
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 11 February 2019
Sec. Rheumatology
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00014
This article is part of the Research Topic Synovial Tissue: Turning the Page to Precision Medicine in Arthritis? View all 16 articles
Psoriatic arthritis (PsA) is an immuno-inflammatory disease with a heterogeneous clinical presentation as affects musculoskeletal tissues (arthritis, enthesitis, spondylitis), skin (psoriasis) and, less frequently, eye (uveitis) and bowel (inflammatory bowel disease). It has been suggested that distinct affected tissues could exhibit different immune-inflammatory pathways so complicating the understanding of the physiopathology of psoriatic disease as well as its treatment. Despite of the key pathogenic and clinical relevance that enthesitis has in PsA, peripheral arthritis is more easily perceived. At the macroscopic level, PsA synovitis has predominantly tortuous, bushy vessels, whereas rheumatoid arthritis (RA) is characterized by mainly straight, branching vessels so reflecting prominent neo-angiogenesis in PsA. Synovial biopsies have demonstrated a similar cellular and molecular picture in PsA and RA, although some differences have been reported at the group level, as higher density of vessels, CD163+ macrophages, neutrophils and mast cells in PsA. In fact, synovial IL-17+ mast cells are significantly increased in PsA and produce more IL-17A compared with RA, and a proof of concept study supports its relevant role in the synovitis of SpA, included PsA. As firstly reported in RA, synovial lymphoid neogenesis is found also in the same proportion of PsA as in RA patients, despite the lack of autoantibodies in PsA. These lymphoid structures are associated with activation of the IL-23/Th17 pathway in RA and seemly in PsA, which could be useful to stratify RA patients. Immunohistochemical and transcriptomic methodologies have still not found synovial biomarkers useful to distinguish psoriatic from rheumatoid synovitis at the patient level. However, modern methodologies, as MALDI-Mass Spectrometry Imaging, applied to the study of synovial tissue have revealed metabolic and lipid signatures which could support clinical decision-making in the diagnosis of PsA and RA and to go further toward the personalized medicine.
Psoriatic arthritis (PsA) is an immune-mediated inflammatory disease with a wide range of clinical manifestations: synovitis, enthesitis, spondylitis, dactylitis, skin, and nail psoriasis. More rarely, it involves the eye (uveitis) and the bowel (Crohn's disease). PsA is included in the spondyloarthritis (SpA) concept, which encompasses a group of diseases sharing immunogenetic, pathophysiological, clinical, and radiological features, which differ from rheumatoid arthritis (RA) (1). McGonagle et al. hypothesized that the primary lesion of SpA is enthesitis, that enthesopathy may be the common link between all forms of SpA, and that enthesitis in SpA synovial joints is frequent (2, 3). The close anatomical relationship between the enthesis, prone to mechanical stress, and the vascular synovium, in contact with a variety of immune mediators, may provide the pathogenic basis for joint inflammation in SpA, including PsA. The functional unit formed by enthesis and adjacent synovium was termed as synovial enthesis complex (SEC). The SEC represents a conceptual framework, which may explain the tissue specificity and highlights the role of mechanical stress in SpA, while at the same time providing a unifying pathophysiological concept for PsA based on the idea that specific tissues may be particularly sensitive to mechanical triggers (4). Paramarta et al. challenged the hypothesis of enthesitis being the primary lesion in SpA leading to a secondary synovitis over time, although the authors recognized some limitations in their study (5). Also, the study of enthesitis pathophysiology is limited by the difficulty to obtaining biopsies from the enthesis due to potential adverse effects.
Arthritis is more easily perceived that enthesitis, as clinical trials and registries of patients with PsA have showed. Peripheral arthritis is a key target of the pathogenic process which may lead to joint destruction and associated impaired function and quality of life (6). Therefore, psoriatic synovitis has been widely studied, generally as part of other peripheral SpA and has been compared with RA, the most prevalent peripheral arthritis (7).
The synovial membrane (synovium) borders the joint cavity and attach to the bone-cartilage interface. A healthy synovium consists of a thin layer (lining) 1-2 cells thick containing synovial fibroblasts and macrophages. Below this layer is the sublining, which is composed of loose connective tissue with blood vessels, lymphoid vessels, fibroblasts, nerve fibers, and few leucocytes. The inflamed synovium (synovitis) has three histological characteristics: lining hyperplasia (proliferation of synovial fibroblasts and accumulation of macrophages); neoangiogenesis (blood vessel proliferation in the sublining), and huge infiltration of the sublining by inflammatory cells, including lymphocytes, macrophages, dendritic cells and mast cells, which produce proinflammatory cytokines, growth factors and metalloproteases contributing to persistent synovitis and joint destruction (1, 7).
