
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mech. Eng., 28 April 2021
Sec. Tribology
Volume 7 - 2021 | https://doi.org/10.3389/fmech.2021.661422
This article is part of the Research TopicBioadhesionView all 14 articles
 Jonas O. Wolff1*
Jonas O. Wolff1* Janek von Byern2
Janek von Byern2 Dakota Piorkowski3
Dakota Piorkowski3 Jian Fang4
Jian Fang4 Xungai Wang5
Xungai Wang5 Lewis Adler6
Lewis Adler6 Donald S. Thomas7
Donald S. Thomas7 James M. Hook7,8
James M. Hook7,8 Sean J. Blamires9*
Sean J. Blamires9*Adhesive snares built from silks are fascinating adaptations that have rarely evolved outside spiders. Glowworms (Arachnocampa spp.) are an iconic part of the fauna of Australia and New Zealand that combine the construction of a sticky snare with a bioluminescent lure. Recently, the structure and biomechanical properties of glowworm silk have been studied in detail, but the chemical composition of its adhesive coating, and how it varies between species of Arachnocampa remained unclear, limiting an understanding of the glue function. Here, we studied the chemical composition of the water-soluble fraction of the adhesive droplets from the snares in cave and epigaeic populations of three species of Arachnocampa from mainland Australia, Tasmania, and New Zealand, using a combination of nuclear magnetic resonance and mass spectrometry. We found that glowworm glues comprise a large variety of small organic compounds, with organic acids, amino acids, amino acid derivates, alcohols, urea, and urea derivates being the major fraction, supplemented by small amounts of sugars, fatty acids, and other organic compounds. While there was a general overlap in the compounds detected in the adhesives of all tested Arachnocampa species and populations, the relative amounts differed considerably. We expect that these differences are a product of diet rather than an adaptive response to different environments, but experiments are needed for clarification. The high amount of polar substances and compounds that are hygroscopic at high humidity explains the adhesive properties of the viscous solution and its stability in damp environments. These results contribute to our understanding of the unique prey capture strategy of glowworms. Further, the comparison with convergent spider webs highlights the use of small polar compounds as plasticizers of macro-molecular bioadhesives as a general principle. This may inspire the biomimetic design of novel pressure sensitive adhesives with high performance under high humidity conditions.
Many invertebrates use viscous fluids or soft solids as reversible adhesives to capture prey, such as in spider capture silk, velvetworm slime, and harvestmen glue (Betz and Kölsch, 2004; Suter and Stratton, 2009; Haritos et al., 2010; Sahni et al., 2010; Wolff et al., 2014; Wolff and Gorb, 2016). Such adhesives have recently come into focus in ecological and biodiversity research (Agnarsson and Blackledge, 2009; Zhang and Weirauch, 2013; Blamires et al., 2014; Wolff et al., 2016; Opell et al., 2018; Diaz et al., 2020). Biological adhesives are often adapted toward special requirements, such as generating adhesion to contaminated substrates or at variable humidity, and show a remarkable performance under conditions that are challenging for artificial adhesives (Wolff et al., 2014; Opell et al., 2018; Diaz et al., 2020). Therefore, they have also been proposed as promising biomimetic models for the design of novel artificial adhesives (von Byern and Grunwald, 2010; Sahni et al., 2011).
Glowworms (Nematocera: Arachnocampa spp.) are the larvae of the fungus gnat, small dipterans that live in the temperate rain forests of Australia and New Zealand. These animals are remarkable in their ability to spin adhesive capture threads and lure prey insects with a bioluminescent organ (Broadley and Stringer, 2001; Meyer-Rochow, 2007). The “web” consists of a horizontal mucous tube that functions as a retreat, and from which a curtain of capture threads hangs (Gatenby and Cotton, 1960). These capture threads bear elliptical mucous droplets that are regularly arranged like beads on a string (Meyer-Rochow, 2007; von Byern et al., 2016). Glowworms spin their snares only in cool, moist, and dark microhabitats, such as caves, the banks of creeks, or shaded canyon walls. Their adhesiveness requires the high humidity to stay hydrated and remain sticky (von Byern et al., 2016; Piorkowski et al., 2018). Under these humid conditions the threads perform remarkably well (Piorkowski et al., 2018; von Byern et al., 2019), where artificial adhesives fail due to water disturbing either the adhesive bonding (Tan et al., 2008) or cohesive strength of the adhesive material itself (Musto et al., 2002).
In a previous study, it was found that adhesion is produced by the salivary gland of the glowworm and is predominantly comprised of water and urea or uric acid, with the addition of trace elements (von Byern et al., 2016), and amino acids (Walker et al., 2015). However, both the exact identity of compounds in the mucous and their variation between species and populations has remained unclear. These aspects are important for advancing our comprehension of the adhesive and hygroscopic functions of this material, to ascertain the relationship between ecological factors and mucous production. Integrating previous results on the function of the glowworm adhesive with a better understanding of the chemical identity and variation of the material could also reveal the principles by which adhesion is enhanced under high humidity, which could help to improve the performance of artificial adhesives and surface coatings.
Here, we comparatively studied the water-soluble fraction of the capture threads of three species and multiple populations of Australian and New Zealand Arachnocampa. We expected the adhesive material to contain salts, which have previously been shown to play an important role in adhesion generation by controlling material hydration in the viscid silk of orb web spiders (Sahni et al., 2014). Further, we expected, the chemical profiles show a high variability and differ between populations and species, either due to diet effects, as observed for spider glues (Blamires et al., 2014, 2017), or as an adaptive response to different habitats, as shown for bioluminescent regulation in glowworms (Sharpe et al., 2015).
We collected adhesive capture silk threads from 10 A. tasmaniensis nests from the ceilings of Mystery Creek and Bradley Chesterman caves, in Southwest National Park, Tasmania, Australia, in October 2017 (see Piorkowski et al., 2017, 2018 for details about the sites). Collection was permitted by the Tasmanian Department of Primary Industries, Parks, Water, and the Environment (permit No. FA15189 and FA17188). We spooled the capture threads around plastic 500 μL pipette tips, which were immediately placed into 3 mL sterile fluid collection tubes for transportation to the Mark Wainwright Analytical centre at the University of New South Wales, Sydney, Australia,. The 3 mL tubes holding the samples wound around pipette tips were all sealed air tight and transported under identical conditions, i.e., taped together and wrapped in foam to prevent temperature variability. All samples were brought to the laboratory at the Mark Wainwright Analytical Centre, UNSW, Sydney, in tact within 2 days of collection, whereupon they were refrigerated at ~4°C.
