
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mech. Eng. , 17 April 2019
Sec. Digital Manufacturing
Volume 5 - 2019 | https://doi.org/10.3389/fmech.2019.00019
This article is part of the Research Topic Advances in 3D Bioprinting View all 4 articles
The pursuit of appropriate, biocompatible materials is one of the primary challenges in translational bioprinting. The requirement to refine a biomaterial into a bioink places additional demands on the criteria for candidate biomaterials. The material must enable extrusion as a liquid bioink and yet be capable of maintaining its shape in the post-printing phase to yield viable tissues, organs and biological materials. Plant-derived biomaterials show great promise in harnessing both the natural strength of plant microarchitecture combined with their natural biological roles as supporters of cell growth. The aim of this review article is to outline the most widely used biomaterials derived from land plants and marine algae: nanocellulose, pectin, starch, alginate, agarose, fucoidan, and carrageenan, with an in-depth focus on nanocellulose and alginate. The properties that render these materials as promising bioinks for three dimensional bioprinting is herein discussed alongside their potential in 3D bioprinting for tissue engineering, drug delivery, wound healing, and implantable medical devices.
3D bioprinting is a rapidly evolving field of biomedicine, merging the disciplines of tissue engineering, materials science and 3D printing technologies to yield viable biological structures in customized spatial arrangements. The implications of the technology are immense, lending itself to fuel advancements in fields such as drug delivery, reconstructive and transplantation surgery, and implantable medical devices (Jovic et al., 2018).
A primary challenge facing the translational potential of bioprinting for medical use is the pursuit of appropriate, biocompatible materials (Malkoc, 2018). Historically, a variety of materials have contributed toward the advancement of biomedicine, with the most noteworthy candidates including alloys, ceramics, metals, and composites (Le May et al., 1975). Although the desired properties for biomedical application may vary between materials, the fundamental requirements for in-vivo implementation remain universal. Successful tissue engineering demands that an optimal scaffold should interface with biological systems to support cell growth whilst displaying biocompatibility, non-toxicity, and providing the mechanical support to emulate natural tissue macro and microarchitecture (Drury and Mooney, 2003; Gungor-Ozkerim et al., 2018). The requirement to refine a biomaterial into a bioink places additional demands on the criteria for candidate biomaterials. The material must enable extrusion as a liquid bioink and yet be capable of maintaining its shape in the post-printing phase to yield viable tissues, organs, and biological materials.
The search for a biomaterial with the appropriate balance of biological and mechanical factors is often fraught with conflict (Chimene et al.). Synthetic materials, such as plastics, traditionally convey superiority in addressing the mechanical properties required to support tissue growth and can be easily modified to augment their printability, viscosity, and strength. The major caveat of such materials lies in their restricted bioactivity: limited cell adhesion and lack of extracellular matrix mimicry translate to a limited capacity to support the biological components of cell growth (O'Brien, 2011). Furthermore, synthetic materials are often non-degradable which presents the risks of extrusion, immunogenicity, and impedance of de novo tissue formation (Sarkar et al., 2017). Of those that are degradable, toxic by-products may be released during the degradation process, presenting a risk of harm when implanted into humans.
With the advent and evolution of nanotechnology, there has been a progressive focus on the use of natural materials for a variety of biomedical applications. Additionally, this rapidly growing industry has further highlighted the need for environmentally friendly biomaterials derived from sources free of ecological burden. Plant-derived biomaterials show great promise in harnessing both the natural strength of plant microarchitecture combined with their natural biological roles as supporters of cell growth (Gershlak et al., 2017). Enhanced bioactivity, biocompatibility, biodegradability, and mechanical stability are perceived advantages of plant derived materials (Yegappan et al., 2018), moreover as potential bioinks owing to their affinity for chemical modulation and facile hydrogel formation. As such, this new generation of abundant, natural, and renewable bioinks have attracted significant attention in the realm of 3D bioprinting research.
The aim of this review article is to outline the most widely used biomaterials derived from land plants and marine algae (Figure 1), with an emphasis on nanocellulose and alginate, as two of the most researched materials in these respective categories. The properties that render these materials as promising bioinks for three dimensional bioprinting will thereafter be discussed.
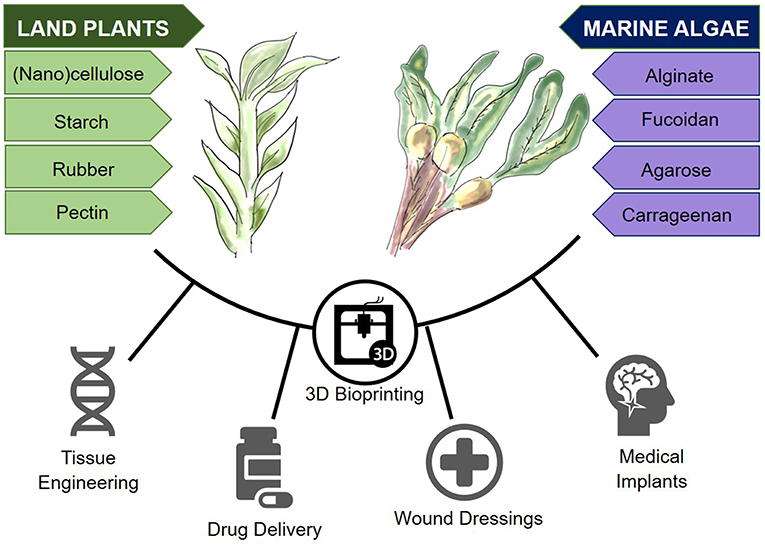
Figure 1. Plant-derived biomaterials from land and marine sources and their potential in 3D bioprinting.
Biological printing “Bioprinting” enables the precise deposition of cells in a viscous biomaterial in a specific spatial arrangement using a computer-aided printer. The evolution of this technology from mainstream 3D printing has demanded increasingly complex printing methods to enable the biological roles of the ink, such as cell adhesion, and proliferation to be served in addition to the mechanical properties required of conventional 3D printing ink. Due to their embedded cellular material, bioinks are printed at much lower temperatures and demand mild, non-cytotoxic crosslinking methods to preserve cellular viability, and function (Malda et al., 2013).
3D bioprinting uses three major types of printing technology: inkjet, laser-assisted and extrusion (Table 1). Inkjet bioprinting uses low viscosity solutions, such as cell suspensions that are deposited at high shear rates as droplets of ~50 μm (micrometer) diameter (Hölzl et al., 2016). In contrast, laser assisted bioprinting focuses a laser toward a laser-absorbing biomaterial layer which in turn produces a local pressure to enable ink deposition (Derakhshanfar et al., 2018). Extrusion based bioprinting, or bioplotting, is the most common subtype, comprising the deposition of cells, and bioink using a nozzle driven by pneumatic, piston or screw forces (Landers et al., 2002; Jakab et al., 2008). Although slower than laser and inkjet printing approaches, extrusion bioprinting enables structural deposition, and solidification as each layer is deposited and is associated with good cell viability (Smith et al., 2004; Derakhshanfar et al., 2018). Each type of printing technology requires bioinks of differing viscosities for successful deposition: droplet and inkjet printers require viscosities of 1–300 mPa·s whereas extrusion-based printers require a minimum of 30–6 × 107 mPa·s (Aljohani et al., 2018). As such, bioinks with tunable viscosity are most likely to offer the versatility required to undergo printing using different technologies.
Bioprinting technology is becoming an increasingly available and affordable technology to support tissue engineering research, with many research groups now opting to customize their own in-house devices owing to the widespread availability of open source software and firmware (Reid et al., 2016). A major limitation of bioprinting for biological constructs is the choice of a suitable bioink: one not only biologically conducive of cell growth but in possession of the necessary rheological properties such as viscosity, shear thinning, and crosslinking to optimize post-printing fidelity (Jessop et al., 2017; Kyle et al., 2017).
In addition to biological and rheological properties, in order to be valid in vivo tissue replacements, bioinks must have adequate mechanical strength to support de novo tissue formation. Common mechanical assessments for bioinks include compressive, storage and elastic moduli, residual, and maximum compressive stresses (Chung et al., 2013). These parameters are of importance when considering the native properties of the target tissue type. Supportive structural tissues such as bone and cartilage, will have significantly greater biomechanical demands to withstand the myriad of load bearing and shear forces exerted in vivo. Articular cartilage for example, has a compressive modulus of ~2 MPa (Beck et al., 2016), whereas human bone has been shown to possess a tensile strength of 34–45 MPa, a compressive strength of 120–160 MPa, and a Young's modulus of 230–540 MPa (Havaldar et al., 2014). The pursuit of a material capable of emulating these structural properties, alongside possessing the rheological demands of a bioink adds to the challenge of material selection. As such, the search for novel materials has naturally progressed to the biologically and mechanically diverse array of plant-derived substances as candidate bioinks.
Alginate is one of the most commonly used materials in 3D bioprinting. Alginate is renewably sourced from brown algae, commonly Laminaria hyperborean, Laminaria digitate, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera (Lee and Mooney, 2012). Structurally, it consists of co-polymers composed of the polysaccharides β-D-Mannuronic acid (M) and α-L-Gluloronic acid (G) (Axpe and Oyen, 2016). Alginates contain blocks of G residues, blocks of M residues, and alternating M and G residues (Stößlein et al., 2019). The M and MG residues of the co-polymer have been shown to increase flexibility, whereas the G residues increase rigidity and the capacity for gelation (Axpe and Oyen, 2016). The natural ratio of M:G varies depending on the seaweed species from which the alginate is extracted (Cardoso et al., 2016) and also exhibits environmental and seasonal variability (Maleki et al., 2016). The various combinations therefore result in anionic compounds with a wide variety of biomedical applications.
Commercially available alginate is acquired via acidification to extract the alginate as alginic acid. Thereafter the material is alkalinized and precipitated with sodium or calcium carbonate to generate an alginate salt. Vacuum filtration can then be used on the precipitated alginate to collect it (Fawzy et al., 2017; Patil et al., 2018). An alternative means of alginate production is through bacterial biosynthesis (Hay et al., 2013). This process is mediated largely by the species of the genera Pseudomonas and Azotobacter (Hay et al., 2013; Moradali et al., 2017; Urtuvia et al., 2017) and is a costly biofabrication process with a theoretical risk of pathogenicity.
Alginate bioinks can be crosslinked readily after extrusion through simple immersion in calcium chloride solution (Wee and Gombotz, 1998). The crosslinking process itself enables structural fixation in a matter of minutes. Despite the speed of crosslinking, the mechanical properties of the crosslinked derivative render it susceptible to diffusion after extrusion, which results in structural deformation. Alternative approaches to rectify this issue include the use of barium chloride (Bajpai and Sharma, 2004) and a calcium chloride nebulizer, which demonstrates a more gradual crosslinking process with less potential for subsequent diffusion (Raddatz et al., 2018).
The rheological properties of alginate-based bioinks must be considered in the context of 3D bioprinting (Ching et al., 2017). The viscosity of an alginate bioink is a product of alginate concentration and molecular weight; as well as the cellular density and phenotype of the laden cells (Axpe and Oyen, 2016). When using a cell laden hydrogel, the density of the biomaterial ideally should be the same as the cells, thus optimizing a homogenous cell suspension; increasing the concentration of alginate, and decreasing the degree of oxidization can increase density to the required level (Jia et al., 2014). Factors affecting viscosity include temperature, where viscosity is inversely proportional to an increase in temperature, and the concept of shear-thinning, where viscosity decreases as shear rate increases (Axpe and Oyen, 2016). In terms of degradation, the ideal solution is that hydrogels should degrade at a rate that mirrors the rate at which the cells produce their own extracellular matrix (Jia et al., 2014).
When considering a biomaterial for 3D bioprinting, it is important to take into account viscoelastic properties. Alginate hydrogels have a propensity to increase the loss modulus and thereafter decrease the storage modulus of a bioink; whilst maintaining a consistent loss tangent through varying concentrations (Gao et al., 2018). However, when using a crosslinked alginate hydrogel, a higher storage modulus compared to loss modulus can be observed (Aguado et al., 2012). When utilizing an extrusion-based method of bioprinting, the extrusion pressure requirement increases as the viscosity increases, with the loss modulus having more of an impact that the storage modulus (Zhang et al., 2017). Unfortunately, there is a negative correlation between extrusion pressure and cell viability, and also loss tangent and structural integrity which must be taken into account when designing bespoke printing parameters using alginate hydrogels (Gao et al., 2018).
Printing parameters of alginate are set to optimize the rheological, mechanical, and physical properties of alginate. As discussed, the viscosity of alginate is an important component to control when 3D bioprinting, it has been shown that a viscosity of 300–30,000 millipascal second (mPa·S) is optimal for printing, an alginate concentration of 2–4% provides this viscosity (He et al., 2016). Alginate can be printed effectively at a range of temperatures in order to achieve the required viscosity (He et al., 2016; Zhu et al., 2018). However, alginate-based hydrogels have been shown to be most effective at 37°C due to: accurate extrusion flow rate, decreased occurrence of obstruction and compatibility with cell viability (Ding et al., 2018). One study showed that for a 7% alginate/8% gelatin hydrogel, optimum printing parameters at 37°C are as follows: nozzle gauge = 30 G, printing pressure = 100 kPa, and printing speed = 4 mm/s (Webb and Doyle, 2017). Storage of alginate can be vast depending upon purpose; for example, cells encapsulated with alginate can be stored comfortably between 0 and 30°C and are fit for purpose, whereas it has been shown that in an alginate/gelatin hydrogel, storage at 3°C prolonged degradation when compared to storage at 37°C (Giuseppe et al., 2018).
As discussed previously, the physicochemical properties of alginate can depend upon the G:M; where higher concentrations of G are more stable when compared to high M content (Axpe and Oyen, 2016). The molecular weight of the crosslinking agent and exposure time can be altered in order to regulate the elasticity (Young's) modulus of a hydrogel; for instance, using increasing concentrations of poly(ethyleneglycol)diamine as a crosslinker has been shown to increase the viscosity and toughness of the hydrogel (Naghieh et al., 2018; Meng et al., 2019). Another study showed an increase in elasticity modulus with increasing concentrations of Ca2+ which enabled an alginate-based hydrogel to improve mechanical properties whilst maintaining high ionic strength (Matyash et al., 2014).
