- 1Polytechnical Institute, Sevastopol State University, Sevastopol, Russia
- 2Institute of Mechanics, Technische Universität Berlin, Berlin, Germany
There is a global tendency to rejuvenate joint diseases, and serious diseases such as arthrosis and arthritis develop in 90% of people over 55 years of age. They are accompanied by degradation of cartilage, joint deformities and persistent pain, which leads to limited mobility and a significant deterioration in the quality of life of patients. For the treatment of these diseases in the late stages, depending on the indications, various methods are used, the most radical of which are methods of joint arthroplasty and, in particular, total arthroplasty. Currently, total arthroplasty is one of the most effective and high-quality surgical operations at the relevant medical indications. However, complications may also arise after it, leading, inter alia, to the need for repeated surgical intervention. In order to minimize the likelihood of complications, the artificial joints used in total arthroplasty and the technology of their fabrication are constantly being improved, which leads to the emergence of new designs and methods for their integration with living tissues. At the same time, at the moment, the improvement of traditional designs and production technologies has almost reached the top of their art, and their further improvements can be insignificantly or are associated with the use of the most up-to-day technologies, allowing for friction couples with low tribological properties to provide for them high ones, for example, gradient increase hardness in the couple titanium alloy on titanium alloy. This paper presents the current state of traditional technical means and technologies for joint arthroplasty. The main attention is paid to the analysis of the latest technologies in the field of joint arthroplasty, such as osseointegration of artificial joints, the improvement of materials with the property of osteoimmunomodulation, the improvement of joint arthroplasty technologies based on the modeling of dynamic osteosynthesis, as well as the identification of possible unconventional designs of artificial joints that contribute to these technologies, predictive assessment of areas for technologies improvement.
Introduction
Cartilage degradation and joint deformities lead to persistent pain, limited mobility, and often to patients with disabilities. For the treatment of these diseases in the late stages, depending on the indications, various methods are used, the most effective of which are rightly considered methods of joint arthroplasty and, in particular, total joint arthroplasty. This method involves the removal of the natural joint and its replacement with an artificial joint in order to restore functions and eliminate pain of the patient for a long period of time.
It is known that even in antiquity, attempts were made to replace natural joint with an artificial joint, but they did not lead to the creation of more or less significant primary technologies of arthroplasty. For a long time, this idea remained relevant, but its implementation was constrained by the current state of materials science and manufacturing technologies for products with complex profiles. Nevertheless, already at the end of the Nineteenth century, one of the first original designs of an artificial joint from ivory was developed and manufactured (first the lower jaw, and then the hip and knee joints), and also the simplest technologies for their implantation were justified (Gluck, 1891). The further history of the artificial joint development has been studied in detail and described in many literary sources (see, for example, Filipenko and Tankut, 2009; Affatato, 2014; Chang, 2014; Affatato et al., 2015; Merola and Affatato, 2019).
Modern artificial joints are high-tech products made from specialized materials using unique technological equipment. Their designs are justified and designed based on system analysis methods, taking into account the accumulated design experience, the physiological state of patients and the possibilities of eliminating the most likely complications (Polyakov et al., 2015). This has made the total joint arthroplasty one of the most common and effective orthopedic surgeries in the world. However, even the most advanced artificial joint (in terms of the capabilities of modern technology) is not able to fully replace natural joint due to a number of objective reasons. Given this fact, developers of the new artificial joint designs seek to minimize this contradiction.
This paper presents the current state of traditional technical means and technologies for joint arthroplasty, which has already been widely studied and described. The main attention is paid to the analysis of the latest technologies in the field of joint arthroplasty, such as osseointegration of artificial joints, the improvement of materials with the property of osteoimmunomodulation, the improvement of joint arthroplasty technologies based on the modeling of dynamic osteosynthesis, as well as the identification of possible unconventional designs of artificial joints that contribute to these technologies, predictive assessment of areas for their improvement.
State of the Problem
From a mechanical point of view, any natural joint is a movable connection of the skeleton elements, ensuring their sliding and/or rolling relative to each other. Still P.F. Lesgaft has noted that by the shape of the articular surfaces it is possible to determine the nature of the joint movements and, conversely, the nature of the movements of this movable joint determines its shape (Lesgaft and Dolbnya, 1987). Hence, one of the main conditions for creating an artificial joint for total joint arthroplasty is to ensure their natural mobility by forming biosimilar contact surfaces. But at the same time (ideally), an artificial joint should optimally integrate into the body and should not be considered by the immune system as a foreign body or, at least, should not be rejected by it for a long period of time. These conditions impose serious restrictions both on the design of an artificial joint, and on the materials from which their elements are made. Considering of these limitations in practice is a very difficult problem for the designer.
In the future, we will focus mainly on current trends in the improvement of the artificial hip joint and the technologies of its arthroplasty (THA), because it is this operation that is performed most often and it is characterized by the same problems that are observed with arthroplasty of the knee, shoulder, elbow, and other joints although structurally they are significantly different. This approach can be considered generally accepted and in many scientific studies the most significant problems characteristic of all artificial joints are not identified with a specific type of joint (see, for example, Römmelt et al., 2019; Shirani et al., 2019; Von Skrbensky et al., 2019; Yang et al., 2019; Zhang L. et al., 2019). Moreover, in most of the studies devoted to artificial joints, artificial hip joints are still used as examples (Bhalekar et al., 2019; Xu H. D. et al., 2019). At the same time, in cases in which the anatomical features, the shape of the contact surfaces and the kinematics of the elements of the joint play an important role, all the features characteristic of a particular artificial joint are taken into account (Stief et al., 2019; Wu et al., 2019; Zhang Q. D. et al., 2019).
It can be argued that modern artificial hip joint designs are the result of a rather long period of their evolution during which solutions to a number of key problems were obtained that ensured the rapid development of THA technologies.
• Firstly, it is the problem of choosing the materials of the artificial hip joint elements that the best meet the clinical and operational requirements.
Theoretical studies and clinical observations have shown that the best of the currently known materials from these points of view are:
– Ti-based alloys (cementless stems and cups for the total artificial hip joints);
– Stainless steels and alloys based on Co, Cr, and Mo (cement stems, in some cases the heads for the total hip joints);
– Alumina (Al2O3) and zirconium (ZrO2) ceramics (heads and liners of cups for the total hip joints);
– Ultra-high molecular weight polyethylene (UHMWPE) (liners of cups for the total hip joints);
– Polyetheretherketone (PEEK).
At the same time, it should be noted that these materials cannot be considered optimal for artificial hip joints, because each of them represents a foreign environment for the body and, to one degree or another, contributes to the appearance of complications after surgery. First of all, it is this circumstance that is the motive for the search for new materials for total hip joints elements and other artificial joints that would be best integrated into the human body, which is a complex biological system.
• Secondly, this is the problem of the optimal artificial hip joint design, which ensures the best fixation of its elements in bone tissues both at the initial stage and during the entire product life cycle. At the same time, optimization of the artificial joint design from the point of view of minimal wear was undertaken, for example (Matsoukas and Kim, 2006), where total hip replacement (THR) optimization is carried out on the basis of Archard's wear law.
As it turned out, to solve this problem only by improving the design is almost impossible, because a possible change in the structure of bone tissue in the area of implant placement eliminates all the advantages obtained as a result of artificial joint optimization. Currently, this problem is considered as multicriteria and inextricably linked with the problem of osseointegration of artificial hip joint elements, the solution of which will significantly reduce the likelihood of their rejection by the body.
• Thirdly, it is the problem of choosing the optimal friction artificial hip joint couple.
In our opinion, this is one of the most important problems, which to the greatest extent determines the reliability and durability of not only artificial hip joint, but also other artificial joints. Its solution is hampered by the fact that it is practically impossible to provide lubrication of rubbing surfaces similar to that observed in natural joint.
At the same time, it should be noted that during the evolution, the best (from the point of view of the state of art engineering and technology) solutions were found that made it possible to ensure the minimum possible wear of the artificial hip joint friction couple surfaces and, accordingly, improve the artificial hip joint quality. According to many well-known specialists in the field of THA, high tribological properties are achieved in friction couples: Co-Cr-Mo/stainless steel on UHMWPE, ceramic on ceramic, sapphire on sapphire. In addition, certain hopes are associated with metal on metal couples of the third generation in the case of using large-diameter heads (more than 32 mm) (Affatato et al., 2007, 2008, 2016; Filipenko and Tankut, 2009; Ihaddadene et al., 2011) as well as with ceramic on metal couples (Williams et al., 2013). Also, the primary results in the use of dual mobility cups (DMC) in THA with third generation cups, but which require longer follow-up, may also be promising (Neri et al., 2018).
Thus, it can be argued that the problems formulated above and the results of their solution generally correspond to the current level of development of science and technology. At the same time, the results achieved in the development of optimal artificial hip joint designs and THA technologies cannot be considered optimal from various points of view. Therefore, despite the impressive results obtained in recent years, the efforts of many researchers, scientific groups and manufacturers are aimed at creating a new generation of artificial hip joint, the use of which would significantly reduce the risk or completely eliminate the complications inherent in THA.
Improvement of Materials for Artificial Hip Joint Friction Coupling Elements
A search for literary sources published over the past 5 years in publications indexed in the Web of Science database using the keywords “friction couples materials of artificial joints or current trends in the development of materials for artificial joints” identified 32 scientific articles, 12 of which were attributed by the authors to the section “Engineering Mechanical,” 10 to the section “Materials Science Multidisciplinary” only 8 to the sections “Engineering BIOMEDICAl,” and “Materials Science Biomaterials”. This indicates that research in the field of creating new materials for elements of artificial joints couples is mostly carried out in material science laboratories that are not directly related to biomedicine, orthopedics, and the development of new biomaterials for orthopedics is a by-product of their activities. In addition, it should be noted that over the past 5 years in the publications indexed in the Web of Science database, not a single review article has been published that would formulate current trends in the development of materials for artificial joints. It is logical to assume that this is due primarily to the fact that such trends (trends) have been identified earlier and that they are decisive in the development of modern biomaterials used in artificial joints. In this regard, the history of the creation of biomaterials for orthopedic use and the current state of this problem are of interest.
There is no doubt that one of the modern directions of artificial hip joint improvement is the search for new materials that provide reduction of the friction moment in the joint and wear of materials of friction couples. From this point of view, the event associated with the fact that J. Charnley proposed using UHMWPE as the liner material for a cup of total hip joint (Charnley, 1961) is especially significant. This event can be considered revolutionary, since for many years it determined the strategy for the selection of friction couples for artificial joint, characterized by a low coefficient of friction.
Subsequently, shortcomings inherent in such couples were identified. For example, it was found that being in an aggressive environment of the human body, UHMWPE undergoes oxidation and delamination, which naturally affects its properties and increases wear, contributing to the development of osteolysis–one of the most serious complications inherent in THA (Harris, 1995; Kim et al., 2012).
It is known that Co, Cr particles and their ions in a friction couple with a metal from CoCr have a toxic nature and, released into the periprosthetic tissues and blood, tend to migrate and accumulate in the vital organs of a person in the future leading to a possible manifestation severe allergic reactions. Wait et al. using the electron microscope technique, were able to show that most of the debris particles extracted from the tissues of patients with damaged metal on metal THR had a maximum size of less than 0.4 μm and exceeded it by less than 0.02 μm (Wait et al., 1995). The wear particle sizes for polyethylene were large in size (Lee et al., 1992; Shanbhag et al., 1994).
