
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 18 January 2017
Sec. Pathology
Volume 3 - 2016 | https://doi.org/10.3389/fmed.2016.00076
This article is part of the Research TopicSecond Line Treatment of Non-Small Cell Lung Cancer: Clinical, Pathological and Molecular Aspects of Novel Promising DrugsView all 10 articles
 Ivana Sullivan
Ivana Sullivan David Planchard*
David Planchard*
Tyrosine kinase inhibitors (TKIs) against the human epidermal growth factor receptor (EGFR) are now standard treatment in the clinic for patients with advanced EGFR mutant non-small-cell lung cancer (NSCLC). First-generation EGFR TKIs, binding competitively and reversibly to the ATP-binding site of the EGFR tyrosine kinase domain, have resulted in a significant improvement in outcome for NSCLC patients with activating EGFR mutations (L858R and Del19). However, after a median duration of response of ~12 months, all patients develop tumor resistance, and in over half of these patients this is due to the emergence of the EGFR T790M resistance mutation. The second-generation EGFR/HER TKIs were developed to treat resistant disease, targeting not only T790M but EGFR-activating mutations and wild-type EGFR. Although they exhibited promising anti-T790M activity in the laboratory, their clinical activity among T790M+ NSCLC was poor mainly because of dose-limiting toxicity due to simultaneous inhibition of wild-type EGFR. The third-generation EGFR TKIs selectively and irreversibly target EGFR T790M and activating EGFR mutations, showing promising efficacy in NSCLC resistant to the first- and second-generation EGFR TKIs. They also appear to have lower incidences of toxicity due to the limited inhibitory effect on wild-type EGFR. Currently, the first-generation gefitinib and erlotinib and second-generation afatinib have been approved for first-line treatment of metastatic NSCLC with activating EGFR mutations. Among the third-generation EGFR TKIs, osimertinib is today the only drug approved by the Food and Drug Administration and the European Medicines Agency to treat metastatic EGFR T790M NSCLC patients who have progressed on or after EGFR TKI therapy. In this review, we summarize the available post-progression therapies including third-generation EGFR inhibitors and combination treatment strategies for treating patients with NSCLC harboring EGFR mutations and address the known mechanisms of resistance.
Over the past decade, scientific advances have progressively improved outcomes for patients diagnosed with lung cancers driven by target oncogene mutations. The first oncogenic driver in non-small-cell lung cancer (NSCLC) was discovered in 2004 with the identification of activating mutations in the kinase domain of the epidermal growth factor receptor (EGFR) among patients with dramatic responses to EGFR tyrosine kinase inhibitors (TKIs) (1–3). EGFR mutations account for 10–17% of NSCLC cases in North America and Europe and 30–50% of NSCLCs in Asian countries and are most common among patients with adenocarcinoma NSCLC and a light or non-smoking history (4, 5). The first-generation TKIs gefitinib (Iressa®, AstraZeneca, London, UK) and erlotinib (Tarceva®, F. Hoffmann-La Roche, Basel, Switzerland), and the second-generation TKI afatinib (Giotrif®, Boehringer Ingelheim, Ingelheim, Germany) have shown higher response rates (RRs), improving progression-free survival (PFS) and quality of life compared to standard platinum-based chemotherapy in patients with good performance status (0–2) whose tumors harbor an activating (sensitizing) EGFR mutation (6–13).
These data established EGFR TKIs as the treatment of choice for patients with newly diagnosed EGFR-mutant advanced NSCLC. Of note, none of these studies demonstrated a benefit in terms of overall survival (OS) due to the high level of crossover. However, an unplanned pooled OS analysis of patients included in the LUX-Lung 3 or LUX-Lung 6 phase III trials demonstrated an OS benefit for afatinib compared to platinum-based chemotherapy in patients whose tumors harbor EGFR Del19 mutations vs. EGFR L858R mutations: 27.3 vs. 24.3 months, respectively [hazard ratio (HR) 0.81; 95% confidence interval (CI), 0.66–0.99; p = 0.037] (14). However, this benefit was not confirmed in the phase IIb LUX-Lung 7 designed to compare head-to-head afatinib with gefitinib in the first-line treatment of patients with EGFR-mutant NSCLC (15). Unfortunately, the majority of patients progress after a median of 12 months treatment with first-line TKIs, and multiple mechanisms of acquired resistance have been identified. Among them, the most common mechanism (~50% of cases) is the acquisition of a missense mutation within exon 20 of EGFR, the T790M mutation (p.Thr790Met) (16).
Until recently, standard chemotherapies were the main treatment option in a post-progression setting. For patients initiating chemotherapy, the role of EGFR TKI maintenance remains controversial. In a retrospective analysis, up to 23% of patients experience a disease flare after TKI discontinuation (17), which led many clinicians to continue EGFR TKIs when starting chemotherapy. It was hypothesized that some clones within a resistant cancer remained sensitive to EGFR inhibition and that withdrawal of the TKI could “let loose” these clones with resultant adverse outcomes. The randomized phase III IMPRESS trial provided the first prospective data to address this clinical question. Patients progressing on first-line gefitinib were randomized to receive cisplatin–pemetrexed with gefitinib or placebo. The trial did not confirm a benefit of maintaining the EGFR TKI, with comparable RRs and PFS in the two arms (18). The final OS analysis was presented recently; patients in the gefitinib arm had significantly lower OS compared to the placebo arm (13.4 vs. 19.5 months, HR = 1.44, p = 0.016), confirming the deleterious effect of maintaining the EGFR inhibition. Of note, this detrimental effect was predominantly observed among patients whose tumors harbored a T790M mutation detected via circulating tumor DNA (ctDNA; HR = 1.49; 95% CI, 1.02–2.21) (19).
To date, many third-generation EGFR TKIs have been developed to target both sensitizing EGFR mutations and EGFR T790M. In this review, we outline available post-progression therapies including osimertinib (previously known as AZD9291) as the only drug approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of patients with metastatic EGFR T790M+ NSCLC who have progressed on or after EGFR TKI therapy (20), and other next-generation irreversible EGFR TKIs in clinical development (Table 1).
For patients whose disease progresses on gefitinib, erlotinib, or afatinib, understanding the major mechanisms of resistance is essential to choosing the optimal post-progression treatment. To date, repeated biopsies are the standard of care; however, this approach comes with some limitations—not all patients are amenable to this procedure, and not all progressing lesions are accessible for biopsy. In addition, there is growing evidence that a single biopsy may not accurately represent the intrinsic heterogeneity of a resistant tumor. Liquid biopsy is a valid alternative to tissue rebiopsy. This approach, which has been validated (21), represents a surrogate DNA source and is a novel strategy for tumor genotyping, mainly applicable at the time of progression for EGFR-mutated patients (22–24). In cases when the T790M mutation is identified in peripheral blood, treatment with third-generation EGFR TKIs is justified (25).
In addition to T790M, other resistance mechanisms have also been identified. Globally, these can be categorized as target gene alterations (i.e., EGFR amplifications and mutations such as T790M), downstream bypass signaling pathway activation (i.e., MET and HER2 amplifications or mutations in BRAF, PIK3CA), and phenotypic changes (including small-cell lung cancer transformation and epithelial to mesenchymal transition) (26, 27).
Following the discovery that T790M is the main resistance mechanism against the first-generation EGFR TKIs gefitinib and erlotinib, many new drugs targeting T790M were developed. Although second-generation EGFR inhibitors such as neratinib, afatinib, and dacomitinib exhibited promising anti-T790M activity in the laboratory, their clinical activity in T790M+ NSCLC was poor, with RR less than 10% among patients resistant to gefitinib or erlotinib (28–30). In addition, increased toxicity, mainly skin and digestive (Table 1), was observed due to EGFR wild-type inhibition at lower concentrations than those required to inhibit T790M. Thus to date, none of the second-generation agents are considered as effective monotherapies in patients progressing on first-generation TKIs.