The study of synovitis in PsA, RA and other chronic arthritis is being driven by mini-arthroscopy and ultrasound-guided biopsies, which are safe and well-tolerated techniques and allow synovial tissue samples to be obtained from large and small joints at any stage of activity of disease: early, established, active or remission, as well as before and after therapeutic interventions. Taken together, easier extraction of synovial tissue together with the application of powerful new methodologies (trancriptomics, single-cell RNA, proteomics, metabolomics, new inmmunohistologic markers, mass spectrometry image analysis) will accelerate the study of synovitis to better understand their diagnostic and prognostic implications (8). Most studies on synovitis have focused on RA, while others comparing RA and SpA, included PsA; however few studies have focused specifically on PsA. We review PsA synovitis from the macroscopic (arthroscopy) and microscopic perspective, highlighting the cellular and molecular characteristics of each of the histological alterations of synovitis mentioned above as compared with RA. Table 1 displays some key clinical and pathogenic differences between PsA and RA.
The morphologic and cellular heterogeneity of synovitis requires review of the macroscopic features, which appear to differ between PsA and RA, and subsequent description of the cellular features according to the key changes that occur in inflammation: lining hyperplasia, neo-angiogenesis and leukocyte infiltration.
Using rheumatologic arthroscopy, Reece et al. (9) found significant differences in the pattern of new blood vessel between psoriatic and rheumatoid synovitis. PsA synovitis is characterized by erythematous villae with dilated, bushy and tortuous vessels (Figure 1A) whereas RA synovitis predominantly shows straight, branched vessels. This distinct pattern probably reflects a distorted proliferation of neovessels (neo-angiogenesis) due to increased expression of pro-angiogenic mediators, such as VEGF and Angiopoietin-2 (Ang-2), in PsA (10). Other studies confirmed these findings in PsA and peripheral SpA, with some differences in the frequency of the straight and branched pattern of RA (11–13). Despite its high sensitivity and specificity, the bushy and tortuous pattern is not diagnostic of PsA, although it may be a useful guide in the diagnostic work-up of undifferentiated arthritis (12).
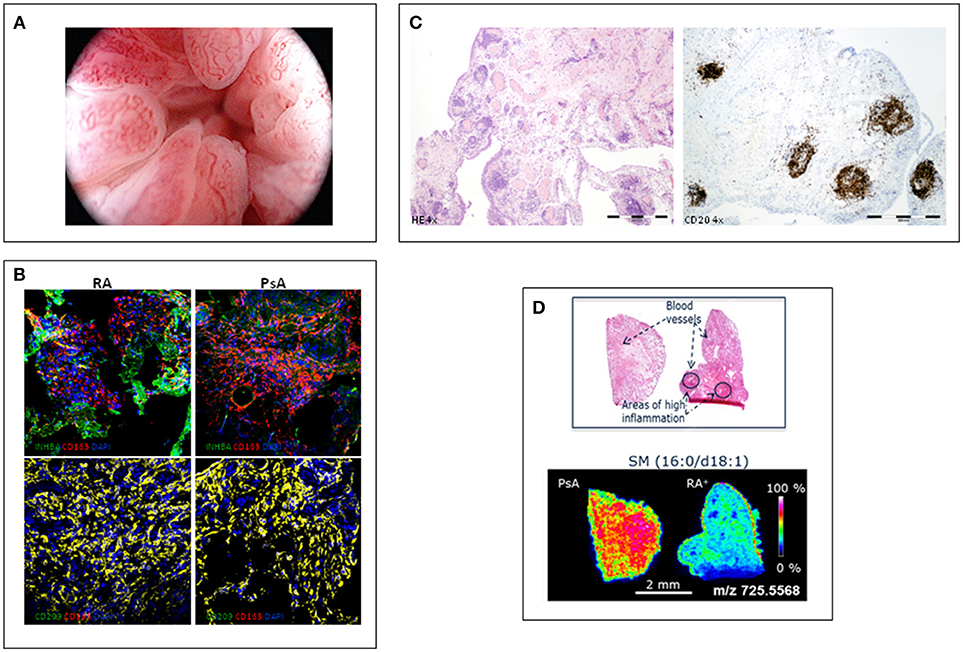
Figure 1. Different features of psoriatic synovitis are represented. (A) Arthroscopic view of psoriatic synovitis with erithematous villae plenty of dilated, tortuous vessels. (B) Immunofluoresence analysis of the expression of macrophage-polarization markers in synovial tissue CD163+ macrophages from RA and PsA patients, as determined by confocal microscopy using anti-INHBA (Activin A) –a GM-CSF induced gene- and CD209 –a M-CSF induced gene- specific antibodies; nuclei were counterstained with DAPI (Courtesy of A Puig-Kröger, PhD, Madrid, Spain). (C) Staining of inflammed synovium from a patient with PsA; left: H-E staining (4x) showing a general view of synovial membrane standing out abundant vessels surrounded by folicular aggregates; right: CD20 staining (4x) highlighting the B-cell folicles in PsA synovitis. (D) Mass Spectometry Image analysis showing spatial mapping positive-lipid ion in synovium sections of PsA and RA. Scale bar shows normalized intensities (Courtesy of Prof. F Blanco, A Coruña, Spain).
