
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 25 September 2018
Sec. Dermatology
Volume 5 - 2018 | https://doi.org/10.3389/fmed.2018.00271
This article is part of the Research Topic Insights into Biomarkers, Cytokines, and Chemokines in Skin Cancer View all 8 articles
Chemokines and their receptors play an important role in the recruitment, activation and differentiation of immune cells. The chemokine receptor, CXCR3, and its ligands, CXCL9, CXCL10, and CXCL11 are key immune chemoattractants during interferon-induced inflammatory responses. Inflammation of the skin resulting from infections or autoimmune disease drives expression of CXCL9/10/11 and the subsequent recruitment of effector, CXCR3+ T cells from the circulation. The relative contributions of the different CXCR3 chemokines and the three variant isoforms of CXCR3 (CXCR3A, CXCR3B, CXCR3alt) to the inflammatory process in human skin requires further investigation. In skin cancers, the CXCR3 receptor can play a dual role whereby expression on tumor cells can lead to cancer metastasis to systemic sites while receptor expression on immune cells can frequently promote anti-tumor immune responses. This review will discuss the biology of CXCR3 and its associated ligands with particular emphasis on the skin during inflammation and carcinogenesis.
Chemokines are secreted, chemotactic cytokines ranging from 8 to 14 kDa in size and are classified into four groups based on the position of the conserved cysteine residues: CXC, CC, C and CX3C (1). Chemokines typically interact with chemokine receptors, which are seven-transmembrane proteins of the G protein-coupled receptor (GPCR) superfamily (2). Through these interactions, chemokines deliver diverse signal transmission to direct leukocyte migration, inflammation and differentiation (3, 4). The importance of chemokines and chemokine receptors in regulating inflammatory processes is underpinned by their involvement in the pathogenesis of several autoimmune diseases, including rheumatoid arthritis (5, 6), inflammatory bowel disease (7), type I diabetes (8, 9), and psoriasis (10, 11).
In some cases, multiple chemokines can interact with the same chemokine receptor resulting in functional redundancy (12, 13). However, some receptors, such as CXCR4, CXCR5, CXCR6, CCR6, CCR9, and CX3CR1 only interact with one known ligand (1). Selective ligand binding directs tissue specific and cell type specific lymphocyte recruitment. For example, CCR9 plays critical role in recruiting lymphocytes to the gut (7) while CCR6 drives Th17 homing to the site of inflammation (14).
More recently, chemokines have been observed to have functions other than simply being leukocyte attractants. Karin et al. termed chemokines that are able to alter the biological functions of recruited leukocytes as “driver chemokines” (15). For example, CXCL12 polarizes CXCR4+ macrophages into IL-10-secreting M2-like macrophages (16) and CXCR4+CD4+ T cells into IL-10-producing regulatory T cells (Tr1) that suppress experimental autoimmune encephalomyelitis (EAE) (17). In addition to inducing immunosuppressive cell types, chemokine signaling can also influence effector T cell polarization. One example is the CXCR3 receptor which can help differentiate naïve T cells into Th1 effector T cells (18).
CXCR3 (GPR9/CD183) is an interferon-inducible chemokine receptor expressed on various cell types, but preferentially monocytes, Th1 T cells, CD8 T cells, NKT cells, NK cells, dendritic cells, and some cancer cells (19–21). Homeostatic proliferation of T cells in immune depleted individuals can also lead to an enrichment of CXCR3+ T cells (22). The CXCR3 receptor reacts with three interferon-inducible chemokines: CXCL9 (MIG), CXCL10 (IP-10) and CXCL11 (I-TAC/IP-9) in addition to CXCL4. Mice have a single isoform of the CXCR3 receptor while three isoforms, CXCR3A, CXCR3B, and CXCR3-alt, exist in humans with distinct roles in cell biology and tumorigenesis (23). CXCR3A plays a traditional CXCR3 role comprising chemotaxis and cell proliferation via calcium mobilization in immune responses induced by IFN-γ (24). With an extension at the N terminus of 52 amino-acids, CXCR3B is an alternatively spliced form of the CXCR3 receptor capable of inhibiting the growth of primary endothelial cell lines (25). CXCR3-B has also been shown to be expressed on endothelial cells within tumors and therefore may be involved in inhibiting angiogenesis through CXCL9/10/11 or CXCL4 signaling (25). The CXCR3-alt receptor results from alternative splicing generated by exon skipping (26). This leads to a truncation at the C-terminus of the protein allowing for binding and mediating the function of CXCL11. Each of these variant CXCR3 receptors can activate different intracellular signaling pathways suggesting that they have non-redundant roles in the immune response (27). A more complete understanding of the regulation and functional activity of these variant receptors during tumorigenesis should aid the design of therapeutic drugs which target the human CXCR3 receptor.
The CXCR3 receptor has become an important marker of a Th1 dominated T cell response. Co-expression of CXCR3 and CCR5 mark Th1 subsets (28), while CCR3 and CCR4 are preferentially expressed on Th2 subsets (29). Interestingly, CXCL11, and to a lesser extent CXCL9/10, is capable of binding and antagonizing the CCR3 receptor thus reducing the Th2 response (30). Xanthou et al. also demonstrated that CXCR3 can bind and sequester the CCR3 ligand, CCL11, again reducing the migration of CD4 Th2 cells. Consequently, CXCR3 and its ligands downregulate Th2 responses while promoting Th1 cell migration. In this context, CXCR3 functions include: (i) the recruitment of activated Th1 cells to inflamed tissues (31–33), (ii) the regulation of skin-homing autoreactive CD8+ T cells in graft-versus-host-disease (GVHD) (34), and (iii) the rapid recruitment of NK cells to antigen-stimulated lymph nodes and the facilitation of Th1 subset priming (35). Transcription factor T-bet, the master regulator controlling Th1 and CTL polarization, is also a direct trans-activator of CXCR3 expression (36). While T-bet upregulation of CXCR3 promotes the migration of Th1 effector cells to inflammatory sites, FoxP3+ regulatory T cells under the influence of IFN-γ can also induce T-bet and subsequently CXCR3 leading to the recruitment of these suppressive T cells into inflammatory sites (37, 38). Ultimately, the timing and number of recruited effector and suppressor cells will dictate the outcome of the immune response.
The key chemokine ligands of CXCR3 (CXCL9, CXCL10, CXCL11) have limited expression under homeostatic conditions but are rapidly up-regulated by cytokine stimulation. While CXCL9 is mostly induced by IFN-γ, CXCL10, and CXCL11 can be induced by both IFN-γ and type I interferons (21). Given the association between the CXCR3 system and inflammation, it is perhaps not surprising that CXCR3 and its ligands also play a role in a variety of autoimmune diseases (39–42). In response to IFN-γ, and synergistically enhanced by TNF-α, many cell types can secrete CXCL9/10/11 including endotheliocytes, fibroblasts, monocytes, and also cancer cells (21). There are two distinct groups of CXC chemokines: one with an ELR (Glu-Leu-Arg) amino acid motif and the other without. Those with the ELR motif can promote angiogenesis while ELR-negative chemokines principally promote lymphocytes migration and repress angiogenesis. CXCL9/10/11 lack the ELR motif thus attenuating angiogenesis to negatively impact on tumor growth (43, 44). Interestingly, CXCR3 and its ligands can also be responsible for tumor growth and metastasis in situations where the tumor cells express the CXCR3 receptor. In a study of CXCR3-expressing colorectal cell lines, metastasis to the liver and lung could be prevented with a small molecule inhibitor of CXCR3, AMG487 (45). In a nude mouse, knock down of human CXCR3A within gastric tumor cells led to reduced metastasis and tumor cell growth in vivo suggesting this receptor variant as the dominant mediator of metastasis in this model (46). Together, these observations suggest that CXCR3 receptor isoform expression and distribution throughout the tumor microenvironment, including on the tumor cells themselves, are important considerations when designing therapeutics that target the human CXCR3 receptor.
