- Department of Life Sciences, Mudanjiang Medical University, Mudanjiang, China
Bacteria are present in the environment around us, including in the air, water, and soil. Moreover, infection-causing bacteria are transmitted indirectly through the air, food, and water, as well as through direct contact. Upon entering the human body, they multiply and cause various discomforts or diseases. To combat such diseases, antibiotics are the current choice of the primary treatment. However, their overuse has led to a major issue referred to as bacterial resistance. Metal NPs possess great potential in microbial detection along with disease diagnosis and treatment. Zinc is an essential trace element crucial for human growth and development, and zinc oxide (ZnO) nanoparticles (NPs) are an inorganic material with broad-spectrum antibacterial activity. Therefore, in this review article, we provide a detailed overview of the antibacterial mechanisms of ZnONPs, thereby providing theoretical support for their application.
1 Introduction
Bacteria consist of a cell wall, cell membrane, and cytoplasm (Shi et al., 2014). They are divided as Gram-positive (G+) and Gram-negative (G−) based on the staining properties and peptidoglycan thickness of the cell wall; the cell wall of G+ strains contains a thick peptidoglycan layer of about 20–80 nm, whereas that of G− strains contains a thin peptidoglycan layer of about 7–8 nm (Singh et al., 2020). The bacterial cytoplasm comprises polysaccharides, proteins, salts, minerals, and water, and supercoiled bacterial DNA functions in cell growth, protein synthesis, and enzyme metabolism (Dorman, 2019). Bacterial infection is one of the key causes of chronic wounds, and wound healing is one of the most important biological processes (Gould et al., 2015; Morton and Phillips, 2016). Various organs and tissues are affected by bacterial infections, thereby resulting in multiple conditions, including inflammation (Lipsky et al., 2012) and sepsis (Corl et al., 2020). Additionally, clinically common chronic wounds such as diabetic, pressure, and vascular always follow these infections (Powers et al., 2016; Zhao et al., 2016). Bacterial infections were effectively reduced only after the discovery and popularization of antibiotics, which are also known as bacterial infection termination factors (Zhu et al., 2014). Antibiotic discovery, commercialization, and management in the mid-20th century completely transformed the medical field; thus, remarkably altering treatment modalities for infectious diseases and providing necessary prevention for the development of new surgical techniques, transplantation, and cancer treatment (Asenjo et al., 2021).
Antibiotics inhibit translation regulation in bacteria by disrupting the assembly of 50S and 30S ribosomal subunits; consequently, this method hinders the formation of specific proteins by inhibiting cellular metabolism (Khiralla and El-Deeb, 2015). However, clinically important antibiotic-resistant pathogens, especially G− bacteria, caused infections that were difficult to treat using conventional or even specialized antibiotics in the past decade, posing a great challenge to clinical anti-infective treatments (Qiu et al., 2021). Owing to increasing antibiotic resistance, treating infections, including wounds, has become challenging (Wangoye et al., 2022). A previous study showed that 90% of Staphylococcus aureus strains isolated from infected wounds in patients admitted to hospitals worldwide were resistant to penicillin (Filius and Gyssens, 2002). Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, the most common pathogens isolated from infected wounds, exhibit very high degrees of resistance to single or multiple drugs (Guan et al., 2021; Puca et al., 2021). Entering the 21st century, antibiotic resistance is one of the top three public health challenges per the World Health Organization (WHO). According to data from the U.S. Department of Health and Human Services in 2019, 2.8 million bacterial infections and 35,000 deaths occur annually in the U.S. because of antibiotic-resistant bacterial infections. By 2050, 10 million people globally are estimated to die annually because of antibiotic-resistant bacterial infections (Dixit et al., 2019). Multidrug-resistant bacteria, including methicillin- and vancomycin-resistant S. aureus, macrolide-resistant pyogenic Streptococci, penicillin-resistant Pneumococci, and multidrug resistant tuberculosis Bacilli, are contributing to increasing mortality and morbidity rates worldwide. In hospitals in the United States and the United Kingdom, more than 40% methicillin-resistant S. aureus strains have been found (Pelgrift and Friedman, 2013). Therefore, identifying new methods and means is imperative for anti-infective treatments.
Since 1978, biomaterials have been categorized into the following three groups based on their biological properties: natural, organic, and inorganic antibacterial materials (Osborn and Weiss, 1978; Bose et al., 2013). Zinc oxide (ZnO) nanoparticles (NPs), inorganic antibacterial materials, have garnered attention owing to their low toxicity and environmental friendliness (Basnet et al., 2018). Currently, ZnONPs are prepared by chemical, physical, and green synthesis, and among these, green synthesis will be regarded as an essential method in the future owing to its minimal environmental impact (Ibrahim, 2015). Thus, in the following sections, we have provided a comprehensive introduction to research on the antibacterial potential of ZnONPs from four perspectives for a complete understanding of ZnONPs.
2 Advantages of ZnONPs
2.1 Advantages compared with those of antibiotics
Bacterial antibiotic resistance mainly arises through intrinsic, acquired, and phenotypic mechanisms. Intrinsic resistance is natural resistance caused by specific microbes through their innate or intrinsic characteristics, and acquired resistance is caused by the mutations or manipulation of the integrity of genetic materials. Furthermore, phenotypic resistance refers to the ability to enhance bacterial tolerance to resistance by altering gene expression or protein levels stimulated by various ecological factors (Farhat et al., 2020). Additionally, bacteria often develop resistance through mechanisms such as drug enzyme inactivation, target protein mutations, bypassing target sites, and preventing drug entry into target sites (Nikaido, 2009). The biggest advantage of ZnONPs over antibiotics is that they offer a practical solution to bacterial resistance. ZnONPs with smaller particle sizes can physically interact with bacterial cell membranes, leading to cell lysis and death or affecting normal bacterial growth via the action of zinc ions. These multiple factors make ZnONPs more advantageous over traditional antibiotics (Ratan et al., 2021).
