
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mech. Eng., 27 January 2021
Sec. Tribology
Volume 6 - 2020 | https://doi.org/10.3389/fmech.2020.635694
This article is part of the Research TopicBioadhesionView all 14 articles
Comprising ca. 28,000, species the Orchidaceae constitute one of the most species-rich plant families. Orchids differ from other monocotyledons i.a., in the formation of so-called pollinaria, which are entities consisting of pollen grains aggregated into compact pollinia and accessory structures, a viscidium and mostly also a pollinium stalk. The viscidium releases an adhesive material that attaches the pollinarium to a pollinator. Pollinaria are part of a complex pollination apparatus that enables the orchids to colonize niches in which only a few individuals of the respective pollinator occur infrequently. Because the aggregated pollen grains are removed from the flower at once, the development of a mechanical barrier ensuring that only suitable pollinators are able to access the flowers and more importantly to remove the pollen are important selective traits. In this paper we describe the functional morphology of the pollination apparatus in two orchid species, Oncidium wentworthianum and O. otogaya, by experimentally mimicking the pollination process. Furthermore, we analyzed the mechanical resistance of this apparatus by means of force measurements and showed that it most probably constitutes a hierarchical two-stage barrier. The first stage consists of the presence of the anther cap that not only protects the pollinia, but also serves to prevent premature removal of young and unripe pollinaria from the flower. As soon as the pollinaria are ripe, the anther cap sheds and the second stage of the mechanical barrier takes effect, a severable bond between pollinarium and rostellum. This bond can be overcome by a potential pollinator, applying a load of at least 10.8 mN (O. otogaya) or 12.6 mN (O. wentworthianum), respectively, on the viscidium which at the same time disengages the pollinarium from its anchorage. The adhesive material produced by the viscidium creates sufficient adhesive contact between pollinarium and pollinator. Potential pollinators, such as Centris spp. or Trigona spp. bees, should be well able to exert such forces by pushing their head/forebody into the orchid flowers. Thus, whether a pollinator is able to detach the pollinarium depends on both how forcefully it can push and how strongly it can pull the orchid pollination apparatus.
Many people are passionate about the splendor of orchid flowers without probably being aware of their functional-morphological peculiarities. Also Darwin was fascinated by these plants (Yam et al., 2009) and dedicated a whole book exclusively to the pollination of orchids by insects (Darwin, 1890). The complex pollination mechanism, which we describe below, was undoubtedly one of the factors responsible for the enormous radiation of the Orchidaceae family (Johnson and Edwards, 2000), resulting in more than 28,000 species currently known (Christenhusz and Byng, 2016). They are organized into the five subfamilies Apostasioideae, Vanilloideae, Cypripedioideae, Orchidoideae, and Epidendroideae (Chase et al., 2015; Givnish et al., 2015): the latter two being the most derived and species-rich subfamilies, and accounting for 98% of all orchids species (Singer and Cocucci, 1997).
Most orchids are characterized by the fact that style and staminal filaments are fused into a column, also called gynostemium, and that part of the stigma, the rostellum, is sterile and involved into pollen transfer. Another key development in orchids is the pollinium, which is a cohesive mass of agglomerated pollen grains that is removed as a unit during the pollination process (Pacini and Hesse, 2002; Harder and Johnson, 2008). In many orchids several pollinia are attached via a caudicle (derived from the anther) and/or a stalk-like stipe (derived from the column), to a sticky pad formed by the rostellum (Dressler, 1993; Stern, 2014). This pad is referred to as viscidium, syn. retinaculum (Schick, 1989), and becomes attached to the pollinator through its adhesive material. The entirety of pollinia, caudicle/stipe, and viscidium is called a pollinarium and is the main part of a complex and sophisticated pollination apparatus that made it possible for the orchids also to colonize niches in which only a few individuals of the respective pollinator occur and thus pollinator visits are infrequent (Johnson and Edwards, 2000). The viscidium thereby sticks to the departing pollinator and the entire pollinarium is removed from the flower and transferred to the stigmatic surface of a conspecific flower. The avoidance of self-pollination is a fascinating topic on its own, which we do not elaborate on here [for more details we refer to, e.g., Borba and Semir (1999) and Johnson and Edwards (2000)]. The fact that all pollen grains are removed at once, however, renders pollination into an “all-or-nothing” process, hence, demonstrating the importance that pollinaria can only be removed by suitable pollinators (Wagenitz, 1981; Jersáková et al., 2006). Filtering for efficient pollinators by the morphological evolutionary adaptation of flowers to prevent a loss of the whole gamet production is particularly pronounced in Epidendroideae, but also widespread among other orchid families (Tremblay, 1992; Pansarin et al., 2017). Filtering is realized by 1) attracting suitable pollinators by either visual or olfactory stimuli (Faegri and Pijl, 1979) and/or 2) morphological traits or mechanical barriers of the flower that only allow legitimate pollinators to access the pollen or a reward of whatever kind (Brantjes, 1981; Dressler, 1981; Claßen-Bockhoff et al., 2004; Reith et al., 2006; Córdoba and Cocucci, 2011). Vice versa, coevolutionary processes lead to morphological, physiological, and behavioral pollinator specialization (Darwin, 1890; Johnson and Anderson, 2010).
Here, we analyze the flower’s mechanical barriers in two Oncidium Sw. species belonging to the subtribe Oncidiinae (Orchidaceae), which represents the most highly derived orchids of the New World (Dressler, 1993; Mosquera-Mosquera et al., 2019). Flowers of Oncidiinae (Figure 1) characteristically have large lips featuring so-called calli. The latter resemble tumors from which the name of the type genus, Oncidium, derives (from the greek word “onkos” = swelling or tumor) (Castro and Singer, 2019). The callus attracts oil gathering bees either by offering true reward produced in epidermal elaiophores or by deceit (Pemberton, 2008). A thickened structure of the column situated below the stigmatic surface, the so-called tabula infrastigmatica, serves female oil-collecting bees as a grip. They can grasp it with their mandibles to free their anterior legs for oil collection (Dressler, 1981; Davies et al., 2014). During this maneuver they come into contact with the viscidium, overcome the mechanical barrier, and establish a sufficiently large contact area with the viscidium that causes the pollinarium to stay attached to the insect and to be removed from the flower when it departs. According to Schick (1989) the adhesive may consist of two phases: a hydrophilic derivative of the cell walls and a lipophilic part, originating from the protoplasm and heterogeneous in nature itself.
