
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 16 August 2017
Sec. Regulatory Science
Volume 4 - 2017 | https://doi.org/10.3389/fmed.2017.00134
This article is part of the Research TopicPublic-Private Partnerships as drivers of innovation in healthcareView all 11 articles
Cellular immunotherapies promise to transform cancer care. However, they must overcome serious challenges, including: (1) the need to identify and characterize novel cancer antigens to expand the range of therapeutic targets; (2) the need to develop strategies to minimize serious adverse events, such as cytokine release syndrome and treatment-related toxicities; and (3) the need to develop efficient production/manufacturing processes to reduce costs. Here, we discuss whether these challenges might better be addressed through forms of public–private research collaborations, including public–private partnerships (PPPs), or whether these challenges are best addressed by way of standard market transactions. We reviewed 14 public–private relationships and 25 underlying agreements for the clinical development of cancer cellular immunotherapies in the US. Most were based on bilateral research agreements and pure market transactions in the form of service contracts and technology licenses, which is representative of the commercialization focus of the field. We make the strategic case that multiparty PPPs may better advance cancer antigen discovery and characterization and improved cell processing/manufacturing and related activities. In the rush toward the competitive end of the translational continuum for cancer cellular immunotherapy and the attendant focus on commercialization, many gaps have appeared in our understanding of cellular biology, immunology, and bioengineering. We conclude that the model of bilateral agreements between leading research institutions and the private sector may be inadequate to efficiently harness the interdisciplinary skills and knowledge of the public and private sectors to bring these promising therapies to the clinic for the benefit of cancer patients.
Public–private partnerships (PPPs) are collaborative efforts to achieve mutually agreed objectives (1). They draw on the respective strengths and resources of the parties involved. In therapeutic product development, PPPs are based on complementary skills, materials, and knowledge along a translational continuum of research and development (R&D) by public/non-profit sector researchers and those in the biotechnology and/or pharmaceutical sectors. While considerable attention has been paid to PPPs engaged in the development of drugs and diagnostics or other devices, this perspective considers the role that PPPs might play in overcoming the clinical development and implementation challenges for cancer cellular immunotherapies. It first identifies the challenges, then takes a case-based approach to review public–private collaborative relationships in the US, and finally expands on the potential for multiparty PPPs to advance this promising field for the benefit of cancer patients.
Cellular immunotherapies have been hailed as transformational for cancer care. In 2013, Science Magazine declared immunotherapy (cellular and checkpoint inhibitors) as its breakthrough of the year (2), and financial markets have generally concurred—2015 was a record year for investment in life sciences companies, with the greatest investment (1,496.49 Mill USD) in the category of immunotherapy/vaccines (3). The excitement stems, in part, from advances in adoptive cellular transfer (ACT), which uses chimeric antigen receptor (CAR-T) cells, tumor-infiltrating lymphocytes (TILs), or T cell receptor (TCR) engineered cells to recognize and target cancer cells (4). ACT promises to improve on the 2.5-month overall gain in survival time reported for cancer drugs approved between 2002 and 2014 (5). For example, clinical trials of CAR-T cells have reported positive results in acute lymphoblastic leukemia (ALL) (6), acute, relapsed refractory chronic lymphocytic leukemia (7), refractory multiple myeloma (8), and pediatric relapsed and refractory B-cell acute lymphocytic leukemia (B-ALL) (9). Similarly, TILs have shown great promise for metastatic melanoma (10–12).
Cellular therapies, in general, and cellular immunotherapies, in particular, face multiple challenges in clinical development and implementation. With respect to clinical development, leading cellular immunotherapy researcher, Dr. Steven A. Rosenberg, has identified lack of suitable targets as a major obstacle for cellular immunotherapies (13). If cellular immunotherapies are to be effective for solid tumors and for hematological malignancies, they must target cancer cells without causing off-target toxicities (14–16). Such toxicities, especially if unpredictable, will be a serious limiting factor for the clinical adoption of cellular immunotherapies. The identification of such cancer-specific antigens is therefore paramount for the future development of the field because most normal tissues, if destroyed by the cellular immunotherapy, cannot be replaced. Clinical trials have reported deaths from cardiopulmonary and neurological toxicities (14–16). Furthermore, cellular immunotherapy for B-cell leukemias that target CD19 may destroy normal B-cells. This can be palliated with immunoglobulin replacement (17). Hematological stem cell transplantation, often performed after these therapies, can also restore normal levels of immune cell subsets. Both, however, are delivered with Intensive Care Unit support, thereby increasing the cost of the therapies.
