with MCI and dementia. Of the remaining thirty-four studies, twenty-eight were excluded for not fulfilling the criteria for this study. The complete list of excluded studies can be found in the Supplementary File. The remaining six studies were considered to have adequate methodological quality and included in the qualitative and quantitative syntheses (meta-analysis). Figure 1 depicts the flowchart of the study selection process.
Study and participant characteristics of the cohort or population-based longitudinal studies are summarized in Table 1. The studies were conducted among community-dwellers in North America (n = 2); Europe (n = 3); and Asia (n = 1). Sample size ranged from 1,575 to 5,480 (total of 14,657 participants). Mean age of study participants was 73.3 years. The overall quality of the studies assessed using NOS was high, with a median score of 8 (Table 1).
There were 936 frail older adults in the 6 studies (14–16, 30–32) investigating the incidence of cognitive disorders over a mean follow-up of 5.33 years (range 3 to 7 years). These subjects were compared with 13,721 non-frail individuals at baseline (Figure 2).
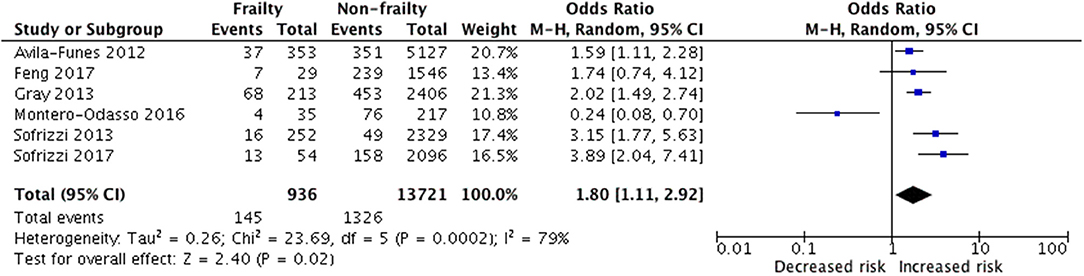
Figure 2. Random-effects meta-analysis of incident cognitive disorder associated with frailty in older adults.
Results showed that baseline frailty was significantly associated with an increased risk of geriatric cognitive disorders (pooled OR = 1.80, 95% CI = 1.11–2.92 p = 0.02; I2 = 79%), as shown in Figure 2. Heterogeneity across the studies was significant (I2 = 79%).
Frail status was most associated with the risk of dementia, particularly non-AD and vascular dementia, even after adjusting for many confounders, as shown in Table 1.
In the Three-City study (14), frailty was a major risk factor for incident dementia and was associated with greater risk of all types of dementia. In the ILSA study (16), physical frailty was associated with a significantly increased risk of overall dementia and vascular dementia over a 3.5-year follow-up, while the risk of AD or other types of dementia did not significantly change in frail individuals compared with robust older adults. Later studies confirm the impact of frailty on incident vascular and overall dementia, but not AD dementia (15, 31). Frail participants did not exhibit a significant risk for incident dementia in the Gait and Brain Study (32).
There were major disparities in definitions of cognitive impairment and assessments of cognitive functioning. Most studies employed different methods (e.g., MMSE, MoCA) and cut-off values for defining cognitive impairment. Several studies evaluated the cognitive performance of participants using screening measures of global cognition. Only two studies adopted a comprehensive neuropsychological test battery (14, 15) and CDR scale (30, 32). Only one study showed that PF was associated with both incident cognitive impairment and greater risk of neurocognitive disorders (NCD) in older adults (30).
In this systematic review and meta-analysis, the relationship between frailty, and cognitive disorders was investigated, summarizing data from longitudinal and cohort studies involving community-dwelling older adults. Our analyses confirmed that frail older adults were at higher risk of incident cognitive disorders, especially vascular dementia, compared with non-frail elders. Previous longitudinal studies have reported that physical frailty may be associated with incident vascular dementia (14, 16). In fact, physical frailty was associated with increased risk of developing vascular dementia in three of the studies included in the present systematic review (14, 16, 31).
