
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 22 January 2018
Sec. Nephrology
Volume 4 - 2017 | https://doi.org/10.3389/fmed.2017.00256
Sleep apnea (SA), a condition associated with increased cardiovascular risk, has been traditionally associated with obesity and aging. However, in patients with fluid-retaining states, such as congestive heart failure and end-stage renal disease, both prevalence and severity of SA are increased. Recently, fluid shift has been recognized to play an important role in the pathophysiology of SA, since the fluid retained in the legs during the day shifts rostrally while recumbent, leading to edema of upper airways. Such simple physics, observed even in healthy individuals, has great impact in patients with fluid overload. Correction of the excess fluid volume has risen as a potential target therapy to improve SA, by attenuation of nocturnal fluid shift. Such strategy has gained special attention, since the standard treatment for SA, the positive airway pressure, has low compliance rates among its users and has failed to reduce cardiovascular outcomes. This review focuses on the pathophysiology of edema and fluid shift, and summarizes the most relevant findings of studies that investigated the impact of treating volume overload on SA. We aim to expand horizons in the treatment of SA by calling attention to a potentially reversible condition, which is commonly underestimated in clinical practice.
Sleep apnea (SA) is a condition characterized by repeated episodes of complete or partial airflow cessation during sleep, typically referred as apnea and hypopnea. Individuals with SA usually present witnessed episodes of snoring, choking, and are more likely to suffer from daytime sleepiness (1), depression (2, 3) and are at increased risk of motor vehicle crash (4), and occupational accidents (5). Other important adverse consequences of SA include neuropsychiatric disorders, such as cognitive impairment (6), abnormal sympathetic activity (7), and cardiovascular abnormalities such as hypertension (8), stroke, and arterial obstruction (9).
The apnea–hypopnea index (AHI), defined as the total number of episodes of apnea and hypopnea per hour of sleep, is routinely used to diagnose SA and to classify it as mild (AHI between 5 and 15), moderate (15–30), or severe (>30) (10). The prevalence of AHI > 5 is 9% in women and 24% in men in the general population (1), not taking into account the presence of symptoms. Nevertheless, the prevalence of SA increases over time, since obesity, one of the most important risk factors, has increased in general population. More recent data suggest that more than 20% of adults have mild SA and up to 7% have moderate or severe SA (11).
Even though aging and obesity are clearly the most relevant associated risk factors, the prevalence of SA is much higher among patients with edematous states, such as end-stage renal disease (ESRD) (12) and congestive heart failure (CHF) (13). Hypervolemia and overnight rostral fluid shift from the legs are the likely cause of the high frequency of SA in edematous states, as indicated by several recent studies of ESRD (14), CHF (13), and nephrotic syndrome (15) (Table 1).
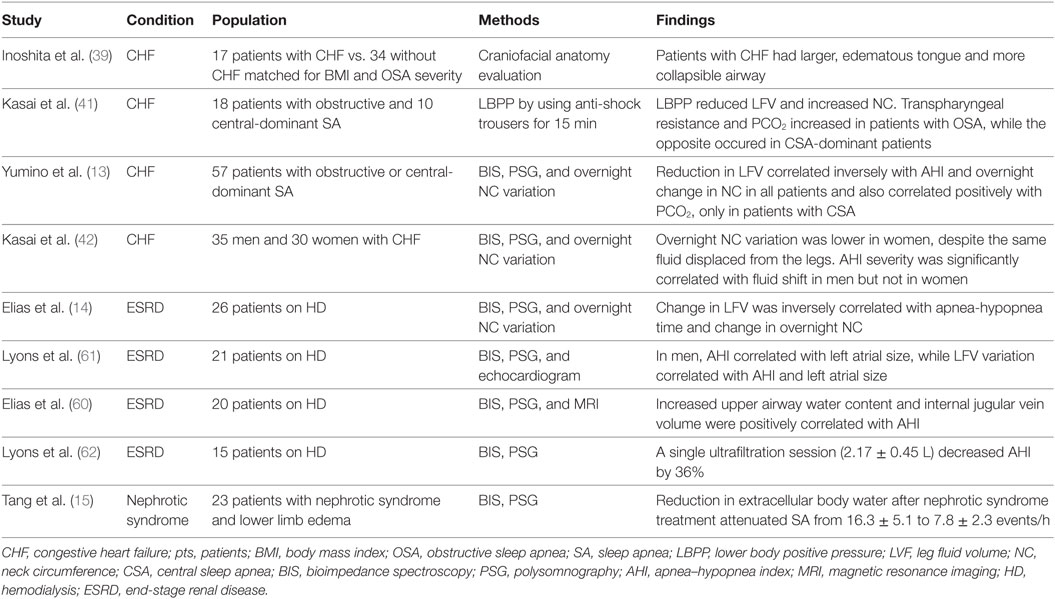
Table 1. Studies on the relationship between volume overload and SA that have included patients with fluid-retaining states.
Despite its high prevalence in edematous patients, SA is often overlooked because of its oligosymptomatic nature (16, 17). Even when SA is adequately diagnosed by polysomnography, management of this condition is of great concern, since the gold standard treatment, the use of continuous positive airway pressure (CPAP) (18–21), presents low compliance rates (22, 23).
Although prevention of fluid accumulation is a plausible alternative strategy to alleviate SA in edematous patients, current guidelines do not include treatment of edema as part of the therapeutic effort against this condition. In this review, we discuss the impact of edema on the pathogenesis of SA in patients with CHF, ESRD and nephrotic syndrome, as well as the corresponding implications for innovative therapeutic strategies.
