
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 08 February 2019
Sec. Nephrology
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00020
Hepatitis C virus (HCV) infection is a systemic disorder that frequently associates with extrahepatic manifestations, including nephropathies. Cryoglobulinemia is a typical extrahepatic manifestation of HCV infection that often involves kidneys with a histological pattern of membranoproliferative glomerulonephritis. Other, less common renal diseases related to HCV infection include membranous nephropathy, focal segmental glomerulosclerosis, IgA nephropathy, fibrillary and immunotactoid glomerulopathy. Over the last decades, the advent of direct-acting antiviral therapies has revolutionized treatment of HCV infection, dramatically increasing the rates of viral clearance. In patients where antiviral therapy alone fails to induce renal disease remission add-on B-cell depleting agents represent an alternative to counteract the synthesis of pathogenic antibodies. Immunosuppressive therapies, such as steroids, alkylating agents, and plasma exchanges, may still represent an effective option to inhibit immune-complex driven inflammatory response, but the potentially associated increase of HCV replication and worsening of liver disease represent a serious limitation to their use.
Hepatitis C virus (HCV), first identified in 1989, is an enveloped positive-stranded RNA virus belonging to the Flaviviridae family (1). HCV is a globally prevalent pathogen and a major health concern. According to the World Health Organization (WHO), in 2015, 71 million people had chronic HCV infection (estimated global prevalence of 1%), putting these individuals at high risk for progressive liver disease including cirrhosis and hepatocellular cancer. HCV infection is generally asymptomatic and only 20–40% of individuals clear the virus spontaneously, therefore most of the subjects that encounter the virus become chronically infected (2). HCV-associated all-cause mortality is double compared to HCV-negative individuals and extrahepatic manifestations represent a major risk factor (3). Lymphoproliferative and autoimmune disorders, ranging from cryoglobulinemia vasculitis to malignant B-cell lymphoma, are the most common extrahepatic conditions associated with HCV infection (4). Large cohort studies have revealed additional extrahepatic manifestations, including cardiovascular, neurological, metabolic, and renal conditions (5) and multiple manifestations often coexist in the same patient. Cacoub et al. (6) have reported that up to 74% of chronically HCV infected patients suffer from at least one extrahepatic manifestation.
Several multi-center survey studies have reported the epidemiology of HCV infection in individuals with end stage renal disease (ESRD): according to the Dialysis Outcomes and Practice Patterns Study (DOPPS) (7), a large observational study including 49,762 ESRD subjects in 12 developed countries, the prevalence of anti-HCV antibody positivity is 9.5%. In developing countries, the prevalence of HCV infections among ESRD patients is less clear and ranges across different reports between 6.1 and 49.6% (8).
In renal transplant recipients, the prevalence of HCV infection varies from 6 to 46% (9) and in most cases the infection occurs before transplant rather than through an infected donor (10). Several studies reported that 74 to 92% of HCV positive renal transplant recipients have detectable HCV RNA levels at the time of transplantation, which persist (11) and rise after antirejection therapy is initiated (12). HCV infection associates with shorter graft and patient survival (13) and renal transplantation increases the risk to develop hepatocellular carcinoma in HCV infected patients (14).
The treatment options for HCV infection have markedly expanded, with a dramatic acceleration since 2001, when the interferon-based regimens were first integrated with and then replaced by direct-acting antiviral drugs (DAAs) (15). Based on the excellent results obtained with the new anti-HCV therapies, one of the goals of the United Nations 2030 Agenda for Sustainable Development is the removal of viral hepatitis as a threat for the public health, with targets including an 80% reduction in the incidence of HCV infections and a 65% decrease in HCV-related mortality (16).
Renal impairment in chronic HCV infection is mostly related to mixed cryoglobulinemia, a systemic vasculitis that mainly affects small-sized vessels and that in the kidney generally leads to membranoproliferative glomerulonephritis (MPGN).
Other glomerular diseases that have been associated with HCV infection include membranous nephropathy, focal segmental glomerulosclerosis (FSGS), fibrillary, or immunotactoid glomerulopathy, and IgA nephropathy (17). Typical renal manifestations in HCV-infected patients include proteinuria, microscopic hematuria, hypertension, and nephrotic syndrome and the triad of purpura, asthenia, and arthralgia is evident in nearly 30% of the cases (18).
HCV evolved in 7 different genotypes and more than 67 subtypes. A community-based prospective study (19) involving 13,805 participants showed an association between HCV genotype 2 and chronic kidney disease. Differently, the REVEAL-HCV study involving 19,984 participants (20), showed that HCV genotype 1 and high serum HCV RNA levels (>167,000 IU/mL) are strong predictors of ESRD. While the impact of the different HCV genotypes on renal outcomes still needs to be completely elucidated, careful renal function evaluation should be part of regular follow-up of individuals with HCV infection, especially if serum levels of HCV RNA are elevated and in case of infection with HCV genotypes 1 or 2. Moreover, in last decade several genome-wide association studies reported many host genetic factors that influence hepatic outcomes and treatment efficacy after HCV infection (21–23). A GWAS among patients with chronic HCV infection found a genome-wide significant association of rs9461776 (HLA-DRB1/DQA1) with cryoglobulin-related vasculitis (24) The same study also identified single nucleotide polymorphisms (SNPs) near NOTCH4 and MHC class II that were strongly associated with this syndrome.
Cryoglobulins are defined as polyclonal immunoglobulin G (IgG) bound to another immunoglobulin that acts as anti-IgG rheumatoid factor, that together precipitate in serum cooled to 4°C. According to Brouet et al. (25), the cryoglobulins can be subdivided into three subgroups: type I contains an isolated monoclonal immunoglobulin, type II comprises IgG and an IgM rheumatoid factor (RF) of monoclonal origin (previously called mixed essential cryoglobulinemia), and type III comprises IgG and a polyclonal IgM RF. Cryoglobulins associated with HCV infection are of type II (26), while type I cryoglobulins are associated with lymphoproliferative disorders (27) and type III cryoglobulins are often related with connective tissue diseases, infections, hepatobiliary diseases, and lymphoproliferative disorders (28).
Cryoglobulinemic glomerulonephritis is caused by cryoglobulin deposits in the glomerular capillary walls (often in the subendothelial space) and in the mesangium, giving an MPGN pattern of injury (29, 30) (Figure 1). The clinical presentation includes hypertension, proteinuria, microscopic hematuria, acute nephritis, or nephrotic syndrome, often associated with C3 and/or C4 complement consumption. All three types of cryoglobulins, including those due to monoclonal or polyclonal immunoglobulins, can cause cryoglobulinemic GN, but it occurs most often with HCV-associated type II cryoglobulinemia (Table 1). Until recent treatment advances, HCV-associated MC was associated with 1-, 3-, 5-, and 10-year survival rates of 96, 86, 75, and 63%, respectively (31).