On the basis of preclinical observations that afatinib plus cetuximab (an anti-EGFR monoclonal antibody) overcame T790M-mediated resistance (31), this combination was evaluated in a phase Ib trial enrolling 126 heavily pretreated patients with advanced EGFR-mutant NSCLC who had developed resistance to erlotinib/gefitinib. The overall response rate (ORR) was 29% and was comparable in both T790M+ and T790M− tumors (32 vs. 25%), and median PFS was 4.7 months (95% CI, 4.3–6.4) (32). However, the dual EGFR inhibition resulted in increased toxicity with various grades 3–4 adverse events (AEs) (mainly rash, diarrhea, and fatigue) reported in up to 46% of patients (32). A randomized phase II/III trial (NCT02438722) of afatinib plus cetuximab vs. afatinib alone is currently open in treatment-naïve patients with advanced EGFR-mutant NSCLC.
Many third-generation EGFR inhibitors are currently being consecutively developed to more effectively target the T790M mutation. Unlike second-generation TKIs, as these drugs exhibit increased specificity for T790M and thus mutant EGFR compared to wild-type EGFR, they are well tolerated resulting in few wild-type EGFR adverse effects. Among them, osimertinib (AZD9291) was the first to receive FDA and EMA approval in November 2015 and February 2016, respectively, for metastatic EGFR T790M+ NSCLC, which has progressed on or after EGFR TKI therapy. Table 2 shows available efficacy data of new-generation EGFR TKIs.
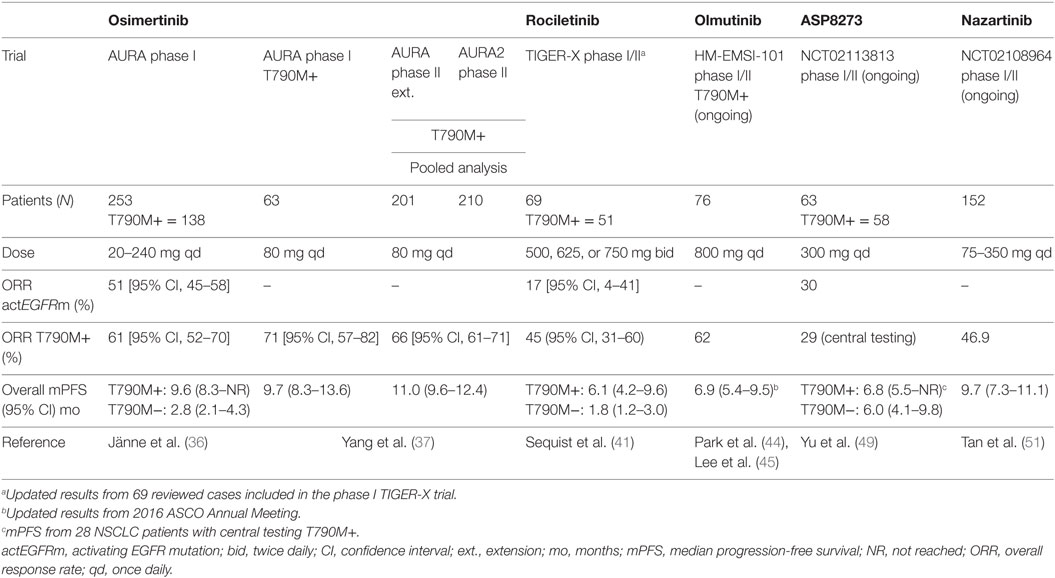
Table 2. Efficacy of third-generation tyrosine kinase inhibitors (TKIs) in activating epidermal growth factor receptor (EGFR) mutations and T790M+ NSCLC patients.
Osimertinib is a mono-anilino-pyrimidine compound that acts as a covalent EGFR TKI. In EGFR recombinant enzyme assays, osimertinib showed potent activity against diverse EGFR mutations (L858R, L858R/T790M, exon 19 deletion, and exon 19 deletion/T790M) and exhibited nearly 200 times greater potency against L858R/T790M than wild-type EGFR. Osimertinib is metabolized to produce at least two circulating metabolites, AZ5104 and AZ7550. In biochemical assays, AZ7550 had a comparable potency and selectivity profile to osimertinib, although AZ5104 showed greater potency against exon 19 deletions, T790M mutants (both ~8-fold) and wild-type (~15-fold) EGFR (33). Its pharmacokinetic exposure did not significantly differ between Asian and non-Asian patients, showing a minimal food effect (34). Additionally, data from a clinical pharmacokinetic study (NCT02163733) showed that osimertinib exposure was not affected by concurrent administration of omeprazole (35). Thus, unlike first- and second-generation TKIs, gastric pH modifying agents can be concomitantly used with osimertinib without restrictions.
A phase I/II dose-escalation study of osimertinib (AURA, NCT01802632) was carried out in patients with locally advanced or metastatic EGFR-mutated NSCLC progressing on first- or second-generation EGFR TKIs. Patients were not preselected according to T790M status (36). The study included 253 patients who received osimertinib at five dose levels ranging from 20 to 240 mg daily and distributed between two cohorts, dose-escalation and dose-expansion cohorts. Among 31 patients enrolled in the dose-escalation cohort, no dose-limiting toxicity (DLT) occurred and the maximum tolerated dose (MTD) was not reached. An additional 222 patients were treated in five dose-expansion cohorts. The EGFR-T790M mutation was detected in tumors from 138 patients (62%) in the expansion cohorts. Of the 253 patients treated across all dose levels, 239 were evaluated for response. The ORR and disease control rate (DCR) in the whole population were 51% [95% CI, 45–58%] and 84% [95% CI, 79–88%], respectively. Among the 138 patients with a centrally confirmed EGFR-T790M mutation, 127 patients were evaluable for response. Outcomes were substantially better in the EGFR T790M+ population compared to T790M− tumor patients with an ORR of 61% [95% CI, 52–70%] vs. 21% [95% CI, 12–34%], a DCR of 95% [95% CI, 90–98%] vs. 61% [95% CI, 47–73%] and median PFS of 9.6 months [95% CI, 8.3–not reached] vs. 2.8 months [95% CI, 2.1 to 4.3], respectively (36). There were no DLTs at any dose level. The most common AE, mostly grade 1–2, were diarrhea (47%), skin toxicity (rash/acne, 40%), nausea (22%), and anorexia (21%). With increased incidence and severity of AEs (rash, dry skin, and diarrhea) in relation to the wild-type EGFR inhibition at higher dose levels (160 and 240 mg), 80 mg daily was selected as the recommended dose for further clinical trials (36).
The efficacy and safety data from the 80 mg expansion cohort in patients with centrally confirmed T790M NSCLC were recently updated (data cutoff: January 4, 2016). Among 63 patients, 61 patients were evaluable for response. The ORR and DCR were 71% [95% CI, 57–82%] and 93% [95% CI, 84–98%], respectively, with a median PFS of 9.7 months [95% CI, 8.3–13.6] (37).
The 80-mg daily dose evaluated in the phase II T790M+ extension cohort of the AURA trial (described above) was evaluated in an additional phase II “AURA2” study (NCT02094261) designed for patients with confirmed EGFR-mutant T790M+ locally advanced or metastatic NSCLC progressing on an approved EGFR TKI. A preplanned pooled analysis of both studies was performed. Among 411 patients (201 from the AURA extension and 210 from AURA2), 397 were evaluable. The ORR and DCR were 66% [95% CI, 61–71%] and 91% [95% CI, 88–94%], respectively. Median PFS was 11.0 [95% CI, 9.6–12.4] months with a median duration of response of 12.5 months [95% CI, 11.1 months to not calculable] (37).