2.2 Advantages compared with other metals
Many natural and organic antibacterial materials are fast-acting and can be decomposed easily (Li et al., 2008). Metals in inorganic antibacterial materials exhibit ideal antibacterial properties. By using nanotechnology to develop materials with a size of 1–100 nm, various metal NPs inhibit the growth of diverse microbes, including viruses, bacteria, and fungi. The size and shape of the nanoparticles also have a great impact on the microorganisms, such as Bruna Lalo da Silva using sol-gel method to prepare ZnO NP, by controlling the reaction time, the preparation of different particle sizes of zinc oxide particles, that zinc oxide nanoparticles size has a great impact on the antibacterial activity, antibacterial activity increases with the decrease of particle size (Lallo da Silva et al., 2019). Babayevska et al. (2022). Synthesized zinc oxide nanoparticles of different sizes and shapes, and concluded that zinc oxide nanoparticles had the strongest antibacterial activity against E. coli and S. aureus due to their high specific surface area. Meanwhile, at the same concentration, zinc oxide nanoparticles had greater harm to cancer cell lines than normal cell lines. S. C. Esparza González et al., 2021. By synthesizing different morphologies of ZnO nanoparticles (NPS), the number of E. coli, S. aureus and HeLa cells decreased linearly by increasing the concentration of nanoparticles. Moreover, spherical ZnO NPS has better capability than hexagonal and rod-shaped ZnO NPS. Rajat K Saha et al., 2020 synthesized floral ZnO and hexagonal massive ZnO by wet chemical method and compared their antimicrobial properties. It was concluded that the geometry of the surface structure is related to the biological characteristics, and FZnO microstructure has a smaller milk content, which is obviously better than its massive structure, and can inhibit gram-negative E. coli microorganisms. This property is related to the size of these particles because smaller NPs can more easily penetrate cells than larger NPs (Chandra et al., 2019; Kamyab et al., 2023). At the nanoscale, these materials acquire unique electrical properties or increased fluidity and reactivity because of quantum effects. These properties make NPs indispensable for manufacturing many products (Higashisaka et al., 2017). Among metal NPs, the most common metal oxide NPs that effectively inhibit fungal and bacterial growth and viral replication are copper oxide, titanium dioxide, ZnO, and nickel oxide. These oxides are used in food packaging and healthcare industries (Garcia et al., 2018).
Silver NPs exert a broad-spectrum inhibitory effect on bacteria, fungi, and viruses. Therefore, researchers are focusing on the antibacterial effects of various metal NPs (Kamyab et al., 2023). However, the release of potentially toxic corrosive byproducts markedly limits the application of some metal materials (Su et al., 2019). For example, the internal environment is a dynamic steady state, and buffering systems may quickly neutralize hydroxide ions produced by Mg degradation (Zeng et al., 2013). Titanium materials exhibit good biocompatibility with host cells and bacteria and increase the likelihood of bacterial infections (Wu et al., 2021). Additionally, toxicity due to the accumulation of silver- or gold-based materials in the human body remains a significant challenge (Liao et al., 2019).
Zinc does not exist in a free state in nature but may be present in other forms such as zinc carbonate, ZnONPs, zinc sulfide, and zinc chloride (ZnCl2). Zinc is primarily obtained through mining and metal smelting. Electrolysis can even place zinc in a sterile environment (Khanam, 2020). Zinc, as a mineral, is essential for bone development and normal growth. Furthermore, it helps recover damaged tissues (Mohamed et al., 2019a). Reduced plasma zinc levels may impair physiological processes and cause various liver diseases, including cirrhosis and hepatitis (Cesur et al., 2005). Nevertheless, recently, ZnONPs have garnered widespread attention in biological research owing to their low toxicity, biocompatibility, and chemical stability (Teow et al., 2018). In addition to meeting needs, these NPs are environmentally friendly. Their multifunctionality allows for their wide application across various industries and disciplines (Basnet et al., 2018).
3 Synthesis approaches for zinc oxide NPs
The most common metal and metal oxide NPs, i.e., zinc, copper, gold, and silver NPs, are currently of interest as broad-spectrum antifungals [40] owing to their antibacterial characteristics. Among various methods for synthesizing ZnONPs introduced in this section, bio-green synthesis is a new one.
3.1 Chemical synthesis methods
ZnONPs can be synthesized using various techniques, and most of them are bottom-up chemical synthesis methods, including chemical reduction, hydrothermal, sol-gel, mechanochemistry, sonochemistry, microfluidics, and microwave-assisted methods (Arya et al., 2021). Chemical reduction is a widely applied chemical synthesis method for preparing ZnONPs (De Matteis et al., 2018). Monami et al. [43] synthesized ZnONPs (46–66 nm) by reducing zinc nitrate hexahydrate using hydrazine and stabilizing it with ethylene glycol. Chemical synthesis methods that can easily control particle size and morphology are widely studied and used for synthesizing ZnONPs. However, some chemical synthesis methods use high temperatures and pressure and toxic reducing agents and stabilizers, which harm humans and the environment. The use of toxic stabilizers results in the adsorption of toxic chemicals on NP surfaces, limiting their use in medical applications (Sabir et al., 2014). Consequently, current studies have focused on developing innovative synthesis methods to overcome the drawbacks of physical and chemical synthesis methods. The sol-gel method is simple and requires a low temperature, which involves the condensation of hydrolyzed organic metal precursors. Hydrolysis is performed in the presence of an alkali or acid in water or alcohol (Arya et al., 2021). Al Abdullah et al. (2017) synthesized NPs with a size of 12–30 nm following the sol-gel method. Sonochemical methods are safe and rapid and use ultrasonic radiations to create cavities in a liquid medium. Nanoparticle production using nanochemistry is divided into the following three steps: cavity formation, growth, and collapse. A liquid is exposed to ultrasonic radiation to increase the energy significantly to around 5,000 K in temperature and 20 MPa in pressure in order to achieve cavity collapse, chemical excitation, and NP formation (Sun et al., 2018). Noman et al. (2019) used a one-pot ultrasonic homogenizer at 20 kHz and 200 W, with ZnCl2 and sodium hydroxide (NaOH) to synthesize quasi-spherical ZnONPs with an average size of 28 nm.
