- 1Center for Research on Ethnicity, Culture, and Health, School of Public Health, University of Michigan, Ann Arbor, MI, United States
- 2Department of Psychiatry, School of Public Health, University of Michigan, Ann Arbor, MI, United States
- 3Medicine and Health Promotion Institute, Tehran, Iran
Background: Non-communicable diseases and associated mortality follow a social gradient and chronic kidney disease is not an exception to this rule. Intermediate behavioral and medical factors that may explain such social gradients are, however, still unknown.
Objectives: Using nationally representative data in the United States, this study was conducted to investigate the mediating effect of medical and behavioral risk factors on the association between socioeconomic status (SES) and renal disease mortality.
Patients and methods: Americans’ Changing Lives Study (ACL), 1986–2011, is a 25-year nationally representative prospective cohort study. ACL followed 3,361 adults for up to 25 years. Income, education, and unemployment were the main predictors of interest. Death due to renal disease was the main outcome. Health behaviors (smoking, drinking, and exercise) and medical risk factors (diabetes, hypertension, and obesity) were the mediators. Cox proportional hazards models were used for data analysis.
Results: Higher income (HR = 0.75; 95% CI = 0.62–0.89) was associated with lower risk of death due to renal disease over the 25-year follow-up period. Although health behaviors and medical risk factors at baseline were also predictors of the outcome, they failed to explain the effect of income on death due to renal disease. That is, income was associated with death due to renal disease above and beyond all potential mediators including behavioral and medical risk factors.
Conclusion: Socioeconomic inequalities in the United States cause disparities in renal disease mortality; however, such differences are not due to health behaviors (smoking and drinking) and medical risk factors (hypertension and diabetes). To reduce disparities in renal disease mortality in the United States, policies should go beyond health behaviors and medical risk factors. While programs should help low-income individuals maintain exercise and avoid smoking, reduction of income disparities should be regarded as a strategy for reduction of disparities in renal disease mortality. By increasing minimum pay and minimizing the income gap, we may reduce disparities in renal disease mortality.
Background
In the United States, socioeconomic status (SES) is a major determinant of mortality due to renal diseases (1–8). While SES indicators such as income, education, employment, wealth, and race determine burden of chronic kidney disease (3–6), it is still unknown whether these disparities are due to health behaviors (e.g., smoking, drinking, and exercise) and medical risk factors (e.g., obesity, diabetes, and hypertension) or not (9–11).
We have previously compared various countries for the effects of SES, health behaviors, and medical risk factors on well-being of populations. Across 15 countries, the United States was the only country in which the effect of income on subjective health is fully explained by medical risk factors. In none of the countries studied, income disparities in subjective health could be fully explained by health behaviors (11). In a recent study, SES (income), health behaviors (smoking, drinking, and exercise), and medical risk factors (diabetes and hypertension) could explain the racial disparities in renal disease mortality in the United States (9). In another study, SES and chronic medical conditions explained racial differences in all-cause mortality in the United States (10). In another study, income was the only SES indicator which universally determined life expectancy across social groups (12). All these studies suggest that income may have a more salient role as a social determinant in the United States compared to other countries.
The effects of SES, health behaviors, and medical risk factors on renal disease mortality are complex (8). Behavioral risk factors may be proximal explanatory factors (i.e., mediators) of SES disparities in renal disease burden. We know that behavioral risk factors such as physical activity, smoking, and drinking—which influence development and progression of renal diseases (13–16)- follow a SES gradient (17–20). Medical risk factors (hypertension, diabetes and obesity) may also explain SES disparities in renal disease. As chronic medical conditions (CMC) such as hypertension and diabetes are leading causes of kidney failure (1), SES disparities in burden of renal disease may be at least in part secondary-to-higher prevalence of CMC among low SES individuals (21, 22). Hypertension (23), diabetes (24), and obesity (25), which are known causes of kidney disease, are all more common in low SES populations (24, 26–28). The role of diabetes (29, 30) and hypertension (31, 32) as etiologic factors in development of chronic kidney disease are well established. Obesity—which is also common among low SES and minorities—may also explain why risk of chronic renal disease is higher among low SES groups (33). Two recent studies have shown that the effect of depression on renal disease mortality was smaller for Whites than Blacks (2). However, medical risk factors (e.g., diabetes, hypertension, obesity) had a stronger long-term effect on renal disease mortality across various SES groups (7).
