
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 20 January 2017
Sec. Intensive Care Medicine and Anesthesiology
Volume 4 - 2017 | https://doi.org/10.3389/fmed.2017.00002
 Sasima Dusitkasem1,2*
Sasima Dusitkasem1,2* Blair H. Herndon1
Blair H. Herndon1 Monsicha Somjit1,3
Monsicha Somjit1,3 David L. Stahl1
David L. Stahl1 Emily Bitticker1,4
Emily Bitticker1,4 John C. Coffman1
John C. Coffman1
Purpose: To compare maternal and fetal effects of intravenous phenylephrine and ephedrine administration during spinal anesthesia for cesarean delivery in high-risk pregnancies.
Source: An extensive literature search was conducted using the US National Library of Medicine, MEDLINE search engine, Cochrane review, and Google Scholar using search terms “ephedrine and phenylephrine,” “preterm and term and spinal hypotension,” “preeclampsia and healthy parturients,” or “multiple and singleton gestation and vasopressor.” Society of Obstetric Anesthesia and Perinatology meeting abstracts for the past 4 years were also searched for relevant studies.
Principle findings: Both phenylephrine and ephedrine can be safely used to counteract hypotension after spinal anesthesia in patients with uteroplacental insufficiency, pregnancy-induced hypertension, and in non-elective cesarean deliveries. Vasopressor requirements before delivery in high-risk cesarean sections are reduced compared to healthy parturients. Among the articles reviewed, there were no statistically significant differences in umbilical arterial pH, umbilical venous pH, incidence of fetal acidosis, Apgar scores, or maternal hypotension when comparing maternal phenylephrine and ephedrine use.
Conclusion: From the limited existing data, phenylephrine and ephedrine are both appropriate selections for treating or preventing hypotension induced by neuraxial blockade in high-risk pregnancies. There is no clear evidence that either medication is more effective at maintaining maternal blood pressure or has a superior safety profile in this setting. Further investigations are required to determine the efficacy, ideal dosing regimens, and overall safety of phenylephrine and ephedrine administration in high-risk obstetric patients, especially in the presence uteroplacental insufficiency.
Maintenance of hemodynamic stability from a sympathetic blockade after neuraxial anesthetic techniques for cesarean delivery remains a significant clinical problem (1). To counteract maternal hypotension, intravenous fluid and vasopressor drugs are required.
Historically, ephedrine was considered the preferred vasopressor for management of spinal-induced hypotension in healthy parturients. Ephedrine has a relatively slow onset and long duration of action compared to phenylephrine and has a predominantly β-agonist effect (2). Studies in pregnant ewes demonstrated that ephedrine was effective in maintaining arterial blood pressure and was associated with greater preservation of uteroplacental blood flow compared with other vasopressors (3, 4). Historically, phenylephrine, a direct α1-agonist, was avoided due to concerns regarding potential uterine blood flow reduction (3). However, more recent clinical evidence has consistently demonstrated that phenylephrine is effective for maintaining blood pressure during elective cesarean deliveries with spinal anesthesia, does not exert an adverse effect on the fetus and is associated with a lower rate of fetal acidosis compared to ephedrine (5, 6).
In 2002, a quantitative systemic review by Lee et al. examined the role of ephedrine and phenylephrine in obstetric patients. The authors reported that phenylephrine use was associated with higher umbilical arterial (UA) pH values compared to ephedrine (2). Subsequent studies conducted in healthy parturient undergoing elective cesarean deliveries have consistently demonstrated that phenylephrine use reduces incidence of fetal acidosis compared to ephedrine (5–9) and is more effective at maintaining maternal blood pressure (7, 8) and preventing intraoperative nausea and vomiting (IONV) (5, 7, 8) compared to ephedrine. It has been demonstrated that ephedrine crosses the placenta to a greater extent than phenylephrine and stimulation of β-adrenergic receptors in the fetus results in an increased fetal metabolic rate (5, 7, 8). Ephedrine-induced fetal tachycardia and acidosis appears to depend on dosage and timing of drug administration prior to delivery (8–10).
There is a paucity of evidence to guide clinical decisions regarding vasopressor use in non-elective cesarean deliveries or high-risk parturients. The majority of data that have shaped current practices of vasopressor use for spinal-induced hypotension have been done in healthy women undergoing elective cesarean deliveries. These results cannot necessarily be extrapolated to patients diagnosed with impaired uteroplacental blood flow or with pregnancy-induced hypertension. This review examines the available evidence regarding the efficacy and safety of phenylephrine and ephedrine administration in high-risk cesarean deliveries.
In order to compare maternal and fetal effects of intravenous phenylephrine and ephedrine administration during spinal anesthesia for cesarean delivery in high-risk pregnancies, an extensive literature search was conducted through the United States National Library of Medicine using the MEDLINE search engine, Cochrane review, and Google Scholar. The search was limited to articles published in English prior to October 11, 2016. Search terms “ephedrine and phenylephrine,” “preterm and term and spinal hypotension,” “preeclampsia and healthy parturients,” or “multiple and singleton gestation and vasopressor” were used to identify relevant articles. Based on our review, 10 articles that investigated vasopressor use in high-risk human pregnancies were located, and 5 of these specifically compared the effects of phenylephrine and ephedrine in high-risk obstetric patients. Society of Obstetric Anesthesia and Perinatology meeting abstracts for the past 4 years were also searched for studies comparing phenylephrine and ephedrine in high-risk parturients.
