- 1Biologie Intégrée du Globule Rouge UMR_S1134, INSERM, Univ. Paris Diderot, Sorbonne Paris Cité, Univ. de la Réunion, Univ. des Antilles, Paris, France
- 2Institut National de la Transfusion Sanguine, Paris, France
- 3Laboratoire d’Excellence GR-Ex, Paris, France
- 4Laboratory of Cellular and Molecular Mechanisms of Hematological Disorders and Therapeutic Implications U1163/CNRS ERL 8254, INSERM, CNRS, Univ Paris Descartes, Sorbonne Paris Cité, Paris, France
- 5Université Paris Descartes, Paris, France
- 6Assistance publique des hôpitaux de Paris, Paris, France
The proportion of transfused red blood cells (RBCs) that remain in circulation is an important surrogate marker of transfusion efficacy and contributes to predict the potential benefit of a transfusion process. Over the last 50 years, most of the transfusion recovery data were generated by chromium-51 (51Cr)-labeling studies and were predominantly performed to validate new storage systems and new processes to prepare RBC concentrates. As a consequence, our understanding of transfusion efficacy is strongly dependent on the strengths and weaknesses of 51Cr labeling in particular. Other methods such as antigen mismatch or biotin-based labeling can bring relevant information, for example, on the long-term survival of transfused RBC. These radioactivity-free methods can be used in patients including from vulnerable groups. We provide an overview of the methods used to measure transfusion recovery in humans, compare their strengths and weaknesses, and discuss their potential limitations. Also, based on our understanding of the spleen-specific filtration of damaged RBC and historical transfusion recovery data, we propose that RBC deformability and morphology are storage lesion markers that could become useful predictors of transfusion recovery. Transfusion recovery can and should be accurately explored by more than one method. Technical optimization and clarification of concepts is still needed in this important field of transfusion and physiology.
Introduction
Each year, more than 85 million red blood cells (RBCs) units are transfused worldwide. This demanding human and organizational task is conducted by national or local organizations. Collection, transformation, storage (for a maximum of 35–49 days), and distribution of blood products are tightly quality controlled, most commonly at the national level. In industrialized countries, most of the transfused RBCs are stored as red cell concentrates (RCC), from which plasma, platelets, and leukocytes have been almost entirely removed, usually using centrifugation and/or leukoreduction filters.
The objective of an RCC transfusion is to increase the oxygenation capacity of the recipient by increasing the number of functional RBC in circulation. Improvement in tissue oxygenation following transfusion is arguably the most relevant marker of transfusion efficacy but measuring it is technically and logistically challenging in patients and impossible in healthy volunteers in whom tissue oxygenation is not altered. Measuring the proportion of RBCs that remain in circulation after transfusion thus appears as a suitable surrogate marker to evaluate the efficacy of a transfusion. That a reasonable proportion of transfused RBC stays in circulation for long enough to operate the expected correction is indeed a prerequisite for transfusion efficacy.
Early studies have identified that, after storage, a variable proportion of transfused RBC is removed from the circulation in the first 24 h following transfusion (1). Then, the remaining transfused RBCs have a normal survival. Although the long-term survival of RBC is an important parameter to evaluate transfusion efficacy, most studies have focused on the measure of the 24 h transfusion recovery.
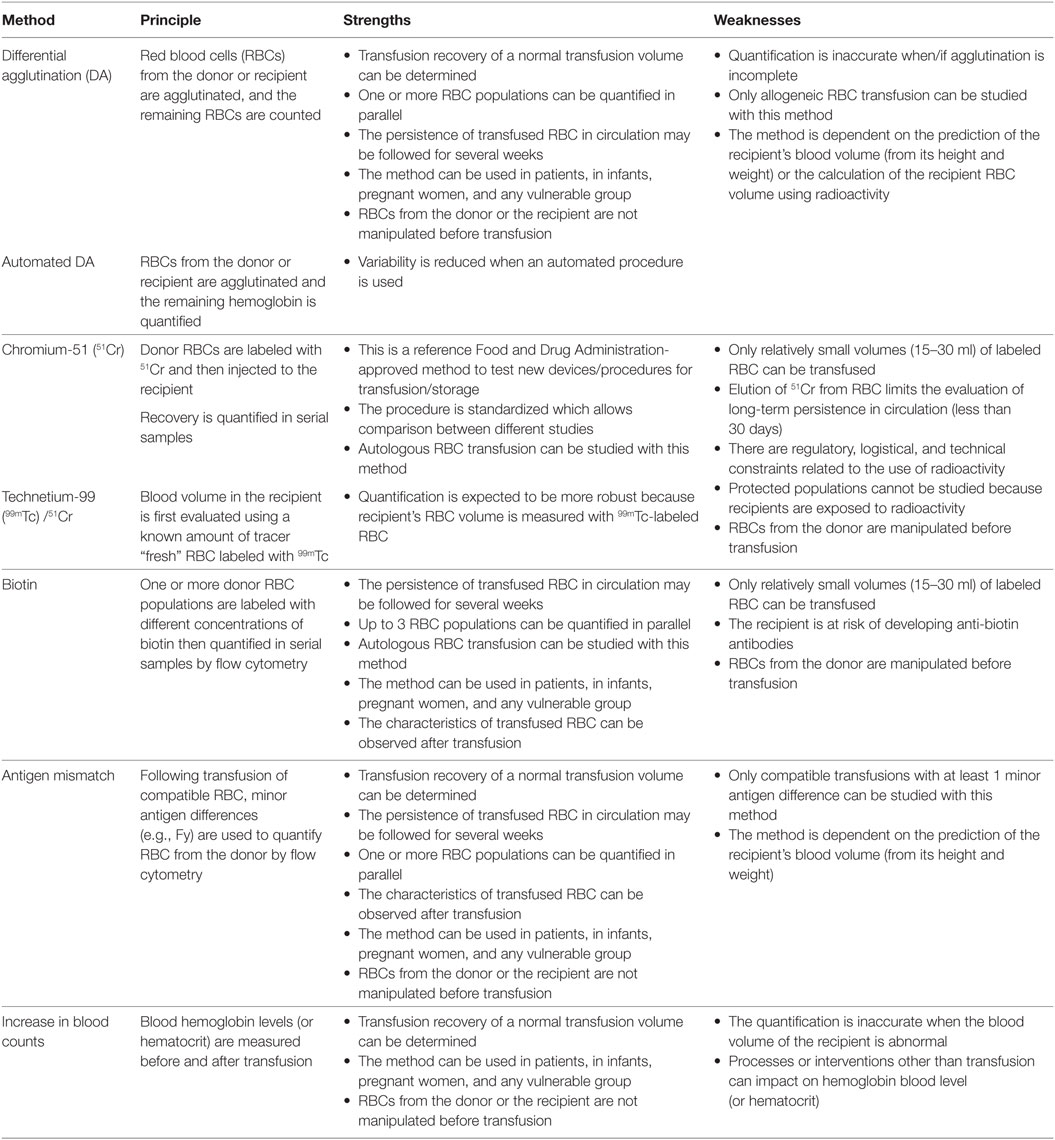
