
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 18 December 2018
Sec. Translational Medicine
Volume 5 - 2018 | https://doi.org/10.3389/fmed.2018.00354
This article is part of the Research Topic Recent Advances in Endothelial Progenitor Cells Toward Their use in Clinical Translation View all 10 articles
Since the discovery of Endothelial Progenitor Cells (EPC) by Asahara and colleagues in 1997, an increasing number of preclinical studies have shown that EPC based therapy is feasible, safe, and efficacious in multiple disease states. Subsequently, this has led to several, mainly early phase, clinical trials demonstrating the feasibility and safety profile of EPC therapy, with the suggestion of efficacy in several conditions including ischemic heart disease, pulmonary arterial hypertension and decompensated liver cirrhosis. Despite the use of the common term “EPC,” the characteristics, manufacturing methods and subset of the cell type used in these studies often vary significantly, rendering clinical translation challenging. It has recently been acknowledged that the true EPC is the endothelial colony forming cells (ECFC). The objective of this review was to summarize and critically appraise the registered and published clinical studies using the term “EPC,” which encompasses a heterogeneous cell population, as a therapeutic agent. Furthermore, the preclinical data using ECFC from the PubMed and Web of Science databases were searched and analyzed. We noted that despite the promising effect of ECFC on vascular regeneration, no clinical study has stemmed from these preclinical studies. We showed that there is a lack of information registered on www.clinicaltrials.gov for EPC clinical trials, specifically on cell culture methods. We also highlighted the importance of a detailed definition of the cell type used in EPC clinical trials to facilitate comparisons between trials and better understanding of the potential clinical benefit of EPC based therapy. We concluded our review by discussing the potential and limitations of EPC based therapy in clinical settings.
The term “putative endothelial cell progenitors” was pioneered by Asahara et al in their seminal publication in Science over two decades ago (1). They showed that this cell population can be successfully isolated from peripheral blood derived mononuclear cells of healthy volunteers, utilizing magnetic bead positive selection of two cell surface antigens, CD34, and Flk1. They also demonstrated that EPCs could home specifically to areas of ischemia. This forms the basis of vasculogenesis, whereby new blood vessels are formed by EPCs or angioblasts, which home, differentiate, proliferate, and incorporate into resident mature vessels in response to various stimuli such as ischemia (2). Prior to the discovery of this cell population, the principal mechanism of vascularization after an ischemic event was thought to be due to the process of angiogenesis, whereby new vessels are formed by direct migration, differentiation, and proliferation of the existing mature endothelial cells (3). Furthermore, the discovery of this novel EPC concept has overturned the previous dogma which suggested that vasculogenesis could only occur during embryogenesis. In fact, both vasculogenesis and angiogenesis may potentially have a synergistic role in postnatal revascularization.
The new paradigm shift in the understanding of vascular regeneration has led to multiple publications using the term “EPC.” Specifically, this term has been widely utilized in many studies as a surrogate biomarker to assess the risk of cardiovascular disease in human subjects (4–6) and as a potential novel therapeutic agent for vascular regeneration (7, 8). The initial nomenclature of EPC encompasses a heterogeneous cell population, including early EPC [or circulating angiogenic cells (CAC), myeloid angiogenic cells (MACs), pro-angiogenic haematopoietic cells (PACs)] and late EPC [or outgrowth endothelial cells, endothelial colony forming cells (ECFC)] (9–12).
Early EPCs typically are cultured on fibronectin coated plates and appear early in culture (4–7 days). They are defined as spindled shaped cells with low proliferative potential, with cells only surviving up to 4 weeks in culture (13, 14). Functionally, early EPCs neither form a colony in culture nor vessels in vitro in 2D Matrigel assays. They also do not integrate into pre-existing vessels. Despite this, they have been found to have pro-angiogenic paracrine capabilities, demonstrated by their ability to increase the number of tubules formed by mature endothelial cells (13). Medina et al. showed that early EPCs possessed an expression profile more similar to monocytes than endothelial cells, with cells expressing haematopoietic markers (RUNX1,WAS,LYN) as well as inflammatory markers (TLRs,CD14,HLAs) (12). Subsequently, Medina et al. showed that early EPCs were of myeloid origin as opposed to endothelial in origin (10). In contrast, late EPCs are cultured on collagen coated plates and tend to appear later in culture (usually after 1 week) as colonies with well circumscribed monolayers of cells pertaining a cobblestone morphology. Late EPCs behave functionally like endothelial cells with the ability to form vessels in 2D Matrigel angiogenesis assays. They also have an expression profile similar to mature endothelial cells (CD34+,VE-Cad+,vWF+) but have a much higher proliferation rate, and survival, with cells surviving up to 12 weeks. These cells secrete less angiogenic factors compared to early EPCs (9, 10, 12, 14). A more in depth comparison of these cell types can be found in a review by Hirschi et al. (15).
A recent Consensus Statement on Nomenclature of endothelial progenitors has discouraged the current liberal use of the term “EPCs,” and recommends the term “ECFC” instead. They have also proposed a more precise characterization of ECFCs based on a pre-defined cellular phenotype and function (10). This is a crucial step in defining the term “EPC” which will lead to the harmonization and standardization of the cell type used in clinical studies, thus allowing comparisons to be made across different studies.
The objective of this review was to search the current literature and critically appraise the current use of EPCs as therapeutic agents by summarizing: (a) clinical trials using EPCs as a therapeutic agent currently registered in www.clinicaltrials.gov; (b) published clinical trials using EPCs as a therapeutic agent; and (c) the efficacy of ECFCs in preclinical and clinical studies. We also highlight the potential and limitations of EPC based therapy in clinical settings.
Three separate literature searches were conducted to generate the data presented here (Appendices 1–3). “EPC” was used as the first search term and this was carried out using the www.clinicaltrials.gov database, focusing specifically on interventional therapies only. The second literature search focused on clinical trials in humans using the term “EPCs” as a therapeutic intervention using the PubMed and Web of Science databases published within the last 10 years. The third literature search (PubMed and Web of Science databases) was performed using the synonymous names of ECFCs, “Endothelial Colony Forming Cells or Outgrowth Endothelial Cells or Blood Outgrowth Endothelial Cells or Endothelial Outgrowth Cells or Late Endothelial Progenitor Cells or Late Outgrowth Endothelial Progenitor Cells,” as defined by Medina et al. (10) as the search term. Results were limited to primary interventional studies carried out within the last 10 years.