CXCL9, 10, and 11 have different binding affinity with CXCR3. Cole et al. showed that human CXCL11 binds to CXCR3 with the highest affinity followed by CXCL10 and CXCL9, although binding to CXCR3 receptor variants was not analyzed (47). This raises the question of whether CXCR3 ligands are redundant or compete during immune responses. The redundancy of CXCL9 and 10 has been demonstrated in a murine model of obliterative bronchiolitis (48). In this study the authors demonstrated that blockade of CXCR3 reduced airway obliteration while single deletion of either CXCL9 or CXCL10 had no effect. However, while CXCL9 and CXCL10 can drive Th1 responses, CXCL11 interaction with CXCR3 can selectively induce regulatory T cells (49, 50). CXCR3 ligands have also been shown to have cooperative effects. For example, murine CXCL9 and 10 cooperatively induce the recruitment of NK cells and CTLs to the spinal cord during herpes simplex virus-2 infection (51). In some cases, CXCR3 ligands can counteract one another. This is seen in a murine MHC-mismatched cardiac transplantation model, where CXCL9 and CXCL10 showed antagonistic effects toward the priming of donor-reactive T cells (52). CXCL9 deficiency decreased the frequency of donor-reactive IFN-γ-producing CD8 T cells, while deficiency of CXCL10 increased the frequency of CD8 T cells in a CXCL9 dependent manner (52). In summary, the interaction of CXCR3 and its ligands is complex and the outcomes will likely be controlled by spatial and temporal patterns of expression that could well be unique to each tissue including the skin.
As post-transcriptional regulators of target genes, multiple microRNAs (miRNAs or miRs) have been reported to regulate CXCR3 ligands. Downregulation of miR-21 in a breast cancer cell line raised secretion of CXCL10, resulting in enhanced recruitment of lymphocytes (53). Interestingly, miR-21 has been shown to be upregulated in cutaneous SCC suggesting that it may reduce CXCL10 recruitment of lymphocytes (54). Similarly, increasing the expression of miR-15a in PBMC results in decreased CXCL10 production (55). In human mesangial cells treated with IFN-γ and TNF-α, the expression of miR-155 was increased resulting in down regulation of CXCL10 while in the inflammatory skin setting of vulvar lichen sclerosus and lichen planus, miR-155 was significantly upregulated but the functional impact of this expression was not fully investigated (56, 57). The expression of CXCL9/10 from psoriatic keratinocytes can also be promoted by the microRNA, miR-17-92 (58). Together this demonstrates that several microRNAs are capable of regulating CXCL9/CXCL10 production in multiple cell types (including skin keratinocytes) and further research will be required to identify factors controlling expression of these miRNAs.
Skin tissue is composed of multiple layers that combine to form a physical barrier to infection and the external environment (59). The epidermis is a non-vascular tissue consisting of keratinocytes at different stages of differentiation, melanocytes, Merkel cells and immune cells (Langerhans cells, T cells). It is separated from the underlying dermis via a basement membrane. In contrast to the epidermis, the dermis is highly vascularized and contains lymphatic vessels and many stromal cells in addition to T cells, macrophages and dendritic cells. Epidermis and dermis can be regarded as different immunological niches as illustrated by resident memory CD8 T cells which typically reside in the epidermis and fail to recirculate to other compartments (60). Chemokine receptors such as CCR4 and CCR10, along with cutaneous lymphocyte antigen, have been associated with skin-specific homing of lymphocytes under homeostatic conditions but the induction of skin inflammation recruits additional T cells with an altered pattern of chemokine receptor expression (61). Immune effector cells such as Th1 CD4 T cells and CD8 T cells that express CXCR3 are frequently involved in inflammatory reactions and therefore it is not surprising that this receptor is often associated with inflammatory skin diseases. However, prior to inflammation, there is some evidence from CXCR3−/− (and CXCL10−/−) mice that memory T cell accumulation within the skin may be dependent on the CXCR3/CXCL10 axis although development of tissue-resident memory CD8 T cells (Trm) in the epidermis appears to be independent of CXCR3 (62, 63). CXCR3 most likely acts not as a skin-specific chemokine receptor but instead attracts immune cells to sites of interferon-mediated inflammation (64). In a variety of inflammatory skin conditions with CXCR3+ T cell infiltrates, CXCL10 and 11 were shown to be produced by activated basal keratinocytes while CXCL9 was produced from dermal cells such as macrophages (65). CXCR3 and its ligands may also control the integrin-dependent adhesion of lymphocytes to the endothelial cell wall and thus control entry into inflamed skin (66). Recruitment of CXCR3+ cells can be enhanced by treatment of the inflammatory site with anti-IL-4 antibodies, leading to increased production of IFN-γ and CXCR3 chemokine ligands (67). IFN-β injections within the skin are also capable of inducing CXCL10/CXCR3 recruitment of T cells and macrophages while TNF-α seems to be a key inducer of CXCL10 from skin fibroblasts (68, 69).
Several inflammatory diseases serve to illustrate the role of CXCR3 in the skin. Autoimmune skin diseases such as alopecia areata (hair loss) that are associated with an IFN-γ gene signature are driven by effector T cells expressing CXCR3. Blockade of CXCR3 with antibodies prevented the development of hair loss in a mouse model of this disease (70). In the acute phase of alopecia areata, hair follicle production of CXCL10 is upregulated suggesting an involvement of this chemokine in attracting CXCR3+ T cells (71). In a second, chronic autoimmune skin disease, psoriasis, typically characterized by scaly, red plaques in patches on the skin, CXCL9/10/11 secretion can be induced by IL-29, produced by Th17 cells, acting on epithelial cells and melanocytes (72, 73). Consistent with the secretion of these chemokines, human psoriatic skin is also characterized by T cells, plasmacytoid dendritic cells and NK cells expressing CXCR3 (74, 75). While CXCR3+ cells may play a role in psoriasis, this is not the only chemokine receptor associated with disease as one study showed that intraepidermal T cells expressing CLA, CCR4 and CCR6 were more prevalent than CXCR3+ T cells (76). Vitiligo is an autoimmune disease that results in destruction of melanocytes and depigmentation of the skin. Active vitiligo in human patients has associated with elevated levels of CXCL10 in both serum and epidermal lesions (77). This matches with the observation of enriched CXCR3+, CD8 resident memory T cells in vitiligo patients (78). Patients receiving anti-PD-1 antibody therapy for metastatic tumor can also develop vitiligo-like skin lesions with infiltration of CXCR3-expressing CD8 T cells (79). Using a mouse model, Rashighi et. al. demonstrated that CXCL10-mediated recruitment, but not CXCL9-mediated recruitment, of CXCR3+ T cells was important for mediating this disease and that antibodies inhibiting CXCL10 might represent a viable treatment option (80). Depleting antibodies directed against CXCR3 were also able to reverse vitiligo in a mouse model (81). Widespread autoimmune diseases such as systemic lupus erythematosus [SLE] can also have skin-related manifestations that involve CXCR3 and its ligands (82–84). These studies show that the skin inflammatory infiltrate in lupus is enriched in CXCR3+ lymphocytes and plasmacytoid dendritic cells, the latter cell producing type I IFNs which result in CXCR3 ligand expression and amplification of the inflammatory response. Similarly, the CXCL9 and CXCL10 chemokines are highly expressed in the skin of patients with systemic sclerosis (85). A skin infiltrate of CXCR3+ T cells is also observed in Bullous pemphigoid, an autoimmune blistering disease, dermatomyositis, an autoimmune reaction in skin and muscle, and interface dermatitis, frequently seen in lichen planus (86–89). The common feature in many of these autoimmune skin diseases is the induction of inflammation and the subsequent production of CXCR3 ligands.