Vascular dementia is caused by cardiovascular disease (CVD). It has been suggested that CVD and vascular cognitive impairment (cerebrovascular disease) in the elderly have the same risk factors (33). Frailty has been associated with an increased odds for hypertension and diabetes (34, 35). Atrial fibrillation (AF) is another major risk factor for cerebrovascular disease. A recent systematic review investigating the association between AF and frailty shows that a higher prevalence of frailty was observed among patients with this CVD (36). Veronese et al. conducted a study showing that frailty is an independent risk factor for any-type of CVD in older adults (37). Moreover, studies have shown that obesity and metabolic disorders are associated with cognitive decline and dementia (38–40). Metabolic Syndrome and insulin resistance are associated with increased risk of frailty (41). However, current evidence on Metabolic Syndrome and risk for cognitive decline in the elderly is conflicting (42).
Physical frailty has been associated with late-life cognitive decline, incident AD and mild cognitive impairment, vascular dementia, and with non-AD dementia in older adults according to findings of previous systematic reviews (19–21). Several studies examining frailty and cognitive impairment suggest these outcomes interact and the existence of a possible bidirectional relationship (23). A pooled prevalence of physical frailty of 32% in patients with AD was reported in a previous systematic review (43).
Cognitive impairment has been considered either a syndrome (e.g., MCI, Subjective Cognitive Decline (SCD), NCD, or cognitive frailty when combined with frailty diagnosis) or a preclinical stage of AD (prodromal AD or preclinical AD) (44). Moreover, studies show a higher prevalence of cognitive impairment among frail older people (45). In our review, we found two studies that considered other outcomes related to cognition (cognitive impairment and cognitive decline) (30, 32). A 5-year longitudinal study revealed that physical impairment in individuals considered cognitively normal could lead to cognitive impairment clinically detectable only later and was associated with a greater risk of developing dementia of the AD type (46). However, it is important to emphasize that the causes of physical frailty and cognitive impairment are not well-established (47).
The etiology of frailty is possibly complex and might be multidimensional, including variables such as cognition, mood, nutrition, mobility, physical activity, strength, balance, endurance, coping, relationship, and social support, among other potential causes (47). Inflammation and oxidative stress are two factors that also play an important role in the development of both frailty and cognitive impairment (48). Frailty components have been linked to typical pathophysiological changes seen in AD (e.g., amyloid deposition) (13). However, it remains unclear whether the association is due to a direct (e.g., amyloid deposits are cause of frailty) or indirect (e.g., amyloid accumulation is related to frailty because they are both age-related conditions) mechanism.
At the same time, improved discrimination of neurodegenerative conditions from disturbances caused by disruption of the homeostatic balance (e.g., frailty; indirectly responsible for cognitive impairment) will impact clinical and research strategies (49). In particular, the impact of several operational definitions of frailty on cognitive decline has been attracting interest in this field of research. Cognitive frailty could be a heterogeneous clinical syndrome, characterized by concomitant physical frailty and MCI, while excluding cases with AD or other dementias (23). More recently, the construct of cognitive frailty proved capable of predicting short- and long-term all-cause mortality and overall dementia, particularly vascular dementia (31).
These comprehensive meta-analysis results advance the literature beyond previously published integrative (50, 51) and/or systematic reviews (19–21) that have explored the relationship between cognitive impairment or dementia and frailty. The present review only included high-quality studies involving a prospective diagnosis of frailty according to validated criteria. Additionally, all studies reported the number of frail participants with incident cognitive disorders, while dementia diagnosis was based on established criteria.
Our data should be interpreted with caution because of potential limitations. First, the number of longitudinal prospective studies was limited. Second, most studies applied modified frailty criteria compared with the original. Third, significant heterogeneity was observed across the studies included in this review.
Lastly, in some studies, it is unclear whether the identification of participants with dementia resulted from a comprehensive assessment of cognitive and functional abilities (as required by current diagnostic criteria) or was merely based on global screening measures (e.g., the MMSE). Therefore, future research is required to understand how different operational definitions of frailty and cognitive impairment are useful and clearly defined as an integral concept.