Samples of A. richardsae capture threads were collected from the Glow Worm Tunnel on the Newnes Plateau, NSW, under the license SL102029 granted by the NSW National Parks and Wildlife Service. The collection method was the same as for A. tasmaniensis.
Samples of A. luminosa capture threads were collected with the same method as above, from populations in Spellbound and Hollow Hill Caves (North Island). Samples were transported on dry ice. Five additional samples were collected in biosilicate glass micro-tubes from a population at the river banks and slopes along the Tatare Tunnels Walk in Franz Josef, Westland (South Island). New Zealand samples were collected under the research permit 39535-RES granted by the Department of Conservation of New Zealand.
The number of threads collected per sample varied for each of the species sampled and from sample to sample as the length of the thread and size of the glue droplets showed immense variation between and among species. In general, between 10 and 20 A. tasmaniensis threads were wound around one pipette tip, between 20 and 30 A. richardsi threads were wound around one pipette tip, and between 10 and 29 A. luminosa threads were wound around a single pipette tip. Ten tips per species and location were collected. However, because the total amount of material extracted per tip was insufficient of itself for the NMR procedures (see below) several (~2–5) tips were pooled prior to processing. The amount of material collected in the field was not weighed. However, the pooled samples were weighed and diluted to standardize their concentrations prior to being prepared for NMR and MS.
All of the glowworm glue samples were washed off the sampling tips with a 150 mM potassium phosphate buffer, pH 6.95, containing an internal reference (deuterated trimethylsilyl propanoate, TMSP), a pH indicator (difluorotrimethylsilanylphosphonic acid, DFTMP), and 99.96% D2O (Cambridge Isotope Laboratories). Single samples were washed with multiple aliquots of buffer adding up to a total volume of 180 μL. Combined samples were prepared from a single aliquot added sequentially to 3 mm NMR tubes (Norell) from the first to last sample in a volume of 180 μL.
Proton (1H) NMR spectroscopy was performed using a Bruker Avance III HD 600 MHz spectrometer (600.13 MHz, 1H; 150.9 MHz 13C) fitted with a 5 mm cryoprobe. Samples were stored in a refrigerated Sample Jet autosampler on the magnet. NMR spectra were acquired using the program TOPSPIN 3.6.0 (Bruker, Preston, Australia). Proton solvent suppression was performed using 1D NOESY pre-saturation (noesy1dpr) and the HOD solvent residual chemical shift. 1H-13C HSQC spectra were acquired using an optimized pulse program in the Bruker library (hsqcedetgpsisp2.4) (Palmer et al., 1991; Kay et al., 1992; Willker et al., 1993; Schleucher et al., 1994; Zwahlen et al., 1997). A sweep width (time domain) of 12 ppm (2k) in the 1H and 240 ppm (512) in the 13C dimension was used over 16 scans. 1H-13C HMBC spectra were acquired using the Bruker pulse program hmbcgplpndqf (Cicero et al., 2001). A sweep width (time domain) of 12 ppm (2k) in the 1H and 195 ppm (512) in the 13C dimension was used over 16 scans. Fourier transformation, phasing, solvent filtering, chemical shift referencing, baseline correction, and reference line shape convolution were performed in TOPSPIN. We compared the relative peak positions of our deconvoluted spectra with a spectral reference database for biological metabolites using BAYESIL (Bovey and Mirau, 1996) to identify the individual organic and inorganic hygroscopic salts, and any other small and large molecular weight compounds, within each species' glues. The relative concentration of each of the compounds identified was calculated upon baseline correction and integration of the peaks using TOPSPIN.
We used mass spectrometry (MS) to verify the presence of compounds identified in the NMR study as follows.
Individual pipette tips containing glowworm glue were washed with 300 uL methanol (HPC grade, Merk, USA) into a 1.5 mL Eppendorf tube. A 7 uL aliquot of each sample was taken for analysis on an Orbitrap LTQ XL (Thermo Fisher Scientific, San Jose Ca, USA) ion trap mass spectrometer using a nanospray (nano-electrospray) ionization source to generate ions from the analytes in solution.
The instrument was calibrated with a standard calibration solution (as outlined in the instrument manual) on each of the analyses. All analyses were carried out in positive ion mode using the orbitrap Fourier Transform MS analyser at a resolution of 100,000. Sample aliquots were injected into a glass needle and inserted onto the nanospray source. Ions generated were measured over the molecular mass range 100–2,000 m/z. Data was acquired in full scan mode over 60 s. The data generated were analyzed using the Qual Browser feature in Xcaliber 2.1 (Thermo Fisher Scientific, San Jose, Ca, USA) and with searches against the ChemSpider (Royal Society of Chemistry) database.
NMR spectra showed peaks that could be assigned to a range of organic molecules, allowing an estimate of the composition of the low mass (i.e., molecules <300 Da) fraction of the glowworm mucous (Table 1, Supplementary Material 1 and 2). The majority of this fraction comprised alcohols (mainly ethanol and methanol), organic acids (e.g., lactic acid, acetic acid, hydroxyisovaleric acid, hydroxybutyric acid), amino acids (dominantly tyrosine, but also glutamine, threonine, alanine, leucine, and others), and amino acid derivates (e.g., betaine and putative methylhistidines). Urea was consistently found across samples, albeit with varying concentration: In the adhesive of A. tasmaniensis urea was the most abundant compound, whereas in the adhesive of A. richardsae it made only a small fraction (Figure 1), with a concentration of only 2.6% of that in A. tasmaniensis (Table 1). Further fractions comprised monosaccharides (predominantly glucose) and other organic compounds such as amines and acetates. Trace amounts of acetate and acetone may be contaminants from glassware that was used for sample processing. NMR showed signs of lipids, which, however, could not be further identified with our NMR approach, as it is limited to smaller weight compounds. However, the MS spectra showed several high abundance peaks that indicate the presence of fatty acids, e.g., at 309 and 360 m/z in South Island A. luminosa, 244 m/z in A. richardsae, and 282, 304, and 585 m/z in A. tasmaniensis (Figure 2). Further peaks at >500 m/z cannot be unequivocally identified, due to the sheer number of possible isomers, and the additional information from NMR is lacking. The MS spectra revealed that, in contrast to the other two species, the adhesive of A. richardsae contained numerous higher mass compounds at higher concentrations. MS also confirmed the presence of urea and urea derivates (see below).