Tensile strength, Young's modulus and elongation at the break are components of the mechanical properties of alginate. It has been shown that uncrosslinked alginate has a tensile strength of 25.8 ± 7.3 megapascal (MPa), a Young's modulus of 12.9 ± 2.6 MPa, and an elongation at the break of 2.50 ± 0.8%. This study showed an increase in tensile strength, Young's modulus and elongation at the break when alginate hydrogels were crosslinked (with d-mannitol and xylitol) (Park et al., 2018).
Nanocellulose, a versatile and abundant natural biopolymer, has shown great promise due to its robust mechanical properties, unique surface chemistry, desirable biological characteristics (high biocompatibility, low biodegradability, and non-toxicity), and cost efficiency.
Generally, its architecture follows a hierarchical order progressing from an intricate arrangement of polymeric cellulose chains [~1 nanometer (nm)] and culminating in macroscopic fibrillar structures of ~5–20 μm (μm) in diameter. A structural hierarchy also exists within the microfibrillar motif itself, consisting of highly organized crystalline regions with alternating amorphous domains (Gumrah Dumanli, 2017). This configuration ultimately dictates the mechanical behavior of cellulose, with the disordered (amorphous) regions conferring flexibility and plasticity, and the ordered (crystalline) fraction contributing to the strength and elasticity of the bulk material (Lin and Dufresne, 2014).
Broadly, nanocellulose can be categorized into three types: (1) Cellulose nanocrystals (CNC), (2) Nanofibrillated cellulose (NFC), and (3) Bacterial cellulose (BC). The plant-derived constituents (CNC & NFC) have recently generated considerable research activity and are largely favored for their sustainability; more-so considering the virtually inexhaustible feedstock from which they are isolated. Popular sources for CNC and NFC extraction include wood, hemp, cotton, potato tuber, and algae (Lin and Dufresne, 2014).
The production of nanocellulose largely involves the chemical and/or mechanical breakdown of lignocellulosic biomass in a “top-down” fashion. Cellulose nanocrystals are most commonly derived through acid hydrolysis; a method which removes the amorphous regions whilst preserving crystalline morphology (crystallinity ~54–88%) (Phanthong et al., 2018) This process also contributes to the hydrophilicity of CNCs and results in a compound with deformation qualities theoretically similar to those of Steel and Kevlar [Young's modulus ~167.5 (gigapascals) GPa] (Tashiro and Kobayashi, 1991). The molecular dimensions can range from 3 to 50 nm in diameter and 50 nm−3 μm in length, dependent on the percentage crystallinity of the primary source (Dumanli et al., 2014). In contrast, high-pressure homogenization (before or after enzymatic treatment) is often the method of choice for the extraction of nanofibrillated cellulose. In NFC, both the amorphous and crystalline sequences of the elementary cellulose structure are conserved, resulting in a product of longer length, high surface area, and extensive hydroxyl groups for surface modification (Lavoine et al., 2012). Although cellulose is widely regarded as a low-cost biomaterial, the intensive processing and modification steps involved in nanocellulose production further contribute to the overall price. With this considered, cost efficient processes for the bulk production of nanocellulose present an area warranting further scientific attention (Klemm et al., 2011). Conversely, the biosynthesis of bacterial cellulose is somewhat distinct to that of plant-based biomaterials in that low molecular weight sugars are naturally assembled in a “bottom-up” process. Furthermore, this mechanism excludes various constituents otherwise found in lignocellulose (i.e., lignin, pectin, and hemicellulose). For this reason, bacterial cellulose has garnered attention as a highly pure and biocompatible resource. Regardless, due to their pre-existing infrastructure for harvesting, pulping, and cost efficient processing, plants undoubtedly offer an optimal source for sustainable nanocellulose derivation (Hao et al., 2015).
From a morphological viewpoint, nanocellulose boasts an array of desirable properties for 3D bioprinting, and tissue engineering. NFC based hydrogels consistently display non-Newtonian, shear thinning (viscoelastic) behavior, generally portrayed by their large storage modulus over the loss modulus at minimal shear rates (Markstedt et al., 2015). More elaborately, the storage (G') and loss (G”) moduli of nanocellulose may be considered accurate indicators of elastic and viscous response, respectively. In a recent study, the storage modulus for NFC, CNC, and Nanocellulose “blends” (NFC and CNC in mixture) was shown to be greater than the loss modulus across all frequencies studied, thus inferring a dominance in elasticity for these biomaterials and demonstrating the presence of robust interconnectivity between nanostructures (Kyle et al., 2018). Furthermore, the examined systems also demonstrated a loss tangent (tan δ) of <1 indicating an inherent propensity for a lower dissipation potential (i.e., greater elastic response) amongst the biomaterials studied.
The flow properties for these samples were also investigated by measuring the loss modulus as a function of shear rate. The retention of viscosity at zero shear and preservation of shape fidelity was especially marked within the nanocellulose blends; possibly due to widespread entanglement between the micro- and nanostructures within the composite hydrogel (Kyle et al., 2018). As detailed by (Chirayil et al., 2014) the concept of shear thinning may be attributed to the orientation of nanocellulose microstructures in the direction of shear flow. At low shear rates, the NC nanoarchitecture exhibits a coiled configuration which progressively disentangles at increasing rates to align its axis with the direction of flow. An amalgamation of these characteristics thus allows for the seamless extrusion of bioink as a liquid phase precursor, with preservation of post-printing shape fidelity within the hydrogel construct. Moreover, the introduction of aldehyde and carboxyl functional groups promotes the formation of readily cross-linkable substrates, thereby overcoming current challenges in the stabilization of pure nanocellulose hydrogels (Markstedt et al., 2015). CaCl2 mediated crosslinking has, however, been shown to disrupt the “macro-pore” appearance of the printed construct. Additionally, the introduction of dialdehyde functional groups through periodate oxidation may also induce pro-inflammatory TNFα gene expression (Kollar et al., 2011). Despite this, further exploration of such novel approaches may yet yield innovative solutions for the relatively poor cross-linking ability of pure nanocellulose. Alternative strategies for hydrogel cross-linking have therefore been revised, with the most noteworthy involving the incorporation of divalent/trivalent cations (i.e., Ca2+, Cu2+, Al3+) (Dong et al., 2012) and covalent cross-linkers (Syverud et al., 2011).
Thermogravimetric analysis of NFC, CNC and bled has, additionally, been used to characterize stability through the measurement of rate and onset of thermal degradation (Kyle et al., 2018). For tissue engineering purposes, all three nanocellulose formulations may be utilized at physiological temperature (37°C) and remain stable above the recommended temperature for autoclaving (121°C). A small degree of moisture loss has, however, noted between 50 and 140°C and subsequently warrants investigation into the effects of heat sterilization on NC nano- and microarchitecture.
Agarose is derived from red seaweed and has garnered interest in the plant derived 3D bioprinting sphere due to its ability to be prepared as a thermal-reversible gel (Zarrintaj et al., 2018). It exhibits many of the properties of alginate, emulating the extracellular matrix with high water uptake, but with the advantage of hydrogen bond-mediated self-gelation without the need for potentially toxic crosslinking agents such as genipin (Campos et al., 2018). It has however been noted to be adversely mechanically affected by the presence of cells: cells within agarose diminish the gel strength due to interference with the hydrogen bonding required for crosslinking and gelation (Shoichet et al., 1996). This limitation can be somewhat surmounted through combination with other polymers and proteins such as collagen, chitosan, and cellulose to increase cell affinity (Annamalai et al., 2016; Awadhiya et al., 2017). Research into the role of agarose composites has been explored for tissue engineering purposes for neural, vascular, bone, and pancreatic tissue (Bhatnagar et al., 2016; Zarrintaj et al., 2018). However, further refinement will be required to produce bioinks possessing the necessary properties for bioprinting purposes.
Young's modulus of agarose is heavily dependent upon gel concentration; one study showed a variance between ~5.6 kPa (0.4% agarose) and ~17.4 kPa (1.2% agarose), whereas another showed values ranging from 130 kPa (1% agarose) to 3,000 kPa (10% agarose) (Ahearne et al., 2005; Walker et al., 2011). Both the storage modulus and loss modulus of agarose are positively correlated with concentration of agarose; and agarose is noted to be significantly elastic (Chen et al., 2003). Compared to hydrogels such as gelatin, collagen, and alginate, agarose displays high viscosity. Even at the highest shear rates, one study noted viscosity of a 4% solution to be 257 mPas·s, which may restrict its use for inkjet printers with high kinetic energies. Furthermore, in order to maintain agarose hydrogels in liquid state, their temperature must be maintained above 37°C: a biologically viable temperature, but one that carries additional considerations for storage and printing (Benning et al., 2018). It has been shown that storage and loss modulus increase with frequency, as well as the loss tangent (Walker et al., 2011). Agarose has been shown to have high mechanical strength with tensile strengths of 31.03 ± 0.74 MPa reported, along with an elongation at break value of 45.2 ± 2.7% (Rhim, 2012).
Carrageenan is a relatively under researched polygalacton derived from the Rhodophyceae members of red algae seaweeds. The material is composed of alternating long chains of α-1, 3 D-galactose, and β-1, 4 3, 6-anhydro-galactose with ester sulfates which emulate the structure of mammalian glycosaminoglycans (Yegappan et al., 2018). Carrageenan can undergo both thermal and ionic gelation, and combined with other materials such as poly(oxyalkylene amine) (Bakarich et al., 2014), methacrylic anhydride (Chimene et al., 2018) and nanosilicates (Wilson et al., 2017; Chimene et al., 2018) to produce a printable bioink capable of extrusion and shape retention, with high fidelity, elasticity and stiffness (Wilson et al., 2017) to enable cross-linkable multi-layered tissue constructs. Young's modulus of carrageenan, like agarose is dependent upon the concentration of carrageenan, with values ranging from 0.10 MPa (1% carrageenan) to 0.66 MPa (3% carrageenan) and becoming increasingly unpredictable as water content increases (In et al., 2014). The tensile strength of carrageenan has been reported as 39.34 ± 0.51 MPa, as well as elongation at break value of 19.5 ± 0.4% (Rhim, 2012). Carrageenan-based bioinks have demonstrated good cell viability and attachment (Chimene et al., 2018), plus an affinity for osteogenesis (Li J. et al., 2015), augmenting the compressive strength of collagen-hydroxyapatite based composite gels for bone tissue engineering (Feng et al., 2017). The presence of the sulfated backbone in carrageenan mimics naturally occurring sulfated glycosaminoglycans in cartilage extracellular matrix (Bhattacharyya et al., 2010; Popa et al., 2015) and has demonstrated chondrogenicity, non-toxicity and mechanical properties similar to that of native cartilage (Popa et al., 2015). The facile crosslinking and glycosaminoglycan mimicry make carrageenan an especially exciting prospect for cartilage bioinks for tissue engineering.
Pectin is a natural constituent of plant cell walls, possessing high molecular weight, and hydrophilicity, making it an ideal candidate for hydrogel formation and subsequently 3D bioprinting. As with other model bioinks, pectin is a highly versatile hydrogel with rheological and viscoelastic properties amenable to modulation, independently of the cell adhesive ligand density (Pereira et al., 2018a). The elastic modulus (G') of pectin may be finely tuned through adjustments in polymer and/or crosslinker concentration, thus allowing for the biomechanical mimicry of a broad range of native human tissue types. Ionic crosslinking with CaCl2 also results in a substantial increase in yield stress (τy) and therefore significantly augments the extrudability of the hydrogel and shape fidelity of the printed construct (Cui et al., 2017).
Industrially, pectin is derived from waste materials from juices, apples, and cider industries through acidic and thermal extraction (Jayani et al., 2005). Gelation is possible through densely concentrated pectin solutions, thereby increasing polymeric entanglements, but also through exposure to acidic conditions and divalent or trivalent cations (Munarin et al., 2012). Similarly to alginate, pectin is readily crosslinked in the presence of divalent calcium ions, which interact through the carboxyl groups to induce bridges between homogalacturonic chains (Fang et al., 2008). Pectin, compared to other natural polymers is largely unexplored in its tissue engineering and regenerative capacity (Munarin et al., 2012). A major limitation, as with alginate amongst other polysaccharides, is its limited capacity for cell adhesion. Nonetheless, the role of pectin as a scaffold for bone tissue engineering has been explored with promising potential (Coimbra et al., 2011; Munarin et al., 2011), with cell adhesion augmented through chemical modification (Kokkonen et al., 2006) and combination with other materials such as polyvinylalcohol (Yao et al., 2009).
Starch is a highly abundant storage polysaccharide derived from cereals and tuber plants such as potatoes (Lu et al., 2009). Starch molecules are typically composed of ~30% amylose and 70% amylopectin, though natural variation from this ratio occurs depending on the source of extraction (Cherian Vengal and Srikumar, 2005). Starches with higher amylose content confer greater crystallinity and subsequent firmness (Ige et al., 2012). Its hygroscopic nature enables reversible hydration and therefore promotes seamless hydrogel ejection for extrusion-based printing methods.