CXCR3 plays an important role in the recruitment of ovalbumin (OVA)-specific CD8 T cells into transgenic mouse skin where keratinocytes express the OVA antigen (34). Lack of CXCR3 on transgenic OVA-specific, CD8 T cells reduced skin infiltration of the T cells and the graft versus host (GVH) –like disease seen when wild-type OT-I cells were transferred. Consistent with this mouse model, cutaneous GVHD in humans is also associated with CXCR3 and its ligands in both acute and chronic forms of the disease (90, 91). Blockade of CXCR3 or CXCL11 also extends skin allograft survival in mice (92, 93). Expression of CXCR3 on non-immune cells of the skin may also play an important role in wound healing as CXCR3 deficient mice have a delayed wound healing response which can be restored by transferring CXCR3+ fibroblasts (94, 95). Expression of CXCR3 on keratinocytes can contribute to re-epithelisation during wound healing (96–98).
Infection of the skin frequently results in inflammation and involvement of the CXCR3 receptor. Inflammation driven by Herpes Simplex virus (HSV) contributes to the recruitment of CXCR3+ CD8 T cells from systemic sites and also trafficking of cells to the infected site within the skin tissue (99, 100). Clearance of epicutaneous vaccinia virus was dependent on CXCR3–expressing CD8 T cells given that transfer of wild type CD8 T cells led to viral resolution in a CXCR3−/−, vaccinia virus infected mouse (101). CD4+ skin resident memory T cells responsive to Leishmania are able to secrete IFN-γ and attract CXCR3+ T cells which aid in parasite clearance (102, 103).
Together, CXCR3 plays an important role in recruitment and function of immune cells in the skin during inflammation resulting from autoimmunity, wounds and infectious disease.
Skin cancers frequently arise in the epidermal layers of the skin where, most commonly, malignant transformation of melanocytes, keratinocytes or Merkel cells results from chronic exposure to ultraviolet light (104). Study of CXCR3 within this group of cancers has mainly focused on melanoma where several lines of evidence suggest that expression of CXCR3 on infiltrating T cells is associated with improved prognosis (Figure 1) (105, 106). Immunohistochemical study of primary human melanomas showed that upregulation of CXCL10 protein via type 1 interferons and the presence of CXCR3 infiltrating T cells was associated with spontaneous tumor regression (107). While it has been established that type I interferons can enhance CXCL10 expression, the source of the interferon in the tumor environment was less clear. A recent mouse study has shown that DNA derived from the tumor was able to activate the stimulator of interferon genes complex (STING) within the cytosol of dendritic cells resulting in the production of IFN-β (108). In addition, plasmacytoid dendritic cells, a key producer of type I interferons, are frequently associated with primary melanoma lesions and can be recruited to the tumor site by CCL20 (109, 110). This interferon may then act on tumor DC subsets such as the CD103+ DCs which have been shown to be key producers of CXCL9/10 in a mouse melanoma model and showed an association with CXCL9/10 in human disease (111). The direct contribution of primary melanoma cells to the secretion of CXCL9/10/11 is not clear although metastatic melanoma cells in vitro can produce CXCL9/10/11 in response to IFN (112). One human study showed that a higher frequency of metastatic melanoma samples expressed the CXCL10 gene relative to primary melanoma samples (113). In a therapeutic setting for melanoma, adjuvant IFN-α therapy of melanomas is known to upregulate CXCL10 production while CXCL9 and CXCL10 were induced in melanomas by chemotherapy agents such as cisplatin (114). Intralesional BCG can also increase production of CXCL9/10/11 which promotes γδ T cell recruitment and regression of melanomas (115). One melanoma study suggested that under IFN-γ stress, melanoma variants which fail to produce CXCL9 could be generated as a mechanism of immune escape (116). In addition to T cells, CXCL10 can also promote the trafficking of adoptively transferred NK cells into melanoma where they cause regression of the tumor mass (117).
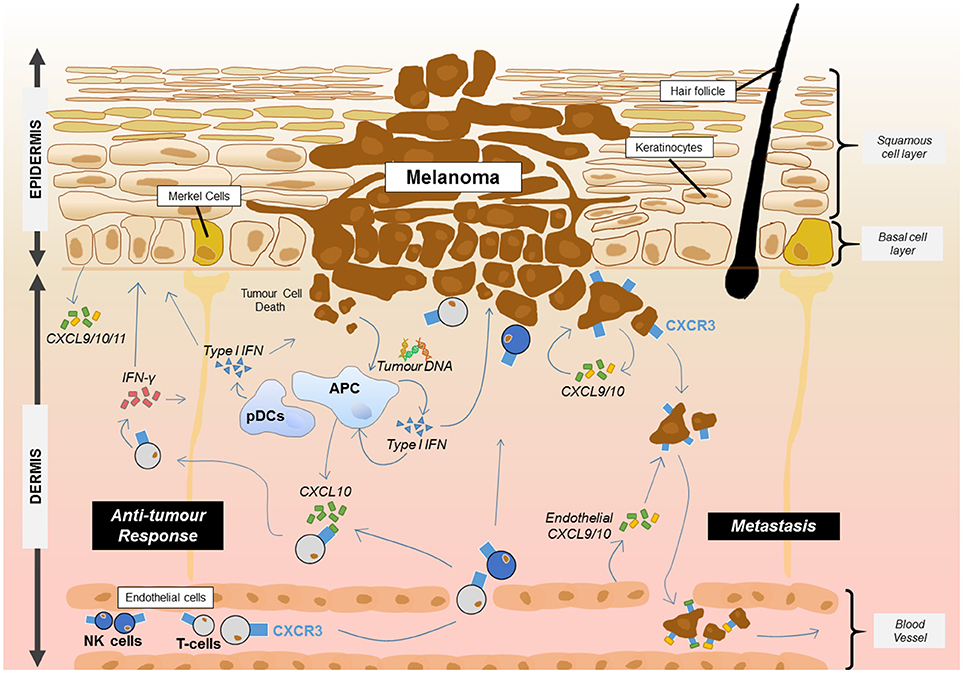
Figure 1. Proposed roles for CXCR3 and its ligands in melanoma. CXCR3 plays at least two key roles in melanoma. The presence of CXCR3 on melanoma cells can lead to metastasis from the primary site through endothelial cell and tumor cell production of CXCL9/10. Meanwhile, the release of DNA from melanoma cells results in uptake by APCs and the activation of the STING pathway resulting in the production of type 1 IFN. Type 1 interferon released from APCs including plasmacytoid dendritic cells (pDCs) then upregulates CXCL10 which can recruit CXCR3+ T cells and NK cells from the blood. Once at the tumor site, T cells and NK cells can produce IFN-γ which acts on keratinocytes, APCs and other skin cells to induce production of CXCL9/10/11 or interact with the tumour to induce cell death. This leads to further recruitment of the adaptive immune cells and anti-tumor immunity.
While CXCR3 expression on infiltrating immune cells generally restrains the melanoma, the expression of this receptor on melanoma cells themselves can lead to metastasis (118, 119). In mouse studies with B16F10 cells, reduction of CXCR3 expression within tumor cells by anti-sense RNA resulted in less frequent metastasis (120). Metastasis can be promoted by endothelial cell secretion of CXCL9 (and CXCL10), assisted by VEGF, within the tumor microenvironment or autocrine CXCL10/CXCR3 interactions on tumor cells (113, 121, 122). Consequently, metastasis represents a situation in which blocking the CXCR3 receptor on tumor cells might be beneficial. In this regard, the design of small molecule antagonists of CXCR3 should be beneficial, particularly if precisely targeted to the tumor cells (123).