As a result, metal on metal couples, creating much smaller volumes of particles than conventional metal on polymer or ceramic on polymer ones, can produce similar active total surface areas of the particles. For example, using a cylindrical wear model and normal density, it can be estimated that 75 μm of an annual linear wear of a metal on polyethylene couple with a diameter of 28 mm corresponds to approximately 44 mg of polymer weight loss per year. In comparison, 5 microns of annual linear wear of a metal on metal couple the same diameter corresponds to approximately 27 mg weight loss per year. If we further assume that the average size of metal particles is 1/10 of those for polyethylene, then the ratio of the surface area of the particles is almost the same as the loss in weight per year. That is, although the linear wear rate of a metal on metal couple is 1/15 (93% reduction) of the metal on polyethylene wear rate, smaller particle sizes provide only about 1/3 (33%) reduction in active surface area. Therefore, couples with polyethylene are still in demand.
The obvious advantages of UHMWPE compared to other materials have contributed to the search for materials similar to UHMWPE, but with less wear. As a result, UHMWPE with a high degree of crosslinking (XLPE) was obtained (Digas et al., 2004). In vivo studies by D. W. Manning et al. showed that its wear rate is reduced by 95% compared to conventional UHMWPE (Manning et al., 2005). However, a little earlier J. M. Martell et al. reported a 45% reduction in wear rate (Martell et al., 2003). A significant difference in these results, apparently, can be explained by a short period of research time. But, at the same time, there is no doubt the fact of decreasing the wear rate of XLPE compared to conventional UHMWPE. In addition, in these and other studies, a decrease in the biological activity of wear particles was found, as well as a sharp decrease in osteolysis (Muratoglu et al., 1999, 2004; Chiesa et al., 2000; Collier et al., 2003).
In addition, to stabilize cross-linked UHMWPEs, there is a technology for adding the antioxidant vitamin E, which allows for increased oxidation resistance and fatigue resistance, but long-term studies are still needed to establish the effectiveness of vitamin E as an effective antioxidant in vivo (Oral and Muratoglu, 2011).
Another remarkable property of XLPE regarding the tribological aspect was discovered by Kyomoto et al. (2007). It turned out that poly (2-methacryloyloxyethyl phosphorylcholine) (PMPC), resulting from photo-induced polymerization, creates a super-lubricating layer similar to articular cartilage (Kyomoto et al., 2009). As a result of tests on a hip simulator, it was found that PMPC grafted onto an XLPE surface significantly reduces wear over 70 million cycles (Ishihara, 2015). But, as noted, for example, in Scemama et al. (2017), long-term observations are still required to guarantee that reduction of XLPE wear will lead to a decrease in the number of cases of osteolysis, leading to aseptic loosening of total hip joint stems. Nevertheless, this direction of total hip joint improving in the scientific environment is considered to be very promising and many studies devoted to it have been carried out recently by various scientific groups (Galliera et al., 2018; Lambert et al., 2019; Massaccesi et al., 2019; Oral et al., 2019; Terkawi et al., 2019; Xu J.-Z. et al., 2019).
In addition, it is also necessary to point out the technology of reinforcing UHMWPE with carbon nanotubes, which allows improving mechanical characteristics and reducing the wear factor compared to that of UHMWPE and improving cytocompatibility comparable to that of UHMWPE. However, several animal studies have noted the adverse effects of carbon nanotubes on lung, liver, and kidney tissue (Ruan et al., 2003; Kanagaraj et al., 2010).
It should also be noted that Polyetheretherketone (PEEK) material, which has a number of advantages that allowed it to occupy a certain place among materials for medical use as an alternative to implants based on titanium and ceramics in cranial, maxillofacial, orthopedic and vertebral operations. In addition, it showed very high tribological properties in in vitro trials (Wang et al., 1998). But clinical data on its use in THR designs experiencing large cyclic and dynamic loads on their elements are absent, probably due to the fact that it has the main drawback in this case in the form of low mechanical strength (Panayotov et al., 2016).
However, as shown in Zhao et al. (2000), Gao et al. (2010), and Pakhaliuk et al. (2016), one can also reduce wear in friction coupling with UHMWPE or XLPE by mechanically modifying the surfaces of friction couples, including by applying regular micro texture in form, for example, of dimples on the surfaces of total hip joint elements. This effect can be explained, for example, from the standpoint of the molecular-mechanical theory of I.V. Kragelsky, according to which, with dry and boundary friction, the friction force consists of two components: molecular (adhesive) and deformation (Kragelsky et al., 1982). For metal surfaces with elastic contact, the molecular component exceeds the mechanical component by almost 100 times, and for polymers this ratio is in the range of 20–30.
It is known that the region of space between the surfaces of interacting solids is filled with a thin film from a few tens to hundreds of nanometers thick, called the “third body,” consisting of an adsorbed layer, chemical compounds, a loosened layer and the main material. With a tangential displacement of one body relative to another, the “third body” is in a continuous formation mode and, like a liquid, as if “flows” in a narrow gap between the bodies.
In accordance with the hypothesis of film starvation, protective layers of the “third body” are simultaneously formed and destroyed on two contacting surfaces in the sliding direction (Kragelsky and Gitis, 1987). If its destruction rate is less than the recovery rate, then there is no film starvation and the surfaces are protected from direct contact, which leads to the bonding of materials. Hence, the longer is the friction path, the higher is the chance of unprotected contact. Given that the film (“third body”) formation time is of the order of 10−8–10−4 seconds (Kragelsky et al., 1982), it can reasonably be assumed that a decrease in the sliding path on the contact surface of the discrete structure will help to prevent depletion of the lubricant layer.
On the other hand, when exposed to an external load, due to the deformation of the contact surface with the micro texture, the volume of the dimples decreases and, if they are completely filled, the liquid inside them is compressed, perceiving a part of the current load, which leads to a decrease in the load perceived directly by the contact surfaces, and, consequently, to a decrease in the intensity of wear, everything else being equal. In addition, separately located dimples closed from the external area of the total hip joint create artificial lubrication pockets. Their presence prevents the adhesive setting of the mating elements of the friction couple, promotes the removal of wear products from the contact zone for a long time inside the dimples, and not into the tissues surrounding the joint, thereby delaying the occurrence of osteolysis, and feeds the frictional contact with a portion of the lubricant as it operates.
Thus, the micro texture in the form of micro dimples, created, for example, on the surface of the total hip joint head, hypothetically contributes to the improvement of the tribological properties of the friction couple, and, thereby, to the increase of its service life. The results of numerical simulations performed by the authors of this work (Pakhaliuk et al., 2016) generally confirm this hypothesis and indicate the relevance of the development of this area of research, focused on reducing the wear of total hip joint friction couples, and as a consequence, the likelihood of complications after THA.
It should be noted that even the minimal wear of materials observed in friction couples of the type metal (ceramic) on UHMWPE or metal (ceramic) on XLPE can lead to a decrease in the durability of total hip joint. This is because osteolysis, in combination with an allergy to metal ions, can lead to loosening of a total hip joint elements and the need for revision arthroplasty. Analysis of the results of THA shows that more than 50% of unsuccessful outcomes are mainly associated with the occurrence of precisely these processes in patients (Abu-Amer et al., 2007).
One of the urgent directions aimed at solving this problem is to develop coatings of the metal surfaces of total hip joint friction couples with very hard biocompatible surface layers, such as diamond-like carbon (DLC) (Narayan, 2005), pure nanocrystalline diamond (Catledge et al., 2011), titanium nitride (TiN), titanium niobium nitride (TiNbN), titanium carbonitride (TiCN) (Pappas et al., 1995; Serro et al., 2009).
It is believed that such an approach eliminates the release of toxic metal ions from Ti-based alloys. However, as it turned out in practice, concomitant problems may arise during its implementation, accompanied by local delamination of the coating, crevice corrosion, wear of the third body (Pappas et al., 1995; Hauert et al., 2012), low adhesion of the diamond coating to the substrate material (Vila et al., 2006), and other processes. But these problems are of a pronounced technological nature and, therefore, in the development of appropriate technologies can be eliminated.
In this case, we can mention as a promising technology of thermohydrogen processing (THP), which is a powerful method for changing the microstructure/properties of deformable, cast and powder metals of titanium alloys, composites and intermetallic compounds. This method is based on the temporary alloying of titanium alloys with hydrogen, which, being a non-stabilizer, modifies the phases and kinetics of phase transformations and allows the development of new thermal and thermomechanical processes to improve machinability and manufacturability, grinding and improving the mechanical properties of the final parts. This can create a gradient structure in the material that provides increased resistance to static and cyclic loads, high resistance to wear and fretting-corrosion, and thereby create a highly efficient friction couple based on a titanium alloy (Froes et al., 2004).
An alternative to a friction couple with UHMWPE or XLPE in the near future may be a ceramic on ceramic couple, the use of which will significantly reduce the number of significant complications of THA and, as a consequence, increase the durability of a total hip joint, which is confirmed by a number of in vivo studies (Kusaba et al., 2011; Murphy et al., 2011). Currently, this couple has been upgraded to fourth-generation ceramics using nanoscale zirconia particles stabilized with yttrium (25%), which are dispersed in an alumina matrix (74%) together with strontium (1%) to suppress crack propagation and, thus, higher strength (Chevillotte et al., 2011). However, despite the undoubted advantages of such a friction couple compared to others, it has a significant lack associated with the high probability of total hip joint revision arthroplasty due to the fragile fracture of its ceramic elements (Filippenko, 2013; Chang, 2014; Kumar et al., 2014), mainly in the tapered connection of the head with the neck of total hip joint stem when significant dynamic loads occur.
It should be noted that despite the fact that a lot of research has been devoted to this problem, its effective solution has not yet been found and is still relevant. From this point of view, various design solutions or technological methods may turn out to be effective, such as the total hip joint design, which provides high-quality fixation of the ceramic head on the cylindrical surface of the stem neck (Korzh et al., 2007), technology, titled as Oxinium, which, by thermal diffusion of oxygen, turns the surface of a metal zirconium alloy (97.5% zirconium and 2.5% niobium) into a durable oxide with a low coefficient of friction. This oxidized layer is not a ceramic coating, but represents a surface transformation of a thickness of 5 to 10 microns, and is much harder and more scratch-resistant than a raw alloy (Good et al., 2003; Smith and Nephew, 2019).
As well as the technology, proposed by the authors of this work, which goes through the process on patenting for an invention. They at first time have developed a combined head for modular THR, in which the external ceramic element (head) and the internal metal element (sleeve) are fixed motionless by soldering, which allows them to be connected securely. Alumina or zirconia ceramics can be used as an external ceramic element, and a Ti-based alloy can be used as an internal metal element, with which the head is then connected to the tapered neck of THR stem. At the same time, the developed brazing technology allows biocompatibility of a brazing alloy. Using a head of this design will allow not only to realize the main advantages of a ceramic-ceramic friction couple in THR, but also ensure that there are no breaking loads for ceramics at the tapered place of the head and stem neck, as well as undesirable physicochemical processes in this conjunction, for example, fretting-corrosion which is also known to be present and in the existing commercial THRs with ceramic head and Ti-based alloy stem (Kurtz, 2013). The indicated advantage is due to the presence of conjugated homogeneous biocompatible metal materials (Ti-based alloy) and the manufacture of a conical head/neck conjunction that provides a sufficient press fit connection under their minimum relative micro motion during operation to prevent crevice fretting-corrosion.