Fibroblast-like synoviocytes (FLS) and macrophages are the cellular components of the lining. Inflammation induces activation, proliferation and/or diminished FLS apoptosis, whereas macrophages increase due to infiltration from the peripheral blood. Studies of FLS in PsA are scarce compared with RA, where it has been shown that FLS undergo epigenetic changes, becoming persistently activated and forming the invasive front of synovial tissue in the joint cartilage (pannus) (14). A study of the effects of Janus kinase inhibitor tofacitinib on synovial fibroblast function suggested that PsA fibroblasts are activated similarly to RA fibroblasts (15). In RA, FLS change phenotypically and functionally at different anatomical sites and contribute to the identity of individual tissue, and they are capable of actively participating and orchestrating inflammation and immunity (14). A single-cell RNA sequencing and immunohistochemistry based study has described three functionally distinct subsets of FLS in RA: lining, immunoregulatory, and pathogenic fibroblasts populations. Pathogenic fibroblasts are located in the sublining around the vessels (CD34-CD90+) and they are the only FLS subset significantly increased in RA compared with osteoarthritis (16). Although several studies have reported increased lining hyperplasia in RA compared with PsA, others have found no differences (7). Using the Hsp47 antibody, a new specific marker of lining and sublining FLS (17), we have found a significant increase of sublining FLS, but no lining FLS, in RA compared with PsA, without between-group differences in systemic inflammation markers (CRP) (18). Lining CD68+ macrophages are functionally heterogeneous and include proinflammatory and tissue resident macrophages, a population not well-defined by lack of markers, but there are no differences in their cellular density between PsA and RA synovitis (19).
In line with the macroscopic hypervascularization that characterizes PsA synovitis, several studies have found an increase of vessels density in PsA compared with RA. Furthermore, different pro-angiogenic factors are expressed in the two diseases, with increased Ang-2 in PsA and Ang-1 in RA (20–22). Successful treatment with anti-TNF therapy in PsA synovitis reduces expression of VEGF and its receptors VGFR1 and VGFR2, but not Ang-2 expression, leading to regression of neovessels, probably by inducing endothelial cell apoptosis (23).
A recent study comparing CD31+ synovial vessels between PsA (n = 38) and RA (n = 40) patients found no significant differences between the two diseases (18).
A vast influx of inflammatory cells of the innate and adaptive immune system populates the inflamed synovial membrane, with the most being macrophages, neutrophils, mast cells, and T and B-lymphocytes. All these cells are activated and produce multiple pro-inflammatory and pro-angiogenic cytokines, chemokines, growth factors, metalloproteases, and other mediators, which contribute to the persistence of synovitis and joint destruction. Global cell infiltration in PsA and RA synovitis in the histologic analysis is similar, although characterization by immunohistochemistry of the infiltrating cells could encounter differences, as synovial infiltration by mast cells, CD15+ neutrophils and CD163+ macrophages is increased in SpA, included PsA, compared with in RA (7).
