- 1Robotics Lab, School of Mathematical and Computer Sciences, Heriot-Watt University, Edinburgh, United Kingdom
- 2Stokes Research Group, School of Engineering, Institute for Integrated Micro and Nano Systems, The University of Edinburgh, Edinburgh, United Kingdom
- 3Graduate Program in Neuroengineering, Edmond and Lily Safra International Institute of Neuroscience, Santos Dumont Institute, Macaiba, Brazil
This work describes the design, fabrication, and initial testing of a Soft Orthotic Physiotherapy Hand Interactive Aid (SOPHIA) for stroke rehabilitation. SOPHIA consists of (1) a soft robotic exoskeleton, (2) a microcontroller-based control system driven by a brain–machine interface (BMI), and (3) a sensorized glove for passive rehabilitation. In contrast to other rehabilitation devices, SOPHIA is the first modular prototype of a rehabilitation system that is capable of three tasks: aiding extension based assistive rehabilitation, monitoring patient exercises, and guiding passive rehabilitation. Our results show that this prototype of the device is capable of helping healthy subjects to open their hand. Finger extension is triggered by a command from the BMI, while using a variety of sensors to ensure a safe motion. All data gathered from the device will be used to guide further improvements to the prototype, aiming at developing specifications for the next generation device, which could be used in future clinical trials.
Introduction
Overview
Strokes are an increasing problem, affecting all ages, genders, and ethnicities (Stroke Helpline Scotland, 2013; Mozaffarian et al., 2015; Scottish Stroke Association, 2016). A lack of resources in providing and monitoring physiotherapy limits the efficacy of conventional rehabilitation techniques (Duncan et al., 2005).
The Soft Orthotic Physiotherapy Hand Interactive Aid (SOPHIA) project focuses on developing systems that can be used for rehabilitation of a patient’s hand after a stroke. We explore the combination of soft robotics and brain–machine interfaces (BMIs), and we have designed this system to be used both in hospital and at home. This new modular interactive tool uses a BMI for control, and soft robotics for actuation, and shows potential for assisting in both passive and assistive (Basteris et al., 2014) stroke rehabilitation.
In contrast to other rehabilitation devices, SOPHIA is the first modular prototype of a rehabilitation system that is capable of three tasks: aiding extension-based assistive rehabilitation, monitoring patient exercises, and guiding passive rehabilitation.
Background
There are approximately 1.2 million stroke survivors in the UK, with this number increasing by over 150,000 annually (Scottish Stroke Association, 2016). Globally, over 20 million people per year suffer a cerebrovascular accident (World Health Organisation, 2011), of which strokes are one of the primary causes (Ward and Cohen, 2004; Strong and Mathers, 2011). Typically, 1 year on from a stroke 65% of those patients remain severely handicapped and dependent on assistance in daily life (Wolfe, 2000). After a stroke occurs, and once the patient has been stabilized, they will begin rehabilitation. Rehabilitation sessions are conducted by a physiotherapist and take the form of supervised daily exercises (Jauch et al., 2013).
Figure 1 shows the sequence of hand motions required to complete two basic grip motions as needed to complete many daily living tasks such as buttoning a shirt or holding a cup: the cylindrical grip (Figure 1A) and the pinch grip (Figure 1B). In some stroke patients, these movements are hindered by involuntary clawing of the hand where the hand is restricted by muscle tension to a tight grasp, which limits full extension of the fingers. Physiotherapy exercises used in rehabilitation seeks to restore these two functions.
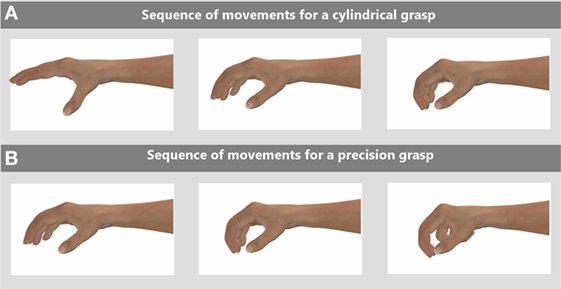
Figure 1. Two exercises people need to perform to complete daily living tasks are cylindrical and pinch grasps, the range of motions required for these tasks are shown in these two sequences for: (A) a cylindrical grasp and (B) a pinch grasp. Rehabilitation exercises for stroke patients aim to help restore these functions.
The likelihood that a full recovery will be made is greater if the intensity of the exercises is increased (Sivenius et al., 1985) and the time taken to start the rehabilitation is decreased (Kwakkel et al., 2006).
Once the patient leaves the hospital, they will see a physiotherapist initially between two and four times a week. When they are not with the physiotherapist they are given a variety of rehabilitation exercises to perform unsupervised, leading to motions being performed incorrectly, infrequently, or not at all, thus reducing the chance of full recovery (Schaechter, 2004). Currently, the physiotherapist relies on the patient for information on how many exercises were performed and this self-reporting can lead to inaccurate data being collected.
Hand rehabilitation is considered to be a lower priority than the recovery of motion in the upper arm, which itself is secondary to the restoration of the motion of the trunk of the body and lower body movement, such as walking through gait relearning. Therefore, when the rehabilitation of the hand begins, it is often after the acute stage—treatment during the first 2 weeks has been shown to hold the greatest potential for recovery (Nakayama et al., 1994; Jørgensen et al., 1995; Feys et al., 1998). Due to this missed window of opportunity, only minor additional measurable improvement occurs after the 6 months following stroke onset (Hussein and Staines, 2013; Bruno-Petrina, 2014; Edwardson and Dromerick, 2016), leading to “less-than-satisfactory results” (Triandafilou and Kamper, 2012).
One way of counteracting these three issues—lack of patient exercise data, incorrect exercise motions, and reduced frequency of exercise—is to use a robotic device to assist with the rehabilitation process (Prange et al., 2006). Robotic rehabilitation can guide the patient’s motions during exercise and ensure that the full movement has been performed. Robotic systems can also display the patient’s progress and, using interactive games or virtual reality, encourage participation (Colombo et al., 2007).
A novel range of technologies—showing great potential in the area of medical robotics—are BMI systems. BMIs have been proposed for motor neurorehabilitation, motor replacement and assistive technologies (de Almeida Ribeiro et al., 2013), and wheelchair control (Li et al., 2013). Previous work by Ramos-Murguialday et al. (2013) with chronic (post 2 weeks) stroke patients showed that patients using-BMIs recovered a wider range of movements than the placebo group. These systems use physiological signals, originating in the brain, to activate or deactivate external devices or computers.
An electroencephalogram (EEG) can record sensorimotor rhythm activities (Yuan and He, 2014; Edelman et al., 2016) and show clear functional specificity during planned, actual, or imagined movements. These imagined movements of extremities cause specific EEG patterns, such as a desynchronization of mu and central beta rhythms at the contralateral sensorimotor area (Pfurtscheller et al., 2006). By using an EEG pattern classifier, it is possible to identify these patterns. There are multiple techniques for designing such classifiers and the choice of algorithm can significantly affect the final level of performance achieved (Bashashati et al., 2007).
Robotic rehabilitation allows the physiotherapist to collect previously unavailable data. These data can allow for greater knowledge of the patient’s progress and can be used to highlight whether any changes are required in their rehabilitation program, such as frequency and duration of the exercises performed. Kwakkel et al. (2003) reported that the length of time from a stroke to full recovery is much longer than was previously thought and that collecting more data gives a better prediction of patient outcomes.
Current Robotic Rehabilitation Systems
There are two main types of robotic system used for rehabilitation: exoskeletons and end effectors. Exoskeleton-based systems are mounted on the patient and control their motion directly. End effector-based systems rely on the patient interacting with the robot through a pointing device, such as a knob in the ReHapticKnob developed by Metzger et al. (2011), or a ball from the SPIDAR-8 developed by Walairacht et al. (2002). These systems are often paired with virtual reality games to allow for greater immersion by the patient.
Following multiple small-scale trials conducted on different robotic rehabilitation systems, the evidence gathered shows all trials produced either an equal or a greater level of recovery of motion than normal non-robotic rehabilitation exercises (Connelly et al., 2009; Stein et al., 2011); the most recent and comprehensive clinical trial involving 127 patients was conducted by Lo et al. (2010).