In normal pregnancy, a low-resistance vascular pathway to the intervillous space develops in order to ensure adequate perfusion to meet the needs of the growing fetus and placenta (11–13). Increases in uterine and UA vascular resistance detected on ultrasound surveillance of high-risk pregnancies are important in predicting fetal hypoxia and optimizing fetal outcomes (14–18). Placental perfusion and fetal well-being can also potentially be impacted by both spinal-induced hypotension and the vasopressors used to prevent this hypotension. Despite evidence in favor of phenylephrine in healthy human pregnancies, there is concern regarding potential reductions in uteroplacental blood flow with administration of α1-adrenergic agonists based on studies in healthy pregnant ewes and also pregnant ewes with uteroplacental insufficiency (3, 19–21). However, there are several limitations to the application of animal study results to clinical medicine. Different species may have dissimilar adrenergic receptor distribution, affinity to vasopressors, or placental transfer of vasopressors. The human placenta is characterized by a thinner hemomonochorial structure that may allow a greater diffusion of ephedrine compared with the synepitheliochorial placenta of sheep (22). Additionally, animal experiments were performed under isoflurane anesthesia, which might enhance the pulmonary vasodilator response to the β-adrenoceptor agonist (23).
Studies on the effects of ephedrine and phenylephrine administration on human uteroplacental blood flow have been limited to elective cesarean deliveries in uncomplicated pregnancies. Increased resistance in uterine and umbilical artery blood flow is associated with higher velocity indices measured by the systolic/diastolic (S/D) ratio, the pulsatility index (PI), and the resistance index (24). Alahuhta et al. compared the effects of phenylephrine and ephedrine infusions on uteroplacental vascular resistance during spinal anesthesia in healthy parturients and observed a significant increase in the PI of uterine and placental arcuate arteries with phenylephrine administration, though no significant change from baseline with ephedrine (18). Recently, Guo et al. examined the effects of phenylephrine or ephedrine infusion on placental vascular resistance during spinal anesthesia and observed no significant differences in the umbilical artery or uterine artery vascular resistance, though the uterine arterial vascular resistance was elevated from baseline in both study groups (25). No significant differences in fetal acid–base status or clinical outcomes were noted between phenylephrine and ephedrine study groups in either study (18, 25). Ngan Kee et al. investigated the effects of ephedrine and metaraminol on uterine vascular resistance in healthy parturients undergoing elective cesarean section. They found that changes in uterine artery PI were similar between groups; however, the patients receiving ephedrine had lower UA and umbilical venous (UV) pH values (26).
Based on our review, there have not been any studies specifically examining the effects of phenylephrine or ephedrine on uteroplacental blood flow in patients with preeclampsia, uteroplacental insufficiency, or other high-risk conditions. Moreover, an association between prenatal stress and changes in Doppler waveform parameters remain inconclusive due to the methodological limitations of available studies (27). Further investigations are necessary to clarify the effect of vasopressors on uteroplacental blood flow and fetal well-being in these high-risk settings.
In elective cesarean deliveries, phenylephrine has been associated with higher fetal pH compared with ephedrine (5–10), suggesting that the effects of phenylephrine on uterine blood flow and the fetus are not harmful in healthy pregnant women (28). However, there is less evidence available in cases of unscheduled cesarean deliveries or in the setting of uteroplacental insufficiency. Based on limited available evidence, clinical outcomes of the neonates in the presence of potential uteroplacental insufficiency show no statistically significant differences in UA and UV pH, incidence of fetal acidosis, or Apgar scores between phenylephrine and ephedrine administration (Table 1).