CD68+ macrophages accumulate in the synovium of RA and PsA joints, where they exhibit destructive and remodeling potential and contribute considerably to joint inflammation and joint destruction (24, 25). In RA and in SpA, including PsA, macrophage density correlates with disease activity (19). Sublining CD68+ macrophages density has been shown to be similar in PsA and RA synovitis (18, 26). A small study comparing RA and PsA synovitis found that synovial p53 expression and CD68+ macrophages density was associated with erosive disease only in RA suggesting that CD68+ macrophages differ in the destructive potential between RA and PsA (27).
Few studies have analyzed macrophage subsets in chronic arthritis, but have shown differences, probably due to the markers used. CD163-positivity has been proposed as a biomarker of anti-inflammatory macrophages and CD163+ macrophages were found overexpressed in SpA synovitis, whereas RA was characterized by overexpression of pro-inflammatory macrophage markers (19). A study using surface markers (CD14, CD163, CD68, CD32, CD64, CD200R, CD80) on synovial tissue macrophages from RA and SpA patients found that macrophages had a mixed M1-proinflammatory/M2-anti-inflammatory phenotype, with M1 predominance in RA and IL-10-expressing macrophages in SpA (28).
The characterization of ex-vivo CD14+ macrophages isolated from the synovial fluid of patients with active RA indicates that they exhibit a transcriptomic and protein profile that is compatible with a GM-CSF-skewed macrophage polarization (29). The proteins encoded by several of the GM-CSF-associated gene markers have also been detected in macrophages from active RA synovial tissue, including activin A, MMP12 and CCR2 (29). We analyzed the expression of markers of GM-CSF derived macrophages (INHBA, MMP12, and TNFα) and M-CSF derived macrophages (CD209) on CD163+ macrophages, and found a similar expression of GM-CSF- and M-CSF-associated markers in synovial tissue of RA and PsA patients (30) (Figure 1B). These results support the presence of similar GM-CSF and M-CSF skewed macrophages in RA and PsA synovitis.
Mast cells have been reported to have a potential sentinel function as innate protective cells which is supported by their strategic location in skin, gut, and airways, and their expression of specific danger signal receptors such as TLR2 and TLR4. Mast cells also have the ability to synthesize and, in addition, release preformed mediators including cytokines, proteases, and anti-microbial defensins (31). Mast cells play a previously- unappreciated role in synovial inflammation in SpA, included PsA, as it has been shown that they are significantly more abundant in PsA than in RA synovitis and, importantly, they are also the main cellular source of IL-17 A in PsA synovial tissue. These findings are independent of the disease stage and anti-TNF therapy (32). However, the absence of IL-17A mRNA in mast cells has also been demonstrated and a novel mechanism whereby mast cells capture and store exogenous IL-17A in specialized intra-cellular vesicles through receptor-mediated endocytosis, releasing bioactive IL-17 A after mast cell stimulation, has been discovered (33).
New findings reporting IL-17A-loaded mast cells in the normal skin and gut, in SpA synovial tissue before and after anti-IL-17 A antibody secukinumab, and in the inflamed gut, support the concept of mast cells as sentinel cells, as IL-17A-positive mast cells are readily available in non-inflamed tissues, and the IL-17A content decreased during inflammation in the gut lamina propria and increased upon anti-inflammatory treatment of SpA synovitis (31). Therefore, the presence of IL-17A-positive mast cells across different SpA target tissues and the inverse correlation between their IL-17A-content and inflammation indicate that the IL-17A content in mast cells can be regulated (31). Understanding how IL-17A can be controlled locally during tissue inflammation may result in novel therapeutic strategies to target IL-17A, a key cytokine in PsA (31).
In RA synovitis, high synovial mast cell counts are associated with local and systemic inflammation, autoantibody positivity and high disease activity. They are located at the outer border of lymphoid aggregates. Furthermore, mast cells promote the activation and differentiation of naïve B cells and induce ACPA production, mainly via contact-dependent interactions (34). Although synovial mast cells are also the main IL-17A positive cells in RA synovitis, its role remains to be studied (35).
Polymorphonuclear cells have been reported to be increased in synovial tissue of axial and peripheral SpA, including PsA synovitis, compared with RA, and correlated with disease activity. Their reduction after treatment was associated with a good therapeutic response, leading to them being defined as a biomarker of response for SpA (36, 37). In fact, after mast cells, neutrophils (CD15+ cells) are the most frequent IL-17 A+ cells in SpA and PsA (32). Neutrophils are scarce in RA synovitis, but a recent study comparing sinovial CD15+ cells (neutrophils) in PsA and RA synovitis found no significant differences between the two diseases (18).