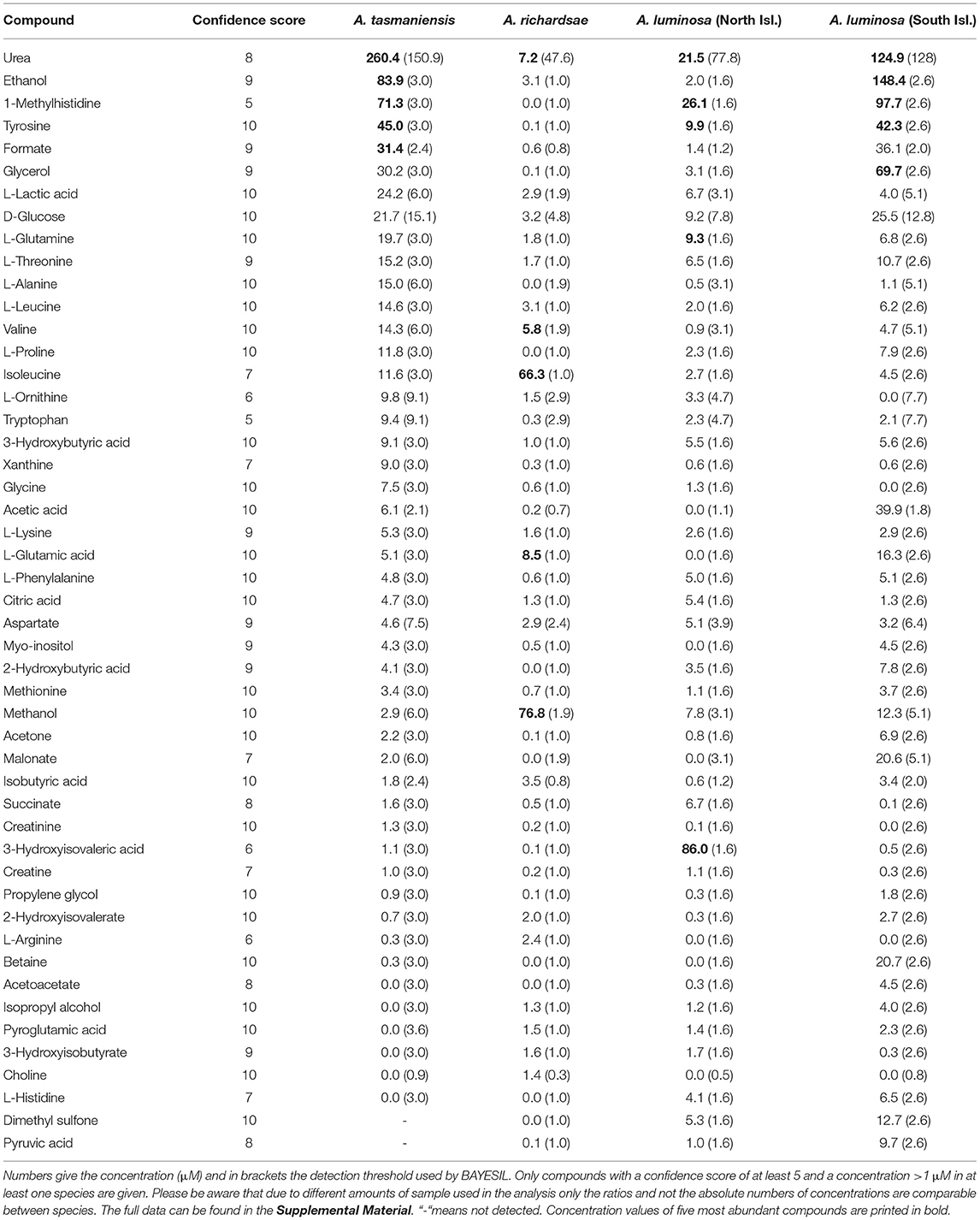
Table 1. Summary of compounds identified from the water soluble fraction of glowworm glue droplets with solution state 1H NMR spectroscopy.
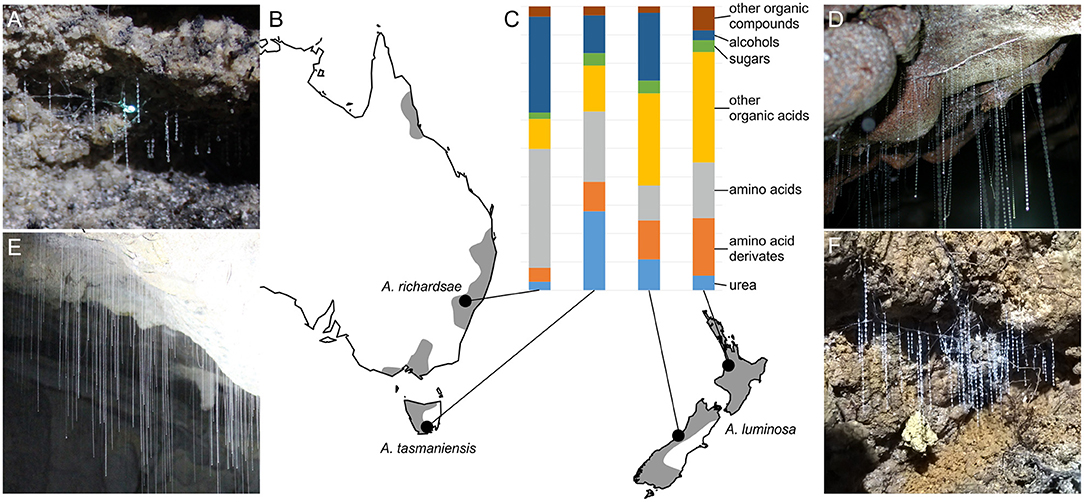
Figure 1. Arachnocampa species and composition of their prey capture adhesive. (A,D,E,F) Morphology of snares of sampled glowworm species. (A) A. richardsae. (D) A. luminosa, North Island. (E) A. tasmaniensis. (F) A. luminosa, South Island. (B) Map with gray shade indicating the known distribution range of the genus (modified from records in the Atlas of Living Australia, https://www.ala.org.au/), map created using the R package maps (Brownrigg, 2013). (C) Bar plot showing the relative abundance of compounds identified in the glowworm adhesive wash with NMR. Note that compounds with unspecific identity, such as fatty acids, or that are not captured by solution state NMR (e.g., peptides) are not shown here.