Edema is defined as an abnormal buildup of fluid anywhere in the body. When utilized with no qualifier, the term “edema” usually refers to the accumulation of plasma transudate in the interstitial space, as in CHF, nephrotic syndrome, and hepatic cirrhosis (24–26).
For fluid to accumulate at the interstitial space, a positive sodium balance must establish. Since the kidneys are ultimately responsible for maintaining sodium balance, it follows that edema formation always demands some degree of renal sodium retention. Nevertheless, impaired sodium excretion is insufficient to ensure fluid accumulation. For instance, in primary hyperaldosteronism, excess sodium reabsorption by the distal nephron translates into hypertension, rather than edema formation. To reach the interstitial space, fluid retained by the kidneys must be driven by an imbalance of Starling forces at the complex interface between the intravascular and interstitial compartments (27).
Under normal conditions, small amounts of fluid do reach the interstitial compartment due to a slight predominance of hydrostatic over oncotic forces. Actually, the normal interstitium contains about 10 L of fluid, an amount kept within narrow limits by three mechanisms (27): the action of lymphatic capillaries, carrying extravasated fluid back to the circulation; the dilution of interstitial protein that results from transcapillary fluid passage; and the tight disposition of the protein molecules that constitute the interstitial matrix—due to this arrangement, substantial elevation of local hydraulic pressure is required to accommodate even small amounts of extra fluid (low interstitial compliance).
An important consequence of these physical characteristics of the normal interstitial matrix is that fluid cannot move freely across the interstitium following gravity and, therefore, will not accumulate in the lower limbs while standing, or in the cervical region after several hours in the recumbent position.
Although the classical view centered on Starling forces still predominates, recent evidence suggests that this theory should be revised taking into account the segmentation of the capillary wall and the adsorption of ions by interstitial macromolecules (28, 29). An overview of the mechanisms evolved in edema formation is summarized in Figure 1.
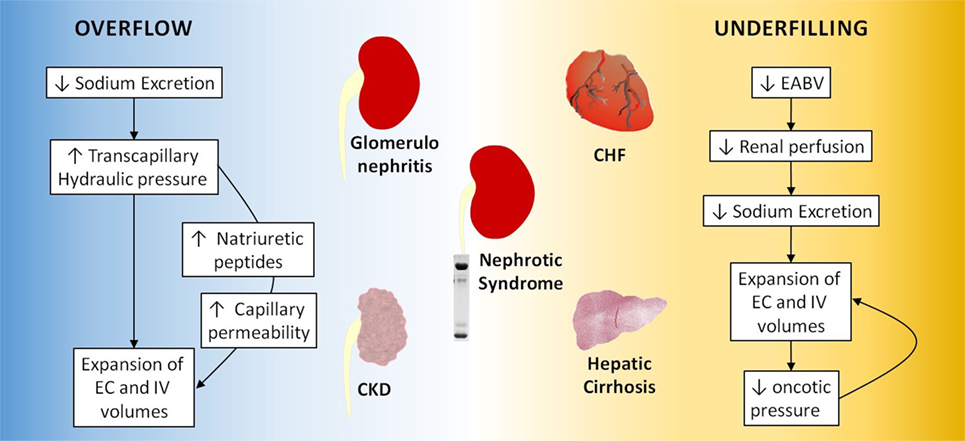
Figure 1. Overflow and underfilling mechanisms in edematous states. CKD, chronic kidney disease; EC, extracellular; IV, intravascular; CHF, congestive heart failure.
When sodium excretion is hindered by intrinsic renal disease, a positive sodium balance establishes, leading to expansion of the extracellular (EC) and intravascular volumes. If Starling equilibrium is not disrupted, sodium retention will cause hypertension, according to Guyton’s theory (27), but not edema. However, if tissue autoregulation fails, capillary hydraulic pressure will rise, and the resulting imbalance of Starling forces will lead to fluid extravasation. This mechanism of fluid retention, known as overflow (also overfill), operates in primary renal disease, such as glomerulonephritis (30) and advanced chronic kidney disease (CKD). As EC volume is expanded, mechanisms that increase sodium excretion are triggered, counteracting the renal limitation and allowing a new sodium balance to be reached. For this reason, edemas accumulated by overflow are relatively modest and confined to the lower limbs and eyelids.
In patients on chronic dialysis, maintenance of fluid balance is entirely dependent on an artificial procedure. Therefore, development of edema in this context usually results from insufficient fluid removal (31) or poor adherence to treatment. Thus, the mechanism of edema formation in these patients can be considered as analogous to overflow.
In a number of situations, effective arterial blood volume (EABV), hence renal perfusion, cannot be maintained despite normal or even increased total blood volume. In this context, the kidneys (assumed to be normal) react to the reduction of EABV by retaining sodium and water, which nevertheless escape the intravascular space because of a disequilibrium of Starling forces, promoting further sodium retention. In this manner, the retained fluid tends to accumulate at the interstitial compartment, instead of recomposing the EABV (32).
This mechanism of edema formation resulting from chronic reduction of the EABV is known as underfilling. Unlike what happens with overflow, here renal dysfunction is not the primary cause of sodium retention. Rather, the kidneys act as expected, responding to hypoperfusion by reabsorbing as much sodium as possible. Underfilling is central to the pathogenesis of edema in CHF, hepatic cirrhosis and some cases of nephrotic syndrome.