Several techniques have been developed and used in the last 100 years to measure transfusion recovery. Transfusion recovery using chromium-51 (51Cr) labeling is now a regulation criterion to license new storage systems or RCC preparation processes by the Food and Drug Administration (FDA). The FDA threshold to approve a preparation and storage process of RBC is a maximum 1% in vitro hemolysis and a 24 h in vivo recovery of at least 75% after reinfusion of autologous 51Cr-labeled RBC in healthy volunteers, at the limit of storage (2). The 51Cr-labeling technique was first used in the early 1950s and became the gold standard in the 1970s when the International Committee for Standardization in Hematology (3) proposed it as the reference technique. The use of a standardized protocol is essential to compare studies distant in space or time. Over the last 50 years, most of the transfusion recovery data were generated by 51Cr-labeling studies, mostly to validate new storage systems and RCC preparation processes. As a consequence, our understanding of transfusion efficacy strongly depends on the strengths and weaknesses of 51Cr labeling. However, other methods have been developed and validated, the advantages and limitations of which deserve careful analysis.
We will provide a brief overview of the methods used to measure transfusion recovery in humans. We will compare their strengths and weaknesses and critically analyze their potential limitations. Also, based on our understanding of the spleen-specific filtration of damaged RBC, we will discuss the relevance of storage lesion markers to predict transfusion recovery. We will finally discuss the current state of knowledge in the RBC transfusion field and propose future directions. Animal studies published in the recent years on this topic are beyond the scope of this analysis.
Methods Used for Transfusion Recovery Studies
Differential Agglutination (DA)
Differential agglutination was the first method used to measure transfusion recovery of a complete RCC (1, 4). In the 24 h post-transfusion blood sample, RBCs from the donor or the recipient are agglutinated with an appropriate antiserum, and the remaining RBCs are counted (5, 6). Similarly, an automated DA technique was developed where agglutinates are removed automatically and the remaining hemoglobin is quantified colorimetrically (7–13). The “100%” initial point is calculated from the prediction of the recipient’s normal blood volume (using its height and weight). Alternatively, the initial point can be obtained from an estimation of the recipient’s red cell volume using radioactive labeling of “fresh” RBC.
Radioactive Labeling Methods With 51Cr and Other Isotopes
Here, 15–30 ml of RBCs from a donor is labeled with 51Cr (14) and injected to the recipient (most of the time the donor himself) (15–31). RBC recovery is quantified after transfusion by taking a blood sample at early time points (5, 7.5, 10, 12.5, and 15 min) and 24 h after injection. In each sample, a radioactivity count number is acquired, and the initial point is extrapolated by linear regression. Alternatively, transfusion recovery can be evaluated using the technetium-99 (99mTc)/51Cr double-labeling technique (32–43). In this method, the recipient’s RBC volume is first evaluated using a known amount of “fresh” RBC labeled with 99mTc (32P and 52Cr were also used in older studies) (44–46), which is then used to calculate transfusion recovery. Similarly, 51Cr labeling can be associated with 125I-labeled albumin to evaluate the recipient’s plasma volume, which is then used in the calculation of transfusion recovery (35, 46–48). A reduced transfusion recovery was observed in some studies (34, 46, 48) that compared the double labeling with the single-labeling method. This is probably due to an undervaluation of the very short-term component of survival when using the single-label method (since some RBCs are rapidly removed from the circulation in the very first minutes following injection) and suggests that the double-label method is worth the extra complexity.
Biotinylation
Red blood cells from a donor (5–30 ml) are labeled with biotin and injected to the recipient. RBC recovery is usually quantified after transfusion by taking a blood sample at an early time point (10 min) and 24 h after injection (49, 50). Fluorescent labeling of biotinylated RBC and flow cytometry detection quantify the proportion of transfused RBC. By labeling RBC with different densities of biotin, it is possible to evaluate the recovery of up to three RBC populations in the same recipient. Care must be taken to avoid too high concentrations of biotin since it has been correlated with increase of transfused RBC clearance and anti-biotin antibodies in the recipient (51). A GMP grade biotin is now available and could be used in countries where radioactive labeling procedures are not authorized (52). This method theoretically allows the determination of transfused RBC characteristics.
