- 14th Department of Internal Medicine, Hippokration University Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 2Department of Academic Rheumatology, King’s College London, London, United Kingdom
- 3Department of Rheumatology, Whittington NHS Health, London, United Kingdom
- 4Arthritis Research UK Centre for Epidemiology, University of Manchester, Manchester, United Kingdom
- 5Department of Rheumatology, Dudley Group NHS Fountation Trust, Dudley, United Kingdom
Rheumatoid arthritis (RA) is an autoimmune, inflammatory disorder associated with excess cardiovascular morbidity and mortality. A complex interplay between traditional risk factors (dyslipidemia, insulin resistance, arterial hypertension, obesity, smoking) and chronic inflammation is implicated in the development of premature atherosclerosis and consequently in the higher incidence of cardiovascular events observed in RA patients. Despite the acknowledgment of elevated cardiovascular risk among RA individuals, its management remains suboptimal. While statin administration has a crucial role in primary and secondary cardiovascular disease prevention strategies as lipid modulating factors, there are limited data concerning the precise benefit of such therapy in patients with RA. Systemic inflammation and anti-inflammatory treatments influence lipid metabolism, leading to variable states of dyslipidemia in RA. Hence, the indications for statin therapy for cardiovascular prevention may differ between RA patients and the general population and the precise role of lipid lowering treatment in RA is yet to be established. Furthermore, some evidence supports a potential beneficial impact of statins on RA disease activity, attributable to their anti-inflammatory and immunomodulatory properties. This review discusses existing data on the efficacy of statins in reducing RA-related cardiovascular risk as well as their potential beneficial effects on disease activity.
Introduction
Rheumatoid arthritis (RA) imparts a significant risk for cardiovascular disease (CVD)-related morbidity and mortality (1, 2). It is well-established that accelerated atherosclerosis and vascular dysfunction in the setting of RA are the result of a complex interplay between traditional CVD risk factors, such as dyslipidemia (3), insulin resistance (4), hypertension (5), limited physical activity (6), and obesity (7), and RA-related characteristics including chronic high grade inflammation and autoimmune activation (8, 9). The recognition of the pivotal role of chronic inflammation in the pathogenesis of accelerated atherosclerosis in RA emphasizes the need for CVD risk management in these patients, to include both inflammation control and modification of traditional CVD risk factors. Therefore, assessment of CVD risk in patients with RA in routine clinical practice is highly recommended (10). However, the proposed CVD risk assessment scores designed for the general population seem to be insufficient in the case of RA patients, leading to an underestimation of CVD risk and, consequently, suboptimal CVD prevention (11).
Dyslipidemia constitutes the leading factor for cardiovascular events in the general population, making it the key target of current primary and secondary CVD prevention strategies (12). In this respect, several randomized clinical trials have firmly demonstrated the beneficial effect of statins, as lipid lowering agents on CVD risk management in many populations (13). However, the precise benefit of lipid lowering treatment in RA remains unclear. The variable prevalence of dyslipidemia in RA as well as the disproportionate association between lipid levels and disease activity, unrepresentative of the actual CVD risk, complicates the setting of low-density lipoprotein cholesterol (LDL-C) treatment targets and the choice of those patients eligible for statin therapy (14–17). Interestingly, experimental and clinical data support that statins may also provide anti-inflammatory and immunomodulatory benefits in RA, due to their pleiotropic therapeutic effects (18).
Cardiovascular events remain the major cause of death in patients with RA, urging a reassessment of the current approach to CVD risk management. Although many lines investigating this topic have been written, the exact benefit from statin treatment in RA needs further clarification. The scope of this manuscript is to undertake a narrative review of existing literature on the efficacy of statins in CVD prevention in RA, to discuss existing and future therapeutic targets arising from their pleiotropic pharmacological properties while addressing knowledge gaps and future research.
Methods
A literature search was conducted using the online databases MEDLINE/PUBMED and EMBASE from January 2000 until July 2017 for original research articles and review articles investigating the effect of statin treatment in patients with RA. Data concerning both CVD management and the impact on several aspects of RA were collected. The combination of the following terms was used to identify relevant publications: statins OR atorvastatin OR simvastatin OR rosuvastatin OR HMG-CoA reductase inhibitor OR cardiovascular disease OR hypercholesterolemia OR dyslipidemia OR cholesterol AND rheumatoid arthritis. We also reviewed the literature for cited articles relevant to the subject in articles identified through the review, to ensure that we did not miss important research data. Full journal articles and published abstracts in English language were included. We used the above terms in ClinicalTrials.gov to search for any recently completed or ongoing relevant pharmaceutical research. Conference proceedings, not accessible abstracts, case reports, or articles not in English, were excluded (19).
Statins and CVD Markers
The reduction of LDL-C by statins leads to a significant decrease in major cardiovascular events in patients at either high or low risk for CVD (20, 21). Such observations in the general population provide the rationale for treating RA patients with statins aiming to reduce the CVD burden (22). On top of the lipid lowering effect, other properties of statins, such as the stabilization and regression of atherosclerotic plaques and the limitation of LDL-C oxidation, could theoretically contribute to the prevention of primary atherosclerosis in RA (23, 24). Lipid profile alterations, displaying an inverse correlation between total cholesterol (TC) levels and disease activity, raise some questions about the exact value of lipid lowering treatment in RA (25). High level inflammation on the one hand and potent antiinflammatory disease-modifying antirheumatic drugs (DMARDs) on the other have significant contrasting effects on lipid levels and could modify the lipid lowering effectiveness and consequent CVD risk reduction impact of statins in RA (26, 27).
Angioprotective Effect
Several clinical studies have attempted to investigate statin benefits in RA patients utilizing non-invasive assessments of vascular function and morphology. The need for early detection of pathological processes in vasculature has led to the development of non-invasive tests which now constitute valuable tools for cardiovascular risk monitoring. The flow-mediated dilatation (FMD) test provides an ultrasonic assessment of nitric oxide-dependent vascular endothelial function, which is disturbed in atheromatous vessels (28). Arterial stiffness, showing an increase in early atheromatosis, can accurately be assessed with aortic pulse wave velocity (PWV) and Augmentation Index (AIx) (29, 30), parameters that are considered to be independent predictors of major cardiovascular events (31). Vascular function and morphology assessed by these markers is impaired in RA relative to healthy controls even in early stages of RA, reflect in the elevated cardiovascular risk (32, 33).