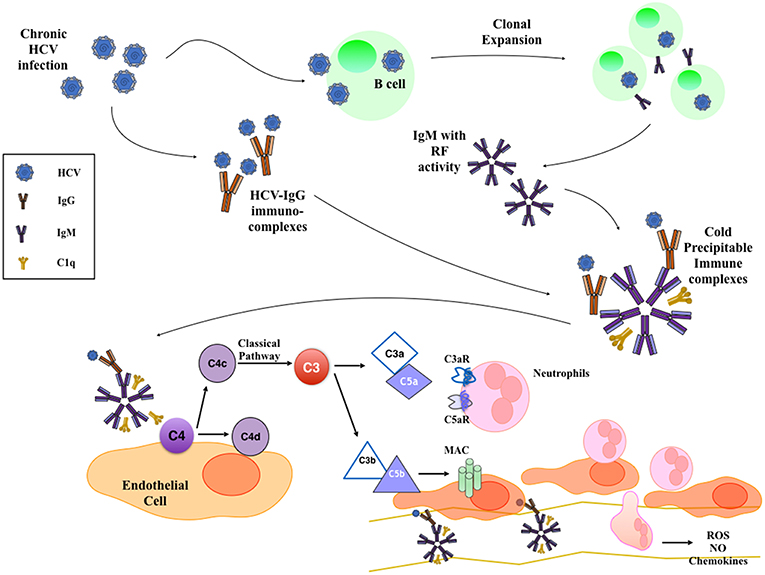
Figure 1. Mechanism of HCV-Induced Cryoglobulinemic Nephropathy. HCV infection of B cells leads to the production of IgM with rheumatoid factor (RF) activity that bind HCV-IgG immune-complexes. These cold-precipitable multimolecular immune-complexes deposit in the subendothelial space and in the mesangium, where they activate classical complement pathway. This leads to the formation of C3a and C5a anaphylatoxins that recruit and activate inflammatory cells and to the deposition of membrane attack complex (MAC) on the endothelium that activates endothelial cell proinflammatory functions.
Histological appearance at light microscopy shows mesangial proliferation and often diffuse and global endocapillary hypercellularity. Cryoglobulins can also deposit in glomerular capillaries as eosinophilic thrombi that usually associate with vasculitis and fibrinoid necrosis of glomeruli. The acute phase often shows neutrophils, while monocyte/macrophages infiltrate in both acute and chronic stages (Figure 1). Arterioles and small arteries may show leukocytoclastic vasculitis, sometimes with cryoglobulin deposits (32). With severe glomerular inflammation and damage of the glomerular capillary wall, cryoglobulins can lead to extracapillary proliferation and crescent formation (33).
At immunofluorescence, capillary walls show significant IgM and C3 staining. Intracapillary thrombi are typically positive for IgM and clonal κ or λ chain staining. Electron microscopy shows subendothelial and mesangial dense deposits, usually with interposed cells and double contours due to new GBM formation beneath subendothelial deposits. Moreover, considerable effacement of podocyte foot processes and endocapillary hypercellularity are often reported (Table 1) (32). Several studies investigated the interaction between HCV and the complement system, establishing an active role of complement in intra- and extrahepatic manifestations of HCV infection. Similarly to autoantibody-initiated kidney glomerulopathies, complement activation in type II cryoglobulinemia occurs prevalently through the classical pathway and promotes injury through the recruitment of inflammatory cells and membrane attack complex formation (34–36).
Cases of membranous nephropathy have been reported in patients with HCV-infection (37), with clinical presentation and histological findings that are similar to primary forms. Yamabe et al. (38) found that 8.3% of membranous nephropathy patients had anti-HCV-antibodies or detectable HCV RNA. The pathogenesis of membranous nephropathy in HCV infected patients is thought to be related to the deposition of immune complexes containing HCV proteins in glomeruli, where viral-like particles have been identified by electron microscopy (39). Glomerular deposition of IgM, IgG, IgA, and complement with the same distribution of HCV (40) strongly support this hypothesis (33, 41).
Fibrillary-immunotactoid glomerulopathy was described in few HCV-infected individuals. It is characterized by extracellular mesangial deposits of microfibrils, positive staining of glomerular capillary walls for IgG4 and C3 (17, 42, 43) and negative for Congo red staining (37). Fibrillary-immunotactoid glomerulopathy typically manifests with clinical and laboratory signs of nephritic syndrome (hematuria, hypertension, and renal failure), but with proteinuria in nephrotic range (44–46).
HCV infection has also been associated with IgA nephropathy (47, 48). Pathogenic link between the two conditions is supported by the evidence that antiviral therapy with IFN-α leads to renal disease remission (49–51). However, due to the reduced hepatic clearance of IgA and IgA-containing immune complexes, IgA deposition in the glomeruli are common in all forms of cirrhosis. Therefore, HCV may not be directly implicated in pathogenesis of the disease (Table 1) (52, 53).
Glomerular lesions associated with HCV infection are mostly sustained by cryoglobulins and immune complex deposits. However, antibody-independent glomerulonephritides have also been described in HCV positive patients that may display features of FSGS. While the pathogenic mechanisms are unclear, it is hypothesized that, similar to human immunodeficiency virus (HIV), HCV directly injures podocytes, leading to glomerulosclerosis (54).
In kidney transplant recipients, HCV infection is associated with increased morbidity and mortality rates, due to hepatic and extra-hepatic complications (55–57). A retrospective study of 706 HCV positive renal transplant recipients found that the presence of HCV antibodies independently predicted reduced patient and graft survival at 10 years. There is no clear evidence of an association between the kind of antirejection therapy and HCV activity in kidney transplant recipients. Some reports suggest a potential benefit of mTOR inhibitors in controlling viral replication (58–60), but this therapeutic approach should be weighed against the increased risk of acute rejections and the poor tolerability of these agents (61).
Extra-hepatic disease associated with HCV infection in transplant recipients includes de novo or recurrence of glomerular diseases, acute rejection, transplant glomerulopathy, and accelerated kidney graft fibrosis (62). MPGN is the most common glomerulopathy in HCV-infected kidney transplant recipients that occurs in 5–54% of patients (63). The presence of anti-HCV antibodies before kidney transplantation is a risk factor for the occurrence of proteinuria and reduced graft survival (64).