Minor Antigen Mismatch
In the minor antigen mismatch method, an antibody directed against a minor antigen (e.g., Fy), which differs between the donor and the recipient, is used to determine, by flow cytometry, the proportion of transfused RBC in the 24 h post-transfusion sample (53, 54). No manipulation of the transfused RBC is necessary, and recovery is evaluated after the transfusion of a complete RCC, but the measure is dependent on the prediction of the recipient blood volume (from its height and weight). Theoretically, the characteristics of transfused RBC can be observed after transfusion.
Increase in Blood Counts
A simple method to evaluate transfusion recovery is to measure the increase in blood count (hemoglobin level or hematocrit) between a pretransfusion sample and post-transfusion samples (55–58). Limitations from such a method include the limited accuracy of the blood count measure and the unknown recipient’s blood volume (and its variable response to transfusion) that both contribute to the inaccuracy of the measure.
Limitations of Existing Recovery Studies
Strengths and weaknesses of the different methods are summarized in Table 1. One of the potential bias of the chromium or biotinylation techniques stems from the labeling protocol necessary to perform these studies. In the normal transfusion setup, RBCs are transfused to the recipient directly from the bag while in these two methods, RBCs are manipulated, centrifuged, and incubated in PBS or saline solution. It is conceivable that these steps modify labeled RBC in a way that affects their ability to stay in circulation, although a recent study showed that some RBC properties are only slightly modified by the biotinylation protocol (59). The situation is different with DA and minor antigen mismatch, as these techniques do not require any RBC manipulation before transfusion, thereby eliminating this potential source of artifact.
Another potential limitation of the accuracy of the 51Cr or biotinylation techniques is the infusion of a relatively small volume (5–30 ml) of RBC rather than a complete RCC. Transfusion recovery may indeed be influenced by the volume of transfused RBC. To explore this possibility and mimic more closely a complete RCC transfusion, 51Cr-labeled or biotinylated RBC could be co-transfused with the rest of the RCC. One study (60) reported a lower transfusion recovery of an entire unit (using automated DA) when compared with a 10–30 ml transfusion volume (using 51Cr labeling). However, it is not possible to ascertain that the “volume of transfusion” was responsible for this difference since other potential factors related to the method used to quantify transfusion recovery method (automated DA vs 51Cr) may have impacted the observation. The exact clearance mechanism(s) of potentially damaged RBC stored for many weeks are not well known. To what extent transfusion recovery data using a small amount of RBC accurately predict the outcome of a complete—or massive—RCC transfusion remains therefore an open question.
To reduce the risk of adverse events including transmission of infectious diseases, most of the transfusion recovery studies are conducted using autologous transfusion of stored RBC to healthy volunteers. In this setup, conditions of transfusion are probably appropriate to evaluate storage and donor effects. However, they do not take into consideration the possible complex interaction between damaged-stored RBC and potential recipient specificities related to its physiopathological condition. Along this line, it has been shown that survival of transfused RBC is abnormally low in some thalassemia patients with splenomegaly (61). Normal survival was restored following splenectomy suggesting that the spleen is where most RBCs that were no longer present in the circulation 24 h after transfusion had been retained. This is an example of how the medical condition of the recipient can impact transfusion recovery.
Storage Lesion, Transfusion Recovery, and Spleen Filtration
Storage Lesion and Transfusion Recovery
Recently, a number of prospective clinical studies have been conducted to evaluate the potential benefit of transfusing RCC stored for a short period (61–66). These studies have shown that transfusion of RCC stored for a short period does not reduce in-hospital morbidity or mortality in adult and children transfused for acute anemia. The “standard of care” collection and storage processes thus appear to be currently adequate when (accurately) assessed on clinical endpoints. However, these complex prospective clinical studies assessing predominantly safety may be difficult to conduct in some cohorts, such as chronically transfused patients, where the long-term impact of transfusions may be even more relevant. In addition, clinical studies of safety did not specifically examine the effect of transfusing RCC stored for a long period (more than 35 days) and did not directly address the efficacy of the procedure. This evaluation could be important in light of the well-documented RBC alterations that accumulate during hypothermic storage (67). The clinical relevance of this storage “lesion” to predict the efficacy and safety of the transfusion for the recipient is still a matter of controversy but suggests that RBC quality does not remain stable during storage.
It has been assumed that the decrease in transfusion recovery related to storage is due to RBC damages that accumulate after several weeks of storage. Studies performed more than 50 years ago have shown indeed that the extent of the storage lesion increases with storage duration while transfusion recovery decreases accordingly (5, 8, 10, 45). Few studies have directly explored the correlation between in vitro markers of storage lesion and in vivo recovery. In these studies, three markers (intracellular ATP, deformability, and morphology) have been shown to correlate with transfusion recovery (Box 1). The proportion of RBC removed from circulation (calculated from transfusion recovery) could correspond to the proportion of RBC, damaged during the storage process, which are over a recipient “clearance threshold.” If this assumption is correct, an optimal marker of storage lesion should identify and quantify the subpopulation of RBC that undergoes early premature clearance.
Box 1 Connecting storage lesion with transfusion recovery.
Intracellular ATP
An inverse correlation between the intracellular content in ATP in the RCC and transfusion recovery has been reported in a number of studies (1, 7, 9, 44). ATP content declines during long-term hypothermic incubation in a non-physiological solution. This is probably at the root of most RBC alterations that accumulate during storage. Intracellular ATP quantification remains, however, difficult to standardize and allows evaluation of the RCC quality at a cell population rather than a single-cell level.