Osimertinib has also demonstrated activity in the first-line setting. Data from two expansion cohorts in treatment-naïve EGFR-mutated advanced NSCLC patients were recently presented. Sixty patients received osimertinib 80 mg (n = 30) or 160 mg (n = 30) once daily and all were evaluable. The confirmed ORR was 77% [95% CI, 64–87%] with a DCR of 98% [95% CI, 89–100%]. Median PFS was 19.3 months [95% CI, 13.7 to not calculable] (38).
A number of phase III trials involving osimertinib in different settings are ongoing. The phase III FLAURA trial (First-Line-AURA; NCT02296125) in EGFR-mutated treatment-naïve NSCLC patients was designed to compare osimertinib 80 mg daily vs. the current standard of care gefitinib or erlotinib. The AURA3 trial (NCT02151981) is an open-label, randomized trial in the second-line setting, designed to compare osimertinib with platinum-based doublet chemotherapy in patients with EGFR T790M+ locally advanced or metastatic NSCLC. In a press release dated July 18, 2016, AstraZeneca announced that the AURA3 trial, which included more than 400 patients, had met its primary endpoint demonstrating superior PFS compared to standard platinum-based chemotherapy.
In the adjuvant setting, the ongoing ADAURA trial (ADjuvant-AURA; NCT02511106) is a double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of osimertinib vs. placebo in patients with EGFR-mutated stage IB–IIIA NSCLC following complete tumor resection. Results are not yet available.
Rociletinib is another oral, irreversible, mutant-selective inhibitor of commonly mutated forms of EGFR, including T790M, with minimal activity against wild-type EGFR in preclinical studies (39). A phase I/II trial (TIGER-X; NCT01526928) of rociletinib was performed in patients with EGFR-mutant NSCLC with acquired resistance to first- or second-generation EGFR TKIs (40). In the expansion (phase II) part of the study, patients with T790M+ NSCLC received rociletinib at doses of 500, 625, or 750 mg twice daily. At the time of report, 130 patients were enrolled. The MTD was not identified. The most common grade 3 AE was hyperglycemia, occurring in 20 of the 92 patients (22%) who received therapeutic doses. Among the 46 evaluable patients with T790M+, the ORR was 59% [95% CI, 45–73%]. For the 17 evaluable patients with T790M− disease, the ORR was 29% [95% CI, 8–51%] (40).
In November 2015, Clovis Oncology issued a press release that contained data from a pooled analysis of TIGER-X and TIGER-2 (NCT02147990), another phase II trial examining rociletinib in second line in patients with EGFR T790M+ NSCLC progressing on at least on EGFR inhibitor. Among 325 patients, the ORR (dose range, 500–750 mg twice daily) was 30.2% [95% CI, 25.2–35.5%]. The ORRs were 32% [95% CI, 25–40%] and 23% [95% CI, 14–34%] in patients receiving 625 mg (n = 170) and 500 mg (n = 79), respectively. The median duration of response for the two treatment doses was 8.8 and 9.1 months, respectively. Due to the different RR, an independent updated analysis was assessed in patients (intention-to-treat population) included in the TIGER-X trial confirming ORRs of 45% [95% CI, 31–60%] and 17% [95% CI, 4–41%] among patients with T790M+ and T790M− disease, respectively (41). Clovis thus decided to halt enrollment in all ongoing rociletinib studies, including the phase III TIGER-3 trial (NCT02322281), and has withdrawn its application for regulatory approval in the European Union.
Olmutinib is an oral EGFR mutant-specific TKI active against mutant EGFR isoforms, including T790M, while sparing wild-type EGFR (42). A phase I/II trial HM-EMSI-101 (NCT01588145) was conducted to evaluate the safety, tolerability, pharmacokinetics, and preliminary activity of olmutinib in Korean patients with EGFR TKI-pretreated NSCLC (43). Patients received olmutinib at doses ranging from 75 to 1,200 mg/day. The ORR was 58.8% in the 34 patients who received olmutinib with a dose more than 650 mg. The most common DLTs involved gastrointestinal symptoms and increased aspartate aminotransferase, alanine aminotransferase, amylase, and lipase levels. The recommended phase II dose was 800 mg/day. In part II of the study, 76 patients with centrally confirmed T790M+ NSCLC were enrolled, 70 of whom were evaluable for response. The ORR was 61% and median PFS (n = 76) was 6.9 months [95% CI, 5.36–9.49]. The most common drug-related AEs (all grades) were diarrhea (59%), pruritus (42%), rash (41%), and nausea (39%) (44). These data validate previous preliminary trial results presented at the European Society for Medical Oncology Asia Congress in December 2015 (45). These results were the basis for Breakthrough Therapy Designation granted by the FDA in 2015 and the first approval for the treatment of patients with EGFR T790M+ NSCLC in South Korea in 2016. Following promising early clinical data, Boehringer Ingelheim launched the ELUXA clinical trial program to investigate olmutinib as a monotherapy in different settings as well as in combination with other anticancer treatments. Nevertheless, due to an unexpected increase in grade 3/4 skin toxicity (epidermolysis) in previous trials Boehringer decided to definitively stop the development of this drug.
ASP8273 is another oral, irreversible TKI that inhibits the kinase activity of EGFR mutations including T790M, with limited activity against EGFR wild-type (46). ASP8273 was further shown to suppress signaling via ERK and Akt. This agent showed activity in mutant EGFR cell lines that are resistant to other EGFR TKIs including osimertinib and rociletinib (47). ASP8273 was evaluated in an open-label phase I/II study (NCT02192697) for safety and efficacy (48). Thirty Japanese patients were enrolled in the phase I dose-escalation cohorts across seven dose levels (25–600 mg/day), and 15 patients were enrolled in the response expansion cohorts across four dose levels (100–400 mg/day). T790M status was 49% positive, 13% negative, and 38% unknown, respectively. Responses were observed in patients enrolled in ≥100 mg/day cohorts. Partial responses were achieved in 50% (18/36) of all evaluable patients and 80% (12/15) of patients with T790M+ NSCLC (including confirmed and unconfirmed). The most common AEs (all grades) were diarrhea (56%), nausea (31%), vomiting (31%), and thrombocytopenia (31%). Based on tolerability and preliminary antitumor activity, the recommended phase II dose selected was 300 mg once daily (48). The safety, tolerability, and antitumor activity for ASP8273 300 mg/day in patients with NSCLC EGFR mutation-positive and previously treated with an EGFR TKI were recently presented in a total of 63 patients, including seven treated in the dose-escalation part, 18 in the response expansion, 19 in recommended phase II dose part, and 19 from the food effect cohort (49). The majority of tumors (>90%) were positive for the T790M mutation based on local testing. All but one patient (98%) had been previously treated with an EGFR TKI, with erlotinib the most common inhibitor. Among the 63 patients treated with ASP8273 300 mg, the ORR was 30% [95% CI, 19.2–43.0%] and the median PFS was 6.0 [95% CI, 4.1–9.8] months. For the subgroups with T790M+ tumors the ORRs, assessed by local or central testing, were similar: 31% [95% CI, 19.5–44.5%] and 29% [95% CI, 13.2–48.7%], respectively. Median PFS for T790M+ patients (local testing) was 6.0 months [95% CI, 5.3–9.8] and 6.8 months [95% CI, 5.5 months to not evaluable] for T790M+ patients (central testing). The most frequent drug-related AEs (all grades) were diarrhea (48%), nausea (27%), hyponatremia (19%), paresthesia (14%), and vomiting (13%). Six patients (10%) discontinued treatment due to treatment-related toxicity (49). Based on this study, the dose of ASP8273 300 mg daily was selected for a recently initiated, large (n = 600), international, randomized, phase III study (SOLAR) to compare the clinical efficacy and safety/tolerability of ASP8273 with erlotinib or gefitinib as initial treatment of advanced EGFR-mutant NSCLC (NCT02588261).