A total of 341 clinical trials were found when the term “EPC” was searched using the www.clinicaltrials.gov registry (22/09/05 to 12/04/17). Many of the registered trials related to the assessment of EPCs across various clinical states and were non-interventional in nature. There are 26 clinical trials which utilized EPCs as a therapeutic agent currently registered in www.clinicaltrials.gov (Table 1) with a total of 1,148 participants expected to be enrolled into the trials. Approximately two thirds of these registered clinical trials involved patients with ischemic conditions such as peripheral artery disease (n = 8), coronary artery disease (n = 7) and ischemic stroke (n = 2). The remaining conditions registered were pulmonary arterial hypertension (n = 4), liver cirrhosis (n = 2), lymphodema (n = 1), erectile dysfunction (n = 1), and traumatic bone defects (n = 1) (Table 1).
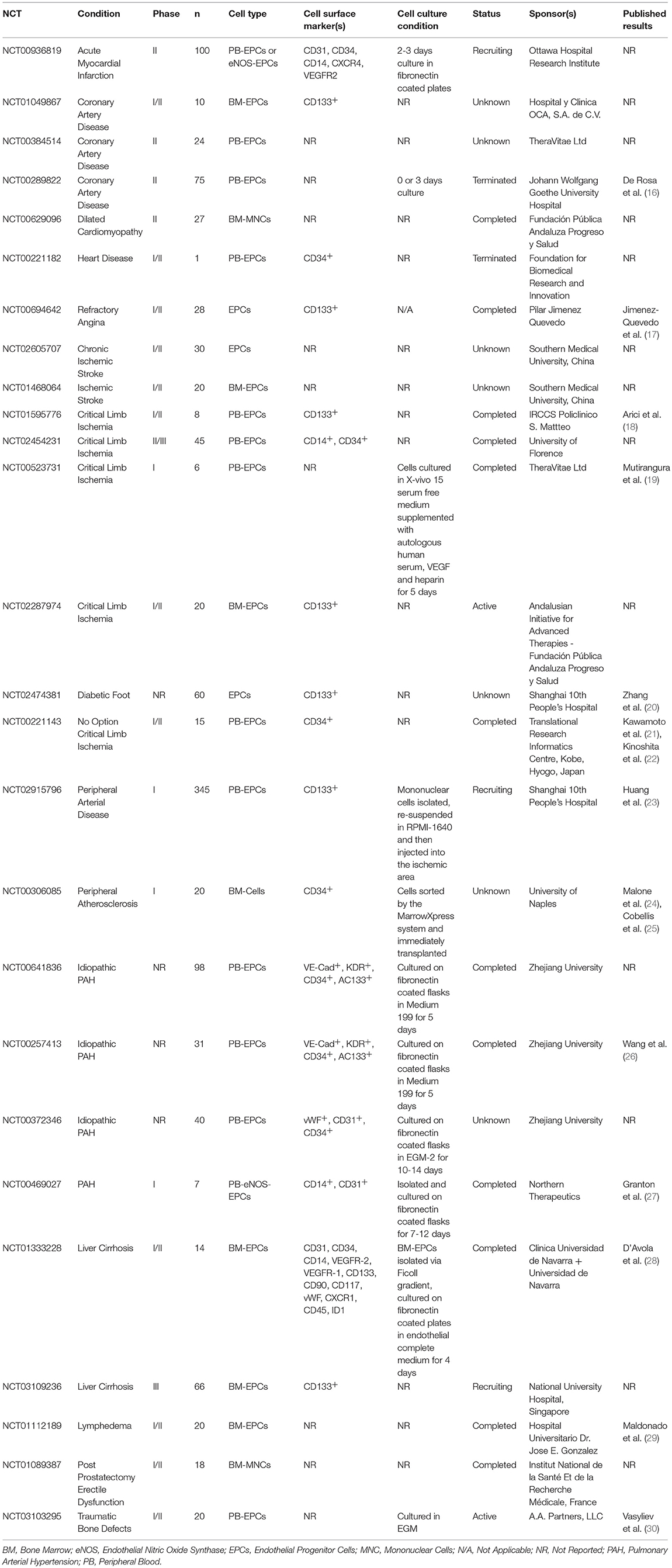
Table 1. Clinical trials using EPCs as therapeutic agent, registered under www.clinicaltrials.gov registry (22nd Sept 2005–12th Apr 2017).
The majority of the 26 registered clinical trials specified the source for EPC in the registry (n = 19), such as bone marrow derived (n = 9) or peripheral blood derived (n = 10), but only 10 trials specified the cell surface markers used for EPC definition in the registry, including CD133+(n = 7), CD34+(n = 2), and CD14−(n = 1). The status of half of these trials were labeled as “complete” (n = 12). Two trials were labeled as “terminated.” Slow recruitment was noted for one of these two trials (NCT00221182). The other trial was subsequently published (NCT00289822) (16). The remaining trials were labeled as “active” (n = 2), “recruiting” (n = 3), and “unknown” (n = 7).
The results of this search showed that the term “EPC” has been widely used to encompass a heterogeneous population of cells. In addition, these results also highlight the paucity of information registered within the www.clinicaltrials.gov for these clinical trials, thereby preventing direct comparison with other trials. A detailed definition of the cell type used in clinical trials is warranted, to facilitate better understanding of the potential clinical benefit of EPC based therapy.
Five relevant published clinical trials using EPCs were found following the PubMed and Web of Science database searches (01/05/2008 to 01/05/2018). These clinical trials involved various disease states including peripheral arterial disease (31, 32), coronary artery disease (33), pulmonary hypertension (34), and liver cirrhosis (28) (Supplementary Table 1). Despite using the term “EPC” as a therapeutic agent, the cell types used in all the five published trials differ, specifically, in their methods of cell isolation and culture, as well as the cell surface markers used for phenotypic characterization. Furthermore, the cell dose, and the route of administration varies between trials.
Lara-Hernandez et al. showed that intramuscular administration of EPCs into the ischemic limbs of 28 patients with no-option critical limb ischemia was safe and feasible (31). They used 50 mls of G-CSF mobilized blood and then selected for CD34+ and CD133+ cells. This treatment resulted in a significant reduction in the pain score of no option CLI patients with increased tissue perfusion and no adverse effects noted after a follow-up of 14 months (31). Tanaka et al. also isolated EPCs from G-CSF mobilized blood, but selected for CD34+ EPCs prior to intramuscular administration into non-healing diabetic feet. They demonstrated the safety of EPC therapy, no serious adverse events noted, coupled with increased vascular perfusion and complete wound closure after ~18 weeks (32).