Cellular immunotherapies must also overcome their potential for other serious adverse events, primarily cytokine release syndrome. There appears to be a correlation between the efficacy of the immunotherapy in destroying cancer cells, and its adverse side effects—high efficacy in killing cancer cells may lead to a cytokine storm, especially in patients with a high disease burden (18). Neurotoxicity poses an additional risk. For example, in November 2016 leading cellular immunotherapy biotechnology company, Juno Therapeutics (Seattle, WA, USA), announced that it is placing a voluntary hold on the Phase II clinical trial of its leading CAR-T cell product, JCAR015, following the death of two participants with relapsed or refractory B cell ALL (19, 20). This voluntary hold for acute irreversible cerebral edema followed a hold placed on the same trial in July 2016 by the US Food and Drug Administration (FDA) due the deaths of three participants also from cerebral edema (21). At the time, Juno Therapeutics blamed the deaths on the addition of the chemotherapy, fludarabine, to eliminate the patient’s existing T-cells, making way for the CAR-T cells. The FDA lifted the hold only 1 week later (22). Not unexpectedly, the new November 2016 (without fludarabine) hold has had a dramatic effect on Juno Therapeutics shares; its stock price plummeted by 44% before trading was halted, and the impact of the deaths has spilled over to negatively impact other CAR-T cell companies (19, 20).
Even if cellular immunotherapy toxicities can be overcome, clinical implementation will be limited by the expected high cost of the therapies ($150,000–$500,000 per dose) that is, in part, determined by the emerging service-based autologous business model for cellular immunotherapies (23). ACT therapies currently derive from the cancer patient’s own circulating lymphocytes. Such autologous therapies incur substantial logistical challenges for scale-up. The circulating lymphocytes must be extracted from the patient, genetically manipulated (CAR or TCR transgenic T cells) or selected for antitumor effect (TILs), expanded, and then reinfused into the patient (12). Current business models suggest processing will occur in a centralized current Good Manufacturing Practice (cGMP) facility, while extraction and infusion will occur at a cancer center. Leading cellular immunotherapy companies, such as Juno Therapeutics and Kite Pharma (Santa Monica, CA, USA), are investing in cGMP infrastructure. The global pharmaceutical giant, Novartis (Basel, Switzerland), initially signaled its intent in the field by opening a Cell and Gene Therapies Unit and purchasing a New Jersey cGMP facility that was originally developed for bankrupt cancer vaccine company, Dendreon (Seattle, WA, USA). However, in February 2016, it closed the Unit to focus on its non-cellular cancer immunotherapy pipeline (24). This shift of Novartis toward its traditional business model cancer therapies, such as checkpoint inhibitors, may indicate continued skepticism in a viable business model for cellular therapies (25). To the detriment of the field, autologous therapies have so far demonstrated greater efficacy than generic allogeneic products. Nevertheless, Cellectis (Paris, France) has advanced an allogeneic CAR-T immunotherapy derived from T cell precursors manipulated using TALEN® technology into Phase I clinical trials (2015-004293-15). The product has been developed in collaboration with Pfizer and Servier and therefore does not represent a PPP. However, Cellectis has entered into a research and development alliance with researchers at MD Anderson Cancer Center (TX, USA), discussed below. The development of allogeneic cellular immunotherapies will be a fruitful area for future PPPs. Advances in all aspects of the service pipeline are therefore central to the clinical success of cellular immunotherapy.
Public–private partnerships are one form of research collaboration based on shared decision making by the public and private sector parties involved with respect to goals, membership, ongoing management, potential expansion of the collaboration, and distribution of benefits (26). Such partnerships harness the complementary skills of the parties along the translational continuum from research laboratory to clinical trials, recognizing that the pathway for most therapies is neither certain nor linear, especially for novel treatment paradigms such as cellular immunotherapy. Rather, the pathway involves iterative research and development as successive challenges in safety and efficacy are identified and sometimes addressed.
Many biomedical PPPs are supportive of the precompetitive portion of the translational continuum wherein they facilitate the sharing of tacit knowledge (27), data, and materials, without limiting the ability of specific actors to appropriate knowledge that is closer to practical application (28–30). As such, PPPs stand in contrast to pure market transactions based on service contracts and technology licensing that more clearly delineate the rights and responsibilities of parties in a competitive environment (26). For example, a research-intensive, precompetitive PPP may be based on a consortium agreement between multiple members that sets out a shared governance structure. In contrast, relationships based on market transactions rarely establish the shared governance models that characterize PPPs. An intermediate form is a hub and spoke model whereby a central party enters into bilateral research agreements with multiple parties to advance its centralized goal. The ordering of research relationships from shared governance structures and collaborative research agreements to service contracts and technology licenses mirrors the translational continuum, from precompetitive to competitive research. The constellation of agreements will depend on the maturity of the technology in question and the state of certainty about its efficacy and market.
The preceding section identified four challenges that might be better addressed by PPPs, given the nascent stage of the field of cellular immunotherapy: (1) the need to identify novel cancer antigens to expand the range of therapeutic targets and minimize both off-target effects and on-target but off-cancer effects; (2) the need to develop strategies to minimize serious adverse events, such as cytokine release syndrome; (3) the need to develop allogeneic therapies; and (4) the need to develop efficient production/manufacturing processes to reduce costs. The issue is whether these challenges might be better addressed through forms of public–private research collaborations, including PPPs, or whether these challenges are best addressed by way of standard market transactions. In this section, we review public–private research relationships in the US. In the next section, we discuss how PPPs might improve the clinical translation of cellular immunotherapies.