Melanoma represents one situation in which metastasis moves cancer cells away from the skin but we must also consider a role of CXCR3 in attracting cancer cells to the skin. Cutaneous T cell lymphoma is an unusual situation where CXCR3 expression on malignant T cells helps recruit and establish this cancer within the skin (124, 125). In this scenario, CXCR3 has been associated with epidermal-trophic cutaneous T lymphomas rather than dermal residing tumors suggesting that CXCR3 ligands may be expressed from the epidermis in this disease setting (126). Interestingly, while T cell lymphomas may use CXCR3 to initially migrate to the skin, the presence of high levels of CXCR3 ligands in the blood, at least partly due to secretion by the lymphoma cells, leads to downregulation of CXCR3 on effector CD8 T cells such that they do not accumulate in the skin (127, 128). This represents a novel form of immune escape in advanced cutaneous T cell lymphoma. Additional cutaneous lymphomas/leukaemias have also reported expression of the CXCR3 receptor on tumor cells including in epidermotrophic B cell lymphoma, lymphomatoid papulosis and skin lesions of leukemic plasmacytoid dendritic cell neoplasms (129–131).
Less well understood is the role of CXCR3 and its ligands in non-melanoma skin cancers such as squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). The lack of studies in this area may reflect the relatively benign nature of many cutaneous BCC/SCCs which are generally removed through surgical procedures (132). However, a subset of SCCs can be metastatic with an estimate of 0.025–20% of premalignant actinic keratosis (AK) lesions progressing to invasive SCC (133). Immunocompromised patients (particularly those undergoing solid organ transplantation or HIV patients) have a 65- to 250-fold increased risk of developing SCC suggesting that immune responses play a role in controlling these tumors (134, 135). Gene profiling of BCC and SCC tumor tissue suggests an increase in IFN related genes including CXCL9 while immunohistochemical staining demonstrated the presence of CXCR3+ immune cells (136). Imiquimod is a clinically approved, topical treatment for SCC/BCC and can induce type 1 IFN signaling through interaction with TLR7 leading to the downstream recruitment of CXCR3+ T cells (137). The expression of CXCL 10/11 and CXCR3 has also been demonstrated in human keratinocytes derived from BCCs (138). In addition, CXCL11 is capable of promoting immunosuppressive indoleamine 2,3-dioxygenase (IDO) expression in human basal cell carcinoma and enhancing keratinocyte proliferation, thus potentially reducing the anti-tumor activity of any infiltrating CXCR3+ effector T cells (138, 139). Consequently, it is still unclear whether the CXCR3 immune infiltrate in human SCC and BCC is associated with tumor regression or progression. In a mouse model of skin epithelial carcinogenesis promoted by DMBA/TPA, gene deletion of CXCR3 produced a lower incidence of skin tumors (140). Both CXCR3-expressing CD4 and CD8 T cells were seen to infiltrate the skin and promoted keratinocyte proliferation (140). The contribution of CXCR3 to tumor development in this mouse model would be consistent with a known role for inflammation in promoting DMBA/TPA tumors (141).
In contrast to this tumor model, we have recently demonstrated that CXCR3 and associated chemokine ligands are important in attracting an effector T cell population to the hyperplastic ear skin of mice transgenic for the human papillomavirus (HPV16) E7 oncogene (142). In this mouse model, HPV16E7 protein is expressed in epithelial cells under the control of a keratin 14 promoter. Intracellular binding of E7 to the retinoblastoma (Rb) protein leads to a dysregulated cell cycle within keratinocytes and the subsequent development of a precancerous, hyperplastic epithelium resembling actinic keratosis, a precursor lesion which can progress to squamous cell carcinoma in human patients (143). One feature of the E7-driven hyperplasia is a chronic inflammatory/wound healing microenvironment associated with elevated levels of IFN-γ and immune cell infiltration (144, 145). Chronic IFN-γ production in the skin, from cells such as infiltrating NKT cells, results in an immunosuppressed microenvironment via mediators such as IDO (146–148). The presence of IFN-γ was also shown to induce CXCL9 and 10 production from CD45− cells in the epidermis and a resulting infiltrate of CXCR3+ T cells (142). These CXCR3+ T cells were shown to be effector cells mediating skin graft rejection in experiments where the E7-expressing graft was devoid of suppressor lymphocytes. Opposing outcomes for the role of CXCR3+ T cells between the HPVE7 transgenic mice and the DMBA/TPA treated mice suggests the need for SCC models which more closely mimic UV-induced cancer in humans to resolve the clinical benefit of attracting CXCR3+ T cells as an immunotherapy. Applying strategies that reduce local immunosuppression will also be important in SCC/BCC.
Within the skin, CXCR3 and associated chemokine ligands form a very important chemotactic response to interferon-mediated inflammation. Recruitment of CXCR3+ immune cells can aid in the response to skin infection while also enhancing autoimmune conditions such as psoriasis. The role of CXCR3 is more complex in skin tumors where expression of this chemokine receptor on tumor cells can assist in tumor metastasis or the suppressive microenvironment of the tumor can overcome recruited CXCR3+ effector T cells. In addition, it is possible that skin CXCR3+ T cells may promote inflammation-driven cancer development in some instances. Consequently, the skin tumor type and a better understanding of the regulation of expression of human CXCR3 isoforms on skin tumor cells and their differential responses to CXCL9/10/11 will dictate if this chemokine receptor/chemokine system can be manipulated to treat skin cancers. Blockade of CXCR3 on tumor cells, thus preventing metastasis, combined with increased skin expression of CXCL9/10/11 might be appropriate in circumstances where CXCR3+ effector T cells can reduce tumor growth. Changes in the expression of CXCR3 and its ligands may be achieved through the manipulation of host miRNAs, an approach that warrants further investigation in animal models. Recruited effector T cells must also overcome the local suppressive environment generated by skin tumors and therefore a combination of chemokine attraction using CXCR3 ligands and modulation of T cell checkpoint molecules (e.g., PD-1) or IDO may be necessary to promote tumor regression.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
GL and JW were supported by grants awarded by the Cancer Council Queensland.
1. Zweemer AJ, Toraskar J, Heitman LH, Ap IJ. Bias in chemokine receptor signalling. Trends Immunol. (2014) 35:243–52. doi: 10.1016/j.it.2014.02.004
2. Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. (2002) 2:106–15. doi: 10.1038/nri722
3. Stoolman LM. Adhesion molecules controlling lymphocyte migration. Cell (1989) 56:907–10. doi: 10.1016/0092-8674(89)90620-X
4. Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. (1998) 338:436–45. doi: 10.1056/NEJM199802123380706
5. Koch AE. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthr Rheum (2005) 52:710–21. doi: 10.1002/art.20932
6. Yellin M, Paliienko I, Balanescu A, Ter-Vartanian S, Tseluyko V, Xu LA, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum (2012) 64:1730–9. doi: 10.1002/art.34330
7. Mcguire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG, et al. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity (2011) 34:602–15. doi: 10.1016/j.immuni.2011.01.021
8. Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Hollander GA, et al. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med. (2002) 8:1414–20. doi: 10.1038/nm1202-792
9. Shehadeh N, Pollack S, Wildbaum G, Zohar Y, Shafat I, Makhoul R, et al. Selective autoantibody production against CCL3 is associated with human type 1 diabetes mellitus and serves as a novel biomarker for its diagnosis. J Immunol. (2009) 182:8104–9. doi: 10.4049/jimmunol.0803348
10. Antonelli A, Fallahi P, Delle Sedie A, Ferrari SM, Maccheroni M, Bombardieri S, et al. High values of alpha (CXCL10) and beta (CCL2) circulating chemokines in patients with psoriatic arthritis, in presence or absence of autoimmune thyroiditis. Autoimmunity (2008) 41:537–42. doi: 10.1080/08916930802170401
11. Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. (2009) 129:2175–83. doi: 10.1038/jid.2009.65
12. Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. (1993) 268:4625–36.
13. Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. (2012) 52:179–97. doi: 10.1146/annurev.pharmtox.010909.105800
14. Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. (2009) 10:514–23. doi: 10.1038/ni.1716
15. Karin N, Wildbaum G, Thelen M. Biased signaling pathways via CXCR3 control the development and function of CD4+ T cell subsets. J Leukoc Biol. (2015) 99:857–62. doi: 10.1189/jlb.2MR0915-441R
16. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. (2008) 13:453–61. doi: 10.2741/2692
17. Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med. (2008) 205:2643–55. doi: 10.1084/jem.20080730
18. Wildbaum G, Netzer N, Karin N. Plasmid DNA encoding IFN-gamma-inducible protein 10 redirects antigen-specific T cell polarization and suppresses experimental autoimmune encephalomyelitis. J Immunol. (2002) 168:5885–92. doi: 10.4049/jimmunol.168.11.5885
19. Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. (2011) 89:207–15. doi: 10.1038/icb.2010.158
20. Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. (2011) 317:620–31. doi: 10.1016/j.yexcr.2010.12.017
21. Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev. (2018) 63:40–7. doi: 10.1016/j.ctrv.2017.11.007
22. Kato A, Takaori-Kondo A, Minato N, Hamazaki Y. CXCR3(high) CD8+ T cells with naïve phenotype and high capacity for IFN-γ production are generated during homeostatic T-cell proliferation. Eur J Immunol. (2018). doi: 10.1002/eji.201747431. [Epub ahead of print].
23. Metzemaekers M, Vanheule V, Janssens R, Struyf S, Proost P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front Immunol. (2017) 8:1970. doi: 10.3389/fimmu.2017.01970
24. Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. (1996) 184:963–9. doi: 10.1084/jem.184.3.963
25. Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. (2003) 197:1537–49. doi: 10.1084/jem.20021897
26. Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM. Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol. (2004) 173:6234–40. doi: 10.4049/jimmunol.173.10.6234
27. Berchiche YA, Sakmar TP. CXC chemokine receptor 3 alternative splice variants selectively activate different signaling pathways. Mol Pharmacol. (2016) 90:483–95. doi: 10.1124/mol.116.105502
28. Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. (1998) 101:746–54. doi: 10.1172/JCI1422
29. Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. (1998) 187:875–83. doi: 10.1084/jem.187.6.875
30. Xanthou G, Duchesnes CE, Williams TJ, Pease JE. CCR3 functional responses are regulated by both CXCR3 and its ligands CXCL9, CXCL10 and CXCL11. Eur J Immunol. (2003) 33:2241–50. doi: 10.1002/eji.200323787
31. Khan IA, Maclean JA, Lee FS, Casciotti L, Dehaan E, Schwartzman JD, et al. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity (2000) 12:483–94. doi: 10.1016/S1074-7613(00)80200-9
32. Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, et al. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol. (2003) 73:771–80. doi: 10.1189/jlb.1102573
33. Campanella GS, Medoff BD, Manice LA, Colvin RA, Luster AD. Development of a novel chemokine-mediated in vivo T cell recruitment assay. J Immunol Methods (2008) 331:127–39. doi: 10.1016/j.jim.2007.12.002
34. Villarroel VA, Okiyama N, Tsuji G, Linton JT, Katz SI. CXCR3-mediated skin homing of autoreactive CD8 T cells is a key determinant in murine graft-versus-host disease. J Invest Dermatol. (2014) 134:1552–60. doi: 10.1038/jid.2014.2
35. Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. (2004) 5:1260–5. doi: 10.1038/ni1138
36. Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood (2005) 106:3432–9. doi: 10.1182/blood-2005-04-1393
37. Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. (2009) 10:595–602. doi: 10.1038/ni.1731
38. Littringer K, Moresi C, Rakebrandt N, Zhou X, Schorer M, Dolowschiak T, et al. Common Features of regulatory T cell specialization during Th1 responses. Front Immunol. (2018) 9:1344. doi: 10.3389/fimmu.2018.01344
39. Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann NY Acad Sci. (2009) 1173:310–7. doi: 10.1111/j.1749-6632.2009.04813.x
40. Lee EY, Lee ZH, Song YW. CXCL10 and autoimmune diseases. Autoimmun Rev. (2009) 8:379–83. doi: 10.1016/j.autrev.2008.12.002
41. Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. (2014) 13:272–80. doi: 10.1016/j.autrev.2013.10.010
42. Karin N, Razon H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine (2018) 109:24–8. doi: 10.1016/j.cyto.2018.02.012
43. Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. (1995) 182:155–62. doi: 10.1084/jem.182.1.155
44. Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. (2001) 107:53–63. doi: 10.1172/JCI9775
45. Cambien B, Karimdjee BF, Richard-Fiardo P, Bziouech H, Barthel R, Millet MA, et al. Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. Br J Cancer (2009) 100:1755–64. doi: 10.1038/sj.bjc.6605078
46. Yang C, Zheng W, Du W. CXCR3A contributes to the invasion and metastasis of gastric cancer cells. Oncol Rep. (2016) 36:1686–92. doi: 10.3892/or.2016.4953
47. Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. (1998) 187:2009–21. doi: 10.1084/jem.187.12.2009
48. Medoff BD, Wain JC, Seung E, Jackobek R, Means TK, Ginns LC, et al. CXCR3 and its ligands in a murine model of obliterative bronchiolitis: regulation and function. J Immunol. (2006) 176:7087–95. doi: 10.4049/jimmunol.176.11.7087
49. Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R, et al. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest. (2014) 124:2009–22. doi: 10.1172/JCI71951
50. Karin N. Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol. (2018) 51:140–5. doi: 10.1016/j.coi.2018.03.004
51. Thapa M, Welner RS, Pelayo R, Carr DJ. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J Immunol. (2008) 180:1098–106. doi: 10.4049/jimmunol.180.2.1098
52. Rosenblum JM, Shimoda N, Schenk AD, Zhang H, Kish DD, Keslar K, et al. CXC chemokine ligand (CXCL) 9 and CXCL10 are antagonistic costimulation molecules during the priming of alloreactive T cell effectors. J Immunol. (2010) 184:3450–60. doi: 10.4049/jimmunol.0903831
53. Wang Z, Han J, Cui Y, Zhou X, Fan K. miRNA-21 inhibition enhances RANTES and IP-10 release in MCF-7 via PIAS3 and STAT3 signalling and causes increased lymphocyte migration. Biochem Biophys Res Commun. (2013) 439:384–9. doi: 10.1016/j.bbrc.2013.08.072
54. Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest. (2012) 122:464–72. doi: 10.1172/JCI57415
55. Liu XF, Wang RQ, Hu B, Luo MC, Zeng QM, Zhou H, et al. MiR-15a contributes abnormal immune response in myasthenia gravis by targeting CXCL10. Clin Immunol. (2016) 164:106–13. doi: 10.1016/j.clim.2015.12.009
56. Imaizumi T, Tanaka H, Tajima A, Yokono Y, Matsumiya T, Yoshida H, et al. IFN-gamma and TNF-alpha synergistically induce microRNA-155 which regulates TAB2/IP-10 expression in human mesangial cells. Am J Nephrol. (2010) 32:462–8. doi: 10.1159/000321365