For at least four reasons, more studies are needed on the complex links between SES, health behaviors, medical risk factors, and disparities in renal disease mortality in the United States. First, there is considerable evidence suggesting that contribution of SES, behaviors, and CMC to health disparities are not universal but vary across countries (11, 34–38). For instance, income may have a stronger role on health disparities in the United States than in other countries (11). Second, only a handful of studies have ever focused on mechanisms that link SES to renal disease mortality (2, 9, 10). Third, not only distribution of the medical risk factors of renal disease depends on SES and race, but also their fatality depends on SES factors (33, 39–42). Last, but not least, most research on this topic has used a local sample, and most prospective studies have used a short-term follow-up period. Thus, there is a need for additional studies that follow a national sample for a long-term period.
Objectives
To better understand mechanisms behind SES gradient in renal disease mortality in the United States, we tested whether or not behavioral and medical risk factors explain SES inequalities in mortality due to renal diseases or not.
Methods
Design and Setting
This is a 25-year longitudinal study. We used data from the Americans’ Changing Lives (ACL), 1986–2011. ACL is a state-of-the-art nationally representative US cohort study conducted by the University of Michigan. Detailed information on the study design is available elsewhere (43, 44).
Participants and Sampling
The ACL used a stratified multistage probability sample of US adults aged 24 or above. The study recruited 3,617 non-institutionalized respondents representing 70% of sampled households and 68% of sample individuals at baseline. The ACL oversampled those 60 years old and older, and Blacks.
Measures
Data on demographic, SES, behavioral, and medical characteristics were measured at baseline (year 1986) during a face-to-face interview.
Socioeconomic Status
The main predictors were two SES indicators, namely, education and income. Education was operationalized as number of schooling years. Income was operationalized as a 10-level categorical variable (<$5K, $5–9K, $10–14K, $15–19K, $20–24K, $25–29K, 30–39K, $40–59K, $60–79K, and $80K+). Education and income were both treated as continuous measures.
Race
In this study, race was defined as non-Hispanic Black or non-Hispanic White based on a coding of self-reported items asking about Hispanic ethnicity, nativity, and racial category.
Demographic Factors
Demographic indicators included age (a continuous variable as number of years since birth) and gender (men as the reference category).
Medical Risk Factors
Self-reported data were collected on history of hypertension and diabetes at baseline. Using separate items, all participants reported whether in their lifetime a health care provider had ever told them that they had hypertension or diabetes (44, 45).
Obesity
Body mass index (BMI) was measured based on self-reports of weights and heights, originally collected in pounds and feet/inches, respectively. We defined obesity as a BMI ≥ 30 kg/m2 (46). Although BMI based on self-reported weight and height results in an underestimation of BMI (47, 48), it strongly correlates with BMI based on direct measures (49).
Health Behaviors
Self-reported data were collected on smoking (current smoker vs. other), drinking (current drinker vs. other), and exercise (frequency of physical activities) using three single-item measures. Item for smoking read, “Do you smoke cigarettes now?”. Item for drinking read, “Do you ever drink beer, wine, or liquor?”. Item for exercise read, “How often do you engage in active sports or exercise—would you say often, sometimes, rarely or never?”. Response items for the first two items were yes and no, and response scale for the third item was (1) often, (2) sometimes, (3) rarely, and (4) never. Smoking and drinking were treated as dichotomous variables; exercise was treated as a continuous measure.
Mortality due to Renal Diseases
The main outcome of interest was time of death from renal diseases. Mortality data from mid-1986 through 2011 were obtained through the National Death Index (NDI), death certificates, or the informants. In majority of our deceased participants, time and cause of death could be verified with a death certificate. In only a handful of cases, death information could not be verified with death certificates. In these cases, we reviewed the information carefully, and actual death was certain in all cases. In only a few cases, the death date was ascertained from the informants or the NDI report in lieu of a death certificate (45, 50). Only in a few cases, cause of death was coded as missing, when death certificate or NDI were not available. Respondents who died due to other causes were censored at the time of death. Time of death was registered as number of months from time of enrollment to the study to time of death, based on the month of death and the month of baseline interview.