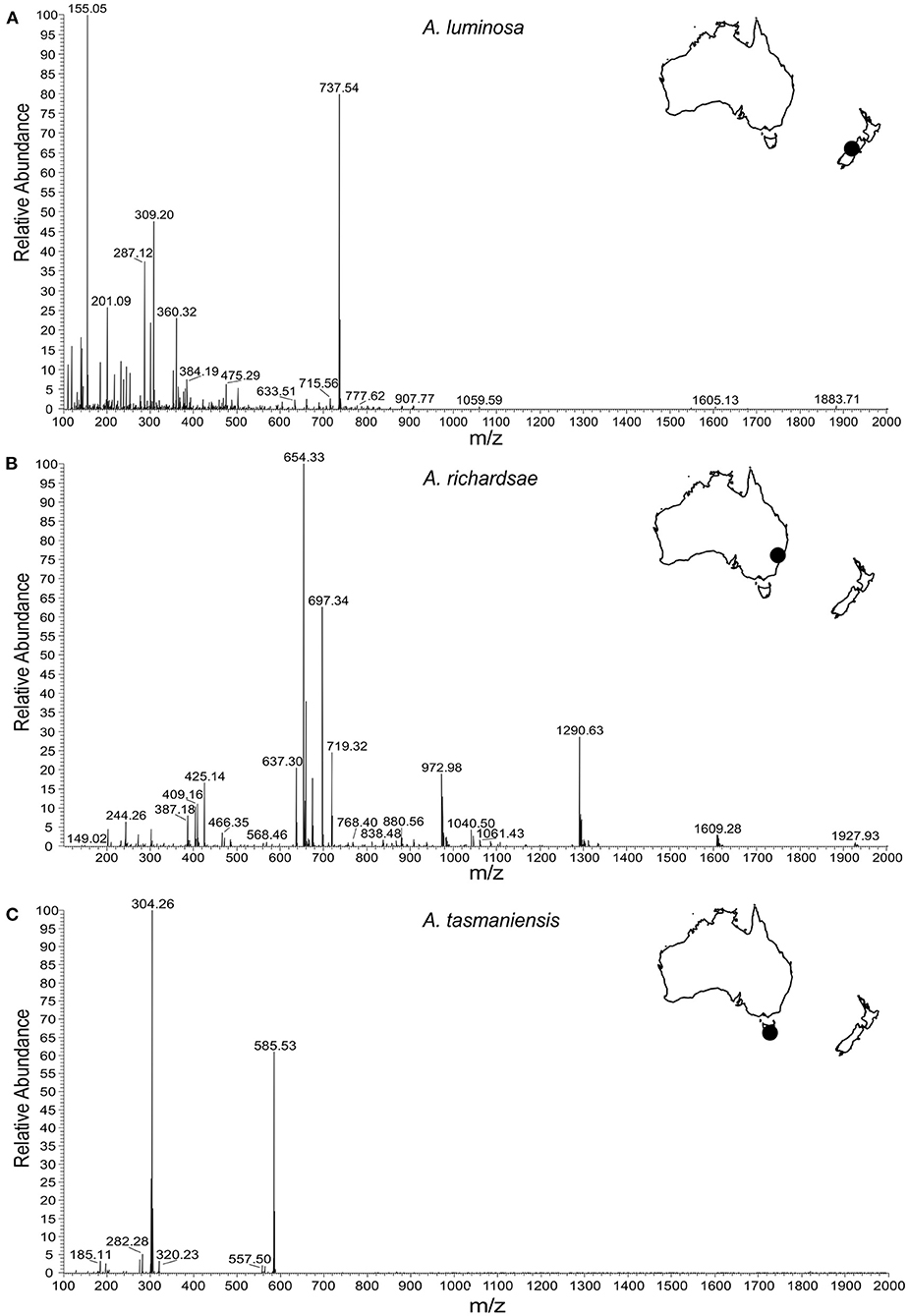
Figure 2. Positive ion nanospray (ESI) high resolution mass spectrometry analysis of glowworm adhesives. (A) Spectrum of isolate from New Zealand glowworms (A. luminosa, South Island). (B) Spectrum of isolate from Australian glowworms (A. richardsae from Newnes, NSW). (C) Spectrum of isolate from Tasmanian glowworms (A. tasmaniensis).
Although there was a high overlap in the compounds detected in the adhesives of all Arachnocampa species the relative amounts of these compounds largely differed (Table 1; Figure 1). The adhesive of A. luminosa contained higher concentrations of organic acids and amino acid derivates, but lower concentrations of amino acids than the other two species. In the adhesive of A. richardsae the organic acid fraction was comparatively small, and instead it contained relatively more alcohols and amino acids. Notably, here the alcohol fraction was comprised almost entirely of methanol, and the most abundant amino acid was isoleucine, whereas in both other species it was ethanol and tyrosine, respectively (Table 1).
Furthermore, some compounds tended to appear in different forms in the different species. For instance, the high peak at 155 m/z in the mass spectrum of A. luminosa is indicative of methylenediurea and 187 m/z a form with additional side branches. A. richardsae showed low amounts of pure urea, but instead the peaks in the MS spectrum at 404, 409, 425, and 637 m/z are indicative of molecules comprised of two to four urea units, possibly with side chains (see Supplementary Material 1 for details). In contrast, these peaks are absent in A. tasmaniensis, which instead showed high peaks indicative of pure urea in the NMR spectrum (Figure 3).