The viscoelastic properties of starch are principally reliant on several mechanical variables, namely concentration, extrusion temperature, storage modulus (G'), yield stress (τy), and flow stress (τf). As a proxy for construct stability, G' and τy refer to the hydrogels potential to withstand its own weight when printed in successive layers whilst concentration, temperature, and τf are markers of extrudability. Intuitively, a moderate to high G' and τy in combination with a low τf constitute the ideal parameters for a highly desirable starch hydrogel for heat extrusion 3D bioprinting (Chen et al., 2019). In their rheological characterization study, Chen et al. (2019) demonstrated that Rice Starch of concentration 15–25% (w/w) extruded at 80°C, Potato Starch [15–20% (w/w)] at 70°C and Corn Starch [20–25% (w/w)] at 75°C expressed ideal τy [32–455 Pascals (Pa)], τf (140–722 Pa), and G' (1150–6909 Pa) values for heat extrusion 3D Bioprinting, thereby conferring excellent printability, shape fidelity and resolution. In a separate study, the use of starch hydrogels with a predominantly elastic response (i.e., tan δ of ~0.1 and 0.2), yield stress between 60 and 730 Pa and dynamic modulus (G*) between 300 and 1,200 Pa at 1 Hertz (Hz) has been extensively advocated (Huang, 2018). It is, however, important to appreciate that although primarily related to the 3D bioprinting of edible products, these printing specifications remain to be translated into application for 3D tissue engineering.
Furthermore, starch molecules may be compounded with other biopolymers to augment properties for bioprinting applications (Ige et al., 2012). Starch is considered highly biocompatible, and as such starch and its compounds have been considered as potential scaffolds for tissue engineering and drug delivery. Indeed cell encapsulation within starch-based hydrogels have been investigated to demonstrate high cell viability and adhesion with rheological properties analogous to a myriad of human tissue types (Dong et al., 2016). More specifically, the rheological versatility of starch allows for the flexible regulation of hydrogel composition to closely emulate the storage modulus of native tissue, thereby satisfying various mechanical requirements as an ideal 3D culture platform for different cell types. Additionally, the effect of hydrogel compositional variations on swelling kinetics and equilibrium swelling ratios has also been examined. In particular, the hydrophilic nature of sulfobetaine-derived starch encourages water retention within the hydrogel; a characteristic which potentiates nutrient, signal factor and metabolic waste transportation and ultimately promotes the differentiation of encapsulated cells (Dong et al., 2016). As such, research into the role of starch-based polymers for 3D bioprinting has been commenced, in particular for bone tissue engineering (Gomes et al., 2002; Salgado et al., 2004; Martins et al., 2009). However, further research needs to be undertaken to ascertain its validity as a viable bioink for multi-tissue bioprinting purposes.
Fucoidan is an anionic sulfated, water-soluble polysaccharide derived from marine brown algae. The material has garnered interest owing to its potential antioxidant, anti-inflammatory (Fitton, 2011), and angiogenic (Purnama et al., 2015; Marinval et al., 2016) properties for tissue engineering purposes. The rheological properties of fucoidan also appear conducive of bioprinting with shear thinning behavior observed below 1.5% weight-volume yet plastic behavior at 2% with a yield value of 2Pa. Dynamic viscoelasticity was also observed: increased by increasing the concentration in solution and through the addition of NaCl and CaCl2, and decreased at higher temperatures (Tako, 2003). Both linear and branched subtypes of fucoidan have been characterized with distinct rheological properties. Indeed the variability in viscosity appears to vary with the species of seaweed from which the fucoidan is derived, reflecting differences in molecular weight, proportion of sulfates, and uronic acids.
As with many of the polysaccharides, combination of this material with chitosan, gelatin, alginate, and hydroxyapaitite has been performed to produce constructs for wound dressings and bone tissue engineering scaffolds (Venkatesan et al., 2014; Lowe et al., 2016). Fucoidan has also been combined with chemotherapeutic agents displaying promise as a drug delivery material in vitro and is believed to possess indirect antimicrobial activity (Lee et al., 2013) which confer excellent biological potential for the development of future bioinks.
The abundancy, gelation capacity, and bioactivity of plant derived biomaterials hold exciting potential as bioinks for 3D bioprinting. In particular, extensive research has been undertaken on the potential role of alginate and nanocellulose as tissue engineering scaffolds, wound healing adjuncts, drug delivery systems, and implantable medical devices. As such, current research on these two biomaterials at the forefront of bioprinting technology will be explored throughout the subsequent section of this review.
Biocompatibility describes a harmonious and non-deleterious relationship between implanted foreign material and host tissue. Hydrogels are designed to mimic the composition of natural extracellular matrix with high cellular biocompatibility (Aljohani et al., 2018), whilst demonstrating the viscoelastic properties to promote fluid dispersion through extrusion-based bioprinting methods. These features are readily exploited in the field of biomedicine; where alginate, nanocellulose, and other plant derived biomaterials are typically employed as model hydrogels (Cardoso et al., 2016) for tissue engineering, pharmaceutical drug delivery, and wound healing (Santana et al., 2013; Patil et al., 2018).
With respect to cellulose and its derivatives, biocompatibility is thought to be heavily reliant on surface chemistry, most notably: topography, wettability, charge and presence of hydrophobic, and hydrophilic domains (Pertile et al., 2010). Intuitively, modification of these parameters has been shown to augment cellular adhesion and bioactivity. In particular, modulation of CNC surface charge is known to increase cellular affinity for the biomaterial through an increase in cell adhesion proteins and subsequent cellular proliferation (Aggarwal et al., 2013). Moreover, nanocellulose also demonstrates good hemocompatability in vitro (Wang et al., 2013) and when implanted into mice models in vivo (Shimotoyodome et al., 2011). The functionalization of polyurethane with CNF has recently shed light on the potential use of nanocellulose as a novel candidate for vascular prosthesis due to its low thrombogenicity and exceptional biochemical versatility (Lin and Dufresne, 2014). Alginate is perceived to be highly biocompatible, non-thrombogenic polymer in its natural state, and thus has been utilized in a plethora of medical treatments (Liberski). However, alginate is amenable to structural modification to render it both thrombogenic and anti-thrombogenic. In its sulfonated form, alginate has been shown to have an anti-thrombogenic effect; alginate sulfate has been shown to be comparable to heparin (also a sulfated polysaccharide) when measuring the activated partial thrombin time (APTT), but shows no significant effect on the prothrombin time (PT), leading to the conclusion that it has an inhibitory effect on the intrinsic coagulation pathway (Ahearne et al., 2005). Whereas, calcium sodium alginate fibers are utilized widely in clinical practice as hemostatic wound dressings (Thomas, 2000). The modifiable properties of alginate undoubtedly augment its applications for clinical use and further examples of clinical trials using alginate include the treatment of pressure sores, post-operative intrauterine adhesions, reflux suppression, weight loss, skin grafts, burns, and surgical wounds (Nowacki et al., 2017).
Overall, nanocellulose, like most plant derived biomaterials, is widely regarded as a biocompatible resource, with negligible immunogenic responses observed in vivo (Miyamoto et al., 1989). The lack of cross reactivity may somewhat be attributed to its inherent resistance to degradation within the human body, largely pertaining to the absence of cellulase enzymes in humans. It, however, still stands that the long-term effects of a non-degradable material may confer a risk of delayed immunogenicity (Lin and Dufresne, 2014).
Closely allied to biocompatibility is the concept of toxicity, with both terms often used synonymously. The cytotoxicity of CNC against nine individual cell lines was determined and exhibited no cytotoxic phenomena in the concentration range and exposure time studied (0–50 μg/ml and 48 h) (Dong et al., 2012). Furthermore, no cytotoxic or inflammatory events were recorded in the assessment of NFC on mouse and human immune cell lines in vitro (Vartiainen et al., 2011). In contrast, various studies have conversely described an increase in cytotoxicity and gene expression modification, more-so at higher CNC concentrations (> ~100 μg/ml). In particular (Pereira et al., 2013), demonstrated a dose-dependent reduction in fibroblast cell viability, with a positive relationship between CNC concentration and the expression of stress related biomarkers (HSP70.1, PRDX1 & BAX). Such dose dependent toxicity has also been illustrated in vivo following inhalation of CNC (Lin and Dufresne, 2014).
Notwithstanding the vast evidence in support of plant derived biomaterials as an inherently safe biomaterial, further research may be required to comprehensively characterize their toxicology for biomedical application. More specifically, the potential risks associated with modification and crosslinking should be thoroughly explored.
Current challenges within the field of tissue engineering focus on the production of biocompatible scaffolds which accurately mimic the native in vivo environment. As previously highlighted, the topography and architecture of natural scaffolds (i.e., surface topology and porosity, fiber density, and network structure) fundamentally dictates the cell-biomaterial interactions and, therewith, cellular behavior (Jorfi and Foster, 2015). With specific reference to 3D bioprinting, a balance between these biological qualities and the requirements for good printability is often difficult to attain.
According to a recent report by Global Market Insights, Inc (Nanocellulose Market Size–Industry Share Analysis Report 2017-20241) the nanocellulose market is projected to exceed USD 1 billion by 2024. In light of such great economic potential, the amount of research activity surrounding the use of lignocellulosic biomaterials continues to grow exponentially (Jorfi and Foster, 2015). For this reason, the applicability of nanocellulose at both microscopic (cell culture) and macroscopic (tissue engineering, repair, implants, drug delivery etc) levels is of significant value to the field of biomedicine.
Alginate has appeal as a candidate biomaterial owing to its low-cost, highly biocompatible, biodegradable, and non-toxic nature plus its ability to be processed into a hydrogel (Ching et al., 2017). Of the plant derived biomaterials, alginate is used most extensively for 3D bioprinting applications. Biocompatible scaffolds using alginate hydrogels have been shown to have great efficacy for tissue engineering and cell culture (Dai et al., 2016), plus the high biodegradability and renewability of alginate is a major advantage over plastic-based compounds in 3D bioprinting (Ching et al., 2017).
The versatility of plant derived biomaterials such as alginate and nanocellulose as isolated and composite scaffold have been demonstrated in the 3D bioprinting of blood vessels, bone, cartilage, and skeletal muscle (Table 2). However, the validity of plant derived biomaterials are not without limitation. Biofabrication of nanocellulose is hindered by poor cross-linking potential and shape fidelity post-printing, whereas alginate alone has only a moderate affinity for cell-adhesion or proliferation (Lee and Mooney, 2012). As such, nanocellulose and alginate hydrogels are often combined with materials that augment the desired characteristics for enhanced 3D bioprinting.
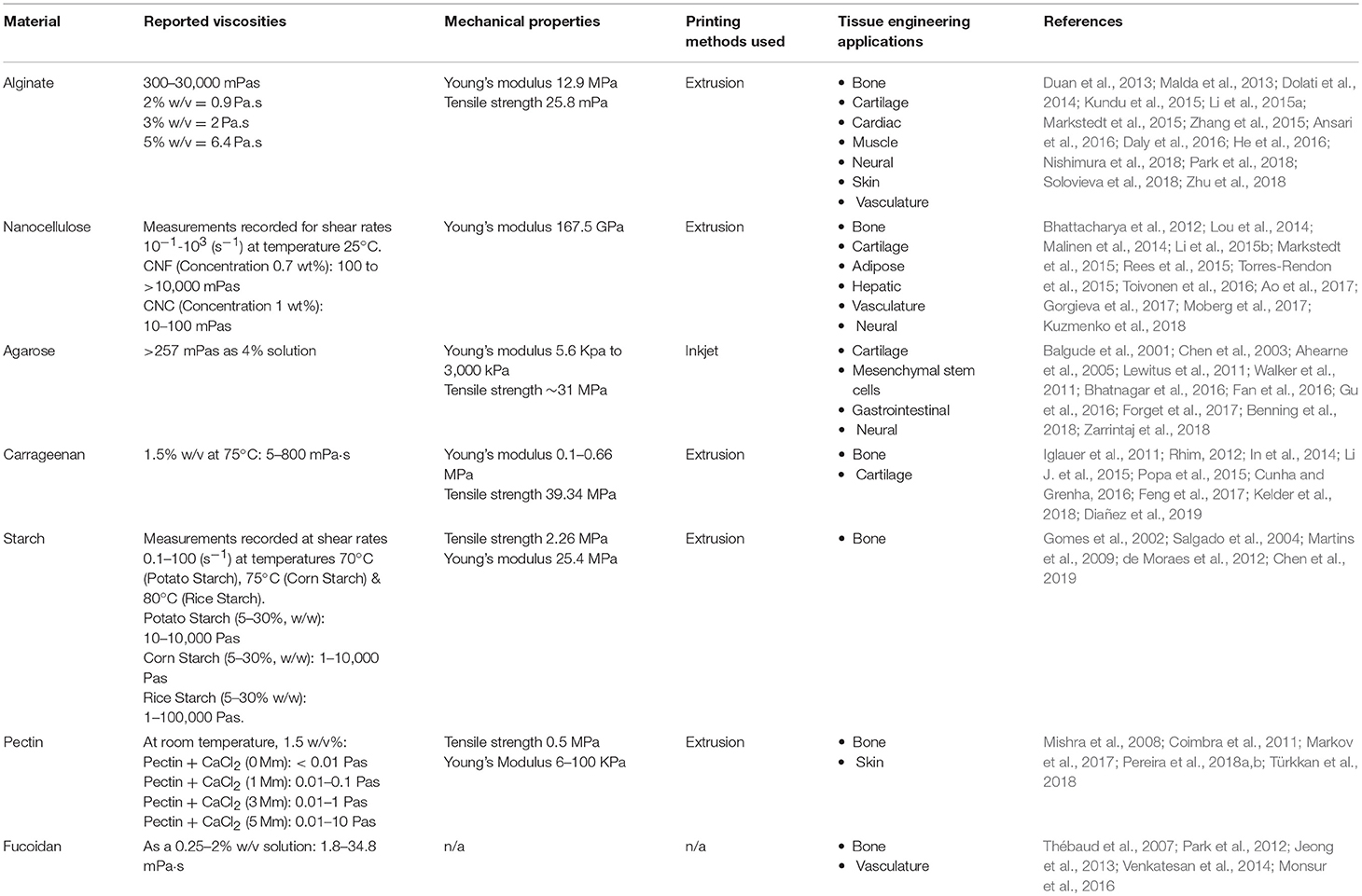
Table 2. Properties and tissue engineering applications for plant-derived biomaterials (n/a = not available).