Compared with traditional chemical synthesis, microwave-assisted synthesis (Garino et al., 2019a; Garino et al., 2019b; He et al., 2020; Wojnarowicz et al., 2020) has received increasing attention owing to its shorter reaction time and better control over size and morphology. Nevertheless, wet chemical precipitation is the most commonly used method for preparing ZnONPs because it is generally cheaper and simpler as well as produces a higher yield. In this method, zinc precursors (usually zinc nitrate (Garino et al., 2019a), zinc sulfate (Wojnarowicz et al., 2020), ZnCl2 (Chittofrati and Matijević, 1990), or zinc acetate (Bharathi et al., 2019)) are reacted with an alkaline solution (such as potassium hydroxide [KOH] or NaOH) under mild conditions such as standard atmospheric pressure and a constant temperature of 80°C (Phan et al., 2020). The resulting precipitate is then collected, washed, and finally dried or calcined (Kadhum et al., 2015).
3.2 Physical synthesis methods
Generally, physical synthesis methods are divided into top-down and bottom-up approaches. The top-down approach involves breaking down larger particles into smaller ones until they reach nanoscale dimensions, whereas the bottom-up approach involves yielding NPs through nucleation from ions, molecules, and atoms. Laser ablation, ball milling, photolithography, mechanical grinding, and ion beam techniques are some of the physical synthesis methods used for ZnONP preparation (Kakiuchi et al., 2006). Laser ablation requires focusing a laser beam on a high-purity zinc plate submerged in a liquid in a vacuum to form a plasma that reacts with the liquid to form NPs (Wang et al., 2010). Vilenchik et al. (Saleh, 2020) used the zinc metal as a precursor and zirconia balls for milling to synthesize spherical ZnONPs with a crystallite size of 68.4 nm. The precursor is milled for 18 h in the presence of an ethanol lubricant, followed by calcination in oxygen at 800°C to form ZnONPs. Saichao Dang et al. (El-Gendy et al., 2022) used photolithography to develop ZnO/Ag and ZnO thin films with two-dimensional periodic apertures. Most physical synthesis methods do not involve hazardous solvents; however, they have other disadvantages such as the requirement of high energy and extended time.
3.3 Environmentally friendly synthesis methods
The abovementioned chemical and physical synthesis methods cause serious environmental pollution. Moreover, a study has shown that NP synthesis using chemical methods is unsafe and has poor biocompatibility (Raouf Hosseini and Nasiri Sarvi, 2015). Further, the clinical and biological applications of such NPs are limited, and they require additional physicochemical analysis to determine characteristics such as their size, shape, surface charge, functional groups, and purity (Król et al., 2017). Thus, investigating specific, cleaner, ecologically safe and viable, and biocompatible methods for NP synthesis is important.
Recently, green compounds have been increasingly used to synthesize nanomaterials and ZnONPs (Gunalan et al., 2012). Compounds isolated from various parts of plants, such as leaves, roots, stems, fruits, and flowers, are used in plant extract biosynthesis. Some plant extracts contain complex phytochemicals, such as phenols, alcohols, terpenes, saponins, and proteins, which act as reducing and stabilizing agents throughout production (Gunalan et al., 2012). For example, plant extracts (Dhandapani et al., 2020) may replace NaOH and KOH as reducing and capping agents. Moreover, they can be functionalized with phytochemicals exhibiting anti-inflammatory, antioxidant, or antibacterial properties, thereby mitigating potential toxicity issues. Serouti et al. (2024) present the successful synthesis of a biogenic ZnO/CuO/Fe2O3 nanocomposite using an aqueous leaf extract of Ocimum Basilicum L. studied the photocatalytic activity of MG-modified ZnO nanoparticles synthesized from biomass (Purslane plant extract) to eliminate two different organic dyes, namely, m-toluidine and p-toluidine. Barani et al. (2023) Photocatalytic activity results showed that the removal rate of organic dyes was significant, and both m-toluidine and p-toluidine could be removed 100% within 60 min. Kir et al. (2023) using the green synthesis method in which lemon peel extract was used as a reducing agent to form ZnO/BaMg2 nanocomposites. The decolorization rates of methyl orange (MO) and Bengal rose (RB) dyes by ZnO/BaMg2 nanocomposites were 90.2% and 98.71%, respectively. Hamza et al. (2024) Used waste lemon peel extract as a bioreducing agent to synthesize zinc oxide (ZnO) -based nano-photocatalysts, including ZnO nanoparticles (NPs) and ZnO/Ag nano-composite heterostructures (NCH). ZnO NPs effectively removed 84% of toluidine