There are four problems with current robotic rehabilitation systems: (1) intimidating esthetics can discourage a patient from using them; (2) not all devices collect the full range of data available; (3) predominantly only physical motion is tracked and not any neural information; and (4) most systems are only deployable in clinics and require supervised use.
Using Soft Robotics in Rehabilitation Systems
To alleviate known problems with existing devices, one approach is to move away from “hard” robotics where the exoskeleton—consists of rigid linkages that can struggle—to mimic human motion, toward “soft” robotics where—through the use of compliant soft material—a more natural and safer motion can be performed.
Polygerinos et al. (2015) developed a hydraulic soft robotic glove to aid grasping tasks in the home environment, their design incorporated fiber-controlled actuators to provide force for the grasping motion. A recently reported soft-rehabilitation system is A Helping Hand (Zhao et al., 2016). A Helping Hand is formed of a single soft exoskeleton and contains individual air chambers for each finger to allow for grasping; in addition, each finger incorporates a novel optical monitoring system. A system that blends both “soft” and “hard” robotics is the Exo-Glove that replaces the inflatable soft sections with a tendon cable system that runs through the soft sections providing the motive force (In et al., 2015).
For further information on stroke rehabilitation systems, an in-depth analysis of the current state of the art and progress is reported by McConnell et al. (2017).
Design of SOPHIA
Exploration of Design Specification
We consulted with physiotherapists, both for design consideration relating to the esthetics and functionality of the device, and for the capability of the testing apparatus. We chose this user-centric design approach because Holt et al. (2007) and Blabe et al. (2015) reported that by including patients and physiotherapists in the design process, the chance of devices being used was increased. The two main points found in our consultations were that: (1) the system should leave as much of the palm and fingers exposed as possible to allow for direct interaction with objects and (2) the exoskeleton must be unobtrusive and lightweight.
The SOPHIA glove design was required to fit a variety of hand sizes; thus, we used the ergonomic data from BSI Standards Publication (BSI Standards, 2011). The BSI data guided our design in terms of the size of the glove and adjustability, for the weight of the glove we were aware that adding any extra weight to a stroke patient’s arm during rehabilitation could prove a burden, therefore, the weight was kept to a minimum with the total glove weighing under 0.4 kg and the control system (not mounted on patient) under 0.5 kg.
The motions and layout of the human hand can be seen clearly in work done by Taylor and Schwarz (1955) where the joint layout is illustrated clearly. The human finger has three degrees of motion per finger, one at the distal interphalangeal (DIP), one at the proximal interphalangeal (PIP), and one at the metacarpophalangeal (MCP). The full extension of the finger and its joint locations is shown by Figure S2 in Supplementary Material. In Figure S2A in Supplementary Material, the finger is shown in a “claw shape” with θ1 at max 45°, θ2 at max 90°, and θ3 at max 90°. When a full extension is performed as shown in Figure S2B in Supplementary Material, all of the joints are in alignment and θ1, θ2, and θ3 should be equal to 0°.
For human hand to complete an extension motion, it requires a torque from the whole finger of 0.94 Nm; this is broken down into 0.03 Nm at the (DIP), 0.75 Nm at the (PIP), and 0.16 Nm at the (MCP) (Milner and Franklin, 1998). This data was from non-stroke individuals. From testing the fiber-reinforced actuators where a force gage was mounted on the distal phalanx and a torque of 1.6 Nm was generated providing a sufficient torque to extend the finger.
Design of the Soft Exoskeleton Glove
For the SOPHIA device, we decided to use multiple pneunet actuators, which build upon the work by Shepherd et al. (2013), in combination with fiber-reinforced actuators (Connolly et al., 2015) to allow for ease of section replacement if one part fails. The SOPHIA device also incorporates BMI technologies. To enhance the patient’s recovery, the inclusion of a BMI in robotic rehabilitation systems has been trialed with positive results (Prasad et al., 2010; Pichiorri et al., 2015).
Figure 2C shows the completed exoskeleton glove, and Figure S3 in Supplementary Material shows a schematic of the actuators.
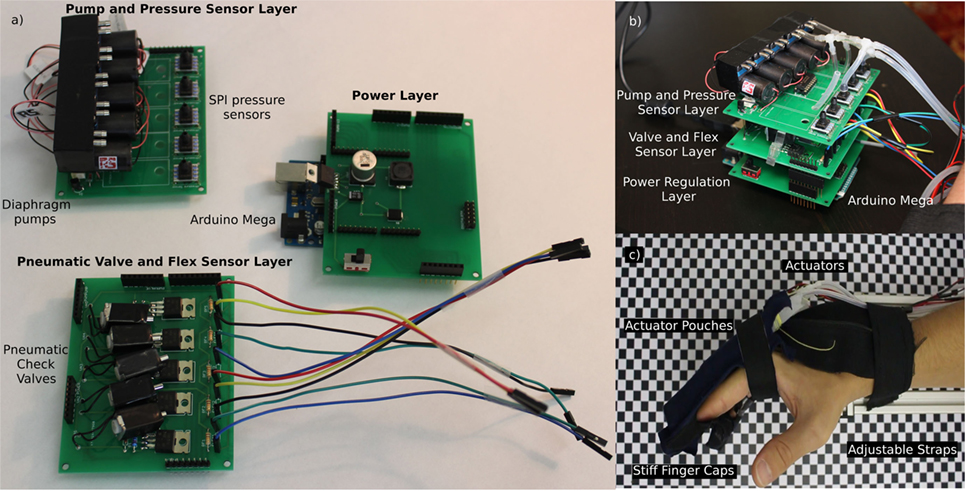
Figure 2. The electropneumatic controller for the Soft Orthotic Physiotherapy Hand Interactive Aid system consists of a modular series of components that connect via a tether to a soft robotic glove. This system is capable of extending a subject’s hand from a closed grasp to an open grasp. This figure shows: (A) the three layers in the electropneumatic control system; (B) the assembled, modular, electropneumatic control system; and (C) the pneunet-based soft exoskeletal glove system mounted on a subject’s hand.
Design of the Fiber-Reinforced Actuators
We designed the mold in a modeling program (solid edge) and then fabricated it by fused deposition manufacturing using an uPrint SE in acrylonitrile butadiene styrene (ABS). We used soft lithography techniques to cast the pneunets in Ecoflex 00-50 (Bentley Advanced Materials), and we used polydimethylsiloxane (PDMS) to form the bottom layer. We wrapped each actuator in Kevlar thread (Tex 40) using a double-helical pattern to control the expansion of the soft section and to allow primarily axial expansion. Each of the four fiber-reinforced actuators was 110 mm long by 20 mm wide to allow for full coverage of each finger. Full technical drawings are provided in Supplementary Material.
Design of the Glove
The glove fully encloses the soft actuators, which reduces the chances of damage, and reduces distraction to the patient. We used a non-elastic textile to create the finger caps and straps for the wrist and palm. The glove securely holds the actuators while leaving the majority of the palm exposed.
Design of the Flexible Sensors
We used piezoresistive flex sensors (Sparkfun; SEN-08606), inside the glove, and we fixed them in place using Ecoflex 00-50.
Design of the Electropneumatic Control System for the Soft Exoskeleton
The control system for the exoskeleton required integration of the pressure sensors, flex sensors, diaphragm pumps, and solenoid valves. A compact design was required to allow for ease of storage and transportation.
Electronic System
To allow for redundancy, we chose a modular design that uses three printed circuit board (PCB) layers. Figures 2A,B shows the complete electrical subsystem, described below:
(1) Control layer: to provide electrical power, digital/analog inputs, and outputs we used a microcontroller (Arduino Mega 2560).
(2) Power regulation layer: we designed a PCB that incorporates a voltage regulator and interconnections for the subsequent layers.
(3) Pneumatic valve and flex sensor layer: we designed this layer to provide electrical connections to the flex sensors, and we used pneumatic check valves to control the actuators (Longykj; LY0520GC).
(4) Pump layer: we use diaphragm pumps (RS Components; 702-6898) and SPI pressure sensors (Mouser; 785-HSCDANN1.6BASA5) in this layer to provide the pneumatic power.
Each layer is 80 mm × 80 mm and the system has a total stacked height of 120 mm. We built a compact box to reduce the chance of damage, and the noise from pumps and valves. Each layer is shown and labeled in (Figure 2C) and the completed modular electropneumatic system is shown by Figure 2B.