ICD-9 and ICD-10 codes (51, 52) were used, depending on which was current at the time death was recorded. For ICD-9 codes, the following codes were considered as renal death: 650 (acute glomeronephritis and nephrotic syndrome), 660 (chronic glomeronephritis, nephritis, and nephropathy, not specified as acute or chronic, and renal sclerosis, unspecified), 670 (renal failure, disorders resulting from impaired renal function, and small kidney of unknown causes), and 680 (infections of kidney). For ICD-10 codes, the following codes were considered: 97 (nephritis, nephrotic syndrome, and nephrosis), 98 (acute/rapidly progressive nephritic and nephrotic syndromes), 99 (chronic glomerulonephritis, nephritis, and nephropathy not specified as acute or chronic, and renal sclerosis unspecified), 100 (renal failure), 101 (other disorders of kidney), 102 (infections of kidney), and 104 (inflammatory diseases of female pelvic organ).
Ethics
The current study followed the tenets of the Declaration of Helsinki. University of Michigan Institutional Review Board (IRB) approved the study protocol. We received written consent from all participants. Data were kept fully confidential.
Statistical Analysis
Stata 13.0 (Stata Corporation, College Station, TX, USA) was used to conduct the univariate, bivariate, and multivariable analyses. We accounted the stratification and clustering of the sample. SEs were estimated using Taylor series linearization. We considered p-values <.05 as statistically significant. We reported adjusted hazard ratios (HRs), associated SEs, and 95% confidence intervals (CIs).
For multivariable analysis, multiple proportional hazards models were run to the pooled sample. Proportional hazards models require a binary outcome (renal death) and time to the event or rime to censoring [number of months between baseline to occurrence of death, loss to follow-up, or end of follow-up (2011)]. Renal death was coded zero if the respondent did not die, or died from any other causes. SES indicators (income, education, and employment) were the main predictors of interest. Health behaviors (smoking, drinking, and exercise) and medical risk factors (hypertension, diabetes, and obesity) were potential mediators.
Results
Descriptive Statistics
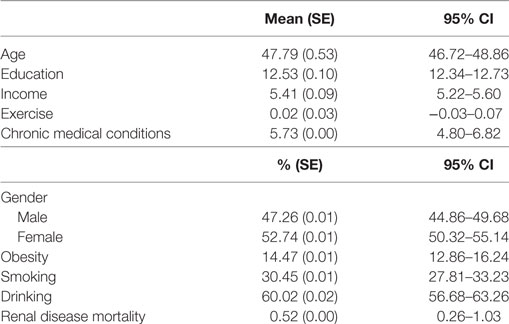
Table 1 summarizes the descriptive statistics for the sample. There were more women in the study than men. Mean age of the participants was 48 years. In total, 14% of the participants were obese, 30% of the participants were smokers, and 60% reported drinking. From all participants, 0.5% died due to renal diseases.
Proportional Hazard Models
Table 2 shows the results of five Cox proportional hazard regression models with renal disease mortality as outcome. As Model 1—a model which only included age, gender, race, and SES—shows, income (HR = 0.75; 95% CI = 0.62–0.89) was associated with risk of death due to renal disease. Models 2–4 show that health behaviors and medical risk factors did not mediate the effect of income on our outcome. However, health behaviors (drinking) and medical risk factors (hypertension and diabetes) did predict the outcome. That is, income was associated with death due to renal disease above and beyond all potential mediators including behavioral and medical risk factors.