Morphology
At least two studies using the 51Cr technique have shown that morphological modifications of RBC in a RCC do correlate with transfusion recovery. In the first study, the proportion of RBC with a discoid shape was positively correlated with transfusion recovery (16), while in the second, the morphology index after rejuvenation correlated with recovery (20). An evaluation of RBC morphology thus seems a good potential predictor of transfusion recovery provided that individual RBC shape can be categorized reliably. However, morphology analyses are low-throughput and operator-dependent making them difficult to standardize and implement. New technologies such as imaging flow cytometry may help circumvent this problem. We have recently identified a subpopulation of small spherocytic RBC that appears and expands during storage with wide variations between donors (68). This spherocytic shift could be a relevant marker as it readily identifies a subpopulation of RBC expected to be cleared rapidly after transfusion. However, direct evidence is lacking that small spherocytic RBC are prematurely cleared following transfusion, hence account for all or part of a suboptimal recovery.
Deformability
A recent study in patients with thalassemia showed that the increase in hemoglobin following transfusion was inversely correlated to the proportion of “less deformable” RBC in the RCC (57). In this study, a cell flow analyzer (69) was used to measure the elongation index of individual RBC that adheres to a polystyrene slide. Deformability can also be evaluated by measuring an RBC elongation index using ektacytometry (70). Both technologies have shown a decrease in RBC deformability during storage but the cell flow analyzer, although not commercially available has the advantage of measuring individual RBC elongation. In principle, the automated rheoscope and cell analyzer would provide interesting individual cell data on the evolution of RBC during storage.
The spleen has a specific filtering function that operates the clearance of damaged or senescent RBC from the circulation. Knowledge on the spleen filtration process is therefore relevant to understand transfusion recovery.
Spleen Filtration Capacity
In the splenic circulation, RBCs engage into two parallel pathways, the fast or slow microcirculations (71). In the fast and “closed” microcirculation, RBCs remain in endothelialized pathways and transit from arterioles to the venous sinus lumen through pathways in the perifollicular zone (72). In the slow and “open” microcirculation, RBCs navigate in tortuous microcirculatory beds of the red pulp, devoid of endothelium, before returning to the venous circulation by squeezing through 1- to 2-μm-wide slits between endothelial cells in the wall of sinuses (71, 73). Macrophages account for approximately half the volume of the cords, and their abundance facilitates direct RBC–macrophage interactions (71). The spleen likely contributes through one or more of these mechanisms to the clearance of transfused RBC. Intensity and kinetics of this clearance depend on the proportion of altered RBC in an RCC and on the intensity of the alterations. In physiologic conditions, the spleen can process at least 20 ml of RBC per day. It is conceivable that its filtration capacity might be overwhelmed by the amount of damaged RBC transfused, potentially leaving in circulation RBC that should normally be removed.
Spleen Filtration Threshold
The spleen-specific filtration process can trigger the clearance of senescent or altered RBC based on the sensing of surface modifications, mechanical alterations, or a combination of both. Inter-endothelial slits in the spleen exert a stringent challenge on RBC and retain least deformable ones (74, 75). Macrophages sense the shape and altered deformability of RBC and phagocytize them (76). In hereditary spherocytosis, morphology and deformability of RBC are linked (77), surface area-to-volume ratio being the main major determinant of RBC ability to cross narrow inter-endothelial slits in the spleen (74, 78). Ex vivo experiments with human spleens have confirmed the correlation between RBC retention in the spleen and the loss in projected surface area (79). Retention was almost complete when more than 17.5% of surface area had been lost. There is therefore a “splenic clearance threshold” that senses biomechanical and morphological changes of RBC which has also been determined by modeling in silico (75). Deformability and morphology of transfused RBC are expected to be very important determinants of transfusion recovery.
Conclusion
Recovery of autologous RBC in healthy non-anemic recipients using 51Cr labeling, 24 h after transfusion, is the method usually performed to determine the validity of the RCC preparation/storage processes. When examining transfusion recovery studies, we identified three parameters, namely transfusion volume, labeling protocol and the recipient pathophysiological state that have been under-evaluated and may impact the determination of transfusion recovery. For example, monocytes and macrophages, that possess a limited clearance capacity (80), could be saturated and leave in circulation damaged RBC when a large volume of RBC is transfused. Such an assumption is supported by data showing that transfusion of more than five RCC leads to a decreased deformability of circulating RBC (81). In the case of the labeling protocol, it has been shown that RBC stored for a long period are “primed” and more sensitive to an incubation in medium at 37°C (82) and may react differently when incubated in non-physiological solutions used in certain labeling protocol. Also, the observation that transfusion recovery is reduced in recipients with a splenomegaly (4, 61, 83) strongly suggests that individual characteristics or a pathological condition in the recipient impacts the recovery and survival of transfused RBC, even in absence of alloantibodies. These technical differences between transfusion recovery studies in healthy volunteers and transfusion of an anemic patient in a medical context suggest that available transfusion recovery data may not reflect transfusion efficacy in anemic recipients in some physiopathological conditions. A better understanding of RBC clearance mechanisms is warranted and could be explored by conducting transfusion recovery studies. In doing so, an appropriate experimental design, considering the strengths, weaknesses of the available methods, should be selected. As such, antigen mismatch method appears to offer a number of theoretical advantages over the other methods but would ideally be coupled with a non-radioactive labeling method to evaluate the RBC volume in the recipient.
That some storage lesion markers correlate with transfusion recovery reinforces the potential relevance of these in vitro studies which may deliver clinically relevant information. However, in the current conditions of blood collection and processing, this correlation between transfusion recovery and storage lesion remains poorly explored. Identification of a marker that could predict transfusion recovery would be a valuable tool for transfusion medicine and help to bridge the gap between storage lesion and the morbi-mortality studies. Future studies that evaluate transfusion recovery should be designed to include selected storage lesion markers to verify potential correlations. Deformability and morphology, preferably at the individual RBC level, appear as key potential markers since both spleen physiology and historical transfusion recovery data identify them as potentially predictive of transfusion recovery.