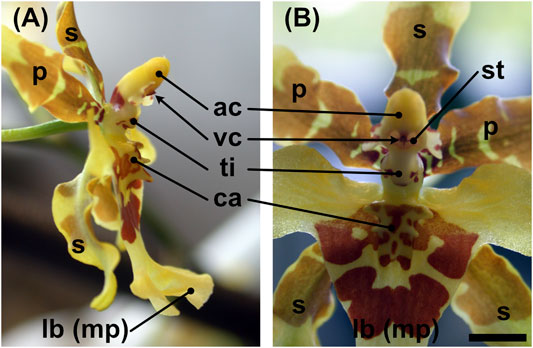
FIGURE 1. Flower of Oncidium wentworthianum in (A) lateral view and (B) frontal view. ac = anther cap, ca = callus, lb (mp) = labellum (modified petal), p = petal, s = sepal, st = (fertile part of the) stigma, ti = tabula infrastigmatica, vc = viscidium. Scale bar: ca. 4 mm.
The detailed pollination process of Oncidium orchids, however, is still largely unknown and also pollinators are rather generically reported. Castro and Singer (2019) summarized that Oncidiinae are mostly pollinated by oil- or perfume-collecting bees. Pollinators of Oncidium spp. are reported to be mainly fairly large apid bees (Hymenoptera, Apidae) belonging to the genera of Centris Fabricius, Trigona Jurine, Tetrapedia Klug, and Epicharis Klug (Dressler, 1993; Parra-Tabla et al., 2000; Singer, 2003; Carmona-Díaz and García-Franco, 2008; Castro and Singer, 2019; Ferreira et al., 2019).
Experiments were performed with Oncidium wentworthianum Bateman ex Lindl. and O. otogaya, both provided by the Zoological-Botanical Garden “Wilhelma,” Stuttgart, Germany. Plants of both species were grown in a mixture of orchid soil and tree bark. From O. otogaya, only an offshoot was available whose roots were wrapped in filter paper towels during the time of the study (approx. 2 weeks) and moistened with tap water each day. O. wentworthianum remained in the flowerpot during the time of measurement; the root ball was dipped in tap water every 2 days for about 1 min. Both orchids were kept in a glass cabinet during the experimental period to assure an elevated relative humidity.
A Leica MZ 125 stereomicroscope (Leica Microsystems, Wetzlar, Germany) was used for sample preparation and to get an in situ impression of flower details. Images were taken using a Nikon Coolpix E995 digital camera adapted to the stereomicroscope with a C-Mount adapter and a MDC 2 relay lens MXA 29005 (Nikon Corporation, Tokyo, Japan).
A Hitachi S-4800 (Hitachi High-Technologies Corp., Tokyo, Japan) cryo-SEM equipped with a Gatan ALTO 2500 cryopreparation system (Gatan, Inc., Abingdon, United Kingdom) was used for imaging the flower surface details. Therefore, the viscidium of a fresh flower was partly removed manually using the head of a pin and mounted on metal holders with Tissue-Tek® O.C.T.™ Compound (Sakura Finetek Europe B. V., Zoeterwoude, The Netherlands). The samples on holders were frozen on a cryostage at 140°C, sputter-coated with gold-palladium (6 nm) in the cryopreparation chamber, and examined in the SEM at −120°C and an accelerating voltage of 1 kV.
To measure the forces necessary to remove pollinaria from Oncidium spp. flowers the natural process was mimicked using a custom-build lab setup (Figure 2A). Living, turgescent individual Oncidium flowers were held with flat-ended tweezers that were mounted on an adjustable stand by a clamp (Figures 2A,B). Flowers, which for morphological reasons could not be grasped with the tweezers without being damaged, were mounted on a piece of stiff Styrofoam using double-sided adhesive tape. Manageability of the flower and accessibility of the viscidium were ensured by trimming the petals and the column wings (staminodia) with a razor blade. Thereby care was taken not to damage the viscidium, rostellum, or anther cap and to keep the flowers turgescent during the measurements (10 min)
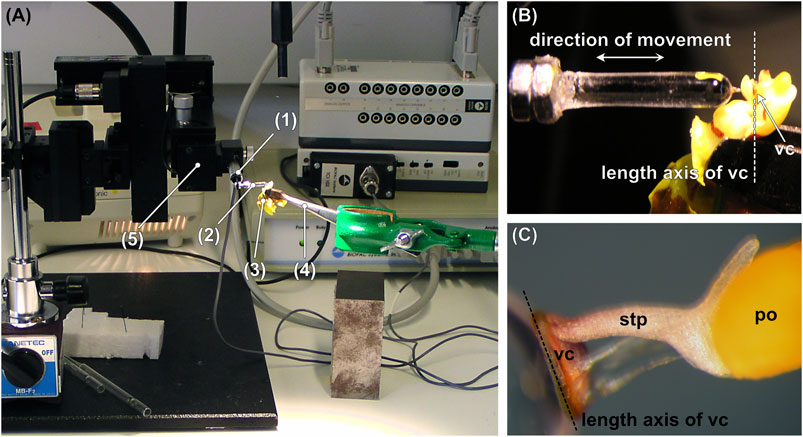
FIGURE 2. (A) Test setup consisting of a pollinator-mimicking glass rod, which is mounted on a force transducer coupled to a motorized micromanipulator. (B) Detail of (A) showing the glass rod that is approached and retracted perpendicular to the length axis of the viscidium. (C) Pollinarium attached to the tip of the glass rod. po = pollinium, stp = stipe, vc = viscidium. 1) Force transducer, 2) glass rod, 3) flower, 4) tweezers, 5) motorized micromanipulator.