Using extrusion-based bioprinting with an alginate scaffold, vascularized bone tissue has been synthesized with high tissue viability (Dhawan et al., 2018). Whole bone organ engineering has been studied using an alginate based bioink with Arg-Gly-Asp adhesion peptides reinforced with polycaprolactone fibers. This combination of materials made it possible to engineer an entire vertebral body. This in vivo supported vascularized endochondral bone with supporting marrow structure, potentially leading to future prospects of bioprinting vertebrae for patients with vertebral osteoporosis/fractures (Daly et al., 2016). Likewise, NFC composite scaffolds for 3D bio-printing of bone tissue have also been successfully constructed from a variety supporting biomaterials, most notably chitin (Torres-Rendon et al., 2015), gelatin (Gorgieva et al., 2017), and hydroxyapatite (Ao et al., 2017).
Cartilage tissue has been successfully bioprinted using an alginate-nanocellulose bioink, in which the study concluded the combination alginate and nanocellulose was conducive to bioprinting live human cartilage (Markstedt et al., 2015). The study successfully combined the rheological properties of NFC (i.e., high printing resolution and shape fidelity) with the increased storage modulus of alginate for cartilage tissue engineering applications. The sheer thinning behavior of the composite hydrogel (NFC-A) enabled printing of anatomically shaped cartilage constructs (e.g., human ear and sheep meniscus), with high human chondrocyte viability (86%) after 7-day culture. It was later determined that the same NFC-A scaffold promotes human nasal chondrocyte redifferentiation, conservation of phenotype and production of cartilage specific ECM components, thus resembling in vivo chondrogenesis (Martínez Ávila et al., 2016), plus when using human nasal chondrocytes, cell proliferation was high and there was enhanced deposition of human collagen II (Möller et al., 2017). For the purposes of cartilage tissue engineering it has also been demonstrated that combining alginate with nanocellulose addresses the issue of cell adhesion, thereby increasing cartilage ECM deposition (Aljohani et al., 2018). Combining alginate with collagen has also surmounted the issue of cell adhesion, demonstrating increased cell viability, tensile strength, cellular proliferation, and an increased capacity for cell adhesion (Yang et al., 2018).
Advances in soft tissue engineering have similarly resulted in NFC and alginate based conductive bio-inks for the generation of neural, dermal and vascular tissue scaffolds, among others. It was discovered that human-derived neuroblastoma cell adhesion, differentiation and proliferation was potentiated by a NFC-carbon nanotube composite with minimal effect on cell viability (Kuzmenko et al., 2018). Furthermore, as validated by Li et al. (2015b) bFGF impregnated CNC-collagen scaffolds were able to demonstrate angiogenic and skin generation capabilities at in vitro and in vivo alike, thus alluding to their infinite potential within the field of regenerative medicine. Fabrication of tissue engineered aortic valve conduits using an alginate/gelatin polymer bioink has been successfully achieved using 3D bioprinting techniques. It was found that by direct encapsulation of smooth-muscle cells and aortic valve leaflet interstitial cells, the tensile strength of the biopolymer held after a 7 day culture (Duan et al., 2013). Alginate based scaffolds have also been tested in vitro as a potential candidate for muscle tissue engineering (Ansari et al., 2016).
A significant challenge in tissue engineering is vascularization. Adequate blood supply to new tissues is a prerequisite for the survival in vivo, and until addressed will limit the scale of viable tissue that can be produced. Using advancements in three dimensional printing technology, it is now possible to synthesize intricate hollow, branched blood vessels and artificial blood vessels have now been created and optimized using 3D printing technology. An initial technique involved using an alginate bioink laced with carbon nanotubes to 3D bioprint mechanically stable vascular conduits. This study concluded that further research should focus on replacing the nanotubes with natural protein nanofibers to allow for large scale tissue production (Dolati et al., 2014). Alginate has been used to encapsulate human umbilical vein smooth muscle cells and thereafter bioprint vasculature conduits in vitro. These conduits showed good proliferative activity and deposition of both smooth muscle matrix and collagen on the peripheral and luminal surface when examined histologically (Zhang et al., 2015). This technique has been further extrapolated to bioprint a perfusable human coronary artery tree using alginate as the primary scaffold material (Hinton et al., 2015). Interestingly, injectable alginate has been shown to promote neovascularization, restore partial blood flow, and tissue function of the heart muscle in-vivo, when injected directly into the ischemic site of patients with myocardial infarction or peripheral arterial disease (Ungerleider and Christman, 2014), alluding to a potential angiogenic role of this natural biomaterial. Bioprinting of alginate microfibers have additionally been combined with theta-glass capillaries to facilitate the formation of vascular architecture with exciting implications for the future of vascularized tissue engineering (Nishimura et al., 2018).
The applicability of NFC as a stand-alone resource has also been fruitfully explored within the realms of cell culture and organoid development, with implications for organ replacement, and drug testing modalities. In a study on 3D organoid development, wood-derived NFC hydrogels were used to successfully expedite the differentiation of HepaRG liver progenitor cell lines in vitro (Bhattacharya et al., 2012). Their results demonstrated the formation of 3D multicellular spheroids with the morphological hallmarks of hepatic tissue, namely functional bile canaliculi and apicobasal polarity. Similarly, the use of NFC hydrogels as a viable medium for human pluripotent stem cell culture ultimately promises a flexible and xeno-free resource for application within drug research and regenerative medicine industries (Lou et al., 2014).
Despite their promise as bioinks for tissue engineering, further characterization of the toxicological properties, immunogenicity, and in vivo durability of these materials needs to be elucidated to determine their validity over widely used synthetic materials such as polylactic acid and polycaprolactone (Pariente et al., 2001). Many of the plant-based materials are being translated from the food industry, where their bioactivity, rheological properties, and facile gelation have garnered significant interest in bioprinting (Seidel et al., 2017; Vancauwenberghe et al., 2017). However, many of the crosslinking methods, especially those involving high temperatures, harsh chemical conditions and ultraviolet radiation, warrant further adaptation to ensure biocompatibility, and cell viability is preserved if these methods are to be used for tissue bioprinting purposes. The degree of heterogeneity in biomaterial blends observed in this review reflects the need to optimize mechanical properties to match the demands of the target tissue type. As such, further refinement and characterization of plant-based composite bioinks is both anticipated and warranted prior to considering their application for clinical use.
Hydrogels hold exciting potential for wound healing applications in maintaining a moist environment conducive of accelerated healing with high biocompatibility to facilitate cell migration, proliferation, and reepithelialisation (Liu et al., 2016; Kamoun et al., 2017). Furthermore, their ability to encapsulate cells, growth factors, and antibacterials holds tremendous promise for the bioprinting of customizable wound dressings (Boateng et al., 2013).
Nanocellulose continues to command significant scientific interest for its role in the homeostatic regulation of wound healing. NFC-based hydrogels and films have been shown to exhibit lower wound adherence, high moisture content, and the ideal swelling properties to closely emulate in vivo tissue repair (Madaghiele et al., 2014). The versatility of nanocellulose is further reflected in its affinity for structural modification and functionalization. The porosity and surface topography of NFC hydrogels may be modified to augment the adsorptive and bacterial anti-adhesion capabilities of the wound dressing (Jack et al., 2017). Two studies (Rees et al., 2015) (Chinga-Carrasco and Syverud, 2014) highlighted the potential benefits of carboxymethylated-periodate oxidized nanocellulose (C-Periodate NC) for 3D bioprinting and wound dressing applications. Following comparative analysis, C-Periodate NC was found to exhibit rheological favourability (i.e., marked shear thinning, low viscosity) over the TEMPO oxidized alternative (Rees et al., 2015). C-Periodate's unique poly-anionic surface chemistry was also shown to encourage the formation of 3D micro-porous structures with pH responsitivity. Such technology may be utilized in chronic wound management for the controlled release of antimicrobial agents into biofilms (Chinga-Carrasco and Syverud, 2014).
Alginate has been produced as sponges, foams, fibers, and hydrogels for wound healing purposes (Sun and Tan, 2013). Its gel-forming ability enables a moist environment surrounding the wound, promoting healing, and leading to a superior cosmetic repair (Zhu et al., 2018). High water absorptivity and optimal water vapor transmission rate make alginate excellent at treating wounds with large volume exudate; in addition, cell-adhesion is limited thereby reducing the occurrence of secondary injury when removing the dressing, making alginate excellent for use in burns patients (Mirzaei et al., 2018). Other useful effects of alginate include a haemostatic effect and stimulation of monocytes to produce IL-6 and TNF-α, thus promoting pro-inflammatory factors which aid in wound healing (Sun and Tan, 2013). Colonization by potentially pathogenic microbes is a perceived disadvantage of conventional alginate dressings. Anti-microbial variants has been created based upon a combination of alginate and chitosan nanoparticles (Karri et al., 2016), high concentration M based alginate aerogels with an amidated pectin and doxycycline core (De Cicco et al., 2016), and combining alginate and calcium fluorine in a nanocomposite hydrogel, which inhibit bacterial growth and promotion cell proliferation for wound healing (Shin et al., 2019). 3D bioprinting personalized wound dressings holds the potential for the dressings to be printed as biomimics of the defect site, this may be achievable by seeding with skin, fat or muscle cells and/growth factors in order to augment the healing process (Aduba and Yang, 2017) and would also enable the controlled microspatial placement of antimicrobial agents.
The potential role of plant derived biomaterials for wound dressings composed of carrageenan (Yegappan et al., 2018), starch (Pal et al., 2006; Kamoun et al., 2017), and pectin (Giusto et al., 2017) have been explored, with evidence of cell migration into chronic wounds, and the incorporation of antimicrobial agents having also been documented (Yegappan et al., 2018).
The safety and efficacy of drug delivery can be enhanced and temporally controlled through the attachment to a biomaterial carrier. Within the 3D bioprinting strategies for versatile drug delivery, plant-derived scaffolds have demonstrated promise in numerous clinical settings. In the pharmaceutical industry, biomaterials such as alginate, and cellulose are used as an excipient to stabilize and protect the active drug compound, especially in non-water-soluble drugs (Crowley et al., 2004; Cardoso et al., 2016). Alginate may also be used as a carrier to immobilize and encapsulate drugs, bioactive molecules, proteins, and cells due to its high biocompatibility and biodegradability (Senna et al., 2018).
Hydrogels, porous scaffolds and microspheres have also been investigated for controlled drug use. Alginate has been used to enable modified/extended release of an active drug within the body (Tønnesen and Karlsen, 2002) and as a component in the construction of capsules used for cell-encapsulation. For cytotherapy, combined with chitosan, the mixture demonstrates high cell viability (Correia et al., 2013) and may be also combined with calcium carbonate to selectively trap of molecules on hydrophilic domains, or polyethylene glycol to protect the microcapsules from acidic environments such as gastric acid (Borvinskaya et al., 2018; Ren et al., 2018). These properties have enabled composites of alginate to be trialed in the treatment of degenerative diseases such as osteoarthritis (Wang et al., 2011). The controlled delivery of TGF-β, using macro-porous alginate scaffolds, have been shown to repair articular cartilage defects in rabbits (Mierisch et al., 2002) and alginate blended with polyethylene glycol has been used as a vector in delivering VEGF to hMSCs to encourage osteogenic differentiation (Miao et al., 2014).
Specific placement of drug molecules in hydrogels such as alginate is attainable using 3D bioprinting techniques (El-Sherbiny and Yacoub, 2013). Layer-by-layer assembly of gelatin and alginate has been used to load insulin-like growth factor-1 (IGF-1) onto neural stem cells (NSCs); the alginate enabled modified release of IGF-1 onto NSCs. The result significantly improved the proliferation and differentiation of NSCs leading to a potential treatment for nervous system disorders such as stroke (Li et al., 2015a).
The combined drug delivery and bioink potential of plant derived materials heralds an exciting future for the development of targeted chemotherapies and wound management strategies. NFC-PNIPAM hybrid microspheres were extensively studied by and exhibited high drug-loading capacity and unique dispersion properties for 5-fluorouracil. In comparison, polyethyleneimine-alginate nanoparticles have been shown to be an effective vector for the transfection of functional DNA and siRNA into target cells, with potential applications within gene silencing of viral loaded and malignant cells (Weinberg and Morris, 2016; Wang et al., 2017).
Nanocellulose materials can also be modified with chitosan oligosaccharide for the prolonged systemic release of cationic drugs such as Imipramine and Procaine (Akhlaghi et al., 2014) whereas the poly-anionic surface chemistry of oxidized nanocellulose demonstrates a micro-porous architecture with extensive drug loading capacity and delivery (Chinga-Carrasco and Syverud, 2014) displaying pH responsivity. As such, dressings with the potential for controlled and intelligent antimicrobial and analgesia release could be bioprinted using this technology. It therefore stands that sustained future research promises a wealth of insight into the design of drug delivery systems for novel and biologically active pharmaceutical technologies.
The degradability of biological materials such as starch and alginate in vivo increases their appeal as a scaffold material for tissue engineering, enabling their replacement with the expanding mass of de novo tissue. These properties do however render them unsuitable for use as a permanent medical device or implant. Nanocellulose however, generally portrays low biodegradability in vivo owing to an absence of cellulase enzymes in vertebrates. With the relatively corrosive internal bodily environment considered, the innate resistance of cellulose to external chemical perturbations makes it an invaluable resource in the development of implant applications. Due to their pseudoplastic nature, nanocellulose bioinks may be intricately extruded to form complex and cell compatible 3D hydrogel constructs with remarkable shape fidelity and definition. In general, higher nanocellulose concentrations have been shown to yield better print resolution (Siqueira et al., 2017). Such malleability also permits for the printing of an infinite number of structural configurations which may be customized to meet a range of patient specific requirements. Nanocellulose has thus been exploited in the generation of 3D bioprinted tissue implants for soft and hard tissue replacement (Lin and Dufresne, 2014). Of note, CNF-polyurethane based vascular prostheses were successfully implanted between the brachiocephalic and right common carotid artery in a 26-year old male patient with Multiple Endocrine Neoplasia 2B (Cherian et al., 2011). Similarly, a viable replacement for native human nucleus palposus was also constructed from a carboxymethylated CNF bio-composite hydrogel prepared using UV polymerization of N-vinyl-2-pyrrolidone (Eyholzer et al., 2011). Other novel approaches have included the exploration of a CNC-Polyvinylalcohol composite biomaterial as an external stimuli responsive neural implant (Shanmuganathan et al., 2010) and the generation of 3D bioprinted cartilaginous models for in vivo application (Monllau et al., 2010; Markstedt et al., 2015).