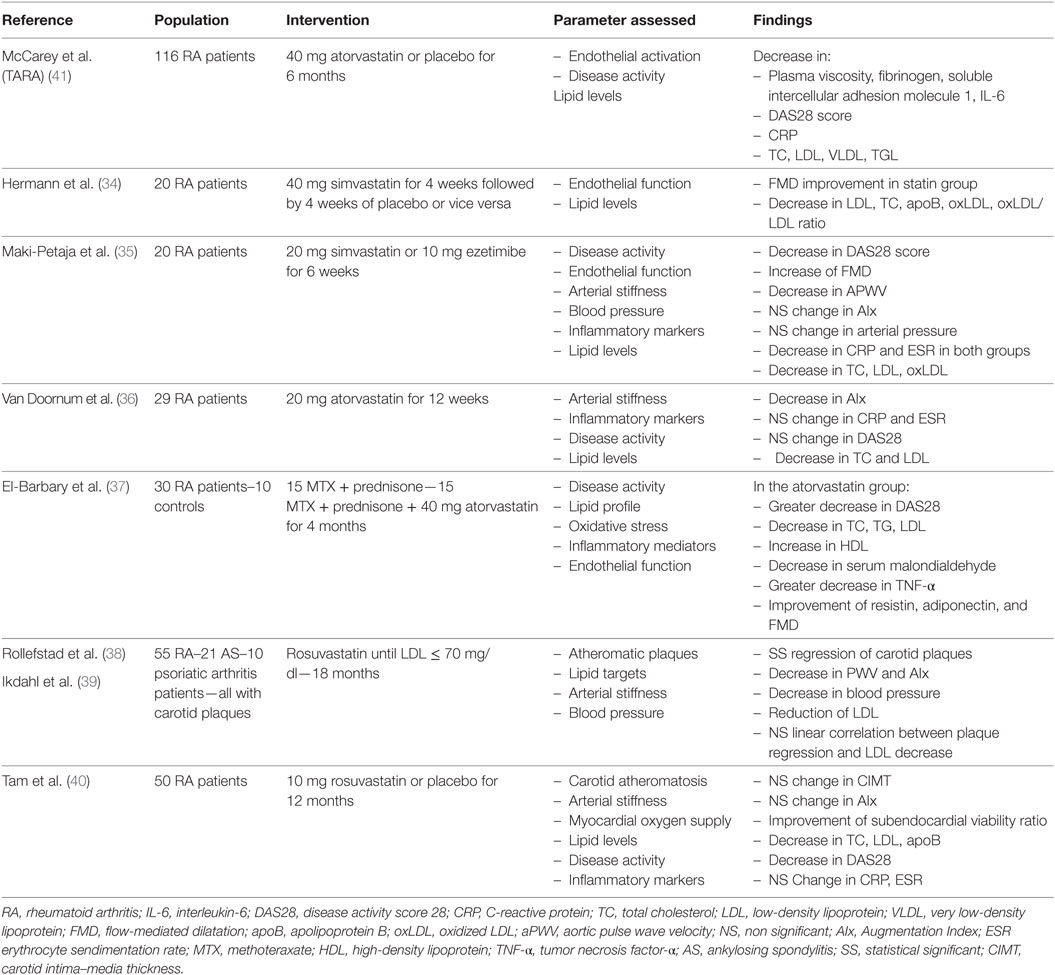
In a trial of 20 RA patients, administration of simvastatin 40 mg/day significantly improved FMD compared with placebo (34). Notably, a higher FMD was observed in patients with higher inflammatory status. A lower dose of simvastatin also induced a parallel reduction in both FMD and aortic PWV (35). A 12-week atorvastatin treatment in the dose of 20 mg/day induced a 12% reduction in arterial stiffness, evaluated with AIx (36). The greatest reductions of AIx occurred in those patients with higher disease activity scores at baseline. Furthermore, it was demonstrated that, in RA patients, atorvastatin had a positive impact on novel serum markers of endocrine function of arterial wall, such as adiponectin, along with a collateral improvement of FMD (37).
The effect of intensive lipid lowering treatment was evaluated in the ROsuvastatin in Rheumatoid Arthritis and Ankylosing Spondylitis study (38, 39). A regression of carotid plaques, inversely associated with disease activity during the study period, was demonstrated after 18 months of increasing dosage of rosuvastatin, to the upper limit of 40 mg/day until LDL-C levels below 70 mg/dL were achieved, highlighting once again the angioprotective properties of statins. In contrast, no significant reduction in carotid intima–media thickness (IMT) and AIx was recorded in 50 RA patients with low-disease activity, randomized to take 10 mg of rosuvastatin or placebo for 12 months (40).
In the context of biomarkers of endothelial activation, the Trial for Atorvastatin in Rheumatoid Arthritis (TARA) included 116 RA patients, randomized to receive 40 mg atorvastatin or placebo additionally to the existing disease-modifying therapy. A significant improvement in intercellular adhesion molecule 1 and fibrinogen levels was observed in the atorvastatin group after six months of treatment (41).
Taking it all into account, statins seem to have a favorable effect on structural, morphological and biochemical parameters of vascular function in RA patients, particularly those with higher disease activity (Table 1). However, the confirmation of these findings in larger and better controlled studies, as well as hard cardiovascular end-point trials, is warranted before specific recommendations can be made for their use in the routine clinical setting.
Lipid Lowering and Antioxidative Effect
Several factors seem to influence the lipid profile of RA patients, including inflammatory disease activity, antirheumatic drugs, and reduced physical activity (16). Despite the demonstrated excessive premature atherosclerosis potentiated by systemic inflammation, some evidence supports a paradoxical decrease of lipid levels in untreated, active RA (42). Given that TC levels are inversely correlated with current risk for CVD, the exact value of an additional lipid lowering effect remains questionable. However, a decrease in TC cannot describe the total lipid alterations characterizing RA. The observed fall in TC levels does not seem to be as marked as that seen in high-density lipoprotein cholesterol (HDL-C) levels and this leads to an increased atherogenic index (TC:HDL ratio), exposing RA patients to higher risk of atherosclerosis (43). Moreover, other lipid components with high predictive value for CVD, such as lipoprotein(a) and apolipoprotein B, are elevated in RA, while the relation between LDL-C and high disease activity remains unclear (44).
An inverse correlation between lipid concentrations and inflammatory markers in RA patients treated with non-biological DMARDS has been observed. While methotrexate therapy combined with prednisolone has been associated with a significant increase in TC and HDL-C levels, a significant decrease in the atherogenic ratio of TC/HDL-C ratio along with a response to treatment has been recorded (45). Similarly, tumor necrosis factor-α (TNF-α) inhibitors have been found to increase TC and HDL-C without affecting LDL-C levels and the atherogenic ratio (46). On the other hand, a significant rise in LDL-C levels with conflicting results regarding the effect on the atherogenic ratio and the net impact on CVD risk has been documented in RA patients treated with the interleukin-6 (IL-6) receptor inhibitor tocilizumab (47, 48). A post hoc analysis of 4,655 patients included in tocilizumab trials found that postbaseline initiation of statins was related to a gradual decrease and stabilization of LDL-C after two years of treatment, in contrast to the patients not receiving statins, where a significant increase in lipid levels was observed (49). Despite the need for further evaluation, these findings may support the administration of statins for the management of unfavorable cholesterol changes attributed to treatment with specific classes of biologic DMARDs.