Design of the Wearable Module for the Passive System
This section covers the design and fabrication of the material sections for the passive system, the block diagram for this system is shown by Figure S1 in Supplementary Material.
Glove Module
We designed the glove to fit an average adult’s hand and we used a modified sports glove to provide both comfort and the durability to have components mounted on to it. The glove fully encloses the hand and has specific mounting sections affixed to the back of the fingers for the flex sensors (Sparkfun; SEN-08606) to be inserted, and a mounting pouch on the back of the hand to hold the PCB.
Lower Arm Module
The wrist module was mounted on top of a lycra layer and held in place with adjustable straps.
Design of the Electronics and Display for the Passive System
The passive control system required integration of flex sensors, IMUs, a bluetooth transmission module, and a battery pack. A compact and lightweight design was required to minimize any restrictions that the user would feel when wearing the device.
Glove Module
We designed this module to gather the motion of the fingers and of the hand by having a flex sensor mounted to each finger and thumb, which were connected to a flexible PCB that is mounted directly to the back of the glove. The PCB also contains an IMU placed centrally to the hand. Each of the flex sensors was coated in Ecoflex 00-50 to reduce the change of damage.
Lower Arm Module
This module collects data about the arm motion, it has bluetooth wireless communications and a battery. It includes an Arduino, bluetooth transmitter, IMU, and a Li-Po battery module.
The passive monitoring system connects to a computer via bluetooth, and this computer is used to record and display the data. We used the Processing IDE both as our compiler, and for our graphical interface (shown by Figure S1C in Supplementary Material). Our embedded microcontroller code implements a complementary filter on the data it receives from the accelerometer and gyroscope data to create a stable signal that is then displayed by the animation of the hand and forearm.
Selection of the BMI System
Recording EEG signals usually requires costly devices along with specialized software to gather the data. Currently, in the market, there are several low cost devices that can be used with open-source software like OpenViBE (Renard et al., 2010). One of the limitations of these systems is that, in general, they only provide data-recording using a limited number of electrodes.
Following the EEG 10-20 standard for electrode placement (Klem et al., 1999), MI-specific brain signals, corresponding to upper limbs, register mainly in two specific areas: C3 and C4 located in the motor cortex. It is common that commercial low cost BMI systems do not cover C3 and C4 specifically, often leading to lower accuracy in the final results of the classifier. EEG signals are user dependent, causing variations in EEG patterns in different subjects. These variations can be seen in the same specific mental tasks (Karthikeyan et al., 2009) and restricting the C3 and C4 electrodes could result in significant data loss (Dias et al., 2009; Arvaneh et al., 2011).
One of the most consumer-oriented devices is the neuroheadset Emotiv Epoc+ (Liu et al., 2012; Duvinage et al., 2013), shown in (Figure 3A), which includes 14 electrodes distributed in the four lobules (Lang, 2012) electrode configuration shown (Figure 3B). In this paper, we compare the Epoc+ with the actiCAP (V-Amp and Easy Cap) from Brain Products, see (Figure 3C) with electrode configuration shown in (Figure 3D). The ActiCap system is best suited for a professional use and has been used in previous healthcare studies (Sefer et al., 2009; Roy et al., 2013). Epoc+ has the advantage of being wireless and easier to place, thus reducing setup time. ActiCAP, by comparison, is wired, bulky, and requires a conductive gel to decrease impedance. These are important aspects that we considered when reviewing the final system design.
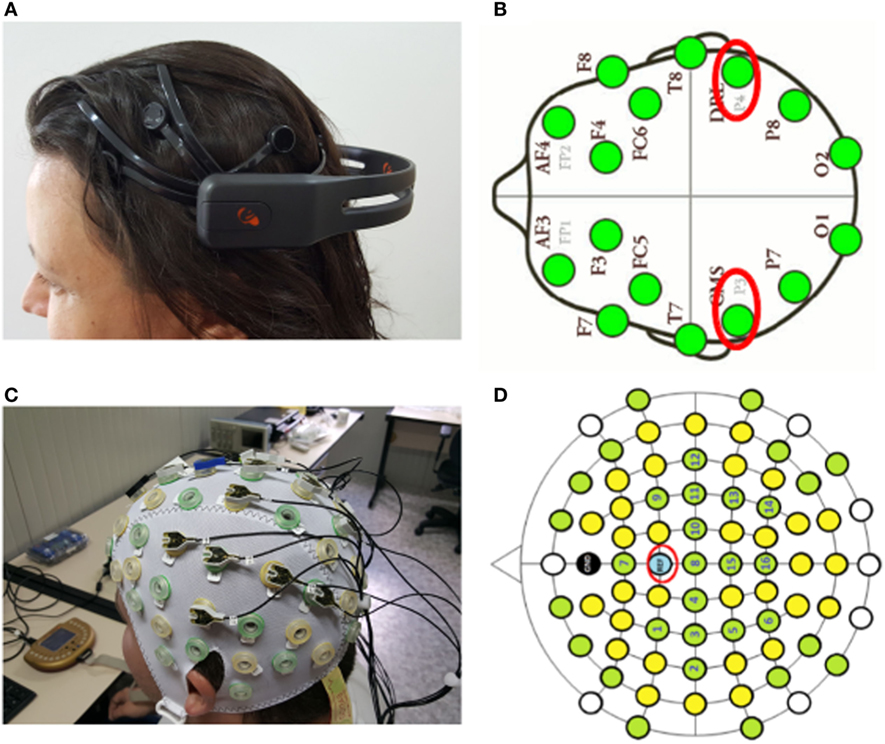
Figure 3. The two brain–machine interface headsets evaluated for use with the Soft Orthotic Physiotherapy Hand Interactive Aid system were: (A) the Emotiv Epoc+ headset and (C) the actiCAP. Insets (B,D) show the number of electrodes in each system, and circled in red are the reference electrodes.
Design of the Software Control System
We integrated the Arduino IDE with OpenViBE using a bespoke communication protocol implemented in C++. Our embedded microcontroller code implements a software proportional–integral–derivative controller loop to control the valve and pump array using feedback from the pressure sensors, the flex sensors, and the received signals from OpenViBE. The embedded software acts as the overall control module that engages and disengages actuation of the rehabilitation system.
The OpenViBE pipeline requires the implementation of different signal processing and machine learning techniques to optimize the interaction between the user and a specific system/software. The raw EEG signals were captured by the BMI system and processed (digitized and amplified), then classified into different movements in OpenViBE, and finally sent to the microcontoller via the C++ communication protocol. For later analysis, all of the sensor data from the glove were saved in real time as well as the raw EEG data. Complete control system for hardware and software shown in (Figure 4).
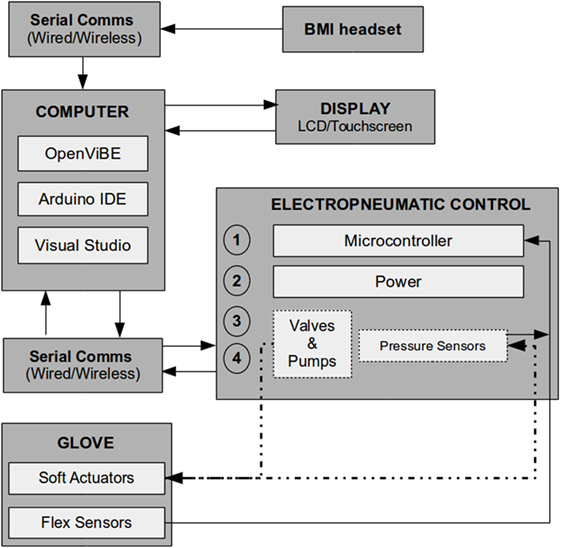
Figure 4. A block diagram showing the hardware and software used in this system. The data flow in the Soft Orthotic Physiotherapy Hand Interactive Aid system begins with the brain–machine interface (BMI) as the initial trigger, the data from is then passed to the software running on the computer, and the outputs are sent to the display and to the electropneumatic controller, and finally the soft robotic glove system applies torque to the fingers.