Zhu et al. demonstrated the safety and feasibility of EPCs by injecting thymosin β1 pre-treated EPCs, into patients with ST segment elevated myocardial infarction, with some efficacy data showing improved exercise capacity and left ventricular function after 6 month follow-up (33). The EPCs used by Zhu et al. were cultured on fibronectin and phenotypically characterized as VE-Cadherin+, KDR+, CD34+, and CD133+ (33) cells. Similarly, Zhu et al demonstrated the safety and feasibility of intravenously administered CD34+, CD133+, and KDR+ EPCs into children with idiopathic pulmonary arterial hypertension (IPAH). Although safety was their primary endpoint, significant increases in exercise capacity and pulmonary hemodynamics were also noted (34).
D'Avola et al. showed that a single administration of EPCs (maximum of 100 × 106 cells) via the hepatic artery in 12 patients with decompensated liver cirrhosis is safe and feasible (28). No treatment-related severe adverse events were seen for up to 1 year of follow up. Extensive characterization of EPCs was performed following 7 days of ex vivo culture of fibronectin adherent mononuclear cells (from 50-100 mls of bone marrow aspirate) using extensive cell surface markers (CD31, CD34, CD14, VEGFR2, VEGFR1, CD133, CD90, CD117, vWF, CXCR4, ID1, and CD45) and functionality assessment with DiL-acetylated-LDL cholesterol uptake and lectin binding capacity as well as demonstration of tube-structure formation over Matrigel matrix (28).
While some of the clinical data from Table 1 was published, several of these papers were not included in this section for overall analysis for the following reasons: one paper did not use the term EPC but instead used the term “angiogenic precursor cells” (19), five papers included cells identified only using a single cell surface marker [e.g., CD133+ (17, 18, 20) or CD34+ (21, 29)], and one did not specifically define the cell type (24). It was concluded that these papers could not be classified according to our search criteria as being interventional EPC studies, and thus excluded. Two papers were published beyond the date range specified in our search (23, 26). Finally, two other papers from Table 1 met our classification criteria did not appear in our literature search (27, 30). Granton et al. used 7–50 × 106 peripheral blood eNOS modified EPCs for pulmonary arterial hypertension and showed an improvement in pulmonary resistance after treatment (27), whereas Vasyliev et al. used 20–60 × 106 autologous peripheral blood derived EPCs in a scaffold of allogeneic bone and fibrin gel for bone fracture repair and demonstrated an improvement in bone regeneration (30). Of note, neither study had a control group to compare against.
Overall, all trials were single arm trials, with four of the five published trials being early phase clinical trials and demonstrated safety and feasibility of EPC therapy. Only Zhu et al. conducted a randomized controlled trial (33).
In the previous sections, we examined human interventional studies using “EPCs” as a therapeutic but had noted that the field is moving toward a cell type that is considered to be the “true EPC.” Thus, we examined the published data on ECFCs, the novel and “bone-fide” EPC, using the PubMed and Web of Science databases. From our search using all the relevant search terms, only 39 out of 1,316 papers retrieved used hECFCs as an interventional therapy in preclinical models (01/05/2008 to 01/05/2018) (Table 2). The majority of the published articles assessed the potential therapeutic role of ECFC in ischemic disease models including hindlimb ischemia (n = 20), cerebral ischemia (n = 4), retinal ischemia (n = 2), myocardial ischemia (n = 2), ischemic acute kidney injury (n = 1) and ischemic reperfusion injury (n = 1). However, ECFCs were also being investigated for other conditions such as islet graft retention (n = 3), vascular injury (n = 2), pulmonary arterial hypertension (n = 1), bronchopulmonary dysplasia (n = 1) and traumatic brain injury (n = 2). In these 39 papers, hECFCs were predominantly derived from the umbilical cord (n = 31), followed by peripheral blood (n = 6) (Table 2). However, despite a robust preclinical evidence to suggest the efficacy of ECFCs in several conditions, this cell type has yet to be tested in human clinical trials.
Medina et al. highlighted the importance of ECFC identification with more than one surface marker (10). Despite the use of the term “ECFC,” four preclinical studies did not confirm the identity of ECFCs using surface expression markers, with eight studies utilizing only one surface expression marker. Of the papers which used multiple markers, the most commonly used surface markers were: CD34+ (n = 11), CD31+ (n = 16), VEGFR2/KDR+ (n = 13), and CD45− (n = 14).
The results of our literature search showed that the term “EPC” has been widely used to encompass a heterogeneous population of cells in both the published and registered clinical studies, thereby, rendering direct comparison amongst studies impossible. Our findings also highlight the paucity of information registered within www.clinicaltrials.gov for these clinical trials, specifically, the cell phenotype that is being tested. Approximately half of the EPC clinical trials (11 out of 26 trials) did not define the culture conditions used for their therapeutic product, and a majority of the trials (17 out of 26 trials) did not specify the cell surface markers used to characterize the cell therapy product used in their trials. Whilst the results from many of the ongoing clinical trials are yet to be published/released, only 12 out of 26 trials have reached the completion phase.
From the published EPC human clinical trials, there is a marked inconsistency in terms of the culture conditions and characterization of EPCs with D'Avola et al. and Vasyliev et al using multiple different cell surface markers to identify the EPCs they used (28, 30), Lara-Hernandez et al. and Granton et al using two cell surface markers (27, 31), Tanaka et al. only utilizing one cell surface marker (32), and Zhu et al. and Zhu et al. did not mention the marker used for defining the EPCs in their studies (33, 34). This lack of standardization makes it difficult to compare the results of different clinical trials and will in the future hinder the translation of these therapies into medical practice. A similar lack of consistency is again observed with the methods to culture EPCs with some reports administering EPCs without prior culture (31, 32), some culturing on fibronectin before re-injection into the patient (27, 28, 33, 34), and Vaysilev et al. culturing cells on uncoated flasks with EGM-2 medium (30). Despite the positive results from the above papers, EPC therapy is still limited by the immunogenicity of allogeneic EPCs, along with its poor definition, isolation and expansion standardization, as outlined above.
From the above data it is clear that a detailed definition of the cell type used in clinical trials is warranted, to facilitate better understanding of the potential clinical benefit of EPC based therapy. Consistent with the recommended nomenclature by Medina et al. the term “ECFC,” a more accurate description of a specific cell type within the EPC population can be carried out, allowing the standardization of cell definition for potential therapeutic use (10).