The pollinator was mimicked by a round-tipped glass rod (diameter = 2.87 mm, length = 14.69 mm) produced by heating and melting the end of a glass rod, which gave a perfectly round tip due to the surface tension of the molten glass (Figure 2C). The glass rod was attached to a Fort 10 force transducer (10 g capacity, World Precision Instruments, Inc., Sarasota, FL, United States) and moved (i.e., approached and retracted) perpendicular to the longitudinal axis of the viscidium at a continuous speed of 190 μm s−1 using a motorized micromanipulator DC3001R combined with a MS314 controller (World Precision Instruments, Inc., Sarasota, FL, United States). Data were recorded using AcqKnowledge 3.7.0 software (Biopac Systems Ltd., Goleta, CA, United States) at a sample rate of 500 s−1. After making contact with the viscidium, the tip of the glass rod was moved forward for between 0.22 and 11.94 s, preloading the sticky viscidium material at forces between 0.045 and 74.087 mN. After keeping the glass rod in contact with the viscidium/flower for several seconds (min. = 1.01 s, max = 6.15 s), the glass rod was pulled off from the flower, either with the pollinarium sticking to it or not.
To determine to what extent the anther cap affects the force required to extract the pollinarium, the anther cap was removed vertically upwards in about half of the flowers shortly before starting the experiment, by using pointed tweezers (in some flowers the anther cap had already fallen off by itself). From 29 O. otogaya flowers, 16 were tested without anther cap. From O. wentworthianum 18 out of 29 were tested without anther cap.
The force measurements were conducted at 23.7 ± 1.69°C temperature and 47.3 ± 9.98% relative humidity. After each measurement, the adhesive residues left by the viscidium on the glass rod were wiped off with acetone-soaked filter paper.
The parameters gained from the raw data (i.e., force-time curves) (Figure 3 and Supplementary Material) using the AcqKnowledge 3.7.0 software (Biopac Systems Ltd., Goleta, CA, United States) were 1) load, 2) duration of load, 3) relaxation time, and 4) pull-off force. Data analysis and statistical evaluation were performed using the software R version 3.2.3 (R Core Team, 2015). The relationship between load and pull-off force was checked using the Spearman rank order correlation test, because tests for normality (Shapiro-Wilk test) revealed nonnormal distribution of the data.
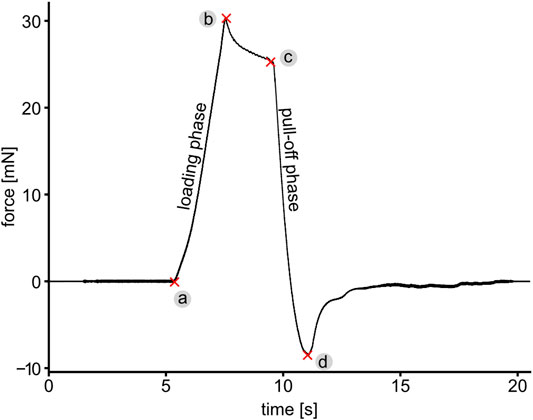
FIGURE 3. A representative force-time curve showing different phases of the force measurement, while pushing and pulling the pollinarium with a glass rod. (a) Contact formation, (a–b) loading phase, (b–c) time in contact showing viscoelastic relaxation similar to pressure sensitive adhesives and (c–d and further until the end of curve) pull-off phase. The maximum pushing and pulling force are denoted as points (b) and (d), respectively. The maximum pulling force is hereinafter referred to as the pull-off force, which characterizes either the detachment force of the viscidium from the glass rod (in the case the pollinarium cannot be removed from the flower) or the force required to pull the whole pollinarium out of the flower.
The adhesive strength was calculated as the ratio of the maximum pulling force over the contact area. The contact area Acwas estimated from the distance d traveled by the glass rod during the loading phase (i.e., loading duration * loading velocity) and the radius r of the glass rod under the assumption that Accorresponds to the lateral area of a spherical dome:
Adhesive strength was only estimated for trials that did not lead to removal of the pollinarium from the flower. It should be noted that the forward movement of the glass rod led not only to deformation of the adhesive material but probably also to deformations of the underlying flower structures. Estimations of the contact area and consequently of the adhesive strength are thus to be interpreted with care and should be considered as first-order approximations.
The viscidium of Oncidium spp. is ellipsoidal, about 1 mm high, 0.4 mm wide, and 0.1 mm thick. It sits in a pouch-shaped structure, formed by the rostellum that is narrowing toward the column (Figure 4A). The stipe is attached to the upper edge of the cushion-shaped viscidium. In case of the presence of an anther cap, it is almost completely covered by this. The anther cap is attached to the flower via a thin band of tissue on either side (Figure 4A) and features a lip-like thickened structure where it touches the stipe (Figure 4B). When the anther cap is removed, the two pollinia and the stipe, which connects the pollinia to the viscidium, become visible (Figure 4C). After manipulation of the pollinarium with a pinhead, in order to (partly) release it from the rostellum/flower, the viscidium is shown to protrude into the rostellar pouch with a 0.2 mm thick half-lobed structure at its backside (Figure 4D). It appears that the cushion-like viscidium completely consists of a translucent adhesive material that is overlaid only by a very thin membrane (Figure 4E). Apparently, this adhesive dries and hardens very quickly as can be seen in Figure 4F by a more than 2 mm long, obviously quite stiff (no drooping caused by gravity) and erect filament of adhesive that remained stuck to the tip of the glass rod after touching and pulling-off from the viscidium and thereby damaging the thin adhesive coverage, but not removing the pollinarium from the flower (Figure 4F). Only the bottom and side parts of the viscidium seem to be connected to the rim of the rostellar pouch by the same (or a similar) sticky material that covers the viscidium, as can be seen from the hardened remnants of the substance pointing toward the pollinia in Figure 2C. Except at the upper end of the pouch, the viscidium seems to have been attached to the rostellum via tiny and distinct tissue connections (Figures 4G,H). At least in the “released” state, the stipe can be described as a semitubular structure with its opening facing the opening of the rostellar pouch (Figure 4I).