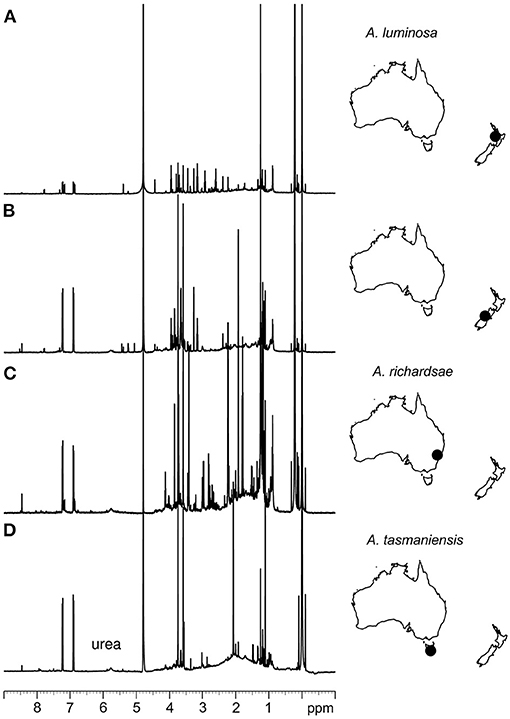
Figure 3. 1H NMR spectroscopic analysis of glowworm adhesives. (A) Spectrum of isolate from New Zealand glowworms (A. luminosa, North Island). (B) Spectrum of isolate from New Zealand glowworms (A. luminosa, South Island). (C) Spectrum of isolate from Australian glowworms (A. richardsae from Newnes, NSW). (D) Spectrum of isolate from Tasmanian glowworms (A. tasmaniensis).
Glue droplets from South Island A. luminosa, i.e., an epigaeic population, generally showed a more balanced composition and higher concentrations of diverse compounds than those of the other species and populations that all originated from cave or cave like habitats. Comparing the composition of the adhesive wash between the cave and epigaeic populations of A. luminosa, the most prominent difference is the much higher abundance of alcohols (ethanol and glycerol) in the adhesive of the epigaeic (South Island) population. The adhesive of the South Island population also showed significant amounts of acetic acid, whereas in the North Island populations, as in the other two species, this compound was almost absent and instead showed lactic acid as the most abundant acid.
Our results revealed that the adhesive produced by Arachnocampa glowworms for prey capture is composed of a much higher diversity of organic compounds than previously thought (von Byern et al., 2016). It confirmed the previous finding that urea is a major compound in Arachnocampa adhesives (von Byern et al., 2016), but only in A. tasmaniensis was pure, unbound, urea the most abundant compound. However, our MS data indicated that urea frequently occurred as part of macromolecules, that might have evaded detection with 1H NMR. Urea, as well as other compounds that were found at high concentrations, such as methylhistidines, are excretory products. It has been assumed that the adhesive secretion originates from the insect's excretory system (von Byern et al., 2016), but confirmatory experiments are needed.
Previous studies suggested that the glowworm adhesive contains acids, with the proposal of oxalic acid (Fulton, 1941) or uric acid (von Byern et al., 2016) as the predominant acidic compound. This was not confirmed by our results. Instead, we found a diversity of acids that may result from excretion processes or anaerobic metabolism. von Byern et al. (2016) found no evidence of the presence of carbohydrates and proteins in the glue droplets of Arachnocampa spp., but found indications of small peptides. Our results showed an abundance of different amino acids, which agrees with Walker et al. (2015). These amino acids could form peptides. Some of the higher range peaks in the MS spectra, such as at 1,290 m/z in the A. richardsae adhesive, and any peaks at >700 m/z across all species may be represented by medium to large peptides (~C30). However, it was difficult to make definitive identifications at this range because there are a wide range of candidate organic compounds and 1H NMR cannot detect many compounds with a molecular weight >300 Da. Additionally - in contrast to von Byern et al. (2016) - we found signals of monosaccharide sugars in the NMR spectra of all three species, albeit at low concentrations.
The capture threads of glowworms can generate high adhesive strength comparable to that of commercial glues on both artificial hydrophilic and hydrophobic surfaces, and on insect surfaces (Piorkowski et al., 2018; von Byern et al., 2019). High adhesion, however, is only observed at high humidity (i.e., close to atmospheric saturation) and at a relative humidity of 60% the adhesive is dry and brittle, and the adhesive properties are lost (Piorkowski et al., 2018; von Byern et al., 2019). This is in stark contrast to artificial adhesives that typically exhibit a loss of adhesion at high humidity, and to the capture threads of some spiders that retain water and stay adhesive across a broad humidity range (~30–100% R.H.) (Opell et al., 2018).
Capture threads act like pressure sensitive adhesives (PSA): these are soft materials that generate adhesive forces by building a high surface area with the substrate, so that short ranging intermolecular attractive forces are active (Creton, 2003). As no covalent bonds are formed, the adhesion of the PSA is reversible and remains efficient over various attachment-detachment cycles. This function requires a high softness of the material (i.e., Young's modulus <100 kPa; Dahlquist, 1969), and a molecular backbone that enables the cohesion (i.e., inner strength) of the adhesive. In the adhesive of spider capture threads, so-called viscid silk, the backbone is formed by large glycoproteins, which are plasticised by water that is retained and dispersed by hygroscopic compounds (Amarpuri et al., 2015). As different compounds differ in their humidity-dependent hygroscopic properties their specific mix determines an optimal environmental humidity at which the adhesive is hydrated to provide optimal softness, while not being too fluid to lose its cohesive strength (Opell et al., 2018). In spiders, this optimum corresponds to the microhabitat conditions that are preferred by the species (Opell et al., 2018).
In the adhesive coating of glowworm capture threads there is, thus far, no evidence for the presence of (glyco-)proteins (von Byern et al., 2016), however, our mass spectrometry results indicate that the abundant urea could serve as a cohesive by forming poly-urea chains and bonds with other compounds, such as carbohydrates. In addition, von Byern et al. (2016) found that the adhesive may contain peptides bound to urea. Such poly-urea based molecules could interact with substrate surfaces via van der Waals forces, or form hydrogen or even covalent bonds with substrate surfaces due to their polar and reactive groups. Pure urea, along with an abundance of other polar compounds, such as tyrosine and glucose, may aid the hydration of the adhesive. Under natural conditions, the glue droplets are comprised mainly of water and other volatile substances (von Byern et al., 2016). Notably, urea exhibits high hygroscopic properties only at high humidity (Werner, 1937). This may explain, why the capture threads of A. tasmaniensis rapidly dry and lose their adhesive properties when removed from their damp cave environments (Piorkowski et al., 2018). However, this was also observed for the capture threads of A. luminosa (von Byern et al., 2019), which did not show a similarly high fraction of pure urea in our analysis, so other compounds of the glowworm adhesives may have similar properties.