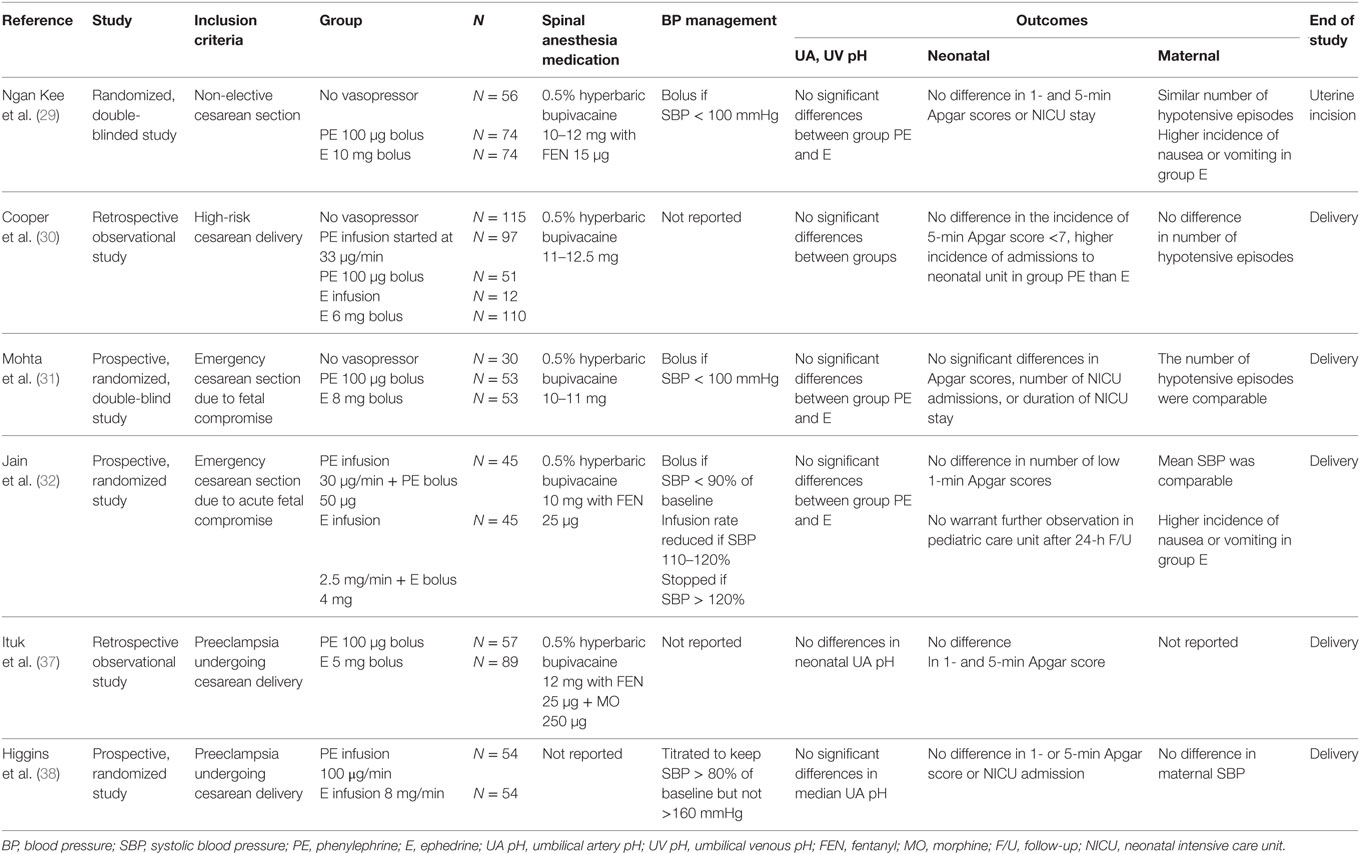
Table 1. Clinical studies comparing the effect of ephedrine and phenylephrine on “fetal outcome” in the setting of uteroplacental hypoperfusion and hypertensive disorders of pregnancy.
A prospective study published by Ngan Kee et al. (29) enrolled 204 patients who were randomized to receive intermittent boluses of either phenylephrine (100 μg) or ephedrine (10 mg) to treat episodes of spinal-induced hypotension (defined as systolic BP ≤100 mmHg) during non-elective cesarean deliveries. Neonatal Apgar scores, UA pH, UV pH, and base excess values were not significantly different between ephedrine and phenylephrine groups, though higher UA and UV lactate concentrations and greater incidence of nausea and vomiting were observed in patients receiving ephedrine (29). It is important to note that this study included patients in which cesarean deliveries were booked on the day of surgery, including patients in the labor ward who eventually underwent cesarean delivery. Potential fetal compromise was noted in 48 patients in this study. The subanalysis of these cases noted similar UA and UV blood gas parameters and lactate concentrations in phenylephrine and ephedrine groups, with the exception of a lower UA PO2 in the phenylephrine group. It is important to note that patients with preexisting hypertension or pregnancy-induced hypertension were excluded from this investigation (29).
In 2010, Cooper et al. presented a retrospective observational study in patients with increased risk of fetal compromise who underwent cesarean delivery under spinal anesthesia (30). Prophylactic infusions of phenylephrine (33 μg/min) or ephedrine and intermittent boluses of either ephedrine (6 mg) or phenylephrine (100 μg) were all utilized as the primary means of preventing or treating spinal-induced hypotension. Similar neonatal Apgar scores and UA pH values were found in patients receiving ephedrine, phenylephrine, or no vasopressors (30). Notably, the incidence of pregnancy-induced hypertension was significantly greater among patients not requiring vasopressors. Multiple regression analysis noted the only variable associated with altered UA pH was a non-reassuring fetal heart rate (HR) tracing prior to cesarean delivery. The authors noted that increased incidence of prematurity and shorter spinal-to-delivery intervals due to the urgent nature of these cases might affect total dose of ephedrine given before delivery. In this study, the ephedrine dose was much lower than ephedrine dose used in low-risk cesarean deliveries (12 vs. 52 mg), thereby reducing potential adverse metabolic effects in the fetus (30). Ngan Kee et al. (29) administered similar low doses of ephedrine (median 10 mg) in their prospective study of non-elective cesarean deliveries compared to the total dose of ephedrine (median 54 mg) reported in their study of elective cesarean deliveries in low-risk patients (8).