It should also be noted a ceramic on metal couple (CoM), in which a ceramic femoral head is connected to a metal acetabulum. This couple was developed with the expectation that it will have advantages both in lower risk of squeaking and in component destruction compared to ceramic on ceramic (CoC) couples, and less acetabular wear and the formation of metal wear debris compared to with a metal on metal couple (MoM). According to in vivo studies, CoM couples showed reduced wear, produce lower levels of metal ions than comparable MoM couples, but both couples are said to be associated with an equivalent increase in serum cobalt and chromium levels and comparable functional outcomes after 6 and 12 months' observations (Schouten et al., 2012; Williams et al., 2013).
It is especially necessary to highlight a couple of single sapphire crystal on single sapphire crystal. The use of sapphire as a friction couple was studied at the Academy of Medical Sciences of Ukraine, Kharkov. They noted that the contact surfaces of sapphire friction couples have a rather low and stable coefficient of friction (0.05–0.10), as well as extremely high wear resistance. They also found that sapphire is biocompatible, inert, and affordable. Sapphire heads implanted in 5 patients showed no complications for 5 years. Reduced friction and wear combined with high biochemical inertness, biocompatibility and low cost make sapphire couple attractive for THA in the future (Mamalis et al., 2006; Chang, 2014; Turmanidze and Popkhadze, 2016).
Further in this study, attention will be paid to the analysis of the latest technologies in the field of joint arthroplasty, such as osseointegration of artificial joints, improvement of materials with the property of osteoimmunomodulation, improvement of joint arthroplasty technologies based on modeling of processes of dynamic osteosynthesis, as well as identification of possible unconventional designs of artificial joints contributing to these technology. The search was performed in the Web of Science Core Collection for the keyword “Osteoimmunomodulation.” As a result, 24 sources were found, published from 1975 to 2019. Of these, 2 were selected for this review, which were review articles on this topic (Chen et al., 2016, 2017a). Given that the study of osseointegration processes has a long history, we limited the search for sources on this topic to 5 years (from 2015 to 2019). The search was performed on the string “Osseointegration. A Review.” In this case, 422 records were identified, of which 47 were selected for the initial analysis as the source belonged to the Materials science biomaterials section. It should be noted that none of these sources analyzed current trends in the development of biomaterials with the property of osseointegration directly for artificial joint elements. Nevertheless, the research results presented in them allow us to make fairly confident predictions regarding the creation of innovative artificial joints that can integrate with bone structures.
To search for non-traditional designs of artificial joints, attention was focused on THR, where implant designs with a closed friction pair, both with lubricant placed inside and without it, were taken as the selection criterion, but the closed design should not allow releasing wear debris into the periprosthetic tissue. A hollow head implant design was also in seeking. Search was carried out among patents for inventions in Google Patents, US Patent Office (US), European Patent Office (EPO), Federal Service on Intellectual Property (RU), and Ukrainian Institute of Intellectual Property (UA). By the specified criterion, only a few patents were found, which are analyzed below.
Improvement of Materials for the Best Osseointegration of Artificial Joints
The operation conditions of artificial joints in the human body suggest that the biomaterials from which their elements are made certainly should not interact with the biological system. However, this is not the only requirement for them. Biomaterials must be non-toxic and biocompatible so as not to cause inflammatory reactions of living tissues to the implant introduction into the body. But on the other hand, they must be bioactive, contribute to the regeneration and integration of living tissues in their structure. And finally, artificial joints biomaterials should ideally have special mechanical characteristics similar to those of bone tissues, which are characterized by a unique structure, relatively low modulus of elasticity, and high static and fatigue strength.
Materials such as titanium, tantalum, magnesium, and a number of others may well be classified as biomaterials, because they are fully biocompatible. They have been widely used for a sufficiently long period of time for the manufacture of load-bearing elements of various artificial joints, including artificial hip joints. However, as the operating experience of such products testifies, in the normal (dense) state they are characterized by low osseointegrating ability, which over time, under the influence of variable dynamic loads on the implant, leads to its loosening and, as a result, to revision arthroplasty. In addition, in the dense state, almost all metal biomaterials have a significantly higher modulus of elasticity than bone tissues, which contributes to the redistribution of stresses and the formation of fibrous tissue on the implant surface (Wen et al., 2002).
In order to eliminate these shortcomings, it is currently proposed to use porous biomaterials, which, in comparison with dense ones, have a number of significant advantages. First of all, this is due to the peculiarities of their structure, which allows bone tissues to grow into the pores and form a single biomechanical system with an implant that is characterized by increased stability. Moreover, such characteristics of the porous material as pore size and porosity are important. The results of a number of experimental studies have shown that the optimal pore size to ensure vascularization and bone ingrowth into the implant should be in the range of 100–400 microns (Bobyn et al., 1980). Moreover, for the formation of a porous structure, it is desirable to use a metal powder with the smallest possible particle size, which allows to increase the surface energy of the material and, as a result, its ability to induce apatite (Chen et al., 2009a).
However, to obtain such a structure by the traditional method of powder sintering is very difficult, since it is necessary to ensure its compression, which leads to a decrease in porosity and quality of the porous biomaterial, since an indicator such as interconnectedness, which plays an important role in ensuring the transport of nutrients through the pores, is deteriorating (Wang et al., 2009). Another problem is that the technological compression necessary to obtain porous biomaterial leads to an increase in its elastic modulus, which, as is known, decreases with increasing porosity (Niu et al., 2010).
The above problems can be avoided by using alternative technologies, the development of which has been intensively carried out in recent years. The production of porous metal biomaterials with a given structure can be implemented based on the Space Holder Method (Wen et al., 2001; Esen and Bor, 2007), Spark Plasma Sintering Method (Miyao et al., 2000; Watari et al., 2002), Microwave Sintering Method (Upadhyaya et al., 2007; Tang et al., 2013), Combustion Synthesis Method (Li et al., 2000; Ryan et al., 2006), Metal Injection Molding Method (Chen et al., 2009b), Capsule-Free Hot Isostatic Pressing Method (Zhang et al., 2007), Solid State Isothermal Foaming Technique (Nugroho et al., 2010) and other methods. Due to this, in recent years, many modifications of porous biomaterials have been created, the use of which in artificial joints has confirmed their advantages over dense materials.
In recent years, Ti-based alloys have been widely used as porous biomaterials (Mediaswanti et al., 2013; Li et al., 2014). Such alloys can have a different internal structure, which allows providing close to the desired mechanical properties, low weight and improved osseointegration. In addition, they are effective for delivering drugs to the field of implantation and metabolism.
At the same time, it is necessary to note that new design solutions are currently being used to achieve these goals, protected by patents on invention. Some of them are a THR friction couple design in the form of a closed bellows made of a biocompatible material with lubricant placed inside. This allows, according to the authors, to improve the lubrication of the implant and virtually eliminate the release of wear debris into the periprosthetic tissues (Anstaett et al., 1998; Pashkov et al., 2016, 2018a,b). But there are also lubrication-free designs in which the bellows consists of a membrane with one-sided penetration, through which the lubricant from the joint capsule penetrates inside, and wear products are retained by it internally (Kevin, 1996; Dietmar, 2000). Moreover, in designs with lubricant to improve the tribological properties of the specified couple in the lubricant may contain graphene, as discussed in Puértolas and Kurtz (2014). But clinical data on the use of these solutions in the design of THR is currently not available.
In addition, the design of a fluidfilled unipolar hip joint replacement is also known (Volkov et al., 2011). The idea of its creation arose after a series of successful laboratory and clinical trials of unipolar hip joint replacement with a hollow head (Poliakov et al., 2012), which showed that the best results of unipolar hip joint arthroplasty using this artificial hip joint design are achieved based on a personalized approach, which involves the implementation of several steps illustrated in Figure 1. It turned out that such artificial hip joint is very effective in the treatment of elderly patients due to minimal trauma during implantation. In addition, a decrease in the mass of the hollow head compared to the solid one improves the dynamics of the patient's walking after joint arthroplasty thereby increasing the lifespan of the implant itself and improving the quality of life of the patient. At the same time, the process of personalized joint arthroplasty includes a number of stages, at each of which local problems of diagnosis, design, modeling, technology, production and implantation of an artificial joint are solved, the sequence of which is illustrated in Figure 2.
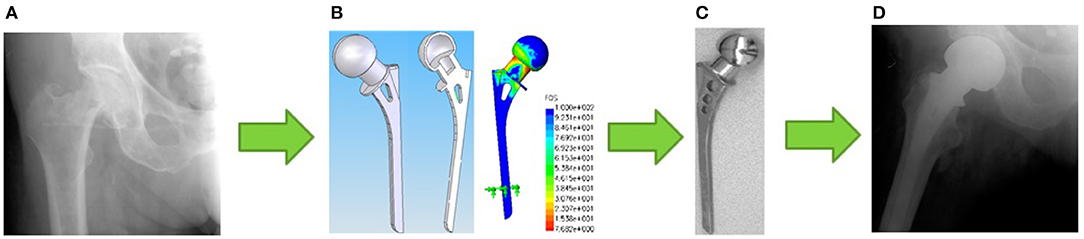
Figure 1. Illustration of the stages of a personalized unipolar hip arthroplasty: (A) the initial stage at which a decision is made on the strategy of surgical intervention; (B) the modeling and development phase of the artificial hip joint personalized design; (C) the manufacturing step of the personalized artificial hip joint; (D) stage of unipolar hip arthroplasty.