In fact, currently, there are an increasing number of preclinical studies which have demonstrated the efficacy of ECFCs in various disease models (36, 40, 56, 59, 61, 65, 68, 73). These studies predominantly focus on ischemic conditions such as limb ischemia, cerebral ischemia, myocardial ischemia, ischemic reperfusion injury, and ischemic kidney injury. The likely reason for this is beyond the scope of this review article but it may be related to the angiogenic effect of these cells, which facilitate the revascularization in these ischemic states; however these cells may have other unexplained therapeutic effects, which may not be related to its angiogenic properties. However, similar to EPC studies, there is a lack of standardization of cell surface markers and culture protocols when producing ECFCs for therapeutic intervention between the different studies. This lack of standardization, while addressed by Medina et al. (10), has not yet been fully adopted by the field of vascular regeneration.
The ability to successfully isolate ECFCs is crucial prior to its consideration for clinical use. We and others have previously published methods for ECFC isolation (74–78). However, the yield of ECFCs varies depending on the method used (9). Most methods used collagen as the matrix molecule for cell seeding, rather than fibronectin, suggesting that the former molecule is a better cell selection method (74–78). Direct comparison between collagen and fibronectin by Colombo et al. showed the contrasting impact of the type of matrix molecule used for cell seeding on the pharmacodynamics of ECFC colonies. Seeding cells on fibronectin, as compared with collagen, resulted in earlier appearance of ECFC colonies. In contrast, ECFC colonies cultured on collagen demonstrated a better cell proliferation and lifespan, which might be IL-6 and IL-8 dependent (79). Interestingly, the immunophenotype and the ability for in vitro tubule formation remains similar despite the type of matrix molecule used for cell seeding (79).
Tasev et al. have further refined the ECFC isolation method with better cell expansion rates using platelet lysate supplemented culture medium, for large scale propagation for potential clinical use (80). Hofmann et al. have also described an easily applicable method for isolating ECFCs directly using adult human blood to generate more than 100 million functional ECFCs (77). They collected 5 ml of peripheral blood from patients and plated directly into a T75 flask discarding supernatants at various time points to remove any blood cells that were not ECFCs. Their method used human platelet lysate, thereby making it a xeno-free protocol, and they were able to consistently isolate and expand ECFCs up to 30 population doublings (81). Moreover, using the culture method outlined in their paper, ECFCs can be cryopreserved without resulting in genomic instability or changes in cell phenotype and function (81).
Siegel et al. have successfully produced an ECFC product by leukapheresis of peripheral blood in accordance with Good Manufacturing Products (GMP) standard (82). Their isolation method can produce approximately 1.44 × 108 ECFC per white blood cell, following leukaparesis of up to 6.8 liters of peripheral blood. These ECFCs showed a significant Dil-AcLDL uptake and showed CD29+, CD31+, CD34+, CD44+, CD105+, CD117+, CD133+, CD144+, CD146+, and VEGFR2+ expression. Furthermore, they showed that their ECFCs could reach up to twelve cumulative population doublings. More importantly, these ECFCs showed no evidence of telomerase activity, as well as capable of in vitro tubule formation and secretion of epidermal growth factor, HGF, VEGF-A, platelet derived growth factor-B, IL-8, and monocyte chemoattractant protein-1 (82).
The major limitations of ECFC therapy are the long culture times to generate a therapeutic dose, due to its low frequency in peripheral and cord blood, and that it can only be administered in an autologous fashion due to its inherent immunogenicity. Furthermore, despite the potential for ECFC cryopreservation for future use, the intrinsic function of autologous ECFC may be impaired due to the underlying diseased state such as diabetes mellitus, with Jarajapu et al. reporting to be able to isolate ECFC from three in every ten diabetic patients, compared to eight out of nine in non-diabetic controls (83). However, ECFCs can be genetically modified to augment their function in vivo which may facilitate correction of disease-induced cell dysfunction. Examples of genetic modification include β1 integrin overexpression to improve blood perfusion in CLI (40), erythropoietin overexpression to promote erythropoiesis (84), or GSK-3β inhibition which improves the angiogenic capabilities of ECFCs (85).
In addition to genetic modification, ECFCs can be combined with other cell types to improve a specific aspect of ECFC therapy, such as combining them with mesenchymal stem cells (MSCs) or mesenchymal progenitor cells to reduce the immunogenic effect of allogeneic ECFCs and to increase cell survival post transplantation (86). This is due to the anti-inflammatory effects of mesenchymal stem cells. Reports have also noted that MSCs can differentiate into pericyte like cells which act to stabilize the vasculature formed (86–90). Based on the results of the above studies, next generation vascular cell therapies will likely consist of genetically modified ECFCs or ECFC combination.
To date, EPC based therapy has been shown to be feasible and safe with suggestion of efficacy. However, it is important to note that only one trial included a control arm. While EPCs have been previously the favored cell type utilized for vascular therapeutics, they consist of a heterogeneous population of cells which will produce challenges in terms of GMP compliant cell manufacturing and definition of a cell product. “ECFCs,” as defined by Medina et al. have several advantages over EPCs as a therapeutic product, as outlined above, including being a more defined cell type with enhanced proliferation, and possessing the ability to form new vessels, while also integrating into pre-existing vasculature. The use of ECFCs facilitates the harmonization and standardization of the cell type used in clinical studies, allowing direct comparison between studies (10). To translate ECFCs into routine clinical practice, issues surrounding their immunogenicity will need to be overcome, along with the issues regarding the standardization of markers used to identify them. To date, there have been no clinical trials using this cell type.
CK, TO, and AL: conception and design of the study. CK, CL, MC, and AL: analysis and interpretation of data. CK, CL, and AL: drafting of the manuscript. All authors revised the manuscript critically for important intellectual content and final approval for the submission of the manuscript.
TB is a founder, director and equity holder in Orbsen Therapeutics Ltd. TB and AL are both authors on a patent entitled ‘Osteopontin for the prediction and treatment of cardiovascular diseases' (US Patent Number: US8323968B2).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Science Foundation Ireland (SFI) Investigator Programme (15/IA/3136).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2018.00354/full#supplementary-material
1. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science (1997) 275:964–7. doi: 10.1126/science.275.5302.964
2. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. (1999) 85:221–8. doi: 10.1161/01.RES.85.3.221
3. Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, et al. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development (1988) 102:471–8.