The focus of our review was on PPPs that had developed products in clinical trials up to December 2015. For our review, we selected 14 US public–private relationships for the clinical development of cancer cellular immunotherapies based on a comprehensive analysis of 1,579 interventional clinical trials from global registries, of which 329 were industry sponsored (31). Of these, 35 companies had products in clinical development beyond Phase I, with verified status as of September 2016. Of these 35 companies, 34 were biotechnology companies operating in Western Europe (n = 16) and North America (n = 17), and one was the pharmaceutical company, Novartis. We reviewed the history of the public–private relationships of Novartis and the 11 North American companies whose clinical trial registry entry indicated that their product was still in clinical development (i.e., not terminated or withdrawn) and listed at least one collaboration with a research institute (Table 1). This is a limitation of our review—we only identified collaborations from the clinical trial record; we did not contact companies or interview investigators associated with all the industry-sponsored clinical trials and may therefore have missed some collaborations with academic centers.
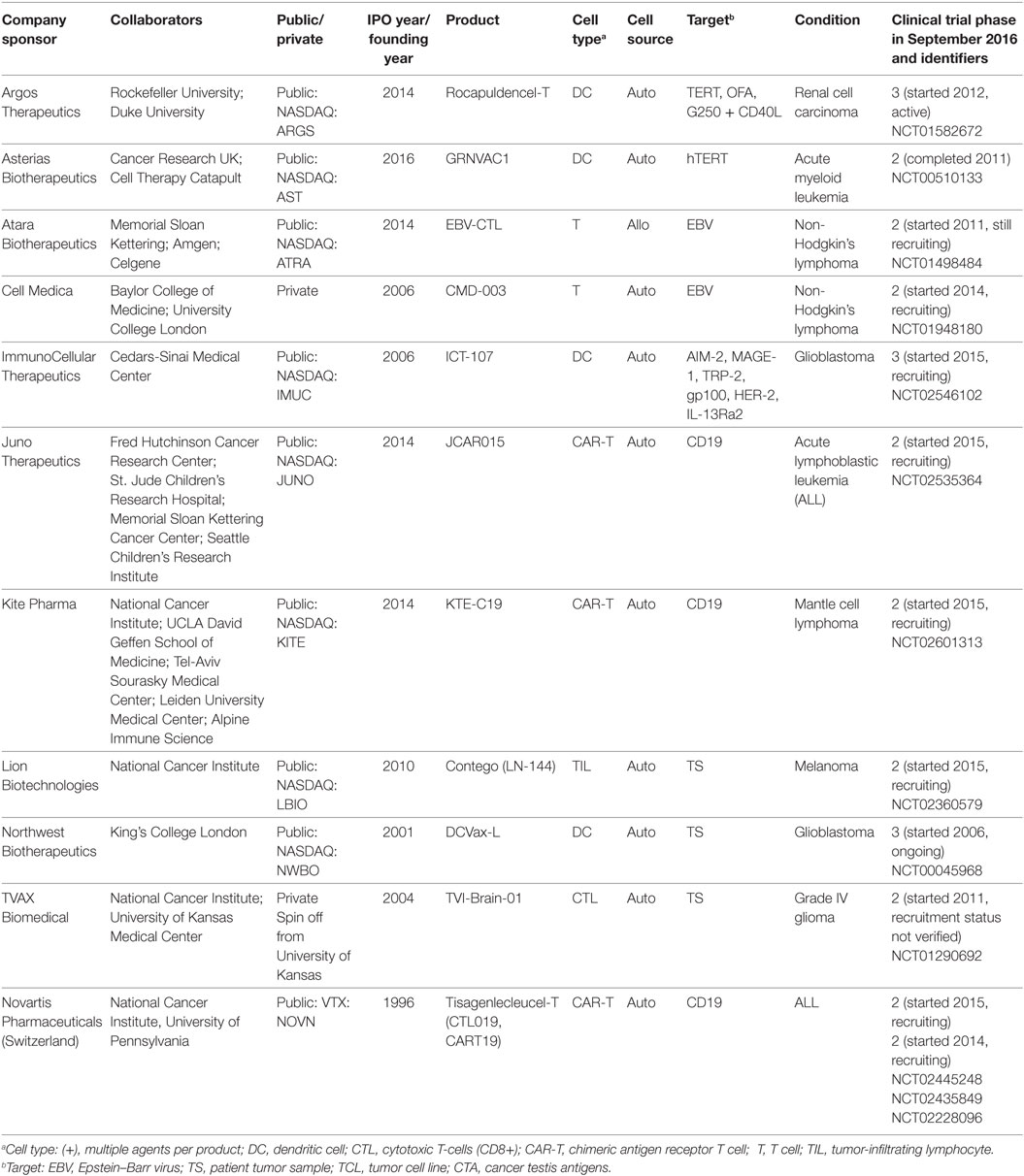
Table 1. Public–private collaborative efforts in the US of cancer cellular immunotherapy in Phase II and III clinical trials.