A recent study by Mohta et al. in patients undergoing cesarean deliveries due to potential fetal compromise reported data from 106 patients randomized to receive either ephedrine (8 mg) or phenylephrine (100 mg) boluses to treat episodes of spinal-induced hypotension (systolic BP ≤100 mmHg) (31). The number of vasopressor boluses and hypotensive episodes were similar between phenylephrine and ephedrine groups. No statistically significant intergroup differences were noted in UA and UV pH, incidence of fetal acidosis, or Apgar scores. The spinal-to-delivery interval in these urgent cesarean deliveries was relatively short (mean 9 min), and the authors suggest that this may have contributed to the reduced ephedrine requirement and decreased fetal drug exposure (31). Patients with preeclampsia were excluded from this investigation (31).
Another recent investigation by Jain et al. prospectively compared prophylactic ephedrine infusion (2.5 mg/min) to phenylephrine infusion (30 μg/min) to manage spinal-induced hypotension in 90 patients undergoing cesarean delivery due to signs of acute fetal compromise in the intrapartum period (32). The authors reported no differences in fetal acidosis with either prophylactic phenylephrine or ephedrine infusion and no adverse neonatal outcomes during the period of observation in the study (32).
Hypertensive disorders in pregnancy are associated with maternal morbidity and mortality, accounting for 9.4% of pregnancy-related deaths in the United States during 2006–2010 (33). Inadequate trophoblast invasion, leading to incomplete remodeling of the uterine spiral arteries, is considered to be a primary cause of the placental ischemia (11–13). The resulting elevated vascular resistance may account for the differences in reactivity of vessels to vasoconstrictor drugs (34). There is concern that administering vasopressors may impair uteroplacental blood flow in preeclamptic patients given the increased responsiveness to vasomotor stimuli.
The incidence of spinal-induced hypotension and the need for vasopressor treatment has been noted to be reduced in patients with preeclampsia in comparison to both healthy term parturients (35) and preterm cesarean deliveries (36). The authors of these investigations conclude that preeclampsia-associated factors likely account for the lower incidence of hypotension (35, 36). Average ephedrine doses of 6–10 mg were administered in preeclamptic patients without reports of adverse maternal or fetal events in these investigations (35, 36). Similarly, a retrospective study by Cooper et al. noted that the patients were less likely to require vasopressor support for hypotension in the setting of pregnancy-induced hypertension (30).
Few studies have directly compared the use of ephedrine and phenylephrine for spinal-induced hypotension in preeclamptic patients. Cooper et al. reported that non-reassuring fetal HR tracing was the only variable associated with lower UA pH on multiple regression analysis, while the use of ephedrine, phenylephrine, or the presence of pregnancy-induced hypertension were not associated with alterations in UA pH (30). Ituk et al. also performed a retrospective study comparing phenylephrine with ephedrine for managing hypotension after spinal anesthesia in preeclamptic patients and reported no difference in UA pH between the two study groups (37). However, the ephedrine group was characterized by significantly lower gestational age at delivery compared to the phenylephrine group (mean 32 vs. 36 weeks gestation), which may have contributed to reduced incidence of hypotension and fewer doses of vasopressors due to reduced aortocaval compression by the smaller fetus (37). Similar to Cooper et al. (30), this study found that non-reassuring fetal heart tracing prior to delivery was the only factor significantly associated with lower UA pH (37). An abstract presented at the Society of Obstetric Anesthesia and Perinatology (SOAP) 2015 conference described a prospective study comparing phenylephrine infusion (100 μg/min) or ephedrine infusion (8 mg/min) for prevention of spinal-induced hypotension in preeclamptic patients. They observed similar UA pH values and incidence of fetal acidosis (UA pH < 7.20) in phenylephrine and ephedrine study groups and concluded that both vasopressors appear to be safe in preeclampsia (38) (Table 1).
Based on the limited data available, it appears that both ephedrine and phenylephrine are suitable options for managing spinal-induced hypotension in patients with hypertensive disorders of pregnancy. In women with preeclampsia, there are no apparent differences between phenylephrine and ephedrine with regard to UA or UV pH, and neonatal outcomes (30, 37, 38). The reduced incidence of spinal-induced hypotension in preeclampsia (35, 36) and correspondingly lower doses of vasopressors may account for these observations.