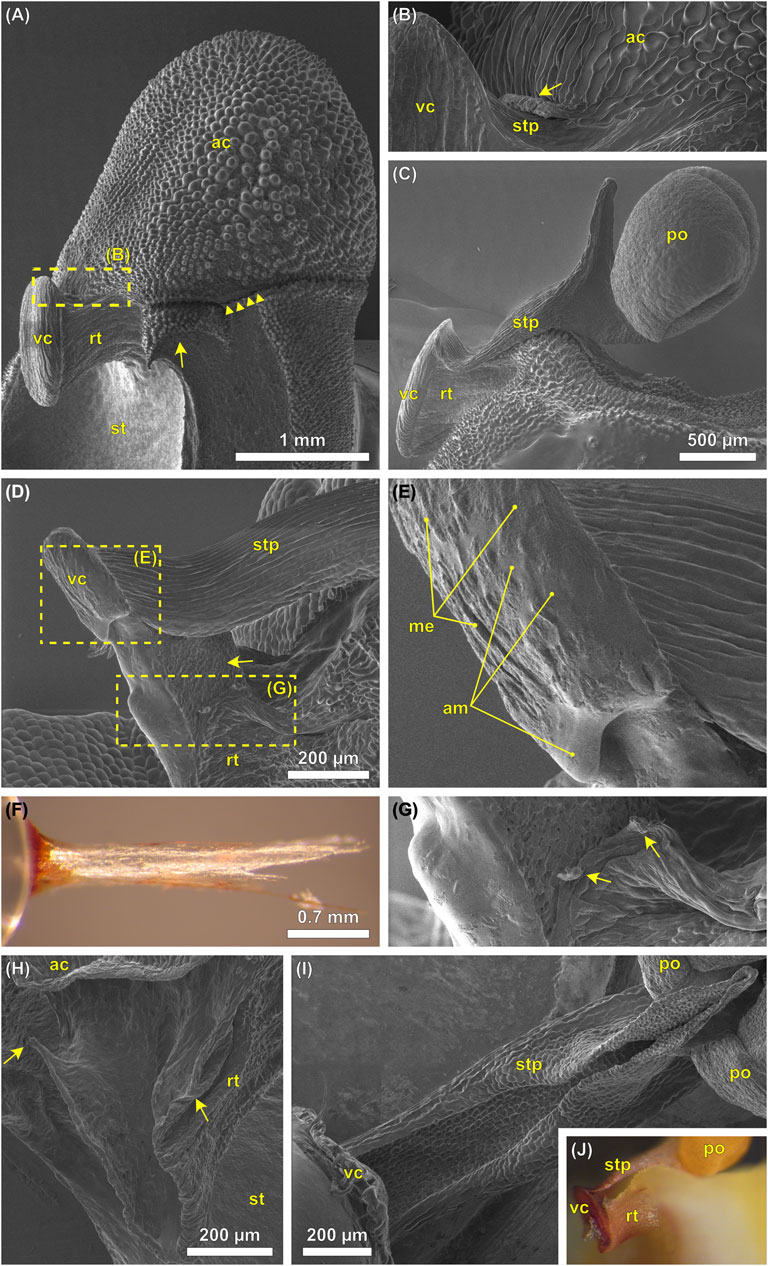
FIGURE 4. (A–E,G–I) Cryo-SEM images and (F,J) digital photographs of the pollination apparatus of Oncidium wentworthianum. (A) A flower with unreleased pollinarium and still attached anther cap. The column wings (staminodia) have been trimmed using a razor blade (arrow) to allow for a lateral perspective. The connective tissue between anther cap and flower is ruptured, probably during sample preparation (arrowheads). (B) Detail of image (A) showing the lip (arrow) of the anther cap that touches the stipe. (C) Lateral view of the pollinarium, which still sits in the rostellar pouch. The anther cap has been removed. The stipe is slightly bent upwards what might have been caused by removal of the anther cap and/or by the freezing process during cryo-SEM. (D) A pollinarium that was manipulated with a pinhead. The viscidium is partly released from its original position revealing the scutellum at its backside (arrow). The rostellar pouch thereby was deformed. The adhesive substance of the viscidium was partly damaged/removed. (E) Detail of image (D) showing the upper part of the viscidium with the adhesive material (am). This substance is covered by a (in this case partly torn) membrane (me). (F) Rod-shaped remnants of the adhesive material sticking to the tip of the glass rod. (G) Detail of image (D) showing the upper part of the rostellar pouch with protruding tissue “bridges” (arrows) probably having been connected to the viscidium. (H) Empty rostellar pouch with its upper edges which probably have the strongest connection to the viscidium. Note that in this case the pollinarium was manually removed from the flower without first removing the anther cap. (I) Artificially released pollinarium seen from below. The viscidium is attached to a pinhead (bottom left). It is not clear whether the stipe has formed into a semitubular shape due to its removal from the flower and a following change of shape due to mechanical stress or if it represents the in situ state. (J) Pollinarium that is partly detached from the flower upon manipulation. Viscidium and stipe have been pushed deeper into the rostellar pouch and upwards. ac, anther cap; am, adhesive material; me, membrane; po, pollinium; rt, rostellum; st, stigma; stp, stipe; vc, viscidium.
By applying load to the viscidium, while mimicking a pollinator visit, the viscidium is pushed deeper into the pocket formed by the rostellum, and at the same time, it is forced upwards. This upward movement causes the stipe to detach from the rostellum (Figure 4J). Once the viscidium was properly attached to the glass rod, and the pollinarium has been removed from the flower; it could not be removed from the glass rod except by scratching it off. Attempts to do so by pulling always resulted in tearing apart the stipe.
From all 29 O. otogaya flowers tested, the pollinarium could be successfully removed in only 8 cases. These were solely flowers, which did not have an anther cap. Among the O. wentworthianum flowers the pollinarium could be removed in 11 out of 29 trials with only one successful removal from a flower with the anther cap still attached (Table 1).
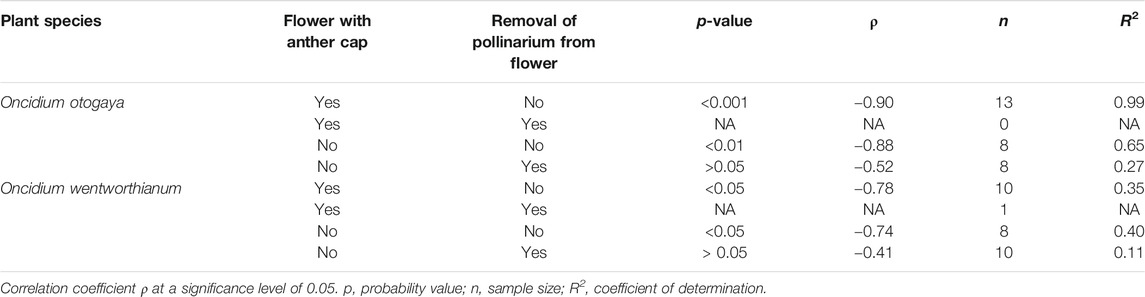
TABLE 1. Correlation analysis between load and pull-off force (Spearman’s rank correlation) in flowers with and without anther cap and removed/nonremoved pollinarium.
