- 1Division of Hematology-Oncology, University of California Irvine, Orange, CA, USA
- 2The Angeles Clinic and Research Institute, Los Angeles, CA, USA
- 3Cedars-Sinai Medical Center, Samuel Oschin Comprehensive Cancer Institute, Los Angeles, CA, USA
The management of anaplastic lymphoma kinase rearranged (ALK+) non-small cell lung cancer (NSCLC) exemplifies the potential of a precision medicine approach to cancer care. The ALK inhibitor crizotinib has led to improved outcomes in the first- and second-line setting; however, toxicities, intracranial activity, and acquired resistance necessitated the advent of later generation ALK inhibitors. A large portion of acquired resistance to ALK inhibitors is caused by secondary mutations in the ALK kinase domain. Alectinib is a second-generation ALK inhibitor capable of overcoming multiple crizotinib-resistant ALK mutations and has demonstrated improved outcomes after crizotinib failure. Favorable toxicity profile and improved intracranial activity have spurred ongoing front-line trials and comparisons to other ALK inhibitors. However, important questions regarding comparability to competitor compounds, acquired alectinib resistance, and ALK inhibitor sequencing remain. Here, we review the key clinical data supporting alectinib in the second-line therapy of ALK+ NSCLC and provide context in comparison to other ALK inhibitors in development.
Background
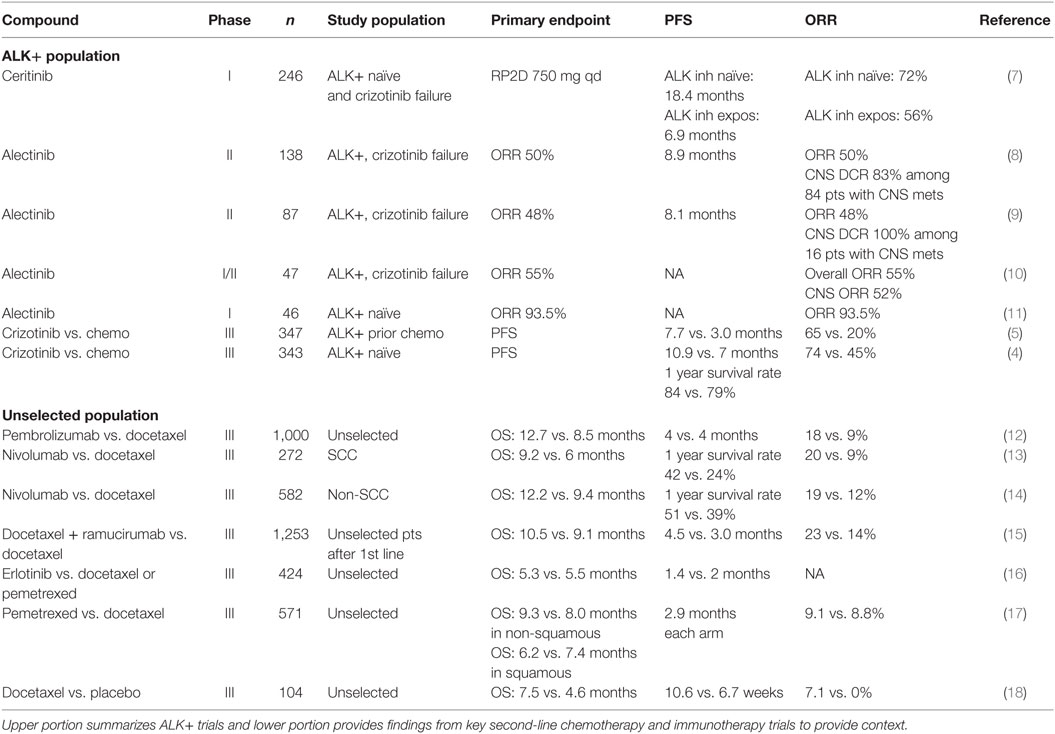
Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer and remains the leading cause cancer-related mortality in both men and women with a 5-year survival rate of less than 20% in US patients (1). Rapid advances in understanding the molecular pathogenesis of NSCLC have demonstrated that NSCLC is a heterogeneous group of diseases. Chromosomal rearrangements involving ALK and ROS1 are present in 3–7% (2) and 2% (3) of patients with NSCLC, respectively. ALK translocations are found nearly exclusively in lung adenocarcinomas. Crizotinib, a first-generation ALK and ROS1 inhibitor, has resulted in improved progression-free survival (PFS) relative to chemotherapy in the first- and second-line settings for ALK-rearranged (ALK+) NSCLC. Compared to chemotherapy in treatment naïve ALK-rearranged patients, crizotinib led to higher objective response rate (ORR) (74 vs. 45%) and median PFS (10.9 vs. 7.0 months) but no difference in overall survival (hazard ratio for death with crizotinib, 0.82; 95% CI, 0.54–1.26; P = 0.36) (Table 1) (4). In ALK-rearranged patients with prior chemotherapy exposure, crizotinib also led to improved ORR (65 vs. 20%) and median PFS (7.7 vs. 3.3 months) (5). Like other oncogene driven tumors, acquired resistance is nearly universal in ALK+ NSCLC, and most develop crizotinib resistance within 1 year of treatment with central nervous system (CNS) metastasis being a major site of progression (6).