Nazartinib is a novel, irreversible mutant-selective EGFR inhibitor that specifically targets both EGFR-activating mutations (L858R, Del19) and the resistant T790M mutation, while sparing wild-type EGFR (50). NCT02108964 (EGF816X2101) is a phase I/II first-in-human study of nazartinib in patients with EGFR-mutated locally advanced or metastatic NSCLC. Updated results from the phase I dose-escalation part were recently presented. Patients were assigned to receive once-daily nazartinib with doses ranging from 75 to 350 mg. At the cutoff date of January 29, 2016, 152 patients had been treated across seven cohorts (51). Among them, 147 patients were evaluable for response. The confirmed ORR was 46.9% [95% CI, 38.7–55.3%] and the DCR was 87.1% [95% CI, 80.6–92.0%]. The estimated median PFS across all dose levels was 9.7 months [95% CI, 7.3–11.1]. Among 69 patients with confirmed responses at the cutoff date, the estimated median duration of response was 9.5 months [95% CI, 9.2–14.7]. The most common toxicities (all grades) were rash (54%), diarrhea (37%), and pruritus (34%). Interestingly, the rashes observed in the study tended to have a different pattern, location, and histology than those seen with other EGFR TKIs that target wild-type EGFR. Diarrhea was the most common grade 3/4 AE (16%), and of note, both incidence of diarrhea and rash tended to increase with increasing nazartinib doses (51). The phase II part, performed in six cohorts, is ongoing (Figure 1). In addition, the drug is being investigated in association with INC280, a specific MET inhibitor (based on the potential escape pathway for third-generation EGFR TKIs) in an ongoing phase Ib/II trial in patients with advanced EGFR mutant NSCLC (NCT02335944), and with nivolumab, an anti-PD-1 monoclonal antibody in a phase II trial in EGFR mutant/T790M+ NSCLC patients who have progressed on first-line EGFR TKI (NCT02323126).
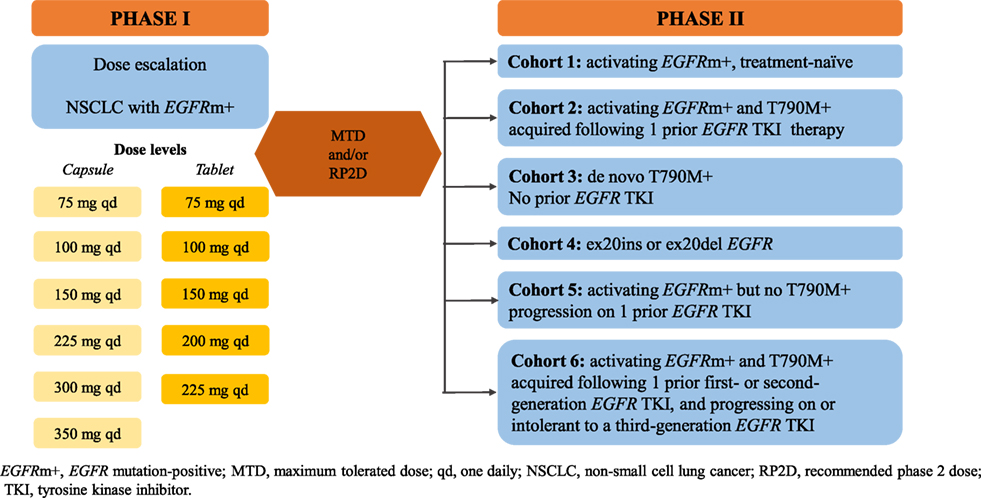
Figure 1. Study design of nazartinib in EGFRm+ NSCLC patients (NCT0210896). EGFRm+, EGFR mutation-positive; MTD, maximum tolerated dose; qd, one daily; NSCLC, non-small-cell lung cancer; RP2D, recommended phase 2 dose.
Avitinib is another new-generation inhibitor of EGFR that, like the abovementioned agents, targets EGFR-activating mutations overcoming T790M-induced mutation with limited activity against wild-type EGFR. Clinical trials were initiated in China and the United States in parallel using avitinib as second-line therapy in NSCLC patients progressing on first-generation EGFR TKIs and who have acquired the gatekeeper T790M mutation. Two trials evaluating the safety, tolerability, pharmacokinetics, and antitumor activity of avitinib are ongoing; a phase I/II trial (NCT02274337) in advanced NSCLC patients progressing on prior therapy with an EGFR TKI agent and a phase I trial (NCT02330367) designed to determine the MTD and/or recommended phase 2 dose in previously treated mutant EGFR NSCLC patients with a T790M resistant mutation.
PF-06747775 is another small molecule inhibitor of EGFR T790M with minimal activity against wild-type EGFR. It is being studied in a phase I/II clinical trial (NCT02349633) in advanced NSCLC patients with EGFR mutations (Del19 or L858R ± T790M). Results are not yet available.
HS-10296 is a small molecule inhibitor of EGFR-activating mutations and T790M-resistant mutation with limited activity against wild-type EGFR. An open-label, multicenter, phase I/II trial of HS-10296 with dose escalation, dose expansion, and extension cohorts in locally advanced or metastatic NSCLC patients who have progressed following prior therapy with an EGFR TKI agent is currently recruiting participants (NCT02981108).
Incidence data of brain and leptomeningeal metastasis in EGFR-mutated NSCLC patients come from retrospective cohorts, reporting 24 and 9%, respectively. Gefitinib, erlotinib, and afatinib have impressive intracranial activity, with RR of 60–80% (52, 53). However, for patients with CNS progression on these first- and second-generation agents, further effective therapies are limited. Among the third-generation EGFR TKIs, osimertinib was the only inhibitor demonstrating sustained tumor regression in both preclinical and clinical models (54). Osimertinib has greater penetration of the mouse blood–brain barrier than gefitinib, rociletinib, or afatinib, and induced sustained tumor regression in an EGFR mutant PC9 mouse brain metastasis model at clinically relevant doses, while rociletinib did not achieve tumor regression (55). CNS activity was confirmed in the AURA study phase II extension cohort (NCT01802632) and the AURA2 phase II study (NCT02094261) (56). The phase I BLOOM trial (NCT02228369) was designed to assess for the first time the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of two third-generation EGFR TKIs, orsimetinib and AZD3759. AZD3759 was the first EGFR TKI primarily designed to effectively across the blood–brain barrier to tackle CNS metastases in patients with EGFR mutant NSCLC (57, 58). The osimertinib cohort of the trial included 21 Asian patients with advanced or metastatic NSCLC harboring the L858R mutation (n = 13) or an exon 19 deletion (n = 9), and a confirmed diagnosis of leptomeningeal metastasis by positive cerebrospinal fluid cytology. At study entry, the T790M mutation was detected in cerebrospinal fluid in two patients and in plasma in six. Patients received osimertinib at 160 mg/day. All patients were evaluable for efficacy; seven (33%) had a confirmed radiologic response, nine (43%) had stable disease, and neurological function improvement was seen in five (24%) patients (59). Preliminary results from the AZD3759 cohort were recently presented (60). Twenty-nine patients with advanced EGFR mutant NSCLC and brain metastases, including leptomeningeal metastasis were treated in escalating dose cohorts of 50–500 mg, twice daily (BID). The pharmacokinetic analysis demonstrated excellent CNS penetration, with a 1:1 ratio with plasma. The tolerability profile of AZD3759 was consistent with EGFR TKI class effects and included grade 3/4 rash (7%), pruritus (7%), diarrhea (3%), and acne (3%). The MTD was 300 mg BID but study investigators recommended 200 mg BID for phase II dosing. AZD3759 demonstrated encouraging intracranial antitumor activity. Among 21 patients with measurable brain metastases, 11 demonstrated tumor shrinkage in the target brain lesion at AZD3759 doses of ≥50 mg BID. In this group, there were six partial responses (three confirmed, three unconfirmed). Among 22 patients with measurable extracranial lesions, eight experienced tumor shrinkage, with one unconfirmed partial response (60). Based on these promising findings, the BLOOM trial is continuing to enroll patients in the AZD3759 brain and leptomeningeal metastasis expansion cohorts.