Although PsA seems to have a partial autoinflammatory pathophysiology whereas RA has a strong autoimmunity component (38), in general sinovial T and B-lymphocytes, and plasma cells have been found to be similar in PsA and RA synovitis (7). However, beyond the number and type of infiltrating leukocytes, their spatial organization in the sinovial microarchitecture may be of pathophysiological relevance (7). Ectopic lymphoid neogenesis (ELN) is characterized by lymphocyte aggregates (Figure 1C) with prototypical features that recapitulate those of germinal centers, such as the presence of high endotelial venules and folicular dendritic cells (39). As ELN resembles secondary lymphoid tissues, it has been proposed that sinovial ELN may play a role in mounting immune responses, and specifically the autoimmune response observed in RA (40). However, sinovial ELN is similarly found in PsA and in RA, and there is no association with the presence of RA-specific autoantibodies (41–43). However, synovial ELN in PsA and RA have been associated with a different cytokine profile characterized by specific expression of the IL-23/Th17 cytokines axis (44, 45). These findings suggest that an important subgroup of RA patients express high IL-23/IL-17 cytokines, introducing the potential of stratification of patients by ELN in exploratory clinical trial for anti-IL23 or anti-IL-17 antibodies.
Comparison of synovial biopsies of patients with RA and SpA, including PsA, to analyse synovial molecular and cellular processes by pan-genomic microarray, has revealed a myogene signature specific for SpA, which was independent of disease duration, treatment and SpA subtype (non-psoriatic vs. psoriatic). These findings were confirmed by qPCR and immunohistochemistry analysis, and the synovial cells expressing myogenes were identified as vimentin-positive, prolil4-hydroxilase-positive, CD90+,CD146+ mesenchimal cells in the lining and sublining layers. This specific myogene signature did not change after anti-TNF therapy (46).
A study of gene array in paired skin and synovial biopsy samples from 12 patients with both PsA and psoriasis, confirmed by PCR and immunohistochemistry, showed that gene expression patterns in psoriatic skin and synovium differed, with a stronger IL-17 signature in skin than synovium, while TNF was higher in synovium (47). These transcriptomic analysis reveal new molecular pathways that open new avenues in the knowledge of the differential pathogenesis of synovitis in PsA and RA as well as between different tissues involved in PsA.
A pionner study used Mass Spectrometry Imaging (MSI) to identify lipid and metabolic profiles in the synovial tissue of 25 patients with PsA, 21 with RA (16 seropositive and 5 seronegative) and 10 with undifferentiated arthritis. Tissue sections were deposited on conductive slides and coated with different matrices for lipid and metabolite extraction. MALDI images were acquired on a rapifleX MALDI Tissuetyper time-of-flight instrument. Multivariate data analysis was used to search for the lipids and metabolites with the highest between-group differences.
MALDI-MSI revealed differentiated lipid and metabolic profiles in all the groups studied. Discriminant analysis of the lipid data acquired in positive ion mode displayed a good separation of patients with PsA and RA, especially seropositive RA (Figure 1D). PsA synovium was characterized by a higher content of phospholipids compared to seronegative and seropositive RA. However, sugar metabolites displayed a stronger intensity in RA than in PsA synovium. Metabolic and lipid signatures reported with this new methodology could support clinical decision-making in the diagnosis of RA and PsA (48).
Globally, PsA synovitis has more similarities than differences when compared with RA at the histologic and immunohistochemical level. However, there is some singularities in PsA that merit more in-depth research: the role of IL-17-positive mast cells in PsA inflammation and in IL-17 A regulation; the role of ectopic lymphoid neogenesis in PsA, and to know if there is distinct functional subsets of synovial FLS in PsA as in RA. New research tools as pan-genomic microarrays and metabolomics/proteomics associated to mass spectrometry image analysis are full of promise to reveal new cellular and molecular features specific to PsA synovitis which improve our diagnostic and prognostic potential.
JC revised the references and wrote the first draft of the manuscript. RC, AC, and JR revised the manuscript and collaborate in the discussion and writing to the last version. All the co-author revised and approved the manuscript.