blue and 77% of Congo red after 120 min, and ZnO/Ag NCH increased the degradation rate of toluidine blue to about 90.5%, and the degradation rate of Congo red to 86%. Gharbi et al. (2023) has successfully prepared a new core-shell nanomaterials ZnO nanoparticles (NPs) and a new core-shell nanomaterials using Calligonum comosum L. Putamen of leaf extract ZnO@SiO2. The degradation efficiency of ZnO@SiO2 for Methylene Blue (MB) and Meng Rose Bengal (RB) is 99.3%, which is higher than that of ZnO NPs (86.6%). The degradation coefficient of Rose Bengal (RB) was 91.7%. Meneceur et al. (2023) used green synthesis method to synthesize nano composite material (NC) from mint leaf extract. The photocatalytic activities of ZnO@Fe3O4 NC on amoxicillin, cefalexin and metronidazole under sunlight were studied. The degradation efficiencies were 71%, 69% and 99%, respectively, indicating that ZnO@Fe3O4 NC has the potential to remove antibiotics from waterways. Tabet et al. (2024) synthesized common ruta plant extracts by green method. By introducing a new ZnO/CuO/Cu2MgO3 nanocomposite (NC), a new method was proposed to solve the pollution of synthetic dyes in wastewater. Photocatalytic degradation experiments demonstrate an impressive 99.66% Bromocresol Green (BCG) removal efficiency through adsorption within 75 min of solar irradiation with a rate constant of 0.091 min−1. Azzi et al. (2024) successfully synthesized ZnO nanoparticles (NPs) using extracts from aquatic leaves of olive. The ZnO NPs demonstrated remarkable efficiency in degrading Cephalexin, achieving a degradation rate of 95% for ZnO (100°C) and 98% for ZnO (450°C). By integrating silver (Ag) and zinc oxide (ZnO) nanoparticles into a potassium polyacrylate hydrogel (PPAH) matrix, Boutalbi et al., 2023 prepared a stable and efficient photocatalytic nanocomposite using a two-step method. The experimental results demonstrate that the PPAH/Ag@ZnO nanocomposite efectively degrades 98.77% of o-toluidine blue (o-TB) and 98.05% of 4-bromophenol (4-Bph). Aouadi et al. (2023) produced chitin-based nanocomposites by reusing shrimp shell waste. Antimicrobial tests revealed synergistic antibacterial effects against gram-positive bacteria, including Pseudomonas aeruginosa and S. aureus. Guerram et al. (2024) synthesized ZnO/SnO2 NC by sol-gel technique. The photocatalytic efficiency was assessed by the degradation of Toluidine Blue (TB) and m-Toluidine Blue (m-TB) dyes. The ZnO/SnO2 NC demonstrated degradation rates of 99% for both TB and m-TB within 120 and 140 min of sunlight exposure, respectively. Hou et al. (2020) found that ZnONPs prepared using carnation leaf extracts exhibited bactericidal properties against multidrug resistant Acinetobacter baumannii. The results indicated that these ZnONPs with a half maximal effective concentration of 0.028 μg/mL killed 90% of multidrug resistant A. baumannii. Another study showed that similarly produced ZnONPs damaged bacterial DNA, causing cell death and inhibiting the biofilm produced by A. baumannii (Steffy et al., 2018). Similarly, Steffy et al. found that ZnONPs synthesized from Strychnos nux-vomica (S. nux-vomica–ZnONPs) inhibited multidrug resistant bacteria and effectively healed wounds. The size range of the S. nux-vomica–ZnONPs was 10–12 nm, and they exhibited bactericidal activity against methicillin-resistant S. aureus (MRSA), E. coli, Pseudomonas aeruginosa, and A. baumannii at concentrations of 100–200 μg/mL. Moreover, the S. nux-vomica–ZnONPs exhibited more than 90% bactericidal activity against multidrug resistant bacteria within 6 h. As mentioned above, S. nux-vomica–ZnONPs effectively healed wounds. Compared with the standard rate (4.49 ± 1.03), the wound closure rates using these nanoparticles were 28.81 ± 1.42 and 22.33 ± 1.58 at 200 μg/mL and 100 μg/mL, respectively. ZnONPs interact with bacterial cells by generating reactive oxygen species (ROS); thus, leading to bacterial cell death via cell and nuclear membrane destruction. Ehsan and Sajjad found that ZnONPs biosynthesized from fig leaf extracts and antibiotics synergistically combatted multidrug resistant bacterial infections. These biosynthesized ZnONPs enhanced the antibacterial activity of antibiotics such as gentamicin, erythromycin, fosfomycin, amikacin, ciprofloxacin, fusidic acid, benzylpenicillin, rifampicin, and tigecycline against the resistant strains of S. aureus, Bacilli, Acinetobacter, Pseudomonas, Pseudomonas aeruginosa, and E. coli (Ehsan and Sajjad, 2017). Therefore, ZnONPS showed strong advantages in bacteriostasis (Table 1), which is worthy of further study.
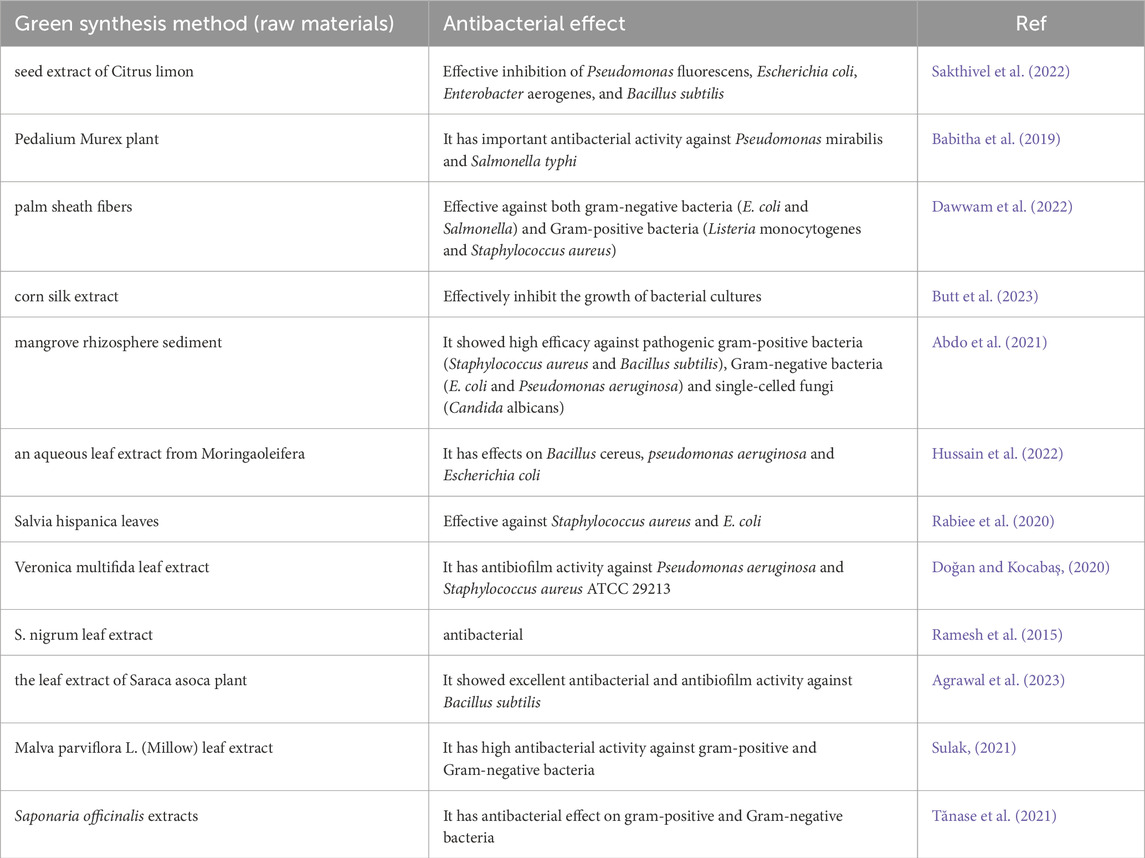
Table 1. Environmentally friendly synthesis method of zinc oxide nanoparticles and their antibacterial effect.