We identified 23 separate agreements. In addition, our review of the academic literature and biotechnology news coverage by STAT News and FierceBiotech of cancer cellular immunotherapy identified four additional agreements by US companies of interest, whose product or technology development fills a gap to an identified challenge: Bellicum Pharmaceuticals (Houston, TX, USA), bluebirdbio (Cambridge, MA, USA), Cellectis (Paris, France), and Adaptimmune Therapeutics (Abington, UK). We further reviewed the history and nature of the research relationships based on documents identified in biotechnology and pharmaceutical trade publications (Factiva database), company websites and SEC filings, and contracts—10 of which had a full-text version available on the Recap database (confidential details redacted).
Our review of the 25 agreements identified a mixture of collaborative research agreements and pure market transactions in the form of service contracts and technology licensing (Table 2). The research agreements for collaborations between companies and research institutions (including universities and hospitals) were based on a hub and spoke model. In other words, when a company listed multiple collaborators on its sponsored clinical trial, all of the research agreements were bilateral between the company and the research institute. Our search only identified two relationships that clearly articulated a shared governance structure (likely an underestimate based on publicly available data we accessed rather than interviews with the parties). Cell Medica’s (London, UK) separate agreements with Baylor College of Medicine and the University College London both stated that the research would be conducted under the guidance of a Joint Steering Committee, with representatives from each party to the respective agreement. In the agreement with University College London, either party could bring novel targets or platform technologies to the collaboration. Note that these agreements specifically stated their stage of research as preclinical and early clinical, after which, Cell Medica had an exclusive license and option agreement to move forward with the codeveloped technologies. Indeed, the majority of the collaborative research agreements we identified additionally provided for options or licenses to the technologies developed.
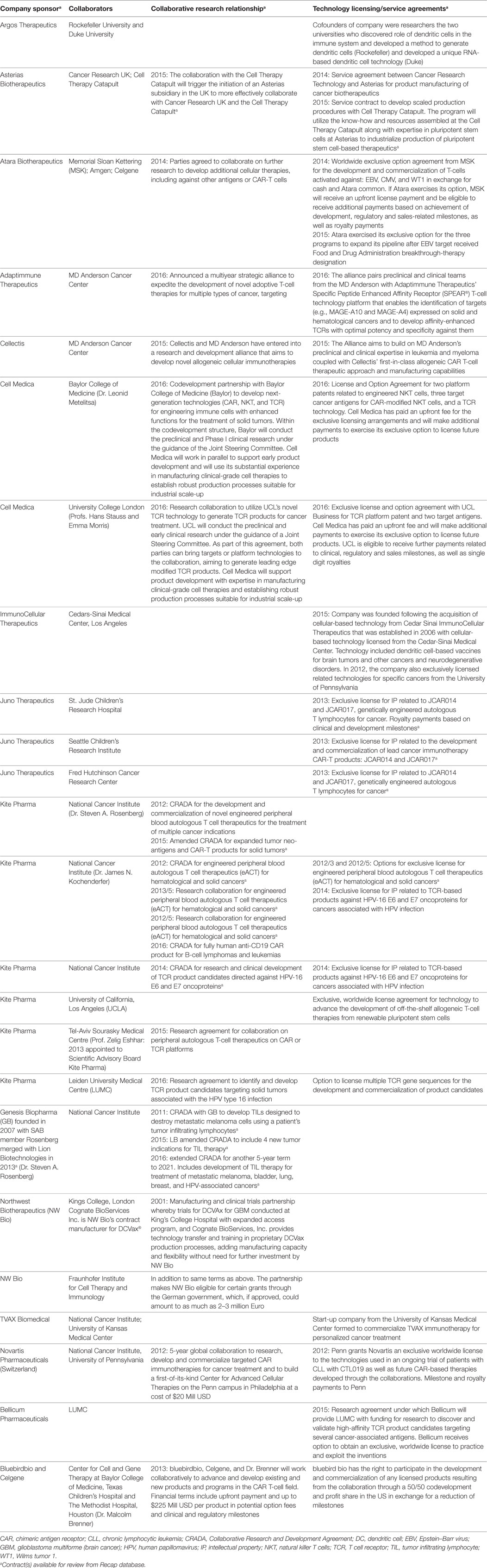
Table 2. Nature of the relationship between companies and research institutions in the development of cancer immunotherapies.