Experimental
The experiments were conducted within a specifically designed arena, shown in (Figure 5A). This area ensured the participant was comfortable and able to perform the required exercises with no external influence, while allowing for the motions to be videorecorded for complete data analysis. The area consists of an adjustable aluminum frame. The armrest has variable height and side position to allow for an ergonomic resting position. We created an adjustable mount for the top of the frame to hold the video camera. To enable positional accuracy, and to act as a point of reference for the camera, we attached a reference grid to the bottom of the area. We enclosed the sides of the frame to create a contained environment. The screen is also adjustable to allow for an ergonomic viewing height for each participant. All of these features create a controlled environment for each participant. A physiotherapist checked that our frame was safe and would not cause any harm or stress to the participants.
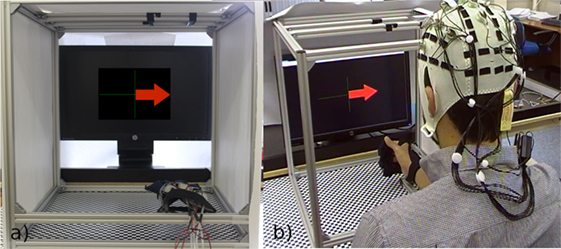
Figure 5. (A) The test environment, which is fully enclosed at either side to avoid distraction, and the glove lying on the arm rest, we have overlayed the arrow from the OpenViBE software to show what the subject would see on the screen. (B) A subject performing the initial training phase.
The experimental procedure was approved by the School of Mathematical and Computer Science at Heriot-Watt University Ethics Board. All participants signed the informed consent forms before participating in the experiments.
Exoskeleton Preparation
Both the glove and the soft robotic actuators were inspected before use and, if there was no damage, then the soft actuators were inserted into the glove and connected to the control system.
BMI Preparation
Each cap requires a different setup procedure as follows.
Epoc+: the sponges that are attached to the sensors are moistened with a saline solution. Then the device is placed on the subject’s head. The Epoc+ software provides a visual display of the contact quality of each electrode and is represented in different colors: absent (black), poor (red), unstable (yellow), and good (green).
ActiCAP: the device is placed on the subject’s head. The calibration of the sensors is carried out by inserting conductive gel into each electrode with a syringe until a low-impedance signal is received. Green LEDs correspond to a correct sensor contact and red LEDs to an insufficient contact between the sensor and the scalp.
Achieving good contact quality of the sensors in both neuroheadsets is a critical requirement for performing successful recordings.
Test Environment
Figure 5B shows the test environment and control boards, also shown are the exoskeleton on the subject’s hand, and the BMI system mounted on the subject’s head.
Protocol for EEG Testing
The BMI system was tested with both the actiCAP and Epoc+ headsets. The experiment was based on the motor imagery task developed by the Graz BCI group (Pfurtscheller and Neuper, 2001), where subjects, in the training phase, were instructed only to imagine open their right hand whenever they saw an arrow pointing to the right side of the screen, or thinking “nothing” when the arrow pointed to the left side. After the training phase, the subjects were able to send the commands to the SOPHIA device to actively open their right hand.
OpenViBE Process
The OpenViBE software was used to map the data from its raw format to a set of numerical clusters that correspond to the different movements being analyzed. Using a series of configurable scenarios, OpenViBE offers a pipeline to perform the acquisition, preprocessing, and classification of raw EEG signals associated with hand motor imagery tasks.
After sampling the data, the next step involves training the linear discriminant analysis (LDA) classifier using the common spatial pattern filter to emphasize the distinction between EEG recordings associated with the right or left hand. Finally, when the training phase is concluded, online sessions can be then run, where the rehabilitation tasks are performed. In this type of session, visual feedback cues (shown in Figure 6A) are shown to the subject while they perform mental tasks.
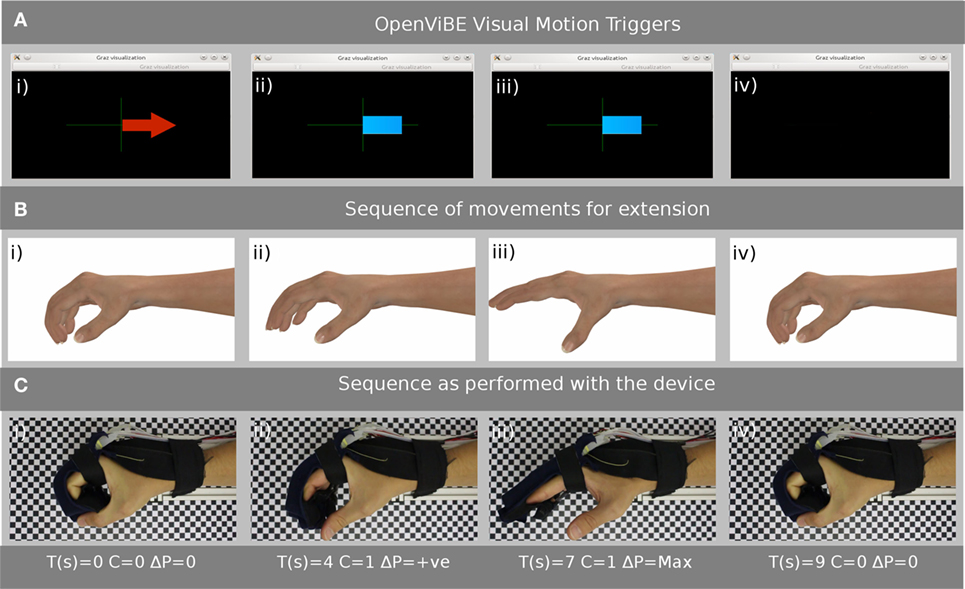
Figure 6. (A) Samples of the Graz Visualization that the subject will see on the display and proceeds from (i) where the arrow indicates the subject should think “open hand” (ii) and (iii) show the classifier signal level (before the binary thresholding), which in this example should stay to the right of the axis, (iv) indicates that the subject should relax and think of “nothing.” (B) An example of the simulated motion the patient should be performing while the exercise is proceeding. (C) Shows the subjects hand, while wearing the glove, and the corresponding time (T), thresholded classifier value (C), and the change in pressure (ΔP) as the exercise proceeds from (i) to (iv).
Experimental Protocol
1. The subject reads and signs ethical and safety forms.
2. The subject sits at the experimental apparatus, which can be adjusted to ensure the correct ergonomic positioning setup.
3. The exoskeleton is affixed to the subject’s hand by the researcher.
4. The BMI is placed on the subject’s head and the connections are all checked by the researcher.
5. OpenViBE is run in training mode for the subject’s data to be gathered and configured to the individual.
6. Once completed, the C++ client and Arduino IDE is run and the OpenViBE test protocol is engaged.
7. The subject is required to visualize the opening of their right hand when the corresponding signal is given on the display and the soft actuators will inflate in response when the threshold signal from the BMI is received.
8. When the opposite signal is shown to the subject, they are required to relax and think of nothing.
9. A brief pause is given between each signal.
10. Points 7, 8, and 9 are repeated for 8 min using 40 random combinations of points 8 and 9.
11. After the test has been completed the system is closed down and the BMI and exoskeleton are removed.
Results and Discussion
Results of Training Experiments Testing EEG Signals and Applied Pressure
This section illustrates the results from the online testing of the different participants. The positive classification output should only be generated when the subject thinks about closing their hand, or should be negative when they provide a neutral thought. Figure 7A shows the ideal pattern of the pressure, classification output versus time that should be generated from the experiments. Under ideal conditions, the pressure should increase to its safe maximum when the classification output is positive or until the exercise time is complete. On receiving a negative signal, the pressure should rapidly drop to the initial state and remain in this state until a positive signal is detected again.