57. Terlou A, Santegoets LA, Van Der Meijden WI, Heijmans-Antonissen C, Swagemakers SM, Van Der Spek PJ, et al. An autoimmune phenotype in vulvar lichen sclerosus and lichen planus: a Th1 response and high levels of microRNA-155. J Invest Dermatol. (2012) 132:658–66. doi: 10.1038/jid.2011.369
58. Zhang W, Yi X, An Y, Guo S, Li S, Song P, et al. MicroRNA-17-92 cluster promotes the proliferation and the chemokine production of keratinocytes: implication for the pathogenesis of psoriasis. Cell Death Dis. (2018) 9:567. doi: 10.1038/s41419-018-0621-y
59. Girardi M. Cutaneous perspectives on adaptive immunity. Clin Rev Allergy Immunol. (2007) 33:4–14. doi: 10.1007/s12016-007-0040-9
60. Gebhardt T, Palendira U, Tscharke DC, Bedoui S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol Rev. (2018) 283:54–76. doi: 10.1111/imr.12650
61. Tan SY, Roediger B, Weninger W. The role of chemokines in cutaneous immunosurveillance. Immunol Cell Biol. (2015) 93:337–46. doi: 10.1038/icb.2015.16
62. Zaid A, Hor JL, Christo SN, Groom JR, Heath WR, Mackay LK, et al. Chemokine receptor-dependent control of skin tissue-resident memory T cell formation. J Immunol. (2017) 199:2451–9. doi: 10.4049/jimmunol.1700571
63. Alanio C, Barreira Da Silva R, Michonneau D, Bousso P, Ingersoll MA, Albert ML. CXCR3/CXCL10 axis shapes tissue distribution of memory phenotype CD8+ T cells in nonimmunized mice. J Immunol. (2018) 200:139–46. doi: 10.4049/jimmunol.1700564
64. Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. (2002) 160:347–55. doi: 10.1016/S0002-9440(10)64378-7
65. Flier J, Boorsma DM, Van Beek PJ, Nieboer C, Stoof TJ, Willemze R, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. (2001) 194:398–405. doi: 10.1002/1096-9896(200108)194:4 < 397::AID-PATH899>3.0.CO;2-S
66. D'ambrosio D, Albanesi C, Lang R, Girolomoni G, Sinigaglia F, Laudanna C. Quantitative differences in chemokine receptor engagement generate diversity in integrin-dependent lymphocyte adhesion. J Immunol. (2002) 169:2303–12. doi: 10.4049/jimmunol.169.5.2303
67. Lazarski CA, Ford J, Katzman SD, Rosenberg AF, Fowell DJ. IL-4 attenuates Th1-associated chemokine expression and Th1 trafficking to inflamed tissues and limits pathogen clearance. PLoS ONE (2013) 8:e71949. doi: 10.1371/journal.pone.0071949
68. Villagomez MT, Bae SJ, Ogawa I, Takenaka M, Katayama I. Tumour necrosis factor-alpha but not interferon-gamma is the main inducer of inducible protein-10 in skin fibroblasts from patients with atopic dermatitis. Br J Dermatol. (2004) 150:910–6. doi: 10.1111/j.1365-2133.2004.05937.x
69. Buttmann M, Goebeler M, Toksoy A, Schmid S, Graf W, Berberich-Siebelt F, et al. Subcutaneous interferon-beta injections in patients with multiple sclerosis initiate inflammatory skin reactions by local chemokine induction. J Neuroimmunol. (2005) 168:175–82. doi: 10.1016/j.jneuroim.2005.07.011
70. Dai Z, Xing L, Cerise J, Wang EH, Jabbari A, De Jong A, et al. CXCR3 blockade inhibits T cell migration into the skin and prevents development of Alopecia Areata. J Immunol. (2016) 197:1089–99. doi: 10.4049/jimmunol.1501798
71. Ito T, Hashizume H, Shimauchi T, Funakoshi A, Ito N, Fukamizu H, et al. CXCL10 produced from hair follicles induces Th1 and Tc1 cell infiltration in the acute phase of alopecia areata followed by sustained Tc1 accumulation in the chronic phase. J Dermatol Sci. (2013) 69:140–7. doi: 10.1016/j.jdermsci.2012.12.003
72. Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. (2014) 32:227–55. doi: 10.1146/annurev-immunol-032713-120225
73. Witte E, Kokolakis G, Witte K, Warszawska K, Friedrich M, Christou D, et al. Interleukin-29 induces epithelial production of CXCR3A ligands and T-cell infiltration. J Mol Med. (2016) 94:391–400. doi: 10.1007/s00109-015-1367-y
74. Ottaviani C, Nasorri F, Bedini C, De Pita O, Girolomoni G, Cavani A. CD56brightCD16(–) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. (2006) 36:118–28. doi: 10.1002/eji.200535243
75. Chen SC, De Groot M, Kinsley D, Laverty M, Mcclanahan T, Arreaza M, et al. Expression of chemokine receptor CXCR3 by lymphocytes and plasmacytoid dendritic cells in human psoriatic lesions. Arch Dermatol Res. (2010) 302:113–23. doi: 10.1007/s00403-009-0966-2
76. Teraki Y, Miyake A, Takebayashi R, Shiohara T. Homing receptor and chemokine receptor on intraepidermal T cells in psoriasis vulgaris. Clin Exp Dermatol. (2004) 29:658–63. doi: 10.1111/j.1365-2230.2004.01638.x
77. Yang L, Yang S, Lei J, Hu W, Chen R, Lin F, et al. Role of chemokines and the corresponding receptors in vitiligo: a pilot study. J Dermatol. (2018) 45:31–8. doi: 10.1111/1346-8138.14004
78. Boniface K, Jacquemin C, Darrigade AS, Dessarthe B, Martins C, Boukhedouni N, et al. Vitiligo skin is imprinted with resident memory CD8 T cells expressing CXCR3. J Invest Dermatol. (2018) 138:355–64. doi: 10.1016/j.jid.2017.08.038
79. Larsabal M, Marti A, Jacquemin C, Rambert J, Thiolat D, Dousset L, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol. (2017) 76:863–70. doi: 10.1016/j.jaad.2016.10.044
80. Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. (2014) 6:223ra223. doi: 10.1126/scitranslmed.3007811
81. Richmond JM, Masterjohn E, Chu R, Tedstone J, Youd ME, Harris JE. CXCR3 depleting antibodies prevent and reverse vitiligo in mice. J Invest Dermatol. (2017) 137:982–5. doi: 10.1016/j.jid.2016.10.048
82. Meller S, Winterberg F, Gilliet M, Muller A, Lauceviciute I, Rieker J, et al. Ultraviolet radiation-induced injury, chemokines, and leukocyte recruitment: an amplification cycle triggering cutaneous lupus erythematosus. Arthr Rheum (2005) 52:1504–16. doi: 10.1002/art.21034
83. Wenzel J, Tuting T. Identification of type I interferon-associated inflammation in the pathogenesis of cutaneous lupus erythematosus opens up options for novel therapeutic approaches. Exp Dermatol. (2007) 16:454–63. doi: 10.1111/j.1600-0625.2007.00556.x
84. Ribero S, Sciascia S, Borradori L, Lipsker D. The cutaneous spectrum of lupus erythematosus. Clin Rev Allergy Immunol. (2017) 53:291–305. doi: 10.1007/s12016-017-8627-2
85. Rabquer BJ, Tsou PS, Hou Y, Thirunavukkarasu E, Haines GK III, Impens AJ, et al. Dysregulated expression of MIG/CXCL9, IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthr Res Ther. (2011) 13:R18. doi: 10.1186/ar3242
86. Kakinuma T, Wakugawa M, Nakamura K, Hino H, Matsushima K, Tamaki K. High level of thymus and activation-regulated chemokine in blister fluid and sera of patients with bullous pemphigoid. Br J Dermatol. (2003) 148:203–10. doi: 10.1046/j.1365-2133.2003.05066.x
87. Wenzel J, Scheler M, Proelss J, Bieber T, Tuting T. Type I interferon-associated cytotoxic inflammation in lichen planus. J Cutan Pathol. (2006) 33:672–8. doi: 10.1111/j.1600-0560.2006.00527.x