The research of JC have been performed by grants from Instituto de Salud Carlos III, Ministry of Economy, Spain, and FEDER, una manera de hacer Europa.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Veale DJ, Fearon U. What makes psoriatic and rheumatoid arthritis so different? RMD Open. (2015) 1:e000025. doi: 10.1136/rmdopen-2014-000025
2. McGonagle D, Gibbon W, O'Connor P, Green M, Pease C, Emery P. Characteristic resonance imaging entheseal changes of knee synovitis in spondyloarthropathy. Arthritis Rheum. (1998) 41:694–700.
3. McGonagle D, Khan MA, Marzo-Ortega H, O'Connor P, Gibbon W, Emery P. Enthesitis in spondyloarthropathy. Curr Opin Rheumatol. (1999) 11:244–50. doi: 10.1097/00002281-199907000-00004
4. McGonagle D, Lories RJ, Tan AL, Benjamin M. The concept of a ‘synovio-entheseal complex' and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. (2007) 56:2482–91. doi: 10.1002/art.22758
5. Paramarta JE, van der Leij C, Gofita I, Yeremenko N, van de Sande MG, de Hair MJ, et al. Peripheral joint inflammation in early onset spondyloarthritis is not specifically related to enthesitis. Ann Rheum Dis. (2014) 73:735–40. doi: 10.1136/annrheumdis-2012-203155
6. Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheum Dis Clin North Am. (2015) 41:569–79. doi: 10.1016/j.rdc.2015.07.003
7. van de Sande MG, Baeten DL. Immunopathology of synovitis: from histology to molecular pathways.Rheumatology. (2016) 55:599–606. doi: 10.1093/rheumatology/kev330
8. Orr C, Vieira-Sousa E, Boyle DL, Buch MH, Buckley CD, Cañete JD, et al. Synovial tissue research: a state-of-the-art review. Nat Rev Rheumatol. (2017) 14:60. doi: 10.1038/nrrheum.2017.206
9. Reece RJ, Cañete JD, Parsons WJ, Emery P, Veale DJ. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum. (1999) 42:1481–4.
10. Fearon U, Griosios K, Fraser A, Reece R, Emery P, Jones PF, et al. Angiopoietins, growth factors, and vascular morphology in early arthritis. J Rheumatol. (2003) 30:260–8.
11. Fiocco U, Cozzi L, Chieco-Bianchi F, Rigon C, Vezzu‘ M, Favero E, et al. Vascular changes in psoriatic knee joint synovitis. J Rheumatol. (2001) 28:2480–6.
12. Cañete JD, Rodríguez JR, Salvador G, Gómez-Centeno A, Muñoz-Gómez J, Sanmartí R. Diagnostic usefulness of synovial vascular morphology in chronic arthritis. A systematic survey of 100 cases. Semin Arthritis Rheum. (2003) 32:378–87. doi: 10.1053/sarh.2002.50004
13. Salvador G, Sanmartí R, Gil-Torregrosa B, García-Peiró A, Rodríguez-Cros JR, Cañete JD. Synovial vascular patterns and angiogenic factors expression in synovial tissue and serum of patients with rheumatoid arthritis. Rheumatology (2006) 45:966–71. doi: 10.1093/rheumatology/kel043
14. Dakin SG, Coles M, Sherlock JP, Powrie F, Carr AJ, Buckley CD. Pathogenic stromal cells as therapeutic targets in joint inflammation. Nat Rev Rheumatol. (2018) 14:714–26. doi: 10.1038/s41584-018-0112-7
15. Gao W, McGarry T, Orr C, McCormick J, Veale DJ, Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis. (2016) 75:311–5. doi: 10.1136/annrheumdis-2014-207201
16. Mizoguchi F, Slowikowski K, Wei K, Marshall JL, Rao DA, Chang SK, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun. (2018) 9:789. doi: 10.1038/s41467-018-02892-y
17. Izquierdo E, Cañete JD, Celis R, Del Rey MJ, Usategui A, Marsal S, et al. Synovial fibroblast hyperplasia in rheumatoid arthritis: clinicopathologic correlations and partial reversal by anti-tumor necrosis factor therapy. Arthritis Rheum. (2011) 63:2575–83. doi: 10.1002/art.30433