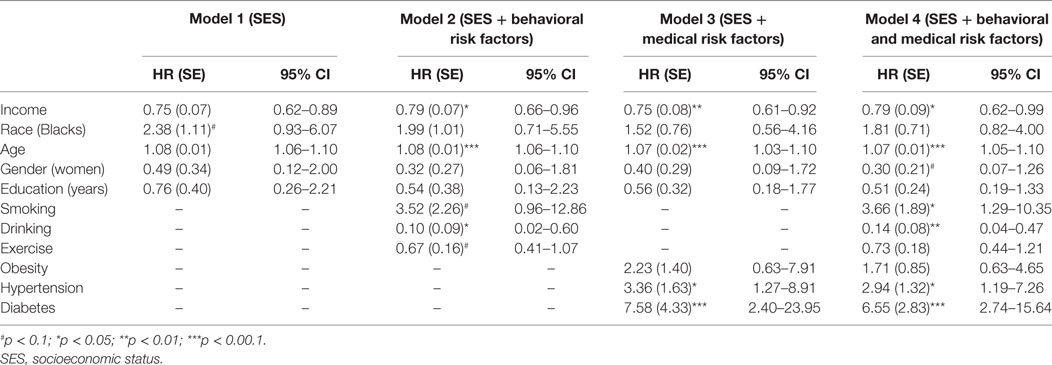
Table 2. Summary of Cox regressions on socioeconomic factors and deaths due to renal disease in the United States.
Discussion
According to our findings, low SES (low income) increases risk of death due to renal disease over a 25-year period, and this effect cannot be explained by SES differences in health behaviors (smoking, drinking, and exercise) and medical risk factors (diabetes, hypertension, and obesity) at baseline. Although health behaviors and medical risk factors influence risk of the outcome, they do not explain the income gradient in renal disease mortality.
Similar to their effects on a wide range of health outcomes (53–55), this study provided support for SES (income) as a distal determinant of renal disease mortality in the United States. According to the Link and Phelan’s Fundamental Cause Theory (FCT), SES is a root cause of health and illness (56–58). This theory provides four essential features for the effect of SES as a fundamental cause of health inequalities (59). First, the effect of SES is not limited to specific health problems as it influences most health outcomes. Second, the effect of SES on health is through a wide range of mechanisms. Third, SES as a proxy of access to materialistic and human resources reduces health risks and delays or minimizes their consequences when they occur. Finally, SES inequalities continually impact health inequalities despite radical changes in causes of morbidity and mortality over decades (59).
According to our findings, SES disparities in health behaviors such as exercise, smoking, and drinking at baseline fail to explain the SES disparities in death due to renal diseases. Distribution of exercise (18), smoking (19), and drinking (20) are under influence of SES, while all have implications for development and progression of renal diseases (13–16). While SES has a large effect on shaping health behaviors of populations (17, 33), our study suggests that healthy behaviors at baseline are not the answer to why low SES groups are at higher risk of renal disease mortality.
While medical risk factors such as hypertension and diabetes also increased risk of renal disease mortality, they did not mediate SES disparities in renal disease mortality. Diabetes and hypertension, which are leading causes of chronic kidney disease (1), are more common among minorities and low SES individuals (23–28). Thus, at least some of the SES gradient in death due to renal disease may be due to chronic medical conditions (21–25). A well-established literature shows that diabetes is associated with chronic kidney disease (29). In fact, one of the major complications of diabetes is chronic kidney disease (30). Hypertension causes chronic kidney disease, and chronic kidney disease also increases risk of hypertension (24, 32).
Our findings that behavioral (smoking, drinking, and exercise) and medical (hypertension and diabetes) risk factors at baseline do not explain the SES disparities in death due to renal disease highlights a need for future research on other potential mechanisms of such effects. Although we did not find a mediational path, our findings have policy and clinical implications for reducing the SES gradient in death due to renal disease. Policies and programs should help people maintain health behaviors and avoid hypertension and diabetes, as they are independent risk factors for renal disease mortality.
Although other mechanisms may also be involved, SES should be considered a root cause of disparities in renal disease mortality in the United States. Factors outside baseline health behaviors and medical risk factors may contribute as mechanisms behind such effects. The health care system and health care use may be one explanation which needs future research.
Our findings suggest that SES shapes disparities in renal disease mortality and this effect is not simply because low-income people have poor health behaviors and additional medical risk factors at baseline. While considerable information exists on SES as a fundamental cause of disparities, less is known about mechanisms by which SES influences mortality across countries (60–62).