The applications of plant derived biomaterials for 3D bioprinting are immense. Enormous appeal lies in their gelation capacity, hydrophilicity, and natural rigidity which appear to emulate the extracellular matrix and render them ideal biological and mechanical candidates for bioprinting tissues, organs, and biomedical adjuncts. Further applications of these natural materials may extend to wound dressings, implantable medical devices and drug delivery systems, potentiated by coupling to bioprinting technologies for truly customizable, and bioactive medical therapies such as wound dressings and drug delivery systems. There is a need to further refine the properties of the bioinks, which appear to be enhanced through combination with other materials to meet the biological and mechanical demands of different tissue types, and a robust investigation into the toxicity of the materials is additionally merited prior to in vivo implementation. Irrespective of the current challenges for translation to clinical practice, this renewable, natural, and abundant set of versatile biological materials hold great promise for revolutionizing the future of bioprinting.
TJ and IW conceived the idea for the article, planned the structure, and edited the final manuscript. TJ, GK, and AM each wrote significant proportions of the body of the final manuscript text.
TJ would like to thank the support of the Welsh Clinical Academic Training Scheme, the Royal College of Surgeons England Research Fellowship and Microtia UK.
The Reconstructive Surgery and Regenerative Medicine Research Group has a joint disclosure agreement for ongoing nanocellulose research with American Process Inc. and a patent pending on nanocellulose bioinks (US Patent Application No. 62/639,38).
1. ^Nanocellulose Market Size—Industry Share Analysis Report 2017-2024. Available online at: https://www.gminsights.com/industry-analysis/nanocellulose-market (accessed December 30, 2018).
Aduba, D., and Yang, H. (2017). Polysaccharide fabrication platforms and biocompatibility assessment as candidate wound dressing materials. Bioengineering 4:E1. doi: 10.3390/bioengineering4010001
Aggarwal, N., Altgärde, N., Svedhem, S., Zhang, K., Fischer, S., and Groth, T. (2013). Effect of molecular composition of heparin and cellulose sulfate on multilayer formation and cell response. Langmuir 29, 13853–13864. doi: 10.1021/la4028157
Aguado, B. A., Mulyasasmita, W., Su, J., Lampe, K. J., and Heilshorn, S. C. (2012). Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 18, 806–815. doi: 10.1089/ten.TEA.2011.0391
Ahearne, M., Yang, Y., El Haj, A. J., Then, K. Y., and Liu, K.-K. (2005). Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface 2, 455–463. doi: 10.1098/rsif.2005.0065
Akhlaghi, S. P., Tiong, D., Berry, R. M., and Tam, K. C. (2014). Comparative release studies of two cationic model drugs from different cellulose nanocrystal derivatives. Eur. J. Pharm. Biopharm. 88, 207–215. doi: 10.1016/J.EJPB.2014.04.012
Aljohani, W., Ullah, M. W., Zhang, X., and Yang, G. (2018). Bioprinting and its applications in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 107, 261–275. doi: 10.1016/j.ijbiomac.2017.08.171
Annamalai, R. T., Mertz, D. R., Daley, E. L. H., and Stegemann, J. P. (2016). Collagen Type II enhances chondrogenic differentiation in agarose-based modular microtissues. Cytotherapy 18, 263–277. doi: 10.1016/J.JCYT.2015.10.015
Ansari, S., Chen, C., Xu, X., Annabi, N., Zadeh, H. H., Wu, B. M., et al. (2016). Muscle tissue engineering using gingival mesenchymal stem cells encapsulated in alginate hydrogels containing multiple growth factors. Ann. Biomed. Eng. 44, 1908–1920. doi: 10.1007/s10439-016-1594-6
Ao, C., Niu, Y., Zhang, X., He, X., Zhang, W., and Lu, C. (2017). Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int. J. Biol. Macromol. 97, 568–573. doi: 10.1016/J.IJBIOMAC.2016.12.091
Awadhiya, A., Kumar, D., Rathore, K., Fatma, B., and Verma, V. (2017). Synthesis and characterization of agarose–bacterial cellulose biodegradable composites. Polym. Bull. 74, 2887–2903. doi: 10.1007/s00289-016-1872-3
Axpe, E., and Oyen, M. L. (2016). Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 17:E1976. doi: 10.3390/ijms17121976
Bajpai, S. K., and Sharma, S. (2004). Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 59, 129–140. doi: 10.1016/J.REACTFUNCTPOLYM.2004.01.002
Bakarich, S. E., Balding, P. III., R, G., Spinks, G. M., and Panhuis, M. in het (2014). Printed ionic-covalent entanglement hydrogels from carrageenan and an epoxy amine. RSC Adv. 4, 38088–38092. doi: 10.1039/C4RA07109C
Balgude, A. P., Yu, X., Szymanski, A., and Bellamkonda, R. V. (2001). Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials 22, 1077–1084. doi: 10.1016/S0142-9612(00)00350-1
Beck, E. C., Barragan, M., Tadros, M. H., Gehrke, S. H., and Detamore, M. S. (2016). Approaching the compressive modulus of articular cartilage with a decellularized cartilage-based hydrogel. Acta Biomater. 38, 94–105. doi: 10.1016/j.actbio.2016.04.019
Benning, L., Gutzweiler, L., Tröndle, K., Riba, J., Zengerle, R., Koltay, P., et al. (2018). Assessment of hydrogels for bioprinting of endothelial cells. J. Biomed. Mater. Res. Part A 106, 935–947. doi: 10.1002/jbm.a.36291
Bertassoni, L. E., Cardoso, J. C., Manoharan, V., Cristino, A. L., Bhise, N. S., Araujo, W. A., et al. (2014). Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 6:024105. doi: 10.1088/1758-5082/6/2/024105
Bhatnagar, D., Simon, M., and Rafailovich, M. H. (2016). “Hydrogels for regenerative medicine,” in Recent Advances in Biopolymers (InTech). doi: 10.5772/62044
Bhattacharya, M., Malinen, M. M., Lauren, P., Lou, Y.-R., Kuisma, S. W., Kanninen, L., et al. (2012). Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 164, 291–298. doi: 10.1016/J.JCONREL.2012.06.039
Bhattacharyya, S., Liu, H., Zhang, Z., Jam, M., Dudeja, P. K., Michel, G., et al. (2010). Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds. J. Nutr. Biochem. 21, 906–913. doi: 10.1016/J.JNUTBIO.2009.07.002
Boateng, J. S., Pawar, H. V., and Tetteh, J. (2013). Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. Int. J. Pharm. 441, 181–191. doi: 10.1016/J.IJPHARM.2012.11.045
Borvinskaya, E., Gurkov, A., Shchapova, E., Baduev, B., Meglinski, I., and Timofeyev, M. (2018). Distribution of PEG-coated hollow polyelectrolyte microcapsules after introduction into the circulatory system and muscles of zebrafish. Biol. Open 7:bio030015. doi: 10.1242/bio.030015
Campos, F., Bonhome-Espinosa, A. B., Vizcaino, G., Rodriguez, I. A., Duran-Herrera, D., López-López, M. T., et al. (2018). Generation of genipin cross-linked fibrin-agarose hydrogel tissue-like models for tissue engineering applications. Biomed. Mater. 13:025021. doi: 10.1088/1748-605X/aa9ad2
Cardoso, M. J., Costa, R. R., and Mano, J. F. (2016). Marine origin polysaccharides in drug delivery systems. Mar. Drugs 14:E34. doi: 10.3390/md14020034
Catros, S., Guillotin, B., Bačáková, M., Fricain, J.-C., and Guillemot, F. (2010). Effect of laser energy, substrate film thickness and bioink viscosity on viability of endothelial cells printed by Laser-Assisted Bioprinting. Appl. Surf. Sci. 257, 5142–5147. doi: 10.1016/j.apsusc.2010.11.049
Chen, H., Xie, F., Chen, L., and Zheng, B. (2019). Effect of rheological properties of potato, rice and corn starches on their hot-extrusion 3D printing behaviors. J. Food Eng. 244, 150–158. doi: 10.1016/J.JFOODENG.2018.09.011
Chen, Q., Suki, B., and An, K.-N. (2003). Dynamic Mechanical Properties of Agarose Gel by a Fractional Derivative Model. Available online at: https://pdfs.semanticscholar.org/ecfb/27677cc4ba385fa67b547c9c41ae796edd97.pdf (accessed February 26, 2019).
Cherian Vengal, J., and Srikumar, M. (2005). Processing and Study of Novel Lignin-Starch and Lignin-Gelatin Biodegradable Polymeric Films. Available online at: http://www.sbaoi.org [accessed December 28, 2018].
Cherian, B. M., Leão, A. L., de Souza, S. F., Costa, L. M. M., de Olyveira, G. M., Kottaisamy, M., et al. (2011). Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydr. Polym. 86, 1790–1798. doi: 10.1016/J.CARBPOL.2011.07.009
Chimene, D., Lennox, K. K., Kaunas, R. R., and Gaharwar, A. K. (2016). Advanced bioinks for 3D printing: a materials science perspective. Ann. Biomed. Eng. 44, 2090–2102. doi: 10.1007/s10439-016-1638-y
Chimene, D., Peak, C. W., Gentry, J. L., Carrow, J. K., Cross, L. M., Mondragon, E., et al. (2018). Nanoengineered Ionic–Covalent Entanglement (NICE) bioinks for 3D bioprinting. ACS Appl. Mater. Interfaces 10, 9957–9968. doi: 10.1021/acsami.7b19808
Ching, S. H., Bansal, N., and Bhandari, B. (2017). Alginate gel particles–a review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 57, 1133–1152. doi: 10.1080/10408398.2014.965773
Chinga-Carrasco, G., and Syverud, K. (2014). Pretreatment-dependent surface chemistry of wood nanocellulose for pH-sensitive hydrogels. J. Biomater. Appl. 29, 423–432. doi: 10.1177/0885328214531511
Chirayil, C. J., Mathew, L., Hassan, P. A., Mozetic, M., and Thomas, S. (2014). Rheological behaviour of nanocellulose reinforced unsaturated polyester nanocomposites. Int. J. Biol. Macromol. 69, 274–281. doi: 10.1016/j.ijbiomac.2014.05.055
Chung, J. H. Y., Naficy, S., Yue, Z., Kapsa, R., Quigley, A., Moulton, S. E., et al. (2013). Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 1:763. doi: 10.1039/c3bm00012e
Coimbra, P., Ferreira, P., de Sousa, H. C., Batista, P., Rodrigues, M. A., Correia, I. J., et al. (2011). Preparation and chemical and biological characterization of a pectin/chitosan polyelectrolyte complex scaffold for possible bone tissue engineering applications. Int. J. Biol. Macromol. 48, 112–118. doi: 10.1016/J.IJBIOMAC.2010.10.006
Correia, C. R., Reis, R. L., and Mano, J. F. (2013). Multilayered hierarchical capsules providing cell adhesion sites. Biomacromolecules 14, 743–751. doi: 10.1021/bm301833z
Crowley, M. M., Schroeder, B., Fredersdorf, A., Obara, S., Talarico, M., Kucera, S., et al. (2004). Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int. J. Pharm. 269, 509–522. doi: 10.1016/J.IJPHARM.2003.09.037
Cui, S., Yao, B., Gao, M., Sun, X., Gou, D., Hu, J., et al. (2017). Effects of pectin structure and crosslinking method on the properties of crosslinked pectin nanofibers. Carbohydr. Polym. 157, 766–774. doi: 10.1016/j.carbpol.2016.10.052
Cunha, L., and Grenha, A. (2016). Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 14:E42. doi: 10.3390/md14030042
Dai, G., Wan, W., Zhao, Y., Wang, Z., Li, W., Shi, P., et al. (2016). Controllable 3D alginate hydrogel patterning via visible-light induced electrodeposition. Biofabrication 8:025004. doi: 10.1088/1758-5090/8/2/025004
Daly, A. C., Cunniffe, G. M., Sathy, B. N., Jeon, O., Alsberg, E., and Kelly, D. J. (2016). 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv. Healthc. Mater. 5, 2353–2362. doi: 10.1002/adhm.201600182
De Cicco, F., Russo, P., Reverchon, E., García-González, C. A., Aquino, R. P., and Del Gaudio, P. (2016). Prilling and supercritical drying: a successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 147, 482–489. doi: 10.1016/j.carbpol.2016.04.031
de Moraes, J. O., Müller, C. M. O., and Laurindo, J. B. (2012). Influence of the simultaneous addition of bentonite and cellulose fibers on the mechanical and barrier properties of starch composite-films. Food Sci. Technol. Int. 18, 35–45. doi: 10.1177/1082013211427622
Derakhshanfar, S., Mbeleck, R., Xu, K., Zhang, X., Zhong, W., and Xing, M. (2018). 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact. Mater. 3, 144–156. doi: 10.1016/J.BIOACTMAT.2017.11.008
Dhawan, A., Kennedy, P. M., Rizk, E. B., and Ozbolat, I. T. (2018). Three-dimensional bioprinting for bone and cartilage restoration in orthopaedic surgery. J. Am. Acad. Orthop. Surg. 27, e215–26. doi: 10.5435/JAAOS-D-17-00632
Diañez, I., Gallegos, C., Brito-de la Fuente, E., Martínez, I., Valencia, C., Sánchez, M. C., et al. (2019). 3D printing in situ gelification of κ-carrageenan solutions: effect of printing variables on the rheological response. Food Hydrocoll. 87, 321–330. doi: 10.1016/J.FOODHYD.2018.08.010
Ding, H., Chang, R., Ding, H., and Chang, R. C. (2018). Printability study of bioprinted tubular structures using liquid hydrogel precursors in a support bath. Appl. Sci. 8:403. doi: 10.3390/app8030403
Dolati, F., Yu, Y., Zhang, Y., De Jesus, A. M., Sander, E. A., and Ozbolat, I. T. (2014). In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology 25:145101. doi: 10.1088/0957-4484/25/14/145101