Existing data demonstrate that statins maintain their lipid lowering effect in patients with RA and active inflammation. The reduction of LDL-C and TC levels is combined with a concurrent decrease in C-reactive protein (CRP) levels (41). Lipid lowering properties of statins remain even in the presence of drugs with an opposite effect on lipid metabolism, such as corticosteroids (50). Conducting a sufficient evaluation of cardiovascular risk in patients with inflammatory joint disease using the systematic coronary risk evaluation (SCORE) (51), 165 RA patients with a SCORE of 5% or greater, received lipid lowering therapy as part of either primary or secondary CVD protection (52). Statin treatment was adjusted, until at least two lipid targets were reached. Overall, statin intervention proved 92.1% successful in achieving lipid goals. Low doses of atorvastatin, 5 and 10 mg/day, in combination with proper control of chronic inflammation and disease activity, were also effective in significantly decreasing lipid levels in 52 RA patients diagnosed with dyslipidemia (53). Besides a reduction of TC and LDL-C levels, 10 mg of rosuvastatin treatment for 12 months resulted in significantly lower apolipoprotein B levels compared to placebo (40).
The oxidized particles of LDL-C, known as oxidized LDL (oxLDL), are recognized as crucial factors in the pathogenesis of atherosclerosis, inducing endothelial dysfunction through stimulation of monocyte infiltration and smooth cell proliferation, impairment of endothelial signaling and generation of reactive oxygen species (54). Atherogenic oxLDL are elevated in RA and correlate with disease activity (55). Interestingly, simvastatin 40 mg/day for 4 weeks led to a reduction of oxLDL/LDL ratio, suggesting a decrease in the formation of reactive oxygen species (34).
Controversial data were presented from a retrospective analysis, addressing an adverse relation between markers of inflammation and the likelihood οf achieving LDL therapeutic targets in RA subjects (56). The precise impact of lipid lowering treatment in RA-related cardiovascular risk can only be evaluated in controlled, long-term, and hard end-point trials. Especially for those subjects with low cholesterol values due to active inflammation, it remains to be determined whether lower cholesterol levels correspond to significant cardiovascular benefit. Nevertheless, up to 25% of RA patients receive suboptimal treatment according to the estimated lipid-associated CVD risk and current guidelines for the general population (17).
Primary and Secondary Prevention
While several reports highlight the improvement of various CVD markers and effectiveness in dyslipidemia management after statin administration in RA, comprehensive assessments on the statins’ potentials on CVD prevention can be obtained through controlled trials with “hard” cardiovascular end points. Aimed at assessing the hypothesis of atorvastatin’s superiority to placebo, the TRACE-RA trial (n = 2,986, median follow-up of 2.53 years) indicates a 34% reduction of major cardiovascular events in the statin group compared to placebo (57). The demonstrated benefit did not reach statistical significance [hazard ratio (HR): 0.66, 95% confidence interval (CI): 0.40–1.11, p = 0.119] likely to be secondary to the study’s premature termination due to the unexpectedly low prevalence of primary events. There was no significant difference in the time to event for the atorvastatin and placebo arms (the medians [IQRs] were 18 [12–24] months and 15 [4–32] months, respectively, p = 0.49, Mann–Whitney test).
The observation of lower lipid values during high disease activity, created a controversy regarding a possible inverse correlation between cholesterol and CVD risk in RA and, accordingly, the benefit of lipid lowering therapy on CVD risk reduction (58). The latter was examined comparing the associations between LDL-C reduction and cardiovascular outcomes in two large cohorts comparing 1,522 RA subjects to 6,511 general controls (GC) and 1,746 RA subjects to 2,554 patients with osteoarthritis, all of whom had dyslipidemia and were treated with statins for primary CVD prevention (59). Effective reduction of LDL-C was associated with a 29 and a 50% decrease in cardiovascular events in the RA/GC and RA/osteoarthritis cohorts, respectively. Despite the fact that the prevalence of traditional CVD risk factors was higher in RA patients, the decrease in cardiovascular events arising from lowering LDL-C concentrations were consistent in RA groups and their matched controls, highlighting the non-inferiority of statins in CVD management in RA.
Among 430 RA patients (181 statin-exposed and 249 statin-unexposed) without previous cardiovascular event, statins induced a 16% decrease in TC levels and were associated with a significant CVD risk reduction (adjusted HR 0.45, 95% CI: 0.20–0.98) and all-cause mortality (adjusted HR: 0.43, 95% CI: 0.20–0.92) (60). Comparable results were reported regarding the reduction of TC levels (15%) after statin intervention for secondary prevention. However, in this smaller group consisting of 78 RA patients, statins failed to offer a significant protection on CVD and all-cause mortality (HR: 0.58, 95% CI: 0.07–4.79 and 0.79, 95% CI: 0.18–3.53, respectively) (60).
Current guidelines encourage the early initiation of statins and the adoption of strict LDL-C treatment goals after the incidence of myocardial infarction (MI) (61, 62). As a result, in contrast to primary CVD prevention in RA, where a decision for statin treatment is complicated by a rather elaborate assessment of cardiovascular risk, treatment options after primary cardiovascular events could be considered more specific. However, a retrospective study indicated lower initiation of statins (HR: 0.69, CI: 0.58–0.82) following MI in 877 RA patients compared to 66,107 controls (63). Furthermore, a higher statin discontinuation was reported in the RA group. The observed unwillingness of physicians to initiate statins for post-MI treatment as well as the lower statin adherence may be attributed to a higher concern for adverse events, such as hepatotoxicity and myopathy, due to the preexisting therapy of RA patients with DMARDs combined with lower cholesterol levels compared to non-RA subjects. As no evidence exist confirming such safety concerns, the non-administration of the evidence-based medication for secondary CVD prevention seems highly irrational. Data regarding the associations between statin therapy and cardiovascular benefit in this indisputably high-risk patient group are limited.
Secondary CVD prevention, with either atorvastatin 80 mg or simvastatin 20–40 mg, was comparable across 87 patients with RA and 8,801 controls, followed for 4.8 years after MI in the Incremental Decrease in Endpoints through Aggressive Lipid lowering (IDEAL) study (64). Cardiovascular events occurred in 26.4% of RA patients and 28.7% of controls (p = 0.70), while no difference was noted considering the lipid lowering effect of treatment, although lower baseline TC and LDL-C values were recorded in the RA group. In addition, a post hoc analysis of the IDEAL trial and data provided by the Treating to New Targets study, also examined the effect of intensive statin therapy on secondary composite cardiovascular outcomes. The study reported a 20% cardiovascular risk reduction with atorvastatin 80 mg in both patients with and those without inflammatory joint disease, compared to less intensive statin treatment (65). However, despite the large number of patients (18,889 patients of whom 199 with RA, 46 with ankylosing spondylitis, and 35 with psoriatic arthritis), the decrease in cardiovascular events through intensive lipid lowering therapy was not significant for patients with inflammatory joint disease. The incidence of cardiovascular events was 37/156 and 35/124 in the intensive and non-intensive statin treatment group, respectively (HR: 0.81, 95% CI: 0.51–1.28).
