- 1Graduate School of Advanced Science and Technology, Kumamoto University, Kumamoto, Japan
- 2Faculty of Advanced Science and Technology, Kumamoto University, Kumamoto, Japan
- 3Faculty of Life Science, Kumamoto University, Kumamoto, Japan
To investigate the effects of microparticles on the immune system, a device was designed for an optimal culture environment. Macrophages phagocytose microparticles and produce inflammatory cytokines. In a culture environment in which macrophages phagocytose microparticles mixed in a culture medium, it remains unclear whether the macrophages can physically access all the microparticles present in the culture medium. In the culturing of fine particles, such as microparticles, it is necessary to devise methods that can realize a close biological contact between the macrophages and the microparticles. To enable macrophages to appropriately phagocytose microparticles, a microchamber with a glenoid hole for cell culturing was designed and manufactured. To clarify the effects of the size, administration amount, and administration time of the microparticles on the production of inflammatory cytokines, a system that can continuously deliver and collect the culture medium was introduced. The results obtained using these systems helped clarify the aforementioned effects. Our study confirms the possibility of employing a system that can optimally adjust the biological contact between macrophages and microparticles in a culture environment.
Introduction
The effects of microparticles on the immune system are widely known. One of the microparticle-formation processes involves microplastics, which are considered to be less than 5 mm in diameter. Fibrous microfibers that cannot be removed by sewage treatment plants from laundered clothes (Browne et al., 2011; De Falco et al., 2018) and plastic products become increasingly finer by mechanical actions, UV radiation, and thermal oxidation (Cole et al., 2011; Auta et al., 2017). Subsequently, they transform into micrometer-sized plastics and diffuse into the environment. These micrometer-sized plastics eventually affect the immune system (Andrady, 2011; Détrée and Gallardo-Escárate, 2018). The effect of these microparticles on the immune system has also been observed with implants in vivo. In the case of artificial joints, micrometer-sized wear particles generated from artificial joints stimulate macrophage activity and promote the secretion of inflammatory cytokines, which cause osteolysis (Matthews et al., 2000; Tipper et al., 2000).
To investigate the effect of microparticles, various culture methods have been proposed. To let macrophages make contact with microparticles that are lighter than the culture medium, inverted incubation, which cultures macrophages inversely with well plates covered by PVC films, was proposed (Motojima et al., 2019; Shen et al., 2018). Although the inverted incubation method is effective for dozens of micrometer-sized particles, few micrometer-sized particles remain suspended in the culture medium and cannot make contact with macrophages. In another culture method, a microfluidic device fabricated based on the microelectromechanical systems technology was proposed (Nakanishi et al., 2020). The microfluidic device consisted of a narrow tube with a cross-section area of 200 × 500 µm2, where the macrophages were cultured with microparticles. However, the device allowed macrophages to access only a few micrometer-sized microparticles, possibly owing to external factors such as shear stress stimulating the macrophages because of the narrow tube. To solve these problems, it is necessary to devise methods that can realize a close biological contact between the macrophages and the microparticles. In addition, the series of flow reactions that lead to the phagocytosis of the macrophages and production of inflammatory cytokines are temporal, so a culture system that can capture these is required.
In this study, a microchamber that minimizes the physical distance between macrophages and microparticles was designed and manufactured so that the macrophages can accurately phagocytose the administered microparticles (Nakashima et al., 2016). To clarify the effects of the size, administration amount, and administration time of the microparticles on the production of inflammatory cytokines, a system that can continuously deliver and collect the culture medium was introduced. With these systems, we conducted experiments using PS fluorescent particles and human monocyte-derived macrophages (HMDMs) and verified the effectiveness and validity of the system.