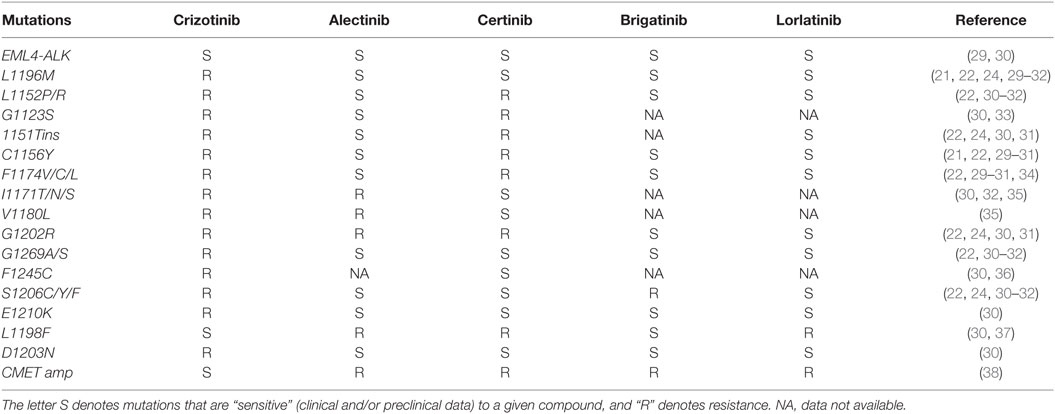
While the propensity for intracranial failure on crizotinib is partly related to lower penetration of blood–brain barrier (19), systemic relapses are mediated by multiple mechanisms including secondary ALK mutations and compensatory bypass pathway activation. In nearly a third of patients, tumors have acquired secondary mutation in the ALK tyrosine kinase domain. The most common resistance mutation is the gatekeeper L1196M mutation, followed by the G1269A (20–22). Additional resistance mutations include C1156Y, L1152R, G1202R, S1206Y, 1151Tins, F1174C, and D1203N, among many others (Table 2) (23–25). These mutations blunt the efficacy of crizotinib by either increasing the ALK kinase affinity for adenosine triphosphate (ATP) (G1269A and 1151Tins), inducing conformational change causing steric hindrance (G1202R and S1206Y) or interfering with the downstream signaling pathway (L1152R) (23). Amplification of the ALK fusion gene was observed either alone or in combination with other resistance mechanisms in both in vitro studies (20) and resistant clinical specimens (26). Beyond the ALK dominant resistance mechanism, preclinical work and progression biopsies from patients on ALK inhibitors have revealed crizotinib resistance from amplification of epidermal growth factor receptor (EGFR) pathway, insulin-like growth factor pathway (IGF-1R), cKIT mutation, and SRC activity (26–28).