Study Limitations
Despite the unique contribution that this paper makes to the literature, the results should be interpreted with consideration of the study limitations. The major limitation of the study was lack of any measure of kidney disease at baseline and over time. In addition, measurement bias is possible, particularly for medical risk factors. Hypertension and diabetes were measured using self-reported data. Furthermore, medical risk factors of renal disease were not comprehensive (63). Further research may use medical records to verify history of medical conditions. Future studies should also assess how changes of SES, behaviors, and CMC over time explain disparities in kidney diseases. Future research may also benefit from biological markers of kidney function. This study did not include access to health care, which may partially explain why low SES individuals and minority people have higher burden of chronic kidney disease (8). While studies have explored the role of the health care system as possible mediators of such disparities (64, 65), we did not have information on health care access or utilization in our study. Some major strengths of this study included large sample size, long-term follow-up, and recruitment of a nationally representative sample.
Conclusions
To conclude, income disparities exist in deaths due to renal diseases over a 25-year period. Such income disparities, however, cannot be easily explained by behavioral (smoking, drinking, and exercise) and medical (diabetes and hypertension) risk factors. These findings extend the existing literature on SES disparities in renal disease in the United States. This is particularly important as the SES gradient in chronic kidney disease is a major challenge in the United States (66).
Ethics Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants included in the study.
Author Contributions
SA designed the study, analyzed the data, and drafted the paper. MML contributed to the paper and revised the manuscript. Both authors approved the final draft.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
SA is partially supported by the Heinz C. Prechter Bipolar Research Fund and the Richard Tam Foundation at the University of Michigan. Thanks to Brianna Preiser for her input to this paper.
Funding
The Americans’ Changing Lives (ACL) study was supported by the National Institute on Aging (DHHS/NIH) (grant number AG018418 from), and the NIH Public Access Policy requires that peer-reviewed research publications generated with NIH support are made available to the public through PubMed Central. NIH is not responsible for the data collection or analyses represented in this article. The ACL study was conducted by the Institute of Social Research, University of Michigan.
References
1. U.S. Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: USRDS (2010).
2. Assari S, Burgard S. Black-White differences in the effect of baseline depressive symptoms on deaths due to renal diseases: 25 year follow up of a nationally representative community sample. J Renal Inj Prev (2015) 4(4):127–34. doi:10.12861/jrip.2015.27
3. Navarro V. Race or class versus race and class: mortality differentials in the United States. Lancet (1990) 336(8725):1238–40. doi:10.1016/0140-6736(90)92846-A
4. Schulz AJ, Mullings L. Gender, Race, Class, and Health: Intersectional Approaches. San Francisco, CA: Jossey-Bass (2006).
5. Williams DR. Race, socioeconomic status, and health the added effects of racism and discrimination. Ann N Y Acad Sci (1999) 896(1):173–88. doi:10.1111/j.1749-6632.1999.tb08114.x
6. Williams DR, Lavizzo-Mourey L, Warren RC. The concept of race and health status in America. Public Health Rep (1994) 109(1):26.
7. Moghani Lankarani M, Assari S. Diabetes, hypertension, obesity and long term risk of renal disease mortality; racial and socioeconomic differences. J Diabetes Invest (2017) 8(4):590–9. doi:10.1111/jdi.12618
8. Assari S. Racial disparities in chronic kidney diseases in the United States; a pressing public health challenge with social, behavioral and medical causes. J Nephropharmacol (2015) 5(1):4–6.