Materials and Methods
Fabrication of Microchamber
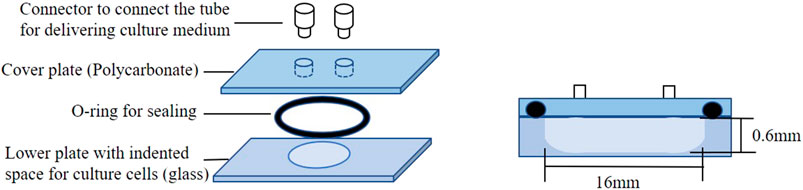
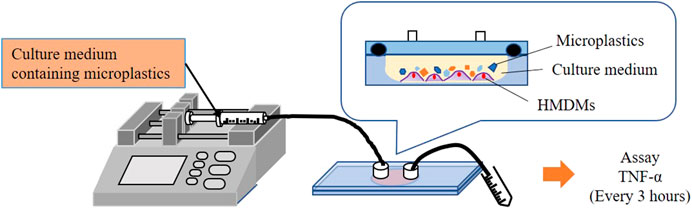
For a close biological contact between macrophages and microparticles, a microchamber comprising a glenoid hole was proposed. Figure 1 shows a schematic of the microchamber. The microchamber comprises a lower plate with a glenoid hole for culturing cells (glass), an O-ring for sealing, a cover plate (polycarbonate), and a connector to connect the tube for delivering the culture medium. The parts used in the microchamber are heat resistant and can therefore withstand sterilization in autoclaves. Figure 2 shows a schematic of the delivery system used for evaluating the inflammatory cytokine production from HMDMs. As shown, the microchamber is connected to a microsyringe pump after seeding the HMDMs in the glenoid hole on the bottom of the microchamber. The culture medium containing the microparticles is delivered to the microchamber using the microsyringe pump, and the inflammatory cytokines are measured using the culture medium collected after the delivery.
Verification Experiment
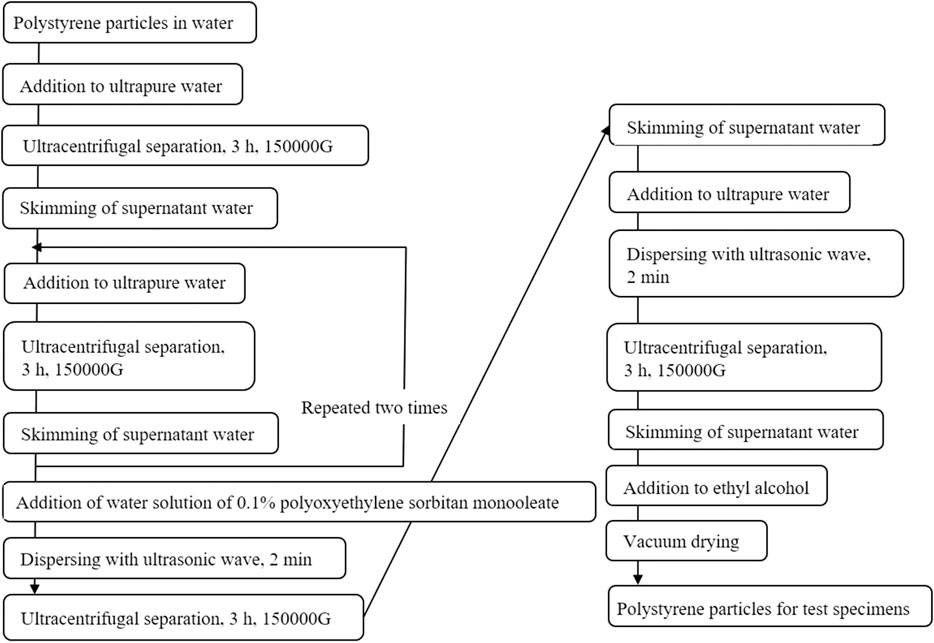
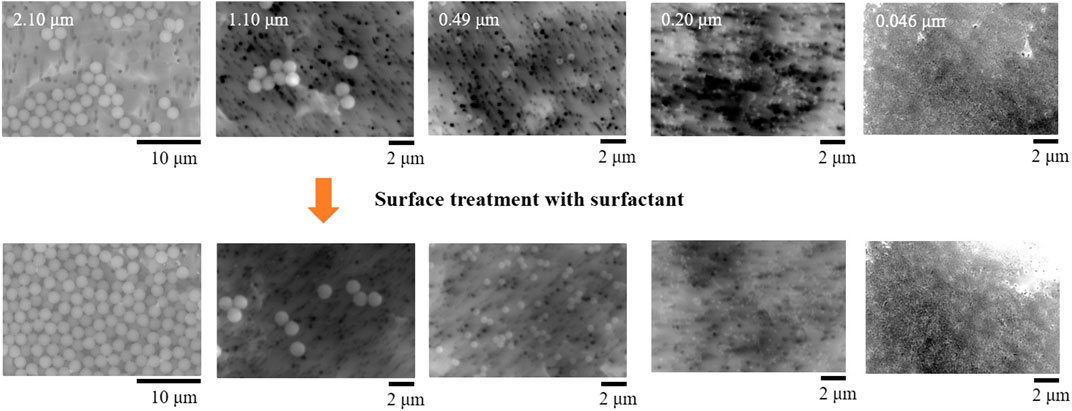
In this study, microparticles made of polystyrene (Fluoro-Max TM, Blue Fluorescent, Thermo Fisher Scientific) were used as the microparticles for the verification experiment. The diameters of the particles were 0.046, 0.2, 0.49, 1.1, and 2.1 μm. Fluoro-Max TM contained microplastics at a concentration of 1% in pure water with small amounts of surfactants and preservatives. The microplastics were manufactured in different ways depending on the particle diameter, and the surface morphology might also vary. Therefore, the surface of the particles was coated with Tween 80 (polyoxyethylene sorbitan monooleate) to make it uniform. Figure 3 shows