Dong, D., Li, J., Cui, M., Wang, J., Zhou, Y., Luo, L., et al. (2016). In situ “Clickable” zwitterionic starch-based hydrogel for 3D cell encapsulation. ACS Appl. Mater. Interfaces 8, 4442–4455. doi: 10.1021/acsami.5b,12141
Dong, S., Hirani, A. A., Colacino, K. R., Lee, Y. W., and Roman, M. (2012). Cytotoxicity and cellular uptake of cellulose nanocrystals. Nano Life 2:1241006. doi: 10.1142/S1793984412410061
Drury, J. L., and Mooney, D. J. (2003). Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24, 4337–4351. doi: 10.1016/S0142-9612(03)00340-5
Duan, B., Hockaday, L. A., Kang, K. H., and Butcher, J. T. (2013). 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 101, 1255–1264. doi: 10.1002/jbm.a.34420
Dumanli, A. G., van der Kooij, H. M., Kamita, G., Reisner, E., Baumberg, J. J., Steiner, U., et al. (2014). Digital color in cellulose nanocrystal films. ACS Appl. Mater. Interfaces 6, 12302–12306. doi: 10.1021/am501995e
El-Sherbiny, I. M., and Yacoub, M. H. (2013). Hydrogel scaffolds for tissue engineering: progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 316–342. doi: 10.5339/gcsp.2013.38
Eyholzer, C., Borges de Couraça, A., Duc, F., Bourban, P. E., Tingaut, P., Zimmermann, T., et al. (2011). Biocomposite hydrogels with carboxymethylated, nanofibrillated cellulose powder for replacement of the nucleus pulposus. Biomacromolecules 12, 1419–1427. doi: 10.1021/bm101131b
Fan, R., Piou, M., Darling, E., Cormier, D., Sun, J., and Wan, J. (2016). Bio-printing cell-laden Matrigel–agarose constructs. J. Biomater. Appl. 31, 684–692. doi: 10.1177/0885328216669238
Fang, Y., Al-Assaf, S., Phillips, G. O., Nishinari, K., Funami, T., and Williams, P. A. (2008). Binding behavior of calcium to polyuronates: comparison of pectin with alginate. Carbohydr. Polym. 72, 334–341. doi: 10.1016/J.CARBPOL.2007.08.021
Fawzy, M. A., Gomaa, M., Hifney, A. F., and Abdel-Gawad, K. M. (2017). Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr. Polym. 157, 1903–1912. doi: 10.1016/j.carbpol.2016.11.077
Feng, W., Qi, Y., Wang, S., Feng, W., Qi, Y., and Wang, S. (2017). Effects of short-range order on the magnetic and mechanical properties of FeCoNi(AlSi)x high entropy alloys. Metals (Basel). 7:482. doi: 10.3390/met7110482
Fitton, J. H. (2011). Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 9, 1731–1760. doi: 10.3390/md9101731
Forget, A., Blaeser, A., Miessmer, F., Köpf, M., Campos, D. F. D., Voelcker, N. H., et al. (2017). Mechanically tunable bioink for 3D bioprinting of human cells. Adv. Healthc. Mater. 6:1700255. doi: 10.1002/adhm.201700255
Gao, T., Gillispie, G. J., Copus, J. S., PR, A. K., Seol, Y. J., Atala, A., et al. (2018). Optimization of gelatin–alginate composite bioink printability using rheological parameters: a systematic approach. Biofabrication 10:034106. doi: 10.1088/1758-5090/aacdc7
Gershlak, J. R., Hernandez, S., Fontana, G., Perreault, L. R., Hansen, K. J., Larson, S. A., et al. (2017). Crossing kingdoms: using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 125, 13–22. doi: 10.1016/J.BIOMATERIALS.2017.02.011
Giuseppe, M. D., Law, N., Webb, B. A, Macrae, R., Liew, L. J., Sercombe, T. B., et al. (2018). Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 79, 150–157. doi: 10.1016/J.JMBBM.2017.12.018
Giusto, G., Vercelli, C., Comino, F., Caramello, V., Tursi, M., and Gandini, M. (2017). A new, easy-to-make pectin-honey hydrogel enhances wound healing in rats. BMC Complement. Altern. Med. 17:266. doi: 10.1186/s12906-017-1769-1
Gomes, M., Godinho, J., Tchalamov, D., Cunha, A., and Reis, R. (2002). Alternative tissue engineering scaffolds based on starch: processing methodologies, morphology, degradation and mechanical properties. Mater. Sci. Eng. C 20, 19–26. doi: 10.1016/S0928-4931(02)00008-5
Gorgieva, S., Girandon, L., and Kokol, V. (2017). Mineralization potential of cellulose-nanofibrils reinforced gelatine scaffolds for promoted calcium deposition by mesenchymal stem cells. Mater. Sci. Eng. C 73, 478–489. doi: 10.1016/J.MSEC.2016.12.092
Groll, J., Boland, T., Blunk, T., Burdick, J. A., Cho, D. W., Dalton, P. D., et al. (2016). Biofabrication: reappraising the definition of an evolving field. Biofabrication 8:013001. doi: 10.1088/1758-5090/8/1/013001
Gu, Q., Tomaskovic-Crook, E., Lozano, R., Chen, Y., Kapsa, R. M., Zhou, Q., et al. (2016). Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mater. 5, 1429–1438. doi: 10.1002/adhm.201600095
Gumrah Dumanli, A. (2017). Nanocellulose and its composites for biomedical applications. Curr. Med. Chem. 24, 512–528. doi: 10.2174/0929867323666161014124008
Gungor-Ozkerim, P. S., Inci, I., Zhang, Y. S., Khademhosseini, A., and Dokmeci, M. R. (2018). Bioinks for 3D bioprinting: an overview. Biomater. Sci. 6, 915–946. doi: 10.1039/c7bm00765e
Hao, X., Shen, W., Chen, Z., Zhu, J., Feng, L., Wu, Z., et al. (2015). Self-assembled nanostructured cellulose prepared by a dissolution and regeneration process using phosphoric acid as a solvent. Carbohydr. Polym. 123, 297–304. doi: 10.1016/J.CARBPOL.2015.01.055
Havaldar, R., Pilli, S. C., and Putti, B. B. (2014). Insights into the effects of tensile and compressive loadings on human femur bone. Adv. Biomed. Res. 3:101. doi: 10.4103/2277-9175.129375
Hay, I. D., Ur Rehman, Z., Moradali, M. F., Wang, Y., and Rehm, B. H. A. (2013). Microbial alginate production, modification and its applications. Microb. Biotechnol. 6, 637–650. doi: 10.1111/1751-7915.12076
He, Y., Yang, F., Zhao, H., Gao, Q., Xia, B., and Fu, J. (2016). Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 6:29977. doi: 10.1038/srep29977
Hinton, T. J., Jallerat, Q., Palchesko, R. N., Park, J. H., Grodzicki, M. S., Shue, H. J., et al. (2015). Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 1:e1500758. doi: 10.1126/sciadv.1500758
Hölzl, K., Lin, S., Tytgat, L., Van Vlierberghe, S., Gu, L., and Ovsianikov, A. (2016). Bioink properties before, during and after 3D bioprinting. Biofabrication 8:032002. doi: 10.1088/1758-5090/8/3/032002
Hopp, B., Smausz, T., Szabó, G., Kolozsvari, L., Nogradi, A., Kafetzopoulos, D., et al. (2012). Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt. Eng. 51:014302. doi: 10.1117/1.OE.51.1.014302
Huang, C. Y. (2018). Extrusion-Based 3D Printing and Characterization of Edible Materials. Available online at: https://uwspace.uwaterloo.ca/handle/10012/12899 (accessed March 10, 2019).
Ige, O. O., Umoru, L. E., and Aribo, S. (2012). Natural products: a minefield of biomaterials. ISRN Mater. Sci. 2012:983062. doi: 10.5402/2012/983062
Iglauer, S., Wu, Y., Shuler, P., Tang, Y., and Goddard, W. A. (2011). Dilute iota- and kappa-Carrageenan solutions with high viscosities in high salinity brines. J. Pet. Sci. Eng. 75, 304–311. doi: 10.1016/J.PETROL.2010.11.025
In, E., Naguib, H., and Haider, M. (2014). Mechanical stability analysis of carrageenan-based polymer gel for magnetic resonance imaging liver phantom with lesion particles. J. Med. Imaging (Bellingham) 1:035502. doi: 10.1117/1.JMI.1.3.035502
Jack, A. A., Nordli, H. R., Powell, L. C., Powell, K. A., Kishnani, H., Johnsen, P. O., et al. (2017). The interaction of wood nanocellulose dressings and the wound pathogen P. aeruginosa. Carbohydr. Polym. 157, 1955–1962. doi: 10.1016/J.CARBPOL.2016.11.080
Jakab, K., Norotte, C., Damon, B., Marga, F., Neagu, A., Besch-Williford, C. L., et al. (2008). Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng. Part A 14, 413–421. doi: 10.1089/tea.2007.0173
Jayani, R. S., Saxena, S., and Gupta, R. (2005). Microbial pectinolytic enzymes: a review. Process Biochem. 40, 2931–2944. doi: 10.1016/J.PROCBIO.2005.03.026
Jeong, H.-S., Venkatesan, J., and Kim, S.-K. (2013). Hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 57, 138–141. doi: 10.1016/J.IJBIOMAC.2013.03.011
Jessop, Z. M., Al-Sabah, A., Gardiner, M. D., Combellack, E., Hawkins, K., and Whitaker, I. S. (2017). 3D bioprinting for reconstructive surgery: principles, applications and challenges. J. Plast. Reconstr. Aesthetic Surg. 70, 1155–1170. doi: 10.1016/j.bjps.2017.06.001
Jia, J., Richards, D. J., Pollard, S., Tan, Y., Rodriguez, J., Visconti, R. P., et al. (2014). Engineering alginate as bioink for bioprinting. Acta Biomater. 10, 4323–4331. doi: 10.1016/j.actbio.2014.06.034
Jorfi, M., and Foster, E. J. (2015). Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 132:41719. doi: 10.1002/app.41719
Jovic, T. H., Jessop, Z. M., Al-Sabah, A., and Whitaker, I. S. (2018). The clinical need for 3D printed tissue in reconstructive surgery. 3D Bioprint. Reconstr. Surg. 235–244. doi: 10.1016/B978-0-08-101103-4.00002-8
Kamoun, E. A., Kenawy, E.-R. S., and Chen, X. (2017). A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 8, 217–233. doi: 10.1016/J.JARE.2017.01.005
Karri, V. V. S. R., Kuppusamy, G., Talluri, S. V., Mannemala, S. S., Kollipara, R., Wadhwani, A. D., et al. (2016). Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int. J. Biol. Macromol. 93, 1519–1529. doi: 10.1016/j.ijbiomac.2016.05.038
Kelder, C., Bakker, A., Klein-Nulend, J., and Wismeijer, D. (2018). The 3D printing of calcium phosphate with K-carrageenan under conditions permitting the incorporation of biological components—a method. J. Funct. Biomater. 9:57. doi: 10.3390/jfb9040057
Klemm, D., Kramer, F., Moritz, S., Lindström, T., Ankerfors, M., Gray, D., et al. (2011). Nanocelluloses: a new family of nature-based materials. Angew. Chemie Int. Ed. 50, 5438–5466. doi: 10.1002/anie.201001273
Kokkonen, H. E., Ilvesaro, J. M., Morra, M., Schols, H. A., and Tuukkanen, J. (2006). Effect of modified pectin molecules on the growth of bone cells. Biomacromolecules 8, 509–515. doi: 10.1021/BM060614H
Kollar, P., Závalová, V., Hošek, J., Havelka, P., Sopuch, T., Karpíšek, M., et al. (2011). Cytotoxicity and effects on inflammatory response of modified types of cellulose in macrophage-like THP-1 cells. Int. Immunopharmacol. 11, 997–1001. doi: 10.1016/j.intimp.2011.02.016
Kundu, J., Shim, J.-H., Jang, J., Kim, S.-W., and Cho, D.-W. (2015). An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 9, 1286–1297. doi: 10.1002/term.1682
Kuzmenko, V., Karabulut, E., Pernevik, E., Enoksson, P., and Gatenholm, P. (2018). Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 189, 22–30. doi: 10.1016/J.CARBPOL.2018.01.097
Kyle, S., Jessop, Z. M., Al-Sabah, A., Hawkins, K., Lewis, A., Maffeis, T., et al. (2018). Characterization of pulp derived nanocellulose hydrogels using AVAP® technology. Carbohydr. Polym. 198, 270–280. doi: 10.1016/j.carbpol.2018.06.091
Kyle, S., Jessop, Z. M., Al-Sabah, A., and Whitaker, I. S. (2017). ‘Printability' of candidate biomaterials for extrusion based 3D printing: state-of-the-art. Adv. Healthc. Mater. 6:1700264. doi: 10.1002/adhm.201700264
Landers, R., Hübner, U., Schmelzeisen, R., and Mülhaupt, R. (2002). Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 23, 4437–4447. doi: 10.1016/S0142-9612(02)00139-4
Lavoine, N., Desloges, I., Dufresne, A., and Bras, J. (2012). Microfibrillated cellulose – its barrier properties and applications in cellulosic materials: a review. Carbohydr. Polym. 90, 735–764. doi: 10.1016/J.CARBPOL.2012.05.026
Le May, I., Lappi, V. G., and White, W. E. (1975). Materials for biomedical applications. Polym. Eng. Sci. 15, 789–794. doi: 10.1002/pen.760151105
Lee, K.-Y., Jeong, M.-R., Choi, S.-M., Na, S.-S., and Cha, J.-D. (2013). Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Arch. Oral Biol. 58, 482–492. doi: 10.1016/j.archoralbio.2012.11.002
Lee, K. Y., and Mooney, D. J. (2012). Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126. doi: 10.1016/j.progpolymsci.2011.06.003
Lewitus, D. Y., Landers, J., Branch, J. R., Smith, K. L., Callegari, G., Kohn, J., et al. (2011). Biohybrid carbon nanotube/agarose fibers for neural tissue engineering. Adv. Funct. Mater. 21, 2624–2632. doi: 10.1002/adfm.201002429
Li, J., Yang, B., Qian, Y., Wang, Q., Han, R., Hao, T., et al. (2015). Iota-carrageenan/chitosan/gelatin scaffold for the osteogenic differentiation of adipose-derived MSCs in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 103, 1498–1510. doi: 10.1002/jbm.b.33339
Li, W., Guan, T., Zhang, X., Wang, Z., Wang, M., Zhong, W., et al. (2015a). The effect of layer-by-layer assembly coating on the proliferation and differentiation of neural stem cells. ACS Appl. Mater. Interfaces 7, 3018–3029. doi: 10.1021/am504456t
Li, W., Lan, Y., Guo, R., Zhang, Y., Xue, W., and Zhang, Y. (2015b). In vitro and in vivo evaluation of a novel collagen/cellulose nanocrystals scaffold for achieving the sustained release of basic fibroblast growth factor. J. Biomater. Appl. 29, 882–893. doi: 10.1177/0885328214547091