Under normal conditions, the heart easily meets the needs of all tissues, keeping cardiac output at physiological levels. In CHF, the weakened myocardium can no longer maintain adequate perfusion of the peripheral territories, including the renal circulation. The consequent fall of EABV stimulates the kidneys to retain sodium. On the other hand, the malfunctioning pump leads to venous damming of blood. Retrograde transmission of the resulting venous hypertension to the capillaries promotes the passage of fluid to the interstitial space. This process is continuously fueled by the renal retention of sodium, which nevertheless fails to restore the EABV. Therefore, the basic mechanism of edema formation in CHF is underfillling (33).
About one-third of patients with nephrotic syndrome exhibit clear signs of hypovolemia despite massive EC fluid expansion. In these patients, edema formation is believed to result from hypoalbuminemia, hence decreased systemic oncotic pressure, leading to an imbalance of starling forces, fluid displacement to the interstitium, EABV reduction, and incessant renal sodium retention. This sequence is fully compatible with the concept of underfilling (25, 34). However, two-thirds of nephrotic patients exhibit clear clinical evidence of fluid overload. It is believed that, in these patients, the basic event is primary sodium retention by the kidneys, with hypoalbuminemia facilitating ultrafiltration through the capillary walls, so that the magnitude of swelling is much higher than in the nephritic syndrome. Therefore, the basic mechanism of edema formation in most cases of nephrotic syndrome is overflow, facilitated by the simultaneous decrease in plasma oncotic pressure (2, 12).
If fluid escape into the interstitium persists, the initially slow accumulation of edema raises gradually the local hydraulic pressure, until it becomes positive. When this happens, the normally tight architecture of the interstitium is disrupted, leading to an abrupt increase of compliance, enabling the interstitium to accommodate increasing amounts of fluid with a small rise of hydraulic pressure. The shift of fluid throughout the interstitium is no longer restricted, being now governed by gravity: during daytime, edema accumulates in the lower limbs; at night, interstitial fluid tends to be redistributed rostrally, reaching the cervical region. These movements largely explain the occurrence of airway obstruction and SA in edematous states.
Sleep apnea can be classified as obstructive sleep apnea (OSA), associated with airway obstruction and, therefore, respiratory effort, or central sleep apnea (CSA), in which the main pathogenic factor is respiratory center instability. The most common sleep disorder is OSA. CSA is far less common although equally as dangerous as OSA.
Both SA modalities are more prevalent in patients with CHF, compared to the general population (1, 35–37), especially in the case of CSA, which affects 21–40% of CHF patients, as compared to less than 1% of the general population (38). In CHF patients, fluid retention, and in particular fluid shift can cause not only upper airway obstruction by local fluid accumulation, but also pulmonary congestion. Despite their different pathogeneses, OSA and CSA can occur simultaneously in patients with CHF. Actually, fluid shift can participate in both SA types and fluid overload can explain the higher prevalence of both OSA and CSA in patients with CHF (13).
In CHF, fluids displaced from the lower body during the night can accumulate at cervical and head areas, thus promoting upper airway obstruction and OSA. It has been postulated that systemic fluid retention, with consequent venous engorgement and mucosal fluid accumulation, can increase tongue volume, facilitating airway obstruction (39). Of note, fluid accumulation in the neck, causing mucosal edema and OSA, was seen in healthy men after IV saline infusion during sleep (40).
In men with CHF and OSA, Kasai and colleagues showed that application of lower body positive pressure (LBPP) in the awake state, thus forcing rostral fluid shift, was accompanied by a significant increase in neck circumference and an increase in upper airway resistance in proportion to the volume of fluid displaced from the legs (41). Interestingly, the relationship between rostral fluid shift and OSA in CHF is less pronounced in women (13, 42).
In CHF patients, fluid retention and fluid shift from the legs can also lead to pulmonary congestion. In this case, however, SA is unrelated to obstruction. Rather, it seems to result from a central respiratory mechanism, thus conforming to the CSA type.
The mechanism underlying the establishment of CSA in these patients has not been fully elucidated, although pulmonary congestion, increased central and peripheral chemosensitivity, and frequent arousals may play a role (43, 44). Pulmonary congestion, a common finding in CHF, can stimulate so-called pulmonary vagal irritant “J” receptors (45), causing reflex inhibition of the respiratory drive through afferent C fibers. The consequent apnea causes PaCO2 to increase, now leading to hyperventilation and generating a Cheynes–Stokes-like pattern (35, 37, 46). In consistency with this concept, PaCO2 in CHF is inversely proportional to pulmonary capillary wedge pressure (47), which is an index of pulmonary congestion (48).
Dietary sodium intake can be associated with the severity of both OSA and CSA in CHF patients, with increased sodium intake presumably resulting in worsening of edema around the upper airway (OSA) and/or pulmonary congestion and CSA, through the mechanisms discussed earlier. Increased leg fluid retention, and consequently nocturnal overnight rostral fluid shift, can also be favored by excessive sodium intake (49).
The presentation of SA in patients with ESRD is quite distinct from that in the general population. First, the typical history of loud snoring and witnessed apnea during sleep is seldom obtained. Second, the association with age, gender, and body mass index is less clearcut (50). Third, even classical symptoms such as daytime sleepiness are infrequent and dissociated from the severity of SA (51). Together, these atypical clinical characteristics can render the diagnosis of SA quite difficult in ESRD patients.