Many single-celled and multicellular organisms, including bacteria, yeast, fungi, viruses, and algae, can be used to biosynthesize metal and metal oxide NPs [67, 68]. Because microorganisms are easy to propagate, they are a better choice for biosynthesis than plants (Thakkar et al., 2010). Moreover, the process is non-toxic, and environmentally friendly. Microorganisms act like small nanofactories, using biomolecules they secrete or produce, including enzymes to reduce metal ions into metal NPs. However, the main drawback is that this synthesis is not cost-effective because it is time-consuming and requires chemicals as a growth medium. Biomolecules derived from microorganisms, including enzymes and proteins are crucial for NP degradation. These proteins can act as capping agents, providing stability for NP production. Nonetheless, not all microorganisms can produce NPs because each microorganism exhibits unique metabolic pathways and enzyme activities. Thus selecting an appropriate microorganism is crucial for NP formation (Mohd Yusof et al., 2019). Additionally, the biological production of metal and metal oxide NPs requires metal precursors, which are usually provided as soluble salts and precipitated in solutions containing microbial cells or extracts (Parveen et al., 2016). Nevertheless, only a few microbes can synthesize ZnONPs. Therefore, more potent microorganisms should be identified to synthesize ZnONPs. In addition to the limitations, there are some drawbacks in isolating and screening potential microorganisms. However, owing to the key environmental benefits, the green synthesis of ZnONPs will become the mainstream method, providing medical benefits for future generations.
4 Antibacterial applications of ZnONPs
Among various NPs, ZnO is extensively used in diverse materials and products, including cement, ceramics, cosmetics, food, glass, rubber, coatings, pigments, plastics, and sealants (Oberdörster et al., 2005). Additionally, it is widely used in the medical field in areas such as artificial bones and cancer treatment. ZnONPs exert antibacterial and anti-inflammatory effects, thereby promoting wound healing (Mousa et al., 2018). Aydin Sevinç et al. (2010) reported that ZnONPs exhibited a bactericidal concentration ranging from 80 µg∙mL−1 to 1.2 mg∙mL−1 against S. aureus within the size range of 8–125 nm. Reddy et al. (2007) reported that for ZnONPs with a particle size of 13 nm, the complete inhibitory concentrations against E. coli and S. aureus were 280 µg∙mL−1 and 80 µg∙mL−1, respectively.
4.1 Artificial bones
Every year, many patients require various types of biomaterials, such as dental fillings and materials needed for hip joint replacements. Moreover, after blood transfusion surgeries, bone graft operations have become the second most common clinical procedure performed [76, 77]. Bone repair or regeneration is a common yet complex clinical event in orthopedic surgeries (Murugan and Ramakrishna, 2004). Moreover, infections and mixed infections are common in orthopedic surgical incisions owing to factors such as proximity to the body surface, long operation time, excessive bleeding during surgery, and the need for internal fixations (Xie et al., 2020).
The hygiene of the operating room is one of the determining factors in avoiding infections of implanted materials. Bacterial load on surgical instruments and implanted materials plays a crucial role (Jones et al., 2017). However, using antimicrobial surface coatings can prevent bacterial adhesion to implants, thus potentially preventing various infections and avoiding biofilm formation on the implant surface. ZnONPs can be coated on implants for antimicrobial action and preservative protection. For instance, AZ31B magnesium alloy implants coated with PEO (the plasma electrolyte oxidation)/ZnONPs actively inhibited S. aureus and E. coli growth at a pH of 13.2 (Seyfi et al., 2021). Additionally, antibacterial properties can be improved by irradiating with ultraviolet (UV) light to generate more electron-hole pairs and free radicals. For example, Jones LC improved the bioactive properties of titanium orthopedic implants against E. coli by binding ZnO with chitosan (Lin et al., 2021). Huang et al. (2017) developed a zinc-doped chitosan/gelatin nanocomposite to reduce surgical site infections. A titanium substrate coated with the nanocomposite exerted antimicrobial effects against E. coli and S. aureus. Laurenti and Cauda (2017) prepared ZnONPs and ZnONP films using hydrothermal methods, and both preparations promoted the growth and bone integration of MC3T3-E1 osteoblast cells. In summary, nanocomposites, especially polymer nanocomposites, coated with ZnONPs have garnered noticeable interest for their applications in biodegradable, dental, and orthopedic implants as well as other biomedical uses.
Orthopedic biomaterials used for implantation should be designed with stringent specifications to ensure surgical success. The improper cleaning and sterilization of the implanted material could result in a risk of infection, ultimately leading to implant failure or even chronic diseases (Jones et al., 2017). The trace element zinc is used in composite materials for bone tissue engineering applications because it enhances bone density and minimizes bone loss (Bhowmick et al., 2018). Moreover, owing to its low toxicity and high biocompatibility, it adds value to the applications of biomaterials. ZnONPs play an important role in bone regeneration, compensating for zinc deficiencies in the bone (0.012–0.025 wt%), and are crucial for bone reconstruction and hypozincemia prevention (Shrestha et al., 2017). Bhowmick A modified montmorillonite clay using chitosan/ZnONPs to mimic the natural bone matrix. The composite material exerted antimicrobial effects against G− and G+ bacterial strains, including E. coli, Clostridium perfringens, and Bacillus cereus. The cytotoxic behavior was determined by performing in vitro proliferation experiments using MG-63 cells. The results indicated that non-toxic substances could be safely used for bone tissue engineering applications (Bhowmick et al., 2018). Heidari F prepared a novel palladium/ZnONPs/hydroxyapatite composite and coated it with varying amounts of chitosan (0.125 and 0.25 g) to achieve higher compressive strength and toughness. The antibacterial activity of the composite was tested against G− bacteria (Pseudomonas aeruginosa), and excellent growth inhibition activity and ability were observed at a concentration of 25 μg/mL (Huang et al., 2017). Atsuo Ito et al., 2000 prepared ZnO/β-TCP/HA composite ceramics at high temperatures and found that MC3T3-E1 osteoblast proliferation was significant when the zinc content was 0.6–1.2 wt%. ZnONPs (7–8 nm) were toxic to human periodontal ligament fibroblasts and mouse dermal fibroblasts when the concentration exceeded 0.05–0.1 mg mL–1 (Şeker et al., 2014). Additionally, ZnONPs were safe at a concentration below 50 mg mL–1; the normal serum zinc concentration in humans is 0.88 ± 0.6 µg∙mL–1 (Kao et al., 2012).