In the studies reviewed, a variety of phenylephrine and ephedrine dosing regimens were used for either prevention of spinal-induced hypotension or to treat hypotension in high-risk obstetric patients, making it difficult to draw conclusions. Based on the limited available evidence, phenylephrine and ephedrine appear to be similarly effective in preventing and treating spinal-induced hypotension in parturients with potential uteroplacental insufficiency (29–32) or hypertensive disorders of pregnancy (30, 37, 38). These findings diverge from studies in healthy parturients, in which phenylephrine has been observed to be more effective at maintaining maternal blood pressure (7, 8) and preventing IONV (5, 7, 8) compared to ephedrine. Compared to healthy pregnancies, there appears to be reduced incidence of hypotension and lower vasopressor requirements in patients with uteroplacental insufficiency or hypertensive disorders of pregnancy (29–32, 37), which may be due to a combination of increased incidence of prematurity, shorter incision-to-delivery intervals in urgent circumstances, or preeclampsia-associated factors.
During spinal anesthesia, preeclampsia patients maintain a high vascular tone and typically display a limited decrease in mean arterial pressure. Preeclampsia-induced endothelial dysfunction leads to increases in endothelin and thromboxane production, decreased vasodilator synthesis, and sensitizes the vasculature to vasoconstrictors (39). Previous studies indicated that spinal anesthesia-induced hypotension was less frequent and less severe in preeclamptic patients when compared to normotensive parturients, and minimal doses of vasopressors are typically required to restore maternal blood pressure to baseline (35, 36, 40) (Table 2). Similarly, Ituk et al. recently conducted a retrospective comparison of phenylephrine and ephedrine for management of spinal-induced hypotension in preeclamptic patients and observed that approximately 50% of patients did not experience hypotension requiring vasopressor treatment following spinal anesthesia (37).
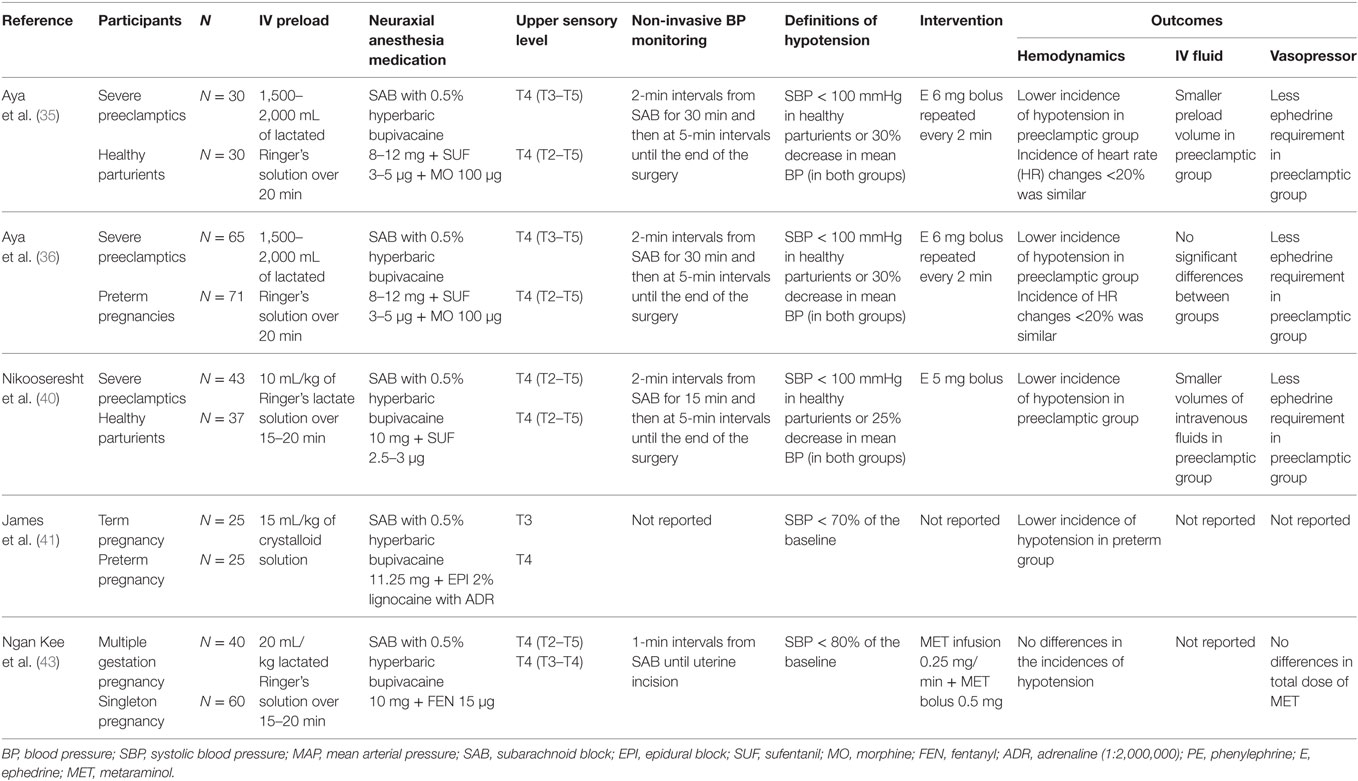
Table 2. Vasopressor use in high risk pregnancy compared to normal pregnancy undergoing Cesarean delivery.