One benefit of PPPs may be in the efficient transfer of tacit knowledge (know-how) through collaborative interactions. However, our review demonstrated that market transactions in cellular immunotherapy may also account for such knowledge transfer (Table 2). For example, the agreement between Asterias Biotherapeutics (Fremont, CA, USA) and Cancer Research UK provided for negotiations to adapt the technology transfer plan associated with a joint development project to use the company’s expertise in cell-manufacturing and industrial scale-up to improve manufacturing/production of the research institute’s cellular immunotherapy candidates. The company committed to the transfer of manufacturing/production know how, including in the form of training of research institute staff. Similarly, the agreement between Northwest Biotherapeutics (NW Bio) (Bethesda, MD, USA) and Kings College London provided for technology transfer and training via its manufacturing service provider, Cognate BioServices (Memphis, TN, USA).
Another stated benefit of the collaborative agreements (especially with UK/European research institutes) was access to public funding, for example access to German funding in the agreements between the Fraunhofer Institute for Cell Therapy and Immunology and NW Bio, and between Asterias Biotherapeutics and Cancer Research UK.
In the US, the preferred model for research agreements between the National Institutes of Health (NIH) and companies is the Collaborative Research and Development Agreement (CRADA). CRADAs provide the legal framework for investigators from these two sectors to conduct research in pursuit of common goals, while leveraging their own research resources: “The purpose of a CRADA is to make Government facilities, intellectual property, and expertise available for collaborative interactions to further the development of scientific and technological knowledge into useful, marketable products” (32). All collaborators must make significant intellectual contributions to the research project or contribute materials and resources not available at the NIH. CRADAs are distinct from sponsored research. CRADAs are not a general funding mechanism, but are specific in their support of the collaborative project. Their terms ensure research freedom and may not unreasonably restrict or constrain the dissemination of research information. Nevertheless, they do support the protection of proprietary materials and intellectual property rights and may grant the industry partner an option to exclusively license intellectual property.
The seven CRADAs we identified also were bilateral in form (Table 2). However, they notably covered the identification of new cancer antigens for targeted cellular immunotherapy. This result is representative of a traditional role of research institutes in target identification for drug discovery. Target identification may be based on review of the peer-reviewed literature followed by early-phase trials to demonstrate safety and proof-of-concept in humans (33). Since most targets will prove neither safe nor efficacious, the public sector plays an important role in de-risking these for later stage development, including through research to enhance understanding of the molecular biology and possible mechanisms of action. Indeed, eight of the research agreements and six of the license agreements we identified explicitly mentioned new antigens/products as a goal.
Finally, we added Bellicum Pharmaceuticals and bluebirdbio to our list of companies because they are explicitly developing technologies that derive from university-based research to mitigate adverse events. These companies are developing molecular switch technologies for programmed cell death of CAR-T or similar cells or to mute CAR-T cell therapy associated adverse events, respectively. We also added Adaptimmune Therapeutics because it is an example of a strategic alliance for target identification. The company has entered into a strategic alliance that combines the companies T-cell technology platform that enables the identification of targets expressed in solid and hematological cancers with MD Anderson Cancer Center’s expertise in preclinical and clinical research (Table 2). Finally, we added Cellectis, which has entered into a research and development alliance, also with MD Anderson Cancer Center, to use the company’s CAR-T cell therapeutic approach and manufacturing technology and MD Anderson Cancer Center’s research expertise to develop allogeneic CAR-T cells. The latter technology has the potential to simplify the business models for manufacture and delivery of CAR-T therapies, thereby reducing costs.
Our review focused on collaborations for clinical development beyond Phase I, which may, in part explain, why we found limited evidence that the products in development resulted from precompetitive PPPs. The public–private relationships we identified were based on bilateral collaborative agreements between companies and research institutions for research based on a common goal. They rarely identified shared governance mechanisms, but rather relied on a hub and spoke model for research relationships. This focus on bilateral agreements and pure market transactions in the form of service contracts and technology licenses is representative of the commercialization focus of the field. The rapid advancement of cellular therapeutics comes with attendant hype with respect to potential efficacy and market size, as evidenced by media and other coverage and a rapid increase in the number of clinical trials and investment in private-sector companies (34).
The market enthusiasm for cellular therapeutics exists in spite of serious concerns about adverse events, business models, and the complexity of cells as therapies (34, 35). Indeed, the latest deaths in Juno Therapeutics’ clinical trial have brought criticism that therapies are being tested in terminal cancer patients without adequate understanding of their biological mechanisms and potential for adverse events (19, 20). This lack of mechanistic understanding presages an expanded role for pre- and early-stage clinical research in the province of research institutions. It may be summed up by the saying “more haste, less speed,” which is defined by the Cambridge English Dictionary as meaning that if you try to do things too quickly, it will take you longer in the end.