4.2 Cancer
Cancer is characterized by uncontrolled cell proliferation (Kim, 2015). Cancer cells usually exist within the body and function similarly to normal cells. Uncontrolled cell division results in unregulated cell proliferation, causing cancer. Cancer cells can create their own blood supply, detach from the original organ, migrate to other organs, and spread during their development (Gruendner et al., 2020). In the past few years, the number of newly diagnosed patients with cancer has remarkably increased, and the disease has affected their physical and mental health, which has led many patients to experience high levels of anxiety, suffering, and depression (Bray et al., 2018). A WHO report estimates that 10 million people worldwidewill die from cancer in 2020; thus, it is a major barrier to increasing life expectancy. In the next 20 years, the number of yearly cancer-related diagnoses is expected to exceed 30 million, with 16.4 million cancer-related deaths (Bray et al., 2021). The use of anti-cancer drugs, lasers, radiation, and hormone therapies often leads to some abnormalities. Furthermore, their toxic effects may damage normal cells and vital organs, deteriorating health and reducing the quality of life (Nabavi et al., 2015).
Metallic NPs hold great promise in cancer detection, diagnosis, and treatment (Ikram et al., 2021). ZnONPs exhibit remarkable anti-cancer activity. Their cellular viability and inhibitory effects increase with their concentration (Jiang et al., 2018). ZnONPs possess electrostatic properties that allow them to intentionally and specifically target cancer cells, excluding normal cells (Zhang et al., 2015; Das et al., 2017). Abundant negatively charged phospholipids on the surface of cancer cells electrostatically interact with ZnONPs, thereby increasing the uptake of NPs by cancer cells and inducing cytotoxicity (Sana et al., 2020). Rajeshkumar et al. (2018) successfully synthesized hexagonal ZnONPs from mango leaf extracts, with an average size of 50 nm, and the NPs exhibited dose-dependent cytotoxicity against the A549 lung cancer cell line. ZnONPs synthesized in the same manner (Zhao et al., 2018; R. et al., 2019) from cashew leaf extracts successfully inhibited the growth of two cancer cell lines, Panc-1 and AsPC-1, similar to the inhibitory effects of Hydrilla verticillata on cervical cancer cells. Therefore, ZnONPs are more likely to destroy cancer cells in the pancreas and cervical lining (siHa cells). The semiconductor properties of biosynthesized ZnONPs generate ROS on the particle surface. Furthermore, direct interactions between such NPs and the cancer cell membrane generate oxidative stress in the cancer cells, ultimately resulting in cell death (Sana et al., 2020). ZnONPs may lead to cell degradation, membrane integrity loss, and DNA damage in cancer cells (Shaw et al., 2020). Nevertheless, the anti-cancer mechanisms of metal NPs are quite complex and under investigation.
4.3 Other applications
Owing to their antimicrobial properties, ZnONPs are used in the food preservation industry (Alavi and Nokhodchi, 2020). Orange juice containers were developed by Emamifar et al. using a nanocomposite material made from ZnONPs and low-density polyethylene (Emamifar et al., 2011). During a storage period of up to 112 days using this nanocomposite, the Lactobacillus plantarum growth rate decreased significantly. Further, star-shaped ZnONPs were synthesized using a simple molten salt method to develop synthetic nanocomposites loaded with either 2 or 4 wt% ZnONPs. When tested against E. coli and Bacillus subtilis, the nanocomposites containing 4 wt% ZnONPs exhibited the highest antimicrobial activity (Esmailzadeh et al., 2016).
ZnONPs have long been used in photoactive ceramic materials owing to their excellent antimicrobial properties (Shi et al., 2014; Wang et al., 2017b; Javidi et al., 2022; Khan et al., 2022). ZnONPs are abundantly present on earth and exhibit good light absorption in the near-UV region, transparent conductivity, non-toxicity, and biocompatibility with human organs; thus, they are particularly attractive for applications in photodetectors, photocatalysts, cosmetics, pigments, and biomedical uses (Look, 2001; Iqbal et al., 2018; Siddiqi et al., 2018; Borysiewicz, 2019; Diguna et al., 2020; Dulta et al., 2022). The antimicrobial coating properties of ZnONPs can be further optimized by performing advanced structural modifications such as doping and nanocomposites (Dulta et al., 2022; Liang et al., 2022; Yavaş et al., 2022).
ZnONPs are used as gas sensors because of their high electron mobility, good chemical stability, excellent electrical properties, and thermal stability (Zhang et al., 2018; Franco et al., 2022). ZnONPs with a basic structure exhibit good biodegradability in bulk and NP forms (Kielbik et al., 2017). Moreover, zinc NPs contain many functional groups on their surface and can be used in disease diagnostics, drug delivery, bio-imaging, and disease treatment (Xiong, 2013). Additionally, advancements in the surface modification of ZnONPs for drug delivery are safe, cost-effective, stable, and eco-friendly (Zhang et al., 2012).