Finally, frailty may represent a novel modifiable target in early cognitive impairment. Identification of modifiable risk factors for cognitive frailty will improve identification of high-risk individuals and help develop interventions to prevent cognitive decline in aging. Physical frailty and cognition together, in the absence of dementia, may have important implications in clinical settings and research scenarios worldwide.
IA: study design, meta-analysis, wrote, and reviewed the manuscript; MB and MarC: database management and search strategies, wrote, and reviewed the manuscript; MatC: study design and reviewed the manuscript.
IA received a National public grant level 2 from the National Council for Scientific and Technological Development (Ministry of Science, Technology, Innovation and Communications, Brazil).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2019.00026/full#supplementary-material
1. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci. (2001) 56:146–57. doi: 10.1093/gerona/56.3.M146
2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet (2013) 381:752–62. doi: 10.1016/S0140-6736(12)62167-9
3. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. (2012) 60:1487–92. doi: 10.1111/j.1532-5415.2012.04054.x
4. Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. (2010) 362:1173–80. doi: 10.1056/NEJMoa0909087
5. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol Ser A Biol Sci Med Sci. (2007) 62:722–7. doi: 10.1093/gerona/62.7.722
6. Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM. In search of an integral conceptual definition of frailty: opinions of experts. J Am Med Dir Assoc. (2010) 11:338–43. doi: 10.1016/j.jamda.2009.09.015
7. Gobbens RJ, van Assen MA, Luijkx KG, Schols JM. Testing an integral conceptual model of frailty. J Adv Nurs. (2012) 68:2047–60. doi: 10.1111/j.1365-2648.2011.05896.x
8. Canevelli M, Cesari M. Cognitive frailty: far from clinical and research adoption. J Am Med Dir Assoc. (2017) 18:816–8. doi: 10.1016/j.jamda.2017.07.004
9. Searle SD, Rockwood K. Frailty and the risk of cognitive impairment. Alzheimer Res Ther. (2015) 7:54. doi: 10.1186/s13195-015-0140-3
10. Panza F, Solfrizzi V, Frisardi V, Maggi S, Sancarlo D, Adante F, et al. Different models of frailty in predementia and dementia syndromes. J Nutr Health Aging (2011) 15:711–9. doi: 10.1007/s12603-011-0126-1
11. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. (2010) 58:248–55. doi: 10.1111/j.1532-5415.2009.02671.x
12. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosomat Med. (2007) 69: 483–9. doi: 10.1097/psy.0b013e318068de1d
13. Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol Ser A Biol Sci Med Sci. (2014) 69:1536–44. doi: 10.1093/gerona/glu117
14. Avila-Funes JA, Carcaillon L, Helmer C, Carrière I, Ritchie K, Rouaud O, et al. Is frailty a prodromal stage of vascular dementia? Results from the Three-City Study. J Am Geriatr Soc. (2012) 60:1708–12. doi: 10.1111/j.1532-5415.2012.04142.x
15. Gray SL, Anderson ML, Hubbard RA, LaCroix A, Crane PK, McCormick W, et al. Frailty and incident dementia. J Gerontol Ser A Biol Sci Med Sci. (2013) 68:1083–90. doi: 10.1093/gerona/glt013
16. Solfrizzi V, Scafato E, Frisardi V, Seripa D, Logroscino G, Maggi S, et al. Frailty syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Aging. Alzheimer Dementia (2013) 9:113–22. doi: 10.1016/j.jalz.2011.09.223
17. Song X, Mitnitski A, Rockwood K. Age-related deficit accumulation and the risk of late-life dementia. Alzheimer Res Ther. (2014) 6:54. doi: 10.1186/s13195-014-0054-5