4. Ricottini E, Madonna R, Grieco D, Zoccoli A, Stampachiacchiere B, Patti G, et al. Effect of high-dose atorvastatin reload on the release of endothelial progenitor cells in patients on long-term statin treatment who underwent percutaneous coronary intervention (from the ARMYDA-EPC Study). Am J Cardiol. (2016) 117:165–71. doi: 10.1016/j.amjcard.2015.10.043
5. Salazar-Martinez E, Rodriguez-Valentin R, Albavera-Hernandez C, Carreon-Rodriguez A, Lazcano-Ponce E. Number of colony-forming unit-Hill colonies among children and teenagers with obesity, dyslipidemia and breastfeeding history. Nutr Metab Cardiovasc Dis. (2016) 26:534–40. doi: 10.1016/j.numecd.2016.03.010
6. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. (2003) 348:593–600. doi: 10.1056/NEJMoa022287
7. Vaughan EE, Liew A, Mashayekhi K, Dockery P, McDermott J, Kealy B, et al. Pretreatment of endothelial progenitor cells with osteopontin enhances cell therapy for peripheral vascular disease. Cell Transplant. (2012) 21:1095–107. doi: 10.3727/096368911X623880
8. Reid E, Guduric-Fuchs J, O'Neill CL, Allen LD, Chambers SEJ, Stitt AW, et al. Preclinical evaluation and optimization of a cell therapy using human cord blood-derived endothelial colony-forming cells for ischemic retinopathies. Stem Cells Transl Med. (2018) 7:59–67. doi: 10.1002/sctm.17-0187
9. Liew A, Barry F, O'Brien T. Endothelial progenitor cells: diagnostic and therapeutic considerations. Bioessays (2006) 28:261–70. doi: 10.1002/bies.20372
10. Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med. (2017) 6:1316–20. doi: 10.1002/sctm.16-0360
11. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood (2004) 104:2752–60. doi: 10.1182/blood-2004-04-1396
12. Medina RJ, O'Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics (2010) 3:18. doi: 10.1186/1755-8794-3-18
13. Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. (2008) 51:660–8. doi: 10.1016/j.jacc.2007.09.059
14. Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. (2004) 24:288–93. doi: 10.1161/01.ATV.0000114236.77009.06
15. Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. (2008) 28:1584–95. doi: 10.1161/ATVBAHA.107.155960
16. De Rosa S Seeger, FH, Honold, J, Fischer-Rasokat U, Lehmann R, Fichtlscherer S, et al. Procedural safety and predictors of acute outcome of intracoronary administration of progenitor cells in 775 consecutive procedures performed for acute myocardial infarction or chronic heart failure. Circ Cardiovasc Interv. (2013) 6:44–51. doi: 10.1161/CIRCINTERVENTIONS.112.971705
17. Jimenez-Quevedo P, Gonzalez-Ferrer JJ, Sabate M, Garcia-Moll X, Delgado-Bolton R, Llorente L, et al. Selected CD133(+) progenitor cells to promote angiogenesis in patients with refractory angina: final results of the PROGENITOR randomized trial. Circ Res. (2014) 115:950–60. doi: 10.1161/CIRCRESAHA.115.303463
18. Arici V, Perotti C, Fabrizio C, Del Fante C, Ragni F, Alessandrino F, et al. Autologous immuno magnetically selected CD133+ stem cells in the treatment of no-option critical limb ischemia: clinical and contrast enhanced ultrasound assessed results in eight patients. J Transl Med. (2015) 13:342. doi: 10.1186/s12967-015-0697-4
19. Mutirangura P, Ruangsetakit C, Wongwanit C, Chinsakchai K, Porat Y, Belleli A, et al. Enhancing limb salvage by non-mobilized peripheral blood angiogenic cell precursors therapy in patients with critical limb ischemia. J Med Assoc Thai. (2009) 92:320-7. Available online at: https://pdfs.semanticscholar.org/83a1/d9c5280b6ce4781840e794fc3c10499f7a58.pdf
20. Zhang X, Lian W, Lou W, Han S, Lu C, Zuo K, et al. Transcatheter arterial infusion of autologous CD133(+) cells for diabetic peripheral artery disease. Stem Cells Int. (2016) 2016:6925357. doi: 10.1155/2016/6925357
21. Kawamoto A, Katayama M, Handa N, Kinoshita M, Takano H, Horii M, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells (2009) 27:2857–64. doi: 10.1002/stem.207
22. Kinoshita M, Fujita Y, Katayama M, Baba R, Shibakawa M, Yoshikawa K, et al. Long-term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor-mobilized CD34 positive cells in patients with critical limb ischemia. Atherosclerosis (2012) 224:440–5. doi: 10.1016/j.atherosclerosis.2012.07.031
23. Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care (2005) 28:2155–60. doi: 10.2337/diacare.28.9.2155
24. Maione C, Botti C, Coppola CA, Silvestroni C, Lillo S, Schiavone V, et al. Effect of autologous transplantation of bone marrow cells concentrated with the MarrowXpress system in patients with critical limb ischemia. Transplant Proc. (2013) 45:402–6. doi: 10.1016/j.transproceed.2012.10.031
25. Cobellis G, Botti C, Taddeo A, Silvestroni A, Lillo S, Da Ponte A, et al. Successful bone marrow transplantation reveals the lack of endothelial progenitor cells mobilization in a patient with critical limb ischemia: a case report. Transplant Proc. (2010) 42:2816–20. doi: 10.1016/j.transproceed.2010.04.047
26. Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. (2007) 49:1566–71. doi: 10.1016/j.jacc.2006.12.037
27. Granton J, Langleben D, Kutryk MB, Camack N, Galipeau J, Courtman DW, Stewart DJ. Endothelial NO-synthase gene-enhanced progenitor cell therapy for pulmonary arterial hypertension: the PHACeT trial. Circ Res. (2015) 117:645–54. doi: 10.1161/CIRCRESAHA.114.305951
28. D'Avola D, Fernandez-Ruiz V, Carmona-Torre F, Mendez M, Perez-Calvo J, Prosper F, et al. Phase 1-2 pilot clinical trial in patients with decompensated liver cirrhosis treated with bone marrow-derived endothelial progenitor cells. Transl Res. (2017) 188:80–91e2. doi: 10.1016/j.trsl.2016.02.009
29. Maldonado GE, Perez CA, Covarrubias EE, Cabriales SA, Leyva LA, Perez JC, et al. Autologous stem cells for the treatment of post-mastectomy lymphedema: a pilot study. Cytotherapy (2011) 13:1249–55. doi: 10.3109/14653249.2011.594791