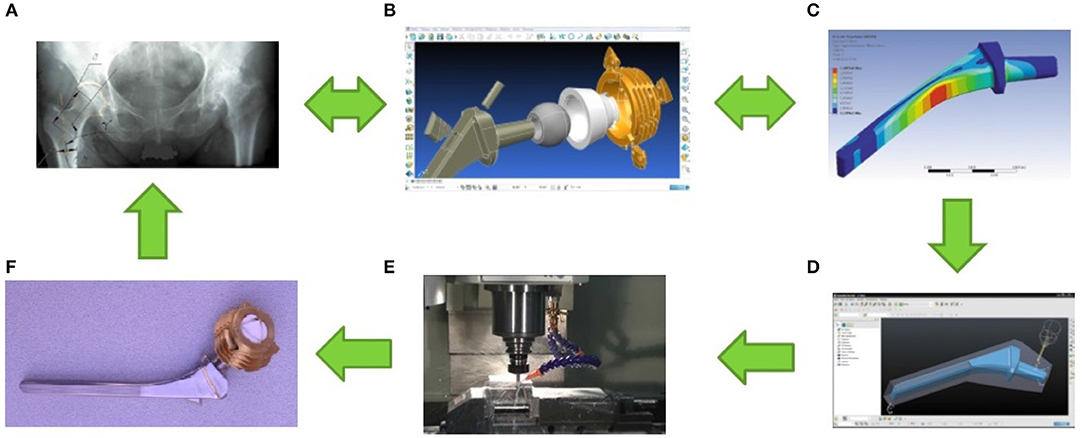
Figure 2. Illustration of the stages of a personalized total hip arthroplasty. (A) The stage of diagnosis and decision-making on the treatment strategy for the disease. At this stage, the overall functional state of the patient, the nature, stage of the disease, condition and geometry of the bone structures in the vicinity of natural joint are evaluated. (B) The design phase. At this stage, the choice of biomaterial that is optimal for the manufacture of artificial joint elements and treatment of the disease in this physiological condition of the patient and his bone tissue is made; personalized artificial joint is being developed in accordance with natural joint geometry. (C) The stage of modeling and analysis of the functioning of artificial joint as part of a biotechnological system. At this stage, the stress-strain state of the artificial joint elements is assessed under extreme loads, taking into account the particular state of the bone structures of a particular patient; if necessary, changes are made to the design of artificial joint in order to increase the strength/stiffness of its elements. (D) Stage of development of manufacturing technology of artificial joint elements. At this stage, the dimensions and manufacturing technology of blanks for artificial joint elements are determined; the allowances necessary to achieve the required product quality are calculated; adjustments are made to the software of technological equipment for the manufacture of all elements of a personalized artificial joint. (E) Production phase of personalized artificial joint. At this stage, all artificial joint elements are manufactured and assembled; tests for compliance with mechanical and toxicological indicators are performed; sterilization of the product is in progress. (F) The stage of implantation of a personalized artificial joint. At this stage, artificial joint implantation is performed, the real state of the bone structures of a particular patient is assessed, and the postoperative procedures necessary for optimal integration of the implant into the bone structures are planned.
Laboratory tests have shown that from the point of view of strength and stability, the shell of the spherical head of the hip replacement can be made so thin that it can change its shape under load, adapting to the shape of the acetabulum. It is this circumstance that predetermined the creation of a hydro filled prosthesis in which the fluid placed inside the head may contain fullerenes that provide the best tribological characteristics of the joint and drugs necessary for better adaptation of the implant in a living organism. The release of these substances from the hollow head can be organized in various ways according to need, including creating even the certain micro holes texturing on the hip head surface.
An analytical review of implant development trends for total arthroplasty of large joints showed that additive technologies for their manufacture have certain prospects and, to a certain extent, this is due to the ability to produce biomaterials with a given structure. So, for example, porous Ti6Al4V implants made by the Selective Laser Melting (SLM) method, after surface treatment, can acquire the desired properties with respect to roughness and wettability. In addition, such materials may have capillarity, which allows transporting fluid along their structure, do not release toxic substances into the body, and therefore provide a suitable environment for cell adhesion and proliferation (Bartolomeu et al., 2019).
The methods of 3D printing of orthopedic products made of polymers by the Fused Deposition Modeling (FDM) method and metals using Direct Metal Laser Sintering (DMLS), developed in recent years, have opened up new possibilities for creating personalized implants (Wong and Scheinemann, 2018; Dall'Ava et al., 2019; Timercan et al., 2019), which, moreover, can be very effective from the point of view of drug delivery to the area of their implantation (Benmassaoud et al., 2019). Despite the fact that additive manufacturing techniques for implants are in the initial stage of development, it can be argued that in the future they will be a powerful means of creating structures with a porous structure, optimal not only in relation to mechanical properties, but also to biocompatibility, bioactivity, and, if necessary and biodegradability (Yuan et al., 2018). Currently, studies of such structures during the replacement of various parts of the body are accumulating, their strengths and weaknesses are being evaluated, and the prospects for their use and improvement from the point of view of orthopedic surgeons are being actively discussed (Han et al., 2019).
The use of porous biomaterials made it possible to significantly increase the osseointegration of artificial hip joint elements; however, as it turned out, it is necessary to carry out a large number of studies aimed at optimizing the internal structure of such materials. From our point of view, such a structure should be partially ordered with mechanical properties varying in depth, i.e., gradient ones (Rabin and Shiota, 1995). When creating it, great importance should be given to the possibility of obtaining materials possessing the property of osteoimmunomodulation (Chen et al., 2016, 2017b).
Improvement of Materials With Osteoimmunomodulation Properties
One of aspects of THA technology is related to bone trauma due to the need to resection the femoral head and prepare surfaces for the installation of artificial hip joint elements. It is assumed that over time, the regeneration of damaged tissues takes place and the implant together with the biological system forms a new biomechanical system. In many cases, such a system is sufficiently reliable and capable of performing its functions for a long time under the influence of multifaceted factors. However, it cannot be considered stable, because even minor disturbances can bring it out of equilibrium and, as a result, lead to all sorts of complications.
First of all, this is due to the fact that at the modern level of technological development, any implant introduced into the biological system (including artificial hip joint) is recognized by the immune system as a foreign body, which leads to the launch of an extensive immune response, which in vivo leads to its rejection (Chen et al., 2017b). This fact determines the motivation for creating a new generation of artificial joint, which ideally would not be rejected by the immune system. It is expected that the most effective solution to this problem can be obtained on the basis of a new generation of biomaterials with the property of osteoimmunomodulation (OIM) and their use in new THA technologies. In this case, the latest advances in cell technology can be used, which are currently already used in bone tissue regeneration.
Usually, when creating biomaterials with indicated properties, many variations are analyzed that affect the osteogenic differentiation of the osteoblast line in vitro, and the potentially osteogenic biomaterials thus determined are investigated in vivo (Chen et al., 2016). But this approach rarely leads to a positive result, because it does not take into account the close relationship of skeletal and immune systems with each other (Takayanagi, 2007).
Effective new generation biomaterials should be able to modulate the local immune environment, which would contribute not only to osseointegration of the implant, but also to osteogenesis in the area of its installation. For their development, it is extremely important to find out the relationship between immune and bone cells, evaluate the effect of the immune system induced by the implant on the process of osteogenesis, and also study the mechanisms underlying the implant-mediated immune response.
Modern osteoimmunology considers osteogenesis mediated by biomaterial as an interactive process in which at least three components participate: the bone and immune cells of the host, as well as the biomaterial proper, taking into account its characteristics and internal structure. It is known that immediately after implant placement, the host immune system includes a universal response in response to damage to living tissues (Brown and Badylak, 2013). In this case, proteins from the blood and intercellular fluids adsorb very quickly on the surface layers of the biomaterial and form a transitional surface matrix. The reaction to this is the activation of coagulation cascade and complement systems, leading to the formation of a blood thrombus and activation of other cell populations (Takayanagi, 2007). As a result, acute inflammation begins in the initial period of blood-biomaterial interaction, including the recruitment and activation of neutrophils or polymorphic-nuclear leukocytes, which, in turn, tend to destroy the biomaterial, releasing proteolytic enzymes and reactive oxygen species that react with the surface of the biomaterial (Kobayashi et al., 2005). But they are quickly depleted, undergo apoptosis and disappear from the implant site (Anderson et al., 2008). In addition, mast cell degranulation affects the inflammatory process, which leads to the release of cytokines and histamine, which enhance the immune response (Tang et al., 1998).
At the same time, it was found that molecules released during the interaction of biomaterial with living tissues can positively regulate osteogenic differentiation by forming a new bone on the surface of the implant, which is actively remodeling in this phase and, in particular, can be destroyed. The main reason for remodeling is functional and mechanical stress. Moreover, signals associated with mechanical stress are translated by osteocytes into biochemical signals that maintain the balance of osteoblasts and osteoclasts, playing a regulatory role at this stage of implant survival (Chen et al., 2014).
Thus, it can be assumed that the observed relationship between the skeletal and immune systems can contribute to different outcomes of implantation of biomaterials, leading to both successful and unsuccessful results. A positive immune response will contribute to the formation of an osteogenic environment in the area of implant placement, a negative one–to extensive inflammation with the formation of a fibrous capsule that separates the implant from living tissues. In this state, the implant may remain in the host biological environment for a sufficiently long time, but it will not be properly integrated into this environment. Contacting with bone tissue under the action of mechanical loads, it will deform them and stimulate an increase in the implantation space, which can subsequently lead to loosening and, as a consequence, to revision arthroplasty.
Obviously, biomaterials themselves with the OIM property cannot be used for the manufacture of artificial hip joint elements, since do not have sufficient mechanical strength. At least there is currently no information on such materials. However, their ability to induce a favorable immune environment in the area of implant placement can be used to provide better survival of artificial joint. In this regard, the promising THA technologies are considering the possibility of combined use of artificial hip joint together with biodegradable extracellular matrices of biomaterials that contribute to the induction of a favorable immune environment in the area of implant placement. It is clear that the biomaterials of the artificial hip joint elements used for this should provide the possibility of sufficient integration of bone tissues into them.
“50 active years after 50.” So say experts from the Institute of Biomedical Engineering of the University of Leeds, John Fisher, Eileen Ingham et al. developing for 5 years a project to find innovative solutions to increase the active life of people who are already over 50. They plan to provide retirees with their own tissues and long-lasting implants. At the same time, the main emphasis is not on the development of new designs of artificial implants from traditionally used materials, improving materials or their design. It is aimed at developing technologies for growing tissues to replace worn out or broken elements of the human body, including joints, based on scaffolds that are neutral for the immune system, for example, tissues of animals that carefully remove their cells using a mixture of enzymes and detergents. This scaffold can then be transplanted to the patient without fear of rejection—the main reason normal grafts wear out and fail. As soon as the scaffold is transplanted, the body takes possession of it and populates it with cells. But according to the project authors, it will take from 30 to 50 years to replace all the necessary tissues using this technology. Each individual product will need to be developed and tested individually (Roberts, 2009).
Improvement of Technologies of Joints Arthroplasty Based on the Simulation of Dynamic Osteosynthesis Processes
In recent years, methods have been developed for modeling processes of osteosynthesis and bone tissue regeneration, for example Viceconti et al. (2000). Among these methods, the most simple and affordable methods for implementation can be distinguished including when exposed to physical stimuli of various nature. The new knowledge gained and their use in the development of new THA technologies will allow us to approach the creation of artificial hip joint close to the “ideal.”
Currently, the fact that osteosynthesis is influenced by dynamic effects applied to the damaged segment of the skeleton is not questioned. Moreover, the greatest effect is achieved under the action of high-frequency alternating loads (Goodship et al., 2009). In addition, as a result of a number of experimental studies, it was found that for the initiation of osteosynthesis processes, it is necessary to ensure deformations in which the formation of new tissues prevails over resorption. These and other studies made it possible to formulate a number of hypotheses about the influence of physical stimuli on the processes of osteosynthesis, the creation and study of computer models that adequately reflect the processes of dynamic osteosynthesis.