the coating method used in the surface treatment with a surfactant (Tween 80). First, to remove the surfactants and preservatives, ultracentrifugal separation was performed using an ultracentrifuge (Optima MAX-XP, BECKMANCOULTER). Second, the supernatant was skimmed. After adding Tween 80, the entire surface of the particles was coated with Tween 80 using ultrasonic wave dispersion. Third, to remove Tween 80 from the solution, the supernatant was skimmed, pure water was added, and another round of ultrasonic wave dispersion was applied. Fourth, after performing ultracentrifugal separation, we skimmed the supernatant and applied ultracentrifugal separation once again. Finally, ethanal was added for sterilization.
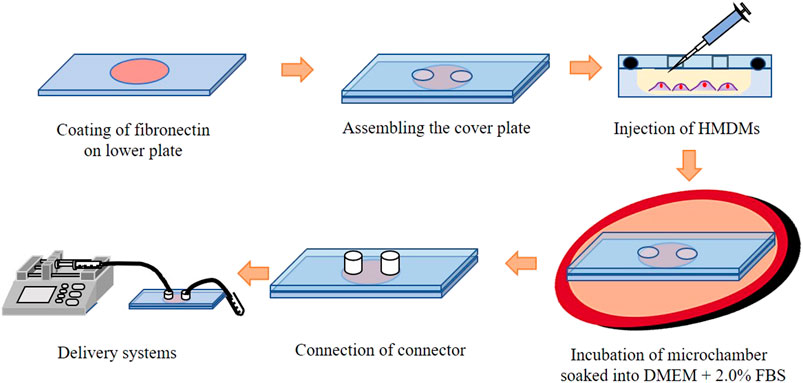
Figure 4 shows a schematic of cell seeding on the microchamber. After coating fibronectin on the lower plate, a cover plate was assembled on the lower plate, and Dulbecco’s Modified Eagle’s Medium (D-MEM, FUJIFILM) with HMDMs was injected into the microchamber. The HMDMs were obtained from healthy volunteer donors. Informed written consent was obtained donors. The concentration of the HMDMs was adjusted to 3.0 × 105 cells/chamber. Thereafter, the microchamber was soaked in the culture medium (D-MEM containing 2% FBS and 100 μg/ml of 100 U/ml penicillin) and incubated for 24 h in an incubator at 37°C. The microparticles of each size were adjusted to
Results and Discussion
Figure 5 shows the SEM images of the microparticles before and after applying the surface treatment with a surfactant. As shown, there is no difference in the surface morphologies before and after the surface treatment. For surface unification, an external force in the form of an ultracentrifugal force was applied. Nevertheless, the original size and shape were appropriately maintained for the experiment.