While crizotinib ushered in a new paradigm for ALK+ NSCLC, the emergence of acquired resistance and rates of intracranial progression suggested ongoing clinical needs in ALK+ disease. The management of crizotinib failure has largely been informed by data from later generation ALK inhibitors including alectinib; however, other recent second-line trials outside ALK+ disease are worth brief contextual mention (Table 1). The phase III REVEL trial demonstrated that the addition of ramucirumab (a vascular endothelial growth factor receptor 2 monoclonal antibody) to docetaxel in unselected advanced NSCLC patients yielded higher response rate (23 vs. 14%), median PFS (4.5 vs. 3 months), and median OS (10.5 vs. 9.1 months) than docetaxel monotherapy (15). Similarly, in the phase III CheckMate 017 trial nivolumab yielded superior ORR (20 vs. 9%), median PFS (3.5 vs. 2.8 months), and median OS (9.2 vs. 6.0 months) compared with docetaxel in heavily pretreated unselected advanced squamous NSCLC patients (13). The CheckMate 057 trial found higher ORR (19 vs. 12%) and median OS (12.2 vs. 9.4 months) in patients with non-squamous NSCLC compared with docetaxel (14). The efficacy of pembrolizumab was demonstrated in phase II/III KEYNOTE-010 trial which compared pembrolizumab vs. docetaxel in more than 1,000 patients (12). Pembrolizumab led to improved median OS in the overall population (12.7 vs. 8.5 months). Among 442 patients with at least 50% PD-L1 expression, the median OS for the pembrolizumab 2 mg/kg, 10 mg/kg, and docetaxel groups was 14.9, 17.3, and 8.2 months, respectively.
Alectinib Overview
The expanding appreciation of crizotinib-resistant ALK mutations spurred development of the second-generation ALK inhibitors. Alectinib is a potent and selective second-generation oral ALK inhibitor. Alectinib exhibits limited inhibitory activity against other protein kinases such as EGFR, fibroblast growth factor receptor 2 (FGFR2), human epidermal growth factor receptor 2 (HER2), hepatocyte growth factor receptor (MET), platelet-derived growth factor subunit B (PDGFB), and Janus kinase 1 (JAK1) (29). In cell free assays, the half maximal inhibitory concentration (IC50) of alectinib for enzyme activity of ALK was 1.9 nM and the dissociation constant (KD) value for ALK in an ATP-competitive manner was 2.4 nM (29). In vitro experiments demonstrated that alectinib induces caspase-mediated apoptosis in EML4-ALK cell lines and results in dose-dependent tumor growth inhibition (ED50 = 0.46 mg/kg) and regression in animal models (29). More importantly, alectinib displayed significant efficacy against crizotinib-resistant ALK L1196M (IC50, 2 nM) and G1269A (IC50, 9 nM) mutations (22, 29). Alectinib was also active against ALK C1156Y, F1174L, 1151Tins, and L1152R but not ALK G1202R (IC50, 70–80 nM) both in vitro and in vivo experiments (Table 2) (22).
Alectininb for Crizotinib Failure
Clinical trials evaluating the safety and efficacy of alectinib have been conducted in Japan and the US as both first-line untreated and ALK+ patient progressing on crizotinib. Support for alectinib activity in crizotinib failure comes from the AF-002JG study in which alectinib at 300–900 mg BID was well tolerated, with the most common adverse events (AEs) being fatigue (30%), myalgia (17%), and peripheral edema (15%) (10). The recommended phase II dose was 600 mg BID. Of the 44 evaluable patients with crizotinib resistance, 24 (55%) patients had response, 16 (36%) had stable disease (SD), and 4 (9%) had progressive disease. Alectinib also demonstrated activity against CNS metastases in 21 patients with an intracranial response rate of 52% [29% complete response (CR), 24% partial response (PR), and 38% SD] (10). Similar results were seen in a North American trial of 87 patients with advanced ALK-rearranged NSCLC who were refractory to crizotinib (9). The ORR for alectinib was 48% with a median PFS of 8.1 months (95% CI, 6.2–12.6). Fifty two patients had brain metastases at enrollment and 21 (40%) patients experienced CNS tumor regression, including 13 (25%) patients who achieved CR. Alectinib 600 mg BID was well tolerated with predominantly low grade constipation (36%), fatigue (33%), myalgia (24%), and peripheral edema (23%). Finally, the large phase II global study (NP2873) examined the ORR of alectinib for crizotinib-refractory ALK+ patients (n = 138) (8). This study is notable for a high rate of CNS metastases (61%) at baseline. The ORR determined by independent review committee was 50% (95% CI, 41–59%) and the median PFS was 8.9 months (95% CI, 5.6–11.3). Alectinib was highly effective for CNS metastases, with ORR of 57% and DCR of 83%. Of the 23 patients with baseline untreated CNS metastases, 10 (43%) had a complete CNS response. The authors note that the cumulative CNS progression rate (24.8%) was lower than the cumulative non-CNS progression rate (33.2%), which suggests that alectinib may delay or prevent the emergence of CNS metastases. Alectinib 600 mg BID was well tolerated with common side effects including low grade constipation (33%), fatigue (26%), and peripheral edema (25%). Overall the similar response rate to alectinib between the US and Japanese patients indicate no ethnic difference in response. Additionally, there was no significant difference in alectinib exposure at 600 mg twice daily among a small subgroup of Caucasian and Asian patients who underwent pharmacokinetic analysis. Based on established activity, the Food and Drug Administration approved alectinib for the treatment of ALK+ NSCLC patients who progressed or were intolerant of crizotinib on December 11, 2015.