One of the first significant results in this area was obtained by Pauwels (1980) on the basis of the development of methods for assessing the stress state of bone tissues under mechanical stimuli, which made it possible to formulate the hypothesis that the shear components of the tensors of elasticity and deformation are a stimulus for the formation of fibrous tissue (collagen fibers), and hydrostatic components determine the formation of cartilage tissue. An important role in understanding the processes of osteosynthesis was made by Carter (1987), Carter and Wong (1988), and Carter et al. (1998). They managed to create plausible mathematical models of osteogenesis that take into account the history of loading, in which the osteogenic stimulus transmitted through chondroepiphyses was calculated in accordance with the hypothesis that cyclic octahedral shear stresses contribute to endochondral ossification and cyclic compression dilatation stresses that prevent ossification. The distributions calculated in this way for the osteogenic stimulus were used by the authors to predict the appearance of the secondary ossific nucleus and the shape of the developing bone epiphysis.
As mechanical stimuli regulating osteosynthesis, D. R. Carter and colleagues used positive hydrostatic stress and alternating octahedral shear stress for each type of load. It was shown that their combination allows the calculation of the so-called “Osteogenesis index,” taking into account the number of loading cycles, hydrostatic and octahedral stresses, normalized using some empirically determined constant. At the hypothesis level, it can be assumed that large index values correspond to the process of transformation of cartilage into bone (the so-called endochondral bone formation), and low ones support the development of cartilage, delaying the formation of bone tissue.
As an alternative, Huiskes et al. (1997) proposed using a “dimensionless mechanical regulatory index” in research models, which allows one to determine the phenotype of tissue formed at a certain point in the environment in response to mechanical stimulation and, accordingly, to predict and control the process of remodeling of undeveloped connective tissue in dense cartilage or bone tissue. To determine the index value, the maximum value of the octahedral shear deformation of the elastic frame of the two-phase medium and the maximum value of the interstitial fluid flow rate in the pores normalized using empirical constants are summarized. These values can be calculated using various mathematical models or obtained experimentally.
The use of the “dimensionless mechanical regulatory index” suggests that cells in each region can differentiate into cells of the main tissue types: fibroblasts, chondrocytes, and osteoblasts depending on the average mechanical state of the studied region and that each cell type produces an elastic matrix of two-phase material with certain elasticity moduli.
It should be noted that in the scientific literature one can still find many attempts to represent and simulate the processes of osteosynthesis under the influence of various types of physical stimuli on biological tissues. But their detailed critical analysis is the subject of a separate article directly devoted to the study of this issue. Here, one cannot fail to note the significant contribution to the modeling of processes of structural restructuring of bone tissue under the action of external mechanical stimuli of a periodic nature, which was made by L. B. Maslov, who developed and studied a generalized dynamic model of a changing poroelastic continuous medium and a mathematical algorithm that conceptually describes this process (Maslov, 2013). One of the most important qualities of this model is that it makes it possible to justify the choice of the optimal periodic effect on damaged tissues with a view to their speedy and sustainable healing. With its help, the author was the first to investigate the influence of the frequency of the stimulating load on the process of tissue remodeling, as well as the effect of early loading on the restoration of the elastic properties of callus. But, as the author himself noted, the analytical solution of the equations of poroelasticity is valid only at constant coefficients, as a result of which the model of structural adjustment presented by him allows one to obtain only a plausible qualitative result. However, Maslov's model quite accurately describes the processes of bone tissue regeneration in the fracture zones and at present it can be considered one of the most adequate among the known ones. Currently, the authors of this review are developing a modification of this Maslov's model, but it will already be the material of another study.
It can be expected that a modification of this model can be successfully used to plan optimal physical effects, aimed at providing the best conditions for osteosynthesis near artificial hip joint and directly in the pores of the biomaterials from which its elements are made. In this context, it is also worth noting the numerical solution algorithms for solving a flat boundary-value problem aimed at predicting the occurrence of ossification zones during reparative regeneration in the area of fracture and healing of bone trauma around an orthopedic implant developed by D.R. Carter with colleagues (Giori et al., 1995).
Thus, a targeted modification of these algorithms, including the development of their spatial versions, will allow you to create the software necessary to develop optimal regenerative and rehabilitation strategies aimed at preventing implant rejection by the immune system.
Conclusion
In this paper, we analyzed the current state of the problem of improving artificial joint designs and their implantation technologies. The solution to this important scientific and technical problem is primarily due to the need to ensure a decent quality of life for a large number of patients who need treatment for diseases and injuries of the joints, the number of which is constantly growing due to many reasons as social, ecological, as man-made.
It should be noted that most artificial joints used in modern THA are high-tech devices that correspond to the latest achievements of science, engineering and technology. This allowed achieving very high indicators of the patient treatment quality and minimizing the number of complications leading to repeated surgical interventions. Total arthroplasty has become a routine surgical operation, the most effective in cases of the impossibility of conservative treatment of the disease.
However, any modern artificial joint is still a foreign body artificially introduced into the biological environment and its presence in a living organism, even in the most favorable cases, imposes certain restrictions on the motor activity of patients. They are not recommended to lift weights, perform sharp motor actions, limit their amplitude, etc. In addition, any artificial joint, unlike natural joint, has a limited resource, and the reasons for its failure are closer to technical than biological ones. Given this fact, the vast majority of artificial joints should be considered precisely as technical devices that are introduced into the biological system and together with it form a new biotechnical system. From this point of view, the artificial joint designer is in a better position than “nature,” since it does not have to fulfill the ontogenetic conditions inherent in living systems. But on the other hand, it is deprived of the ability to ensure the natural artificial joint operating, which, in the end, causes certain inconvenience to patients and can lead to premature failure of an artificial joint.
Nevertheless, it can be argued that the level of modern technology and the capabilities of technological equipment make it possible to manufacture artificial joints from virtually any material and provide it with any shape. This allows you to develop personalized technology for joint arthroplasty, focused on providing the best treatment conditions for a particular patient, taking into account his physiological condition. However, it should be recognized that personalized joint arthroplasty technologies can be accessible only to a limited number of patients, therefore, the trends in the development of THA technologies are more focused on the mass consumer, on the standardization of biomaterials, structures, methods, and means of postoperative rehabilitation. From this point of view, one of the most important is the creation of new biomaterials, which should ensure the strength of artificial joint, be biocompatible, bioactive and facilitate the integration of bone structures with its elements.
If you imagine some technology of arthroplasty close to ideal, then it must lead to the reproduction of the new natural joint. At first glance, this is a fantastic idea, but, nevertheless, quite feasible. An example here is the technology of tooth regeneration from stem cells, which have now received quite clear outlines (Thesleff, 2018; Balic, 2019; Rajabzadeh et al., 2019).
Imagine that in the long term some artificial joint can be created that replaces the damaged natural joint, made in the form of scaffold of bioresorbable high-strength biomaterial, capable of absorbing the effects of external loads at the initial stage. We also assume that under certain conditions, cells inducing bone growth in the desired direction can proliferate on the walls of this scaffold, which ultimately leads to resorption of the scaffold material and the formation of a new natural joint in its place. This idea, which is fantastic in its essence, is already finding practical application in THA technologies, but in a different quality. It is being implemented and will continue to develop on the basis of the creation of porous biomaterials with a special internal structure, mechanical and physicochemical properties. The characteristics of the porous materials based on titanium and tantalum that have already been created to date suggest that biomaterials with a porous gradient structure can also be created that have the OIM property and promote tissue regeneration in the area of implant placement. In new THA technologies, such materials can already be used now, at least in the form of artificial extracellular matrices, to populate stem cells that promote bone tissue regeneration.
No less important are the tribological properties of biomaterials, which to the greatest extent determine the quality of the artificial joint operating process and its durability. An analysis of the literature carried out in the framework of this work showed that friction couples in metal-metal or ceramic-ceramic type of an artificial joint have the best prospects, despite the undoubted advantages of the metal/ceramic-UHMWPE/XLPE couples currently widely used. At the same time, it should be noted that this friction couple will be in demand for a long time for THA, since it turns out to be the easiest to manufacture and, as a result, the artificial joints created on the basis of this couple have the greatest reliability compared to other designs. In addition, the durability of the metal/ceramic on UHMWPE/XLPE friction couple can be increased by modifying the surfaces, for example, by applying regular or partially regular texture on them.
In conclusion, we note that the artificial joints used to replace natural joints play an important role in THA. However, even the most technologically advanced artificial joint design alone does not determine the quality of THA. From this point of view, the quality of artificial joint implantation is particularly important, the provision of which to a large extent depends on the qualifications of the surgeon. In this regard, specialized devices play an important role in THA, allowing standardizing the technological operations of the surgical process, while reducing the risk of subjective errors of the surgeon. It is expected that the process of developing such devices will take place in a direction that minimizes the participation of the surgeon in THA, up to the complete robotization of this procedure.
Author Contributions
AP outlined the structure of this review. AP, VP, and VLP completed this review and drafted the manuscript.
Funding
The reported study was funded by Russian Fund for Basic Research (grant No. 20-03-00046A).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are deeply grateful to the researchers at the State Institute of Spine and Joint Pathology of the National Academy of Medical Sciences of Ukraine named after Sitenko to Professor A. N. Korzh and Professor V. A. Tankut, as well as Honored Doctor of Ukraine G. D. Olinichenko for predictive assessments of the development trends of joint arthroplasty based on the vast experience of their practical activities in this direction. The authors acknowledge support by the Open Access Publication Fund of TU Berlin.
References
Abu-Amer, Y., Darwech, I., and Clohisy, J. C. (2007). Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 9:S6. doi: 10.1186/ar2170
Affatato, S. (2014). Perspectives in Total Hip Arthroplasty, 1st Edition—Advances in Total Hip Arthroplasty (THA) Techniques and Biomaterials. Woodhead Publishing. ISBN: 9781782420316.
Affatato, S., Freccero, N., and Taddei, P. (2016). The biomaterials challenge: a comparison of polyethylene wear using a hip joint simulator. J. Mech. Behav. Biomed. Mater. 53, 40–48. doi: 10.1016/j.jmbbm.2015.08.001
Affatato, S., Leardini, W., Jedenmalm, A., Ruggeri, O., and Toni, A. (2007). Larger diameter bearings reduce wear in metal-on-metal hip implants. Clin. Orthop. Relat. Res. 456, 153–158. doi: 10.1097/01.blo.0000246561.73338.68
Affatato, S., Ruggiero, A., and Merola, M. (2015). Advanced biomaterials in hip joint arthroplasty. A review on polymer and ceramics composites as alternative bearings. Compos. Part B Eng. 83, 276–283. doi: 10.1016/j.compositesb.2015.07.019
Affatato, S., Spinelli, M., Zavalloni, M., Mazzega-Fabbro, C., and Viceconti, M. (2008). Tribology and total hip joint replacement: current concepts in mechanical simulation. Med. Eng. Phys. 30, 1305–1317. doi: 10.1016/j.medengphy.2008.07.006
Anderson, J. M., Rodriguez, A., and Chang, D. T. (2008). Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100. doi: 10.1016/j.smim.2007.11.004
Anstaett, G., Klardle, M. R., Folsom, A. C., and Czuwala, P. J. (1998). Implant module unit and rotating seal for prosthetic joint. US Patent No 5,755,807. Felham, AL: US Patent Office.