9. Assari S. Distal, intermediate, and proximal mediators of racial disparities in renal disease mortality in the United States. J Nephropathol (2016) 5(1):51–9. doi:10.15171/jnp.2016.09
10. Assari S. Number of chronic medical conditions fully mediates the effects of race on mortality; 25-year follow-up of a nationally representative sample of Americans. J Racial Ethn Health Disparities (2017) 4(4):623–31. doi:10.1007/s40615-016-0266-4
11. Assari S, Lankarani MM. Does multi-morbidity mediate the effect of socioeconomics on self-rated health? Cross-country differences. Int J Prev Med (2015) 6:85. doi:10.4103/2008-7802.164413
12. Assari S, Lankarani MM. Race and urbanity alter the protective effect of education but not income on mortality. Front Public Health (2016) 4:100. doi:10.3389/fpubh.2016.00100
13. Knap B, Buturović-Ponikvar J, Ponikvar R, Bren AF. Regular exercise as a part of treatment for patients with end-stage renal disease. Ther Apher Dial (2005) 9(3):211–3. doi:10.1111/j.1774-9987.2005.00256.x
14. Koning SH, Gansevoort RT, Mukamal KJ, Rimm EB, Bakker SJ, Joosten MM, et al. Alcohol consumption is inversely associated with the risk of developing chronic kidney disease. Kidney Int (2015) 87(5):1009–16. doi:10.1038/ki.2014.414
15. Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol (2008) 3(1):226–36. doi:10.2215/CJN.03740907
16. Stöckmann A, Conradt C, Ritz E, Ferro M, Kreusser W, Piccoli G, et al. Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int (1998) 54(3):926–31. doi:10.1046/j.1523-1755.1998.00067.x
17. Sorkin DH, Billimek J. Dietary behaviors of a racially and ethnically diverse sample of overweight and obese Californians. Health Educ Behav (2012) 39(6):737–44. doi:10.1177/1090198111430709
18. August KJ, Sorkin DH. Racial/ethnic disparities in exercise and dietary behaviors of middle-aged and older adults. J Gen Intern Med (2011) 26(3):245–50. doi:10.1007/s11606-010-1514-7
19. Kandel DB, Kiros GE, Schaffran C, Hu MC. Racial/ethnic differences in cigarette smoking initiation and progression to daily smoking: a multilevel analysis. Am J Public Health (2004) 94(1):128–35. doi:10.2105/AJPH.94.1.128
20. Gilman SE, Breslau J, Conron KJ, Koenen KC, Subramanian SV, Zaslavsky AM. Education and race-ethnicity differences in the lifetime risk of alcohol dependence. J Epidemiol Community Health (2008) 62(3):224–30. doi:10.1136/jech.2006.059022
21. Cabassa LJ, Humensky J, Druss B, Lewis-Fernández R, Gomes AP, Wang S, et al. Do race, ethnicity, and psychiatric diagnoses matter in the prevalence of multiple chronic medical conditions? Med Care (2013) 51(6):540–7. doi:10.1097/MLR.0b013e31828dbb19
22. Johnson-Lawrence VD, Griffith DM, Watkins DC. The effects of race, ethnicity and mood/anxiety disorders on the chronic physical health conditions of men from a national sample. Am J Men Health (2013) 7(4S):58S–67S. doi:10.1177/1557988313484960
23. Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr (2007) 18(4):241–7.
24. Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, et al. Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health (2007) 97(12):2260–7. doi:10.2105/AJPH.2006.094482
25. Jackson CL, Szklo M, Yeh HC, Wang NY, Dray-Spira R, Thorpe R, et al. Black-white disparities in overweight and obesity trends by educational attainment in the United States, 1997-2008. J Obes (2013) 2013:140743. doi:10.1155/2013/140743
26. Abate N, Chandalia M. The impact of ethnicity on type 2 diabetes. J Diabetes Complications (2003) 17:39–58. doi:10.1016/S1056-8727(02)00190-3
27. Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults: the third national health and nutrition examination survey, 1988–1994. Diabetes Care (1998) 21(4):518–24. doi:10.2337/diacare.21.4.518
28. Sampson UK, Edwards TL, Jahangir E, Munro H, Wariboko M, Wassef MG, et al. Factors associated with the prevalence of hypertension in the southeastern United States: insights from 69,211 blacks and whites in the southern community cohort study. Circ Cardiovasc Qual Outcomes (2014) 7(1):33–54. doi:10.1161/CIRCOUTCOMES.113.000155
29. Laville M, Lengani A, Serme D, Fauvel J, Ouandaogo B, Zech P. Epidemiological profile of hypertensive disease and renal risk factors in black Africa. J Hypertens (1994) 12(7):839–43. doi:10.1097/00004872-199407000-00017
30. Seedat YK. Hypertension in black South Africans. J Hum Hypertens (1999) 13(2):96–103. doi:10.1038/sj.jhh.1000773
31. Richardson AD, Piepho RW. Effect of race on hypertension and antihypertensive therapy. Int J Clin Pharmacol Ther (2000) 38(2):75–9. doi:10.5414/CPP38075