Based on promising second-line data and potential superiority over crizotinib, alectinib is being investigated in the first-line setting. In the phase I/II AF-001JP study conducted in Japan, patients with ALK inhibitor-naïve ALK+ NSCLC were treated with alectinib (11). Alectinib at 300 mg BID daily was well tolerated with few grade 3 toxicities or dose-limiting toxicities (DLTs) and ORR was observed in 43 out of 46 patients (93.5%) at this dose. On the other hand, the response rate for first-line crizotinib reported by Solomon et al. was 74% (4). Two phase III trials, ALEX (NCT02075840), and JapicCTI-132316, are currently comparing alectinib and crizotinib in ALK inhibitor-naive patients with ALK-rearranged NSCLC. Recently updated clinical data among 207 randomized patients in the J-ALEX trial were presented at the ASCO 2016 annual meeting (39). The primary endpoint was PFS and secondary endpoints included OS, ORR, CNS PFS, safety, and quality of life. In the alectinib arm, constipation (36%) was the only common event, while in the crizotinib arm nausea (74%), diarrhea (73%), vomiting (59%), visual disturbance (55%), dysgeusia (52%), constipation (46%), ALT elevation (32%), and AST elevation (31%) were seen in >30% patients. Alectinib was more tolerable than crizotinib with fewer grade 3/4 AEs (26.2 vs. 51.9%) which translated to a lower discontinuation rate (8.7 vs. 20.2%). The ORRs of the alectinib and crizotinib arms were 91.6 and 78.9%, respectively. The median PFS was not reached (CI, 20.3 to NR) but significantly higher than crizotinib 10.2 (CI, 8.2–12.0) with HR 0.34 (0.17–0.71). Complete data sets from first-line trials are eagerly awaited and may lead to additional indications for alectinib.
Additional Second- and Third-Generation ALK Inhibitors
The second-generation ALK inhibitor ceritinib has in vitro activity against crizotinib-resistant mutations. Results from the open label multicenter ASCEND-1 trial showed that ceritinib yielded ORR of 72% (95% CI, 61–82) in 83 ALK inhibitor-naive patients and 56% (49–64) in 163 ALK inhibitor-resistant patients (7). Median PFS was 18.4 months in ALK inhibitor-naive patients and 6.9 months (5.6–8.7) in ALK inhibitor-pretreated patients. Among 94 patients with brain metastases, intracranial disease control was reported in 15 of 19 (79%) ALK inhibitor-naïve patients and in 49 of 75 (65%) ALK inhibitor-pretreated patients. In ALK inhibitor-resistant patients with CNS metastasis, the rates of intracranial CR, PR, and SD were 5, 13, and 47%, respectively. Common toxicities included diarrhea (80%), nausea (77%), vomiting (57%), fatigue (38%), abdominal pain (37%), decreased appetite (36%), constipation (30%), cough (29%), abdominal pain (23%), and dyspnea (21%). In April 2014, ceritinib 750 mg daily was approved by the US FDA for ALK+ previously treated with crizotinib.
Although both alectinib and ceritinib have shown promising systemic and CNS activity they are unlikely to be compared head to head in clinical trials. While ceritinib appears to have similar systemic response to alectinib, the intracranial response rate appears inferior to alectinib in crizotinib-resistant patients with CNS metastases. Accepting cross-trial comparison caveats the absolute median PFS is numerically shorter for ceritinib (6.9 months in the ASCEND-1 trial) than alectinib (8.9 months in the global NP2873 trial) in ALK inhibitor-resistant patients.