In vitro studies have shown that marked RBC alterations appear and worsen during storage, but a paradox remains since clinical studies have not found correlations between using RCC stored for a short time (generally less than 7–10 days) and improved clinical outcome. This apparent discrepancy is a source of interrogation in the transfusion community. Clinical studies were appropriately designed to guide transfusion policy. The current conclusion is that there would be no benefit at keeping “fresh blood” for specific situations. The impact of studying storage “lesion” has been questioned as well as the medical relevance of cellular alterations that do not translate into any negative outcome. Is storage “lesion” merely a misnomer, to be replaced advantageously by storage “changes”? This is not so sure yet. Many have argued that clinical studies did not assess the effect of transfusing RCC stored for more than 28 days, while storage lesion studies indicate that the extent of damage rapidly increases after 4 weeks of storage (84). Furthermore, clinical studies were not designed to assess transfusion efficacy and particularly the influence of storage duration on transfusion recovery. On the other hand, in vitro studies of the storage lesion are not often correlated with in vivo recovery. In vitro studies of the storage lesion and clinical studies deliver complementary information while addressing different questions. Studies that explore both dimensions of knowledge are difficult to implement since their designs differ. Large safety studies collect simple data from many patients while transfusion recovery collects complex repetitive samples, which are analyzed using relatively sophisticated methods. The way forward is probably to set-up ancillary recovery studies in the context of large safety trials.
Author Contributions
All the authors listed have made a contribution to the work and approved it for publication.
Conflict of Interest Statement
PA and PB have a sponsored research agreement with Zimmer Biomet. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
CR was supported by a fellowship from the Laboratory of excellence GR-Ex. The Labex GR-Ex, reference ANR-11-LABX-0051, is funded by the program “Investissements d’avenir” of the French National Research Agency, reference ANR-11-IDEX-0005-02.
References
1. Ashby W. The determination of the length of life of transfused blood corpuscles in man. J Exp Med (1919) 29(3):267–81. doi:10.1084/jem.29.3.267
2. Hess JR. Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Scientific problems in the regulation of red blood cell products. Transfusion (2012) 52(8):1827–35. doi:10.1111/j.1537-2995.2011.03511.x
4. Klein HG, Anstee DJ. Mollison’s Blood Transfusion in Clinical Medicine. 11th ed. Chichester: Wiley-Blackwell (2008). 912 p.
5. Mollison PL, Young IM. Failure of in vitro tests as a guide to the value of stored blood. Br Med J (1941) 2(4222):797–800. doi:10.1136/bmj.2.4222.797
6. Mollison PL, Sloviter HA, Chaplin H. Survival of transfused red cells previously stored for long periods in the frozen state. Lancet (1952) 2(6733):501–5. doi:10.1016/S0140-6736(52)90290-0
7. Szymanski IO, Valeri CR, McCallum LE, Emerson CP, Rosenfield RE. Automated differential agglutination technic to measure red cell survival. I. Methodology. Transfusion (1968) 8(2):65–73. doi:10.1111/j.1537-2995.1968.tb02397.x
8. Szymanski IO, Valeri CR. Automated differential agglutination technic to measure red cell survival. II. Survival in vivo of preserved red cells. Transfusion (1968) 8(2):74–83. doi:10.1111/j.1537-2995.1968.tb02398.x
9. Valeri CR, Landrock RD, Pivacek LE, Gray AD, Fink JG, Szymanski IO. Quantitative differential agglutination method using the Coulter Counter to measure survival of compatible but identifiable red blood cells. Vox Sang (1985) 49(3):195–205. doi:10.1111/j.1423-0410.1985.tb00793.x
10. Szymanski IO, Dean HM, Valeri CR, Bougas JA, Desforges JF. Measurement of erythrocyte survival during open-heart surgery. Transfusion (1970) 10(4):163–70. doi:10.1111/j.1537-2995.1970.tb00726.x
11. Szymanski IO, Valeri CR. Lifespan of preserved red cells. Vox Sang (1971) 21(2):97–108. doi:10.1111/j.1423-0410.1971.tb00566.x
12. Szymanski IO, Valeri CR. Evaluation of double 51Cr technique. Vox Sang (1968) 15(4):287–92. doi:10.1159/000467074
13. Valeri CR. Factors influencing the 24-hour posttransfusion survival and the oxygen transport function of previously frozen red cells preserved with 40 per cent W-V glycerol and frozen at –80°C. Transfusion (1974) 14(1):1–15. doi:10.1111/j.1537-2995.1974.tb04478.x
14. Moroff G, Sohmer PR, Button LN. Proposed standardization of methods for determining the 24-hour survival of stored red cells. Transfusion (1984) 24(2):109–14. doi:10.1046/j.1537-2995.1984.24284173339.x
15. Deverdier CH, Garby L, Hjelm M, Hoegman C. Adenine in blood preservation: posttransfusion viability and biochemical changes. Transfusion (1964) 4:331–8. doi:10.1111/j.1537-2995.1964.tb02883.x
16. Haradin AR, Weed RI, Reed CF. Changes in physical properties of stored erythrocytes relationship to survival in vivo. Transfusion (1969) 9(5):229–37. doi:10.1111/j.1537-2995.1969.tb04929.x
17. Herve P, Lamy B, Peters A, Toubin M, Bidet AC. Preservation of human erythrocytes in the liquid state: biological results with a new medium. Vox Sang (1980) 39(4):195–204. doi:10.1111/j.1423-0410.1980.tb01857.x
18. Beutler E, Kuhl W, West C. The osmotic fragility of erythrocytes after prolonged liquid storage and after reinfusion. Blood (1982) 59(6):1141–7.