Preterm women have less aortocaval compression due to a smaller uterine mass, which has been observed to decrease the incidence of hypotension and requires smaller doses of vasopressors than those in term pregnancy (41). However, the frequency and magnitude of spinal hypotension in preeclampsia patients has been observed to be less than in women with preterm pregnancies (36). The risk of hypotension among the preeclamptic group was almost two times lower than that in the preterm group (relative risk = 0.603; 95% confidence interval, 0.362–1.003; P = 0.044), and the ephedrine requirement to restore blood pressure to baseline level was less than in the preterm group (9.8 ± 4.6 vs. 15.8 ± 6.2 mg, respectively, P = 0.031) (36).
On the other hand, multiple gestations do not appear to be a risk factor for developing hypotension after regional anesthesia for cesarean deliveries, despite having a greater uterine mass (42). Ngan Kee et al. (43) conducted a prospective study comparing vasopressor requirements in twin gestations vs. singletons. There were no differences in the incidences of hypotension, nausea, vomiting, or vasopressor dosage between study groups (Table 2).
The maternal HR is significantly higher after ephedrine administration (29, 31, 32), while the incidence of maternal bradycardia is significantly greater after phenylephrine administration (29, 31, 32), though these differences do not appear to significantly impact clinical outcomes in high-risk obstetric patients. As a selective α1-adrenergic receptor agonist, phenylephrine-induced increases in blood pressure activate the baroreceptor reflex and cause bradycardia. However, these bradycardic events can be minimized or prevented by careful bolus dosing of phenylephrine, or minimizing the infusion rate. Furthermore, with these measures in place, no detrimental effects on maternal and neonatal outcome have been observed in normal (44) or in high-risk parturients (29, 31, 32).
The management of hypotension during cesarean delivery under spinal anesthesia remains challenging. The administration of vasopressors is often necessary, despite other measures such as intravenous crystalloid infusions. Phenylephrine is currently the vasopressor of choice for preventing or treating spinal-induced hypotension in many practices, as many studies in elective cesarean deliveries have demonstrated phenylephrine to be associated with more favorable fetal acid–base status and greater effectiveness in preventing hypotension and IONV. However, phenylephrine use in the presence of preexisting fetal compromise continues to be controversial due to concern of potential uterine blood flow reduction.
Based on limited available evidence, it appears that phenylephrine and ephedrine are similarly safe and effective, and both may be used to prevent or treat spinal-induced hypotension in the setting of potential uteroplacental insufficiency and preeclampsia. The current literature does not show any statistically significant differences in terms of maternal and neonatal outcomes between phenylephrine and ephedrine in these high-risk patients. Further randomized double-blind studies are required to further clarify the safety and efficacy and determine effective vasopressor dosing regimens in high-risk obstetric patients.
SD and BH conducted a review of the literature. SD, MS, EB, BH, DS, and JC prepared the body of the manuscript. JC critically reviewed the publication. All the authors endorsed the final form of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge Nicolaus Turner of The Ohio State University Wexner Medical Center, Department of Anesthesiology.
The authors declare that their work on this submitted manuscript was not financially supported by funding from any entity within their institution or by any outside organization.
1. Rout CC, Rocke DA. Prevention of hypotension following spinal anesthesia for cesarean section. Int Anesthesiol Clin (1994) 32(2):117–36. doi: 10.1097/00004311-199432020-00010
2. Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg (2002) 94(4):920–6. doi:10.1097/00000539-200204000-00028
3. Ralston DH, Shnider SM, DeLorimier AA. Effects of equipotent ephedrine, metaraminol, mephentermine, and methoxamine on uterine blood flow in the pregnant ewe. Anesthesiology (1974) 40(4):354–70. doi:10.1097/00000542-197404000-00009
4. James FM III, Greiss FC Jr, Kemp RA. An evaluation of vasopressor therapy for maternal hypotension during spinal anesthesia. Anesthesiology (1970) 33(1):25–34. doi:10.1097/00000542-197007000-00010
5. Cooper DW, Carpenter M, Mowbray P, Desira WR, Ryall DM, Kokri MS. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology (2002) 97(6):1582–90. doi:10.1097/00000542-200212000-00034