18. McGough EL, Cochrane BB, Pike KC, Logsdon RG, McCurry SM, Teri L. Dimensions of physical frailty and cognitive function in older adults with amnestic mil cognitive impairment. Ann Phys Rehabil Med. (2013) 56:329–41. doi: 10.1016/j.rehab.2013.02.005
19. Panza F, Solfrizzi V, Barulli MR, Santamato A, Seripa D, Pilotto A, et al. Cognitive frailty: a systematic review of epidemiological and neurobiological evidence of an age-related clinical condition. Rejuvenat Res. (2015) 18:389–412. doi: 10.1089/rej.2014.1637
20. Kojima G, Taniguchi Y, Iliffe S, Walters K. Frailty as a predictor of Alzheimer disease, vascular dementia, and all dementia among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. (2016) 17:881–8. doi: 10.1016/j.jamda.2016.05.013
21. Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. Meta-analysis of modifiable risk factors for Alzheimer's disease. J Neurol Neurosurg Psychiatr. (2015) 86:1299–306. doi: 10.1136/jnnp-2015-310548
22. Panza F, Seripa D, Solfrizzi V, Tortelli R, Greco A, Pilotto A, et al. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimer Dis. (2015) 47:793–813. doi: 10.3233/JAD-150358
23. Ruan Q, Yu Z, Chen M, Bao Z, Li J, He W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res Rev. (2015) 20:1–10. doi: 10.1016/j.arr.2014.12.004
24. Eden J, Levit L, Berg A, Morton S. Institute of Medicine (US). Committee on Standards for Systematic Reviews of Comparative Effectiveness Research. Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: National Academies Press (2011).
25. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
26. American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed. Washington, DC: American Psychiatry Association (2013).
27. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA Work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology (1984) 4:939–44.
28. Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology (1993) 43:250–60.
29. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May 20, 2018).
30. Feng L, Nyunt MS, Gao Q, Feng L, Lee TS, Tsoi T, et al. Physical frailty, cognitive impairment, and the risk of neurocognitive disorder in the singapore longitudinal ageing studies. J Gerontol Ser A Biol Sci Med Sci. (2017) 72:369–75. doi: 10.1093/gerona/glw050
31. Solfrizzi V, Scafato E, Seripa D, Lozupone M, Imbimbo BP, D'Amato A, et al. Reversible cognitive frailty, dementia, and all-cause mortality. The Italian longitudinal study on aging. J Am Med Dir Assoc. (2017) 18:89.e1–89.e8. doi: 10.1016/j.jamda.2016.10.012
32. Montero-Odasso MM, Barnes B, Speechley M, Muir Hunter SW, Doherty TJ, Duque G, et al. Disentangling cognitive-frailty: results from the gait and brain study. J Gerontol Ser A Biol Sci Med Sci. (2016) 71:1476–82. doi: 10.1093/gerona/glw044
33. Leritz EC, McGlinchey RE, Kellison I, Rudolph JL, Milberg WP. Cardiovascular disease risk factors and cognition in the elderly. Curr Cardiovasc Risk Factors Rep. (2011) 5:407–12. doi: 10.1007/s12170-011-0189-x
34. Vetrano DL, Palmer KM, Galluzzo L, Giampaoli S, Marengoni A, Bernabei R, et al. Hypertension and frailty: a systematic review and meta-analysis. BMJ Open (2018) 8:e024406. doi: 10.1136/bmjopen-2018-024406
35. Assar ME, Laosa O, Rodríguez Mañas L. Diabetes and frailty. Curr Opin Clin Nutr Metab Care (2019) 22:52–7. doi: 10.1097/MCO.0000000000000535
36. Villani ER, Tummolo AM, Palmer K, Gravina EM, Vetrano DL, Bernabei R, et al. Frailty and atrial fibrillation: a systematic review. Eur J Intern Med. (2018) 56:33–8. doi: 10.1016/j.ejim.2018.04.018