19. Högman CF, Hedlund K. Storage of red cells in a CPD/SAGM system using Teruflex PVC. Vox Sang (1985) 49(3):177–80. doi:10.1111/j.1423-0410.1985.tb00790.x
20. Högman CF, de Verdier CH, Ericson A, Hedlund K, Sandhagen B. Studies on the mechanism of human red cell loss of viability during storage at +4 degrees C in vitro. I. Cell shape and total adenylate concentration as determinant factors for posttransfusion survival. Vox Sang (1985) 48(5):257–68. doi:10.1111/j.1423-0410.1985.tb00181.x
21. AuBuchon JP, Estep TN, Davey RJ. The effect of the plasticizer di-2-ethylhexyl phthalate on the survival of stored RBCs. Blood (1988) 71(2):448–52.
22. Davey RJ, Carmen RA, Simon TL, Nelson EJ, Leng BS, Chong C, et al. Preparation of white cell-depleted red cells for 42-day storage using an integral in-line filter. Transfusion (1989) 29(6):496–9. doi:10.1046/j.1537-2995.1989.29689318446.x
23. Moore GL, Hess JR, Ledford ME. In vivo viability studies of two additive solutions in the postthaw preservation of red cells held for 3 weeks at 4 degrees C. Transfusion (1993) 33(9):709–12. doi:10.1046/j.1537-2995.1993.33994025017.x
24. Greenwalt TJ, Dumaswala UJ, Rugg N. Studies in red blood cell preservation 10. 51Cr recovery of red cells after liquid storage in a glycerol-containing additive solution. Vox Sang (1996) 70(1):6–10. doi:10.1111/j.1423-0410.1996.tb00988.x
25. Hess JR, Rugg N, Knapp AD, Gormas JF, Silberstein EB, Greenwalt TJ. Successful storage of RBCs for 9 weeks in a new additive solution. Transfusion (2000) 40(8):1007–11. doi:10.1046/j.1537-2995.2000.40081007.x
26. Hess JR, Rugg N, Gormas JK, Knapp AD, Hill HR, Oliver CK, et al. RBC storage for 11 weeks. Transfusion (2001) 41(12):1586–90. doi:10.1046/j.1537-2995.2001.41121586.x
27. Valeri CR, Ragno G, Pivacek L, O’Neill EM. In vivo survival of apheresis RBCs, frozen with 40-percent (wt/vol) glycerol, deglycerolized in the ACP 215, and stored at 4 degrees C in AS-3 for up to 21 days. Transfusion (2001) 41(7):928–32. doi:10.1046/j.1537-2995.2001.41070928.x
28. Hess JR, Rugg N, Joines AD, Gormas JF, Pratt PG, Silberstein EB, et al. Buffering and dilution in red blood cell storage. Transfusion (2006) 46(1):50–4. doi:10.1111/j.1537-2995.2005.00672.x
29. Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion (2008) 48(6):1053–60. doi:10.1111/j.1537-2995.2008.01642.x
30. Cancelas JA, Dumont LJ, Maes LA, Rugg N, Herschel L, Whitley PH, et al. Additive solution-7 reduces the red blood cell cold storage lesion. Transfusion (2015) 55(3):491–8. doi:10.1111/trf.12867
31. Rapido F, Brittenham GM, Bandyopadhyay S, La Carpia F, L’Acqua C, McMahon DJ, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest (2017) 127(1):375–82. doi:10.1172/JCI90837
32. Mishler JM, Darley JH, Haworth C, Mollison PL. Viability of red cells stored in diminished concentration of citrate. Br J Haematol (1979) 43(1):63–7. doi:10.1111/j.1365-2141.1979.tb03720.x
33. Card RT, Mohandas N, Mollison PL. Relationship of post-transfusion viability to deformability of stored red cells. Br J Haematol (1983) 53(2):237–40. doi:10.1111/j.1365-2141.1983.tb02016.x
34. Heaton WA, Keegan T, Holme S, Momoda G. Evaluation of 99mtechnetium/51chromium post-transfusion recovery of red cells stored in saline, adenine, glucose, mannitol for 42 days. Vox Sang (1989) 57(1):37–42. doi:10.1159/000460998
35. Moroff G, Holme S, Heaton WA, Kevy S, Jacobson M, Popovsky M. Effect of an 8-hour holding period on in vivo and in vitro properties of red cells and factor VIII content of plasma after collection in a red cell additive system. Transfusion (1990) 30(9):828–32. doi:10.1046/j.1537-2995.1990.30991048790.x
36. Heaton WA, Holme S, Smith K, Brecher ME, Pineda A, AuBuchon JP, et al. Effects of 3–5 log10 pre-storage leucocyte depletion on red cell storage and metabolism. Br J Haematol (1994) 87(2):363–8. doi:10.1111/j.1365-2141.1994.tb04923.x
37. Reid TJ, Babcock JG, Derse-Anthony CP, Hill HR, Lippert LE, Hess JR. The viability of autologous human red cells stored in additive solution 5 and exposed to 25 degrees C for 24 hours. Transfusion (1999) 39(9):991–7. doi:10.1046/j.1537-2995.1999.39090991.x
38. Hess JR, Rugg N, Knapp AD, Gormas JF, Silberstein EB, Greenwalt TJ. Successful storage of RBCs for 10 weeks in a new additive solution. Transfusion (2000) 40(8):1012–6. doi:10.1046/j.1537-2995.2000.40081012.x
39. Hess JR, Hill HR, Oliver CK, Lippert LE, Rugg N, Joines AD, et al. Twelve-week RBC storage. Transfusion (2003) 43(7):867–72. doi:10.1046/j.1537-2995.2003.00442.x
40. Yoshida T, AuBuchon JP, Tryzelaar L, Foster KY, Bitensky MW. Extended storage of red blood cells under anaerobic conditions. Vox Sang (2007) 92(1):22–31. doi:10.1111/j.1423-0410.2006.00860.x