In addition to an enhanced role for academic-industry collaborations in overcoming adverse events, we identified one case—Asterias Biotherapeutics—that exemplified the role of PPPs in the development of production/manufacturing (Table 2). This implies a greater role for not only the clinical research community, but also for bioengineers that specialize in cell processing, manipulation, sorting, and expansion to clinical dosage levels (25, 36). The research agreements we identified were focused on clinical partnerships and therefore raise opportunities for an expanded set of interdisciplinary partners. At this juncture, there is an important role for industry expertise, as evidenced by some agreements for cGMP scale-up for clinical application.
Finally, there is a clear convergence of interests between research institutions and industry in the identification and preclinical characterization of novel cancer antigens, both to expand the types of tumors that may be targeted by cellular immunotherapies and reduce on-target, off-cancer adverse effects. As stated above, the de-risking of novel targets falls within the purview of research institutions and target identification has been the subject of successful PPPs. The best known of these is the Structural Genomics Consortium (SGC) that creates an open collaborative network of scientists across sectors to identify druggable protein targets and develop chemical probes for drug discovery (29, 37). The differences between the SGC and the research collaborations we identified are the large number of partners within the SGC and its commitment to open science (38). Its open science model and common governance structure stands in contrast to a proprietary model based on options to license codeveloped intellectual property. PPPs such as the SGC bring the added benefit of enabling systematic, high-throughput research that avoids duplication of effort and reduces costs.
While the SGC is built on an open science model, other PPPs enable commercialization based on formal intellectual property rights within an open innovation platform. Such a model may be more palatable in the context of cellular immunotherapy, given the rapid advance to clinical translation in the field and the fact that the field is dominated by biotechnology rather than larger pharmaceutical companies. One example is the European Lead Factory, a pan-European drug discovery project of 30 partners established in 2013, which has received E196 million in funding from the Innovative Medicines Initiative and other sources (39). The European Lead Factory supports the generation of a compound library and an industry-standard screening center, providing free access to around 500,000 novel compounds. Any researcher from a European academic center or a small- and medium-sized enterprise (SME) can apply to screen a drug target of interest and to which the researcher/SME has intellectual property rights. If a screening application is accepted by the European Lead Factory, the parties enter into a standard contract that ensures confidentiality of the screening program and resulting data. Researchers/SMEs receiving the results are able to manage them as they see fit, but are given the option to partner with one of the participating pharmaceutical companies. Researchers are free to make results public, following the PPP’s publication guidelines. However, if the screening program results in patent rights, there is an obligation to share benefits with the European Lead Factory. The researcher/SME can pay the PPP a fixed amount while filing the patent, a higher amount 2 years following filing, or a percentage of royalties generated by the patent.
Given that cellular immunotherapies are highly personalized, autologous therapies, it is expected that there might be an additional convergence in the discovery of cancer targets for cellular immunotherapies and precision medicine initiatives. The latter are building PPPs focused on the identification and development clinical protein-based biomarkers. For example, the Personalized Medicine Partnership for Cancer is a public–private consortium, in part funded by the Government of Québec, Canada (http://pmpc-org.com/en/). It partners a Quebec-based multidisciplinary network of clinicians, academic scientists and other members of the translational research community with private-sector partners: Caprion, a Montreal-based biotechnology company, Oncozyme Pharma (Montreal, QC, Canada), Pfizer Canada (Kirkland, QC, Canada), and Sanofi Canada (Laval, QC, Canada). Exemplifying the convergence between biomarker and cancer antigen discovery, in 2016, Caprion presented results on the use of its platform to identify neo-epitopes for cancer vaccines and adoptive T-cell therapies (40). Similarly, in 2012, the German Ministry for Education and Research granted 1.2 Mill Euro over 3 years to a public–private Consortium of Individualized Vaccines for Cancer (41).
In conclusion, a strategic case may be made to establish multiparty PPPs with governance structures to advance two areas that are crucial to the safe and effective translation of cellular immunotherapies for cancer: cancer antigen discovery and characterization and improved cell processing/manufacturing and related activities. This conclusion is supported in the Recommendations of the Blue Ribbon Panel on Research Opportunities for the Vice President’s Cancer “Moonshot,” which may still proceed in some form under the new US administration, identified a strategic need for better coordination for data and tumor samples from cancer patients that may benefit from a series of PPPs (42). To advance immunotherapies, it recommended the integration of methods and sequencing data, especially with respect to proteins that are uniquely expressed in pediatric cancers, supported by the integration of PPPs “to develop the right immunotherapeutic tools (drugs) to exploit these targets” (42).
In the rush toward the competitive end of the translational continuum for cancer cellular immunotherapy and the attendant focus on commercialization of research, many gaps have appeared in our understanding of cellular biology, immunology, and bioengineering. In the US, the model of bilateral agreements between leading research institutions and the private sector may be inadequate to efficiently harness the interdisciplinary skills and knowledge of the public and private sectors to bring these promising therapies to the clinic for the benefit of cancer patients.