In contrast to the viscid silk of spiders we found that the glowworm adhesive contained significant amounts of alcohols, predominantly ethanol (in A. luminosa and A. tasmaniensis) and methanol (A. richardsae). These compounds may act as solvents and aid in plasticizing the adhesive. The mechanism with which a volatile compound such as methanol or ethanol is retained in the glue droplet remains unclear.
The abundance of acids in the glowworm droplet, especially those of A. luminosa, could have a function in prey ingestion. This was previously speculated (Fulton, 1941), but has not yet been experimentally tested.
Our analysis uncovered remarkable differences in the composition of adhesives both between species, as well as between cave and epigaeic populations of A. luminosa. A. tasmaniensis and North Island A. luminosa were collected from true cave environments, A. richardsae were collected from a cave-like environment (abandoned railway tunnel) in an otherwise dry area and the South Island A. luminosa were collected from the creek banks in a temperate rain forest. None of the three species are troglobiont and epigaeic populations exist in suitable, damp microhabitats. It could be expected that epigaeic populations are exposed to a higher variation in humidity, and accordingly show enhanced hygroscopic properties. This supposition nevertheless remains to be experimentally tested. However, the chemical profiles of adhesives of South Island A. luminosa or A. richardsae did not show a higher abundance of hygroscopic compounds (in A. richardsae the adhesive even exhibited a high concentration the non-polar isoleucine).
Different chemical profiles could result from diet influencing the chemical mix within the adhesive secretion. From spider capture threads it is known that the relative abundance of small mass molecular compounds, such as choline and potassium and phosphate salts, varies with spider diet (Higgins et al., 2001; Blamires et al., 2017). As different compounds may fulfill the same function within the adhesives (e.g., different types of hygroscopic substances, different types of solvents, or different forms of urea forming the adhesive backbone, such plastic effects might not necessarily reflect an adaptive function). Furthermore, the threads may also be prone to contamination with foreign substances (e.g., by aerosols), which may also affect their composition after secretion.
More and more materials designers are looking to biological materials for inspiration for new products. This is because biological materials often show a high performance, are synthetized under environmentally benign conditions, and are biodegradable. Moreover, some materials, such as spider silks exhibit a high biocompatibility, which renders them excellent candidates for the design of biomaterials for biomedical applications (Vepari and Kaplan, 2007; Widhe et al., 2012).
A range of bioadhesives have received high attention due to their specific properties that remain challenging to achieve for artificial adhesives, such as the generation of adhesive bonds in marine environments (Bandara et al., 2013) or the reversibility of strong adhesive bonds (Cho et al., 2019). Glowworm glues are different from other bioadhesives in being adapted to work best under extremely humid conditions. Therefore, they have the potential to serve as models for specialized moisture activated adhesives such as tissue adhesives (Mehdizadeh and Yang, 2013).
At the same time, our study has shown some similarities to spider adhesives. The study of such convergent adhesive systems can be a powerful way to separate functional principles from the effects of evolutionary history in biological systems (Wolff et al., 2017). The function of common artificial adhesives is severely affected by water. This is especially true for pressure sensitive adhesives, which lose their stickiness at high humidity. In contrast, the adhesives produced by glowworms and many spiders retain their stickiness at high humidity. In both cases this seems to be based on a on similar principle: the plasticising of an otherwise dry and stiff material with water by the ubiquitous distribution of small organic compounds with hygroscopic properties. An experimental investigation of the functional chemistry in glowworm capture thread adhesives will help us to identify compounds that may be useful for designing specialized adhesives for applications in humidity environments.
In summary, here we showed for the first time that the adhesive droplets of Arachnocampa glowworms are composed of a complex mix of organic compounds. Our analyses were qualitative and revealed the presence of many of the same compounds or classes of substances between different species and populations of glowworms, albeit with different relative abundance. We assume that such differences rather reflect differences in the diet and remnants of different preys than adaptations to different habitats and functions. Similar to the adhesive coatings of spider capture threads, glowworm glues are characterized by an abundance of low molecular weight organic compounds with hygroscopic properties, which could act as plasticizers and transform the stiff and brittle silk thread into a soft and tacky contact adhesive. More research is required to understand the functional significance of the different compounds, in order to extract design principles for specialized moisture activated adhesives for biomedical and other applications.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
SB and JW conceived the study. SB, JH, DT, and LA designed the chemical analysis. JW, DP, JB, and SB organized the logistics of sample collection, including the applications for research permits. SB, JW, JB, DP, JF, and XW collected the samples. DT processed the samples and performed the NMR analysis. LA performed the MS analysis. DT, LA, SB, and JH interpreted the spectroscopy data. JW and SB drafted the manuscript, and all authors contributed in its revisions.