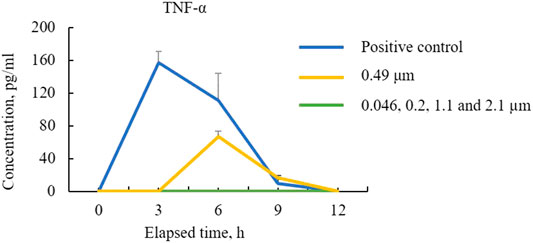
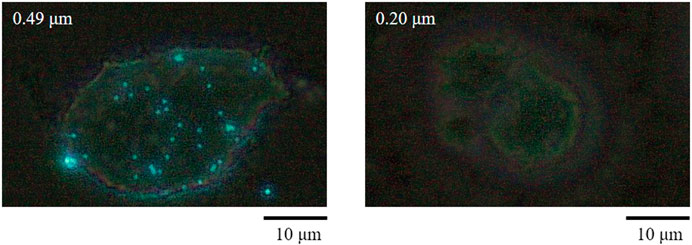
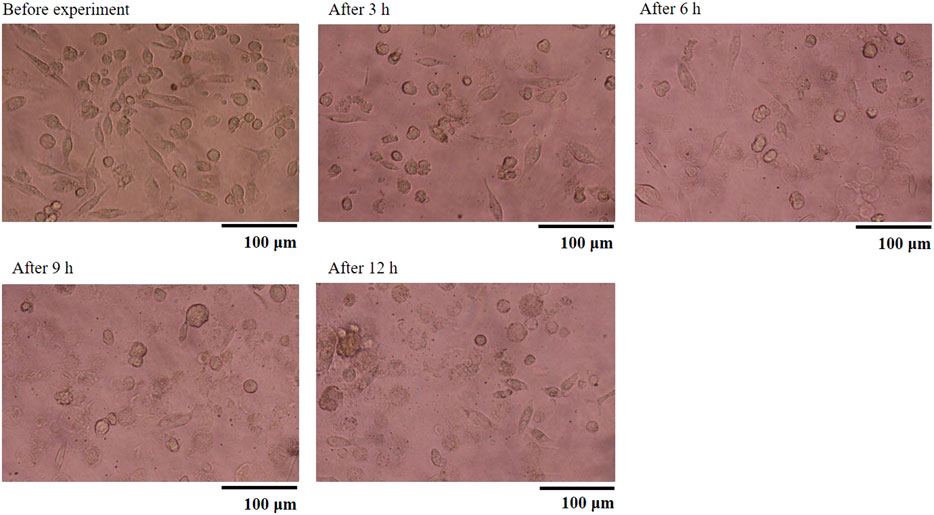
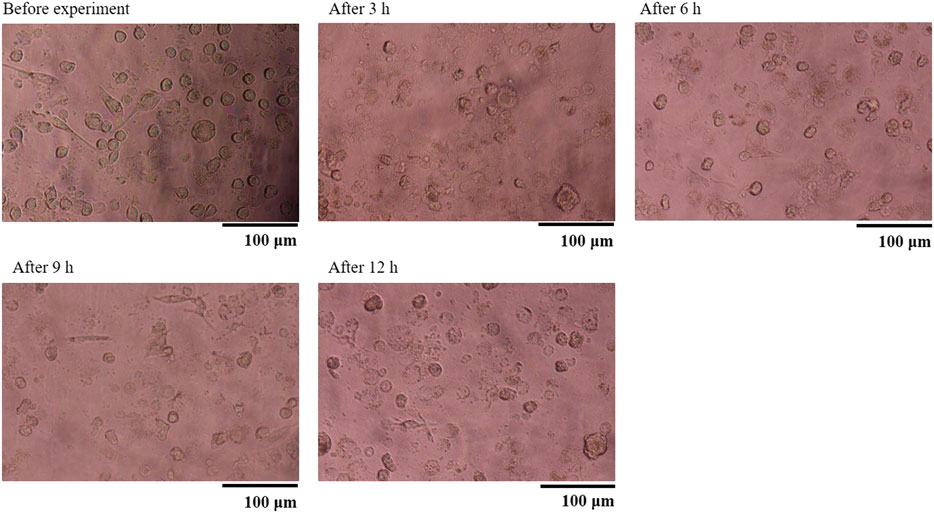
Figure 6 shows the TNF-α concentration (Yoshioka et al., 2016). As shown, the HMDMs react only to microparticles with a size of 0.49 μm in this experiment. Moreover, there is a peak in the inflammatory cytokine production 6 h after administering the microparticles. Regarding the positive control, there is a peak after 3 h. These results show that the HMDMs phagocytosed microparticles of a specific size and that there is a peak corresponding to the occurrence of the phagocytosis reaction. Figure 7 shows an observation example of the HMDMs after the experiment. The microparticles used in this study were PS fluorescent particles, which became luminescent under ultraviolet radiation. As shown in Figure 7, the microchamber delivers particles with a size of 0.49 μm, resulting in the production of inflammatory cytokines and phagocytosis of the HMDMs. The microchamber, which did not produce inflammatory cytokines, did not phagocytose the HMDMs, when injecting particles with a size of 0.046 μm. These results show that the production of inflammatory cytokines can be attributed to the phagocytosis of the microparticles by the HMDMs. Figures 8 and 9 show examples of the HMDMs observed every 3 h in the microchamber that delivered particles with a size of 0.49 and 0.046 μm, respectively. The HMDMs in the microchamber that delivered particles with the size of 0.49 µm were alive even after peak inflammatory cytokine production. In addition, no difference in the status of HMDMs was observed in this case, compared to the HMDMs in the microchamber that delivered particles with the size of 0.046 µm, and did not produce inflammatory cytokine. Therefore, no inflammatory cytokine was produced after the peak because the HMDMs did not phagocytose.
From these results, we can infer that if the size of wear particles can be controlled, inflammatory cytokine production may be suppressed in the case of artificial joints, even if the wear rate is high. In addition, a previous experiment has been reported, where HMDMs reacted with microparticles with a size of 0.8 µm (Chikaura et al., 2016). In that experiment, polymethylmethacrylate particles were used, whereas in the experiment in this study, polystyrene particles were used. Currently, we cannot confirm whether the peak in the inflammatory cytokine production with different particle sizes is due to particles of different materials or different culture environments. In the future, we would like to investigate various experimental conditions to confirm the usefulness of this microchamber.
Conclusion
A microchamber was proposed for a close biological contact between macrophages and microparticles. The microchamber can be considered a potential evaluation system to improve the accuracy of the relationship between the microparticles and the inflammatory cytokine production. Using the microchamber, we found that macrophages can phagocytose microparticles of specific sizes and that this phagocytosis process varies with time.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical review committee, Faculty of Life Science, Kumamoto University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MH and YNs designed this research work and wrote the manuscript. MH, HK, FY and KY conducted the experiments. Both YNs managed and supervised all aspects of this work.