Sleep apnea can exert a high impact on CKD mortality (52), given is very high prevalence (up to 80%) among these patients (14, 53, 54), and its well-known association with cardiovascular events (8, 55, 56). Therefore, recognizing SA in this population is imperative.
Uremia has been implicated as a possible cause of SA (57, 58). This concept, based on anecdotal reports of symptom improvement following renal transplantation, have been disputed (59). It must be noted that, even if these observations were confirmed by large clinical trials, interpretation would be problematic, given the plethora of factors that can be ameliorated after kidney transplantation.
Rostral fluid shift may exert a similar influence in CKD as in CHF. In a study of 26 patients on conventional hemodialysis, Elias and coworkers (14) showed that SA, present in 46.1% of subjects, was associated with age, male gender, and time spent in the sitting position during the day. Rostral fluid shift correlated significantly with the severity of SA and with the overnight increase of neck circumference. In a related study, fluid shift was shown to correlate with the increase of internal jugular vein volume, mucosal water content, and AHI (60). Likewise, Lyons et al. (61) showed a correlation between the magnitude of rostral fluid shift and the severity of both OSA and left atrial size in 40 patients on conventional hemodialysis, reinforcing the view that fluid shift may have an impact on both OSA and cardiac dysfunction in ESRD. The proof of concept that fluid overload can impact in the severity of OSA in patient on dialysis was demonstrated by Lyons and coworkers, who showed that AHI fell by 36% after removal of an average of 2.2 L by ultrafiltration alone in ESRD patients (62).
The importance of fluid retention in the pathogenesis of SA is not restricted to CKD and ESRD. In patients with nephrotic syndrome, even with normal renal function, the treatment of hypervolemia, with contraction of EC volume and disappearance of lower limb edema, was shown to alleviate SA (15).
In summary, patients with kidney disease, particularly those on dialysis, and patients with nephrotic syndrome are more prone to have SA. The role of fluid overload and overnight fluid shift as risk factors for SA were well demonstrated in these settings (14, 15, 54, 60, 62–66). The data presented in these cited studies suggest that kidney disease and nephrotic syndrome might cause SA independently of confounding factors. Fluid overload per se contributed to the presence of SA in patients on hemodialysis that can be partly reversible through fluid removal by ultrafiltration (67).
Below, we describe different treatment options for the management of OSA. All these treatments are summarized in Figure 2.
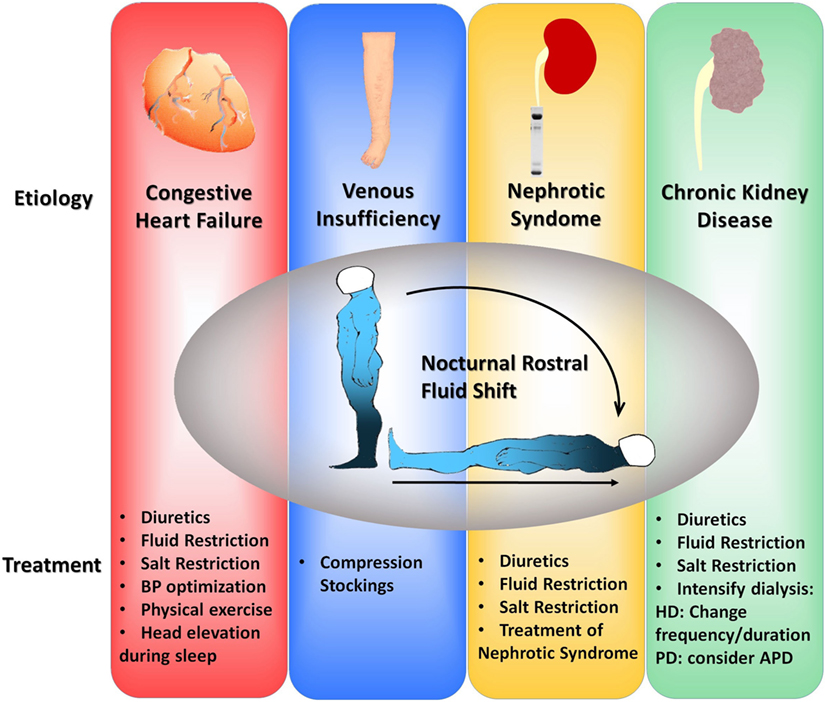
Figure 2. Flowchart of suggested therapeutic interventions to alleviate fluid shift in four different clinical scenarios: congestive heart failure, venous insufficiency, nephrotic syndrome, and chronic kidney disease. BP, blood pressure; HD, hemodialysis; PD, peritoneal dialysis; APD, automatic peritoneal dialysis.
Basically, the therapeutic action of CPAP is to mechanically impede the collapse of the upper airways, thus preventing OSA. In addition, CPAP prevents CSA because it maintains a continuous airflow. CPAP seems to have no effect on overnight fluid shift in patients on hemodialysis (68), although is considered the mainstream treatment for OSA regardless of volume overload given its mechanism of action.
Targeted therapy for fluid retention and/or rostral fluid shift has been tested in several studies. In CHF patients with left ventricular diastolic dysfunction and severe OSA, intensive diuretic therapy increased upper airway cross-sectional area and lowered AHI by 24% (69). Increased physical activity during cardiac rehabilitation has also been associated with attenuation of both OSA and CSA (70, 71), possibly by preventing lower body fluid accumulation.