JW was supported by a Macquarie University Research Fellowship of Macquarie University and a Discovery Early Career Researcher Award of the Australian Research Council (DE190101338). JB was supported by COST Action CA15216 (European Network of Bioadhesion Expertise). XW acknowledges the Australian Research Council (ARC) for support through an Industry Transformation Research Hub (IH140100018). SB was supported by a Hermon Slade Foundation grant (HSF 17/6). DP was supported by a grant from the Ministry of Science and Technology, Taiwan (MOST 108-2811-B-029-500). SB was funded by a Hermon Slade Foundation grant (HSF/16).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Niall Doran and Hamish Craig for assistance collecting A. tasmaniensis threads and Deb Kane for discussions on A. richardsae. Thanks to Dr. Chowdhury Sarowar for his assistance in preparing and running MS samples. Access to instrumentation in the BMSF and NMR Facilities of the Mark Wainwright Analytical Centre, UNSW, is gratefully appreciated.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmech.2021.661422/full#supplementary-material
Agnarsson, I., and Blackledge, T. A. (2009). Can a spider web be too sticky? Tensile mechanics constrains the evolution of capture spiral stickiness in orb-weaving spiders. J. Zool. 278, 134–140. doi: 10.1111/j.1469-7998.2009.00558.x
Amarpuri, G., Chaurasia, V., Jain, D., Blackledge, T. A., and Dhinojwala, A. (2015). Ubiquitous distribution of salts and proteins in spider glue enhances spider silk adhesion. Sci. Rep. 5:9030. doi: 10.1038/srep09030
Bandara, N., Zeng, H., and Wu, J. (2013). Marine mussel adhesion: biochemistry, mechanisms, and biomimetics. J. Adh. Sci. Technol. 27, 2139–2162. doi: 10.1080/01694243.2012.697703
Betz, O., and Kölsch, G. (2004). The role of adhesion in prey capture and predator defence in arthropods. Arthropod. Struct. Devel. 33, 3–30. doi: 10.1016/j.asd.2003.10.002
Blamires, S. J., Hasemore, M., Martens, P. J., and Kasumovic, M. M. (2017). Diet-induced co-variation between architectural and physicochemical plasticity in an extended phenotype. J. Exp. Biol. 220, 876–884. doi: 10.1242/jeb.150029
Blamires, S. J., Sahni, V., Dhinojwala, A., Blackledge, T. A., and Tso, I. M. (2014). Nutrient deprivation induces property variations in spider gluey silk. PLoS ONE 9:e88487. doi: 10.1371/journal.pone.0088487
Broadley, R. A., and Stringer, I. A. (2001). Prey attraction by larvae of the New Zealand glowworm, Arachnocampa luminosa (Diptera: Mycetophilidae). Inv. Biol. 120, 170–177. doi: 10.1111/j.1744-7410.2001.tb00121.x
Brownrigg, M. R. (2013). Package ‘maps'. Available online at: https://cran.r-project.org/web/packages/maps/ (accessed January 13, 2021).
Cho, H., Wu, G., Jolly, J. C., Fortoul, N., He, Z., Gao, Y., et al. (2019). Intrinsically reversible superglues via shape adaptation inspired by snail epiphragm. Proc. Natl. Acad. Sci. U. S.A. 116, 13774–13779. doi: 10.1073/pnas.1818534116
Cicero, D. O., Barbato, G., and Bazzo, R. (2001). Sensitivity enhancement of a two-dimensional experiment for the measurement of heteronuclear long-range coupling constants, by a new scheme of coherence selection by gradients. J. Magn. Reson. 148, 209–213. doi: 10.1006/jmre.2000.2234
Creton, C. (2003). Pressure-sensitive adhesives: an introductory course. MRS Bull. 28, 434–439. doi: 10.1557/mrs2003.124
Dahlquist, C. A. (1969). “Pressure-sensitive adhesives,” in Treatise on Adhesion and Adhesives, Vol. 2, ed R. L. Patrick (New York, NY: Marcel Dekker), 219–260.
Diaz, C., Maksuta, D., Amarpuri, G., Tanikawa, A., Miyashita, T., Dhinojwala, A., et al. (2020). The moth specialist spider Cyrtarachne akirai uses prey scales to increase adhesion. J. R. Soc. Interface 17:20190792. doi: 10.1098/rsif.2019.0792
Fulton, B. B. (1941). A luminous fly larva with spider traits (Diptera, Mycetophilidae). Ann. Entomol. Soc. Am. 34, 289–302. doi: 10.1093/aesa/34.2.289
Gatenby, J. B., and Cotton, S. (1960). Snare building and pupation in Bolitophila luminosa. Transact. R. Soc. New Zealand 88, 149–156.
Haritos, V. S., Niranjane, A., Weisman, S., Trueman, H. E., Sriskantha, A., and Sutherland, T. D. (2010). Harnessing disorder: onychophorans use highly unstructured proteins, not silks, for prey capture. Proc. R. Soc. B Biol. Sci. 277, 3255–3263. doi: 10.1098/rspb.2010.0604
Higgins, L. E., Townley, M. A., Tillinghast, E. K., and Rankin, M. A. (2001). Variation in the chemical composition of orb webs built by the spider Nephila clavipes (Araneae, Tetragnathidae). J. Arachnol. 29, 82–94. doi: 10.1636/0161-8202(2001)029[0082:VITCCO]2.0.CO;2
Kay, L., Keifer, P., and Saarinen, T. (1992). Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J. Amer. Chem. Soc. 114, 10663–10665. doi: 10.1021/ja00052a088
Mehdizadeh, M., and Yang, J. (2013). Design strategies and applications of tissue bioadhesives. Macromol. Biosci. 13, 271–288. doi: 10.1002/mabi.201200332
Meyer-Rochow, V. B. (2007). Glowworms: a review of Arachnocampa spp. and kin. Luminescence 22, 251–265. doi: 10.1002/bio.955
Musto, P., Ragosta, G., Scarinzi, G., and Mascia, L. (2002). Probing the molecular interactions in the diffusion of water through epoxy and epoxy–bismaleimide networks. J. Polym. Sci. B 40, 922–938. doi: 10.1002/polb.10147
Opell, B. D., Jain, D., Dhinojwala, A., and Blackledge, T. A. (2018). Tuning orb spider glycoprotein glue performance to habitat humidity. J. Exp. Biol. 221:jeb161539. doi: 10.1242/jeb.161539
Palmer, A. G., Cavanagh, J., Wright, P. E., and Rance, M. (1991). Sensitivity improvement in proton-detected two-dimensional heteronuclear correlation NMR spectroscopy. J. Magnet. Res. 93, 151–170. doi: 10.1016/0022-2364(91)90036-S