As is the case with first and second-generation EGFR TKIs, mutations mediating resistance to third-generation EGFR TKIs are emerging (61–65). Among them, while the C797S mutation in exon 20 of EGFR was the most common mechanism responsible for resistance to osimertinib (62), it occurs in less than 3% of patients treated with rociletinib (66). The C797S mutation was also reported in one case that led to resistance to olmutinib (65). Very recently, two novel tertiary EGFR mutations were described. The acquired L798I mutation was observed in cis with T790M in one patient following rociletinib therapy (66). Subsequently, another mutation in the same codon (L798Q) was reported in one patient at the time of progression under osimertinib (67).
The acquired resistance associated with the EGFR T790M mutation can occur by selection of preexisting EGFR T790M+ clones or via genetic evolution of initially EGFR T790M− drug-tolerant cells, suggesting that cancer cells that survive third-generation TKIs may serve as a key reservoir from which acquired resistance can emerge during treatment (68).
Additional EGFR-independent mechanisms of resistance have been reported. NRAS mutations, including a novel E63K mutation, and amplifications of wild-type NRAS or KRAS have been described as mechanisms of acquired resistance to osimertinib but also to gefitinib and afatinib (69). Amplifications in HER2 and MET genes were also described as potential mechanisms of acquired resistance to osimertinib and rociletinib in EGFR T790M+ NSCLC patients (66, 70). Additionally, loss of T790M at the time of progression may be mediated by overgrowth of cells harboring HER2 amplification, or BRAF V600E or PIK3CA mutations, as was recently detected in plasma of patients included in the phase I AURA trial (71).
Finally, small-cell lung cancer transformation was seen in two cases of rociletinib resistance and one osimertinib-resistant patient; the T790M was lost while the original EGFR mutation was maintained in the small cell transformed cancer in each case (72, 73).
The favorable toxicity profiles of the third-generation EGFR TKIs make them particularly attractive candidates for combination therapy, and many trials are currently planned or ongoing (Table 3).
Preclinical EGFR L858R/T790M/C797S mutation cell models exhibited in vitro sensitivity to cetuximab, an antibody that blocks EGFR dimerization (74, 75), but this was not confirmed in in vivo analyses. However, the allosteric inhibitor EAI045 in combination with cetuximab exhibited mechanistic synergy and was effective in mouse models of lung cancer driven by EGFR L858R/T790M and by EGFR L858R/T790M/C797S (76). Interestingly, the allelic context in which C797S was acquired may predict responsiveness to subsequent TKI treatments. For example, if the C797S and T790M mutations are in trans, cells will be resistant to third-generation EGFR TKIs but are sensitive to a combination of first and third-generation TKIs, and when C797S develops in T790 wild-type cells, this results in resistance to third-generation TKIs, while sensitivity to first-generation TKIs is retained (61). These data are of great clinical value in sequencing for this mutation in patients with acquired resistance to osimertinib.
Navitoclax (ABT-263), a BCL-2 family inhibitor, enhances the apoptotic response of late-resistant EGFR T790M cells with decreased sensitivity to EGFR inhibition. The combination of navitoclax with the third-generation EGFR TKI WZ4002 (in preclinical development) induced more apoptosis compared to WZ4002 alone in both in vivo and in vitro analyses. This approach could be an effective strategy for treating EGFR T790M-positive cancers that have a decreased apoptotic response to EGFR inhibition (68). Additionally, the combination of WZ4002 with trametinib, another MEK inhibitor, prevents the development of acquired resistance in EGFR-mutant lung cancer models (77). A phase Ib trial is ongoing to evaluate the safety and tolerability of the osimertinib/navitoclax combination in patients with EGFR-mutant NSCLC following resistance to prior EGFR TKIs (NCT02520778).
In vitro, a combination of osimertinib with the MEK 1/2 inhibitor selumetinib prevented emergence of resistance in PC9 (Ex19del) cells and delayed resistance in NCI-H1975 (L858R/T790M) cells. In vivo, concomitant osimertinib with selumetinib caused regression of osimertinib-resistant tumors in an EGFR-mutant/T790M transgenic model (69). This association, among others, is being evaluated in the phase Ib TATTON trial (NCT02143466) designed to evaluate the safety, tolerability, and preliminary antitumor activity of osimertinib in combination with durvalumab (an anti-PD-L1 monoclonal antibody), savolitinib (MET inhibitor) or selumetinib in patients with advanced EGFR-mutant NSCLC who have progressed on an EGFR TKI. Preliminary results from the osimertinib/durvalumab arm were recently presented (78). The investigator-assessed ORR was 67% in nine patients with T790M+ tumors, compared to 21% in 14 T790M− NSCLC. Interstitial lung disease was reported in 38% (13/34) of patients, which is higher than would be expected with either drug alone, including five grade 3/4 events (78). Thus, recruitment in the osimertinib + durvalumab arm was stopped but expansion cohorts of the MET and MEK inhibitor combinations are ongoing. In addition, the phase III CAURAL trial (NCT02454933) is being conducted in second-line metastatic EGFR-mutant T790M+ NSCLC patients testing osimertinib plus durvalumab vs. osimertinib monotherapy. This study was also stopped prematurely due to the pulmonary toxicity observed in the TATTON trial.
Dual EGFR blockage is being evaluated in a phase I trial (NCT02496663) combining osimertinib with the anti-EGFR monoclonal antibody necitumumab to assess safety and determine the optimal dose in patients with EGFR-mutant advanced NSCLC who have progressed on a previous EGFR TKI.
As was reported, the dual vascular endothelial growth factor receptor (VEGFR) and EGFR blockade inhibit tumor growth in EGFR TKI resistance xenograft models (79). This hypothesis was confirmed in two phase II clinical trials in EGFR-mutant NSCLC treatment-naïve patients, the randomized Japanese (JO25567) trial comparing erlotinib plus bevacizumab vs. erlotinib alone, and the single-arm Caucasian (BELIEF) trial. Median PFS was similar and encouraging in both trials supporting the combination in the first-line setting (80, 81). Following this strategy, a phase I trial was designed to evaluate the safety of two osimertinib-based combination strategies, with necitumumab or ramucirumab (an anti-VEGFR2 monoclonal antibody) in patients with advanced EGFR T790M+ NSCLC after progression on first-line EGFR TKI therapy (NCT02789345). Finally, the osimertinib/bevacizumab combination will be evaluated in another phase I/II 3 + 3 dose-escalation study (NCT02803203) to test the safety of combining these drugs.
For patients whose tumors undergo small-cell lung cancer transformation, platinum-based plus etoposide chemotherapy is recommended.