32. Weisstuch JM, Dworkin LD. Does essential hypertension cause end-stage renal disease? Kidney Int (1992) 36:S33–7.
33. Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int (2005) 68(3):914–24. doi:10.1111/j.1523-1755.2005.00485.x
34. Assari S. Cross-country differences in the additive effects of socioeconomics, health behaviors and medical comorbidities on disability among older adults with heart disease. J Tehran Heart Cent (2015) 10(1):24–33.
35. Assari S. Cross-country variation in additive effects of socio-economics, health behaviors, and comorbidities on subjective health of patients with diabetes. J Diabetes Metab Disord (2014) 13(1):36. doi:10.1186/2251-6581-13-36
36. Assari S, Lankarani RM, Lankarani MM. Cross-country differences in the association between diabetes and disability. J Diabetes Metab Disord (2014) 13(1):3. doi:10.1186/2251-6581-13-3
37. Assari S, Lankarani MM. Association between heart disease and subjective health in ten north, middle, and South American countries. Int J Travel Med Global Health (2014) 2(4):141–7.
38. Lankarani MM, Shah S, Assari S. Gender difference in vulnerability to socio-economic status on self-rated health in 15 countries. Womens Health Bull (2017) 4(3):e45280. doi:10.5812/whb-45280
39. Hall YN. Racial and ethnic disparities in end stage renal disease: access failure. Clin J Am Soc Nephrol (2012) 7(2):196–8. doi:10.2215/CJN.13021211
40. Peralta CA, Katz R, DeBoer I, Ix J, Sarnak M, Kramer H, et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol (2011) 22(7):1327–34. doi:10.1681/ASN.2010090960
41. Gómez-Puerta JA, Feldman CH, Alarcón GS, Guan H, Winkelmayer WC, Costenbader KH. Racial and ethnic differences in mortality and cardiovascular events among patients with end-stage renal disease due to lupus nephritis. Arthritis Care Res (Hoboken) (2015) 67(10):1453–62. doi:10.1002/acr.22562
42. Norris KC, Kalantar-Zadeh K, Kopple JD. The role of race in survival among patients undergoing dialysis. Nephrol News Issues (2011) 25(13):13–4.
43. House JS, Lepkowski JM, Kinney AM, Mero RP, Kessler RC, Herzog AR. The social stratification of aging and health. J Health Soc Behav (1994) 35(3):213–34. doi:10.2307/2137277
44. House JS, Kessler RC, Herzog AR. Age, socioeconomic status, and health. Milbank Q (1990) 68(3):383–411. doi:10.2307/3350111
45. Houle JN. Depressive symptoms and all-cause mortality in a nationally representative longitudinal study with time-varying covariates. Psychosom Med (2013) 75(3):297–304. doi:10.1097/PSY.0b013e31828b37be
46. Assari S. Additive effects of anxiety and depression on body mass index among blacks: role of ethnicity and gender. Int Cardiovasc Res J (2014) 8(2):44–51.
47. Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry (2006) 63(7):824–30. doi:10.1001/archpsyc.63.7.824
48. Taylor AW, Dal Grande E, Gill TK, Chittleborough CR, Wilson DH, Adams RJ, et al. How valid are self-reported height and weight? A comparison between CATI self-report and clinic measurements using a large cohort study. Aust N Z J Public Health (2006) 30(3):238–46. doi:10.1111/j.1467-842X.2006.tb00864.x
49. Gavin AR, Rue T, Takeuchi D. Racial/ethnic differences in the association between obesity and major depressive disorder: findings from the comprehensive psychiatric epidemiology surveys. Public Health Rep (2010) 125(5):698–708. doi:10.1177/003335491012500512
50. Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA (1998) 279(21):1703–8. doi:10.1001/jama.279.21.1703
51. Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep (2001) 49(2):1–32.