The general consensus is that statin treatment in RA is not inferior regarding both the achievement of lipid targets and cardiovascular risk management, compared to the general population. To provide additional support, poor compliance in RA patients already assigned to statin treatment was associated with 67% increased risk of acute MI (HR: 1.67; 95% CI: 1.24–2.25), regardless of the timing of first statin prescription or prior CVD status (66). Last but not least, no significant difference in the frequency of adverse events has been reported in RA patients, while considerations on possible negative interactions between statins and DMARDs were not confirmed in large cohort studies (67, 68). Trials evaluating the efficacy of statins in CVD management in RA are summarized in Table 2.
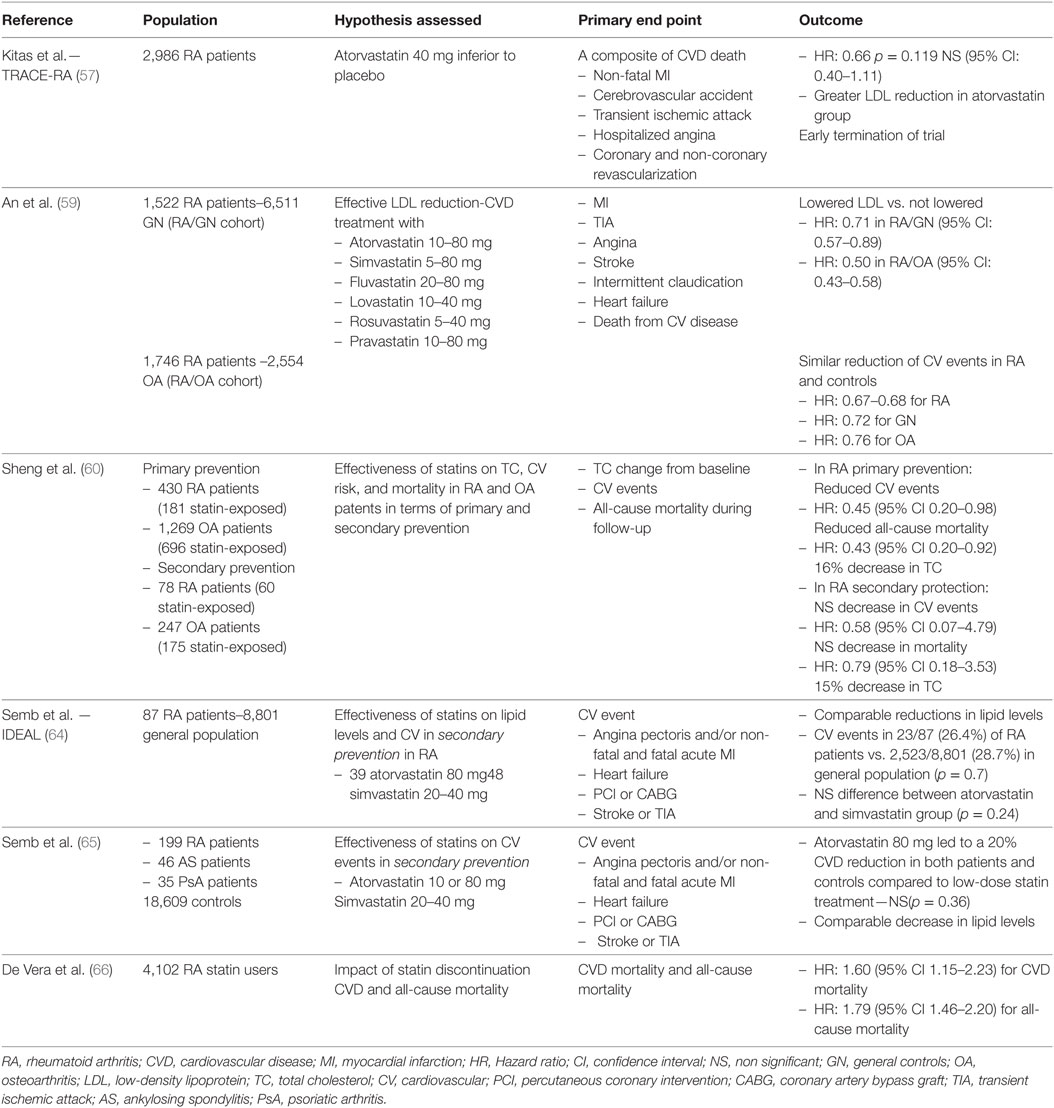
Table 2. Controlled trials on the efficacy of statins on cardiovascular risk management in rheumatoid arthritis.
Ideally, more prospective, large controlled trials investigating the impact of statins on hard cardiovascular outcomes in RA patients are needed. Ethical considerations, and the very large sample sizes and long follow-up periods required to reach statistical significance, make such trials extremely difficult. Therefore, a reevaluation and elaborate approach of existing data becomes more necessary in order to redefine the role of statins in CVD management in RA.
Disease Activity
Although in the general population the cardioprotective effect of statins is almost entirely attributed to their lipid-lowering properties, there is some evidence supporting that their therapeutic properties expand beyond these and may be particularly elevant in inflammatory states such as RA. Their so-called pleiotropic effects include anti-inflammatory, antiproliferative, antithrombotic, antioxidative, and immunomodulatory properties (69) (Figure 1). Hence, there is growing interest regarding their potential influence on the inflammatory process characterizing autoimmune diseases. Beside their administration as cardiovascular risk modifiers, attempting role as adjuvant therapy for the control of inflammation in RA has stimulated experimental and clinical research.