30. Vasyliev RG, Oksymets VM, Rodnichenko AE, Zlatska AV, Gubar OS, Gordiienko IM, et al. Tissue-engineered bone for treatment of combat related limb injuries. Exp Oncol. (2017) 39:191–6. Available online at: http://exp-oncology.com.ua/article/10281
31. Lara-Hernandez R, Lozano-Vilardell P, Blanes P, Torreguitart-Mirada N, Galmes A, Besalduch J. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann Vasc Surg. (2010) 24:287–94. doi: 10.1016/j.avsg.2009.10.012
32. Tanaka R, Masuda H, Kato S, Imagawa K, Kanabuchi K, Nakashioya C, et al. Autologous G-CSF-mobilized peripheral blood CD34+ cell therapy for diabetic patients with chronic nonhealing ulcer. Cell Transplant. (2014) 23:167–79. doi: 10.3727/096368912X658007
33. Zhu J, Song J, Yu L, Zheng H, Zhou B, Weng S, Fu G. Safety and efficacy of autologous thymosin beta4 pre-treated endothelial progenitor cell transplantation in patients with acute ST segment elevation myocardial infarction: a pilot study. Cytotherapy (2016) 18:1037–42. doi: 10.1016/j.jcyt.2016.05.006
34. Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, et al. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant. (2008) 12:650–5. doi: 10.1111/j.1399-3046.2007.00863.x
35. Bennis Y, Sarlon-Bartoli G, Guillet B, Lucas L, Pellegrini L, Velly L, et al. Priming of late endothelial progenitor cells with erythropoietin before transplantation requires the CD131 receptor subunit and enhances their angiogenic potential. J Thromb Haemost. (2012) 10:1914–28. doi: 10.1111/j.1538-7836.2012.04835.x
36. Sarlon G, Zemani F, David L, Duong Van Huyen JP, Dizier B, Grelac F, et al. Therapeutic effect of fucoidan-stimulated endothelial colony-forming cells in peripheral ischemia. J Thromb Haemost. (2012) 10:38–48. doi: 10.1111/j.1538-7836.2011.04554.x
37. Flex A, Biscetti F, Iachininoto MG, Nuzzolo ER, Orlando N, Capodimonti S, et al. Human cord blood endothelial progenitors promote post-ischemic angiogenesis in immunocompetent mouse model. Thromb Res. (2016) 141:106–11. doi: 10.1016/j.thromres.2016.03.012
38. Mena HA, Lokajczyk A, Dizier B, Strier SE, Voto LS, Boisson-Vidal C, et al. Acidic preconditioning improves the proangiogenic responses of endothelial colony forming cells. Angiogenesis (2014) 17:867–79. doi: 10.1007/s10456-014-9434-5
39. Kim BR, Jang IH, Shin SH, Kwon YW, Heo SC, Choi EJ, et al. Therapeutic angiogenesis in a murine model of limb ischemia by recombinant periostin and its fasciclin I domain. Biochim Biophys Acta. (2014) 1842:1324–32. doi: 10.1016/j.bbadis.2014.05.004
40. Goto K, Takemura G, Takahashi T, Okada H, Kanamori H, Kawamura I, et al. Intravenous administration of endothelial colony-forming cells overexpressing integrin beta1 augments angiogenesis in ischemic legs. Stem Cells Transl Med. (2016) 5:218–26. doi: 10.5966/sctm.2015-0096
41. Odent Grigorescu G, Preda MB, Radu E, Rosca AM, Tutuianu R, Mitroi DN, et al. Combinatorial approach for improving the outcome of angiogenic therapy in ischemic tissues. Biomaterials (2015) 60:72–81. doi: 10.1016/j.biomaterials.2015.05.002
42. Lee JH, Lee SH, Yoo SY, Asahara T, Kwon SM. CD34 hybrid cells promote endothelial colony-forming cell bioactivity and therapeutic potential for ischemic diseases. Arterioscler Thromb Vasc Biol. (2013) 33:1622–34. doi: 10.1161/ATVBAHA.112.301052
43. Schwarz TM, Leicht SF, Radic T, Rodriguez-Arabaolaza I, Hermann PC, Berger F, et al. Vascular incorporation of endothelial colony-forming cells is essential for functional recovery of murine ischemic tissue following cell therapy. Arterioscler Thromb Vasc Biol. (2012) 32:e13–21. doi: 10.1161/ATVBAHA.111.239822
44. Mena HA, Zubiry PR, Dizier B, Schattner M, Boisson-Vidal C, Negrotto S. Acidic preconditioning of endothelial colony-forming cells (ECFC) promote vasculogenesis under proinflammatory and high glucose conditions in vitro and in vivo. Stem Cell Res Ther. (2018) 9:120. doi: 10.1186/s13287-018-0872-7
45. d'Audigier C, Susen S, Blandinieres A, Mattot V, Saubamea B, Rossi E, et al. Egfl7 Represses the vasculogenic potential of human endothelial progenitor cells. Stem Cell Rev. (2018) 14:82–91. doi: 10.1007/s12015-017-9775-8
46. Fraineau S, Palii CG, McNeill B, Ritso M, Shelley WC, Prasain N, et al. Epigenetic activation of pro-angiogenic signaling pathways in human endothelial progenitors increases vasculogenesis. Stem Cell Rep. (2017) 9:1573–87. doi: 10.1016/j.stemcr.2017.09.009
47. Poitevin S, Cussac D, Leroyer AS, Albinet V, Sarlon-Bartoli G, Guillet B, et al. Sphingosine kinase 1 expressed by endothelial colony-forming cells has a critical role in their revascularization activity. Cardiovasc Res. (2014) 103:121–30. doi: 10.1093/cvr/cvu104
48. Palii CG, Vulesevic B, Fraineau S, Pranckeviciene E, Griffith AJ, Chu A, et al. Trichostatin A enhances vascular repair by injected human endothelial progenitors through increasing the expression of TAL1-dependent genes. Cell Stem Cell (2014) 14:644–57. doi: 10.1016/j.stem.2014.03.003
49. Heo SC, Kwon YW, Jang IH, Jeong GO, Yoon JW, Kim CD, et al. WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony-forming cells. Stem Cells (2014) 32:779–90. doi: 10.1002/stem.1578
50. Bouvard C, Gafsou B, Dizier B, Galy-Fauroux I, Lokajczyk A, Boisson-Vidal C, et al. alpha6-integrin subunit plays a major role in the proangiogenic properties of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. (2010) 30:1569–75. doi: 10.1161/ATVBAHA.110.209163
51. Smadja DM, Bieche I, Silvestre JS, Germain S, Cornet A, Laurendeau I, et al. Bone morphogenetic proteins 2 and 4 are selectively expressed by late outgrowth endothelial progenitor cells and promote neoangiogenesis. Arterioscler Thromb Vasc Biol. (2008) 28:2137–43. doi: 10.1161/ATVBAHA.108.168815
52. Cubbon RM, Yuldasheva NY, Viswambharan H, Mercer BN, Baliga V, Stephen SL, et al. Restoring Akt1 activity in outgrowth endothelial cells from South Asian men rescues vascular reparative potential. Stem Cells (2014) 32:2714–23. doi: 10.1002/stem.1766