Over the last decade, we have seen considerable advances in the treatment of patients with EGFR mutant NSCLC. Three EGFR TKIs are currently FDA and EMA approved for first-line treatment of patients with sensitizing EGFR mutations in metastatic NSCLC. Despite this progress, the development of acquired resistance is an unfortunate reality and remains an important challenge in the clinical setting. No second-generation TKIs have been successfully developed, and to date, osimertinib is the only third-generation EGFR mutant/T790M+ TKI approved by the FDA and EMA for patients with advanced T790M NSCLC who progress on a first-line EGFR TKI. Osimertinib has demonstrated strong efficacy and safety data in phase I and II studies, mainly in a second- or post-second-line setting but also as first-line treatment, placing it as a very attractive drug in this scenario. The clinical development of osimertinib represents one of the fastest cancer drug development programs, taking just 2 years, 8 months, and 1 week from the first patient dosed to the first approved indication. Until recently, patients with advanced NSCLC with EGFR-activating mutations who progress on a first-line EGFR TKI have traditionally been treated with a platinum-doublet chemotherapy. These combinations show ORRs of approximately 30%, marginally higher than those observed in the T790M− populations, but significantly lower than those reported in T790M+ cohorts across osimertinib phase I–III trial development. In addition, given the encouraging CNS efficacy, osimertinib is also attractive as frontline treatment for patients with brain and/or leptomeningeal metastases. The phase III FLAURA (NCT02296125) trial will hopefully soon answer the issue of where osimertinib should be positioned. Among the other new-generation EGFR TKIs and considering that the development of rociletinib and olmutinib as monotherapies has been stopped, ASP8273 is now the most advanced agent in the clinic.
Figure 2 illustrates potential post-progression treatment algorithms for EGFR-mutated advanced NSCLC patients. The heterogeneity of resistant cancers seems to play an important role in both efficacy and resistance to these novel T790M-specific agents, and combination strategies could be effective in delaying and/or preventing resistance. Finally, in an era of personalized medicine, the analysis of both tumor tissue and ctDNA should be a priority to improve our knowledge to the benefit of our patients.
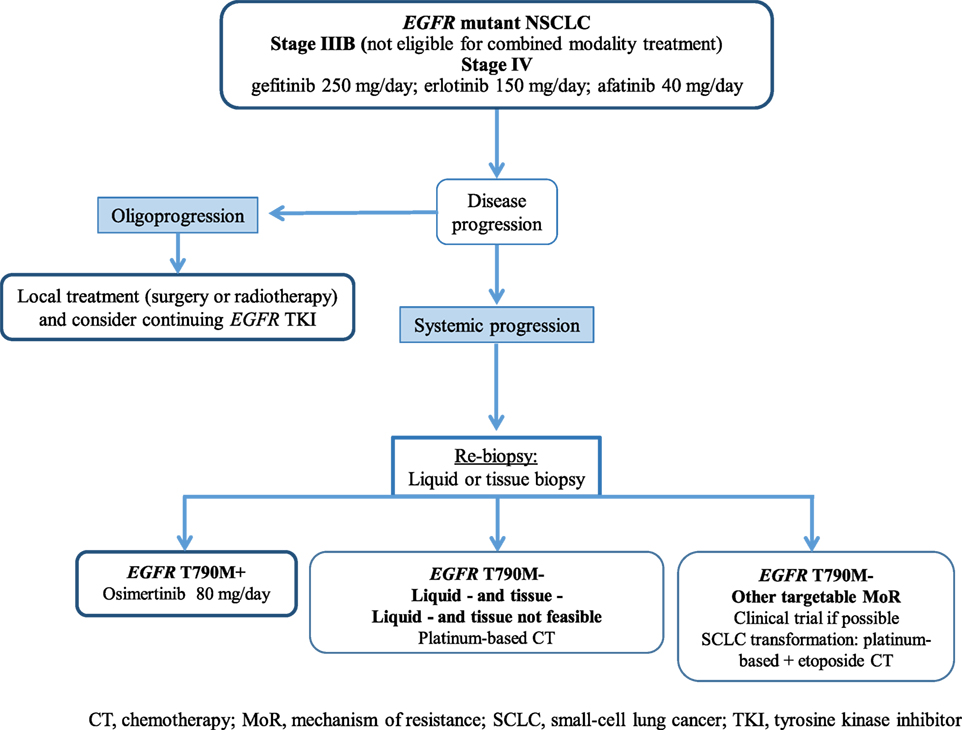
Figure 2. Potential treatment algorithm for advanced EGFR-mutated NSCLC patients. CT, chemotheraphy; EGFR, epidermal growth factor receptor; MoR, mechanism of resistance; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer; TKI, tyrosine kinase inhibitor.
IS prepared the manuscript. DP supervised and accepted the final version.
IS reports no conflicts of interest. DP: Advisory Boards for AstraZeneca, Pfizer, Novartis, Clovis, and Roche.
The authors thank Sarah MacKenzie PhD for English editing.
No funding sources were used to assist with the preparation of this review.
1. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med (2004) 350:2129–39. doi: 10.1056/NEJMoa040938
2. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A (2004) 101:13306–11. doi:10.1073/pnas.0405220101
3. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (2004) 304:1497–500. doi:10.1126/science.1099314
4. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA (2014) 311:1998–2006. doi:10.1001/jama.2014.3741
5. Barlesi F, Mazieres J, Merlio J-P, Debieuvre D, Mosser J, Lena H, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet (2016) 387:1415–26. doi:10.1016/S0140-6736(16)00004-0
6. Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med (2009) 361:947–57. doi:10.1056/NEJMoa0810699
7. Han J-Y, Park K, Kim S-W, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol (2012) 30:1122–8. doi:10.1200/JCO.2011.36.8456
8. Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol (2013) 24:54–9. doi:10.1093/annonc/mds214
9. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med (2010) 362:2380–8. doi:10.1056/NEJMoa0909530
10. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol (2010) 11:121–8. doi:10.1016/S1470-2045(09)70364-X
11. Sequist LV, Yang JC-H, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol (2013) 31:3327–34. doi:10.1200/JCO.2012.44.2806
12. Wu Y-L, Zhou C, Hu C-P, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol (2014) 15:213–22. doi:10.1016/S1470-2045(13)70604-1
13. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13:239–46. doi:10.1016/S1470-2045(11)70393-X
14. Yang JC-H, Wu Y-L, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol (2015) 16:141–51. doi:10.1016/S1470-2045(14)71173-8
15. Paz-Ares L, Tan E-H, Zhang L, Hirsh V, O’Byrne K, Boyer M, et al. Afatinib vs gefitinib in patients with EGFR mutation-positive NSCLC: overall survival data from the phase IIb trial LUX-lung 7. Ann Oncol (2016) 27(Suppl 6):vi552–87. doi:10.1093/annonc/mdw435.42
16. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res (2013) 19:2240–7. doi:10.1158/1078-0432.CCR-12-2246
17. Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res (2011) 17:6298–303. doi:10.1158/1078-0432.CCR-11-1468
18. Soria J-C, Wu Y-L, Nakagawa K, Kim S-W, Yang J-J, Ahn M-J, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol (2015) 16:990–8. doi:10.1016/S1470-2045(15)00121-7
19. Soria J-C, Kim S-W, Wu Y-L, Nakagawa K, Yang J-J, Ahn M-J, et al. Gefitinib/chemotherapy vs chemotherapy in EGFR mutation-positive NSCLC after progression on 1st line gefitinib (IMPRESS study): final overall survival (OS) analysis. Ann Oncol (2016) 27(Suppl 6):vi416–54. doi:10.1093/annonc/mdw383.01
20. Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2016) 27(Suppl 5):v1–27. doi:10.1093/annonc/mdw326
21. Douillard J-Y, Ostoros G, Cobo M, Ciuleanu T, McCormack R, Webster A, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer (2014) 110:55–62. doi:10.1038/bjc.2013.721
22. Marchetti A, Del Grammastro M, Felicioni L, Malatesta S, Filice G, Centi I, et al. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS One (2014) 9:e103883. doi:10.1371/journal.pone.0103883
23. Marchetti A, Palma JF, Felicioni L, De Pas TM, Chiari R, Del Grammastro M, et al. Early prediction of response to tyrosine kinase inhibitors by quantification of EGFR mutations in plasma of NSCLC patients. J Thorac Oncol (2015) 10:1437–43. doi:10.1097/JTO.0000000000000643