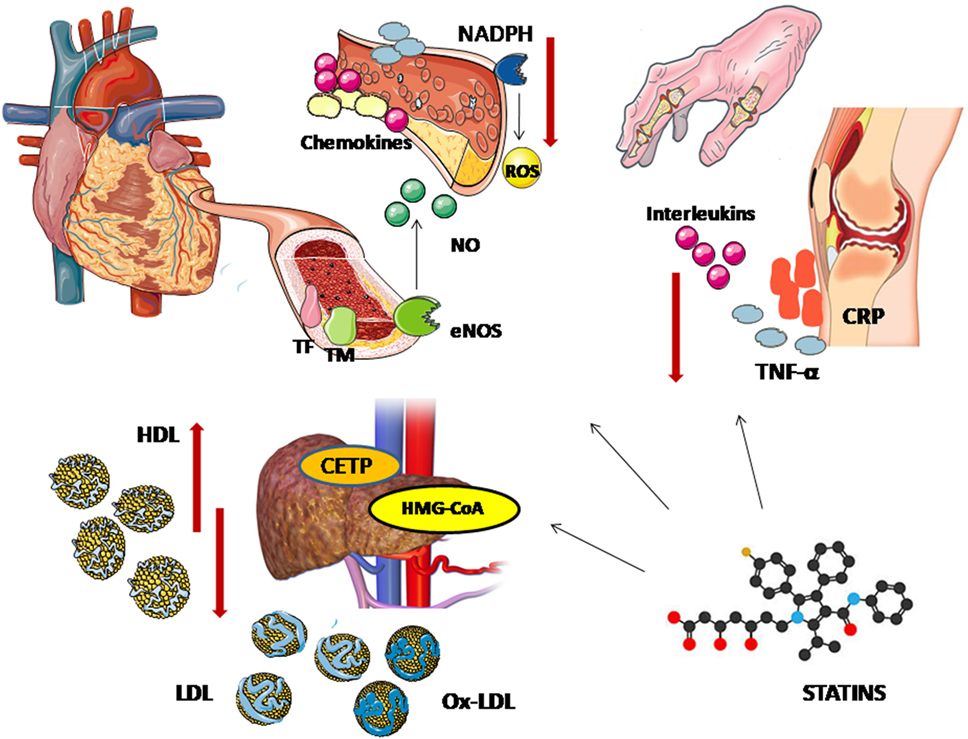
Figure 1. Pleiotropic effects of statins. The lipid lowering effects of statins is attributed to their action on 3-hydroxy-methylglutaryl coenzyme A (HMG-CoA reductase). Statins block the pathway for synthesizing cholesterol in liver by competitively inhibiting HMG-CoA reductase, the rate-controlling enzyme of the mevalonate pathway, leading to lower circulating low-density lipoprotein (LDL) cholesterol levels. A decrease in cholesteryl ester transfer protein (CETP) results in a modest increase in apolipoprotein A-I and high-density lipoprotein (HDL) cholesterol levels. Through the upregulation of endothelial nitric oxide synthase (eNOS), statins promote nitric oxide production and enhance endothelium-dependent vasodilatation. Statins also modulate the endothelial expression of cytokines, chemokines and leukocyte adhesion molecules, decreasing vascular inflammation—an important contributor to the vascular atherogenetic process. Furthermore, affecting both the endothelial production of inflammatory factors and cholesterol uptake, statins stabilize the atheromatic plaques. Their benefit on vascular function is also associated with the downregulation of nicotinamide adenine dinucleotide phosphate (NADPH) which results in lower levels of reactive oxygen species (ROS). Their antioxidative effects are also reflected on a decrease in oxidized LDL (oxLDL) levels. Statins elicit downregulation of tissue factor (TF) and overexpression of thrombomodulin, showing antithrombotic properties. Finally, the potential systematic beneficial effect of statins on systemic inflammatory diseases can be attributed to their ability to reduce inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukins, and C-reactive protein (CRP).
In a study of 118 RA patients on DMARD therapy, atorvastatin 40 mg provided a significant improvement on disease activity score 28 (DAS28) and acute-phase reactants compared with placebo after 6 months of treatment (41). Greater response rates were also recorded in the atrovastatin group versus placebo, among 111 patients receiving the oral Janus kinase inhibitor, tofacitinib (70). Mowla et al. confirmed a suppression of disease activity, as evaluated by inflammatory markers and clinical assessment after three months treatment with atovastatin 40 mg as an adjunct to current disease-modifying antirheumatic drugs (71). A recent meta-analysis of 11 relevant studies reported a standardized mean difference in DAS28 of −0.55 (95% CI: −0.83 to −0.26, p = 0.0002), with an I2 value of 68%, documenting the positive effect of statins on RA activity (72). Interestingly, statin treatment tended to be more beneficial in those cases with higher disease activity. However, the observed association is to be critically evaluated, as no adjustment for DMARD therapy was obtainable in the analysis, due to high heterogeneity of DMARD use among the included studies.
The contribution of statins to the improvement of clinical aspects of RA could be attributed to their ability to affect multiple steps in the pathogenesis of the disease. Chronic joint inflammation results from the overexpression of several inflammatory mediators, including cytokines, growth factors, adhesion molecules and T lymphocytes (73). Given that TNF-α and ILs are key factors in the process of chronic arthritis (74, 75), the ability of statins to decrease such mediators may mediate an anti-inflammatory benefit in RA (76). Simvastatin treatment has been found to decrease TNF-α levels in RA patients, accompanied by reduction of serum markers of inflammation (77). In that respect statins proved effective in diminishing CRP levels in the general population, along with a recorded suppression of inflammatory mediators, such as IL-6 (78). In line with such observations a recent meta-analysis confirmed the favorable outcome of statins on several clinical parameters of RA such as erythrocyte sedimentation rate, CRP, tender, and swollen joint count, highlighting the potential dual benefit of statins on both joint and vascular inflammation (79).
On the other hand, the reduction in CRP levels following statin treatment might not necessarily be accompanied by improvement in the overall disease status, as shown in a study evaluating the effects of 6-month rosuvastatin therapy in 50 RA patients (80). In contrast to CRP, a trend toward worsening in IL-6 levels in the rosuvastatin group in the same study suggested a rather direct effect of statins on CRP liver production rather than a global suppression of inflammation.
The impact of simvastatin on RA disease activity was evaluated in a cohort study, including 100 RA patients under DMARD treatment. With respect to recommendations for statin treatment, 50 patients were chosen to receive simvastatin 20 mg/day additionally to the existing DMARD therapy, forming the intervention group. Simvastatin treatment for 6 months resulted in a more pronounced, but not statistically significant reduction in CRP levels, DAS28, early morning stiffness and tender joint count compared to the control group. In contrast to the clinical and biological variables, the global assessment of disease activity performed by a non-blinded evaluator was the only parameter that significantly differed between the two groups after 6 months (difference between groups: −9.54; 95% CI: −15.913 to −3.184; p = 0.007) (81).
Most of the observations concerning the anti-inflammatory effects of statins were made on study populations already receiving a multitude of disease suppressing drugs, so any impact on systemic inflammation cannot be attributed solely to a statin effect. Although some of the evidence points to a potential anti-inflammatory statin effect in RA, this “additional” effect has not been consistent or accurately quantified and its clinical significance (in the context of high-grade rheumatic inflammation) remains undetermined. However, the lack of significant adverse events in RA patient cohorts receiving several other potentially hepatotoxic medications is reassuring and their significant clinical effect at reducing cardiovascular risk make them an attractive choice in suitable patients.