Liberski, A. R. (2016). Three-dimensional printing of alginate: From seaweeds to heart valve scaffolds. QScience Connect 2016:3. doi: 10.5339/connect.2016.3
Lin, N., and Dufresne, A. (2014). Nanocellulose in biomedicine: current status and future prospect. Eur. Polym. J. 59, 302–325. doi: 10.1016/J.EURPOLYMJ.2014.07.025
Liu, J., Chinga-Carrasco, G., Cheng, F., Xu, W., Willför, S., Syverud, K., et al. (2016). Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 23, 3129–3143. doi: 10.1007/s10570-016-1038-3
Lou, Y.-R., Kanninen, L., Kuisma, T., Niklander, J., Noon, L. A., Burks, D., et al. (2014). The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev. 23, 380–392. doi: 10.1089/scd.2013.0314
Lowe, B., Venkatesan, J., Anil, S., Shim, M. S., and Kim, S.-K. (2016). Preparation and characterization of chitosan-natural nano hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 93, 1479–1487. doi: 10.1016/j.ijbiomac.2016.02.054
Lu, D. R., Xiao, C. M., and Xu, S. J. (2009). Starch-based completely biodegradable polymer materials. eXPRESS Polym. Lett. 3, 366–375. doi: 10.3144/expresspolymlett.2009.46
Madaghiele, M., Sannino, A., Ambrosio, L., and Demitri, C. (2014). Polymeric hydrogels for burn wound care: advanced skin wound dressings and regenerative templates. Burn. Trauma 2:153. doi: 10.4103/2321-3868.143616
Malda, J., Visser, J., Melchels, F. P., Jüngst, T., Hennink, W. E., Dhert, W. J. A., et al. (2013). 25th Anniversary article: engineering hydrogels for biofabrication. Adv. Mater. 25, 5011–5028. doi: 10.1002/adma.201302042
Maleki, S., Almaas, E., Zotchev, S., Valla, S., and Ertesvåg, H. (2016). Alginate biosynthesis factories in pseudomonas fluorescens: localization and correlation with alginate production level. Appl. Environ. Microbiol. 82, 1227–1236. doi: 10.1128/AEM.03114-15
Malinen, M. M., Kanninen, L. K., Corlu, A., Isoniemi, H. M., Lou, Y.-R., Yliperttula, M. L., et al. (2014). Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. Biomaterials 35, 5110–5121. doi: 10.1016/J.BIOMATERIALS.2014.03.020
Malkoc, V. (2018). Challenges and the Future of 3D Bioprinting. Available online at: http://www.alliedacademies.org/articles/challenges-and-the-future-of-3d-bioprinting.pdf (accessed December 31, 2018)
Mandrycky, C., Wang, Z., Kim, K., and Kim, D.-H. (2016). Research review paper 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 34, 422–434. doi: 10.1016/j.biotechadv.2015.12.011
Marinval, N., Saboural, P., Haddad, O., Maire, M., Bassand, K., Geinguenaud, F., et al. (2016). Identification of a pro-angiogenic potential and cellular uptake mechanism of a LMW highly sulfated fraction of fucoidan from Ascophyllum nodosum. Mar. Drugs 14:185. doi: 10.3390/md14100185
Markov, P. A., Krachkovsky, N. S., Durnev, E. A., Martinson, E. A., Litvinets, S. G., and Popov, S. V. (2017). Mechanical properties, structure, bioadhesion, and biocompatibility of pectin hydrogels. J. Biomed. Mater. Res. A 105, 2572–2581. doi: 10.1002/jbm.a.36116
Markstedt, K., Mantas, A., Tournier, I., Martínez Ávila, H., Hägg, D., and Gatenholm, P. (2015). 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 16, 1489–1496. doi: 10.1021/acs.biomac.5b00188
Martínez Ávila, H., Schwarz, S., Rotter, N., and Gatenholm, P. (2016). 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 1–2, 22–35. doi: 10.1016/J.BPRINT.2016.08.003
Martins, A., Chung, S., Pedro, A. J., Sousa, R. A., Marques, A. P., Reis, R. L., et al. (2009). Hierarchical starch-based fibrous scaffold for bone tissue engineering applications. J. Tissue Eng. Regen. Med. 3, 37–42. doi: 10.1002/term.132
Matyash, M., Despang, F., Ikonomidou, C., and Gelinsky, M. (2014). Swelling and mechanical properties of alginate hydrogels with respect to promotion of neural growth. Tissue Eng. Part C. Methods 20, 401–411. doi: 10.1089/ten.TEC.2013.0252
Meng, C., Li, H., Zhu, G., Cao, S., Zhang, H., Liu, Y., et al. (2019). Improvement in mechanical and hygroscopic properties of modified SA fiber crosslinking with PEGDE. J. Appl. Polym. Sci. 136:47155. doi: 10.1002/app.47155
Miao, T., Rao, K. S., Spees, J. L., and Oldinski, R. A. (2014). Osteogenic differentiation of human mesenchymal stem cells through alginate-graft-poly(ethylene glycol) microsphere-mediated intracellular growth factor delivery. J. Control. Release 192, 57–66. doi: 10.1016/j.jconrel.2014.06.029
Mierisch, C. M., Cohen, S. B., Jordan, L. C., Robertson, P. G., Balian, G., Diduch, D. R., et al. (2002). Transforming growth factor-beta in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. Arthroscopy 18, 892–900. doi: 10.1053/jars.2002.36117
Mirzaei, B., Etemadian, S., Goli, H. R., Bahonar, S., Gholami, S. A., Karami, P., et al. (2018). Construction and analysis of alginate-based honey hydrogel as an ointment to heal of rat burn wound related infections. Int. J. Burns Trauma 8, 88–97. Available online at: https://europepmc.org/articles/pmc6146165
Mishra, R. K., Datt, M., and Banthia, A. K. (2008). Synthesis and Characterization of Pectin/PVP hydrogel membranes for drug delivery system. AAPS PharmSciTech 9, 395–403. doi: 10.1208/s12249-008-9048-6
Miyamoto, T., Takahashi, S., Ito, H., Inagaki, H., and Noishiki, Y. (1989). Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 23, 125–133. doi: 10.1002/jbm.820230110
Moberg, T., Sahlin, K., Yao, K., Geng, S., Westman, G., Zhou, Q., et al. (2017). Rheological properties of nanocellulose suspensions: effects of fibril/particle dimensions and surface characteristics. Cellulose 24, 2499–2510. doi: 10.1007/s10570-017-1283-0
Möller, T., Amoroso, M., Hägg, D., Brantsing, C., Rotter, N., Apelgren, P., et al. (2017). In vivo chondrogenesis in 3D bioprinted human cell-laden hydrogel constructs. Plast. Reconstr. Surg. Glob. Open 5:e1227. doi: 10.1097/GOX.0000000000001227
Monllau, J. C., Pelfort, X., and Tey, M. (2010). “Collagen meniscus implant: technique and results,” in The Meniscus, eds P. Beaufils, and R. Verdonk (Berlin; Heidelberg: Springer), 373–382. doi: 10.1007/978-3-642-02450-4_47
Monsur, H. A., Jaswir, I., Simsek, S., Amid, A., and Alam, Z. (2016). International Journal of Food Properties Chemical structure of sulfated polysaccharides from brown seaweed (Turbinaria turbinata) Chemical structure of sulfated polysaccharides from brown seaweed (Turbinaria turbinata). Int. J. Food Prop. 20, 1457–1469. doi: 10.1080/10942912.2016.1211144
Moradali, M. F., Ghods, S., and Rehm, B. H. A. (2017). Activation mechanism and cellular localization of membrane-anchored alginate polymerase in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 83:e03499-16. doi: 10.1128/AEM.03499-16
Munarin, F., Guerreiro, S. G., Grellier, M. A., Tanzi, M. C., Barbosa, M. A., Petrini, P., et al. (2011). Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 12, 568–577. doi: 10.1021/bm101110x
Munarin, F., Tanzi, M. C., and Petrini, P. (2012). Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 51, 681–689. doi: 10.1016/J.IJBIOMAC.2012.07.002
Murphy, S. V., and Atala, A. (2014). 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785. doi: 10.1038/nbt.2958
Naghieh, S., Karamooz-Ravari, M. R., Sarker, M., Karki, E., and Chen, X. (2018). Influence of crosslinking on the mechanical behavior of 3D printed alginate scaffolds: Experimental and numerical approaches. J. Mech. Behav. Biomed. Mater. 80, 111–118. doi: 10.1016/J.JMBBM.2018.01.034
Nishimura, K., Morimoto, Y., Mori, N., and Takeuchi, S. (2018). Formation of branched and chained alginate microfibers using theta-glass capillaries. Micromachines 9:303. doi: 10.3390/mi9060303
Nowacki, M., Pietkun, K., Kloskowski, T., Pokrywczynska, M., Tyloch, D., Rasmus, M., et al. (2017). Are agricultural and natural sources of bio-products important for modern regenerative medicine? A Rev. Ann. Agric. Environ. Med. 24, 207–212. doi: 10.5604/12321966.1235171
O'Brien, F. J. (2011). Biomaterials & scaffolds for tissue engineering. Mater. Today 14, 88–95. doi: 10.1016/S1369-7021(11)70058-X
Pal, K., Banthia, A., and Majumdar, D. (2006). Starch based hydrogel with potential biomedical application as artificial skin. Afr. J. Biomed. Res. 9, 23–29. doi: 10.4314/ajbr.v9i1.48769
Pariente, J.-L., Kim, B.-S., and Atala, A. (2001). In vitro biocompatibility assessment of naturally derived and synthetic biomaterials using normal human urothelial cells. J. Biomed. Mater. Res. 55, 33–39. doi: 10.1002/1097-4636(200104)55:1<33::AID-JBM50>3.0.CO;2-7
Park, S., Lee, K. W., Lim, D.-S., and Lee, S. (2012). The sulfated polysaccharide fucoidan stimulates osteogenic differentiation of human adipose-derived stem cells. Stem Cells Dev. 21, 2204–2211. doi: 10.1089/scd.2011.0521
Park, S. Y., Kim, W.-J., Choi, J. B., and Kim, S. (2018). Physical and mechanical properties of alginate-based hydrogel film as carrier for release of acetylthiocholine. Int. J. Precis. Eng. Manuf. 19:129. doi: 10.1007/s12541-018-0015-1
Patil, N. P., Le, V., Sligar, A. D., Mei, L., Chavarria, D., Yang, E. Y., et al. (2018). Algal polysaccharides as therapeutic agents for atherosclerosis. Front. Cardiovasc. Med. 5:153. doi: 10.3389/fcvm.2018.00153
Pereira, M. M., Raposo, N. R. B., Brayner, R., Teixeira, E. M., Oliveira, V., Quintão, C. C. R., et al. (2013). Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology 24:075103. doi: 10.1088/0957-4484/24/7/075103
Pereira, R. F., Barrias, C. C., Bártolo, P. J., and Granja, P. L. (2018a). Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 66, 282–293. doi: 10.1016/j.actbio.2017.11.016
Pereira, R. F., Sousa, A., Barrias, C. C., Bártolo, P. J., and Granja, P. L. (2018b). A single-component hydrogel bioink for bioprinting of bioengineered 3D constructs for dermal tissue engineering. Mater. Horizons 5, 1100–1111. doi: 10.1039/C8MH00525G
Pertile, R. A. N., Andrade, F. K., Alves, C., and Gama, M. (2010). Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohydr. Polym. 82, 692–698. doi: 10.1016/J.CARBPOL.2010.05.037
Phanthong, P., Reubroycharoen, P., Hao, X., Xu, G., Abudula, A., and Guan, G. (2018). Nanocellulose: extraction and application. Carbon Resour. Convers. 1, 32–43. doi: 10.1016/J.CRCON.2018.05.004
Popa, E. G., Caridade, S. G., Mano, J. F., Reis, R. L., and Gomes, M. E. (2015). Chondrogenic potential of injectable κ -carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J. Tissue Eng. Regen. Med. 9, 550–563. doi: 10.1002/term.1683
Purnama, A., Aid-Launais, R., Haddad, O., Maire, M., Mantovani, D., Letourneur, D., et al. (2015). Fucoidan in a 3D scaffold interacts with vascular endothelial growth factor and promotes neovascularization in mice. Drug Deliv. Transl. Res. 5, 187–197. doi: 10.1007/s13346-013-0177-4
Raddatz, L., Lavrentieva, A., Pepelanova, I., Bahnemann, J., Geier, D., Becker, T., et al. (2018). Development and application of an additively manufactured calcium chloride nebulizer for alginate 3D-bioprinting purposes. J. Funct. Biomater. 9:63. doi: 10.3390/jfb9040063
Rees, A., Powell, L. C., Chinga-Carrasco, G., Gethin, D. T., Syverud, K., Hill, K. E., et al. (2015). 3D bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. Biomed Res. Int. 2015:925757. doi: 10.1155/2015/925757
Reid, J. A., Mollica, P. A., Johnson, G. D., Ogle, R. C., Bruno, R. D., and Sachs, P. C. (2016). Accessible bioprinting: adaptation of a low-cost 3D-printer for precise cell placement and stem cell differentiation. Biofabrication 8:025017. doi: 10.1088/1758-5090/8/2/025017
Ren, B., Chen, X., Du, S., Ma, Y., Chen, H., Yuan, G., et al. (2018). Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Int. J. Biol. Macromol. 118, 1257–1266. doi: 10.1016/j.ijbiomac.2018.06.200
Rhim, J.-W. (2012). Physical-mechanical properties of agar/κ-carrageenan blend film and derived clay nanocomposite film. J. Food Sci. 77, N66–N73. doi: 10.1111/j.1750-3841.2012.02988.x
Salgado, A. J., Coutinho, O. P., and Reis, R. L. (2004). Novel starch-based scaffolds for bone tissue engineering: cytotoxicity, cell culture, and protein expression. Tissue Eng. 10, 465–474. doi: 10.1089/107632704323061825
Santana, B. P., Nedel, F., Piva, E., de Carvalho, R. V., Demarco, F. F., and Carreño, N. L. V. (2013). Preparation, modification, and characterization of alginate hydrogel with nano-/microfibers: a new perspective for tissue engineering. Biomed Res. Int. 2013:307602. doi: 10.1155/2013/307602
Sarkar, K., Xue, Y., and Sant, S. (2017). “Host response to synthetic versus natural biomaterials,” in The Immune Response to Implanted Materials and Devices, ed C. Bruna (Cham: Springer International Publishing), 81–105.