24. Sueoka-Aragane N, Katakami N, Satouchi M, Yokota S, Aoe K, Iwanaga K, et al. Monitoring EGFR T790M with plasma DNA from lung cancer patients in a prospective observational study. Cancer Sci (2016) 107:162–7. doi:10.1111/cas.12847
25. Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, et al. 135O_PR: plasma genotyping for predicting benefit from osimertinib in patients (pts) with advanced NSCLC. J Thorac Oncol (2016) 11(Suppl 4):S154. doi:10.1016/S1556-0864(16)30328-8
26. Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol (2013) 31:3987–96. doi:10.1200/JCO.2012.45.2029
27. Cortot AB, Jänne PA. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur Respir Rev (2014) 23:356–66. doi:10.1183/09059180.00004614
28. Sequist LV, Besse B, Lynch TJ, Miller VA, Wong KK, Gitlitz B, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol (2010) 28:3076–83. doi:10.1200/JCO.2009.27.9414
29. Miller VA, Hirsh V, Cadranel J, Chen Y-M, Park K, Kim S-W, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol (2012) 13:528–38. doi:10.1016/S1470-2045(12)70087-6
30. Reckamp KL, Giaccone G, Camidge DR, Gadgeel SM, Khuri FR, Engelman JA, et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer (2014) 120:1145–54. doi:10.1002/cncr.28561
31. Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest (2009) 119:3000–10. doi:10.1172/JCI38746
32. Janjigian YY, Smit EF, Groen HJM, Horn L, Gettinger S, Camidge DR, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov (2014) 4:1036–45. doi:10.1158/2159-8290.CD-14-0326
33. Cross DAE, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov (2014) 4:1046–61. doi:10.1158/2159-8290.CD-14-0337
34. Planchard D, Brown KH, Kim D-W, Kim S-W, Ohe Y, Felip E, et al. Osimertinib Western and Asian clinical pharmacokinetics in patients and healthy volunteers: implications for formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother Pharmacol (2016) 77:767–76. doi:10.1007/s00280-016-2992-z
35. Vishwanathan K, Dickinson PA, Bui K, Weilert D, So K, Thomas K, et al. Effect of food and gastric pH modifiers on the pharmacokinetics of AZD9291. AACR. Mol Cancer Ther (2015) 14(Suppl 2):abstract B153. doi:10.1158/1535-7163.TARG-15-B153
36. Jänne PA, Yang JC-H, Kim D-W, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med (2015) 372:1689–99. doi:10.1056/NEJMoa1411817
37. Yang J, Ramalingam SS, Jänne PA, Cantarini M, Mitsudomi T. LBA2_PR: osimertinib (AZD9291) in pre-treated pts with T790M-positive advanced NSCLC: updated Phase 1 (P1) and pooled Phase 2 (P2) results. J Thorac Oncol (2016) 11(Suppl 4):S152–3. doi:10.1016/S1556-0864(16)30325-2
38. Ramalingam S, Yang JC-H, Lee CK, Kurata T, Kim D-W, John T, et al. LBA1_PR: osimertinib as first-line treatment for EGFR mutation-positive advanced NSCLC: updated efficacy and safety results from two Phase I expansion cohorts. J Thorac Oncol (2016) 11(Suppl 4):S152. doi:10.1016/S1556-0864(16)30324-0
39. Walter AO, Sjin RTT, Haringsma HJ, Ohashi K, Sun J, Lee K, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov (2013) 3:1404–15. doi:10.1158/2159-8290.CD-13-0314
40. Sequist LV, Soria J-C, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med (2015) 372:1700–9. doi:10.1056/NEJMoa1413654
41. Sequist LV, Soria J-C, Camidge DR. Update to rociletinib data with the RECIST confirmed response rate. N Engl J Med (2016) 374:2296–7. doi:10.1056/NEJMc1602688
42. Lee K-O, Cha M, Kim M, Song J, Lee J-H, Kim Y, et al. Discovery of HM61713 as an orally available and mutant EGFR selective inhibitor. Clin Cancer Res (2014) 74(Suppl 19):LB–100. doi:10.1158/1538-7445.AM2014-LB-100
43. Park K, Lee J-S, Lee KH, Kim J-H, Min YJ, Cho JY, et al. Updated safety and efficacy results from phase I/II study of HM61713 in patients (pts) with EGFR mutation positive non-small cell lung cancer (NSCLC) who failed previous EGFR-tyrosine kinase inhibitor (TKI). J Clin Oncol (2015) 33:abstract 8084.
44. Park K, Lee J-S, Lee KH, Kim J-H, Cho BC, Min YJ, et al. Olmutinib (BI 1482694; HM61713), an EGFR mutant-specific inhibitor, in T790M+ NSCLC: efficacy and safety at the RP2D. J Clin Oncol (2016) 34:abstract 9055.
45. Lee J-S, Park K, Han J-Y, Lee KH, Kim J-H, Cho EK, et al. 425PDClinical activity and safety of the EGFR mutant-specific inhibitor, BI1482694, in patients (pts) with T790M-positive NSCLC. Ann Oncol (2015) 26(Suppl 9):ix125–47. doi:10.1093/annonc/mdw532.09
46. Sakagami H, Konagai S, Yamamoto H, Tanaka H, Matsuya T, Mori M, et al. ASP8273, a novel mutant-selective irreversible EGFR inhibitor, inhibits growth of non-small cell lung cancer (NSCLC) cells with EGFR activating and T790M resistance mutations. Cancer Res (2014) 74(Suppl 19):abstract 1728. doi:10.1158/1538-7445.AM2014-1728
47. Konagai S, Sakagami H, Yamamoto H, Tanaka H, Matsuya T, Mimasu S, et al. ASP8273 selectively inhibits mutant EGFR signal pathway and induces tumor shrinkage in EGFR mutated tumor models. Cancer Res (2015) 75(Suppl 15):abstract 2586. doi:10.1158/1538-7445.AM2015-2586
48. Goto Y, Nokihara H, Murakami H, Shimizu T, Seto T, Krivoshik A, et al. ASP8273, a mutant-selective irreversible EGFR inhibitor in patients (pts) with NSCLC harboring EGFR activating mutations: preliminary results of first-in-human phase I study in Japan. J Clin Oncol (2015) 33:abstract 8014.
49. Yu HA, Spira A, Horn L, Weiss J, Jack H, Giaccone G, et al. Antitumor activity of ASP8273 300 mg in subjects with EGFR mutation-positive non-small cell lung cancer: interim results from an ongoing phase 1 study. J Clin Oncol (2016) 34:abstract 9050.
50. Lelais G, Epple R, Marsilje TH, Long YO, McNeill M, Chen B, et al. Discovery of (R,E)-N-(7-Chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imidazol-2-yl)-2-methylisonicotinamide (EGF816), a novel, potent, and WT sparing covalent inhibitor of oncogenic (L858R, ex19del) and resistant (T790M) EGFR mutants for the treatment of EGFR mutant non-small-cell lung cancers. J Med Chem (2016) 59:6671–89. doi:10.1021/acs.jmedchem.5b01985
51. Tan DSW, Yang JC-H, Leighl NB, Riely G, Sequist LV, Felip E, et al. Updated results of a phase 1 study of EGF816, a third-generation, mutant-selective EGFR tyrosine kinase inhibitor (TKI), in advanced non-small cell lung cancer (NSCLC) harboring T790M. J Clin Oncol (2016) 34:abstract 9044.