53. Leicht SF, Schwarz TM, Hermann PC, Seissler J, Aicher A, Heeschen C. Adiponectin pretreatment counteracts the detrimental effect of a diabetic environment on endothelial progenitors. Diabetes (2011) 60:652–61. doi: 10.2337/db10-0240
54. Rossi E, Smadja D, Goyard C, Cras A, Dizier B, Bacha N, et al. Co-injection of mesenchymal stem cells with endothelial progenitor cells accelerates muscle recovery in hind limb ischemia through an endoglin-dependent mechanism. Thromb Haemost. (2017) 117:1908–18. doi: 10.1160/TH17-01-0007
55. Medina RJ, O'Neill CL, O'Doherty TM, Chambers SE, Guduric-Fuchs J, Neisen J, et al. Ex vivo expansion of human outgrowth endothelial cells leads to IL-8-mediated replicative senescence and impaired vasoreparative function. Stem Cells (2013) 31:1657–68. doi: 10.1002/stem.1414
56. Tan Q, Qiu L, Li G, Li C, Zheng C, Meng H, et al. Transplantation of healthy but not diabetic outgrowth endothelial cells could rescue ischemic myocardium in diabetic rabbits. Scand J Clin Lab Invest. (2010) 70:313–21. doi: 10.3109/00365511003774593
57. Kim SW, Jin HL, Kang SM, Kim S, Yoo KJ, Jang Y, et al. Therapeutic effects of late outgrowth endothelial progenitor cells or mesenchymal stem cells derived from human umbilical cord blood on infarct repair. Int J Cardiol. (2016) 203:498–507. doi: 10.1016/j.ijcard.2015.10.110
58. Kang KT, Coggins M, Xiao C, Rosenzweig A, Bischoff J. Human vasculogenic cells form functional blood vessels and mitigate adverse remodeling after ischemia reperfusion injury in rats. Angiogenesis (2013) 16:773–84. doi: 10.1007/s10456-013-9354-9
59. Burger D, Vinas JL, Akbari S, Dehak H, Knoll W, Gutsol A, et al. Human endothelial colony-forming cells protect against acute kidney injury: role of exosomes. Am J Pathol. (2015) 185:2309–23. doi: 10.1016/j.ajpath.2015.04.010
60. Wang SH, Lin SJ, Chen YH, Lin FY, Shih JC, Wu CC, et al. Late outgrowth endothelial cells derived from Wharton jelly in human umbilical cord reduce neointimal formation after vascular injury: involvement of pigment epithelium-derived factor. Arterioscler Thromb Vasc Biol. (2009) 29:816–22. doi: 10.1161/ATVBAHA.109.184739
61. Xia WH, Chen L, Liang JW, Zhang XY, Su C, Tong X, et al. BMP4/Id2 signaling pathway is a novel therapeutic target for late outgrowth endothelial progenitor cell-mediated endothelial injury repair. Int J Cardiol. (2017) 228:796–804. doi: 10.1016/j.ijcard.2016.11.027
62. Huang XT, Zhang YQ, Li SJ, Li SH, Tang Q, Wang ZT, et al. Intracerebroventricular transplantation of ex vivo expanded endothelial colony-forming cells restores blood-brain barrier integrity and promotes angiogenesis of mice with traumatic brain injury. J Neurotrauma. (2013) 30:2080–8. doi: 10.1089/neu.2013.2996
63. Zhang Y, Li Y, Wang S, Han Z, Huang X, Li S, et al. Transplantation of expanded endothelial colony-forming cells improved outcomes of traumatic brain injury in a mouse model. J Surg Res. (2013) 185:441–9. doi: 10.1016/j.jss.2013.05.073
64. Garrigue P, Hache G, Bennis Y, Brige P, Stalin J, Pellegrini L, et al. Erythropoietin pretreatment of transplanted endothelial colony-forming cells enhances recovery in a cerebral ischemia model by increasing their homing ability: a SPECT/CT study. J Nucl Med. (2016) 57:1798–804. doi: 10.2967/jnumed.115.170308
65. Pellegrini L, Bennis Y, Guillet B, Velly L, Garrigue P, Sabatier F, et al. Therapeutic benefit of a combined strategy using erythropoietin and endothelial progenitor cells after transient focal cerebral ischemia in rats. Neurol Res. (2013) 35:937–47. doi: 10.1179/1743132813Y.0000000235
66. Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, Sabatier F, et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. (2011) 7:208–20. doi: 10.1007/s12015-010-9157-y
67. Ding J, Zhao Z, Wang C, Wang CX, Li PC, Qian C, et al. Bioluminescence imaging of transplanted human endothelial colony-forming cells in an ischemic mouse model. Brain Res. (2016) 1642:209–18. doi: 10.1016/j.brainres.2016.03.045
68. Coppens V, Heremans Y, Leuckx G, Suenens K, Jacobs-Tulleneers-Thevissen D, Verdonck K, et al. Human blood outgrowth endothelial cells improve islet survival and function when co-transplanted in a mouse model of diabetes. Diabetologia (2013) 56:382–90. doi: 10.1007/s00125-012-2754-3
69. Kim JH, Oh BJ, Lee HN, Park HS, Park SG, Park KS. Endothelial colony-forming cell coating of pig islets prevents xenogeneic instant blood-mediated inflammatory reaction. Cell Transplant. (2011) 20:1805–15. doi: 10.3727/096368911X566154
70. Jung HS, Kim MJ, Hong SH, Lee YJ, Kang S, Lee H, et al. The potential of endothelial colony-forming cells to improve early graft loss after intraportal islet transplantation. Cell Transplant. (2014) 23:273–83. doi: 10.3727/096368912X661364
71. Ormiston ML, Deng Y, Stewart DJ, Courtman DW. Innate immunity in the therapeutic actions of endothelial progenitor cells in pulmonary hypertension. Am J Respir Cell Mol Biol. (2010) 43:546–54. doi: 10.1165/rcmb.2009-0152OC
72. Alphonse RS, Vadivel A, Fung M, Shelley WC, Critser PJ, Ionescu L, et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation (2014) 129:2144–57. doi: 10.1161/CIRCULATIONAHA.114.009124
73. Aide N, Desmonts C, Beauregard JM, Beyer T, Kinross K, Roselt P, et al. High throughput static and dynamic small animal imaging using clinical PET/CT: potential preclinical applications. Eur J Nucl Med Mol Imaging (2010) 37:991–1001. doi: 10.1007/s00259-009-1352-1
74. Martin-Ramirez J, Hofman M, van den Biggelaar M, Hebbel RP, Voorberg J. Establishment of outgrowth endothelial cells from peripheral blood. Nat Protoc. (2012) 7:1709–15. doi: 10.1038/nprot.2012.093
75. Liew A, O'Brien T. Isolation of Endothelial Progenitor Cells (EPCs). In: Slevin M, McDowell G, editors. Handbook of Vascular Biology Techniques. Dordrecht: Springer Netherlands (2015). p. 45–54.