88. Wenzel J, Schmidt R, Proelss J, Zahn S, Bieber T, Tuting T. Type I interferon-associated skin recruitment of CXCR3+ lymphocytes in dermatomyositis. Clin Exp Dermatol. (2006) 31:576–82. doi: 10.1111/j.1365-2230.2006.02150.x
89. Wenzel J, Tuting T. An IFN-associated cytotoxic cellular immune response against viral, self-, or tumor antigens is a common pathogenetic feature in “interface dermatitis”. J Invest Dermatol. (2008) 128:2392–402. doi: 10.1038/jid.2008.96
90. Piper KP, Horlock C, Curnow SJ, Arrazi J, Nicholls S, Mahendra P, et al. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood (2007) 110:3827–32. doi: 10.1182/blood-2006-12-061408
91. Croudace JE, Inman CF, Abbotts BE, Nagra S, Nunnick J, Mahendra P, et al. Chemokine-mediated tissue recruitment of CXCR3+ CD4+ T cells plays a major role in the pathogenesis of chronic GVHD. Blood (2012) 120:4246–55. doi: 10.1182/blood-2012-02-413260
92. Jiankuo M, Xingbing W, Baojun H, Xiongwin W, Zhuoya L, Ping X, et al. Peptide nucleic acid antisense prolongs skin allograft survival by means of blockade of CXCR3 expression directing T cells into graft. J Immunol. (2003) 170:1556–65. doi: 10.4049/jimmunol.170.3.1556
93. Li B, Xu W, Xu L, Jiang Z, Wen Z, Li K, et al. I-TAC is a dominant chemokine in controlling skin intragraft inflammation via recruiting CXCR3+ cells into the graft. Cell Immunol. (2010) 260:83–91. doi: 10.1016/j.cellimm.2009.09.004
94. Yates CC, Whaley D, Kulasekeran P, Hancock WW, Lu B, Bodnar R, et al. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol. (2007) 171:484–95. doi: 10.2353/ajpath.2007.061092
95. Yates CC, Whaley D, Wells A. Transplanted fibroblasts prevents dysfunctional repair in a murine CXCR3-deficient scarring model. Cell Transplant. (2012) 21:919–31. doi: 10.3727/096368911X623817
96. Yates CC, Whaley D, Chen A-C, Kulesekaran P, Hebda PA, Wells A. ELR-negative CXC chemokine CXCL11 (IP-9/I-TAC) facilitates dermal and epidermal maturation during wound repair. Am J Pathol. (2008) 173:643–52. doi: 10.2353/ajpath.2008.070990
97. Huen AC, Wells A. The beginning of the end: CXCR3 signaling in late-stage wound healing. Adv Wound Care (2012) 1:244–8. doi: 10.1089/wound.2011.0355
98. Kroeze KL, Boink MA, Sampat-Sardjoepersad SC, Waaijman T, Scheper RJ, Gibbs S. Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J Invest Dermatol. (2012) 132:216–25. doi: 10.1038/jid.2011.245
99. Ariotti S, Beltman JB, Borsje R, Hoekstra ME, Halford WP, Haanen JB, et al. Subtle CXCR3-dependent chemotaxis of CTLs within infected tissue allows efficient target localization. J Immunol. (2015) 195:5285–95. doi: 10.4049/jimmunol.1500853
100. Hensel MT, Peng T, Cheng A, De Rosa SC, Wald A, Laing KJ, et al. Selective expression of CCR10 and CXCR3 by circulating human herpes simplex virus-specific CD8 T cells. J Virol. (2017) 91:e00810-17. doi: 10.1128/JVI.00810-17
101. Hickman HD, Reynoso GV, Ngudiankama BF, Cush SS, Gibbs J, Bennink JR, et al. CXCR3 chemokine receptor enables local CD8+ T cell migration for the destruction of virus-infected cells. Immunity (2015) 42:524–37. doi: 10.1016/j.immuni.2015.02.009
102. Geiger B, Wenzel J, Hantschke M, Haase I, Stander S, Von Stebut E. Resolving lesions in human cutaneous leishmaniasis predominantly harbour chemokine receptor CXCR3-positive T helper 1/T cytotoxic type 1 cells. Br J Dermatol. (2010) 162:870–4. doi: 10.1111/j.1365-2133.2009.09573.x
103. Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med. (2015) 212:1405–14. doi: 10.1084/jem.20142101
104. Lai V, Cranwell W, Sinclair R. Epidemiology of skin cancer in the mature patient. Clin Dermatol. (2018) 36:167–76. doi: 10.1016/j.clindermatol.2017.10.008
105. Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, et al. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. (2004) 64:7697–701. doi: 10.1158/0008-5472.CAN-04-2059
106. Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. (2015) 6:7458. doi: 10.1038/ncomms8458
107. Wenzel J, Bekisch B, Uerlich M, Haller O, Bieber T, Tuting T. Type I interferon-associated recruitment of cytotoxic lymphocytes: a common mechanism in regressive melanocytic lesions. Am J Clin Pathol. (2005) 124:37–48. doi: 10.1309/4EJ9KL7CGDENVVLE
108. Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity (2014) 41:830–42. doi: 10.1016/j.immuni.2014.10.017
109. Vermi W, Bonecchi R, Facchetti F, Bianchi D, Sozzani S, Festa S, et al. Recruitment of immature plasmacytoid dendritic cells (Plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol. (2003) 200:255–68. doi: 10.1002/path.1344
110. Charles J, Di Domizio J, Salameire D, Bendriss-Vermare N, Aspord C, Muhammad R, et al. Characterization of circulating dendritic cells in melanoma: role of CCR6 in plasmacytoid dendritic cell recruitment to the tumor. J Invest Dermatol. (2010) 130:1646–56. doi: 10.1038/jid.2010.24
111. Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell (2017) 31:711–23.e714. doi: 10.1016/j.ccell.2017.04.003
112. Dengel LT, Norrod AG, Gregory BL, Clancy-Thompson E, Burdick MD, Strieter RM, et al. Interferons induce CXCR3-cognate chemokine production by human metastatic melanoma. J Immunother. (2010) 33:965–74. doi: 10.1097/CJI.0b013e3181fb045d
113. Wightman SC, Uppal A, Pitroda SP, Ganai S, Burnette B, Stack M, et al. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br J Cancer (2015) 113:327–35. doi: 10.1038/bjc.2015.193
114. Mohty AM, Grob JJ, Mohty M, Richard MA, Olive D, Gaugler B. Induction of IP-10/CXCL10 secretion as an immunomodulatory effect of low-dose adjuvant interferon-alpha during treatment of melanoma. Immunobiology (2010) 215:113–23. doi: 10.1016/j.imbio.2009.03.008
115. Yang J, Jones MS, Ramos RI, Chan AA, Lee AF, Foshag LJ, et al. Insights into Local tumor microenvironment immune factors associated with regression of cutaneous melanoma metastases by mycobacterium bovis Bacille Calmette-Guerin. Front Oncol. (2017) 7:61. doi: 10.3389/fonc.2017.00061
116. Petro M, Kish D, Guryanova OA, Ilyinskaya G, Kondratova A, Fairchild RL, et al. Cutaneous tumors cease CXCL9/Mig production as a result of IFN-gamma-mediated immunoediting. J Immunol. (2013) 190:832–41. doi: 10.4049/jimmunol.1201906
117. Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother. (2015) 64:225–35. doi: 10.1007/s00262-014-1629-5
118. Monteagudo C, Martin JM, Jorda E, Llombart-Bosch A. CXCR3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: correlation with clinicopathological prognostic factors. J Clin Pathol. (2007) 60:596–9. doi: 10.1136/jcp.2005.032144
119. Jenkins MH, Brinckerhoff CE, Mullins DW. CXCR3 signaling in BRAFWT melanoma increases IL-8 expression and tumorigenicity. PLoS ONE (2015) 10:e0121140. doi: 10.1371/journal.pone.0121140
120. Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. (2004) 64:4010–7. doi: 10.1158/0008-5472.CAN-03-1757