37. Veronese N, Cereda E, Stubbs B, Solmi M, Luchini C, Manzato E, et al. Risk of Cardiovascular disease morbidity and mortality in frail an pre-frail older adults: results from a meta-analysis and exploratory meta-regression analysis. Ageing Res Rev. (2017) 35:63–73. doi: 10.1016/j.arr.2017.01.003
38. Gorelich PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecol C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke (2011) 42:2672–713. doi: 10.1161/STR.0b013e3182299496
39. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. (2011) 12:723–38. doi: 10.1038/nrn3114
40. Porter Starr KN, McDonald SR, Bales CW. Obesity and physical frailty in older adults: a scoping review of lifestyle intervention trials. J Am Med Dir Assoc. (2014) 15:240–50. doi: 10.1016/j.jamda.2013.11.008
41. Pérez-Tasigchana RF, León-Muñoz LM, Lopez-Garcia E, Gutierrez-Fisac JL, Laclaustra M, Rodríguez-Artalejo F, et al. Metabolic syndrome and insulin resistance are associated with frailty in older adults: a prospective cohort study. Age Ageing (2017) 46:807–12. doi: 10.1093/ageing/afx023
42. Assuncao N, Sudo FK, Drummond C, Felice FG, Mattos P. Metabolic Syndrome and cognitive decline in the elderly: a systematic review. PLoS ONE (2018) 13:e0194990. doi: 10.1371/journal.pone.0194990
43. Kojima G, Liljas A, Iliffe S, Walters K. Prevalence of frailty in mild to moderate Alzheimer's disease: a systematic review and meta-analysis. Curr Alzheimer Res. (2017) 14:1256–63. doi: 10.2174/1567205014666170417104236
44. Morley JE, Morris JC, Berg-Weger M, Borson S, Carpenter BD, Del Campo N, et al. Brain health: the importance of recognizing cognitive impairment: an IAGG consensus conference. J Am Med Dir Assoc. (2015) 16:731–9. doi: 10.1016/j.jamda.2015.06.017
45. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment: a review of the evidence and causal mechanisms. Ageing Res Rev. (2013) 12:840–51. doi: 10.1016/j.arr.2013.06.004
46. Wilkins CH, Roe CM, Morris JC, Galvin JE. Mild physical impairment predicts future diagnosis of dementia of the Alzheimer's type. J Am Geriatr Soc. (2013) 61:1055–9. doi: 10.1111/jgs.12255
47. Panza F, Lozupone M, Solfrizzi V, Stallone R, Bellomo A, Greco A, et al. Cognitive frailty: a potential target for secondary prevention of dementia. Expert Opin Drug Metab Toxicol. (2017) 13:1023–7. doi: 10.1080/17425255.2017.1372424
48. Mulero J, Zafrilla P, Martinez-Cacha A. Oxidative stress, frailty and cognitive decline. J Nutr Health Aging (2011) 15:756–60. doi: 10.1007/s12603-011-0130-5
49. Canevelli M, Cesari M, Remiddi F, Trebbastoni A, Quarata F, Vico C, et al. Promoting the assessment of frailty in the clinical approach to cognitive disorders. Front Aging Neurosci. (2017) 9:36. doi: 10.3389/fnagi.2017.00036
50. Sargent L, Brown R. Assessing the current state of cognitive frailty: measurement properties. J Nutr Health Aging (2017) 21:152–60. doi: 10.1007/s12603-016-0735-9
Keywords: mild cognitive impairment, cognitive decline, dementia, cognitive disorders, comorbidity, elderly, meta-analysis
Citation: Borges MK, Canevelli M, Cesari M and Aprahamian I (2019) Frailty as a Predictor of Cognitive Disorders: A Systematic Review and Meta-Analysis. Front. Med. 6:26. doi: 10.3389/fmed.2019.00026
Received: 11 December 2018; Accepted: 28 January 2019;
Published: 19 February 2019.
Edited by:
Nicola Veronese, Consiglio Nazionale delle Ricerche - Area della Ricerca di Padova, ItalyReviewed by:
Francesco Panza, Università degli Studi di Bari, ItalyCopyright © 2019 Borges, Canevelli, Cesari and Aprahamian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivan Aprahamian, aXZhbi5hcHJhaGFtaWFuQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.