Co-infection with HIV seems to be an independent risk factor for graft failure and patient survival compared to HCV infection alone (65). As recently showed by Rallòn et al. (66), HCV related immune defects accelarate HIV disease progression, supporting early anti-HCV treatment in case of combined HIV/HCV infection.
A better understanding of the pathophysiology of HCV-associated nephropathies has progressively opened the door to more targeted, hypothesis-driven approaches: (a) antiviral treatment to avoid the formation of cryoglobulins, immune complexes and direct viral injury to the kidney; (b) B-cell depletion, aimed at reducing cryoglobulin production, and (c) immunosuppressive treatments targeting glomerular inflammation.
Differently from HBV and HIV, HCV infection can be completely and permanently cured by antiviral treatment as HCV has no long-term reservoir in the body. The definitive cure of HCV infection is commonly reflected by the sustained virologic response (SVR), defined as no-viremia for 24 weeks after ending antiviral therapy. Attaining an SVR has been associated with decreased all-cause mortality and need for liver transplantation, even among patients with advanced liver fibrosis (67, 68).
Interferon and ribavirin still represent the standard of care for recent HCV infection, but the management of subjects with chronic infection has been revolutionized by the development of HCV-specific antiviral drugs (direct acting antivirals–DAAs). HCV-encoded proteins (NS3/4A protease, NS5A protein, and NS5B polymerase) are fundamental for virus replication and represent the main target of the DAAs [for more details see (69)]. Combining two or more DAAs from different classes has increased SVR from ~50% (70) to over 90% (71) and shortened treatment duration to only 8–12 weeks in most populations with chronic HCV infection (71–75).
According to the 2015 guidelines by the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) (76), all of the approved DAA regimens can be used in patients with estimated glomerular filtration rate (eGFR) >30 ml/min/1.73 m2 (77). In subjects with eGFR < 30 ml/min/1.73 m2 or in individuals on dialysis, the three approved regimens are: (1) ritonavir-boosted paritaprevir, ombitasvir, and dasabuvir (2) ribavirin, elbasvir and grazoprevir, and (3) glecaprevir + pibrentasvir (78–81).
Reports on DAAs treatments in subjects with HCV-GN are limited. Saadoun and colleagues. (82) treated five HCV-MC subjects with sofosbuvir and RBV for 24 weeks and showed that eGFR increased, while proteinuria declined in four cases. Similarly, Sise et al. (83) reported that four out of seven HCV-positive patients with renal impairment had a reduction of proteinuria and/or eGFR increase after sofosbuvir-based treatment. More recently, Saadoun et al. (84) showed that sofosbuvir and daclatasvir induced complete remission in five patients with renal involvement. Collectively, over 60% of the studies that evaluated response of MC-GN patients to DAAs treatment, showed complete resolution of GN or improvement in renal parameters (Table 2) (82–93). Despite DAAs treatment in MC-GN implies higher medication costs compared to classical treatments, Cacoub et al. (94) have elegantly demonstrated how costs related to hospitalizations and supportive therapies decreased. The cost/effectiveness of DAAs therapies may become more advantageous in the future, in light of recent indications for reducing DAAs treatment durations and lowering price (95).
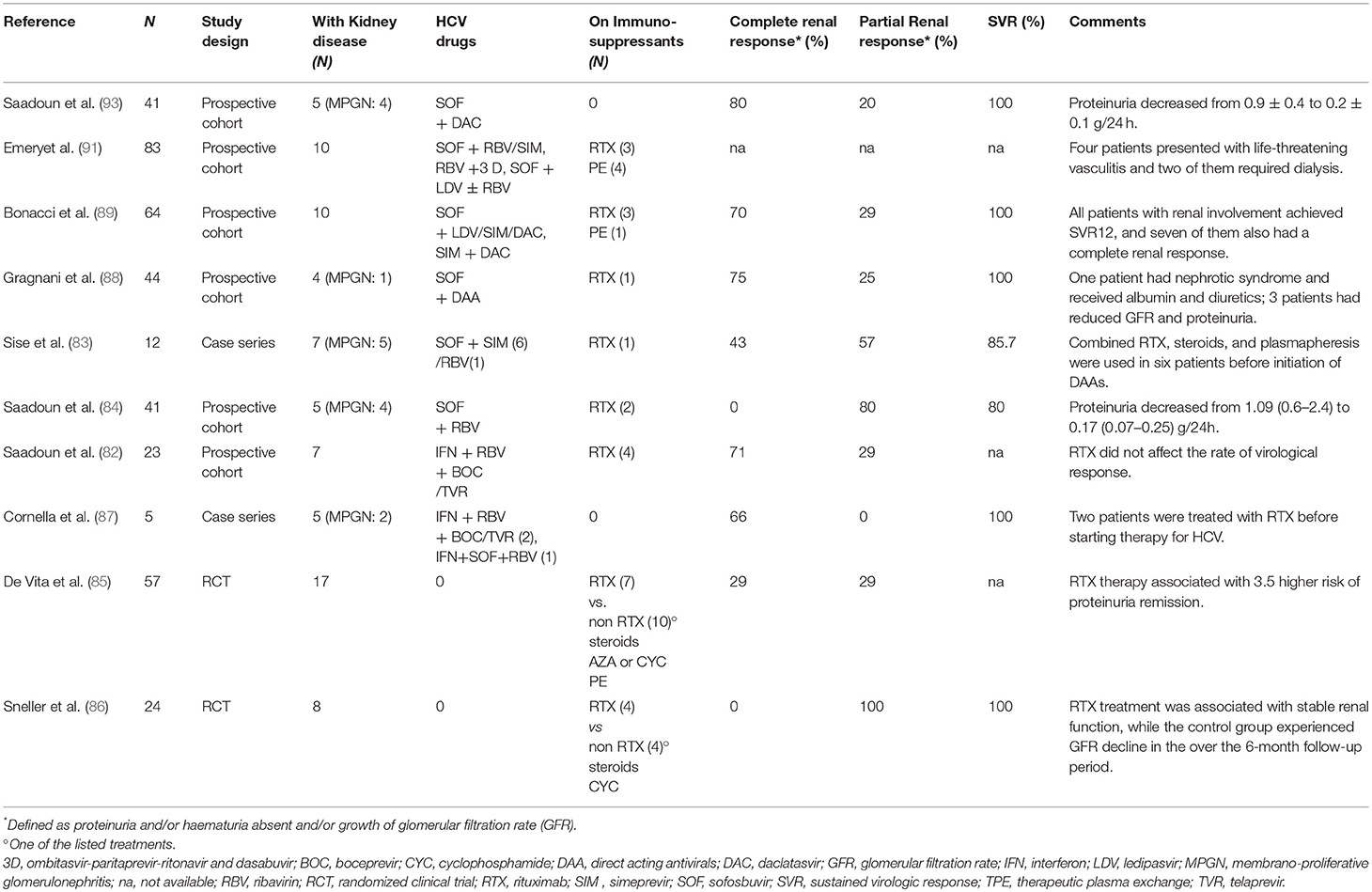
Table 2. Major published clinical studies of antiviral and immunosuppressive treatments for HCV-related glomerulonephritis.