Balic, A. (2019). Isolation of dental stem cell-enriched populations from continuously growing mouse incisors. Methods Mol. Biol. 1922, 29–37. doi: 10.1007/978-1-4939-9012-2_4
Bartolomeu, F., Dourado, N., Pereira, F., Alves, N., Miranda, G., and Silva, F. S. (2019). Additive manufactured porous biomaterials targeting orthopedic implants: a suitable combination of mechanical, physical and topological properties. Mater. Sci. Eng. C Mater. Biol. Appl. 107:110342. doi: 10.1016/j.msec.2019.110342
Benmassaoud, M. M., Kohama, C., Kim, T. W. B., Kadlowec, J. A., Foltiny, B., Mercurio, T., et al. (2019). Efficacy of eluted antibiotics through 3D printed femoral implants. Biomed. Microdevices 21:51. doi: 10.1007/s10544-019-0395-8
Bhalekar, R. M., Smith, S. L., and Joyce, T. J. (2019). Wear at the taper-trunnion junction of contemporary ceramic-on-ceramic hips shown in a multistation hip simulator. J. Biomed. Mater. Res. Part B Appl. Biomater. 107, 1199–1209. doi: 10.1002/jbm.b.34213
Bobyn, J. D., Pilliar, R. M., Cameron, H. U., and Weatherly, G. C. (1980). The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin. Orthop. Relat. Res. 150, 263–270. doi: 10.1097/00003086-198007000-00045
Brown, B. N., and Badylak, S. F. (2013). Expanded applications, shifting paradigms and an improved understanding of host–biomaterial interactions. Acta Biomater. 9, 4948–4955. doi: 10.1016/j.actbio.2012.10.025
Carter, D. R. (1987). Mechanical loading history and skeletal biology. J. Biomech. 20, 1095–1109. doi: 10.1016/0021-9290(87)90027-3
Carter, D. R., Beaupre, G. S., Giori, N. J., and Helms, J. A. (1998). Mechanobiology of skeletal regeneration. Clin. Orthop. Suppl. 355, 41–55. doi: 10.1097/00003086-199810001-00006
Carter, D. R., and Wong, M. (1988). The role of mechanical loading histories in the development of diarthrodial joints. J. Orthop. Res. 6, 804–816. doi: 10.1002/jor.1100060604
Catledge, S. A., Vaid, R., Diggins, P., Weimer, J. J., Koopman, M., and Vohra, Y. K. (2011). Improved adhesion of ultra-hard carbon films on cobalt-chromium orthopaedic implant alloy. J. Mater. Sci. Mater. Med. 22, 307–316. doi: 10.1007/s10856-010-4207-1
Chang, J.-D. (2014). Future bearing surfaces in total hip arthroplasty. Clin. Orthop. Surg. 6, 110–116. doi: 10.4055/cios.2014.6.1.110
Charnley, J. (1961). Arthroplasty of the hip: a new operation. Lancet 1, 1129–1132. doi: 10.1016/s0140-6736(61)92063-3
Chen, L. J., Li, T., Li, Y. M., He, H., and Hu, Y. H. (2009a). Porous titanium implants fabricated by metal injection molding. Trans. Nonferr. Met. Soc. China 19, 1174–1179. doi: 10.1016/S1003-6326(08)60424-0
Chen, X. B., Li, Y. C., Hodgson, P. D., and Wen, C. (2009b). The importance of particle size in porous titanium and nonporous counterparts for surface energy and its impact on apatite formation. Acta Biomater. 5, 2290–2302. doi: 10.1016/j.actbio.2009.02.027
Chen, Z., Bachhuka, A., Wei, F., Wang, X., Liu, G., Vasilev, K., et al. (2017a). Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration. Nanoscale 9, 18129–18152. doi: 10.1039/c7nr05913b
Chen, Z., Klein, T., Murray, R. Z., Crawford, R., Chang, J., Wu, C., et al. (2016). Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 19, 304–321. doi: 10.1016/j.mattod.2015.11.004
Chen, Z., Wu, C., and Xiao, Y. (2017b). “Convergence of osteoimmunology and immunomodulation for the development and assessment of bone biomaterials,” in The Immune Response to Implanted Materials and Devices, ed B. Corradetti (Springer), 107–124. doi: 10.1007/978-3-319-45433-7_6
Chen, Z., Wu, C., Yuen, J., Klein, T., Crawford, R., and Xiao, Y. (2014). Influence of osteocytes in the in vitro and in vivo β-tricalcium phosphate-stimulated osteogenesis. – J. Biomed. Mater. Res. Part A 102, 2813–2823. doi: 10.1002/jbm.a.34954
Chevillotte, C., Pibarot, V., Carret, J. P., Bejui-Hugues, J., and Guyen, O. (2011). Nine years follow-up of 100 ceramic-on-ceramic total hip arthroplasty. Int. Orthop. 35, 1599–1604. doi: 10.1007/s00264-010-1185-3
Chiesa, R., Tanzi, M. C., Alfonsi, S., Paracchini, L., Moscatelli, M., Cigada, A., et al. (2000). Enhanced wear performance of highly crosslinked UHMWPE for artificial joints. J. Biomed. Mater. Res. 50, 381–387. doi: 10.1002/(sici)1097-4636(20000605)50:3<381::aid-jbm12>3.0.co;2-p
Collier, J. P., Currier, B. H., Kennedy, F. E., Currier, J. H., Timmins, G. S., Jackson, S. K., et al. (2003). Comparison of cross-linked polyethylene materials for orthopaedic applications. Clin. Orthop. Relat. Res. 414, 289–304. doi: 10.1097/01.blo.0000073343.50837.03
Dall'Ava, L., Hothi, H., Di Laura, A., Henckel, J., and Hart, A. (2019). 3D printed acetabular cups for total hip arthroplasty: a review article. Metals 9:729. doi: 10.3390/met9070729
Digas, G., Kärrholm, J., Thanner, J., Malchau, H., and Herberts, P. (2004). Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin. Orthop. Relat. Res. 429, 6–16. doi: 10.1097/01.blo.0000150314.70919.e3
Esen, Z., and Bor, S. (2007). Processing of titanium foams using magnesium spacer particles. Scr. Mater. 56, 341–344. doi: 10.1016/j.scriptamat.2006.11.010
Filipenko, V. A., and Tankut, A. V. (2009). Evolution of the problem of joint grafting. Int. Med. J. Orthopaedics (Kharkiv). 1, 70–74. (in Russian).
Filippenko, V. A. (2013). To help a practicing doctor. Lectures. - orthopaedics, traumatology and prosthetics (Ukraine). 3, 66–69. (in Russian).
Froes, F. H., Senkov, O. N., and Qazi, J. I. (2004). Hydrogen as a temporary alloying element in titanium alloys: thermohydrogen processing. Int. Mater. Rev. 49, 227–245. doi: 10.1179/095066004225010550
Galliera, E., Ragone, V., Marazzi, M. G., Selmin, F., Banci, L., and Romanelli, M. M. (2018). Vitamin E-stabilized UHMWPE: biological response on human osteoblasts to wear debris. Clin. Chim. Acta. 486, 18–25. doi: 10.1016/j.cca.2018.07.012
Gao, L., Yang, P., Dymond, I., Fisher, J., and Jin, Z. (2010). Effect of surface texturing on the elastohydrodynamic lubrication analysis of metal-on-metal hip implants. Tribol. Int. 43, 1851–1860. doi: 10.1016/j.triboint.2010.02.006
Giori, N. J., Ryd, L., and Carter, D. R. (1995). Mechanical influence on tissue differentiation at bone-cement interfaces. J. Arthroplasty. 10, 514–522. doi: 10.1016/S0883-5403(05)80154-8
Gluck, T. (1891). Referat uber die durch das moderne chirugische experiment gewonnen positiven resultate. Arch. Klin. Chir. 41:87.
Good, V., Ries, M., Barrack, R. L., Widding, K., Hunter, G., and Heuer, D. (2003). Reduced wear with oxidized zirconium femoral heads. J. Bone Joint Surg. Am. 85, 105–110. doi: 10.2106/00004623-200300004-00013
Goodship, A. E., Lawes, T. J., and Rubin, C. T. (2009). Low-magnitude high-frequency mechanical signals accelerate and augment endochondral bone repair: preliminary evidence of efficacy. Orthop. Res. 27, 922–930. doi: 10.1002/jor.20824
Han, Q., Wang, C. Y., Chen, H., Zhao, X., and Wang, J. C. (2019). Porous tantalum and titanium in orthopedics: a review. ACS Biomater. Sci. Eng. 5, 5798–5824. doi: 10.1021/acsbiomaterials.9b00493
Hauert, R., Falub, C. V., Thorwarth, G., Thorwarth, K., Affolter, C., Stiefel, M., et al. (2012). Retrospective lifetime estimation of failed and explanted diamond-like carbon coated hip joint balls. Acta Biomater. 8, 3170–3176. doi: 10.1016/j.actbio.2012.04.016
Huiskes, R., Van Driel, W. D., Prendergast, P. J., and Søballe, K. (1997). A biomechanical model for periprosthetic fibrous-tissue differentiation. J. Mater. Sci. Mater. Med. 8, 785–788. doi: 10.1023/a:1018520914512
Ihaddadene, R., Affatato, S., Zavalloni, M., Bouzid, S., and Viceconti, M. (2011). Femoral head diameter and carbon composition on wear of metal-on-metal hip replacements. Comput. Methods Biomech. Biomed. Engin. 14, 31–32. doi: 10.1080/10255842.2011.591531
Ishihara, K. (2015). Highly lubricated polymer interfaces for advanced artificial hip joints through biomimetic design. Polym. J. 47, 585–597. doi: 10.1038/pj.2015.45
Kanagaraj, S., Mathew, M. T., Fonseca, A., Oliveira, M. S., Simoes, J. A., and Rocha, L. A. (2010). Tribological characterisation of carbon nanotubes/ ultrahigh molecular weight polyethylene composites: the effect of sliding distance. Int. J. Surf. Sci. Eng. 4, 305–321. doi: 10.1504/IJSURFSE.2010.035138
Kevin, G. S. (1996). Prosthetic joint with semipermeable capsule with reinforcing ribs. US Patent No 5,514,182. Salt Lake City: US Patent Office.
Kim, Y. H., Kim, J. S., Park, J. W., and Joo, J. H. (2012). Periacetabular osteolysis is the problem in contemporary total hip arthroplasty in young patients. J. Arthroplast. 27, 74–81. doi: 10.1016/j.arth.2011.03.022
Kobayashi, S. D., Voyich, J. M., Burlak, C., and DeLeo, F. R. (2005). Neutrophils in the innate immune response. Arch. Immunol. Ther. Exp. 53, 505–517.
Korzh, M. O., Filipenko, V. A., Radchenko, V. O., Litvinov, L. A., Voloshin, O. V., Sliunin, Y. V., et al. (2007). Hip Endoprosthesis. UA Patent No 79,551. Kharkiv: Ukrainian Institute of Intellectual Property.
Kragelsky, I. V., Dobychin, M. N., and Kombalov, V. S. (1982). Friction and Wear: Calculation Methods. New York, NY: Pergamon Press.
Kumar, N., Arora, G. N., and Datta, B. (2014). Bearing surfaces in hip replacement - evolution and likely future. Med. J. Armed Forces India 70, 371–376. doi: 10.1016/j.mjafi.2014.04.015
Kurtz, S. M. (2013). Taper corrosion update: what is the role of ceramic femoral ball heads? CeraNews 1, 11–13.