Head-elevated patient positioning can ameliorate OSA in CHF by preventing cervical fluid accumulation (72). Interestingly, this maneuver can also prevent CSA in CHF patients. Similar results were obtained in CHF patients with predominant OSA (72), with no effect on thoracic fluid content or left ventricular hemodynamics (73). This effect was attributed to increased venous return and dilation of the left heart while in the supine position, although lung congestion may also play a role.
As remarked earlier in this review, overflow is the mechanism of fluid retention in patients on hemodialysis. Accordingly, amelioration of SA in this population is expected to be proportional to the efficiency of fluid removal. In patients on conventional hemodialysis (three 4-h sessions/week), the SA severity tends to increase during interdialytic periods, reaching a maximum immediately before each session (12, 16, 51). Therefore, increasing the duration and/or frequency of sessions, thus mimicking more faithfully the operation of normal kidneys, may be a sound strategy to prevent SA in ESRD. In a study of 14 patients transferred from conventional to intensive hemodialysis (five 6-h sessions/week), AHI decreased by 68%, with marked improvement of oxygen saturation (63). A similar trend was observed in patients treated with peritoneal dialysis (PD) (74). Tang et al. (65) showed that in patients undergoing automated nocturnal PD, in which fluid removal was more efficient than with the manual procedure, a greater reduction of AHI was achieved, in association with less airway obstruction.
The importance of fluid retention is highlighted by the behavior of SA in patients with nephrotic syndrome, even when renal function is normal. In patients with steroid-responsive nephropathy, Tang et al. (15) showed that the severity of SA was reduced after kidney disease remission, in association with disappearance of lower limb edema and reductions in body water content.
Reducing leg swelling by wearing compression stockings during the day attenuated SA in patients with venous insufficiency (75, 76) and ESRD (68). This beneficial effect was observed in a general OSA population (77), highlighting the impact that even small amounts of fluid retained in the legs during the day might have on SA.
Table 2 summarizes several studies in which the efficacy of the aforementioned therapies was tested.
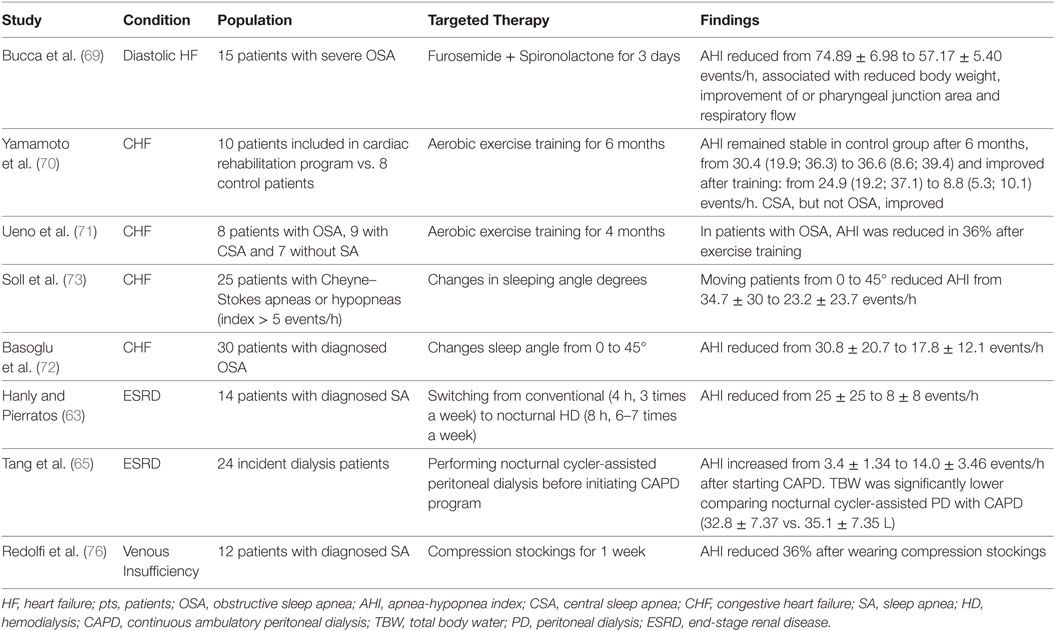
Table 2. Studies that evaluated the impact of target therapies on SA in patients with fluid overload conditions.
In the search for alternatives to CPAP, it is imperative to understand the pathogenic role of fluid overload and overnight fluid shift. The association of CPAP with strategies aimed at limiting edema and rostral fluid shift may reduce the need for high airway pressure, thus improving tolerance. Further work is required in order to assess cardiovascular outcomes of treating SA by interference on fluid overload/redistribution. Additionally, CPAP alone has failed to improve mortality among patients with OSA. Nevertheless, it is unclear if adding a fluid restriction strategy would change such outcomes.
Concept and design: BS and RE; data interpretation: BS, TK, FC, RZ, and RE; manuscript writing: BS, TK, RZ, and RE; final approval of manuscript: BS, TK, FC, RZ, and RE.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AHI, apnea–hypopnea index; CHF, congestive heart failure; CKD, chronic kidney disease; CPAP, continuous positive airway pressure; CSA, central sleep apnea; EABV, effective arterial blood volume; ESRD, end-stage renal disease; LBPP, lower body positive pressure; OSA, obstructive sleep apnea; PCWP, pulmonary capillary wedge pressure; SA, sleep apnea.
1. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med (1993) 328(17):1230–5. doi:10.1056/NEJM199304293281704
2. Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med (2006) 166(16):1709–15. doi:10.1001/archinte.166.16.1709
3. Hayley AC, Williams LJ, Venugopal K, Kennedy GA, Berk M, Pasco JA. The relationships between insomnia, sleep apnoea and depression: findings from the American National Health and Nutrition Examination Survey, 2005–2008. Aust N Z J Psychiatry (2015) 49(2):156–70.
4. Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med (2009) 5(6):573–81.
5. Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of occupational accidents in workers with obstructive sleep apnea: systematic review and meta-analysis. Sleep (2016) 39(6):1211–8. doi:10.5665/sleep.5834
6. Bruin PF, Bagnato Mda C. [Cognitive impairment in obstructive sleep apnea syndrome]. J Bras Pneumol (2010) 36(Suppl 2):32–7. doi:10.1590/S1806-37132010001400010
7. Abboud F, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest (2014) 124(4):1454–7. doi:10.1172/JCI70420
8. Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension (2011) 58(5):811–7. doi:10.1161/HYPERTENSIONAHA.111.179788
9. Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis (2013) 229:489–95. doi:10.1016/j.atherosclerosis.2013.04.026
10. Redline S, Budhiraja R, Kapur V, Marcus CL, Mateika JH, Mehra R, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med (2007) 3(2):169–200.
11. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med (2002) 165(9):1217–39. doi:10.1164/rccm.2109080
12. Merlino G, Piani A, Dolso P, Adorati M, Cancelli I, Valente M, et al. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant (2006) 21(1):184–90. doi:10.1093/ndt/gfi144
13. Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE, et al. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation (2010) 121(14):1598–605. doi:10.1161/CIRCULATIONAHA.109.902452
14. Elias RM, Bradley TD, Kasai T, Motwani SS, Chan CT. Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transplant (2012) 27(4):1569–73. doi:10.1093/ndt/gfr605
15. Tang SC, Lam B, Lam JC, Chan CK, Chow CC, Ho YW, et al. Impact of nephrotic edema of the lower limbs on obstructive sleep apnea: gathering a unifying concept for the pathogenetic role of nocturnal rostral fluid shift. Nephrol Dial Transplant (2012) 27(7):2788–94. doi:10.1093/ndt/gfr759
16. Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol (2011) 6(5):986–94. doi:10.2215/CJN.05720710
17. Redeker NS, Hilkert R. Sleep and quality of life in stable heart failure. J Card Fail (2005) 11(9):700–4. doi:10.1016/j.cardfail.2005.07.003
18. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int (1993) 43(5):1134–9. doi:10.1038/ki.1993.159
19. Owada T, Yoshihisa A, Yamauchi H, Iwaya S, Suzuki S, Yamaki T, et al. Adaptive servoventilation improves cardiorenal function and prognosis in heart failure patients with chronic kidney disease and sleep-disordered breathing. J Card Fail (2013) 19(4):225–32. doi:10.1016/j.cardfail.2013.03.005
20. Yogasundaram H, Oudit GY. Increased mortality associated with adaptive servo-ventilation therapy in heart failure patients with central sleep apnea in the halted SERVE-HF trial. Can J Cardiol (2015) 31(9):1202–3. doi:10.1016/j.cjca.2015.07.712
21. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med (2016) 375(10):919–31. doi:10.1056/NEJMoa1606599
22. Chai-Coetzer CL, Luo YM, Antic NA, Zhang XL, Chen BY, He QY, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep (2013) 36(12):1929–37. doi:10.5665/sleep.3232
23. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev (2011) 15(6):343–56. doi:10.1016/j.smrv.2011.01.003
24. Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med (2006) 119(7 Suppl 1):S47–53. doi:10.1016/j.amjmed.2006.05.007
25. Ellis D. Pathophysiology, evaluation, and management of edema in childhood nephrotic syndrome. Front Pediatr (2016) 3:111. doi:10.3389/fped.2015.00111
26. Bekheirnia MR, Schrier RW. Pathophysiology of water and sodium retention: edematous states with normal kidney function. Curr Opin Pharmacol (2006) 6(2):202–7. doi:10.1016/j.coph.2005.09.008
28. Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res (2010) 87(2):198–210. doi:10.1093/cvr/cvq062
29. Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth (2012) 108(3):384–94. doi:10.1093/bja/aer515
31. Akonur A, Guest S, Sloand JA, Leypoldt JK. Automated peritoneal dialysis prescriptions for enhancing sodium and fluid removal: a predictive analysis of optimized, patient-specific dwell times for the day period. Perit Dial Int (2013) 33(6):646–54. doi:10.3747/pdi.2012.00261
32. Abbadi AC, Deldime P, Van Espen D, Simon M, Rosoux P. The spontaneous aortocaval fistula: a complication of the abdominal aortic aneurysm. Case report and review of the literature. J Cardiovasc Surg (Torino) (1998) 39(4):433–6.