There was significant evidence (p < 0.05) suggesting a negative linear correlation between pull-off force and load, only for trials in which the pollinarium was not removed from the flower. This is true for both O. otogaya and O. wentworthianum (Table 1; Figure 5). The absolute pull-off forces, necessary to remove the pollinaria from the flowers, were comparatively low and ranged between 2.95 and 8.29 mN for O. otogaya and between 3.46 and 12.19 mN (3.46 and 7.46 mN, if only flowers without anther cap were considered) for O. wentworthianum.
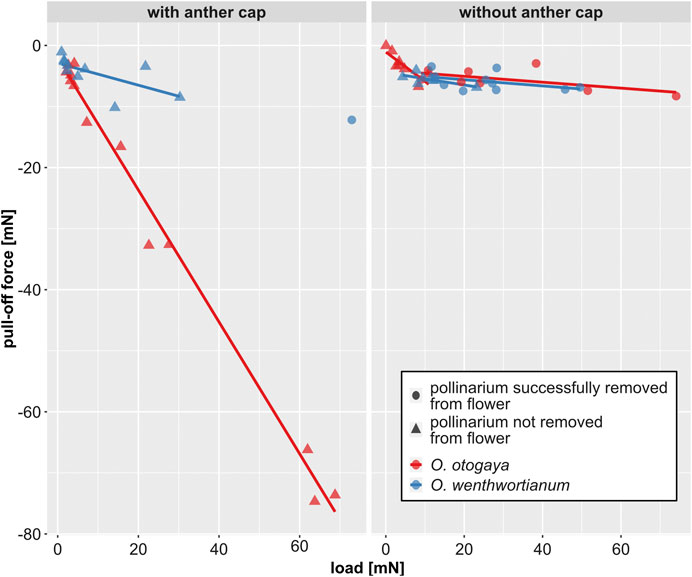
FIGURE 5. Pull-off force vs. load, measured in Oncidium otogaya and O. wentworthianum flowers with (left) and without anther cap (right) and grouped by species (color of the symbol) and success of pollinarium removal (shape of the symbol). Statistical details for the linear regression lines are displayed in Table 1.
For flowers without anther cap, the minimal load needed to start a successful removal process of the pollinarium was 8.99 mN in O. otogaya and 11.66 mN in O. wentworthianum. After applying loads higher than 10.82 mN (O. otogaya) and 12.58 mN (O. wentworthianum), respectively, the pollinarium could always be removed in flowers without anther cap (Figures 6A,B).
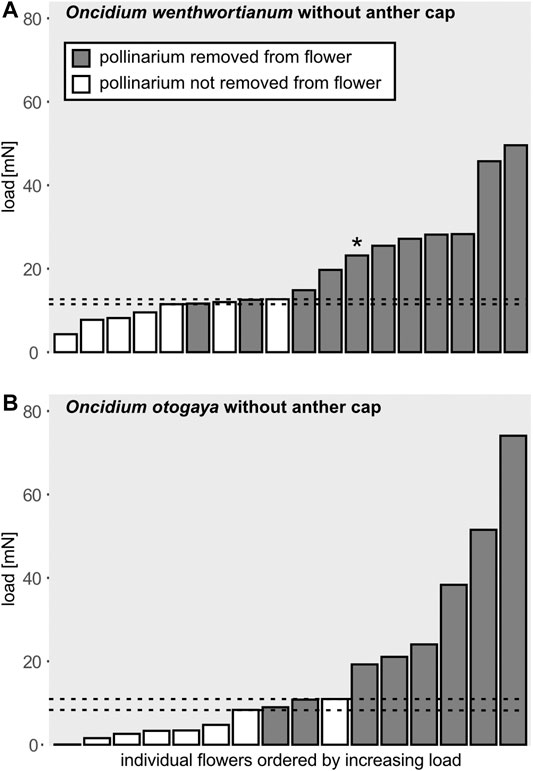
FIGURE 6. Applied load and related success of removal of the pollinarium in (A)Oncidium wentworthianum and (B)O. otogaya for flowers without anther cap. The gray bars show the applied load that leads to successful pull-off of the pollinarium from the flower. The white bars correspond to the load that was not sufficient to separate the pollinarium from the flower. The dotted lines indicate the threshold of the load sufficient to overcome the initial attachment of the pollinarium to the flower. When the applied load was higher than the upper threshold, the pollinarium could be removed from the flower in all attempts. When the applied load was lower than the lower threshold, the pollinarium could be removed from the flower in no attempt. (*This pollinarium was successfully and completely separated; however, after a short distance of pull-off it got stuck and detached from the glass rod. The pollinarium was thus rated as successfully removed from the flower.)
The adhesive strength of the viscidia was calculated from the estimated contact area and the maximum pull-off force. For obvious reasons this could only be done for trials in which the pollinarium itself remained attached to the flower. For O. otogaya the mean adhesive strength is 5.62 ± 4.01 kPa (max. 15.38 kPa), and 4.07 ± 2.08 kPa (max. 8.96 kPa) for O. wentworthianum.
The experiments mimicking the first stage of the pollination process of Oncidium flowers, i.e., the removal of the pollinarium from the flower, reveal that a successful pollinarium removal depends on whether the anther cap is present or not. As anther caps in Oncidium sp. seem to be shed with an increasing flower age, which is for example reported for Oncidium sphacelatum (Pemberton, 2008), their presence or absence is an indirect indicator of pollen “ripeness.” This is related to the developmental stage of any other flower structure contributing to a fully functional pollination apparatus. When mature, the backside of the viscidium, referred to as scutellum, consists of dead cells with thickened walls, and while still in contact with the rostellum in its central part, the peripheral tissue already separates from the rostellum as observed by Schick (1989) in O. hastatum, except for the tissue strands seen in Figure 4G at the upper edge of the rostellar pouch. Concurrently the stipe differentiates from the rostellum tissue by insertion of a separation layer and mechanical stresses progressively build up in the stipe due to thickening of the cell walls (Schick, 1989).