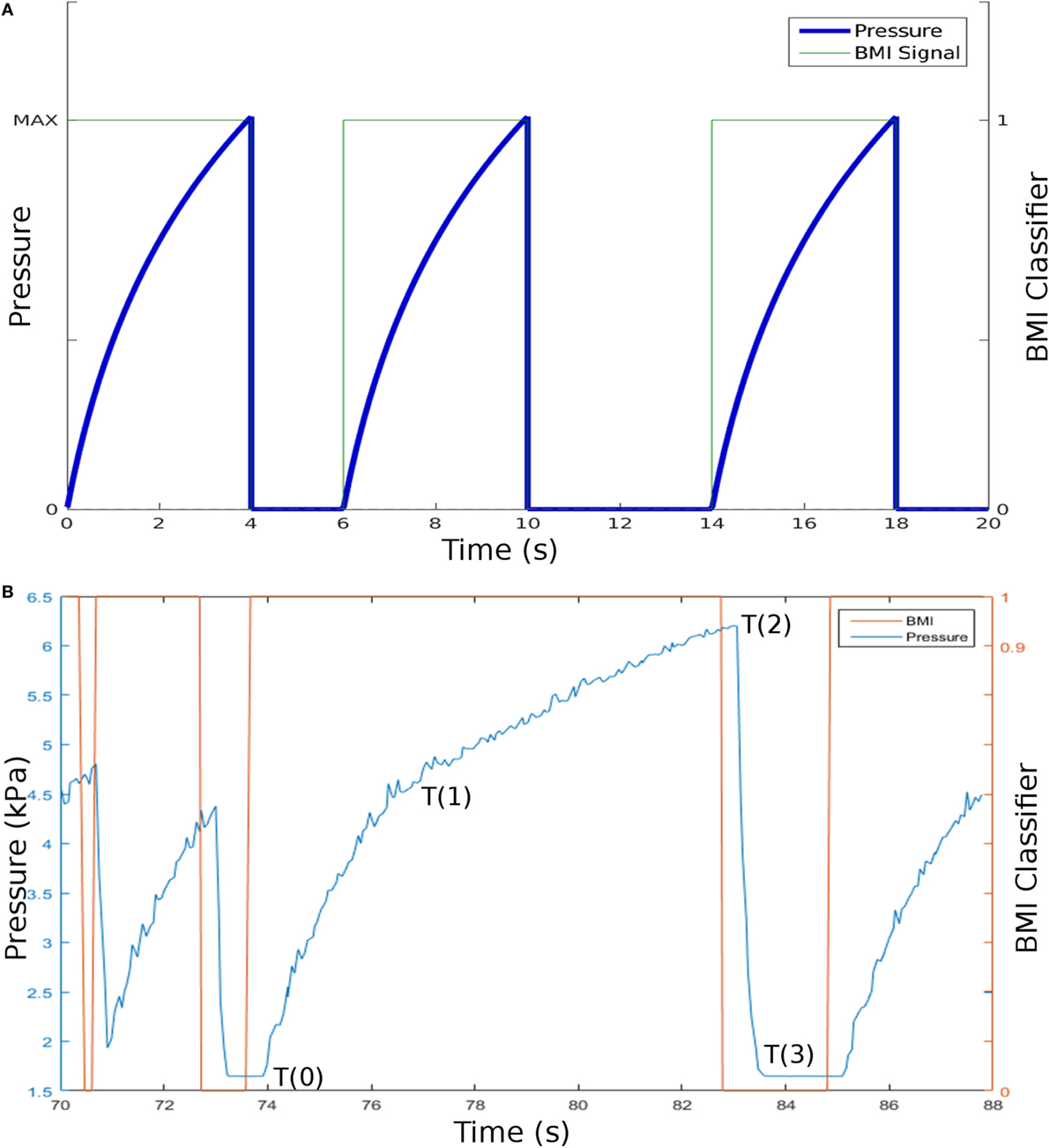
Figure 7. (A) Model data showing the electroencephalogram data generated from a brain–machine interface (BMI) device linked to the Soft Orthotic Physiotherapy Hand Interactive Aid system and the pneumatic pressure in one of the soft actuators. The pneumatic pressure increases (to a safe maximum) when the classifier is positive, and this pressure is released by opening a valve when the classifier is zero. (B) Example experimentally collected data showing how the BMI classifier data correlates to the increase in pneumatic pressure. T(0) to T(2) shows the pressure increase while the classifier is positive, and T(2) to T(3) shows the drop in pressure when the classifier is zero.
Figure 7B shows an example of the data collected. We mapped the classification output into two categories by thresholding. 0 and 1 represent the subject thinking of opening their hand or not. In Figure 7B, the signal showing applied pressure clearly correlates well with the classifier output for this subject. To put an objective figure of merit on these data, we developed a metric called “average reliability,” this is a percentage score to show the relationship between the BMI signal received from the headset and the correct response resulting in actuation of the electropneumatic system. We analyzed the datasets from five subjects, three with large hands, and two with medium-sized hands. For each, we thresholded the classifier data to give a binary response, positive (1) and negative (0); we then thresholded the pressure data to ensure that the output response was above the noise level. We compared these two pieces of information to compare the number of times the system responded correctly (system actuates when patient intends to open their hand and system does not actuate when the patient does not intend to open their hand) versus the number of times the system responded incorrectly. For these five patients, the average reliability was 83.8%. Future studies will be clinically validated with a larger sample size.
Comparison of Simulated Motion with Directed Motion of the Hand by the Exoskeleton
Figure 6A–C show the display that the subject follows as the experiment takes place. Four simulations of the intended motion of the subject’s hand are displayed on the video screen, and a corresponding photograph shows the directed motion of the subject’s hand as a result of pressure being applied by the SOPHIA device. The time (T) starting at 0 s, classifier data (C), and the change in pressure (P) are provided in each case. The directed hand extension clearly matches the simulated hand motion, and this visual feedback gives the subject confidence that the rehabilitation exercise was performed correctly.
Comparison between Epoc+ and ActiCAP
Choice of BMI Headset
When selecting an appropriate BMI headset, we evaluated the following points: setup time, cost, and comfort.
The Epoc+ headset required approximately 10 min to set up, while the actiCAP could take 20 min. This shorter setup time was due to the Epoc+ requiring only the pads to be moistened in a saline solution first before being placed on the device, and mounted on the subject’s head. The actiCAP required careful placement of each sensor and, once mounted, a conductive gel injected between the scalp and the sensor, the impedance level checked and then more gel injected if required. The Epoc+ sensors cannot be adjusted to cover other parts of the head and are fixed in their mountings, while the actiCAP has greater flexibility and its sensor placement can be adjusted depending on the sensor requirements.
There is a substantial cost difference between the systems, and this difference could be a key point for use in a clinical setting with the Epoc+ retailing for approximately £600 and the actiCAP system for £10,000.
Subjects noted less discomfort with the Epoc+ as its wireless design gave reduced weight and less pull on the subject’s head as compared to the actiCAP.
Analysis of Standard OpenViBE Classifier for the ActiCAP and Epoc+ System
We found a noticeable difference between the accuracy of the classifier generated from the different systems, the results of these experiments are shown in Figure 8. The actiCAP had an average cross validated accuracy of 82.5% over the tests performed, as compared to 66.8% from the Epoc+. OpenViBE recommend that any experiments resulting in an accuracy of less than 65% are redone until a suitable accuracy is achieved (Renard et al., 2010). Although the standard Epoc+ classifier accuracy is low, it is still above the minimum acceptable level.
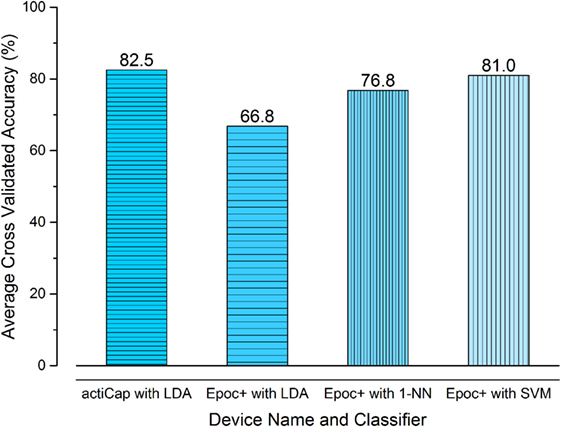
Figure 8. The results of using the actiCAP and Epoc+ brain–machine interface systems. The average cross validated accuracy for four tests is shown. The actiCAP and raw Epoc+ data [with standard openViBE preprocessing and linear discriminant analysis (LDA) classifier] show that the actiCAP gives 82.5% accuracy versus 66.8% for the Epoc+. On applying the preprocessing procedure (detailed in section 4) with two different classifiers, then the percentage cross-validated accuracy for the Epoc+ system was improved to 76.8% (1-NN) and 81.0% (SVM). These results show that with appropriate preprocessing and classifier techniques then the cheaper Epoc+ system can return data which is comparable to the much more expensive actiCAP system.