Clearing the viral trigger of HCV-associated glomerular diseases is the ideal treatment for MC-GN. However, B-cell depleting therapies are also widely used, either alone or in combination with antiviral therapies, to prevent immune complex formation and control disease progression. Rituximab is a chimeric monoclonal depleting antibody targeting the CD20 antigen expressed on B cells (96). Rituximab depletes naïve and memory B cells through the induction of apoptosis, antibody-dependent cell-mediated cytotoxicity, phagocytosis and complement-mediated cytotoxicity (97).
In a single-center, open-label trial, 24 patients with HCV-associated cryoglobulinemic vasculitis in whom antiviral therapy had failed to induce remission, were randomized to immunosuppressive therapy + rituximab or immunosuppressive therapy alone (Table 2). After 6 months, ten patients in the rituximab group and only one in the non-rituximab group were in remission (86). In a multicenter, phase III, randomized controlled trial (85), 59 patients with cryoglobulinemic vasculitis (associated to HCV infection in 53 cases) were allocated to rituximab vs. immunosuppressive treatment (glucocorticoids with azathioprine, cyclophosphamide, or plasmapheresis). Among the 16 patients with glomerulonephritis, four out of seven of those in the rituximab group had complete or partial response, while none of those in the non-rituximab group reported a significative reduction in proteinuria.
Rituximab has also been tested as add-on to anti-viral therapy (Table 2). A prospective controlled trial showed a higher rate of complete kidney response (81 vs. 40%) and a good safety profile in 31 patients with severe HCV-associated cryoglobulinemic vasculitis randomized to rituximab plus PEG-IFN/RBV combined therapy vs. PEG-IFN/RBV alone therapy (98). Similar data were obtained in the subset of 9 patients with kidney involvement (99). In line with the above studies, other reports showed the safety of rituximab in HCV-infected individuals, which have shown no increase in HCV viremia and stable liver function tests after rituximab therapy (86, 98) (Table 2).
Plasma exchange has been considered for years the treatment of choice for subjects with cryoglobulinemic vasculitis, with or without renal involvement, with the aim of removing circulating cryoglobulins and preventing the deposition of immune complexes (100). Cyclophosphamide has also been used to suppress B cell function and cryoglobulins production, but this treatment should be used with caution because it can induce flare-ups of HCV infection (101). Compared to cyclophosphamide, mycophenolate mofetil is a more selective treatment to inhibit lymphocyte proliferation and function and represents a safer alternative to induce remission in cryoglobulinemic vasculitis (102). Steroid pulses or low doses of steroids have been used to treat glomerular infiltration (100), but steroids may favor HCV replication and worsen liver disease (37). Due to the lack of strong evidence-based recommendations for the treatment of HCV-related glomerular disease, plasma exchange and conventional immunosuppression still represent an option before starting specific HCV antiviral treatment.
According to the DOPPS study (103), <2% of HCV-positive ESRD patients and < 5% of those wait-listed for renal transplant receive treatments for HCV eradication. The main reason for not treating these patients before transplant is to provide them with the opportunity to obtain kidneys from HCV-positive donors, which may reduce the time on the waiting-list. This could be a reasonable approach in countries such as the United States, where HCV-positive donors represent more than 20% of the donor pool (104) and are often young and with limited comorbidities (105). On the contrary, in European countries, where percentage of HCV positive donors is more limited, this theoretical advantage would be lost (57) and treatment of HCV patients in dialysis should not be further delayed.
Importantly, preliminary data indicate that DAAs therapy may provide the opportunity to allocate renal transplant from HCV-positive to HCV-negative subjects. In the THINKER (Transplanting Hepatitis C Kidneys into Negative Kidney Recipients) trial (106), a 12-week regimen of elbasvir/grazoprevir achieved SVR and renal function improvement in 10 HCV-negative subjects receiving kidneys from HCV-positive donors. Despite these and other encouraging results (107), the evidence on the safety/efficacy profile of DAAs treatment in patients on renal replacement therapies is limited and does not support its widespread use.
Available data regarding the treatment of HCV positive kidney transplant recipients with DAAs are still few, but several reports support a favorable safety/efficacy profile and available evidence indicates that such therapies do not affect the risk of acute rejection (108). Recent studies demonstrate that DAAs therapies effectively cured HCV in 406 of 418 kidney transplant recipients (97%) at 12 months, without affecting the risk of acute rejection (108). However, in a multicenter trial, Colombo et al. (109) randomized 114 kidney transplant recipients with HCV infection to a 12- or 24-week course of ledipasvir/sofosbuvir. All patients achieved SVR (primary endpoint) at 12 and 24 weeks, respectively, and treatment-related reduction of eGFR was reported in three patients, but with no evidence of acute rejection.
Chronic HCV infection affects more than 170 million people worldwide and is responsible for over 350,000 deaths every year. Besides chronic liver disease, HCV infection is associated with extrahepatic manifestations including cryoglobulinemia, lymphoproliferative disorders, and renal diseases.
HCV is associated with a large spectrum of renal lesions and clinical sequelae, in both native and transplanted kidneys. The most common HCV-associated renal disease is cryoglobulinemic glomerulopathy, but HCV may associate also with membranous, IgA, and fibrillary nephropathy, amongst others.
Recent data from relatively small studies show promise of the novel antiviral therapies in HCV-associated glomerulopathies. These treatments, together with B-cell depleting agents, may improve outcomes of affected patients. However, the still limited number of studies in this area prevents a clear assessment of the safety/efficacy profile of these treatments.