Due to narrowing of the rostellar pouch, pushing back of the viscidium automatically leads to its upward movement, which leads to rupturing of the connection between viscidium and rostellum and between rostellum and stipe (Figures 4G,H,J, 7B). Furthermore, the rostellar pouch is also widened during this process, additionally helping to release the stipe from the rostellum. The semitubular shape of the stipe is probably induced by previously build-up stresses that are released, when the connection between rostellum and stipe breaks, and increases its ex situ bending stiffness, ensuring precise positioning of the pollinia in relation to the viscidium (Schick, 1989) and thereby also an exact deposit on the stigma in the next visited flower. The probability of self-pollination is drastically reduced because the stipe of the pollinarium attached to the pollinator reconfigures over a time period of several minutes or hours, which eventually brings the pollinia into a position that allows them to come into contact with the stigma (Johnsson and Edwards, 2000). The mature pollination apparatus finds itself in a rather delicate mechanical state of equilibrium. In compliance with the development of mechanical stresses reported by Schick (1989), our cryo-SEM analysis suggests that the mature stipe is prestressed transversely to its longitudinal axis (Figure 4I), which in combination with the deformation caused by a pollinator pushing against the viscidium causes the stipe to curl and to consequently detach and lift off from the rostellum. When the anther cap is still present its lip blocks this relaxation and the back and upward movement caused by a pollinator and thus forestalls a premature release of the pollinium (Figures 4B, 7A).
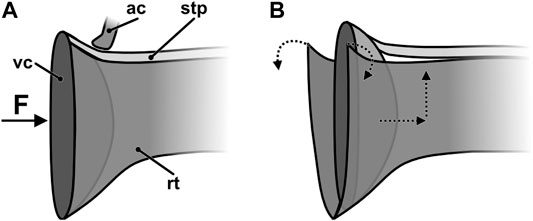
FIGURE 7. Schematic drawing of the viscidium sitting in the rostellar pouch (A) before and (B) after applying a sufficient force, e.g., by a pollinator. The dotted arrows indicate direction of movement and of deformation. ac, anther cap (only the lip is shown); F, load; rt, rostellum; stp, stipe; vs, viscidium.
A load slightly higher than 10 mN, that is required to successfully remove the pollinarium from the flowers of the two Oncidium species, serves 1) for the establishment of a sufficient contact between the adhesive of the viscidium and the glass rod (or the pollinator) and 2) for the release of the mechanical anchoring of the pollinarium. The linear correlation between the load and pull-off force indicates a dependence of the viscidium’s adhesive force on the load, which let us hypothesize a pressure sensitive property of the adhesive. This is also supported by the viscoelastic relaxation under load (Figure 3), which is typical for pressure sensitive adhesives (Feldstein, 2009). Calculations of the adhesive strength yielded values that are considerably lower than those of artificial pressure sensitive adhesives, like for example styrenic block copolymers (∼200–800 kPa) (Pandey et al., 2020) or comparable biological pressure sensitive adhesives. Trichome secretions of the flypaper trap of the protocarnivorous plant Roridula gorgonias for example have a median adhesive strength between 17.5 and 156.2 kPa, depending on trichome length and corresponding functional adaptation (Voigt et al., 2009). The values determined here, however, are based on in vivo experiments; i.e., the viscidium was attached to the rostellum and not to an uncompliant, stiff support. It is thus likely that the established contact area is overestimated which in turn entails an underestimation of the adhesive strength. In any case, a correlation between load and pull-off force is beneficial to the plant in that insects that fail to remove the pollinarium, either because the anther cap is still present or because they are not strong enough to overcome the mechanical barrier, can at least free themselves and do not remain stuck to the flower and block it against suitable pollinators. The pull-off forces required to fully withdraw the pollinarium from the flower are rather low (7.46 and 8.29 mN), as compared to the loading forces required to detach the pollinarium, and are needed to overcome the connection between scutellum and rostellum, as well as the friction between pollinarium and the flower structure.
Potential pollinators of Oncidium spp. have been reported to be fairly large apid bees, which, deducing from their size [Centris Fabricius: 9–19 mm, Trigona Jurine: 4–9 mm, Tetrapedia Klug: 8–13 mm, and Epicharis Klug: 15–25 mm (Michener, 2000)], probably are able to easily produce forces well above 10 mN. Information on forces insects in general and bees in particular are able to apply by pushing is scarce. The forces measured by Reith et al. (2006) for Bombus terrestris L. (Hymenoptera, Apidae, size: 12–19 mm) are 24.6 ± 14.8 mN (max. 59 mN) for workers and 46.8 ± 25.5 mN (max. 90 mN) for queens. For Apis mellifera L., a mean value of 14 ± 7.4 mN (max. 29 mN) was measured, which is approximately half the force measured by Córdoba and Cocucci (2011) for A. mellifera (26.26 ± 3.89 mN). For different Bombus species the latter authors measured forces of well over 200 mN. It should however be noted that Reith et al. (2006) and Córdoba and Cocucci (2011) used different experimental setups to assess the insects pushing forces. Whereas in the approach of Córdoba and Cocucci (2011) the force insects exert for opening a trap door in a kind of escape reaction was recorded, in the approach of Reith et al. (2006) the pushing forces invested by insects to get access to a food source in an artificial flower were measured. As the pollinaria are dislodged by the bee rather accidentally (at least from the bee’s point of view) while collecting oil from the elaiophores (Singer, 2003; Pansarin et al., 2017), it seems evident that they will not push on the viscidium with their full strength. Also Córdoba and Cocucci (2011) reported that pollinators are able to exert a lot higher maximum force than actually needed to operate the flower’s mechanism. While only female Centris bees collect oil, there are reports of male Centris bees that may be involved in the pollination process of Oncidium as well. When the flowers of Oncidium move or "dance" in the wind (hence their name “dancing ladies”), they may be mistakenly perceived by male Centris bees as enemies and tempt them to attack in a behavior known as pseudoantagonism (Dressler, 1993; Castro and Singer, 2019). While attachment of the pollinia would be in principle possible also to male Centris bees, regarding the remarkably quick-setting properties of the glue of the viscidium (Dressler, 1961) (Figure 4F), Castro and Singer (2019) doubt the existence of such an attachment mechanism of rapidly striking a flower while attempting to attack an enemy as extremely unlikely. The impossibility of adhesion generation by fast movement may be explained by the viscoelastic property of the viscidium material. The fast contact formation will not generate large contact and sufficient pull-off force, because at short relaxation times it is hardly possible. Only the slow motion or large contact times will potentially generate large contact area and strong pull-off force. It may be hypothesized that viscoelastic properties of the viscidium material might represent a safety mechanism preventing occasional detachment of the pollinium due to short fast contacts caused for example by wing actions.