During testing, we used both a wireless Epoc+ and a wired actiCAP to test the system, and we found that both were capable of providing the required signals. We found notable differences in the quality of the signals from the two caps; the positive and negative values for each test should be equal, i.e., −10 to 10 or −3.5 to 3.5 for an ideal test. These values are unitless. For the actiCAP, the blank signal ranged between −3.29 to −12.77 and the “open” signal range was 4.05 to 11.29. These small differences at either end of the range (Δ0.76 and Δ1.49) led to little noticeable control loss.
While using the Epoc+ system the blank signal range was −4.12 to −9.60 and the “open” signal range was 8.02–5.10. These large differences at either end of the range (Δ3.90 and Δ4.5) illustrate a strong bias toward either the positive or the negative end of the range, leading to false positives or negatives in applied pressure.
Improving the Classification Accuracy of Epoc+ and ActiCAP Devices
Comparing the performance of different neuroheadsets is a challenging task, since no exhaustive classification-based assessments that quantitatively compare medical devices exist so far. In a survey released in 2010, Stamps and Hamam (2010) claimed that, in terms of usability, the best low cost EEG recorder was the Emotiv Epoc headset.
van Vliet et al. (2012) assessed actiCAP and Epoc headsets by gathering steady-state visually evoked potential signals and using these data to control a tactical video-game. After limiting the number of nodes in the actiCAP device to eight, located over the occipital area and under sitting conditions, the researchers showed that the Emotiv Epoc headset provides decreased performance compared to the actiCAP system, but the performance achieved was good enough to robustly detect the signals. The study did not give details of the software or algorithms used in the classification, which are also critical aspects to take into account.
Other studies also corroborate this enhancement in the classification accuracy of the actiCAP. In the comparative research conducted again with both devices, focusing on the distinction of patterns of brain activity formed by imagining pictures (Bobrov et al., 2011), the percentage of correctly recognized states was always higher in the case of actiCAP but with a difference less than 10%. The application of advanced machine learning algorithms can notably increase the performance of the Epoc+ system. Following this approach, for stroke rehabilitation, Munoz et al. (2014) claimed to reach 98% of accuracy applying a type of support vector machine (SVM) algorithm to distinguish between two states (open and close the hand) using Epoc+ headset.
Knowing these differences in the quality of the signal recorded by both devices, the question is if improvements to the default OpenViBE pipeline can sufficiently aid in enhancing the final classification performance.
We applied further signal processing and machine learning techniques, including filtering using the non-recursive finite impulse response, electrooculographic artifacts removal based on a second-order blind inference method, and extraction of features with the discrete wavelet transform approach. Afterward, two advanced classification techniques were applied to distinguish hand movements: k-nearest neighbor (k-NN) and SVM. These types of techniques have been used previously to classify signals for stroke rehabilitation (Munoz et al., 2014; Zhang et al., 2015). We provide links to our software that implements these algorithms in Supplementary Material.
We performed proof-of-concept experiments using five healthy subjects. Due to the small sample size, the results showed here should not be considered statistically significant and future work will focus on increasing the sample size. Our preliminary data, however, do give some information about expected performance in larger scale experiments. Our results are shown by Figure 8, where the final classification accuracy is depicted according to the different algorithms used (k-NN and SVM). Figure 8 illustrates the results from each of the algorithms, applying both the OpenViBE preprocessing steps and our improved pipeline. The figure shows that the use of the actiCAP, with sensors allocated in the motor cortex, achieves marginally higher performance (82.5%) than the Epoc+, with our modified OpenViBE preprocessing and SVM (81%), as was concluded in previous studies. The difference in performance between both BMI systems, however, is not quantitatively significant and therefore, we conclude that the low cost Epoc+ is able to distinguish between two states—open and relaxed—with comparable accuracy to the high-cost actiCAP system.
Monitoring of Passive Exercises
We tested the passive system on three subjects and found that it works consistently on each person when interfaced with a laptop computer. The GUI on the laptop displays the motions that are being performed, and an example image of the CAD representation is shown by Figure S1C in Supplementary Material. The joint angle between the wrist and the hand is computed by taking a differential measurement of the data from the two IMUs, and the finger flexion data come from monitoring the outputs from the flex sensors.
We explored the possibility of running the GUI on a low cost computer with a small display. We tried a Rapsberry Pi 3 and we found that even with the Raspberry Pi 3’s increased GPU power over its predecessors the CAD output from the GUI lagged behind the actual real-time motion, and this lag created frustration in the subjects. Future work will focus on improving the 3D-performance of a low cost mobile display unit.
Challenges and Scope for Future Designs
We created a prototype of a soft robotic system that is capable of safely opening a human hand using a BMI system. We note four challenges and scope for iteration in future designs:
(1) We designed the glove for a large hand, meaning that for people of a smaller hand size the device did not function optimally.
(2) The noise and “feel” of the exoskeleton proved to be a distraction for the participants, and these factors lead to initial difficulties in concentration.
(3) We demonstrated results using either the actiCAP and Epoc+, initially the stability of the EEG signal from the actiCAP generated less instabilities and false positives. This was partially corrected when our modified pipeline was applied to the Epoc+.
(4) When using the passive exercise system, the feedback from the study group reported that the 3D representation of the hand was not as lifelike as the subjects would have liked.
Conclusion
Soft Orthotic Physiotherapy Hand Interactive Aid consists of (1) a soft robotic exoskeleton, (2) a microcontroller-based control system driven by a BMI, and (3) a sensorized glove for passive rehabilitation. This first prototype of our modular soft robotic exoskeleton system can provide guided rehabilitation exercises, and we have demonstrated how it assists a subject to extend their hand from the claw shape using information received from a BMI. We show that by collecting data from EEG, pressure, and video, we can quantify the performance of the system, and we have identified areas that are important for future development.
There were issues with our system, such as (1) the variety of hand sizes when testing the system on multiple people and (2) the Epoc+ system could not provide as stable readings as the actiCAP using the standard openViBE workflow, leading to issues of a bias in the motion of the exoskeleton (opening when no motion should happen). This issue was reduced but not fully removed when we implemented preprocessing and the k-NN and SVN algorithms.
Taking into account the extra time that is required to use the £10,000 actiCAP arrangement, and the cost difference, the £600 Epoc+ proves to be a suitable alternative. We note that there is a market opportunity for an adjustable electrode, wireless BMI system that covers the motor cortex, and which is priced similar to the Epoc+.
The work shown illustrates the potential for further development in the expansion of the soft system to other body areas while using BMI to complement the rehabilitation. This paper reports on a proof-of-concept system that will enable us to develop specifications for a subsequent device, which could be used in future clinical trials.
Ethics Statement
This study was carried out in accordance with the recommendations of “Research Ethics Policy and Procedures, School of Mathematical and Computer Science, Heriot-Watt University Ethics Board” with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the “School of Mathematical and Computer Science at Heriot-Watt University Ethics Board.”
Author Contributions
AM created the system and is the lead author of all sections of the work. MV is an expert on the data analysis and classifier work done in the project and for this manuscript. RM and FB are experts in the BMI field of research providing the insight and direct contribution to the BMI setup, protocol, and experimental work. NS directly contributed to the design and build of the sensorized glove and the 3D rendering of its motion and the corresponding sections of this paper. CR directly contributed to the classifier and modified BMI testing section. DC contributed to the data analysis and document formation. PV advised on document style, document formation, and overviewed the project. AS is an expert in soft robotics contributing to the fabrication of the soft glove as well as advising on paper construction, layout, and contributed directly to all sections as primary editor.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Iain Gold of the Edinburgh University for his expertise in electronics fabrication, the members of The Stokes Research Group at The University of Edinburgh, the members of the Edmond and Lily Safra International Institute of Neuroscience, and the members of the Heriot-Watt Robotics Lab.
Funding
We would like to thank The Royal Society (Newton International Exchange Award, NI140250) for funding, ERASMUS+ for allowing Nicola Secciani to be a part of this work, and support from the EPSRC via a Studentship Award (1373260) for Alistair McConnell, the Robotarium Capital Equipment and CDT Capital Equipment Grants (EP/L016834/1). Markus Nemitz gratefully acknowledges support from the CDT in Integrative Sensing and Measurement (EP/L016753/1). Fabricio Brasil and Renan Moioli acknowledge the support from the Santos Dumont Institute and the Brazilian Ministry of Education (MEC).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fmech.2017.00003/full#supplementary-material.