Conclusion
Optimal cardiovascular risk management remains a challenging theme in RA. Inflammation and primary atherosclerosis are highly associated and cannot be treated as independent factors. Statins have a beneficial impact on RA-related cardiovascular risk, due to their lipid lowering but also possibly angioprotective, antioxidative and anti-inflammatory effects. This, and their safety profile, makes them an ideal choice in the overall armamentarium aimed at complete control of RA disease and associated vascular morbidity. Despite this, the blanket use of statins on all RA patients cannot be supported by evidence, thus appropriate CV risk assessment tools for this population are necessary, which in addition to established risk factors could include non-invasive vascular assessments, particularly carotid IMT (82). Whether any anti-inflammatory statin effect is clinically important in the context of RA activity outcomes is intriguing and needs to be further evaluated in studies designed specifically for this purpose.
Author Contributions
SS: literature search and writing of the manuscript. EN: design and revision of the manuscript. TD: literature search, writing, and revision of the manuscript. GK: design and final revision of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol (2015) 11:693–704. doi:10.1038/nrrheum.2015.112
2. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis (2012) 71:1524–9. doi:10.1136/annrheumdis-2011-200726
3. Toms TE, Panoulas VF, Kitas GD. Dyslipidaemia in rheumatological autoimmune diseases. Open Cardiovasc Med J (2011) 5:64–75. doi:10.2174/1874192401105010064
4. Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Douglas KMJ, Nevill AM, Jamurtas AZ, et al. Associations of obesity with modifiable risk factors for the development of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis (2009) 68:242–5. doi:10.1136/ard.2008.095596
5. Panoulas VF, Metsios GS, Pace AV, John H, Treharne GJ, Banks MJ, et al. Hypertension in rheumatoid arthritis. Rheumatology (2008) 47:1286–98. doi:10.1093/rheumatology/ken159
6. Fenton SAM, Kitas GD. Rheumatoid arthritis: sedentary behaviour in RA – a new research agenda. Nat Rev Rheumatol (2016) 12:698–700. doi:10.1038/nrrheum.2016.179
7. Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, Sandoo A, Toms TE, Nevill AM, et al. Rheumatoid cachexia and cardiovascular disease. Clin Exp Rheumatol (2009) 27:985–8.
8. Sandoo A, Dimitroulas T, Hodson J, Smith JP, Douglas KM, Kitas GD. Cumulative inflammation associates with asymmetric dimethylarginine in rheumatoid arthritis: a 6 year follow-up study. Rheumatology (Oxford) (2015) 54:1145–52. doi:10.1093/rheumatology/keu349
9. Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis (2011) 70:8–14. doi:10.1136/ard.2010.142133
10. Peters MJL, Symmons DPM, McCarey D, Dijkmans BAC, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis (2010) 69:325–31. doi:10.1136/ard.2009.113696
11. Hasson CJ, Caldwell GE, Van Emmerik REA. Changes in muscle and joint coordination in learning to direct forces. Hum Mov Sci (2009) 27:590–609. doi:10.1016/j.humov.2008.02.015
12. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet (2004) 364:937–52. doi:10.1016/S0140-6736(04)17018-9
13. Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments. J Am Coll Cardiol (2008) 52:1769–81. doi:10.1016/j.jacc.2008.08.039
14. Semb AG, Rollefstad S, van Riel P, Kitas GD, Matteson EL, Gabriel SE. Cardiovascular disease assessment in rheumatoid arthritis: a guide to translating knowledge of cardiovascular risk into clinical practice. Ann Rheum Dis (2014) 73:1284–8. doi:10.1136/annrheumdis-2013-204792
15. Crowson CS, Rollefstad S, Ikdahl E, Kitas GD, van Riel PLCM, Gabriel SE, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis (2017) 77:48–54. doi:10.1136/annrheumdis-2017-211735
16. Toms TE, Symmons DP, Kitas GD. Dyslipidaemia in rheumatoid arthritis: the role of inflammation, drugs, lifestyle and genetic factors. Curr Vasc Pharmacol (2010) 8:301–26. doi:10.2174/1570209197581151611
17. Toms TE, Panoulas VF, Douglas KMJ, Griffiths H, Sattar N, Smith JP, et al. Statin use in rheumatoid arthritis in relation to actual cardiovascular risk: evidence for substantial undertreatment of lipid-associated cardiovascular risk? Ann Rheum Dis (2010) 69:683–8. doi:10.1136/ard.2009.115717
18. Paraskevas KI. Statin treatment for rheumatoid arthritis: a promising novel indication. Clin Rheumatol (2008) 27:281–7. doi:10.1007/s10067-007-0806-8
19. Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int (2011) 31:1409–17. doi:10.1007/s00296-011-1999-3
20. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet (2012) 380:581–90. doi:10.1016/S0140-6736(12)60367-5
21. Minder CM, Blumenthal RS, Blaha MJ. Statins for primary prevention of cardiovascular disease. Curr Opin Cardiol (2013) 28:554–60. doi:10.1097/HCO.0b013e32836429e6
22. Gazi IF, Boumpas DT, Mikhailidis DP, Ganotakis ES. Clustering of cardiovascular risk factors in rheumatoid arthritis: the rationale for using statins. Clin Exp Rheumatol (2007) 25:102–11.
23. Banach M, Serban C, Sahebkar A, Mikhailidis DP, Ursoniu S, Ray KK, et al. Impact of statin therapy on coronary plaque composition: a systematic review and meta-analysis of virtual histology intravascular ultrasound studies. BMC Med (2015) 13:229. doi:10.1186/s12916-015-0459-4
24. Rosenson RS. Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis (2004) 173:1–12. doi:10.1016/S0021-9150(03)00239-9
25. Peters MJL, Voskuyl AE, Sattar N, Dijkmans BAC, Smulders YM, Nurmohamed MT. The interplay between inflammation, lipids and cardiovascular risk in rheumatoid arthritis: why ratios may be better. Int J Clin Pract (2010) 64:1440–3. doi:10.1111/j.1742-1241.2009.02220.x
26. Sheng X, Murphy MJ, MacDonald TM, Wei L. The comparative effectiveness of statin therapy in selected chronic diseases compared with the remaining population. BMC Public Health (2012) 12:712. doi:10.1186/1471-2458-12-712
27. Liao KP, Playford MP, Frits M, Coblyn JS, Iannaccone C, Weinblatt ME, et al. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc (2015) 4:1–8. doi:10.1161/JAHA.114.001588
28. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the international brachial artery reactivity task force. J Am Coll Cardiol (2002) 39:257–65. doi:10.1016/S0735-1097(01)01746-6
29. Sandoo A, Hodson J, Douglas KM, Smith JP, Kitas GD. The association between functional and morphological assessments of endothelial function in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther (2013) 15:R107. doi:10.1186/ar4287
30. Sandoo A, Chanchlani N, Hodson J, Smith JP, Douglas KM, Kitas GD. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: a six-year prospective study. Arthritis Res Ther (2013) 15:R203. doi:10.1186/ar4396
31. McEniery CM, Wallace S, MacKenzie IS, McDonnell B, Yasmin , Newby DE, et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension (2006) 48:602–8. doi:10.1161/01.HYP.0000239206.64270.5f
32. Sandoo A. Important considerations for examining endothelial dysfunction in rheumatoid arthritis. Meditter J Rheumatol (2017) 28:14–7.