52. Centers for Disease Control and Prevention, National Center for Health Statistics. Instruction Manual, Part 9. ICD-10 Cause-of-Death Lists for Tabulating Mortality Statistics (updated October 2002 to Include ICD Codes for Terrorism Deaths for Data Year 2001 and WHO Updates to ICD-10 for Data Year 2003). (2017). Available from: https://www.cdc.gov/nchs/nvss/instruction_manuals.htm (accessed October 24, 2017).
53. Malekahmadi MR, Rahimzadeh S, Dezfuli Nejad ML, Lankarani MM, Einollahi B, Assari S. Importance of socioeconomic, clinical, and psychological factors on health-related quality of life in adolescents after kidney transplant. Exp Clin Transplant (2011) 9(1):50–5.
54. Assari S, Ahmadi K, Rezazade M. Socio-economic status determines risk of receptive syringe sharing behaviors among iranian drug injectors; a national study. Front Psychiatry (2015) 23:194. doi:10.3389/fpsyt.2014.00194
55. Assari S, Rezazade M, Ahmadi K, Sehat M. Socio-economic status may suppress the effect of knowledge on sexual risk among female sex workers. Int J Health Allied Sci (2014) 3(2):84. doi:10.4103/2278-344X.132691
56. Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav (2010) 51(Suppl):S28–40. doi:10.1177/0022146510383498
57. Freese J, Lutfey K. Fundamental causality: challenges of an animating concept for medical sociology. Handbook of the Sociology of Health, Illness, and Healing. Springer (2011). p. 67–81.
58. Link BG, Phelan J. Social conditions as fundamental causes of health inequalities. Handbook of Medical Sociology. (2010). p. 3–17.
59. Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav (1995) 35(Spec No):80–94. doi:10.2307/2626958
60. Drake KA, Galanter JM, Burchard EG. Race, ethnicity and social class and the complex etiologies of asthma. Pharmacogenomics (2008) 9(4):453–62. doi:10.2217/14622416.9.4.453
61. Krause JS, Broderick LE, Saladin LK, Broyles J. Racial disparities in health outcomes after spinal cord injury: mediating effects of education and income. J Spinal Cord Med (2006) 29(1):17–25. doi:10.1080/10790268.2006.11753852
62. Brown SA, Saunders LL, Krause JS. Racial disparities in depression and life satisfaction after spinal cord injury: a mediational model. Top Spinal Cord Inj Rehabil (2012) 18(3):232–40. doi:10.1310/sci1803-232
63. Boyer GS, Templin DW, Goring WP, Cornoni-Huntley JC, Everett DF, Lawrence RC, et al. Discrepancies between patient recall and the medical record. Potential impact on diagnosis and clinical assessment of chronic disease. Arch Intern Med (1995) 155(17):1868–72. doi:10.1001/archinte.1995.00430170060007
64. Saha S, Arbelaez JJ, Cooper LA. Patient-physician relationships and racial disparities in the quality of health care. Am J Public Health (2003) 93(10):1713–9. doi:10.2105/AJPH.93.10.1713
65. Perneger TV, Whelton PK, Klag MJ. Race and end-stage renal disease. Socioeconomic status and access to health care as mediating factors. Arch Intern Med (1995) 155(11):1201–8. doi:10.1001/archinte.1995.00430110121013
Keywords: socioeconomic status, hypertension, diabetes, obesity, deaths, renal diseases
Citation: Assari S and Lankarani MM (2017) Income Gradient in Renal Disease Mortality in the United States. Front. Med. 4:190. doi: 10.3389/fmed.2017.00190
Received: 14 March 2017; Accepted: 20 October 2017;
Published: 06 November 2017
Edited by:
Narinder K. Mehra, All India Institute of Medical Sciences, IndiaReviewed by:
Rakesh Malhotra, University of California, San Diego, United StatesTheodoros Kasimatis, Guy’s and St Thomas’ NHS Foundation Trust, United Kingdom
Kirk Campbell, Icahn School of Medicine at Mount Sinai, United States
Copyright: © 2017 Assari and Lankarani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shervin Assari, YXNzYXJpQHVtaWNoLmVkdQ==
 Shervin Assari
Shervin Assari Maryam Moghani Lankarani
Maryam Moghani Lankarani