Kusaba, A., Nagese, K., Kondo, S., Kuroki, Y., Maeda, A., and Scholz, J. (2011). 5 to 10-Year results of cementless CoC THA in young and active patients with dysplastic coxarthrosis. CeraNews 1, 19–21.
Kyomoto, M., Moro, T., Iwasaki, Y., Miyaji, F., Kawaguchi, H., Takatori, Y., et al. (2009). Superlubricious surface mimicking articular cartilage by grafting poly (2-methacryloyloxyethyl phosphorylcholine) on orthopaedic metal bearings. J. Biomed. Mater. Res. Part A. 91, 730–741. doi: 10.1002/jbm.a.32280
Kyomoto, M., Moro, T., Konno, T., Takadama, H., Yamawaki, N., Kawaguchi, H., et al. (2007). Enhanced wear resistance of modified cross-linked polyethylene by grafting with poly(2-methacryloyloxyethyl phosphorylcholine). J. Biomed. Mater. Res. Part A 82, 10–17. doi: 10.1002/jbm.a.31134
Lambert, B., Neut, D., van der Veen, H. C., and Bulstra, S. K. (2019). Effects of vitamin E incorporation in polyethylene on oxidative degradation, wear rates, immune response, and infections in total joint arthroplasty: a review of the current literature. Intern. Orthop. 43, 1549–1557. doi: 10.1007/s00264-018-4237-8
Lee, J. M., Salvati, E. A., Betts, F., DiCarlo, E. F., Doty, S. B., and Bullough, P. G. (1992). Size of metallic and polyethylene debris particles in failed cemented total hip replacements. J. Bone Joint Surg. Br. 74, 380–384. doi: 10.1302/0301-620X.74B3.1587882
Lesgaft, P. F., and Dolbnya, I. P. (1987). “Theory of simple joints,” in Proceedings of the St. Petersburg Biological Laboratory (St. Petersburg), Vol. 2, 22–44.
Li, B. Y., Rong, L. J., Li, Y. Y., and Gjunter, V. E. (2000). Synthesis of porous Ni-Ti shape-memory alloys by self-propagating high-temperature synthesis: reaction mechanism and anisotropy in pore structure. Acta Mater. 48, 3895–3904. doi: 10.1016/S1359-6454(00)00184-1
Li, Y., Yang, C., Zhao, H., Qu, S., Li, X., and Li, Y. (2014). New developments of Ti-based alloys for biomedical applications. Materials 7, 1709–1800. doi: 10.3390/ma7031709
Mamalis, A. G., Ramsden, J. J., Grabchenko, A. I., Lytvynov, L. A., Filipenko, V. A., and Lavrynenko, S. N. (2006). A novel concept for the manufacture of individual sapphire-metallic hip joint endoprostheses. J. Biol. Phys. Chem. 6, 113–117. doi: 10.4024/30601.jbpc.06.03
Manning, D. W., Chiang, P. P., Martell, J. M., Galante, J. O., and Harris, W. H. (2005). In vivo comparative wear study of traditional and highly cross-linked polyethylene in total hip arthroplasty. J. Arthroplast. 20, 880–886. doi: 10.1016/j.arth.2005.03.033
Martell, J. M., Verner, J. J., and Incavo, S. J. (2003). Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J. Arthroplast. 18(7 Suppl. 1), 55–59. doi: 10.1016/s0883-5403(03)00341-3
Maslov, L. B. (2013). Mathematical model of structural adjustment of bone tissue. Russ. J. Biomech. 17, 39–63.
Massaccesi, L., Ragone, V., Papini, N., Goi, G., Romanelli, M. M., and Galliera, E. (2019). Effects of vitamin E-stabilized ultra high molecular weight polyethylene on oxidative stress response and osteoimmunological response in human osteoblast. Front. Endocrinol. 10:203. doi: 10.3389/fendo.2019.00203
Matsoukas, G., and Kim, I. Y. (2006). “Shape optimization of an Un-cemented Total Hip Replacement Prosthesis Considering Volumetric Wear,” in 11th AIAA/ISSMO Multidisciplinary Analysis and Optimization Conference (Portsmouth). doi: 10.2514/6.2006-6904
Mediaswanti, K., Wen, C., Ivanova, E. P., Berndt, C. C., and Malherbe, F. (2013). A review on bioactive porous metallic biomaterials. J. Biomim. Biomater. Tissue Eng. 18:104. doi: 10.4172/1662-100X.1000104
Merola, M., and Affatato, S. (2019). Materials for hip prostheses: a review of wear and loading considerations. Materials 12:495. doi: 10.3390/ma12030495
Miyao, R., Omori, M., Watari, F., Yokoyama, A., Matsuno, H., Hirai, T., et al. (2000). Fabrication of functionally graded implants by spark plasma sintering and their properties. J. Jpn. Soc. Powder Metall. 47, 1239–1242. doi: 10.2497/jjspm.47.1239
Muratoglu, O. K., Bragdon, C. R., O'Connor, D. O., Jasty, M., Harris, W. H., Gul, R., et al. (1999). Unified wear model for highly cross-linked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials 20, 1463–1470. doi: 10.1016/S0142-9612(99)00039-3
Muratoglu, O. K., Greenbaum, E. S., Bragdon, C. R., Jasty, M., Freiberg, A. A., and Harris, W. H. (2004). Surface analysis of early retrieved acetabular polyethylene liners: A comparison of conventional and highly crosslinked polyethylenes. J. Arthroplasty 19, 68–77. doi: 10.1016/j.arth.2003.08.003
Murphy, A. C., Steppacher, S. D., Tannast, M., and Murphy, S. B. (2011). Outcome of ceramic-ceramic total hip arthroplasty in patients younger than 50 years. CeraNews. 1:7.
Narayan, R. J. (2005). Nanostructured diamondlike carbon thin films for medical applications. Mater. Sci. Eng. C 25, 405–416. doi: 10.1016/j.msec.2005.01.026
Neri, T., Philippot, R., Klasan, A., Putnis, S., Leie, M., Boyer, B., et al. (2018). Dual mobility acetabular cups for total hip arthroplasty: advantages and drawbacks. Expert Rev. Med. Devices 15, 835–845. doi: 10.1080/17434440.2018.1538781
Niu, W. J., Gill, S. P., Dong, H. B., and Bai, C. G. (2010). A two-scale model for predicting elastic properties of porous titanium formed with space-holders. Comput. Mater. Sci. 50, 172–178. doi: 10.1016/j.commatsci.2010.07.022
Nugroho, A. W., Leadbeater, G., and Davies, I. J. (2010). Processing of a porous titanium alloy from elemental powders using a solid state isothermal foaming technique. J. Mater. Sci. Mater. Med. 21, 3103–3107. doi: 10.1007/s10856-010-4162-x
Oral, E., and Muratoglu, O. K. (2011). Vitamin E diffused, highly crosslinked UHMWPE: a review. Int. Orthop. 35, 215–223. doi: 10.1007/s00264-010-1161-y
Oral, E., Wannomae, K. K., Bichara, D. A., Micheli, B., Doshi, B. N., O'Brien, C., et al. (2019). An antioxidant stabilized, chemically cross-linked UHMWPE with superior toughness. J. Biomed. Mater. Res. Part B Appl. Biomater. 107, 1945–1952. doi: 10.1002/jbm.b.34287
Pakhaliuk, V., Polyakov, A., Kalinin, M., and Bratan, S. (2016). Evaluating the impact and norming the parameters of partially regular texture on the surface of the articulating ball head in a total hip joint prosthesis. Tribol. Online 11, 527–539. doi: 10.2474/trol.11.527
Panayotov, I. V., Orti, V., Cuisinier, F., and Yachouh, J. (2016). Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 27:118. doi: 10.1007/s10856-016-5731-4
Pappas, M. J., Makris, G., and Buechel, F. F. (1995). Titanium nitride ceramic film against polyethylene: a 48-million cycle wear test. Clin. Orthop. Relat. Res. 317, 64–70.
Pashkov, E. V., Kalinin, M. I., and Olinichenko, G. D. (2018a). Biarticular Fluid-Circulation Anatomically Adaptable Hip Prosthesis. RU Patent No 2,662,710. Sevastopol: Federal Service on Intellectual Property.
Pashkov, E. V., Kalinin, M. I., and Pakhaliuk, V. I. (2018b). Biarticular Anatomically Adaptable Hip Prosthesis with Fluid. RU Patent No 2,653,273. Sevastopol: Federal Service on Intellectual Property.
Pashkov, E. V., Pashkova, E. V., Evstigneev, M. P., and Kalinin, M. I. (2016). The Closed Hip Replacement. RU Patent No 2,605,149. Sevastopol: Federal Service on Intellectual Property.
Pauwels, F. (1980). “A new theory concerning the influence of mechanical stimuli on the differentiation of the supporting tissue,” in The Biomechanics of the Locomotor Apparatus, eds. P. Maquet, R. Furlong (Berlin: Springer-Verlag), 375–407. doi: 10.1007/978-3-642-67138-8_14
Poliakov, O., Olinichenko, G., Pashkov, Y., Kalinin, M., Kramar, V., and Burkov, D. (2012). “A new design of a unipolar hip endoprosthesis focused on the best quality of life of the patients during the postoperative period,” in International Conference on Biomedical Engineering (ICoBE) (Penang), 27–28. doi: 10.1109/ICoBE.2012.6178948
Polyakov, A., Pakhaliuk, V., Kalinin, M., Kramar, V., Kolesova, M., and Kovalenko, O. (2015). System analysis and synthesis of total hip joint endoprosthesis. Proc. Eng. 100, 530–538. doi: 10.1016/j.proeng.2015.01.400
Puértolas, J. A., and Kurtz, S. M. (2014). Evaluation of carbon nanotubes and graphene as reinforcements for UHMWPE-based composites in arthroplastic applications: A review. J. Mech. Behav. Biomed. Mater. 39, 129–145. doi: 10.1016/j.jmbbm.2014.06.013
Rabin, B., and Shiota, I. (1995). Functionally gradient materials. MRS Bull. 20, 14–18. doi: 10.1557/S0883769400048855
Rajabzadeh, N., Fathi, E., and Farahzadi, R. (2019). Stem cell-based regenerative medicine. Stem cell Invest. 6:19. doi: 10.21037/sci.2019.06.04
Roberts, M. (2009). Science to 'stop age clock at 50'. Available online at: http://news.bbc.co.uk/2/hi/health/8314442.stm (accessed September 29, 2019).