Schuurman, W., Levett, P. A., Pot, M. W., van Weeren, P. R., Dhert, W. J. A., Hutmacher, D. W., et al. (2013). Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 13, 551–561. doi: 10.1002/mabi.201200471
Seidel, J., Ahlfeld, T., Adolph, M., Kümmritz, S., Steingroewer, J., Krujatz, F., et al. (2017). Green bioprinting: extrusion-based fabrication of plant cell-laden biopolymer hydrogel scaffolds. Biofabrication 9:045011. doi: 10.1088/1758-5090/aa8854
Senna, J. P., Barradas, T. N., Cardoso, S., Castiglione, T. C., Serpe, M. J., de Holanda eSilva, K. G., et al. (2018). Dual alginate-lipid nanocarriers as oral delivery systems for amphotericin B. Colloids Surf. B Biointerfaces 166, 187–194. doi: 10.1016/j.colsurfb.2018.03.015
Shanmuganathan, K., Capadona, J. R., Rowan, S. J., and Weder, C. (2010). Stimuli-responsive mechanically adaptive polymer nanocomposites. ACS Appl. Mater. Interfaces 2, 165–174. doi: 10.1021/am9006337
Shimotoyodome, A., Suzuki, J., Kumamoto, Y., Hase, T., and Isogai, A. (2011). Regulation of postprandial blood metabolic variables by TEMPO-oxidized cellulose nanofibers. Biomacromolecules 12, 3812–3818. doi: 10.1021/bm2010609
Shin, D.-Y., Cheon, K.-H., Song, E.-H., Seong, Y.-J., Park, J.-U., Kim, H.-E., et al. (2019). Fluorine-ion-releasing injectable alginate nanocomposite hydrogel for enhanced bioactivity and antibacterial property. Int. J. Biol. Macromol. 123, 866–877. doi: 10.1016/j.ijbiomac.2018.11.108
Shoichet, M. S., Li, R. H., White, M. L., and Winn, S. R. (1996). Stability of hydrogels used in cell encapsulation: an in vitro comparison of alginate and agarose. Biotechnol. Bioeng. 50, 374–381. doi: 10.1002/(SICI)1097-0290(19960520)50:4<374::AID-BIT4>3.0.CO;2-I
Siqueira, G., Kokkinis, D., Libanori, R., Hausmann, M. K., Gladman, A. S., Neels, A., et al. (2017). Cellulose nanocrystal inks for 3D printing of textured cellular architectures. Adv. Funct. Mater. 27:1604619. doi: 10.1002/adfm.201604619
Smith, C. M., Stone, A. L., Parkhill, R. L., Stewart, R. L., Simpkins, M. W., Kachurin, A. M., et al. (2004). Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 10, 1566–1576. doi: 10.1089/ten.2004.10.1566
Solovieva, E. V., Fedotov, A. Y., Mamonov, V. E., Komlev, V. S., and Panteleyev, A. A. (2018). Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomed. Mater. 13:025007. doi: 10.1088/1748-605X/aa9089
Stößlein, S., Grunwald, I., Stelten, J., and Hartwig, A. (2019). In-situ determination of time-dependent alginate-hydrogel formation by mechanical texture analysis. Carbohydr. Polym. 205, 287–294. doi: 10.1016/j.carbpol.2018.10.056
Sun, J., and Tan, H. (2013). Alginate-based biomaterials for regenerative medicine applications. Materials (Basel). 6, 1285–1309. doi: 10.3390/ma6041285
Syverud, K., Kirsebom, H., Hajizadeh, S., and Chinga-Carrasco, G. (2011). Cross-linking cellulose nanofibrils for potential elastic cryo-structured gels. Nanoscale Res. Lett. 6:626. doi: 10.1186/1556-276X-6-626
Tako, M. (2003). Rheological characteristics of fucoidan isolated from commercially cultured Cladosiphon okamuranus. Bot. Mar. 46, 461–465. doi: 10.1515/BOT.2003.047
Tashiro, K., and Kobayashi, M. (1991). Theoretical evaluation of three-dimensional elastic constants of native and regenerated celluloses: role of hydrogen bonds. Polymer (Guildf). 32, 1516–1526. doi: 10.1016/0032-3861(91)90435-L
Thébaud, N.-B., Pierron, D., Bareille, R., Le Visage, C., Letourneur, D., and Bordenave, L. (2007). Human endothelial progenitor cell attachment to polysaccharide-based hydrogels: a pre-requisite for vascular tissue engineering. J. Mater. Sci. Mater. Med. 18, 339–345. doi: 10.1007/s10856-006-0698-1
Thomas, D. J., Jessop, Z. M., and Whitaker, I. S. (2018). 3D Bioprinting for Reconstructive Surgery : Techniques and Applications. Woodhead Publishing; an imprint of Elsevier. Available online at: https://www.sciencedirect.com/book/9780081011034/3d-bioprinting-for-reconstructive-surgery#book-info
Thomas, S. (2000). Alginate dressings in surgery and wound management—part 1. J. Wound Care 9, 56–60. doi: 10.12968/jowc.2000.9.2.26338
Toivonen, S., Malinen, M. M., Küblbeck, J., Petsalo, A., Urtti, A., Honkakoski, P., et al. (2016). Regulation of human pluripotent stem cell-derived hepatic cell phenotype by three-dimensional hydrogel models. Tissue Eng. Part A 22, 971–984. doi: 10.1089/ten.tea.2016.0127
Tønnesen, H. H., and Karlsen, J. (2002). Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 28, 621–630. doi: 10.1081/DDC-120003853
Torres-Rendon, J. G., Femmer, T., De Laporte, L., Tigges, T., Rahimi, K., Gremse, F., et al. (2015). Bioactive gyroid scaffolds formed by sacrificial templating of nanocellulose and nanochitin hydrogels as instructive platforms for biomimetic tissue engineering. Adv. Mater. 27, 2989–2995. doi: 10.1002/adma.201405873
Türkkan, S., Atila, D., Akdag, A., and Tezcaner, A. (2018). Fabrication of functionalized citrus pectin/silk fibroin scaffolds for skin tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 106, 2625–2635. doi: 10.1002/jbm.b.34079
Ungerleider, J. L., and Christman, K. L. (2014). Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl. Med. 3, 1090–1099. doi: 10.5966/sctm.2014-0049
Urtuvia, V., Maturana, N., Acevedo, F., Peña, C., and Díaz-Barrera, A. (2017). Bacterial alginate production: an overview of its biosynthesis and potential industrial production. World J. Microbiol. Biotechnol. 33:198. doi: 10.1007/s11274-017-2363-x
Vancauwenberghe, V., Baiye Mfortaw Mbong, V., Vanstreels, E., Verboven, P., Lammertyn, J., and Nicolai, B. (2017). 3D printing of plant tissue for innovative food manufacturing: encapsulation of alive plant cells into pectin based bio-ink. J. Food Eng. doi: 10.1016/J.JFOODENG.2017.12.003
Vartiainen, J., Pöhler, T., Sirola, K., Pylkkänen, L., Alenius, H., Hokkinen, J., et al. (2011). Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 18, 775–786. doi: 10.1007/s10570-011-9501-7
Venkatesan, J., Bhatnagar, I., and Kim, S.-K. (2014). Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 12, 300–316. doi: 10.3390/md12010300
Walker, J. M., Myers, A. M., Schluchter, M. D., Goldberg, V. M., Caplan, A. I., Berilla, J. A., et al. (2011). Nondestructive evaluation of hydrogel mechanical properties using ultrasound. Ann. Biomed. Eng. 39, 2521–2530. doi: 10.1007/s10439-011-0351-0
Wang, C.-C., Yang, K.-C., Lin, K.-H., Liu, H.-C., and Lin, F.-H. (2011). A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials 32, 7118–7126. doi: 10.1016/j.biomaterials.2011.06.018
Wang, G., Tan, Y., Wang, H., and Zhou, P. (2017). Autophagy promotes degradation of polyethyleneimine–alginate nanoparticles in endothelial progenitor cells. Int. J. Nanomed. 12, 6661–6675. doi: 10.2147/IJN.S141592
Wang, M., Yuan, J., Huang, X., Cai, X., Li, L., and Shen, J. (2013). Grafting of carboxybetaine brush onto cellulose membranes via surface-initiated ARGET-ATRP for improving blood compatibility. Colloids Surf. B Biointerfaces 103, 52–58. doi: 10.1016/J.COLSURFB.2012.10.025
Wang, X., Ao, Q., Tian, X., Fan, J., Wei, Y., Hou, W., et al. (2016). 3D bioprinting technologies for hard tissue and organ engineering. Mater. (Basel, Switzerland) 9:802. doi: 10.3390/ma9100802
Webb, B., and Doyle, B. J. (2017). Parameter optimization for 3D bioprinting of hydrogels. Bioprinting 8, 8–12. doi: 10.1016/J.BPRINT.2017.09.001
Wee, S., and Gombotz, W. R. (1998). Protein release from alginate matrices. Adv. Drug Deliv. Rev. 31, 267–285.
Weinberg, M. S., and Morris, K. V. (2016). Transcriptional gene silencing in humans. Nucleic Acids Res. 44, 6505–6517. doi: 10.1093/nar/gkw139
Wilson, S. A., Cross, L. M., Peak, C. W., and Gaharwar, A. K. (2017). Shear-thinning and thermo-reversible nanoengineered inks for 3D bioprinting. ACS Appl. Mater. Interfaces 9, 43449–43458. doi: 10.1021/acsami.7b13602
Xiong, Z., Yan, Y., Wang, S., Zhang, R., and Zhang, C. (2002). Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scr. Mater. 46, 771–776. doi: 10.1016/S1359-6462(02)00071-4
Yang, X., Lu, Z., Wu, H., Li, W., Zheng, L., and Zhao, J. (2018). Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 83, 195–201. doi: 10.1016/j.msec.2017.09.002
Yao, N., Huang, C., and Jin, D. (2009). Evaluation of biocompatibility of a pectin/polyvinyl alcohol composite hydrogel as a new nucleus material. Orthop. Surg. 1, 231–237. doi: 10.1111/j.1757-7861.2009.00036.x
Yegappan, R., Selvaprithiviraj, V., Amirthalingam, S., and Jayakumar, R. (2018). Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 198, 385–400. doi: 10.1016/J.CARBPOL.2018.06.086
Zarrintaj, P., Manouchehri, S., Ahmadi, Z., Saeb, M. R., Urbanska, A. M., Kaplan, D. L., et al. (2018). Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 187, 66–84. doi: 10.1016/J.CARBPOL.2018.01.060
Zhang, K., Fu, Q., Yoo, J., Chen, X., Chandra, P., Mo, X., et al. (2017). 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 50, 154–164. doi: 10.1016/J.ACTBIO.2016.12.008
Zhang, Y., Yu, Y., Akkouch, A., Dababneh, A., Dolati, F., and Ozbolat, I. T. (2015). In vitro study of directly bioprinted perfusable vasculature conduits. Biomater. Sci. 3, 134–143. doi: 10.1039/C4BM00234B
Keywords: plants, algae, 3D bioprinting, biomaterial, tissue engineering, drug delivery, wound healing
Citation: Jovic TH, Kungwengwe G, Mills AC and Whitaker IS (2019) Plant-Derived Biomaterials: A Review of 3D Bioprinting and Biomedical Applications. Front. Mech. Eng. 5:19. doi: 10.3389/fmech.2019.00019
Received: 31 December 2018; Accepted: 29 March 2019;
Published: 17 April 2019.
Edited by:
Amit Bandyopadhyay, Washington State University, United StatesReviewed by:
Subhadip Bodhak, Central Glass and Ceramic Research Institute (CSIR), IndiaCopyright © 2019 Jovic, Kungwengwe, Mills and Whitaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas H. Jovic, dC5oLmpvdmljQHN3YW5zZWEuYWMudWs=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.