121. Boulday G, Haskova Z, Reinders ME, Pal S, Briscoe DM. Vascular endothelial growth factor-induced signaling pathways in endothelial cells that mediate overexpression of the chemokine IFN-gamma-inducible protein of 10 kDa in vitro and in vivo. J Immunol. (2006) 176:3098–107. doi: 10.4049/jimmunol.176.5.3098
122. Amatschek S, Lucas R, Eger A, Pflueger M, Hundsberger H, Knoll C, et al. CXCL9 induces chemotaxis, chemorepulsion and endothelial barrier disruption through CXCR3-mediated activation of melanoma cells. Br J Cancer (2011) 104:469–79. doi: 10.1038/sj.bjc.6606056
123. Pease JE. Designing small molecule CXCR3 antagonists. Expert Opin Drug Discov. (2017) 12:159–68. doi: 10.1080/17460441.2017.1268597
124. Kallinich T, Muche JM, Qin S, Sterry W, Audring H, Kroczek RA. Chemokine receptor expression on neoplastic and reactive T cells in the skin at different stages of mycosis fungoides. J Invest Dermatol. (2003) 121:1045–52. doi: 10.1046/j.1523-1747.2003.12555.x
125. Maliniemi P, Hahtola S, Ovaska K, Jeskanen L, Vakeva L, Jantti K, et al. Molecular characterization of subcutaneous panniculitis-like T-cell lymphoma reveals upregulation of immunosuppression- and autoimmunity-associated genes. Orphanet J Rare Dis. (2014) 9:160. doi: 10.1186/s13023-014-0160-2
126. Lu D, Duvic M, Medeiros LJ, Luthra R, Dorfman DM, Jones D. The T-cell chemokine receptor CXCR3 is expressed highly in low-grade mycosis fungoides. Am J Clin Pathol. (2001) 115:413–21. doi: 10.1309/3N7P-J84L-JQ9K-G89R
127. Yagi H, Seo N, Ohshima A, Itoh T, Itoh N, Horibe T, et al. Chemokine receptor expression in cutaneous T cell and NK/T-cell lymphomas: immunohistochemical staining and in vitro chemotactic assay. Am J Surg Pathol. (2006) 30:1111–9. doi: 10.1097/01.pas.0000213267.92349.59
128. Winter D, Moser J, Kriehuber E, Wiesner C, Knobler R, Trautinger F, et al. Down-modulation of CXCR3 surface expression and function in CD8+ T cells from cutaneous T cell lymphoma patients. J Immunol. (2007) 179:4272–82. doi: 10.4049/jimmunol.179.6.4272
129. Bendriss-Vermare N, Chaperot L, Peoc'h M, Vanbervliet B, Jacob MC, Briere F, et al. (2004). In situ leukemic plasmacytoid dendritic cells pattern of chemokine receptors expression and in vitro migratory response. Leukemia 18:1491–8. doi: 10.1038/sj.leu.2403452
130. Yamaguchi T, Ohshima K, Karube K, Kawano R, Nakayama J, Suzumiya J, et al. Expression of chemokines and chemokine receptors in cutaneous CD30+ lymphoproliferative disorders. Br J Dermatol. (2006) 154:904–9. doi: 10.1111/j.1365-2133.2005.07039.x
131. Magro CM, Momtahen S, Lee BA, Swanson DL, Pavlovic MD. Epidermotropic B-cell lymphoma: a unique subset of CXCR3-positive marginal zone lymphoma. Am J Dermatopathol. (2016) 38:105–12. doi: 10.1097/DAD.0000000000000401
132. Neville JA, Welch E, Leffell DJ. Management of nonmelanoma skin cancer in 2007. Nat Clin Pract Oncol. (2007) 4:462–9. doi: 10.1038/ncponc0883
133. Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. (2001) 344:975–83. doi: 10.1056/NEJM200103293441306
134. Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. (2000) 143:513–9. doi: 10.1046/j.1365-2133.2000.03703.x
135. Rangwala S, Tsai KY. Roles of the immune system in skin cancer. Br J Dermatol. (2011) 165:953–65. doi: 10.1111/j.1365-2133.2011.10507.x
136. Wenzel J, Tomiuk S, Zahn S, Kusters D, Vahsen A, Wiechert A, et al. Transcriptional profiling identifies an interferon-associated host immune response in invasive squamous cell carcinoma of the skin. Int J Cancer (2008) 123:2605–15. doi: 10.1002/ijc.23799
137. Wenzel J, Uerlich M, Haller O, Bieber T, Tueting T. Enhanced type I interferon signaling and recruitment of chemokine receptor CXCR3-expressing lymphocytes into the skin following treatment with the TLR7-agonist imiquimod. J Cutan Pathol. (2005b) 32:257–62. doi: 10.1111/j.0303-6987.2005.00297.x
138. Lo BK, Yu M, Zloty D, Cowan B, Shapiro J, Mcelwee KJ. CXCR3/ligands are significantly involved in the tumorigenesis of basal cell carcinomas. Am J Pathol. (2010) 176:2435–46. doi: 10.2353/ajpath.2010.081059
139. Lo BK, Jalili RB, Zloty D, Ghahary A, Cowan B, Dutz JP, et al. CXCR3 ligands promote expression of functional indoleamine 2,3-dioxygenase in basal cell carcinoma keratinocytes. Br J Dermatol. (2011) 165:1030–6. doi: 10.1111/j.1365-2133.2011.10489.x
140. Winkler AE, Brotman JJ, Pittman ME, Judd NP, Lewis JSJr, Schreiber RD, et al. CXCR3 enhances a T-cell-dependent epidermal proliferative response and promotes skin tumorigenesis. Cancer Res. (2011) 71:5707–16. doi: 10.1158/0008-5472.CAN-11-0907
141. Kwong BY, Roberts SJ, Silberzahn T, Filler RB, Neustadter JH, Galan A, et al. Molecular analysis of tumor-promoting CD8+ T cells in two-stage cutaneous chemical carcinogenesis. J Invest Dermatol. (2010) 130:1726–36. doi: 10.1038/jid.2009.362
142. Kuo P, Tuong ZK, Teoh SM, Frazer IH, Mattarollo SOE, Leggatt GR. HPV16E7-induced hyperplasia promotes CXCL9/10 expression and induces CXCR3+ T-cell migration to skin J Invest Dermatol. (2017) 138:1349–59. doi: 10.1016/j.jid.2017.12.021
143. Tuong ZK, Noske K, Kuo P, Bashaw AA, Teoh SM, Frazer IH. Murine HPV16 E7-expressing transgenic skin effectively emulates the cellular and molecular features of human high-grade squamous intraepithelial lesions. Papillomavirus Res. (2018) 5:6–20. doi: 10.1016/j.pvr.2017.10.001
144. Choyce A, Yong M, Narayan S, Mattarollo SR, Liem A, Lambert PF, et al. Expression of a single, viral oncoprotein in skin epithelium is sufficient to recruit lymphocytes. PLoS ONE (2013) 8:e57798. doi: 10.1371/journal.pone.0057798
145. Jazayeri SD, Kuo PT, Leggatt GR, Frazer IH. HPV16-E7-specific activated CD8 T cells in E7 transgenic skin and skin grafts. Front Immunol. (2017) 8:524. doi: 10.3389/fimmu.2017.00524
146. Mattarollo SR, Rahimpour A, Choyce A, Godfrey DI, Leggatt GR, Frazer IH. Invariant NKT cells in hyperplastic skin induce a local immune suppressive environment by IFN-gamma production. J Immunol. (2010) 184:1242–50. doi: 10.4049/jimmunol.0902191
147. Mittal D, Kassianos AJ, Tran LS, Bergot AS, Gosmann C, Hofmann J, et al. Indoleamine 2,3-dioxygenase activity contributes to local immune suppression in the skin expressing human papillomavirus oncoprotein e7. J Invest Dermatol. (2013) 133:2686–94. doi: 10.1038/jid.2013.222
Keywords: CXCR3, skin cancer, CXCL9/10/11, melanoma, squamous cell carcinoma
Citation: Kuo PT, Zeng Z, Salim N, Mattarollo S, Wells JW and Leggatt GR (2018) The Role of CXCR3 and Its Chemokine Ligands in Skin Disease and Cancer. Front. Med. 5:271. doi: 10.3389/fmed.2018.00271
Received: 13 July 2018; Accepted: 04 September 2018;
Published: 25 September 2018.
Edited by:
Ivan V. Litvinov, McGill University, CanadaReviewed by:
Unni Samavedam, University of Cincinnati, United StatesCopyright © 2018 Kuo, Zeng, Salim, Mattarollo, Wells and Leggatt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Graham R. Leggatt, Zy5sZWdnYXR0QHVxLmVkdS5hdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.