Piorkowski, D., Blackledge, T. A., Liao, C. P., Doran, N. E., Wu, C. L., Blamires, S. J., et al. (2018). Humidity-dependent mechanical and adhesive properties of Arachnocampa tasmaniensis capture threads. J. Zool. 305, 256–266. doi: 10.1111/jzo.12562
Piorkowski, D., Blamires, S. J., Doran, N., Liao, C. P., Wu, C. L., and Tso, I. M. (2017). Ontogenetic shift towards stronger, tougher silk in a web building cave spider. J. Zool. 304, 81–89. doi: 10.1111/jzo.12507
Sahni, V., Blackledge, T. A., and Dhinojwala, A. (2010). Viscoelastic solids explain spider web stickiness. Nature Comm. 1, 1–4. doi: 10.1038/ncomms1019
Sahni, V., Blackledge, T. A., and Dhinojwala, A. (2011). A review on spider silk adhesion. J. Adhes. 87, 595–614. doi: 10.1080/00218464.2011.583588
Sahni, V., Miyoshi, T., Chen, K., Jain, D., Blamires, S. J., Blackledge, T. A., et al. (2014). Direct solvation of glycoproteins by salts in spider silk glues enhances adhesion and helps to explain the evolution of modern spider orb webs. Biomacromolecules 15, 1225–1232. doi: 10.1021/bm401800y
Schleucher, J., Schwendinger, M., Sattler, M., Schmidt, P., Schedletzky, O., Glaser, S. J., et al. (1994). A general enhancement scheme in heteronuclear multidimensional NMR employing pulsed field gradients. J. Biomol. NMR 4, 301–306. doi: 10.1007/BF00175254
Sharpe, M. L., Dearden, P. K., Gimenez, G., and Krause, K. L. (2015). Comparative RNA seq analysis of the New Zealand glowworm Arachnocampa luminosa reveals bioluminescence-related genes. BMC Genomics 16, 1–15. doi: 10.1186/s12864-015-2006-2
Suter, R. B., and Stratton, G. E. (2009). Spitting performance parameters and their biomechanical implications in the spitting spider, Scytodes thoracica. J. Insect Sci. 9:62. doi: 10.1673/031.009.6201
Tan, K. T., Vogt, B. D., White, C. C., Steffens, K. L., Goldman, J., Satija, S. K., et al. (2008). On the origins of sudden adhesion loss at a critical relative humidity: examination of bulk and interfacial contributions. Langmuir 24, 9189–9193. doi: 10.1021/la800632r
Vepari, C., and Kaplan, D. L. (2007). Silk as a biomaterial. Progr. Polymer Sci. 32, 991–1007. doi: 10.1016/j.progpolymsci.2007.05.013
von Byern, J., Chandler, P., Merritt, D., Adlassnig, W., Stringer, I., Meyer-Rochow, V. B., et al. (2019). Biomechanical properties of fishing lines of the glowworm Arachnocampa luminosa (Diptera; Keroplatidae). Sci. Rep. 9, 1–14. doi: 10.1038/s41598-019-39098-1
von Byern, J., Dorrer, V., Merritt, D. J., Chandler, P., Stringer, I., Marchetti-Deschmann, M., et al. (2016). Characterization of the fishing lines in Titiwai (=Arachnocampa luminosa Skuse, 1890) from New Zealand and Australia. PLoS ONE 11:e0162687. doi: 10.1371/journal.pone.0162687
von Byern, J., and Grunwald, I. (2010). Biological Adhesive Systems. From Nature to Technical and Medical Application. Wien, New York: Springer, 305. doi: 10.1007/978-3-7091-0286-2
Walker, A. A., Weisman, S., Trueman, H. E., Merritt, D. J., and Sutherland, T. D. (2015). The other prey-capture silk: fibres made by glow-worms (Diptera: Keroplatidae) comprise cross-β-sheet crystallites in an abundant amorphous fraction. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 187, 78–84. doi: 10.1016/j.cbpb.2015.05.008
Widhe, M., Johansson, J., Hedhammar, M., and Rising, A. (2012). Current progress and limitations of spider silk for biomedical applications. Biopolymers 97, 468–478. doi: 10.1002/bip.21715
Willker, W., Leibfritz, D., Kerssebaum, R., and Bermel, W. (1993). Gradient selection in inverse heteronuclear correlation spectroscopy. Magnet. Res. Chem. 31, 287–292. doi: 10.1002/mrc.1260310315
Wolff, J., and Gorb, S. N. (2016). “Attachment structures and adhesive secretions in arachnids,” in Biologically Inspired Systems, Vol. 7, ed S. N. Gorb (Cham: Springer), 1–184. doi: 10.1007/978-3-319-45713-0_8
Wolff, J. O., Schönhofer, A. L., Martens, J., Wijnhoven, H., Taylor, C. K., and Gorb, S. N. (2016). The evolution of pedipalps and glandular hairs as predatory devices in harvestmen (Arachnida, Opiliones). Zool. J. Linn. Soc. 177, 558–601. doi: 10.1111/zoj.12375
Wolff, J. O., Schönhofer, A. L., Schaber, C. F., and Gorb, S. N. (2014). Gluing the ‘unwettable': soil-dwelling harvestmen use viscoelastic fluids for capturing springtails. J. Exp. Biol. 217, 3535–3544. doi: 10.1242/jeb.108852
Wolff, J. O., Wells, D., Reid, C. R., and Blamires, S. J. (2017). Clarity of objectives and working principles enhances the success of biomimetic programs. Bioinspir. Biomimet. 12:051001. doi: 10.1088/1748-3190/aa86ff
Zhang, G., and Weirauch, C. (2013). Sticky predators: a comparative study of sticky glands in harpactorine assassin bugs (Insecta: Hemiptera: Reduviidae). Acta Zoologica 94, 1–10. doi: 10.1111/j.1463-6395.2011.00522.x
Keywords: biological adhesive, adhesive secretion, chemical ecology, silk, water-based adhesive
Citation: Wolff JO, von Byern J, Piorkowski D, Fang J, Wang X, Adler L, Thomas DS, Hook JM and Blamires SJ (2021) Adhesive Droplets of Glowworm Snares (Keroplatidae: Arachnocampa spp.) Are a Complex Mix of Organic Compounds. Front. Mech. Eng. 7:661422. doi: 10.3389/fmech.2021.661422
Received: 30 January 2021; Accepted: 26 March 2021;
Published: 28 April 2021.
Edited by:
Ken Nakano, Yokohama National University, JapanReviewed by:
Pierangiola Bracco, University of Turin, ItalyCopyright © 2021 Wolff, von Byern, Piorkowski, Fang, Wang, Adler, Thomas, Hook and Blamires. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonas O. Wolff, am9uYXMud29sZmZAbXEuZWR1LmF1; Sean J. Blamires, c2Vhbi5ibGFtaXJlc0B1bnN3LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.