6. Ngan Kee WD, Lee A. Multivariate analysis of factors associated with umbilical arterial pH and standard base excess after caesarean section under spinal anaesthesia. Anaesthesia (2003) 58(2):125–30. doi:10.1046/j.1365-2044.2003.02888.x
7. Ngan Kee WD, Khaw KS, Tan PE, Ng FF, Karmakar MK. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology (2009) 111(3):506–12. doi:10.1097/ALN.0b013e3181b160a3
8. Ngan Kee WD, Lee A, Khaw KS, Ng FF, Karmakar MK, Gin T. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: the effects on fetal acid-base status and hemodynamic control. Anesth Analg (2008) 107(4):1295–302. doi:10.1213/ane.0b013e31818065bc
9. Cooper DW, Gibb SC, Meek T, Owen S, Kokri MS, Malik AT, et al. Effect of intravenous vasopressor on spread of spinal anaesthesia and fetal acid-base equilibrium. Br J Anaesth (2007) 98(5):649–56. doi:10.1093/bja/aem056
10. Ngan Kee WD, Khaw KS, Lee BB, Lau TK, Gin T. A dose-response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg (2000) 90(6):1390–5. doi:10.1097/00000539-200006000-00024
11. Espinoza J, Romero R, Mee Kim Y, Kusanovic JP, Hassan S, Erez O, et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med (2006) 34(6):447–58. doi:10.1515/JPM.2006.089
12. Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation (2011) 123(24):2856–69. doi:10.1161/CIRCULATIONAHA.109.853127
13. Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol (1967) 93(2):569–79. doi:10.1002/path.1700930218
14. Gudmundsson S, Dubiel M. Doppler velocimetry in the evaluation of fetal hypoxia. J Perinat Med (2001) 29(5):399–407. doi:10.1515/JPM.2001.056
15. Maulik D, Mundy D, Heitmann E, Maulik D. Evidence-based approach to umbilical artery Doppler fetal surveillance in high-risk pregnancies: an update. Clin Obstet Gynecol (2010) 53(4):869–78. doi:10.1097/GRF.0b013e3181fbb5f5
16. Chien PF, Arnott N, Gordon A, Owen P, Khan KS. How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An overview. BJOG (2000) 107(2):196–208. doi:10.1111/j.1471-0528.2000.tb11690.x
17. Ghosh G, Breborowicz A, Brazert M, Maczkiewicz M, Kobelski M, Dubiel M, et al. Evaluation of third trimester uterine artery flow velocity indices in relationship to perinatal complications. J Matern Fetal Neonatal Med (2006) 19(9):551–5. doi:10.1080/14767050600852510
18. Alahuhta S, Räsänen J, Jouppila P, Jouppila R, Hollmén AI. Ephedrine and phenylephrine for avoiding maternal hypotension due to spinal anaesthesia for caesarean section. Effects on uteroplacental and fetal haemodynamics. Int J Obstet Anesth (1992) 1(3):129–34. doi:10.1016/0959-289X(92)90016-W
19. Erkinaro T, Mäkikallio K, Kavasmaa T, Alahuhta S, Räsänen J. Effects of ephedrine and phenylephrine on uterine and placental circulations and fetal outcome following fetal hypoxaemia and epidural-induced hypotension in a sheep model. Br J Anaesth (2004) 93(6):825–32. doi:10.1093/bja/aeh273
20. Erkinaro T, Mäkikallio K, Acharya G, Päkkilä M, Kavasmaa T, Huhta JC, et al. Divergent effects of ephedrine and phenylephrine on cardiovascular hemodynamics of near-term fetal sheep exposed to hypoxemia and maternal hypotension. Acta Anaesthesiol Scand (2007) 51(7):922–8. doi:10.1111/j.1399-6576.2007.01327.x
21. Erkinaro T, Kavasmaa T, Päkkilä M, Acharya G, Mäkikallio K, Alahuhta S, et al. Ephedrine and phenylephrine for the treatment of maternal hypotension in a chronic sheep model of increased placental vascular resistance. Br J Anaesth (2006) 96(2):231–7. doi:10.1093/bja/aei305
22. Leiser R, Kaufmann P. Placental structure: in a comparative aspect. Exp Clin Endocrinol (1994) 102(3):122–34. doi:10.1055/s-0029-1211275
23. Lennon PF, Murray PA. Isoflurane and the pulmonary vascular pressure-flow relation at baseline and during sympathetic alpha- and beta-adrenoreceptor activation in chronically instrumented dogs. Anesthesiology (1995) 82(3):723–33. doi:10.1097/00000542-199503000-00014
24. Adamson SL, Morrow RJ, Bascom PA, Mo LY, Ritchie JW. Effect of placental resistance, arterial diameter, and blood pressure on the uterine arterial velocity waveform: a computer modeling approach. Ultrasound Med Biol (1989) 15(5):437–42. doi:10.1016/0301-5629(89)90096-3
25. Guo R, Xue Q, Qian Y, Hu Y, Tan J. The effects of ephedrine and phenylephrine on placental vascular resistance during cesarean section under epidual anesthesia. Cell Biochem Biophys (2015) 73(3):687–93. doi:10.1007/s12013-015-0676-7
26. Ngan Kee WD, Lau TK, Khaw KS, Lee BB. Comparison of metaraminol and ephedrine infusions for maintaining arterial pressure during spinal anesthesia for elective cesarean section. Anesthesiology (2001) 95(2):307–13. doi:10.1097/00000542-200108000-00009