TB designed the review, drafted, edited, and submitted manuscript; KB, SL, J-SD, and EG contributed to the review, commented on draft manuscript, and approved submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
TB and EG’s contributions were supported by a grant from the BioCanRx NCE: Clinical, Social and Economic Impact Program (PI: TB); a Stem Cell Network Strategic Core Grant: Public Policy & ELSI Research in the Stem Cell Field (PI: TB); and the PACEOMICS project (co-lead investigators: C. McCabe and TB) funded by Genome Canada, Genome Alberta, the Canadian Institutes for Health Research, and Alberta Innovates-Health Solutions. KB, SL, and J-SD’s contributions were supported by Genome Canada’s Personalized Cancer ImmunoTherapy Program (www.pcitp.org) and its partners Génome Québec and the Canadian Institutes for Health Research. The authors wish to thank Dr. Marc Lussier (PCITP) for helpful feedback and Zackariah Breckenridge, Katherine Fu, Jaclyn Hutchinson, and Yael Mansour for research assistance.
1. Robinson H, Carrillo P, Anumba CJ, Patel M. Governance & Knowledge Management for Public-Private Partnerships. Oxford, UK: Wiley-Blackwell (2010). 247 p.
3. Huggett B. Biotech’s wellspring – a survey of the health of the private sector in 2015. Nat Biotechnol (2016) 34:608–15. doi:10.1038/nbt.3600
4. June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med (2015) 7:280s7. doi:10.1126/scitranslmed.aaa3643
5. Fojo T, Mailankody S, Lo A. Unintended consequences of expensive cancer therapeutics-the pursuit of marginal indications and a me-too mentality that stifles innovation and creativity: the John Conley Lecture. JAMA Otolaryngol Head Neck Surg (2014) 140:1225–36. doi:10.1001/jamaoto.2014.1570
6. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med (2014) 371:1507–17. doi:10.1056/NEJMoa1407222
7. Porter DL, Hwang W-T, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med (2015) 7:303ra139. doi:10.1126/scitranslmed.aac5415
8. Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood (2016) 128:1688–700. doi:10.1182/blood-2016-04-711903
9. Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med (2015) 373:1040–7. doi:10.1056/NEJMoa1504542
10. Dudley ME, Gross CA, Somerville RP, Hong Y, Schaub NP, Rosati SF, et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol (2013) 31:2152–9. doi:10.1200/JCO.2012.46.6441
11. Hinrichs CS, Rosenberg SA. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol Rev (2014) 257:56–71. doi:10.1111/imr.12132
12. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science (2015) 348:62–8. doi:10.1126/science.aaa4967
13. Rosenberg SA. Finding suitable targets is the major obstacle to cancer gene therapy. Cancer Gene Ther (2014) 21:45–7. doi:10.1038/cgt.2014.3
14. Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med (2013) 5:197ra03. doi:10.1126/scitranslmed.3006034
15. Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood (2013) 122:863–71. doi:10.1182/blood-2013-03-490565
16. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther (2010) 18:843–51. doi:10.1038/mt.2010.24
17. Barrett DM, Grupp SA, June CH. Chimeric antigen receptor- and TCR-modified T cells enter main street and wall street. J Immunol (2015) 195:755–61. doi:10.4049/jimmunol.1500751
18. Kohrt HE, Tumeh PC, Benson D, Bhardwaj N, Brody J, Formenti S, et al. Immunodynamics: a cancer immunotherapy trials network review of immune monitoring in immuno-oncology clinical trials. J Immunother Cancer (2016) 4:15. doi:10.1186/s40425-016-0118-0
19. Adams B. Juno CAR-T study put on hold, again, after cerebral edemas and fatality, again. FierceBiotech (2016). Available from: http://www.fiercebiotech.com/biotech/juno-car-t-study-put-hold-again-after-cerebral-edemas-and-fatality-again
20. Garde D, Keshavan M. Two Patient Deaths Halt Trial of Juno’s New Approach to Treating Cancer. STAT News (2016). Available from: https://www.statnews.com/2016/11/23/juno-cancer-immunotherapy-deaths-2/
21. Garde D, Keshavan M. Juno Halts Its Immunotherapy Trial for Cancer after Three Patient Deaths. STAT News (2016). Available from: https://www.statnews.com/2016/07/07/juno-cancer-immunotherapy-deaths/
22. Lawrence S. FDA Clears Juno to Resume CAR-T Trial. FierceBiotech (2016). Available from: http://www.fiercebiotech.com/biotech/fda-clears-juno-to-resume-car-t-trial
23. Hettle R, Corbett M, Hinde S, Hodgson R, Jones-Diette J, Woolacott N, et al. Exploring the Assessment and Appraisal of Regenerative Medicines and Cell Therapy Products. York, UK: University of York (2015). Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/Science%20policy%20and%20research/final-york-report-march-16.pdf
24. Hallam K, Paton J. Novartis Dissolves Its Cell Therapy Unit, Cutting 120 Positions. Bloomberg (2016). Available from: https://www.bloomberg.com/news/articles/2016-08-31/novartis-dissolves-its-cell-therapy-unit-cutting-120-positions
25. Brindley DA, Mason C. Cell therapy commercialisation. In: Alta A, editor. Progenitor and Stem Cell Technologies and Therapies. Oxford, UK: Woodhead Publishing (2012). p. 169–205.