33. Sandoo A, Veldhuijzen van Zanten JJCS, Metsios GS, Carroll D, Kitas GD. Vascular function and morphology in rheumatoid arthritis: a systematic review. Rheumatology (2011) 50:2125–39. doi:10.1093/rheumatology/ker275
34. Hermann F, Forster A, Chenevard R, Enseleit F, Hürlimann D, Corti R, et al. Simvastatin improves endothelial function in patients with rheumatoid arthritis. J Am Coll Cardiol (2005) 45:461–4. doi:10.1016/j.jacc.2004.11.006
35. Maki-Petaja KM, Booth AD, Hall FC, Wallace SML, Brown J, McEniery CM, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol (2007) 50:852–8. doi:10.1016/j.jacc.2007.04.076
36. Van Doornum S, McColl G, Wicks IP. Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis. Ann Rheum Dis (2004) 63:1571–5. doi:10.1136/ard.2003.018333
37. El-Barbary AM, Hussein MS, Rageh EM, Hamouda HE, Wagih AA, Ismail RG. Effect of atorvastatin on inflammation and modification of vascular risk factors in rheumatoid arthritis. J Rheumatol (2011) 38:229–35. doi:10.3899/jrheum.100582
38. Rollefstad S, Ikdahl E, Hisdal J, Olsen IC, Holme I, Hammer HB, et al. Rosuvastatin-induced carotid plaque regression in patients with inflammatory joint diseases: the rosuvastatin in rheumatoid arthritis, ankylosing spondylitis and other inflammatory joint diseases study. Arthritis Rheumatol (2015) 67:1718–28. doi:10.1002/art.39114
39. Ikdahl E, Rollefstad S, Hisdal J, Olsen IC, Pedersen TR, Kvien TK, et al. Sustained improvement of arterial stiffness and blood pressure after long-term rosuvastatin treatment in patients with inflammatory joint diseases: results from the RORA-AS study. PLoS One (2016) 11:e0153440. doi:10.1371/journal.pone.0153440
40. Tam L-S, Li EK, Shang Q, Tomlinson B, Lee VW, Lee KK, et al. Effects of rosuvastatin on subclinical atherosclerosis and arterial stiffness in rheumatoid arthritis: a randomized controlled pilot trial. Scand J Rheumatol (2011) 40:411–21. doi:10.3109/03009742.2011.586649
41. McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, et al. Trial of atorvastatin in rheumatoid arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet (2004) 363:2015–21. doi:10.1016/S0140-6736(04)16449-0
42. Steiner G, Urowitz MB. Lipid profiles in patients with rheumatoid arthritis: mechanisms and the impact of treatment. Semin Arthritis Rheum (2009) 38:372–81. doi:10.1016/j.semarthrit.2008.01.015
43. Park YB, Lee SK, Lee WK, Suh CH, Lee CW, Lee CH, et al. Lipid profiles in untreated patients with rheumatoid arthritis. J Rheumatol (1999) 26:1701–4.
44. Toms TE, Smith JP, Panoulas VF, Blackmore H, Douglas KMJ, Kitas GD. Apolipoprotein E gene polymorphisms are strong predictors of inflammation and dyslipidemia in rheumatoid arthritis. J Rheumatol (2012) 39:218–25. doi:10.3899/jrheum.110683
45. Georgiadis AN, Papavasiliou EC, Lourida ES, Alamanos Y, Kostara C, Tselepis AD, et al. Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: effect of early treatment – a prospective, controlled study. Arthritis Res Ther (2006) 8:R82. doi:10.1186/ar1952
46. Daïen CI, Duny Y, Barnetche T, Daurès J-P, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis (2012) 71:862–8. doi:10.1136/annrheumdis-2011-201148
47. Kim SC, Solomon DH, Rogers JR, Gale S, Klearman M, Sarsour K, et al. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi-database cohort study. Arthritis Rheumatol (2017) 69:1154–64. doi:10.1002/art.40084
48. Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet (2008) 371:987–97. doi:10.1016/S0140-6736(08)60453-5
49. Soubrier M, Pei J, Durand F, Gullestad L, John A. Concomitant use of statins in tocilizumab-treated patients with rheumatoid arthritis: a post hoc analysis. Rheumatol Ther (2017) 4:133–49. doi:10.1007/s40744-016-0049-8
50. Okamoto H, Koizumi K, Kamitsuji S, Inoue E, Hara M, Tomatsu T, et al. Beneficial action of statins in patients with rheumatoid arthritis in a large observational cohort. J Rheumatol (2007) 34:964–8.
51. Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J (2003) 24:987–1003. doi:10.1016/S0195-668X(03)00114-3
52. Rollefstad S, Kvien TK, Holme I, Eirheim AS, Pedersen TR, Semb AGP. Treatment to lipid targets in patients with inflammatory joint diseases in a preventive cardio-rheuma clinic. Ann Rheum Dis (2013) 72:1968–74. doi:10.1136/annrheumdis-2012-202789
53. Akiyama M, Mawatari T, Nakashima Y, Miyahara H, Yamada H, Okazaki K, et al. Prevalence of dyslipidemia in Japanese patients with rheumatoid arthritis and effects of atorvastatin treatment. Clin Rheumatol (2015) 34:1867–75. doi:10.1007/s10067-015-3049-0
54. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res (2000) 87:840–4. doi:10.1161/01.RES.87.10.840
55. Nowak B, Madej M, Łuczak A, Małecki R, Wiland P. Disease activity, oxidized-LDL fraction and anti-oxidized LDL antibodies influence cardiovascular risk in rheumatoid arthritis. Adv Clin Exp Med (2016) 25:43–50. doi:10.17219/acem/29847
56. Myasoedova E, Gabriel SE, Green AB, Matteson EL, Crowson CS. Impact of statin use on lipid levels in statin-naive patients with rheumatoid arthritis versus non-rheumatoid arthritis subjects: results from a population-based study. Arthritis Care Res (Hoboken) (2013) 65:1592–9. doi:10.1002/acr.22029
57. Kitas GD, Nightingale P, Armitage J, Sattar N; Trace RA Consortium, Belch J, et al. SAT0105 trial of atorvastatin for the primary prevention of cardiovascular events in patients with rheumatoid arthritis (TRACE RA). Ann Rheum Dis (2015) 74:688. doi:10.1136/annrheumdis-2015-eular.3071
58. Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-gibbon PD, Therneau TM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis (2012) 70:482–7. doi:10.1136/ard.2010.135871.LIPID
59. An JJ, Alemao E, Reynolds K, Kawabata H, Solomon DH, Liao KP, et al. Cardiovascular outcomes associated with lowering low-density lipoprotein cholesterol in rheumatoid arthritis and matched nonrheumatoid arthritis. J Rheumatol (2016) 43:1989–96. doi:10.3899/jrheum.160110
60. Sheng X, Murphy MJ, MacDonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol (2012) 39:32–40. doi:10.3899/jrheum.110318
61. O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, De Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol (2013) 61:78–140. doi:10.1016/j.jacc.2012.11.019
62. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation (2014) 130:e344–426. doi:10.1161/CIR.0000000000000134
63. Lindhardsen J, Ahlehoff O, Gislason GH, Madsen OR, Olesen JB, Torp-Pedersen C, et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis (2012) 71:1496–501. doi:10.1136/annrheumdis-2011-200806
64. Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: an explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology (2011) 50:324–9. doi:10.1093/rheumatology/keq295
65. Semb AG, Kvien TK, Demicco DA, Fayyad R, Wun CC, Larosa JC, et al. Effect of intensive lipid-lowering therapy on cardiovascular outcome in patients with and those without inflammatory joint disease. Arthritis Rheum (2012) 64:2836–46. doi:10.1002/art.34524
66. De Vera MA, Choi H, Abrahamowicz M, Kopec J, Lacaille D. Impact of statin discontinuation on mortality in patients with rheumatoid arthritis: a population-based study. Arthritis Care Res (2012) 64:809–16. doi:10.1002/acr.21643
67. Arts EEA, Jansen TL, Den Broeder A, Vonkeman HE, Dutmer E, Van de Laar MAFJ, et al. Statins inhibit the antirheumatic effects of rituximab in rheumatoid arthritis: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Ann Rheum Dis (2011) 70:877–8. doi:10.1136/ard.2010.136093
68. Lehane PB, Lacey S, Hessey EW, Jahreis A. Effect of concomitant statins on rituximab efficacy in patients with rheumatoid arthritis. Ann Rheum Dis (2014) 73:1906–8. doi:10.1136/annrheumdis-2014-205474
69. Bedi O, Dhawan V, Sharma PL, Kumar P. Pleiotropic effects of statins: new therapeutic targets in drug design. Naunyn Schmiedebergs Arch Pharmacol (2016) 389:695–712. doi:10.1007/s00210-016-1252-4
70. McInnes IB, Kim H-Y, Lee S-H, Mandel D, Song Y-W, Connell CA, et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study. Ann Rheum Dis (2014) 73:124–31. doi:10.1136/annrheumdis-2012-202442
71. Mowla K, Rajai E, Ghorbani A, Dargahi-Malamir M, Bahadoram M, Mohammadi S. Effect of atorvastatin on the disease activity and severity of rheumatoid arthritis: double-blind randomized controlled trial. J Clin Diagn Res (2016) 10:32–6. doi:10.7860/JCDR/2016/16538.7814
72. Xing B, Yin Y-F, Zhao L-D, Wang L, Zheng W-J, Chen H, et al. Effect of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor on disease activity in patients with rheumatoid arthritis: a meta-analysis. Medicine (Baltimore) (2015) 94:e572. doi:10.1097/MD.0000000000000572
73. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol (2007) 7:429–42. doi:10.1038/nri2094
74. Matsuno H, Yudoh K, Katayama R, Nakazawa F, Uzuki M, Sawai T, et al. The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): a study using a human RA/SCID mouse chimera. Rheumatology (Oxford) (2002) 41:329–37. doi:10.1093/rheumatology/41.3.329
75. Burska A, Boissinot M, Ponchel F. Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm (2014) 2014:545493. doi:10.1155/2014/545493
76. Pereira MC, Cardoso PRG, Da Rocha LF, Rêgo MJBM, Gonçalves SMC, Santos FA, et al. Simvastatin inhibits cytokines in a dose response in patients with rheumatoid arthritis. Inflamm Res (2014) 63:309–15. doi:10.1007/s00011-013-0702-4
77. Tikiz C, Utuk O, Pirildar T, Bayturan O, Bayindir P, Taneli F, et al. Effects of angiotensin-converting enzyme inhibition and statin treatment on inflammatory markers and endothelial functions in patients with long term rheumatoid arthritis. J Rheumatol (2005) 32:2095–101.
78. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet (2009) 373:1175–82. doi:10.1016/S0140-6736(09)60447-5
79. Lv S, Liu Y, Zou Z, Li F, Zhao S, Shi R, et al. The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol (2015) 33:69–76.
80. Kumar P, Kennedy G, Khan F, Pullar T, Belch JJF. Rosuvastatin might have an effect on C-reactive protein but not on rheumatoid disease activity: Tayside randomized controlled study. Scott Med J (2012) 57:80–3. doi:10.1258/smj.2012.012004
81. Cojocaru L, Rusali AC, Şuţa C, Radulescu AM, Şuţa M, Craiu E. The role of simvastatin in the therapeutic approach of rheumatoid arthritis. Autoimmune Dis (2013) 2013:326258. doi:10.1155/2013/326258
82. Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJL, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis (2017) 76:17–28. doi:10.1136/annrheumdis-2016-209775
Keywords: statins, rheumatoid arthritis, cardiovascular risk, lipid lowering action, anti-inflammatory effect
Citation: Soulaidopoulos S, Nikiphorou E, Dimitroulas T and Kitas GD (2018) The Role of Statins in Disease Modification and Cardiovascular Risk in Rheumatoid Arthritis. Front. Med. 5:24. doi: 10.3389/fmed.2018.00024
Received: 22 October 2017; Accepted: 24 January 2018;
Published: 08 February 2018
Edited by:
Kayo Masuko, Sanno Medical Center, JapanReviewed by:
Ioannis Parodis, Karolinska Institutet (KI), SwedenNicolai Leuchten, Technical University Dresden, Germany
Copyright: © 2018 Soulaidopoulos, Nikiphorou, Dimitroulas and Kitas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stergios Soulaidopoulos, c291bGFpZG9wb3Vsb3NAaG90bWFpbC5jb20=
 Stergios Soulaidopoulos
Stergios Soulaidopoulos Elena Nikiphorou
Elena Nikiphorou Theodoros Dimitroulas
Theodoros Dimitroulas George D. Kitas
George D. Kitas