Some potential applications of ZnONPs include therapeutic carriers, biosensors, gene transfer, nanomedical discoveries, biomarkers, medical implant coatings, electronic sensors, wastewater treatment, and communication (Wiesmann et al., 2020; Qin et al., 2021; Shaba et al., 2021). Recent research on ZnO–Au nanocomposites has led to electrochemical DNA biosensor development [126]. Further, ZnONPs have been used in plant tracing studies (Nath et al., 2018) and as materials for electrochemical sensors to detect the food additive aspartame (Xu et al., 2019). ZnONPs affect horizontal gene transfer, thereby affecting Bacillus subtilis transformation efficiency (Eymard-Vernain et al., 2018). Additionally, ZnO–Ag NPs decrease biofilm formation and gene expression rates in S. aureus at sub-minimal inhibitory concentrations (Shakerimoghaddam et al., 2020). Moreover, ZnONPs reduce parameters that lead to liver fibrosis (hydroxyproline) and renal toxicity (creatinine, urea, and uric acid) (Bashandy et al., 2018). Additionally, they can alleviate gonadal toxicity induced by cyclophosphamide, an anti-cancer and immunosuppressive drug, through their antioxidant and anti-apoptotic functions (Anan et al., 2018). They can attenuate cancer cell death through autophagy induction, supported by zinc ion release and ROS generation (Hu et al., 2019).
Stainless steel is a fundamental material for door handles, utensils, surgical tables, furniture, pipes, faucets, and containers in medical facilities, which often serve as media for bacterial and viral growth and transmission. Moreover, disinfecting their surfaces with alcohol or alkaline solutions does not provide a lasting reduction effect with respect to bacterial growth or kill all viruses.
Owing to the strong luminescence of ZnO in the visible spectrum, it can be used as a phosphor, and because of its nonlinear optical response, it can be used in frequency converters. Moreover, its high thermal conductivity results in its use as an additive in rubber (Chavali and Nikolova, 2019). The transparent properties and good UV radiation absorption capabilities of ZnO make it suitable for use as a UV-protective agent in cosmetics and textiles (Sasani Ghamsari et al., 2016).
Thin-film antimicrobial ZnONP coatings can reliably decrease bacterial growth; however, their toxicity needs careful evaluation. Xim et al. studied the toxicity of ZnO nanowall thin films on the skin by performing elution tests (Shim et al., 2017). Short-term contact with ZnO was relatively safe for the skin; thus, it was considered non-irritating based on the primary irritation index. However, contact with ZnO for 24 and 72 h caused skin irritation, manifesting as inflammation, erythema, and rashes. Moreover, in a key study, both zinc ions and ZnONPs exerted cytotoxic effects on the gastrointestinal tract of earthworms, affecting the intestinal epithelium and chloragogenous tissue (Świątek et al., 2020).
ZnONPs can combat diseases caused by multidrug-resistant bacteria in various animals and birds. Usama T et al. found that ZnONPs combatted multidrug-resistant S. aureus (MRSA), a bacteria that causes pododermatitis in broiler chickens. ZnONP addition to their dietary supplements improved the standing, feeding, and resting activities of the broiler chickens compared with the activities of the infected broiler chickens consuming a diet without ZnONPs. The dose-dependent administration of ZnONPs improved their inhibitory effects on microbial growth (Mahmoud et al., 2021). Mohd Yusuf et al. studied the effectiveness of biosynthesized ZnONPs against multidrug-resistant bacteria such as Salmonella, E. coli, and S. aureus, which cause foodborne diseases in poultry. The ZnONPs inhibited the formation of a biofilm of these drug-resistant bacteria by generating ROS and leading to cell death. A recent study showed that ZnONPs inhibited 60%–80% of multidrug-resistant bacterial biofilms within 24 h and 60%–70% within 48 h (Mohd Yusof et al., 2021).
5 Antimicrobial mechanism of ZnONPs
Since inorganic materials have been used as antimicrobial agents, their material properties and antibacterial mechanisms are being investigated. Silver, gold, ZnO, selenium, copper, cobalt/cobalt oxide, iron/iron oxide, and aluminum oxide NPs have been extensively studied for activities against bacterial infections (Guerrero Correa et al., 2020). These metal ions primarily inhibit bacterial growth via mechanisms such as disrupting bacterial cell membrane potential and integrity, inhibiting biofilms,
Generating ROS, enhancing host immune responses, and inhibiting RNA and protein synthesis by inducing intracellular processes (Wang et al., 2017a; Lee et al., 2019).
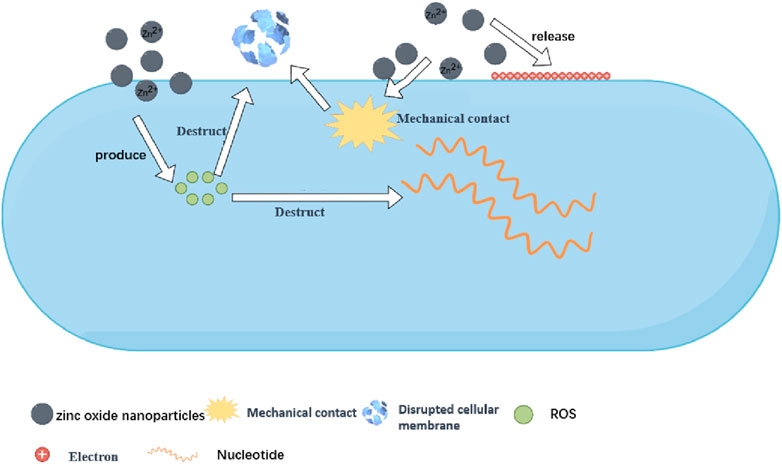
ZnONPs exert their antibacterial effects via three main mechanisms (Figure 1). Release of Zn2+ ions: These ions bind to the thiol groups of bacterial respiratory enzymes and impair their activity (Mishra et al., 2017). They inhibit the active transmembrane transport and amino acid metabolism in bacteria (Sirelkhatim et al., 2015).