AA, CC, and PC conceived the article contents, prepared the manuscript, and endorsed the final draft submitted.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bukh J. The history of hepatitis C virus (HCV): basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol. (2016) 65(1 Suppl):S2–21. doi: 10.1016/j.jhep.2016.07.035
2. Li HC, Lo SY. Hepatitis C virus: virology, diagnosis and treatment. World J Hepatol. (2015) 7:1377–89. doi: 10.4254/wjh.v7.i10.1377
3. Uto H, Stuver SO, Hayashi K, Kumagai K, Sasaki F, Kanmura S, et al. Increased rate of death related to presence of viremia among hepatitis C virus antibody–positive subjects in a community-based cohort study. Hepatology (2009) 50:393–9. doi: 10.1002/hep.23002
4. Zignego AL, Giannini C, Ferri C. Hepatitis C virus-related lymphoproliferative disorders: an overview. World J Gastroenterol. (2007) 13:2467–78. doi: 10.3748/wjg.v13.i17.2467
5. Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB. Italian association of the study of liver commission on extrahepatic manifestations of HCVi. extrahepatic manifestations of hepatitis c virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. (2007) 39:2–17. doi: 10.1016/j.dld.2006.06.008
6. Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis. (2014) 46 Suppl 5:S165–73. doi: 10.1016/j.dld.2014.10.005
7. Canaud B, Bragg-Gresham JL, Marshall MR, Desmeules S, Gillespie BW, Depner T, et al. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: european results from the DOPPS. Kidney Int. (2006) 69:2087–93. doi: 10.1038/sj.ki.5000447
8. Fabrizi F, Donato FM, Messa P. Hepatitis C and its metabolic complications in kidney disease. Ann Hepatol. (2017) 16:851–61. doi: 10.5604/01.3001.0010.5275
9. Papafragkakis H, Fabrizi F, Martin P. Viral hepatitis in renal transplantation. Clin Nephrol. (2011) 76:29–39. doi: 10.5414/CN106851
10. Nampoory MR, Gupta RK, Johny KV, Costandi JN, Samhan M, Ninan VT, et al. Organ-transmitted HCV infection in kidney transplant recipients from an anti-HCV negative donor. Transplant Proc. (1999) 31:3207–8. doi: 10.1016/S0041-1345(99)00691-0
11. Behzad-Behbahani A, Mojiri A, Tabei SZ, Farhadi-Andarabi A, Pouransari R, Yaghobi R, et al. Outcome of hepatitis B and C virus infection on graft function after renal transplantation. Transplant Proc. (2005) 37:3045–7. doi: 10.1016/j.transproceed.2005.07.039
12. Zucker K, Cirocco R, Roth D, Burke G, Nery J, Esquenazi V, et al. Prospective longitudinal assessment of hepatitis C virus infection after renal transplantation. Transplant Proc. (1995) 27:943–4.
13. Corouge M, Vallet-Pichard A, Pol S. HCV and the kidney. Liver Int. (2016) 36(Suppl. 1):28–33. doi: 10.1111/liv.13022
14. Hoffmann CJ, Subramanian AK, Cameron AM, Engels EA. Incidence and risk factors for hepatocellular carcinoma after solid organ transplantation. Transplantation (2008) 86:784–90. doi: 10.1097/TP.0b013e3181837761
15. Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV–related cirrhosis treated with direct-acting antivirals. J Hepatol. (2016) 65:727–33. doi: 10.1016/j.jhep.2016.06.015
16. Duffell EF, Hedrich D, Mardh O, Mozalevskis A. Towards elimination of hepatitis B and C in European union and european economic area countries: monitoring the World Health Organization's global health sector strategy core indicators and scaling up key interventions. Euro Surveill. (2017) 22:30476. doi: 10.2807/1560-7917.ES.2017.22.9.30476
17. Markowitz GS, Cheng JT, Colvin RB, Trebbin WM, D'Agati VD. Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol. (1998) 9:2244–52.
18. Monti G, Galli M, Invernizzi F, Pioltelli P, Saccardo F, Monteverde A, et al. Cryoglobulinaemias: a multi-centre study of the early clinical and laboratory manifestations of primary and secondary disease. GISC: Italian group for the study of cryoglobulinaemias. QJM (1995) 88:115–26.
19. Lai TS, Lee MH, Yang HI, You SL, Lu SN, Wang LY, et al. High hepatitis C viral load and genotype 2 are strong predictors of chronic kidney disease. Kidney Int. (2017) 92:703–9. doi: 10.1016/j.kint.2017.03.021
20. Lai TS, Lee MH, Yang HI, You SL, Lu SN, Wang LY, et al. Hepatitis C viral load, genotype, and increased risk of developing end-stage renal disease: reveal-HCV study. Hepatology (2017) 66:784–93. doi: 10.1002/hep.29192
21. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature (2009) 461:399–401. doi: 10.1038/nature08309
22. Rau M, Baur K, Geier A. Host genetic variants in the pathogenesis of hepatitis C. Viruses (2012) 4:3281–302. doi: 10.3390/v4123281
23. Enache EL, Sin A, Enache LS, Bancu L. Triplex high-resolution melting assay for the simultaneous assessment of IFNL3 rs12979860, ABCB11 rs2287622, and RNF7 rs16851720 genotypes in chronic hepatitis C patients. J Mol Diagn. (2017) 19:857–869. doi: 10.1016/j.jmoldx.2017.07.005
24. Zignego AL, Wojcik GL, Cacoub P, Visentini M, Casato M, Mangia A, et al. Genome-wide association study of hepatitis C virus- and cryoglobulin-related vasculitis. Genes Immun. (2014) 15:500–5. doi: 10.1038/gene.2014.41
25. Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins: a report of 86 cases. Am J Med. (1974) 57:775–88. doi: 10.1016/0002-9343(74)90852-3
26. Gulli F, Santini SA, Napodano C, Bottoni P, Pocino K, Rapaccini GL, et al. Cryoglobulin test and cryoglobulinemia hepatitis C-virus related. Mediterr J Hematol Infect Dis. (2017) 9:e2017007. doi: 10.4084/MJHID.2017.007
27. Terrier B, Karras A, Kahn JE, Le Guenno G, Marie I, Benarous L, et al. The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases. Medicine (2013) 92:61–8. doi: 10.1097/MD.0b013e318288925c
29. Dammacco F, Sansonno D. Therapy for hepatitis C virus-related cryoglobulinemic vasculitis. N Engl J Med. (2013) 369:1035–45. doi: 10.1056/NEJMra1208642
30. Roccatello D, Saadoun D, Ramos-Casals M, Tzioufas AG, Fervenza FC, Cacoub P, et al. Cryoglobulinaemia. Nat Rev Dis Primers (2018) 4:11. doi: 10.1038/s41572-018-0009-4
31. Terrier B, Semoun O, Saadoun D, Sene D, Resche-Rigon M, Cacoub P. Prognostic factors in patients with hepatitis c virus infection and systemic vasculitis. Arthritis Rheum. (2011) 63:1748–57. doi: 10.1002/art.30319
32. Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD atlas of renal pathology: cryoglobulinemic glomerulonephritis. Am J Kidney Dis. (2016) 67:e5–7. doi: 10.1053/j.ajkd.2015.12.007
33. Barsoum RS. Hepatitis C virus: from entry to renal injury–facts and potentials. Nephrol Dial Transplant. (2007) 22:1840–8. doi: 10.1093/ndt/gfm205
34. Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. (2016) 12:383–401. doi: 10.1038/nrneph.2016.70
35. Angeletti A, Reyes-Bahamonde J, Cravedi P, Campbell KN. Complement in non-antibody-mediated kidney diseases. Front Med. (2017) 4:99. doi: 10.3389/fmed.2017.00099
36. El-Shamy A, Branch AD, Schiano TD, Gorevic PD. The complement system and c1q in chronic hepatitis c virus infection and mixed cryoglobulinemia. Front Immunol. (2018) 9:1001. doi: 10.3389/fimmu.2018.01001
37. Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. (2014) 20:7544–54. doi: 10.3748/wjg.v20.i24.7544
38. Yamabe H, Johnson RJ, Gretch DR, Fukushi K, Osawa H, Miyata M, et al. Hepatitis C virus infection and membranoproliferative glomerulonephritis in Japan. J Am Soc Nephrol. (1995) 6:220–3.