Other ALK inhibitors including brigatinib (AP26113) and lorlatinib (PF-06463922) have shown activity in crizotinib failure and highlight the non-overlapping resistance mutation coverage among current ALK inhibitors (Table 2). Briefly, brigatinib is a potent dual inhibitor of ALK and EGFR, including ALK L1196M and EGFR T790M mutants, shown in preclinical studies (40, 41). In the phase II ALTA study, 222 heavily pretreated ALK-rearranged patients were randomized to receive brigatinib 90 mg PO (arm A) vs. 180 mg PO qd (arm B) (42). The investigator-assessed ORRs of arm A and B patients were 46% (95% CI, 36–55%) and 54% (95% CI, 44–63%), respectively. Median PFS in arms A and B was 8.8 and 11.1 months, respectively. However, the median follow-up was only 8.3 months and longer follow-up is needed to confirm the higher PFS observed in arm B. Among patients with active brain metastases at baseline, intracranial ORRs, as assessed by independent review committee, in A and B were 37% (7/19) and 73% (11/15), respectively. Most common AEs in arms A/B included nausea (33/40%), diarrhea (19/38%), headache (28/27%), cough (18/34%), dyspnea (21/21%), fatigue (20/27%), constipation (19/15%), abdominal pain (17/8%), and vomiting (24/23%). Grade ≥ 3 treatment-emergent AEs (A/B) included: increased CPK (3/8%), hypertension (4/5%), pneumonia (3/5%), rash (1/4%), and pneumonitis (2/3%). Discontinuations and dose reductions due to AEs (A/B) were 3/6% and 7/18%, respectively. Due to the favorable efficacy and toxicity profile, brigatinib 180 mg PO daily was chosen as the optimal dose and is moving forward in the phase III ALTA-1L vs. crizotinib in the first-line setting.
Lorlatinib (PF-06463922) is a third-generation reversible, potent ATP-competitive small molecule, inhibitor of ALK and ROS1. Lorlatinib has demonstrated activity against the majority of known resistant ALK mutations, except for L1198F (Table 2) (31, 37). Early data from an ongoing phase I/II study of lorlatinib in mostly pretreated patients with ALK+ and ROS1+ NSCLC were presented at the ASCO 2016 annual meeting (43). Among the 54 evaluable patients who received dose escalation from 10 mg to 200 mg, the overall response rate was 50% and intracranial response rate was 44% for target and non-target lesions and 60% for target lesions. The most common treatment-related AEs were hypercholesterolemia (54%) and peripheral edema (37%). Hypercholesterolemia was the most common (9%) grade (G) ≥ 3 treatment-related AE and most frequent reason for dose delay/reduction. No patient was discontinued due to a treatment-related AEs. The phase II dose was identified as 100 mg once daily. Pharmacokinetic analysis of four patients revealed that the unbound CSF to plasma drug ratio ranged from 0.61 to 0.96, indicative of good CSF penetration. In contrast, the ratio of CNS to serum concentration of crizotinib has been in the range of 0.0006–0.001 in previous reports (19, 44). Lorlatinib is effective against the G1202R mutation (Table 2).
Conclusion/Future Directions
Over the past decade, there has been a remarkable progress in the target therapy for the management of ALK-rearranged NSCLC. Second- and third-generation inhibitors demonstrate broader coverage against crizotinib-resistant ALK mutations and often more favorable side effect profiles. As discussed elsewhere in this issue, we are approaching a paradigm in which understanding the exact resistance mechanism will inform the optimal choice and perhaps sequencing of ALK inhibitors. The approval of alectinib for crizotinib failure highlights major areas of focus in ALK+ disease; toxicity profile, intracranial activity, and resistance mutation coverage. While alectinib compares favorably in these areas, ongoing results from first-line trials and direct comparison against current and emerging ALK inhibitors will be important to refine optimal alectinib usage. Here we have provided a review of the clinical data supporting the activity of alectinib in the management of ALK+ NSCLC with a focus on the second-line setting in advanced disease.
Author Contributions
PT and SK are involved in the conception/design and drafting the manuscript. All the authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GP and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
The authors would like to recognize the important contributions from researchers whose work could not be cited due to space constraints.