18. Cuervo A, Celis R, Ramírez J, Hernández MV, Ruíz Esquide V, Inciarte-Mundo J, et al. Immunohistologic study of synovitis from patients with undifferentiated arthritis who evolved to rheumatoid arthritis or psoriatic arthritis after follow-up- Ann Rheum Dis. (2017) 76:128. doi: 10.1136/annrheumdis-2017-eular.4850
19. Baeten D, Kruithof E, De Rycke L, Boots AM, Mielants H, Veys EM. Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res Ther. (2005) 7:R359–69. doi: 10.1186/ar1501
20. Fraser A, Fearon U, Reece R, Emery P, Veale DJ. Matrix metalloproteinase 9, apoptosis, and vascular morphology in early arthritis. Arthritis Rheum. (2001) 44:2024–8. doi: 10.1002/1529-0131(200109)44:9<2024::AID-ART351>3.0.CO;2-K
21. van de Sande MG, de Launay D, de Hair MJ, García S, van de Sande GP, Wijbrandts CA, et al. Local synovial engagement of angiogenic TIE-2 is associated with the development of persistent erosive rheumatoid arthritis in patients with early arthritis. Arthritis Rheum. (2013) 65:3073–83. doi: 10.1002/art.38128
22. Kruithof E, Baeten D, De Rycke L, Vandooren B, Foell D, Roth J, et al. Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res Ther. (2005) 7:R569–80. doi: 10.1186/ar1698
23. Cañete JD, Pablos JL, Sanmartí R, Mallofré C, Marsal S, Maymó J, et al. Antiangiogenic effects of anti-tumor necrosis factor alpha therapy with infliximab in psoriatic arthritis. Arthritis Rheum. (2004) 50:1636–41. doi: 10.1002/art.20181
24. van Kuijk AW, Reinders-Blankert P, Smeets TJ, Dijkmans BA, Tak PP. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann Rheum Dis. (2006) 65:1551–7. doi: 10.1136/ard.2005.050963
25. Baeten D, Demetter P, Cuvelier C, Van Den Bosch F, Kruithof E, Van Damme N, et al. Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: influence of disease duration and activity. Ann Rheum Dis. (2000) 59:945–53. doi: 10.1136/ard.59.12.945
26. Baeten D, Kruithof E, De Rycke L, Vandooren B, Wyns B, Boullart L, et al. Diagnostic classification of spondylarthropathy and rheumatoid arthritis by synovial histopathology: a prospective study in 154 consecutive patients. Arthritis Rheum. (2004) 50:2931–41. doi: 10.1002/art.20476
27. Salvador G, Sanmarti R, Garcia-Peiró A, Rodríguez-Cros JR, Muñoz-Gómez J, Cañete JD. p53 expression in rheumatoid and psoriatic arthritis synovial tissue and association with joint damage. Ann Rheum Dis. (2005) 64:183–7. doi: 10.1136/ard.2004.024430
28. Ambarus CA, Noordenbos T, de Hair MJ, Tak PP, Baeten DL. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res Ther. (2012) 14:R74. doi: 10.1186/ar3796
29. Soler Palacios B, Estrada-Capetillo L, Izquierdo E, Criado G, Nieto C, Municio C, et al. Macrophages from the synovium of active rheumatoid arthritis exhibit an activin A-dependent pro-inflammatory profile. J Pathol. (2015) 235:515-26. doi: 10.1002/path.4466
30. Cuervo Aguilera A, Fuentelsaz-Romero S, Estrada-Capetillo L, Celis R, Samaniego R, Ramírez J, et al. Synovial tissue macrophages polarisation (M1, M2) in patients with undifferentiated arthritis meeting diagnostic criteria for rheumatoid arthritis or psoriatic arthritis along the follow up. Ann Rheum Dis. (2018) 77(Suppl 2):1240–1. doi: 10.1136/annrheumdis-2018-eular.4846
31. Chen S, Noordenbos T, Blijdorp I, van Mens L, Ambarus CA, Vogels E, et al. Histologic evidence that mast cells contribute to local tissue inflammation in peripheral spondyloarthritis by regulating IL-17A-content. Rheumatology (2018). doi: 10.1093/rheumatology/key331. [Epub ahead of print].