41. Yoshida T, AuBuchon JP, Dumont LJ, Gorham JD, Gifford SC, Foster KY, et al. The effects of additive solution pH and metabolic rejuvenation on anaerobic storage of red cells. Transfusion (2008) 48(10):2096–105. doi:10.1111/j.1537-2995.2008.01812.x
42. Cancelas JA, Slichter SJ, Rugg N, Pratt PG, Nestheide S, Corson J, et al. Red blood cells derived from whole blood treated with riboflavin and ultraviolet light maintain adequate survival in vivo after 21 days of storage. Transfusion (2017) 57(5):1218–25. doi:10.1111/trf.14084
43. Cancelas JA, Gottschall JL, Rugg N, Graminske S, Schott MA, North A, et al. Red blood cell concentrates treated with the amustaline (S-303) pathogen reduction system and stored for 35 days retain post-transfusion viability: results of a two-centre study. Vox Sang (2017) 112(3):210–8. doi:10.1111/vox.12500
44. Gabrio BW, Donohue DM, Finch CA. Erythrocyte preservation. V. Relationship between chemical changes and viability of stored blood treated with adenosine. J Clin Invest (1955) 34(10):1509–12. doi:10.1172/JCI103202
45. Shields CE. Effect of adenine on stored erythrocytes evaluated by autologous and homologous transfusions. Transfusion (1969) 9(3):115–9. doi:10.1111/j.1537-2995.1969.tb05528.x
46. Heaton WA, Keegan T, Hanbury CM, Holme S, Pleban P. Studies with nonradioisotopic sodium chromate. II. Single- and double-label 52Cr/51Cr posttransfusion recovery estimations. Transfusion (1989) 29(8):703–7. doi:10.1046/j.1537-2995.1989.29890020444.x
47. Simon TL, Marcus CS, Myhre BA, Nelson EJ. Effects of AS-3 nutrient-additive solution on 42 and 49 days of storage of red cells. Transfusion (1987) 27(2):178–82. doi:10.1046/j.1537-2995.1987.27287150195.x
48. Valeri CR, Pivacek LE, Palter M, Dennis RC, Yeston N, Emerson CP, et al. A clinical experience with ADSOL preserved erythrocytes. Surg Gynecol Obstet (1988) 166(1):33–46.
49. Strauss RG, Mock DM, Widness JA, Johnson K, Cress G, Schmidt RL. Posttransfusion 24-hour recovery and subsequent survival of allogeneic red blood cells in the bloodstream of newborn infants. Transfusion (2004) 44(6):871–6. doi:10.1111/j.1537-2995.2004.03393.x
50. Peters AL, Beuger B, Mock DM, Widness JA, de Korte D, Juffermans NP, et al. Clearance of stored red blood cells is not increased compared with fresh red blood cells in a human endotoxemia model. Transfusion (2016) 56(6):1362–9. doi:10.1111/trf.13595
51. Mock DM, Widness JA, Veng-Pedersen P, Strauss RG, Cancelas JA, Cohen RM, et al. Measurement of posttransfusion red cell survival with the biotin label. Transfus Med Rev (2014) 28(3):114–25. doi:10.1016/j.tmrv.2014.03.003
52. Ohlmann P, Kemperman G, Basten J, Viaud-Massuard M, Ravanat C. Synthesis of the first GMP grade biotin-3-sulfo hydroxysuccinimide is now available to label blood cells intended for human transfusion studies. In: Devine DA, editor. Vox Sanguinis. Proceedings of the 28th Regional Congress of the ISBT; 2017 Nov 25–28; Guangzhou, China. Copenhagen: Wiley-Blackwell (2017). p. 5–191.
53. Zeiler T, Müller JT, Kretschmer V. Flow-cytometric determination of survival time and 24-hour recovery of transfused red blood cells. Transfus Med Hemother (2003) 30(1):14–9. doi:10.1159/000069340
54. Luten M, Roerdinkholder-Stoelwinder B, Schaap NPM, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion (2008) 48(7):1478–85. doi:10.1111/j.1537-2995.2008.01734.x
55. Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, Ddungu H, Kyeyune D, Musisi E, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the TOTAL Randomized Clinical Trial. JAMA (2015) 314(23):2514–23. doi:10.1001/jama.2015.13977
56. Pilania RK, Saini SS, Dutta S, Das R, Marwaha N, Kumar P. Factors affecting efficacy of packed red blood cell transfusion in neonates. Eur J Pediatr (2017) 176(1):67–74. doi:10.1007/s00431-016-2806-7
57. Barshtein G, Goldschmidt N, Pries AR, Zelig O, Arbell D, Yedgar S. Deformability of transfused red blood cells is a potent effector of transfusion-induced hemoglobin increment: a study with β-thalassemia major patients. Am J Hematol (2017) 92(9):E559–60. doi:10.1002/ajh.24821
58. Barshtein G, Pries AR, Goldschmidt N, Zukerman A, Orbach A, Zelig O, et al. Deformability of transfused red blood cells is a potent determinant of transfusion-induced change in recipient’s blood flow. Microcirculation (2016) 23(7):479–86. doi:10.1111/micc.12296
59. de Back DZ, Vlaar R, Beuger B, Daal B, Lagerberg J, Vlaar APJ, et al. A method for red blood cell biotinylation in a closed system. Transfusion (2018) 58(4):896–904. doi:10.1111/trf.14535
60. Valeri CR, Zaroulis CG. Rejuvenation and freezing of outdated stored human red cells. N Engl J Med (1972) 287(26):1307–13. doi:10.1056/NEJM197212282872601
61. Smith CH, Schulman I, Ando RE, Stern G. Studies in Mediterranean (Cooley’s) anemia. I. Clinical and hematologic aspects of splenectomy, with special reference to fetal hemoglobin synthesis. Blood (1955) 10(6):582–99.