Römmelt, C., Munsch, T., Drynda, A., Lessmann, V., Lohmann, C. H., and Bertrand, J. (2019). Periprosthetic hypoxia as consequence of TRPM7 mediated cobalt influx in osteoblasts. J. Biomed. Mater. Res. Part B Appl. Biomater. 107, 1806–1813. doi: 10.1002/jbm.b.34273
Ruan, S. L., Gao, P., Yang, X. G., and Yu, T. X. (2003). Toughening high performance ultrahigh molecular weight polyethylene using multiwalled carbon nanotubes. Polymer 44, 5643–5654. doi: 10.1016/S0032-3861(03)00628-1
Ryan, G., Pandit, A., and Apatsidis, D. P. (2006). Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 27, 2651–2670. doi: 10.1016/j.biomaterials.2005.12.002
Scemama, C., Anract, P., Dumaine, V., Babinet, A., Courpied, J. P., and Hamadouche, M. (2017). Does vitamin E-blended polyethylene reduce wear in primary total hip arthroplasty: a blinded randomised clinical trial. Int. Orthop. 41, 1113–1118. doi: 10.1007/s00264-016-3320-2
Schouten, R., Malone, A. A., Tiffen, C., Frampton, C. M., and Hooper, G. (2012). A prospective, randomised controlled trial comparing ceramic-on-metal and metal-on-metal bearing surfaces in total hip replacement. J. Bone Joint Surg. Br. 94, 1462–1467. doi: 10.1302/0301-620X.94B11.29343
Serro, A. P., Completo, C., Colaco, R., dos santos, F., Lobatoda Silva, C., Cabral, J. M. S., et al. (2009). A comparative study of titanium nitrides, TiN, TiNbN and TiCN, as coatings for biomedical applications. Surf. Coat. Technol. 203, 3701–3707. doi: 10.1016/j.surfcoat.2009.06.010
Shanbhag, A. S., Jacobs, J. J., Giant, T. T., Gilbert, J. L., Black, J., and Galante, J. O. (1994). Composition and morphology of wear debris in failed uncemented total hip replacement. J. Bone Joint Surg. Br. 76, 60–67. doi: 10.1302/0301-620X.76B1.8300684
Shirani, A., Hu, Q., Su, Y., Joy, T., Zhu, D., and Berman, D. (2019). Combined tribological and bactericidal effect of nanodiamonds as a potential lubricant for artificial joints. ACS Appl. Mater. Interfaces 11, 43500–43508. doi: 10.1021/acsami.9b14904
Smith Nephew. (2019). OXINIUM: Oxidized Zirconium. Available at: http://www.smith-nephew.com/professional/products/all-products/oxinium/ (accessed September 29, 2019).
Stief, F., van Drongelen, S., Brenneis, M., Tarhan, T., Fey, B., and Meurer, A. (2019) Influence of hip geometry reconstruction on frontal plane hip knee joint moments during walking following primary total hip replacement. J. Arthroplasty 34, 3106–3113. doi: 10.1016/j.arth.2019.07.027
Takayanagi, H. (2007). Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304. doi: 10.1038/nri2062
Tang, C. Y., Wong, C. T., Zhang, L. N., Choy, M. T., Chow, T. W., Chan, K. C., et al. (2013). In situ formation of Ti alloy/TiC porous composites by rapid microwave sintering of Ti6Al4V/MWCNTs powder. J. Alloy. Compd. 557, 67–72. doi: 10.1016/j.jallcom.2012.12.147
Tang, L., Jennings, T. A., and Eaton, J. W. (1998). Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc. Natl. Acad. Sci. U.S.A. 95, 8841–8846. doi: 10.1073/pnas.95.15.8841
Terkawi, M. A., Kadoya, K., Takahashi, D., Tian, Y., Hamasaki, M., Matsumae, G., et al. (2019). Identification of IL-27 as potent regulator of inflammatory osteolysis associated with vitamin E-blended ultra-high molecular weight polyethylene debris of orthopedic implants. Acta Biomater. 89, 242–251. doi: 10.1016/j.actbio.2019.03.028
Thesleff, I. (2018). From understanding tooth development to bioengineering of teeth. – Eur. J. Oral. Sci. 126, 67–71. doi: 10.1111/eos.12421
Timercan, A., Brailovski, V., Petit, Y., Lussier, B., and Séguin, B. (2019). Personalized 3D-printed endoprostheses for limb sparing in dogs: modeling and in vitro testing. Med. Eng. Phys. 71, 17–29. doi: 10.1016/j.medengphy.2019.07.005
Turmanidze, R., and Popkhadze, G. (2016). New materials for implants of the human hip joint and technology of their machining with the achievement of high precision and quality of spherical surfaces. Mater. Sci. Nonequilib. Phase Transform. 3, 12–16. doi: 10.20535/2305-9001.2015.75.54304
Upadhyaya, A., Tiwari, S. K., and Mishra, P. (2007). Microwave sintering of W-Ni-Fe alloy. Scr. Mater. 56, 5–8. doi: 10.1016/j.scriptamat.2006.09.010
Viceconti, M., Muccini, R., Bernakiewicz, M., Baleani, M., and Cristofolini, L. (2000). Large-sliding contact elements accurately predict levels of bone-implant micromotion relevant to osseointegration. J. Biomech. 33, 1611–1618. doi: 10.1016/s0021-9290(00)00140-8
Vila, M., Amaral, M., Oliveira, F. J., Silva, R. F., Fernandes, A. J. S., and Soares, M. R. (2006). Residual stress minimum in nanocrystalline diamond films. Appl. Phys. Lett. 89, 93–109. doi: 10.1063/1.2339042
Volkov, V. V., Kalinin, M. I., Pakhaliuk, V. I., Kovalenko, O. V., Olinichenko, G. D., and Polyakov, O. M. (2011). Unipolar Hip Endoprosthesis. UA Patent No 93,823. Sevastopol: Ukrainian Institute of Intellectual Property.
Von Skrbensky, G., Mühlbacher, K., Benca, E., Kolb, A., Windhager, R., Reischl, G., et al. (2019). Evaluation of aerosol electrospray analysis of metal-on-metal wear particles from simulated total joint replacement. Sensors 19:3751. doi: 10.3390/s19173751
Wait, M., Ealker, P., and Blunn, G. (1995). Tissue reactions to CoCr wear debris from metal on metal total hip replacements. Trans. Europ. Orthop. Res. Soc. 5:160.
Wang, A., Lin, R., Polineni, V. K., Essner, A., Stark, C., and Dumbleton, J. H. (1998). Carbon fiber reinforced polyether ether ketone composite as a bearing surface for total hip replacement. Tribol. Int. 31, 661–667. doi: 10.1016/S0301-679X(98)00088-7
Wang, X., Li, Y., Xiong, J., Hodgson, P. D., and Wen, C. (2009). Porous TiNbZr alloy scaffolds for biomedical applications. Acta Biomater. 5, 3616–3624. doi: 10.1016/j.actbio.2009.06.002
Watari, F., Yokoyama, A., Omori, M., Hirai, T., Kondo, H., and Uo, M. (2002). Effect of spark plasma sintering pressure on the properties of functionally graded implant and its biocompatibility. J. Jpn. Soc. Powder Metall. 49, 1063–1069. doi: 10.2497/jjspm.49.1063
Wen, C. E., Mabuchi, M., Yamada, Y., Shimojima, K., Chino, Y., and Asahina, T. (2001). Processing of biocompatible porous Ti and Mg. Scr. Mater. 45, 1147–1153. doi: 10.1016/S1359-6462(01)01132-0
Wen, C. E., Yamada, Y., Shimojima, K., Chino, Y., Asahina, T., and Mabuchi, M. (2002). Processing and mechanical properties of autogenous titanium implant materials. J. Mater. Sci. Mater. Med. 13, 397–401. doi: 10.1023/a:1014344819558
Williams, S., Al-Hajjar, M., Isaac, G. H., and Fisher, J. (2013). Comparison of ceramic-on-metal and metal-on-metal hip prostheses under adverse conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 101, 770–775. doi: 10.1002/jbm.b.32880
Wong, K. C., and Scheinemann, P. (2018). Additive manufactured metallic implants for orthopaedic applications. Sci. China Mater. 61, 440–454. doi: 10.1007/s40843-017-9243-9
Wu, M. J., Chen, K., Xu, L. M., Qi, J. W., Luo, Y., and Zhang, D. K. (2019). Swing-torsion composite friction behavior of CoCrMo/UHMWPE contact interface of artificialknee joint prosthesis implantation materials. Mater. Res. Express 6:055402. doi: 10.1088/2053-1591/ab04d3
Xu, H. D., Zhang, D. K., Chen, K., and Zhang, T. (2019). Taper fretting behavior of PEEK artificial hip joint. Tribol. Int. 137, 30–38. doi: 10.1016/j.triboint.2019.04.027
Xu, J.-Z., Muratoglu, O. K., and Oral, E. (2019). Improved oxidation and wear resistance of ultrahigh molecular weight polyethylene using cross-linked powder reinforcement. J. Biomed. Mater. Res. Part B, Appl. Biomater. 107, 716–723. doi: 10.1002/jbm.b.34165
Yang, C., Li, J., Zhu, K., Yuan, X., Cheng, T., Qian, Y., et al. (2019). Puerarin exerts protective effects on wear particle-induced inflammatory osteolysis. Front. Pharmacol. 10, 1113, doi: 10.3389/fphar.2019.01113
Yuan, L., Ding, S., and Wen, C. (2018). Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: a review. Bioact. Mater. 4, 56–70. doi: 10.1016/j.bioactmat.2018.12.003
Zhang, L., Huang, Y., Chen, G. D., Xu, M. R., Xia, W. H., and Fu, Y. F. (2019). Experimental study of coverage constraint abrasive flow machining of titanium alloy artificial joint surface. Proc. Inst Mech. Eng. Part B 233, 2399–2409. doi: 10.1177/0954405419840553
Zhang, Q., Chen, Z., Zhang, J., Hu, J., Peng, Y., Fan, X., et al. (2019). Insert conformity variation affects kinematics and wear performance of total knee replacements. Clin. Biomech. 65, 19–25. doi: 10.1016/j.clinbiomech.2019.03.016
Zhang, Y. P., Yuan, B., Zeng, M. Q., Chung, C. Y., and Zhang, X. P. (2007). High porosity and large pore size shape memory alloys fabricated by using pore-forming agent (NH4HCO3) and capsule-free hot isostatic pressing. J. Mater. Process. Technol. 192–193, 439–442. doi: 10.1016/j.jmatprotec.2007.04.069
Keywords: natural joint, artificial joint, total joint arthroplasty, biomaterials, bone tissue regeneration, osseointegration, osteoimmunomodulation, dynamic osteosynthesis
Citation: Poliakov A, Pakhaliuk V and Popov VL (2020) Current Trends in Improving of Artificial Joints Design and Technologies for Their Arthroplasty. Front. Mech. Eng. 6:4. doi: 10.3389/fmech.2020.00004
Received: 20 October 2019; Accepted: 16 January 2020;
Published: 05 February 2020.
Edited by:
Alessandro Ruggiero, University of Salerno, ItalyReviewed by:
Saverio Affatato, Rizzoli Orthopedic Institute (IRCCS), ItalyEmile Van Der Heide, University of Twente, Netherlands
Copyright © 2020 Poliakov, Pakhaliuk and Popov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vladimir Pakhaliuk, cGFoYWx1ayYjeDAwMDQwO3NldnN1LnJ1; Valentin L. Popov, di5wb3BvdiYjeDAwMDQwO3R1LWJlcmxpbi5kZQ==
 Aleksandr Poliakov
Aleksandr Poliakov Vladimir Pakhaliuk
Vladimir Pakhaliuk Valentin L. Popov
Valentin L. Popov