26. OECD Working Party on Biotechnology. Collaborative Mechanisms for Intellectual Property in the Life Sciences. Paris, France: OECD (2011). Available from: https://www.oecd.org/sti/biotech/48665248.pdf
27. Polanyi M. Personal Knowledge: Towards a Post-Critical Philosophy. Expanded Edition. Chicago, IL: University of Chicago Press (2015). 428 p.
28. Bubela T, FitzGerald GA, Gold ER. Recalibrating intellectual property rights to enhance translational research collaborations. Sci Transl Med (2012) 4:122cm3. doi:10.1126/scitranslmed.3003490
29. Edwards AM, Bountra C, Kerr DJ, Willson TM. Open access chemical and clinical probes to support drug discovery. Nat Chem Biol (2009) 5:436–40. doi:10.1038/nchembio0709-436
30. Friend SH. The need for precompetitive integrative bionetwork disease model building. Clin Pharmacol Ther (2010) 87:536–9. doi:10.1038/clpt.2010.40
31. Bonter K, Breckenridge Z, Lachance S, Delisle J-S, Bubela T. Opportunities and challenges for the cellular immunotherapy sector: a global landscape of clinical trials. Regen Med (2017) (in press).
32. Office of Intramural Research, Office of Technology Transfer. CRADAs. Wachington, DC: National Institutes of Health (2016). Available from: https://www.ott.nih.gov/cradas
33. Strovel J, Sittampalam GS, Coussens NP, Hughes M, Inglese J, Kurtz A, et al. Early drug discovery and development guidelines: for academic researchers, collaborators, and start-up companies. In: Sittampalam GS, Coussens NP, Nelson H, Arkin M, Auld D, Austin C, et al., editors. Assay Guidance Manual. Bethesda, MD: Eli Lilly & Company, National Center for Advancing Translational Sciences (2012). Available from: https://www.ncbi.nlm.nih.gov/books/NBK92015/
34. EP Vantage. Not for the Faint of CAR-T: The CAR-T Therapy Landscape in 2015. London, UK: Evaluate (2015). Available from: http://www.evaluategroup.com/public/Reports/EPVantage-CAR-T-Therapy-Landscape-in-2015.aspx
35. EP Vantage. EP Vantage’s 2016 Asco Backgrounder. Boston, MA: EvaluatePharma (2016). Available from: http://www.evaluategroup.com/public/reports/EPVantage-ASCO-2016-Backgrounder.aspx
36. Kaiser AD, Assenmacher M, Schroder B, Meyer M, Orentas R, Bethke U, et al. Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer Gene Ther (2015) 22:72–8. doi:10.1038/cgt.2014.78
37. Arrowsmith CH, Audia JE, Austin C, Baell J, Bennett J, Blagg J, et al. The promise and peril of chemical probes. Nat Chem Biol (2015) 11:536–41. doi:10.1038/nchembio.1867
38. Structural Genomics Consortium. (2016). Available from: http://www.thesgc.org/
39. The Euroopean Lead Factory. (2017). Available from: https://www.europeanleadfactory.eu/
40. Caprion. (2016). Available from: http://www.caprion.com/medias/doc/news/Caprion-Press-Release-20160202.pdf
41. Consortium of Individualized Vaccines for Cancer (IVAC). IVAC Consortium Wins BMBF Grant to Develop Individualized Cancer Vaccine. (2012). Available from: http://tron-mainz.de/blog/2012/02/15/ivac-consortium-wins-bmbf-grant/
42. Blue Ribbon Panel. Cancer Moonshot Blue Ribbon Panel Report. (2016). Available from: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel/blue-ribbon-panel-report-2016.pdf
Keywords: cellular immunotherapy, cancer, adoptive cellular transfer, CAR-T cell, public–private partnerships, Collaborative Research and Development Agreements, technology licensing
Citation: Bubela T, Bonter K, Lachance S, Delisle J-S and Gold ER (2017) More Haste, Less Speed: Could Public–Private Partnerships Advance Cellular Immunotherapies? Front. Med. 4:134. doi: 10.3389/fmed.2017.00134
Received: 22 December 2016; Accepted: 25 July 2017;
Published: 16 August 2017
Edited by:
Michel Goldman, Free University of Brussels, BelgiumReviewed by:
Bruno Laugel, Cardiff University, United KingdomCopyright: © 2017 Bubela, Bonter, Lachance, Delisle and Gold. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tania Bubela, dGJ1YmVsYUBzZnUuY2E=
†Present address: Tania Bubela, Faculty of Health Sciences, Simon Fraser University, Burnaby, BC, Canada
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.