32. Noordenbos T, Yeremenko N, Gofita I, van de Sande M, Tak PP, Cañete JD, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. (2012) 64:99–109. doi: 10.1002/art.33396
33. Noordenbos T, Blijdorp I, Chen S, Stap J, Mul E, Cañete JD, et al. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J Leukoc Biol. (2016) 100:453–62. doi: 10.1189/jlb.3HI1215-542R.
34. Rivellese F, Mauro D, Nerviani A, Pagani S, Fossati-Jimack L, Messemaker T, et al. Mast cells in early rheumatoid arthritis associate with disease severity and support B cell autoantibody production. Ann Rheum Dis. (2018) 77:1773–81. doi: 10.1136/annrheumdis-2018-213418
35. Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. (2010) 184:3336-40. doi: 10.4049/jimmunol.0903566
36. Appel H, Maier R, Wu P, Scheer R, Hempfing A, Kayser R, et al. Analysis of IL-17(+) cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res Ther. (2011) 20 13:R95. doi: 10.1186/ar3370
37. Appel H, Maier R, Bleil J, Hempfing A, Loddenkemper C, Schlichting U, et al. In situ analysis of interleukin-23- and interleukin-12-positive cells in the spine of patients with ankylosing spondylitis. Arthritis Rheum. (2013) 65:1522–9. doi: 10.1002/art.37937
38. McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. (2006) 3:e297. doi: 10.1371/journal.pmed.0030297
39. Bombardieri M, Lewis M, Pitzalis C. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat Rev Rheumatol. (2017) 13:141–54. doi: 10.1038/nrrheum.2016.217
40. Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. (2009) 6:e1. doi: 10.1371/journal.pmed.0060001
41. Cañete JD, Santiago B, Cantaert T, Sanmartí R, Palacin A, Celis R, et al. Ectopic lymphoid neogenesis in psoriatic arthritis. Ann Rheum Dis. (2007) 66:720–6. doi: 10.1136/ard.2006.062042
42. Cañete JD, Celis R, Moll C, Izquierdo E, Marsal S, Sanmartí R, et al. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. (2009) 68:751–6. doi: 10.1136/ard.2008.089284
43. Cantaert T, Kolln J, Timmer T, van der Pouw Kraan TC, Vandooren B, Thurlings RM, et al. B lymphocyte autoimmunity in rheumatoid synovitis is independent of ectopic lymphoid neogenesis. J Immunol. (2008) 181:785–94 doi: 10.4049/jimmunol.181.1.785
44. Celis R, Planell N, Fernández-Sueiro JL, Sanmartí R, Ramírez J, González-Álvaro I, et al. Synovial cytokine expression in psoriatic arthritisand associations with lymphoid neogenesis and clinical features. Arthritis Res Ther. (2012) 14:R93. doi: 10.1186/ar3817
45. Cañete JD, Celis R, Yeremenko N, Sanmartí R, van Duivenvoorde L, Ramírez J, et al. Ectopic lymphoid neogenesis is strongly associated with activation of the IL-23 pathway in rheumatoid synovitis. Arthritis Res Ther. (2015) 17:173. doi: 10.1186/s13075-015-0688-0
46. Yeremenko N, Noordenbos T, Cantaert T, van Tok M, van de Sande M, Cañete JD, et al. Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum. (2013) 65:174–85. doi: 10.1002/art.37704
47. Belasco J, Louie JS, Gulati N, Wei N, Nograles K, Fuentes-Duculan J, et al. Comparative genomic profiling of synovium versus skin lesions in psoriatic arthritis.Arthritis Rheumatol. (2015) 67:934–44. doi: 10.1002/art.38995
Keywords: psoriatic arthritis, rheumatoid arthritis, synovitis, immunohistochemistry, macrophage-polarization, microarrays, mass spectrometry image
Citation: Celis R, Cuervo A, Ramírez J and Cañete JD (2019) Psoriatic Synovitis: Singularity and Potential Clinical Implications. Front. Med. 6:14. doi: 10.3389/fmed.2019.00014
Received: 03 December 2018; Accepted: 17 January 2019;
Published: 11 February 2019.
Edited by:
Burkhard Franz Leeb, Karl Landsteiner Institute for Clinical Rheumatology, AustriaReviewed by:
Garifallia Sakellariou, University of Pavia, ItalyCopyright © 2019 Celis, Cuervo, Ramírez and Cañete. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan D. Cañete, amNhbmV0ZUBjbGluaWMudWIuZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.