Funding
This work was supported by JSPS KAKENHI (Grant Number 19KK0096), the Environment Research and Technology Development Fund (1-1908) of the Environmental Restoration and Conservation Agency of Japan, and the Interdisciplinary Research Project of the Institute of Industrial Nanomaterials (IINa), Kumamoto University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Andrady, A. L. (2011). Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605. doi:10.1016/j.marpolbul.2011.05.030
Auta, H. S., Emenike, C. U., and Fauziah, S. H. (2017). Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ. Int. 102, 165–176. doi:10.1016/j.envint.2017.02.013
Browne, M. A., Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., et al. (2011). Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ. Sci. Technol. 45, 9175–9179. doi:10.1021/es201811s
Chikaura, H., Nakashima, Y., Fujiwara, Y., Komohara, Y., Takeya, M., and Nakanishi, Y. (2016). Effect of particle size on biological response by human monocyte-derived macrophages, Biosurf. Biotribol. 2, 18–25. doi:10.1016/j.bsbt.2016.02.003
Cole, M., Lindeque, P., Halsband, C., and Galloway, T. S. (2011). Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62, 2588–2597. doi:10.1016/j.marpolbul.2011.09.025
Détrée, C., and Gallardo-Escárate, C. (2018). Single and repetitive microplastics exposures induce immune system modulation and homeostasis alteration in the edible mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 83, 52–60. doi:10.1016/j.fsi.2018.09.018
De Falco, F., Gullo, M. P., Gentile, G., Di Pace, E., Cocca, M., Gelabert, L., et al. (2018). Evaluation of microplastic release caused by textile washing processes of synthetic fabrics. Environ. Pollut. 108, 916–925. doi:10.1016/j.envpol.2017.10.057
Matthews, J. B., Besong, A. A., Green, T. R., Stone, M. H., Wloblewski, B. M., Fisher, J., et al. (2000). Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J. Biomed. Mater. Res. 52, 296–307. doi:10.1002/1097-4636(200011)52:2<296:AID-JBM8>3.0.CO;2-9
Motojima, N., Nakashima, Y., Fujiwara, Y., Komohara, Y., Takeya, M., Miura, H., et al. (2019). Relationship between wear behaviour of ultra-high-molecular-weight polyethylene and surface profile of Co–Cr–Mo alloy in artificial joint. Biosurf. Biotribol. 5, 1–7. doi:10.1049/bsbt.2018.0029
Nakanishi, Y., Nakashima, Y., Fujiwara, Y., Komohara, Y., Hino, K., Miura, H., et al. (2020). Microfluidic device used for the secretion of inflammatory cytokines from human monocyte-derived macrophages stimulated by ultra-high molecular weight polyethylene particles. Biotribol 23, 100–137. doi:10.1016/j.biotri.2020.100137
Nakashima, Y., Yamamoto, Y., Hikichi, Y., and Nakanishi, Y. (2016). Creation of cell micropatterns using a newly developed gel micromachining technique, Biofabrication. 8, 035006. doi:10.1088/1758-5090/8/3/035006
Shen, W., Tomita, N., Niikura, M., and Sugino, T. (2018). Amount of TNF-α released from macrophages reacting with polyethylene particles showed dose-dependent relationship to the total surface area of added particles. Biosurf. Biotribol. 4, 122–127. doi:10.1049/bsbt.2018.0026
Tipper, J. L., Ingham, E., Hailey, J. L., Besong, A. A., Fisher, J., Wroblewski, B. M., et al. (2000). Quantitative analysis of polyethylene wear debris, wear rate and head damage in retrieved charnley hip prostheses, J. Mater. Sci. Mater. Med. 11, 117–124. doi:10.1023/a:1008901302646
Keywords: microparticles, microchamber, macrophages, inflammatory cytokine, phagocytosis
Citation: Miyamoto H, Higuchi K, Nakashima Y, Fujiwara Y, Komohara Y and Nakanishi Y (2021) Culture System for a Closer Biological Contact Between Macrophages and Microparticles. Front. Mech. Eng 7:631128. doi: 10.3389/fmech.2021.631128
Received: 19 November 2020; Accepted: 14 January 2021;
Published: 17 February 2021.
Edited by:
Jacqueline Krim, North Carolina State University, United StatesReviewed by:
T. V. V. L. N. Rao, SRM Institute of Science and Technology, IndiaAbhishek Srivastava, Western Digital, United States
Copyright © 2021 Miyamoto, Higuchi, Nakashima, Fujiwara, Komohara and Nakanishi.. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshitaka Nakanishi, eS1uYWthQG1lY2gua3VtYW1vdG8tdS5hYy5qcA==
 Haruki Miyamoto
Haruki Miyamoto Katsunori Higuchi1
Katsunori Higuchi1 Yuta Nakashima
Yuta Nakashima Yukio Fujiwara
Yukio Fujiwara Yoshihiro Komohara
Yoshihiro Komohara Yoshitaka Nakanishi
Yoshitaka Nakanishi