27. Levine TA, Alderdice FA, Grunau RE, McAuliffe FM. Prenatal stress and hemodynamics in pregnancy: a systematic review. Arch Womens Ment Health (2016) 19(5):721–39. doi:10.1007/s00737-016-0645-1
28. Ngan Kee WD. Phenylephrine infusions for maintaining blood pressure during spinal anesthesia for cesarean delivery: finding the shoe that fits. Anesth Analg (2014) 118(3):496–8. doi:10.1213/ANE.0000000000000111
29. Ngan Kee WD, Khaw KS, Lau TK, Ng FF, Chui K, Ng KL. Randomised double-blinded comparison of phenylephrine vs ephedrine for maintaining blood pressure during spinal anaesthesia for non-elective caesarean section. Anaesthesia (2008) 63(12):1319–26. doi:10.1111/j.1365-2044.2008.05635.x
30. Cooper DW, Sharma S, Orakkan P, Gurung S. Retrospective study of association between choice of vasopressor given during spinal anaesthesia for high-risk caesarean delivery and fetal pH. Int J Obstet Anesth (2010) 19(1):44–9. doi:10.1016/j.ijoa.2009.06.002
31. Mohta M, Aggarwal M, Sethi AK, Harisinghani P, Guleria K. Randomized double-blind comparison of ephedrine and phenylephrine for management of post-spinal hypotension in potential fetal compromise. Int J Obstet Anesth (2016) 27:32–40. doi:10.1016/j.ijoa.2016.02.004
32. Jain K, Makkar JK, Subramani Vp S, Gander S, Kumar P. A randomized trial comparing prophylactic phenylephrine and ephedrine infusion during spinal anesthesia for emergency cesarean delivery in cases of acute fetal compromise. J Clin Anesth (2016) 34:208–15. doi:10.1016/j.jclinane.2016.03.015
33. Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol (2015) 125(1):5–12. doi:10.1097/AOG.0000000000000564
34. Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol (1997) 37(3):240–9. doi:10.1111/j.1600-0897.1997.tb00222.x
35. Aya AG, Mangin R, Vialles N, Ferrer JM, Robert C, Ripart J, et al. Patients with severe preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: a prospective cohort comparison. Anesth Analg (2003) 97(3):867–72. doi:10.1213/01.ANE.0000073610.23885.F2
36. Aya AG, Vialles N, Tanoubi I, Mangin R, Ferrer JM, Robert C, et al. Spinal anesthesia-induced hypotension: a risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg (2005) 101(3):869–75. doi:10.1213/01.ANE.0000175229.98493.2B
37. Ituk US, Cooter M, Habib AS. Retrospective comparison of ephedrine and phenylephrine for the treatment of spinal anesthesia induced hypotension in pre-eclamptic patients. Curr Med Res Opin (2016) 32(6):1083–6. doi:10.1185/03007995.2016.1159953
38. Higgins N, McCarthy RJ, Wong CA. Phenylephrine versus ephedrine for the management of spinal anesthesia-induced hypotension in preeclamptic patients during cesarean delivery. Abstracts from the Society of Obstetric Anesthesia and Perinatology Annual Meeting at October 26th 2015. San Diego, CA (2015). Available from: https://soap.org/display_2015_abstract.php?id=O2-03
39. Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol (2002) 283(1):R29–45. doi:10.1152/ajpregu.00762.2001
40. Nikooseresht M, Seif Rabiei MA, Hajian P, Dastaran R, Alipour N. Comparing the hemodynamic effects of spinal anesthesia in preeclamptic and healthy parturients during cesarean section. Anesth Pain Med (2016) 6(3):e11519. doi:10.5812/aapm.11519
41. James KS, McGrady E, Patrick A. Combined spinal-extradural anaesthesia for preterm and term caesarean section: is there a difference in local anaesthetic requirements? Br J Anaesth (1997) 78(5):498–501. doi:10.1093/bja/78.5.498
42. Ngan Kee WD. Prevention of maternal hypotension after regional anaesthesia for caesarean section. Curr Opin Anaesthesiol (2010) 23(3):304–9. doi:10.1097/ACO.0b013e328337ffc6
43. Ngan Kee WD, Khaw KS, Ng FF, Karmakar MK, Critchley LA, Gin T. A prospective comparison of vasopressor requirement and hemodynamic changes during spinal anesthesia for cesarean delivery in patients with multiple gestation versus singleton pregnancy. Anesth Analg (2007) 104(2):407–11. doi:10.1213/01.ane.0000252461.97656.3e
Keywords: phenylephrine, ephedrine, hypotension, preeclampsia, uteroplacental insufficiency, fetal compromise
Citation: Dusitkasem S, Herndon BH, Somjit M, Stahl DL, Bitticker E and Coffman JC (2017) Comparison of Phenylephrine and Ephedrine in Treatment of Spinal-Induced Hypotension in High-Risk Pregnancies: A Narrative Review. Front. Med. 4:2. doi: 10.3389/fmed.2017.00002
Received: 10 November 2016; Accepted: 06 January 2017;
Published: 20 January 2017
Edited by:
César Aldecoa, Hospital Universitario Río Hortega, SpainReviewed by:
Yoshinori Ohta, Hyogo College of Medicine, JapanCopyright: © 2017 Dusitkasem, Herndon, Somjit, Stahl, Bitticker and Coffman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sasima Dusitkasem, c2FzaW1hMTMwOEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.