Funding
The authors received no funding for this manuscript.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin (2016) 66(1):7–30. doi: 10.3322/caac.21332
2. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature (2007) 448(7153):561–6. doi:10.1038/nature05945
3. Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol (2012) 30(8):863–70. doi:10.1200/JCO.2011.35.6345
4. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med (2014) 371(23):2167–77. doi:10.1056/NEJMoa1408440
5. Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med (2013) 368(25):2385–94. doi:10.1056/NEJMoa1214886
6. Costa DB, Shaw AT, Ou S-HI, Solomon BJ, Riely GJ, Ahn M-J, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non–small-cell lung cancer and brain metastases. J Clin Oncol (2015) 33:1881–90. doi:10.1200/JCO.2014.59.0539
7. Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol (2016) 17(4):452–63. doi:10.1016/S1470-2045(15)00614-2
8. Ou SH, Ahn JS, De Petris L, Govindan R, Yang JC, Hughes B, et al. Alectinib in crizotinib-refractory ALK-rearranged non–small-cell lung cancer: a phase II global study. J Clin Oncol (2015) 34(7):661–8. doi:10.1200/JCO.2015.63.9443
9. Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol (2015) 17(2):234–42. doi:10.1016/S1470-2045(15)00488-X
10. Gadgeel SM, Gandhi L, Riely GJ, Chiappori AA, West HL, Azada MC, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol (2014) 15(10):1119–28. doi:10.1016/S1470-2045(14)70362-6
11. Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M, Inoue A, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol (2013) 14(7):590–8. doi:10.1016/S1470-2045(13)70142-6
12. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2015) 387(10027):1540–50. doi:10.1016/S0140-6736(15)01281-7
13. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med (2015) 373(2):123–35. doi:10.1056/NEJMoa1504627
14. Borghaei H, Paz-Ares L, Horn L, Spigel D, Steins M, Ready N, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi:10.1056/NEJMoa1507643
15. Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet (2014) 384(9944):665–73. doi:10.1016/S0140-6736(14)60845-X
16. Ciuleanu T, Stelmakh L, Cicenas S, Miliauskas S, Grigorescu AC, Hillenbach C, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol (2012) 13(3):300–8. doi:10.1016/S1470-2045(11)70385-0
17. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non–small-cell lung cancer previously treated with chemotherapy. J Clin Oncol (2004) 22(9):1589–97. doi:10.1200/JCO.2004.08.163
18. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non–small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol (2000) 18(10):2095–103. doi:10.1200/JCO.2000.18.10.2095
19. Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol (2011) 29(15):e443–5. doi:10.1200/JCO.2010.34.1313
20. Doebele RC, Pilling AB, Aisner DL, Kutateladze TG, Le AT, Weickhardt AJ, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non–small cell lung cancer. Clin Cancer Res (2012) 18(5):1472–82. doi:10.1158/1078-0432.CCR-11-2906
21. Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med (2010) 363(18):1734–9. doi:10.1056/NEJMoa1007478
22. Kodama T, Tsukaguchi T, Yoshida M, Kondoh O, Sakamoto H. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett (2014) 351(2):215–21. doi:10.1016/j.canlet.2014.05.020
23. Rolfo C, Passiglia F, Castiglia M, Raez LE, Germonpre P, Gil-Bazo I, et al. ALK and crizotinib: after the honeymoon… what else? Resistance mechanisms and new therapies to overcome it. Transl Lung Cancer Res (2014) 3(4):250. doi:10.3978/j.issn.2218-6751.2014.03.01
24. Katayama R, Shaw AT, Khan TM, Mino-Kenudson M, Solomon BJ, Halmos B, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med (2012) 4(120):120ra17. doi:10.1126/scitranslmed.3003316
25. Sasaki T, Koivunen J, Ogino A, Yanagita M, Nikiforow S, Zheng W, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res (2011) 71(18):6051–60. doi:10.1158/0008-5472.CAN-11-1340
26. Katayama R, Khan TM, Benes C, Lifshits E, Ebi H, Rivera VM, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A (2011) 108(18):7535–40. doi:10.1073/pnas.1019559108