Besides the force that the pollinator is able to generate “accidentally,” its body dimension is also an important factor. The bee or more general the pollinator must be big and forceful enough to hit the viscidium, when sitting on the labellum. Further aspects, potentially essential for a comprehensive understanding of the pollinator-flower-interactions, concern properties of the surface structures (e.g., papillae or cuticular folds, cell shapes, and dimensions), the nature and position of the attachment of the viscidium to the insect integument, the chemical and physical properties of the adhesive substance, and the process of detachment deposition of the pollinia on the stigma. These could be addressed in future studies to further enhance the understanding of the form-structure-function relationships in the pollination apparatus of Oncidium species and their coevolution with Centris bees.
In the studied Oncidium species, the complex pollination apparatus constitutes a two-stage barrier preventing risk of erroneous removal of pollinium from the flower. At the first stage, the presence of an anther cap efficiently prevents visiting pollinators from removing the pollinaria before these reach maturity. At the second stage, a mechanical barrier formed by a severable interconnection between the pollinaria and another part of the flower, the rostellum, ensures that only legitimate pollinators, able to push and then pull the pollination apparatus strongly enough, are able to remove the pollinaria which can be hypothesized as effect of coevolution between plants and their pollinators.
Summarized raw data are included in the articel Supplementary Material, further inquiries can be directed to the corresponding author.
SG and TS initiated the research. SG, DV, and FG designed and supervised the study. Data collection, data assessment, and statistical analyses were carried out by MT. Data evaluation and discussion of results were a joint effort by all authors. MT contributed to the first draft of the manuscript. All authors improved further versions of the manuscript and gave final approval for publication.
This project was financed by institutional budgets. Partial support was provided by the European Network of Bioadhesion Expertise: Fundamental Knowledge to Inspire Advanced Bonding Technologies (COST Action CA15216“ENBA”). The article processing charge was funded by the Baden-Württemberg Ministry of Science, Research and Art and the University of Freiburg in the funding program Open Access Publishing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors want to thank F. Lô-Kockel† and B. Schäfer from the Zoological-Botanical Garden “Wilhelma” in Stuttgart, Germany, for kindly providing the orchids analyzed in this project. Also, the help of Jan Schuppert (Max Planck Institute for Metals Research, Stuttgart) with cryo-SEM imaging is acknowledged.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmech.2020.635694/full#supplementary-material.
Borba, E. L., and Semir, J. (1999). Temporal variation in pollinarium size after its removal in species of Bulbophyllum: a different mechanism preventing self-pollination in Orchidaceae. Plant Systemat. Evol. 217, 197–204. doi:10.1007/BF00984365
Brantjes, N. B. M. (1981). Floral mechanics in phlomis (lamiaceae). Ann. Bot. 47, 279–282. doi:10.1093/oxfordjournals.aob.a086018
Cameron, L. D., and Johnson, S. D. (2008). Function and evolution of aggregated pollen in angiosperms. Int. J. Plant Sci. 169, 59–78. doi:10.1086/523364
Carmona-Díaz, G., and García-Franco, J. G. (2008). Reproductive success in the Mexican rewardless Oncidium cosymbephorum (Orchidaceae) facilitated by the oil-rewarding Malpighia glabra (Malpighiaceae). Plant Ecol. 203, 253–261. doi:10.1007/s11258-008-9543-6
Castro, J. B., and Singer, R. B. (2019). A literature review of the pollination strategies and breeding systems in Oncidiinae orchids. Acta Bot. Bras. 33, 618–643. doi:10.1590/0102-33062019abb0111
Chase, M. W., Cameron, K. M., Freudenstein, J. V., Pridgeon, A. M., Salazar, G., Berg, C., et al. (2015). An updated classification of Orchidaceae. Bot. J. Linn. Soc. 177, 151–174. doi:10.1111/boj.12234
Christenhusz, M. J. M., and Byng, J. W. (2016). The number of known plants species in the world and its annual increase. Phytotaxa. 261, 201. doi:10.11646/doi.org/phytotaxa.261.3.1
Claßen-Bockhoff, R., Speck, T., Tweraser, E., Wester, P., Thimm, S., and Reith, R. (2004). The staminal lever mechanism in Salvia L. (Lamiaceae): a key innovation for adaptive radiation?. Org. Divers. Evol. 4, 189–205. doi:10.1016/j.ode.2004.01.004
Córdoba, S. A., and Cocucci, A. A. (2011). Flower power: its association with bee power and floral functional morphology in papilionate legumes. Ann. Bot. 108, 919–931. doi:10.1093/aob/mcr196
Darwin, C. (1890). The various contrivances by which orchids are fertilised by insects. London, UK: John Murray. doi:10.5962/bhl.title.37883
Davies, K. L., Stpiczyńska, M., and Rawski, M. (2014). Comparative anatomy of floral elaiophores in vitekorchis romowicz & szlach., cyrtochilum kunth and a florally dimorphic species of Oncidium Sw. (Orchidaceae: Oncidiinae). Ann. Bot. 113, 1155–1173. doi:10.1093/aob/mcu045
Dressler, R. L. (1981). The orchids: natural history and classification. Cambridge, MA: Harvard University Press.
Dressler, R. L. (1993). Phylogeny and classification of the orchid family. Cambridge, UK: Cambridge University Press.
Faegri, K., and Pijl, L. v. d. (1979). The principles of pollination ecology. 3rd Edn. Oxford, UK: Pergamon Press.