39. Appel GB. Immune-complex glomerulonephritis–deposits plus interest. N Engl J Med. (1993) 328:505–6. doi: 10.1056/NEJM199302183280711
40. Sansonno D, Lauletta G, Montrone M, Grandaliano G, Schena FP, Dammacco F. Hepatitis C virus RNA and core protein in kidney glomerular and tubular structures isolated with laser capture microdissection. Clin Exp Immunol. (2005) 140:498–506. doi: 10.1111/j.1365-2249.2005.02778.x
41. Spatola L, Generali E, Angelini C, Badalamenti S, Selmi C. HCV-negative mixed cryoglobulinemia and kidney involvement: in-depth review on physiopathological and histological bases. Clin Exp Med. (2018) 18:465–71. doi: 10.1007/s10238-018-0514-5
42. Coroneos E, Truong L, Olivero J. Fibrillary glomerulonephritis associated with hepatitis C viral infection. Am J Kidney Dis. (1997) 29:132–5. doi: 10.1016/S0272-6386(97)90020-2
43. Guerra G, Narayan G, Rennke HG, Jaber BL. Crescentic fibrillary glomerulonephritis associated with hepatitis C viral infection. Clin Nephrol. (2003) 60:364–8. doi: 10.5414/CNP60364
44. Duffy JL, Khurana E, Susin M, Gomez–Leon G, Churg J. Fibrillary renal deposits and nephritis. Am J Pathol. (1983) 113:279–90.
45. Alpers CE, Rennke HG, Hopper J Jr, Biava CG. Fibrillary glomerulonephritis: an entity with unusual immunofluorescence features. Kidney Int. (1987) 31:781–9. doi: 10.1038/ki.1987.66
46. Iskandar SS, Falk RJ, Jennette JC. Clinical and pathologic features of fibrillary glomerulonephritis. Kidney Int. (1992) 42:1401–7. doi: 10.1038/ki.1992.433
47. Gonzalo A, Navarro J, Barcena R, Quereda C, Ortuno J. IgA nephropathy associated with hepatitis C virus infection. Nephron (1995) 69:354. doi: 10.1159/000188494
48. Ji FP, Li ZX, Tian CY, Ge H, Cai ZF, Pan GY. [IgA nephropathy associated with hepatitis C virus infection. a case report]. Zhonghua Gan Zang Bing Za Zhi (2010) 18:718–9. doi: 10.3760/cma.j.issn.1007-3418.2010.09.021
49. Matsumoto S, Nakajima S, Nakamura K, Etani Y, Hirai H, Shimizu N, et al. Interferon treatment on glomerulonephritis associated with hepatitis C virus. Pediatr Nephrol. (2000) 15:271–3. doi: 10.1007/s004670000467
50. Ji F, Li Z, Ge H, Deng H. Successful interferon-alpha treatment in a patient with IgA nephropathy associated with hepatitis C virus infection. Intern Med. (2010) 49:2531–2. doi: 10.2169/internalmedicine.49.4365
51. Dey AK, Bhattacharya A, Majumdar A. Hepatitis C as a potential cause of IgA nephropathy. Indian J Nephrol. (2013) 23:143–5. doi: 10.4103/0971-4065.109443
52. Roccatello D, Picciotto G, Torchio M, Ropolo R, Ferro M, Franceschini R, Quattrocchio G, et al. Removal systems of immunoglobulin A and immunoglobulin A containing complexes in IgA nephropathy and cirrhosis patients: the role of asialoglycoprotein receptors. Lab Invest. (1993) 69:714–23.