52. Park SJ, Kim HT, Lee DH, Kim KP, Kim S-W, Suh C, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer (2012) 77:556–60. doi:10.1016/j.lungcan.2012.05.092
53. Fan Y, Xu X, Xie C. EGFR-TKI therapy for patients with brain metastases from non-small-cell lung cancer: a pooled analysis of published data. Onco Targets Ther (2014) 7:2075–84. doi:10.2147/OTT.S67586
54. Kim D, Yang J, Cross D, Ballard P, Yang P, Yates J. Preclinical evidence and clinical cases of AZD9291 activity in EGFR-mutant non-small cell lung cancer (NSCLC) brain metastases (BM). Ann Oncol (2014) 25(Suppl 4):146–64.
55. Ballard P, Yates JWT, Yang Z, Kim D-W, Yang JC-H, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res (2016) 22:5130–40. doi:10.1158/1078-0432.CCR-16-0399
56. Ahn M-J, Tsai C-M, Yang JC-H, Shepherd FA, Satouchi M, Kim D-W, et al. AZD9291 activity in patients with EGFR-mutant advanced non-small cell lung cancer (NSCLC) and brain metastases: data from Phase II studies. Eur J Cancer (2015) 51(Suppl 3):S625–6. doi:10.1016/S0959-8049(16)31724-5
57. Zeng Q, Wang J, Cheng Z, Chen K, Johnström P, Varnäs K, et al. Discovery and evaluation of clinical candidate AZD3759, a potent, oral active, central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor. J Med Chem (2015) 58:8200–15. doi:10.1021/acs.jmedchem.5b01073
58. Kim D-W, Yang C-H, Chen K, Cheng Z, Yin L, Martin P, et al. AZD3759, an EGFR inhibitor with blood brain barrier (BBB) penetration for the treatment of non-small cell lung cancer (NSCLC) with brain metastasis (BM): preclinical evidence and clinical cases. J Clin Oncol (2015) 33:abstract 8016.
59. Yang JC-H, Kim D-W, Kim S-W, Cho BC, Lee J-S, Yin XYX, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): updated results from BLOOM, a phase I study. J Clin Oncol (2016) 34:abstract 9002.
60. Ahn M-J, Kim D-W, Kim TM, Lin C-C, Ratnayake J, Carlie D, et al. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). J Clin Oncol (2016) 34:abstract 9003.
61. Niederst MJ, Hu H, Mulvey HE, Lockerman EL, Garcia AR, Piotrowska Z, et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res (2015) 21:3924–33. doi:10.1158/1078-0432.CCR-15-0560
62. Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med (2015) 21:560–2. doi:10.1038/nm.3854
63. Yu HA, Tian SK, Drilon AE, Borsu L, Riely GJ, Arcila ME, et al. Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol (2015) 1:982–4. doi:10.1001/jamaoncol.2015.1066
64. Costa DB, Kobayashi SS. Whacking a mole-cule: clinical activity and mechanisms of resistance to third generation EGFR inhibitors in EGFR mutated lung cancers with EGFR-T790M. Transl Lung Cancer Res (2015) 4:809–15. doi:10.3978/j.issn.2218-6751.2015.05.05
65. Song H-N, Jung KS, Yoo KH, Cho J, Lee JY, Lim SH, et al. Acquired C797S mutation upon treatment with a T790M-specific third-generation EGFR inhibitor (HM61713) in non-small cell lung cancer. J Thorac Oncol (2016) 11:e45–7. doi:10.1016/j.jtho.2015.12.093
66. Chabon JJ, Simmons AD, Lovejoy AF, Esfahani MS, Newman AM, Haringsma HJ, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun (2016) 7:11815. doi:10.1038/ncomms11815
67. Bersanelli M, Minari R, Bordi P, Gnetti L, Bozzetti C, Squadrilli A, et al. L718Q mutation as new mechanism of acquired resistance to AZD9291 in EGFR-mutated NSCLC. J Thorac Oncol (2016) 11:e121–3. doi:10.1016/j.jtho.2016.05.019
68. Hata AN, Niederst MJ, Archibald HL, Gomez-Caraballo M, Siddiqui FM, Mulvey HE, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med (2016) 22:262–9. doi:10.1038/nm.4040
69. Eberlein CA, Stetson D, Markovets AA, Al-Kadhimi KJ, Lai Z, Fisher PR, et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res (2015) 75:2489–500. doi:10.1158/0008-5472.CAN-14-3167
70. Planchard D, Loriot Y, André F, Gobert A, Auger N, Lacroix L, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol (2015) 26:2073–8. doi:10.1093/annonc/mdv319
71. Oxnard GR, Thress C, Paweletz CP, Stetson D, Dougherty B, Markovets A, et al. Mechanisms of acquired resistance to AZD9291 in EGFR T790M positive lung cancer. J Thorac Oncol (2015) 10(Suppl 9):Oral 17.07.
72. Piotrowska Z, Niederst MJ, Karlovich CA, Wakelee HA, Neal JW, Mino-Kenudson M, et al. Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Cancer Discov (2015) 5:713–22. doi:10.1158/2159-8290.CD-15-0399
73. Kim TM, Song A, Kim D-W, Kim S, Ahn Y-O, Keam B, et al. Mechanisms of acquired resistance to AZD9291: a mutation-selective, irreversible EGFR inhibitor. J Thorac Oncol (2015) 10:1736–44. doi:10.1097/JTO.0000000000000688
74. Li S, Schmitz KR, Jeffrey PD, Wiltzius JJW, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell (2005) 7:301–11. doi:10.1016/j.ccr.2005.03.003
75. Ercan D, Choi HG, Yun C-H, Capelletti M, Xie T, Eck MJ, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res (2015) 21:3913–23. doi:10.1158/1078-0432.CCR-14-2789
76. Jia Y, Yun C-H, Park E, Ercan D, Manuia M, Juarez J, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature (2016) 534:129–32. doi:10.1038/nature17960
77. Tricker EM, Xu C, Uddin S, Capelletti M, Ercan D, Ogino A, et al. Combined EGFR/MEK inhibition prevents the emergence of resistance in EGFR-mutant lung cancer. Cancer Discov (2015) 5:960–71. doi:10.1158/2159-8290.CD-15-0063
78. Ahn M-J, Yang JC-H, Yu HA, Saka H, Ramalingam S, Huang X. LBA2_PR osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON Phase Ib trial. Eur J Cancer (2016) 51:S625–6. doi:10.1016/S0959-8049(16)31724-5
79. Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res (2009) 15:3484–94. doi:10.1158/1078-0432.CCR-08-2904
80. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol (2014) 15:1236–44. doi:10.1016/S1470-2045(14)70381-X
81. Stahel R, Dafni U, Gautschi O, Felip E, Curioni-Fontecedro A, Peters S, et al. A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced non-small-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T790M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial. Eur J Cancer (2015) 51(Suppl 3):S711–12. doi:10.1016/S0959-8049(15)30068-X
Keywords: EGFR, T790M, NSCLC, osimertinib, third generation, brain metastasis
Citation: Sullivan I and Planchard D (2017) Next-Generation EGFR Tyrosine Kinase Inhibitors for Treating EGFR-Mutant Lung Cancer beyond First Line. Front. Med. 3:76. doi: 10.3389/fmed.2016.00076
Received: 16 November 2016; Accepted: 28 December 2016;
Published: 18 January 2017
Edited by:
Pierlorenzo Pallante, Consiglio Nazionale delle Ricerche (CNR), ItalyReviewed by:
Francesco Trapasso, Magna Græcia University, ItalyCopyright: © 2017 Sullivan and Planchard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Planchard, ZGF2aWQucGxhbmNoYXJkQGd1c3RhdmVyb3Vzc3kuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.