References
Arvaneh, M., Guan, C., Ang, K. K., and Quek, C. (2011). Optimizing the channel selection and classification accuracy in EEG-based BCI. IEEE Trans. Biomed. Eng. 58, 1865–1873. doi: 10.1109/TBME.2011.2131142
Bashashati, A., Fatourechi, M., Ward, R. K., and Birch, G. E. (2007). A survey of signal processing algorithms in brain–computer interfaces based on electrical brain signals. J. Neural Eng. 4, R32–R57. doi:10.1088/1741-2560/4/2/R03
Basteris, A., Nijenhuis, S. M., Stienen, A. H. A., Buurke, J. H., Prange, G. B., and Amirabdollahian, F. (2014). Training modalities in robot-mediated upper limb rehabilitation in stroke: a framework for classification based on a systematic review. J. Neuroeng. Rehabil. 11, 111. doi:10.1186/1743-0003-11-111
Blabe, C. H., Gilja, V., Chestek, C. A., Shenoy, K. V., Anderson, K. D., and Henderson, J. M. (2015). Assessment of brain–machine interfaces from the perspective of people with paralysis. J. Neural Eng. 12, 43002. doi:10.1088/1741-2560/12/4/043002
Bobrov, P., Frolov, A., Cantor, C., Fedulova, I., Bakhnyan, M., and Zhavoronkov, A. (2011). Brain-computer interface based on generation of visual images. PLoS ONE 6:e20674. doi:10.1371/journal.pone.0020674
Bruno-Petrina, A. (2014). Motor Recovery in Stroke: Recovery Considerations, Theories of Recovery, Mechanisms of Recovery. Available at: http://emedicine.medscape.com/article/324386-overview
BSI Standards. (2011). Basic Human Body Measurements for Technological Design Part 1: Body Measurement Definitions and Landmarks. Brussels, Belgium: BSI.
Colombo, R., Pisano, F., Mazzone, A., Delconte, C., Micera, S., Carrozza, M. C., et al. (2007). Design strategies to improve patient motivation during robot-aided rehabilitation. J. Neuroeng. Rehabil. 4, 3. doi:10.1186/1743-0003-4-3
Connelly, L., Stoykov, M. E., Jia, Y., Toro, M. L., Kenyon, R. V., and Kamper, D. G. (2009). Use of a pneumatic glove for hand rehabilitation following stroke. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2434–2437. doi:10.1109/IEMBS.2009.5335400
Connolly, F., Polygerinos, P., Walsh, C. J., and Bertoldi, K. (2015). Mechanical programming of soft actuators by varying fiber angle. Soft Robot. 2, 26–32. doi:10.1089/soro.2015.0001
de Almeida Ribeiro, P. R., Brasil, F. L., Witkowski, M., Shiman, F., Cipriani, C., Vitiello, N., et al. (2013). Controlling assistive machines in paralysis using brain waves and other biosignals. Adv. Hum. Comput. Interact. 2013, 1–9. doi:10.1155/2013/369425
Dias, N. S., Mendes, P. M., and Correia, J. H. (2009). “Feature selection for brain-computer interface,” in 4th European Conference of the International Federation for Medical and Biological Engineering, 318–321.
Duncan, P. W., Zorowitz, R., Bates, B., Choi, J. Y., Glasberg, J. J., Graham, G. D., et al. (2005). Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke 36, e100–e143. doi:10.1161/01.STR.0000180861.54180.FF
Duvinage, M., Castermans, T., Petieau, M., Hoellinger, T., Cheron, G., and Dutoit, T. (2013). Performance of the Emotiv Epoc headset for P300-based applications. Biomed. Eng. Online 12, 56. doi:10.1186/1475-925X-12-56
Edelman, B. J., Baxter, B., and He, B. (2016). EEG source imaging enhances the decoding of complex right-hand motor imagery tasks. IEEE Trans. Biomed. Eng. 63, 4–14. doi:10.1109/TBME.2015.2467312
Edwardson, M. A., and Dromerick, A. W. (2016). Ischemic Stroke Prognosis in Adults, ed. T. W. Post (UpToDate). Available at: http://www.uptodate.com/contents/ischemic-stroke-prognosis-in-adults
Feys, H. M., De Weerdt, W. J., Selz, B. E., Cox Steck, G. A., Spichiger, R., Vereeck, L. E., et al. (1998). Effect of a therapeutic intervention for the hemiplegic upper limb in the acute phase after stroke: a single-blind, randomized, controlled multicenter trial. Stroke 29, 785–792. doi:10.1161/01.STR.29.4.785
Holt, R., Makower, S., Jackson, A., Culmer, P., Levesley, M., Richardson, R., et al. (2007). “User involvement in developing rehabilitation robotic devices: an essential requirement,” in IEEE 10th International Conference on Rehabilitation Robotics (IEEE), 196–204.
Hussein, N., and Staines, E. (2013). “The rehabilitation of younger stroke patients,” in Evidence-Based Review of Stroke Rehabilitation, eds R. Teasell, N. Hussein, N. C. Foley, and A. Cotoi 17th ed. (London: Evidence-Based Review of Stroke Rehabilitation), 1–63.
In, H., Kang, B. B., Sin, M. K., and Cho, K.-J. (2015). Exo-glove: a wearable robot for the hand with a soft tendon routing system. IEEE Robot. Autom. Mag. 22, 97–105. doi:10.1109/MRA.2014.2362863
Jauch, E. C., Saver, J. L., Adams, H. P. Jr., Bruno, A., Connors, J. J., Demaerschalk, B. M., et al. (2013). Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44, 870–947. doi:10.1161/STR.0b013e318284056a
Jørgensen, H. S., Nakayama, H., Raaschou, H. O., Vive-Larsen, J., Støier, M., and Olsen, T. S. (1995). Outcome and time course of recovery in stroke. Part II: time course of recovery. The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 76, 406–412. doi:10.1016/S0003-9993(95)80568-0
Karthikeyan, G., Sheet, D., and Manjunatha, M. (2009). “An electroencephalogram signal based triggering circuit for controlling hand grasp in neuroprosthetics,” in 13th International Conference on Biomedical Engineering (Berlin, Heidelberg: Springer Berlin Heidelberg), 691–693.
Klem, G. H., Lüders, H. O., Jasper, H. H., and Elger, C. (1999). The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 52, 3–6.
Kwakkel, G., Kollen, B., and Twisk, J. (2006). Impact of time on improvement of outcome after stroke. Stroke 37, 2348–2353. doi:10.1161/01.STR.0000238594.91938.1e
Kwakkel, G., Kollen, B. J., van der Grond, J., and Prevo, A. J. (2003). Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 34, 2181–2186. doi:10.1161/01.STR.0000087172.16305.CD
Lang, M. (2012). Investigating the Emotiv EPOC for Cognitive Control in Limited Training Time. University of Canterbury.
Li, Y., Pan, J., Wang, F., and Yu, Z. (2013). A hybrid BCI system combining P300 and SSVEP and its application to wheelchair control. IEEE Trans. Biomed. Eng. 60, 3156–3166. doi:10.1109/TBME.2013.2270283
Liu, Y., Jiang, X., Cao, T., Wan, F., Mak, P. U., Mak, P.-I., et al. (2012). “Implementation of SSVEP based BCI with Emotiv EPOC,” in 2012 IEEE International Conference on Virtual Environments Human-Computer Interfaces and Measurement Systems (VECIMS) Proceedings (IEEE), 34–37.
Lo, A. C., Guarino, P. D., Richards, L. G., Haselkorn, J. K., Wittenberg, G. F., Federman, D. G., et al. (2010). Robot-assisted therapy for long-term upper-limb impairment after stroke. N. Engl. J. Med. 362, 1772–1783. doi:10.1056/NEJMoa0911341
McConnell, A. C., Moioli, R. C., Brasil, F. L., Vallejo, M., Corne, D. W., Vargas, P. A., et al. (2017). Robotic devices and brain machine interfaces for hand rehabilitation post-stroke: current state and future potentials. J. Rehabil. Med.
Metzger, J.-C., Lambercy, O., Chapuis, D., and Gassert, R. (2011). “Design and characterization of the ReHapticKnob, a robot for assessment and therapy of hand function,” in IEEE/RSJ International Conference on Intelligent Robots and Systems, 3074–3080.