33. Schrier RW, Gurevich AK, Cadnapaphornchai MA. Pathogenesis and management of sodium and water retention in cardiac failure and cirrhosis. Semin Nephrol (2001) 21(2):157–72. doi:10.1053/snep.2001.20933
34. Palmer BF, Alpern RJ. Pathogenesis of edema formation in the nephrotic syndrome. Kidney Int Suppl (1997) 59:S21–7.
35. Kasai T. Sleep apnea and heart failure. J Cardiol (2012) 60(2):78–85. doi:10.1016/j.jjcc.2012.05.013
36. Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol (2011) 57(2):119–27. doi:10.1016/j.jacc.2010.08.627
37. Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation (2012) 126(12):1495–510. doi:10.1161/CIRCULATIONAHA.111.070813
38. Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med (1998) 157(1):144–8. doi:10.1164/ajrccm.157.1.9706079
39. Inoshita A, Kasai T, Takahashi M, Inoshita H, Kasagi S, Kawana F, et al. Craniofacial anatomical risk factors in men with obstructive sleep apnea and heart failure: a pilot study. Sleep Breath (2014) 18(2):439–45. doi:10.1007/s11325-013-0906-4
40. Yadollahi A, Gabriel JM, White LH, Taranto Montemurro L, Kasai T, Bradley TD. A randomized, double crossover study to investigate the influence of saline infusion on sleep apnea severity in men. Sleep (2014) 37(10):1699–705. doi:10.5665/sleep.4084
41. Kasai T, Motwani SS, Yumino D, Gabriel JM, Montemurro LT, Amirthalingam V, et al. Contrasting effects of lower body positive pressure on upper airways resistance and partial pressure of carbon dioxide in men with heart failure and obstructive or central sleep apnea. J Am Coll Cardiol (2013) 61(11):1157–66. doi:10.1016/j.jacc.2012.10.055
42. Kasai T, Motwani SS, Yumino D, Mak S, Newton GE, Bradley TD. Differing relationship of nocturnal fluid shifts to sleep apnea in men and women with heart failure. Circ Heart Fail (2012) 5(4):467–74. doi:10.1161/CIRCHEARTFAILURE.111.965814
43. Solin P, Roebuck T, Johns DP, Walters EH, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med (2000) 162(6):2194–200. doi:10.1164/ajrccm.162.6.2002024
44. Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis (1993) 148(2):330–8. doi:10.1164/ajrccm/148.2.330
45. Tkacova R, Hall MJ, Liu PP, Fitzgerald FS, Bradley TD. Left ventricular volume in patients with heart failure and Cheyne-Stokes respiration during sleep. Am J Respir Crit Care Med (1997) 156(5):1549–55. doi:10.1164/ajrccm.156.5.9612101
46. Yumino D, Bradley TD. Central sleep apnea and Cheyne-Stokes respiration. Proc Am Thorac Soc (2008) 5(2):226–36. doi:10.1513/pats.200708-129MG
47. Lorenzi-Filho G, Azevedo ER, Parker JD, Bradley TD. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J (2002) 19(1):37–40. doi:10.1183/09031936.02.00214502
48. Schober OH, Meyer GJ, Bossaller C, Creutzig H, Lichtlen PR, Hundeshagen H. Quantitative determination of regional extravascular lung water and regional blood volume in congestive heart failure. Eur J Nucl Med (1985) 10(1–2):17–24. doi:10.1007/BF00261757
49. Kasai T, Arcand J, Allard JP, Mak S, Azevedo ER, Newton GE, et al. Relationship between sodium intake and sleep apnea in patients with heart failure. J Am Coll Cardiol (2011) 58(19):1970–4. doi:10.1016/j.jacc.2011.08.012
50. Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Hammond TC, et al. Sleep apnea in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol (2006) 17(12):3503–9. doi:10.1681/ASN.2006060659
51. Hanly P. Sleep apnea and daytime sleepiness in end-stage renal disease. Semin Dial (2004) 17(2):109–14. doi:10.1111/j.0894-0959.2004.17206.x
52. Xu J, Yoon IY, Chin HJ. The effect of sleep apnea on all-cause mortality in nondialyzed chronic kidney disease patients. Sleep Med (2016) 2(7–28):32–8. doi:10.1016/j.sleep.2016.07.026
53. de Oliveira Rodrigues CJ, Marson O, Tufic S, Kohlmann O Jr, Guimarães SM, Togeiro P, et al. Relationship among end-stage renal disease, hypertension, and sleep apnea in nondiabetic dialysis patients. Am J Hypertens (2005) 18(2 Pt 1):152–7. doi:10.1016/j.amjhyper.2004.08.028
54. Santos RS, Motwani SS, Elias RM. Chronic kidney disease and sleeping disordered breathing (SDB). Curr Hypertens Rev (2016) 12(1):43–7. doi:10.2174/1573402112666160114094222
55. Masuda T, Murata M, Honma S, Iwazu Y, Sasaki N, Ogura M, et al. Sleep-disordered breathing predicts cardiovascular events and mortality in hemodialysis patients. Nephrol Dial Transplant (2011) 26(7):2289–95. doi:10.1093/ndt/gfq756
56. Tang SC, Lam B, Yao TJ, Leung WS, Chu CM, Ho YW, et al. Sleep apnea is a novel risk predictor of cardiovascular morbidity and death in patients receiving peritoneal dialysis. Kidney Int (2010) 77(11):1031–8. doi:10.1038/ki.2010.76
57. Lee JJ, Kim GS, Kim JA, Kim SJ, Kang JG, Kim GH, et al. Improvement of sleep-related breathing disorder in patients with end-stage renal disease after kidney transplantation. Clin Transplant (2011) 25(1):126–30. doi:10.1111/j.1399-0012.2009.01174.x