27. Crystal AS, Shaw AT, Sequist LV, Friboulet L, Niederst MJ, Lockerman EL, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science (2014) 346(6216):1480–6. doi:10.1126/science.1254721
28. Lovly CM, McDonald NT, Chen H, Ortiz-Cuaran S, Heukamp LC, Yan Y, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med (2014) 20(9):1027–34. doi:10.1038/nm.3667
29. Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell (2011) 19(5):679–90. doi:10.1016/j.ccr.2011.04.004
30. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov (2016) 6(10):1118–33. doi:10.1158/2159-8290.CD-16-0596
31. Zou HY, Friboulet L, Kodack DP, Engstrom LD, Li Q, West M, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell (2015) 28(1):70–81. doi:10.1016/j.ccell.2015.05.010
32. Friboulet L, Li N, Katayama R, Lee CC, Gainor JF, Crystal AS, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non–small cell lung cancer. Cancer Discov (2014) 4(6):662–73. doi:10.1158/2159-8290.CD-13-0846
33. Toyokawa G, Inamasu E, Shimamatsu S, Yoshida T, Nosaki K, Hirai F, et al. Identification of a novel ALK G1123S mutation in a patient with ALK-rearranged non–small-cell lung cancer exhibiting resistance to ceritinib. J Thorac Oncol (2015) 10(7):e55–7. doi:10.1097/JTO.0000000000000509
34. Ou S-H, Milliken JC, Azada MC, Miller VA, Ali SM, Klempner SJ, et al. ALK F1174V mutation confers sensitivity while ALK I1171 mutation confers resistance to alectinib. The importance of serial biopsy post progression. Lung Cancer (2016) 91:70–2. doi:10.1016/j.lungcan.2015.09.006
35. Katayama R, Friboulet L, Koike S, Lockerman EL, Khan TM, Gainor JF, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res (2014) 20(22):5686–96. doi:10.1158/1078-0432.CCR-14-1511
36. Kodityal S, Elvin JA, Squillace R, Agarwal N, Miller VA, Ali SM, et al. A novel acquired ALK F1245C mutation confers resistance to crizotinib in ALK-positive NSCLC but is sensitive to ceritinib. Lung Cancer (2016) 92:19–21. doi:10.1016/j.lungcan.2015.11.023
37. Shaw AT, Friboulet L, Leshchiner I, Gainor JF, Bergqvist S, Brooun A, et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med (2016) 374(1):54–61. doi:10.1056/NEJMoa1508887
38. Awad MM, Shaw AT. ALK inhibitors in non–small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol (2014) 12(7):429.
39. Nokihara H, Hida T, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): primary results from the J-ALEX study. Oral presentation ASCO 2016. J Clin Oncol (2016) 34(Suppl):abstr 9008.
40. Camidge DR, Bazhenova L, Salgia R, Weiss GJ, Langer CJ, Shaw AT, et al. First-in-human dose-finding study of the ALK/EGFR inhibitor AP26113 in patients with advanced malignancies: updated results. J Clin Oncol (2013) 31(Suppl):8031.
41. Zhang S, Anjum R, Squillace R, Nadworny S, Zhou T, Keats J, et al. The potent ALK inhibitor AP26113 can overcome mechanisms of resistance to first- and second-generation ALK TKIs in preclinical models. Cancer Res (2015) 75(15 Suppl):781–781. doi:10.1158/1538-7445.AM2015-781
42. Kim D-W, Tiseo M, Ahn M-J, Reckamp KL, Hansen KH, Kim S-W, et al. Brigatinib (BRG) in patients (pts) with crizotinib (CRZ)-refractory ALK+ non-small cell lung cancer (NSCLC): first report of efficacy and safety from a pivotal randomized phase (ph) 2 trial (ALTA). ASCO 2016 meeting oral presentation. J Clin Oncol (2016) 34(Suppl):abstr 9007.
43. Solomon BJ, Bauer TM, Felip E, Besse B, James LP, Clancy JS, et al. Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). J Clin Oncol (2016) 34(Suppl):abstr 9009.
Keywords: alectinib, NSCLC, ALK, second line, crizotinib, resistance
Citation: Tran PN and Klempner SJ (2016) Focus on Alectinib and Competitor Compounds for Second-Line Therapy in ALK-Rearranged NSCLC. Front. Med. 3:65. doi: 10.3389/fmed.2016.00065
Received: 25 September 2016; Accepted: 17 November 2016;
Published: 30 November 2016
Edited by:
Umberto Malapelle, University of Naples Federico II, ItalyReviewed by:
Giovanna Maria Pierantoni, University of Naples Federico II, ItalyDario De Biase, University of Bologna, Italy
Copyright: © 2016 Tran and Klempner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel J. Klempner, c2tsZW1wbmVyQHRoZWFuZ2VsZXNjbGluaWMub3Jn
 Phu N. Tran
Phu N. Tran Samuel J. Klempner
Samuel J. Klempner