Milner, T. E., and Franklin, D. W. (1998). Characterization of multijoint finger stiffness: dependence on finger posture and force direction. IEEE Trans. Biomed. Eng. 45, 1363–1375. doi:10.1109/10.725333
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2015). Heart disease and stroke statistics: 2015 update: a report from the American Heart Association. Circulation 131, e29–e322. doi:10.1161/CIR.0000000000000157
Munoz, J. E., Rios, L. H., and Henao, O. A. (2014). Low cost implementation of a motor imagery experiment with BCI system and its use in neurorehabilitation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2014, 1230–1233. doi:10.1109/EMBC.2014.6943819
Nakayama, H., Jørgensen, H. S., Raaschou, H. O., and Olsen, T. S. (1994). Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 75, 394–398. doi:10.1016/0003-9993(94)90161-9
Pfurtscheller, G., Brunner, C., Schlögl, A., and da Silva, F. H. L. (2006). Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. Neuroimage 31, 153–159. doi:10.1016/j.neuroimage.2005.12.003
Pfurtscheller, G., and Neuper, C. (2001). Motor imagery and direct brain-computer communication. Proc. IEEE 89, 1123–1134. doi:10.1109/5.939829
Pichiorri, F., Morone, G., Petti, M., Toppi, J., Pisotta, I., Molinari, M., et al. (2015). Brain-computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol. 77, 851–865. doi:10.1002/ana.24390
Polygerinos, P., Wang, Z., Galloway, K. C., Wood, R. J., and Walsh, C. J. (2015). Soft robotic glove for combined assistance and at-home rehabilitation. Rob. Auton. Syst. 73, 135–143. doi:10.1016/j.robot.2014.08.014
Prange, G. B., Jannink, M. J., Groothuis-Oudshoorn, C. G., Hermens, H. J., and IJzerman, M. J. (2006). Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J. Rehabil. Res. Dev. 43, 171–184. doi:10.1682/JRRD.2005.04.0076
Prasad, G. B., Herman, P., Coyle, D., McDonough, S., and Crosbie, J. (2010). Applying a brain-computer interface to support motor imagery practice in people with stroke for upper limb recovery: a feasibility study. J. Neuroeng. Rehabil. 7, 60. doi:10.1186/1743-0003-7-60
Ramos-Murguialday, A., Broetz, D., Rea, M., Läer, L., Yilmaz, Ö, Brasil, F. L., et al. (2013). Brain-machine interface in chronic stroke rehabilitation: a controlled study. Ann. Neurol. 74, 100–108. doi:10.1002/ana.23879
Renard, Y., Lotte, F., Gibert, G., Congedo, M., Maby, E., Delannoy, V., et al. (2010). OpenViBE: an open-source software platform to design, test, and use brain–computer interfaces in real and virtual environments. Presence Teleoper. Virtual Environ. 19, 35–53. doi:10.1162/pres.19.1.35
Roy, R. N., Bonnet, S., Charbonnier, S., and Campagne, A. (2013). Mental fatigue and working memory load estimation: interaction and implications for EEG-based passive BCI. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 6607–6610. doi:10.1109/EMBC.2013.6611070
Schaechter, J. D. (2004). Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog. Neurobiol. 73, 61–72. doi:10.1016/j.pneurobio.2004.04.001
Scottish Stroke Association. (2016). State of the Nation: Stroke Statistics. London: Stroke Association.
Sefer, A. B., Krbot, M., Isgum, V., and Cifrek, M. (2009). “Movement related evoked potentials in Parkinson’s disease patients and healthy controls,” in IFMBE Proceedings, eds W. C. Schlegel and O. Dössel (Munich: Springer, Berlin, Heidelberg), 2158–2161. doi:10.1007/978-3-642-03882-2_573
Shepherd, R. F., Stokes, A. A., Nunes, R. M., and Whitesides, G. M. (2013). Soft machines that are resistant to puncture and that self seal. Adv. Mater. 25, 6709–6713. doi:10.1002/adma.201303175
Sivenius, J., Pyorala, K., Heinonen, O. P., Salonen, J. T., and Riekkinen, P. (1985). The significance of intensity of rehabilitation of stroke. A controlled trial. Stroke 16, 928–931. doi:10.1161/01.STR.16.6.928
Stamps, K., and Hamam, Y. (2010). “Towards inexpensive BCI control for wheelchair navigation in the enabled environment – a hardware survey,” in Proceedings International Conference on Brain Informatics, Toronto, ON, Canada, August 28–30, 2010, eds Y. Yao, R. Sun, T. Poggio, J. Liu, N. Zhong, and J. Huang (Berlin, Heidelberg: Springer Berlin Heidelberg), 336–345.
Stein, J., Bishop, J., Gillen, G., and Helbok, R. (2011). A pilot study of robotic-assisted exercise for hand weakness after stroke. IEEE Int. Conf. Rehabil. Robot. 2011, 1–4. doi:10.1109/ICORR.2011.5975426
Strong, K., and Mathers, C. (2011). “The global burden of stroke,” in Stroke, ed. H. J. M. Barnett 279–89. (Saunders, Saint Louis: Elsevier), 279–289. doi:10.1016/B978-1-4160-5478-8.10019-3
Taylor, C. L., and Schwarz, R. J. (1955). The anatomy and mechanics of the human hand. Artif. Limbs 2, 22–35.
Triandafilou, K. M., and Kamper, D. G. (2012). Investigation of hand muscle atrophy in stroke survivors. Clin. Biomech. (Bristol, Avon) 27, 268–272. doi:10.1016/j.clinbiomech.2011.10.002
van Vliet, M., Robben, A., Chumerin, N., Manyakov, N. V., Combaz, A., and Van Hulle, M. M. (2012). “Designing a brain-computer interface controlled video-game using consumer grade EEG hardware,” in Proceedings Biosignals and Biorobotics Conference (BRC) (IEEE), 1–6.
Walairacht, S., Yamada, K., Hasegawa, S., Koike, Y., and Sato, M. (2002). 4 + 4 fingers manipulating virtual objects in mixed-reality environment. Presence Teleoper. Virtual Environ. 11, 134–143. doi:10.1162/1054746021470586
Ward, N. S., and Cohen, L. G. (2004). Mechanisms underlying recovery of motor function after stroke. Arch. Neurol. 61, 1844–1848. doi:10.1001/archneur.61.12.1844
World Health Organisation. (2011). World Report on Disability. Geneva, Switzerland: World Health Organization. doi:10.1136/ip.2007.018143
Yuan, H., and He, B. (2014). Brain-computer interfaces using sensorimotor rhythms: current state and future perspectives. IEEE Trans. Biomed. Eng. 61, 1425–35. doi:10.1109/TBME.2014.2312397
Zhang, Z., Fang, Q., and Xudong, G. (2015). Objective assessment of upper limb mobility for post-stroke rehabilitation. IEEE Trans. Biomed. Eng. 63, 859–868. doi:10.1109/TBME.2015.2477095
Keywords: brain–machine interface, soft robotics, rehabilitation, stroke, exoskeleton
Citation: McConnell AC, Vallejo M, Moioli RC, Brasil FL, Secciani N, Nemitz MP, Riquart CP, Corne DW, Vargas PA and Stokes AA (2017) SOPHIA: Soft Orthotic Physiotherapy Hand Interactive Aid. Front. Mech. Eng. 3:3. doi: 10.3389/fmech.2017.00003
Received: 10 February 2017; Accepted: 04 May 2017;
Published: 02 June 2017
Edited by:
Thrishantha Nanayakkara, Imperial College London, United KingdomReviewed by:
Marcello Calisti, Sant’Anna School of Advanced Studies, ItalyXinyu Liu, McGill University, Canada
Copyright: © 2017 McConnell, Vallejo, Moioli, Brasil, Secciani, Nemitz, Riquart, Corne, Vargas and Stokes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam A. Stokes, YS5hLnN0b2tlc0BlZC5hYy51aw==
 Alistair C. McConnell
Alistair C. McConnell Marta Vallejo
Marta Vallejo Renan Cipriano Moioli
Renan Cipriano Moioli Fabricio L. Brasil3
Fabricio L. Brasil3 Nicola Secciani
Nicola Secciani Cecile P. Riquart
Cecile P. Riquart Patricia A. Vargas
Patricia A. Vargas Adam A. Stokes
Adam A. Stokes