76. Mead LE, Prater D, Yoder MC, Ingram DA. Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol. (2008) Chapter 2:Unit 2C 1. doi: 10.1002/9780470151808.sc02c01s6
77. Hofmann NA, Reinisch A, Strunk D. Endothelial colony-forming progenitor cell isolation and expansion. Methods Mol Biol. (2012) 879:381–7. doi: 10.1007/978-1-61779-815-3_23
78. Alphonse RS, Vadivel A, Zhong S, McConaghy S, Ohls R, Yoder MC, Thebaud B. The isolation and culture of endothelial colony-forming cells from human and rat lungs. Nat Protoc. (2015) 10:1697–708. doi: 10.1038/nprot.2015.107
79. Colombo E, Calcaterra F, Cappelletti M, Mavilio D, Della Bella S. Comparison of fibronectin and collagen in supporting the isolation and expansion of endothelial progenitor cells from human adult peripheral blood. PLoS ONE (2013) 8:e66734. doi: 10.1371/journal.pone.0066734
80. Tasev D, van Wijhe MH, Weijers EM, van Hinsbergh VW, Koolwijk P. Long-term expansion in platelet lysate increases growth of peripheral blood-derived endothelial-colony forming cells and their growth factor-induced sprouting capacity. PLoS ONE (2015) 10:e0129935. doi: 10.1371/journal.pone.0129935
81. Reinisch A, Hofmann NA, Obenauf AC, Kashofer K, Rohde E, Schallmoser K, et al. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood (2009) 113:6716–25. doi: 10.1182/blood-2008-09-181362
82. Siegel G, Fleck E, Elser S, Hermanutz-Klein U, Waidmann M, Northoff H, et al. Manufacture of endothelial colony-forming progenitor cells from steady-state peripheral blood leukapheresis using pooled human platelet lysate. Transfusion (2018) 58:1132–42. doi: 10.1111/trf.14541
83. Jarajapu YPR, Hazra S, Segal M, LiCalzi S, Jhadao C, Qian K, et al. Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLOS ONE (2014) 9:e93965. doi: 10.1371/journal.pone.0093965
84. Lin R-Z, Dreyzin A, Aamodt K, Li D, Jaminet S-CS, Dudley AC, et al. Induction of erythropoiesis using human vascular networks genetically engineered for controlled erythropoietin release. Blood (2011) 118:5420. doi: 10.1182/blood-2011-08-372946
85. Choi JH, Hur J, Yoon CH, Kim JH, Lee CS, Youn SW, et al. Augmentation of therapeutic angiogenesis using genetically modified human endothelial progenitor cells with altered glycogen synthase kinase-3beta activity. J Biolog Chem. (2004) 279:49430–8. doi: 10.1074/jbc.M402088200
86. Souidi N, Stolk M, Rudeck J, Strunk D, Schallmoser K, Volk HD, et al. Stromal cells act as guardians for endothelial progenitors by reducing their immunogenicity after co-transplantation. Stem Cells (2017) 35:1233–45. doi: 10.1002/stem.2573
87. Allen P, Kang KT, Bischoff J. Rapid onset of perfused blood vessels after implantation of ECFCs and MPCs in collagen, PuraMatrix and fibrin provisional matrices. J Tissue Eng Regen Med. (2015) 9:632–6. doi: 10.1002/term.1803
88. Kang KT, Lin RZ, Kuppermann D, Melero-Martin JM, Bischoff J. Endothelial colony forming cells and mesenchymal progenitor cells form blood vessels and increase blood flow in ischemic muscle. Sci Rep. (2017) 7:770. doi: 10.1038/s41598-017-00809-1
89. Lin RZ, Moreno-Luna R, Zhou B, Pu WT, Melero-Martin JM. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis (2012) 15:443–55. doi: 10.1007/s10456-012-9272-2
90. Shafiee A, Patel J, Wong HY, Donovan P, Hutmacher DW, Fisk NM, et al. Priming of endothelial colony-forming cells in a mesenchymal niche improves engraftment and vasculogenic potential by initiating mesenchymal transition orchestrated by NOTCH signaling. FASEB J. (2017) 31:610–24. doi: 10.1096/fj.201600937
Keywords: endothelial colony forming cells (ECFCs), Endothelial progenitor cell (EPC), clinical translation, clinical trial, preclinical study
Citation: Keighron C, Lyons CJ, Creane M, O'Brien T and Liew A (2018) Recent Advances in Endothelial Progenitor Cells Toward Their Use in Clinical Translation. Front. Med. 5:354. doi: 10.3389/fmed.2018.00354
Received: 11 September 2018; Accepted: 03 December 2018;
Published: 18 December 2018.
Edited by:
David M. Smadja, Université Paris Descartes, FranceReviewed by:
Nicholas Ekow Thomford, University of Cape Coast, GhanaCopyright © 2018 Keighron, Lyons, Creane, O'Brien and Liew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aaron Liew, YWFyb24ubGlld0BudWlnYWx3YXkuaWU=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.