53. Rutledge SM, Chung RT, Sise ME. Treatment of hepatitis C virus infection in patients with mixed cryoglobulinemic syndrome and cryoglobulinemic glomerulonephritis. Hemodial Int. (2018) 22(Suppl. 1):S81–96. doi: 10.1111/hdi.12649
54. Gupta A, Quigg RJ. Glomerular diseases associated with hepatitis B and C. Adv Chronic Kidney Dis. (2015) 22:343–51. doi: 10.1053/j.ackd.2015.06.003
55. Xia Y, Friedmann P, Yaffe H, Phair J, Gupta A, Kayler LK. Effect of HCV, HIV and coinfection in kidney transplant recipients: mate kidney analyses. Am J Transplant. (2014) 14:2037–47. doi: 10.1111/ajt.12847
56. Del Bello D, Ross MJ, Huprikar SS. Hepatitis C virus infection and kidney transplantation. newer options and a brighter future ahead? Kidney Int. (2015) 88:223–5. doi: 10.1038/ki.2015.180
57. La Manna G. HCV and kidney transplant in the era of new direct-acting antiviral agents (DAAs). J Nephrol. (2018) 31:185–7. doi: 10.1007/s40620-018-0476-4
58. Gallego R, Henriquez F, Oliva E, Camacho R, Hernandez R, Hortal L, et al. Switching to sirolimus in renal transplant recipients with hepatitis c virus: a safe option. Transplant Proc. (2009) 41:2334–6. doi: 10.1016/j.transproceed.2009.06.064
59. Marino M, Iemmolo RM, Montalti R, Bertolotti M, Di Benedetto F, De Ruvo N, et al. predictive factors of lack of response to antiviral therapy among in patients with recurrent hepatitis C after liver transplantation. Transplant Proc. (2010) 42:1223–5. doi: 10.1016/j.transproceed.2010.03.052
60. Pacheco LS, Garcia VD, Pra RLD, Cardoso BD, Rodrigues MF, Zanetti HK, et al. Effect of conversion from calcineurin inhibitors to everolimus on hepatitis C viremia in adult kidney transplant recipients. J Bras Nefrol. (2018) 40:143–50 doi: 10.1590/2175-8239-jbn-3860
61. Cravedi P, Ruggenenti P, Remuzzi G. Sirolimus to replace calcineurin inhibitors? too early yet. Lancet (2009) 373:1235–6. doi: 10.1016/S0140-6736(09)60709-1
62. Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. (2015) 11:172–82. doi: 10.1038/nrneph.2015.5
63. Roth D, Cirocco R, Zucker K, Ruiz P, Viciana A, Burke G, et al. De novo membranoproliferative glomerulonephritis in hepatitis c virus-infected renal allograft recipients. Transplantation (1995) 59:1676–82. doi: 10.1097/00007890-199506270-00006
64. Hestin D, Guillemin F, Castin N, Le Faou A, Champigneulles J, Kessler M. Pretransplant hepatitis C virus infection: a predictor of proteinuria after renal transplantation. Transplantation (1998) 65:741–4. doi: 10.1097/00007890-199803150-00024
65. Kayler LK, Xia Y, Friedmann P. Effect of HCV/HIV coinfection versus HCV monoinfection in kidney transplant recipients. Am J Transplant. (2015) 15:849–50. doi: 10.1111/ajt.13080
66. Rallon N, Garcia M, Garcia-Samaniego J, Rodriguez N, Cabello A, Restrepo C, et al. HCV coinfection contributes to hiv pathogenesis by increasing immune exhaustion in CD8 T-cells. PLoS ONE (2017) 12:e0173943. doi: 10.1371/journal.pone.0173943
67. Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. (2007) 147:677–84. doi: 10.7326/0003-4819-147-10-200711200-00003
68. Russo MW. Antiviral therapy for hepatitis C is associated with improved clinical outcomes in patients with advanced fibrosis. Expert Rev Gastroenterol Hepatol. (2010) 4:535–9. doi: 10.1586/egh.10.60
69. Kiser JJ, Flexner C. Direct-acting antiviral agents for hepatitis C virus infection. Annu Rev Pharmacol Toxicol. (2013) 53:427–49. doi: 10.1146/annurev-pharmtox-011112-140254
70. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa−2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. (2002) 347:975–82. doi: 10.1056/NEJMoa020047
71. Feld JJ. Direct-acting antivirals for hepatitis C virus (HCV): the progress continues. Curr Drug Targets (2017) 18:851–62. doi: 10.2174/1389450116666150825111314
72. Feld JJ, Foster GR. Second generation direct-acting antivirals - do we expect major improvements? J Hepatol. (2016) 65(1 Suppl):S130–42. doi: 10.1016/j.jhep.2016.07.007
73. Ladino M, Pedraza F, Roth D. Hepatitis C virus infection in chronic kidney disease. J Am Soc Nephrol. (2016) 27:2238–46. doi: 10.1681/ASN.2016010030
74. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. (2017) 166:637–48. doi: 10.7326/M16-2575
75. Ladino M, Roth D. Hepatitis C virus infection in ESKD patients. Clin J Am Soc Nephrol. (2018) 13:1735–7. doi: 10.2215/CJN.03700318
76. AIHG Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology (2015) 62:932–54. doi: 10.1002/hep.27950
77. Jadoul M, Martin P. Hepatitis C treatment in chronic kidney disease patients: the kidney disease improving global outcomes perspective. Blood Purif. (2017) 43:206–9. doi: 10.1159/000452730
78. Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet (2015) 386:1537–45. doi: 10.1016/S0140-6736(15)00349-9
79. Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, et al. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology (2016) 150:1590–8. doi: 10.1053/j.gastro.2016.02.078
80. Gane E, Lawitz E, Pugatch D, Papatheodoridis G, Brau N, Brown A, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med. (2017) 377:1448–55. doi: 10.1056/NEJMoa1704053
81. Fabrizi F, Messa P. Treatment choices for hepatitis C in patients with kidney disease. Clin J Am Soc Nephrol. (2018) 13:793–5. doi: 10.2215/CJN.12621117
82. Saadoun D, Resche Rigon M, Pol S, Thibault V, Blanc F, Pialoux G, et al. PegIFNalpha/ribavirin/protease inhibitor combination in severe hepatitis C virus-associated mixed cryoglobulinemia vasculitis. J Hepatol. (2015) 62:24–30. doi: 10.1016/j.jhep.2014.08.015
83. Sise ME, Bloom AK, Wisocky J, Lin MV, Gustafson JL, Lundquist AL, et al. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology (2016) 63:408–17. doi: 10.1002/hep.28297
84. Saadoun D, Thibault V, Si Ahmed SN, Alric L, Mallet M, Guillaud C, et al. Sofosbuvir plus ribavirin for hepatitis C virus-associated cryoglobulinaemia vasculitis: vascuvaldic study. Ann Rheum Dis. (2016) 75:1777–82. doi: 10.1136/annrheumdis-2015-208339
85. De Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, Campanini M, et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. (2012) 64:843–53. doi: 10.1002/art.34331