Feldstein, M. M. (2009). Contribution of relaxation processes to adhesive-joint strength of viscoelastic polymers. Polym. Sci. 51, 1341–1354. doi:10.1134/S0965545X09110194
Ferreira, N. P., Chiavelli, L. U. R., Savaris, C. R., Oliveira, S. M., Lucca, D. L., Milaneze-Gutierre, , et al. (2019). Chemical study of the flowers of the orchid Oncidium baueri Lindley and their visiting bees Trigona spinipes Fabricius. Biochem. Systemat. Ecol. 86, 103918. doi:10.1016/jdoi.org/.bse.2019.103918
Givnish, T. J., Spalink, D., Ames, M., Lyon, S. P., Hunter, S. J., Zuluaga, A., et al. (2015). Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proc. Biol. Sci. 282, 20151553. doi:10.1098/rspb.2015.1553
Jersáková, J., Johnson, S. D., and Kindlmann, P. (2006). Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 81, 219–235. doi:10.1017/S1464793105006986
Johnson, S. D., and Anderson, B. (2010). Coevolution between food-rewarding flowers and their pollinators. Evo. Edu. Outreach. 3, 32–39. doi:10.1007/s12052-009-0192-6
Johnson, S. D., and Edwards, T. J. (2000). The structure and function of orchid pollinaria. Plant Systemat. Evol. 222, 243–269. doi:10.1007/BF00984105
Mosquera-Mosquera, H. R., Valencia-Barrera, R. M., and Acedo, C. (2019). Variation and evolutionary transformation of some characters of the pollinarium and pistil in Epidendroideae (Orchidaceae). Plant Systemat. Evol. 305, 353–374. doi:10.1007/s00606-019-01575-5
Pacini, E., and Hesse, M. (2002). Types of pollen dispersal units in orchids, and their consequences for germination and fertilization. Ann. Bot. 89, 653–664. doi:10.1093/aob/mcf138
Pandey, V., Fleury, A., Villey, R., Creton, C., and Ciccotti, M. (2020). Linking peel and tack performances of pressure sensitive adhesives. Soft Matter. 16, 3267–3275. doi:10.1039/C9SM02172H
Pansarin, E. R., Alves-Dos-Santos, I., and Pansarin, L. M. (2017). Comparative reproductive biology and pollinator specificity among sympatric Gomesa (Orchidaceae: Oncidiinae). Plant Biol. 19, 147–155. doi:10.1111/plb.12525
Parra-Tabla, V., Vargas, C. F., Magaña-Rueda, S., and Navarro, J. (2000). Female and male pollination success of Oncidium ascendens Lindey (Orchidaceae) in two contrasting habitat patches: forest vs agricultural field. Biol. Conserv. 94, 335–340. doi:10.1016/S0006-3207(99)00187-1
Pemberton, R. W. (2008). Pollination of the ornamental orchid Oncidium sphacelatum by the naturalized oil-collecting bee (Centris nitida) in Florida. Selbyana. 29, 87–91.
R Core Team (2015). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Reith, M., Claßen-Bockhoff, R., and Speck, T. (2006). “Biomechanics in Salvia flowers: the role of lever and flower tube in specialization on pollinators,” in Ecology and Biomechanics - a mechanical approach to the ecology of animals and plants. Editors A. Herrel, T. Speck, and N. P. Rowe (Boca Raton, FL: CRC Press), 123–146.
Schick, B. (1989). “I: dactylorhiza majalis (Rchb.) Hunt & Summerh., Disa uniflora Bergius und Oncidium hastatum Lindl,” in Zur Anatomie und Biotechnik des Bestäubungsapparates der Orchideen.Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie. Editors J. Grau, B. Hiepko, and P. Leins (Stuttgart, Germany: Schweizerbart’sche Verlagsbuchhandlung (Nägele u. Obermiller), 215–262.
Singer, R. B. (2003). Orchid pollination: recent developments from Brazil. Lankesteriana. 7, 111–114.
Singer, R. B., and Cocucci, A. A. (1997). Eye attached Hemipollinaria in the hawkmoth and settling moth pollination of Habenaria (Orchidaceae): a study on functional morphology in 5 species from subtropical South America. Bot. Acta. 110, 328–337. doi:10.1111/j.1438-8677.1997.tb00648.x
Stern, W. L. (2014). Anatomy of the monocotyledons, Vol. X: Orchidaceae. Oxford, UK: Oxford University Press.
Tremblay, R. L. (1992). Trends in the pollination ecology of the Orchidaceae: evolution and systematics. Can. J. Bot. 70, 642–650.doi:10.1139/b92-083
Voigt, D., Gorb, E., and Gorb, S. (2009). Hierarchical organisation of the trap in the protocarnivorous plant Roridula gorgonias (Roridulaceae). J. Exp. Biol. 212 (19), 3184–3191. doi:10.1242/jeb.034280
Wagenitz, G. (1981). Orchideen und Compositen, Vergleich zweier Familien und Evolutionsstrategien. Ber. Deutsch. Bot. Ges. 94, 229–247.
Keywords: adhesion, biomechanics, Oncidium, orchid, pollinarium, pollination, viscidium
Citation: Thielen M, Voigt D, Gallenmüller F, Speck T and Gorb S (2021) “Push and Pull”: Biomechanics of the Pollination Apparatus of Oncidium spp.. Front. Mech. Eng 6:635694. doi: 10.3389/fmech.2020.635694
Received: 30 November 2020; Accepted: 22 December 2020;
Published: 27 January 2021.
Edited by:
Yu Tian, Tsinghua University, ChinaReviewed by:
Antonio Papangelo, Politecnico di Bari, ItalyCopyright © 2021 Thielen, Voigt, Gallenmüller, Speck and Gorb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc Thielen, bWFyYy50aGllbGVuQGJpb2xvZ2llLnVuaS1mcmVpYnVyZy5kZQ==
†ORCID: Marc Thielen, orcid.org/0000-0002-7773-6724; Dagmar Voigt, orcid.org/0000-0003-2772-8504; Friederike Gallenmüller, orcid.org/0000-0001-9891-094X; Thomas Speck, orcid.org/0000-0002-2245-2636; Stanislav Gorb, orcid.org/0000-0001-9712-7953
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.