62. Cooper DJ, McQuilten ZK, Nichol A, Ady B, Aubron C, Bailey M, et al. Age of red cells for transfusion and outcomes in critically ill adults. N Engl J Med (2017) 377(19):1858–67. doi:10.1056/NEJMoa1707572
63. Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med (2016) 375(20):1937–45. doi:10.1056/NEJMoa1609014
64. Lacroix J, Hébert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, et al. Age of transfused blood in critically ill adults. N Engl J Med (2015) 372(15):1410–8. doi:10.1056/NEJMoa1500704
65. Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med (2015) 372(15):1419–29. doi:10.1056/NEJMoa1414219
66. Cook RJ, Heddle NM, Lee KA, Arnold DM, Crowther MA, Devereaux PJ, et al. Red blood cell storage and in-hospital mortality: a secondary analysis of the INFORM randomised controlled trial. Lancet Haematol (2017) 4(11):e544–52. doi:10.1016/S2352-3026(17)30169-2
67. Fergusson DA, Hébert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA (2012) 308(14):1443–51. doi:10.1001/2012.jama.11953
68. Roussel C, Dussiot M, Marin M, Morel A, Ndour PA, Duez J, et al. Spherocytic shift of red blood cells during storage provides a quantitative whole cell-based marker of the storage lesion. Transfusion (2017) 57(4):1007–18. doi:10.1111/trf.14015
69. Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion (2008) 48(1):136–46. doi:10.1111/j.1537-2995.2007.01491.x
70. Mohandas N, Clark MR, Jacobs MS, Shohet SB. Analysis of factors regulating erythrocyte deformability. J Clin Invest (1980) 66(3):563–73. doi:10.1172/JCI109888
71. Groom AC, Schmidt EE, MacDonald IC. Microcirculatory pathways and blood flow in spleen: new insights from washout kinetics, corrosion casts, and quantitative intravital videomicroscopy. Scanning Microsc (1991) 5(1):159–173; discussion 173–174.
72. Buffet PA, Safeukui I, Deplaine G, Brousse V, Prendki V, Thellier M, et al. The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood (2011) 117(2):381–92. doi:10.1182/blood-2010-04-202911
73. Buffet PA, Milon G, Brousse V, Correas J-M, Dousset B, Couvelard A, et al. Ex vivo perfusion of human spleens maintains clearing and processing functions. Blood (2006) 107(9):3745–52. doi:10.1182/blood-2005-10-4094
74. Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood (2008) 112(10):3939–48. doi:10.1182/blood-2008-07-161166
75. Pivkin IV, Peng Z, Karniadakis GE, Buffet PA, Dao M, Suresh S. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc Natl Acad Sci U S A (2016) 113(28):7804–9. doi:10.1073/pnas.1606751113
76. Sosale NG, Rouhiparkouhi T, Bradshaw AM, Dimova R, Lipowsky R, Discher DE. Cell rigidity and shape override CD47’s “self”-signaling in phagocytosis by hyperactivating myosin-II. Blood (2015) 125(3):542–52. doi:10.1182/blood-2014-06-585299
77. Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet (2008) 372(9647):1411–26. doi:10.1016/S0140-6736(08)61588-3
78. Waugh RE, Narla M, Jackson CW, Mueller TJ, Suzuki T, Dale GL. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood (1992) 79(5):1351–8.
79. Safeukui I, Buffet PA, Deplaine G, Perrot S, Brousse V, Ndour A, et al. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood (2012) 120(2):424–30. doi:10.1182/blood-2012-01-404103
80. Noyes WD, Bothwell TH, Finch CA. The role of the reticulo-endothelial cell in iron metabolism. Br J Haematol (1960) 6:43–55. doi:10.1111/j.1365-2141.1960.tb06216.x
81. Frank SM, Abazyan B, Ono M, Hogue CW, Cohen DB, Berkowitz DE, et al. Decreased erythrocyte deformability after transfusion and the effects of erythrocyte storage duration. Anesth Analg (2013) 116(5):975–81. doi:10.1213/ANE.0b013e31828843e6
82. Burger P, Kostova E, Bloem E, Hilarius-Stokman P, Meijer AB, van den Berg TK, et al. Potassium leakage primes stored erythrocytes for phosphatidylserine exposure and shedding of pro-coagulant vesicles. Br J Haematol (2013) 160(3):377–86. doi:10.1111/bjh.12133
83. Greenberg MS, Jandl JH. The selective destruction of transfused compatible normal red cells in two patients with splenomegaly. J Lab Clin Med (1957) 49(2):233–45.
Keywords: transfusion recovery, red blood cell, spleen, red blood cell morphology, red blood cell deformability, storage lesion
Citation: Roussel C, Buffet PA and Amireault P (2018) Measuring Post-transfusion Recovery and Survival of Red Blood Cells: Strengths and Weaknesses of Chromium-51 Labeling and Alternative Methods. Front. Med. 5:130. doi: 10.3389/fmed.2018.00130
Received: 15 January 2018; Accepted: 19 April 2018;
Published: 15 May 2018
Edited by:
Michel Prudent, Transfusion Interrégionale CRS SA, SwitzerlandReviewed by:
Dirk De Korte, Sanquin, NetherlandsMaxime Desmarets, Université Bourgogne Franche-Comté, France
Copyright: © 2018 Roussel, Buffet and Amireault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pascal Amireault, cGFtaXJlYXVsdEBpbnRzLmZy
 Camille Roussel
Camille Roussel Pierre A. Buffet
Pierre A. Buffet Pascal Amireault
Pascal Amireault