86. Sneller MC, Hu Z, Langford CA. A randomized controlled trial of rituximab following failure of antiviral therapy for hepatitis C virus-associated cryoglobulinemic vasculitis. Arthritis Rheum. (2012) 64:835–42. doi: 10.1002/art.34322
87. Cornella SL, Stine JG, Kelly V, Caldwell SH, Shah NL. Persistence of mixed cryoglobulinemia despite cure of hepatitis C with new oral antiviral therapy including direct-acting antiviral sofosbuvir: a case series. Postgrad Med. (2015) 127:413–7. doi: 10.1080/00325481.2015.1021660
88. Gragnani L, Visentini M, Fognani E, Urraro T, De Santis A, Petraccia L, et al. Prospective study of guideline-tailored therapy with direct–acting antivirals for hepatitis C virus-associated mixed cryoglobulinemia. Hepatology (2016) 64:1473–82. doi: 10.1002/hep.28753
89. Bonacci M, Lens S, Londono MC, Marino Z, Cid MC, Ramos-Casals M, et al. Virologic, clinical, and immune response outcomes of patients with hepatitis c virus-associated cryoglobulinemia treated with direct-acting antivirals. Clin Gastroenterol Hepatol. (2017) 15:575–83.e571. doi: 10.1016/j.cgh.2016.09.158
90. Comarmond C, Garrido M, Pol S, Desbois AC, Costopoulos M, Le Garff-Tavernier M, et al. Direct-acting antiviral therapy restores immune tolerance to patients with hepatitis C virus-induced cryoglobulinemia vasculitis. Gastroenterology (2017) 152:2052–62.e2052. doi: 10.1053/j.gastro.2017.02.037
91. Emery JS, Kuczynski M, La D, Almarzooqi S, Kowgier M, Shah H, W et al. Efficacy and safety of direct acting antivirals for the treatment of mixed cryoglobulinemia. Am J Gastroenterol. (2017) 112:1298–308. doi: 10.1038/ajg.2017.49
92. Gragnani L, Piluso A, Urraro T, Fabbrizzi A, Fognani E, Petraccia L, et al. Virological and clinical response to interferon-free regimens in patients with HCV-related mixed cryoglobulinemia: preliminary results of a prospective pilot study. Curr Drug Targets (2017) 18:772–85. doi: 10.2174/1389450117666160208145432
93. Saadoun D, Pol S, Ferfar Y, Alric L, Hezode C, Si Ahmed SN, de Saint Martin L, et al. Efficacy and safety of sofosbuvir plus daclatasvir for treatment of HCV-associated cryoglobulinemia vasculitis. Gastroenterology (2017) 153:49–52.e45. doi: 10.1053/j.gastro.2017.03.006
94. Cacoub P, Vautier M, Desbois AC, Lafuma A, Saadoun D. Effectiveness and cost of hepatitis C virus cryoglobulinaemia vasculitis treatment: from interferon-based to direct-acting antivirals era. Liver Int. (2017) 37:1805–13. doi: 10.1111/liv.13465
95. Wisloff T, White R, Dalgard O, Amundsen EJ, Meijerink H, Lovlie AL, K et al. Economic evaluation of direct-acting antivirals for hepatitis C in Norway. Pharmacoeconomics (2018) 36:591–601. doi: 10.1007/s40273-017-0604-3
96. Cravedi P, Angeletti A, Remuzzi G. New biologics in the treatment of rare glomerular diseases of childhood. Curr Opin Pharmacol. (2017) 33:27–33. doi: 10.1016/j.coph.2017.03.010
97. Datta SK. Anti-CD20 antibody is an efficient therapeutic tool for the selective removal of autoreactive T cells. Nat Clin Pract Rheumatol. (2009) 5:80–2. doi: 10.1038/ncprheum0983
98. Saadoun D, Resche Rigon M, Sene D, Terrier B, Karras A, Perard L, et al. Rituximab plus peg-interferon-alpha/ribavirin compared with peg-interferon-alpha/ribavirin in hepatitis C-related mixed cryoglobulinemia. Blood (2010) 116:326–34; quiz 504–325. doi: 10.1182/blood-2009-10-248518
99. Dammacco F, Tucci FA, Lauletta G, Gatti P, De Re V, Conteduca V, et al. Pegylated interferon-alpha, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: a long-term study. Blood (2010) 116:343–53. doi: 10.1182/blood-2009-10-245878
100. Fabrizi F, Colucci P, Ponticelli C, Locatelli F. Kidney and liver involvement in cryoglobulinemia. Semin Nephrol. (2002) 22:309–18. doi: 10.1053/snep.2002.33672
101. Quigg RJ, Brathwaite M, Gardner DF, Gretch DR, Ruddy S. Successful cyclophosphamide treatment of cryoglobulinemic membranoproliferative glomerulonephritis associated with hepatitis C virus infection. Am J Kidney Dis. (1995) 25:798–800. doi: 10.1016/0272-6386(95)90557-X
102. Reed MJ, Alexander GJ, Thiru S, Smith KG. Hepatitis C–associated glomerulonephritis–a novel therapeutic approach. Nephrol Dial Transplant. (2001) 16:869–71. doi: 10.1093/ndt/16.4.869-a
103. Goodkin DA, Bieber B, Jadoul M, Martin P, Kanda E, Pisoni RL. Mortality, hospitalization, and quality of life among patients with hepatitis c infection on hemodialysis. Clin J Am Soc Nephrol. (2017) 12:287–97. doi: 10.2215/CJN.07940716
104. Sawinski D, Bloom RD. Novel hepatitis C treatment and the impact on kidney transplantation. Transplantation (2015) 99:2458–66. doi: 10.1097/TP.0000000000000847
105. Li AA, Cholankeril G, Cheng XS, Tan JC, Kim D, Toll AE, et al. Underutilization of hepatitis C virus seropositive donor kidneys in the united states in the current opioid epidemic and direct-acting antiviral era. Diseases (2018) 6:E62. doi: 10.3390/diseases6030062
106. Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR, et al. Trial of transplantation of HCV-infected kidneys into uninfected recipients. N Engl J Med. (2017) 376:2394–5. doi: 10.1056/NEJMc1705221
107. Kadatz M, Klarenbach S, Gill J, Gill JS. Cost-effectiveness of using kidneys from hepatitis C nucleic acid test-positive donors for transplantation in hepatitis C-negative recipients. Am J Transplant. (2018) 18:2457–64. doi: 10.1111/ajt.14929
108. Chute DF, Chung RT, Sise ME. Direct-acting antiviral therapy for hepatitis C virus infection in the kidney transplant recipient. Kidney Int. (2018) 93:560–7. doi: 10.1016/j.kint.2017.10.024
Keywords: direct acting antivirals, HCV, cryoglobulinemia, rituximab, kidney transplant
Citation: Angeletti A, Cantarelli C and Cravedi P (2019) HCV-Associated Nephropathies in the Era of Direct Acting Antiviral Agents. Front. Med. 6:20. doi: 10.3389/fmed.2019.00020
Received: 11 August 2018; Accepted: 23 January 2019;
Published: 08 February 2019.
Edited by:
Azreen Syazril Adnan, University of Science, Malaysia, MalaysiaReviewed by:
Gurvinder Kaur, Laboratory Oncology, All India Institute of Medical Sciences, IndiaCopyright © 2019 Angeletti, Cantarelli and Cravedi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo Cravedi, cGFvbG8uY3JhdmVkaUBtc3NtLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.