58. Rodrigues CJ, Marson O, Togeiro SM, Tufik S, Ribeiro AB, Tavares A. Sleep-disordered breathing changes after kidney transplantation: a polysomnographic study. Nephrol Dial Transplant (2010) 25(6):2011–5. doi:10.1093/ndt/gfp752
59. Beecroft JM, Zaltzman J, Prasad R, Meliton G, Hanly PJ. Impact of kidney transplantation on sleep apnoea in patients with end-stage renal disease. Nephrol Dial Transplant (2007) 22(10):3028–33. doi:10.1093/ndt/gfm309
60. Elias RM, Chan CT, Paul N, Motwani SS, Kasai T, Gabriel JM, et al. Relationship of pharyngeal water content and jugular volume with severity of obstructive sleep apnea in renal failure. Nephrol Dial Transplant (2013) 28(4):937–44. doi:10.1093/ndt/gfs473
61. Lyons OD, Chan CT, Elias RM, Bradley TD. Relationship of left atrial size to obstructive sleep apnea severity in end-stage renal disease. Sleep Med (2014) 15(11):1314–8. doi:10.1016/j.sleep.2014.07.001
62. Lyons OD, Chan CT, Yadollahi A, Bradley TD. Effect of ultrafiltration on sleep apnea and sleep structure in patients with end-stage renal disease. Am J Respir Crit Care Med (2015) 191(11):1287–94. doi:10.1164/rccm.201412-2288OC
63. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med (2001) 344(2):102–7. doi:10.1056/NEJM200101113440204
64. Lyons OD, Bradley TD, Chan CT. Hypervolemia and sleep apnea in kidney disease. Semin Nephrol (2015) 35(4):373–82. doi:10.1016/j.semnephrol.2015.06.008
65. Tang SC, Lam B, Ku PP, Leung WS, Chu CM, Ho YW, et al. Alleviation of sleep apnea in patients with chronic renal failure by nocturnal cycler-assisted peritoneal dialysis compared with conventional continuous ambulatory peritoneal dialysis. J Am Soc Nephrol (2006) 17(9):2607–16. doi:10.1681/ASN.2005090936
66. Tang SC, Lam B, Lai AS, Pang CB, Tso WK, Khong PL, et al. Improvement in sleep apnea during nocturnal peritoneal dialysis is associated with reduced airway congestion and better uremic clearance. Clin J Am Soc Nephrol (2009) 4(2):410–8. doi:10.2215/CJN.03520708
67. Padwal RS, Hemmelgarn BR, Khan NA, Grover S, McAlister FA, McKay DW, et al. The 2008 Canadian Hypertension Education Program recommendations for the management of hypertension: Part 1 – blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol (2008) 24(6):455–63. doi:10.1016/S0828-282X(08)70619-6
68. Silva BC, Santos RSS, Drager LF, Coelho FM, Elias RM. Impact of compression stockings vs. continuous positive airway pressure on overnight fluid shift and obstructive sleep apnea among patients on hemodialysis. Front Med (2017) 4:57. doi:10.3389/fmed.2017.00057
69. Bucca CB, Brussino L, Battisti A, Mutani R, Rolla G, Mangiardi L, et al. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest (2007) 132(2):440–6. doi:10.1378/chest.07-0311
70. Yamamoto U, Mohri M, Shimada K, Origuchi H, Miyata K, Ito K, et al. Six-month aerobic exercise training ameliorates central sleep apnea in patients with chronic heart failure. J Card Fail (2007) 13(10):825–9. doi:10.1016/j.cardfail.2007.08.001
71. Ueno LM, Drager LF, Rodrigues AC, Rondon MU, Braga AM, Mathias W Jr, et al. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep (2009) 32(5):637–47. doi:10.1093/sleep/32.5.637
72. Basoglu OK, Keskin B, Tasbakan MS, Gurgun C. Effect of semi-recumbent sleep position on severity of obstructive sleep apnea in heart failure patients. J Card Fail (2015) 21(10):842–7. doi:10.1016/j.cardfail.2015.06.004
73. Soll BA, Yeo KK, Davis JW, Seto TB, Schatz IJ, Shen EN. The effect of posture on Cheyne-Stokes respirations and hemodynamics in patients with heart failure. Sleep (2009) 32(11):1499–506. doi:10.1093/sleep/32.11.1499
74. Tang SC, Lai KN. Sleep disturbances and sleep apnea in patients on chronic peritoneal dialysis. J Nephrol (2009) 22(3):318–25.
75. Redolfi S, Arnulf I, Pottier M, Bradley TD, Similowski T. Effects of venous compression of the legs on overnight rostral fluid shift and obstructive sleep apnea. Respir Physiol Neurobiol (2011) 175(3):390–3. doi:10.1016/j.resp.2011.01.001
76. Redolfi S, Arnulf I, Pottier M, Lajou J, Koskas I, Bradley TD, et al. Attenuation of obstructive sleep apnea by compression stockings in subjects with venous insufficiency. Am J Respir Crit Care Med (2011) 184(9):1062–6. doi:10.1164/rccm.201102-0350OC
Keywords: sleep apnea, fluid overload, edema, fluid shift, continuous positive airway pressure, congestive heart failure, chronic kidney disease
Citation: Silva BC, Kasai T, Coelho FM, Zatz R and Elias RM (2018) Fluid Redistribution in Sleep Apnea: Therapeutic Implications in Edematous States. Front. Med. 4:256. doi: 10.3389/fmed.2017.00256
Received: 30 November 2017; Accepted: 22 December 2017;
Published: 22 January 2018
Edited by:
Rolando Claure-Del Granado, Universidad Mayor de San Simon, BoliviaReviewed by:
Zaid A. Abassi, Technion – Israel Institute of Technology, IsraelCopyright: © 2018 Silva, Kasai, Coelho, Zatz and Elias. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bruno Caldin da Silva, YnJ1bm9jYWxkaW5AaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.