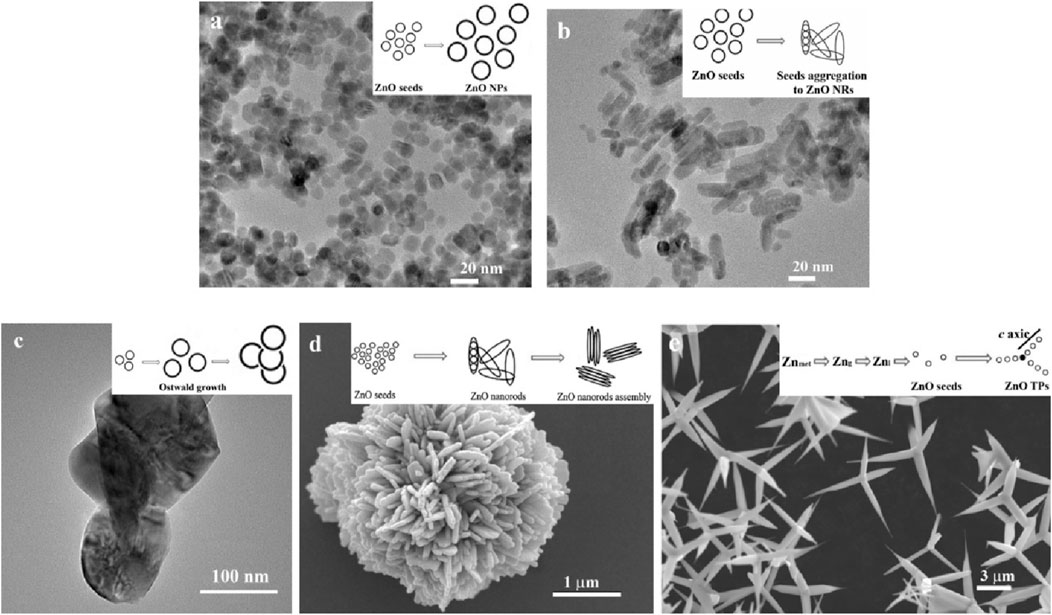
Figure 1. TEM and SEM images of ZnO nano- and microparticles: nanoparticles (A), nanorods (B), particles (C), hierarchical structures (D), tetrapods (E). Copyright © (Babayevska et al., 2022).
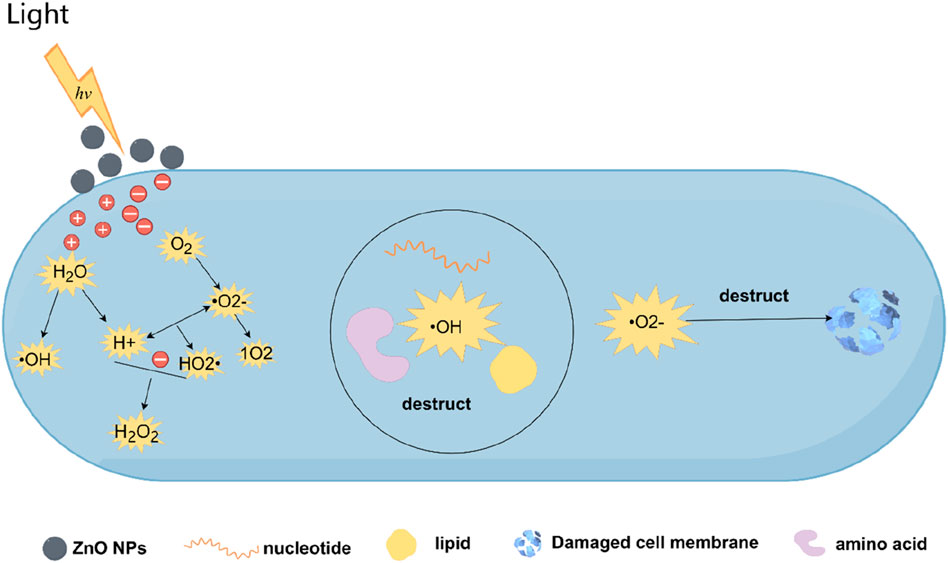
Generation of ROS: ZnONPs act as a direct bandgap semiconductor with a gap of 3.3 eV. Therefore, when exposed to UV radiation, electron-hole pairs are generated on their conduction and valence bands. In the presence of water and oxygen, these carriers subsequently initiate redox reactions on the surface of the material, leading to ROS generation, such as hydroxyl radicals (OH·), hydrogen peroxide (H2O2), and superoxide anions (O2−). The high reactivity of these species causes fatal oxidative damage and DNA, protein, and lipid destruction, ultimately leading to cell death (Sirelkhatim et al., 2015; Jiang et al., 2018).
Direct contact between the cell membrane and particles: ZnONPs accumulate near bacterial cells, and electrostatic interactions facilitate contact between the two. Bacterial cells carry a negative charge, whereas ZnONPs usually carry a positive charge in aqueous suspensions, which leads to membrane depolarization and deformation and wear and tear caused by the sharp edges of ZnONPs, ultimately resulting in cell death (Sirelkhatim et al., 2015).
5.1 Release of Zn2+ ions
Ions often lead to hazardous situations. When metal ions in a culture medium interact with metal ions present in bacterial cells, they become nonspecifically concentrated and uniformly dispersed in bacterial cell surroundings. Conversely, NPs
That bind to the bacterial cell wall form concentrated ions that are released continuously to increase their cytotoxicity (McQuillan et al., 2012). The resulting high ion concentration aids in cell penetration. However, NP dissolution is limited to the cell membrane vicinity, and the dissolution rate is determined by the shape and size of the NPs. The surface morphology of NPs markedly affects their function; for example, the rougher the surface of the NPs, the faster the dissolution (Kim et al., 2007). Furthermore, the smaller the NPs, the higher the surface area-to-volume ratio and the faster the decomposition. Zn2+ ions adsorb on the bacterial surface, disrupt the cell membrane, and penetrate cellular components. Inside the cell, Zn2+ ions interact with organic functional groups, leading to metabolic imbalances and ultimately destroying the bacterium (Liu et al., 2019).
ZnO antimicrobial activity and toxicity are closely associated with Zn2+ ion dissolution. Thus, Zn2+ ions play a crucial role in ion signaling between different cells and intracellular organelles as well as catalyst and protein arrangement (Wahab et al., 2014). Zn2+ ions play a key role in inhibiting active transport, amino acid metabolism, and enzymatic degradation (Sirelkhatim et al., 2015). Huang et al. found an interesting phenomenon during their study on the bactericidal activity of ZnONPs against Streptococcus lactis and S. aureus, where dissolved ions exerted a strong destructive effect on the bacterial cells and cell membranes, thereby increasing the possibility of ZnONPs penetrating the cells (Huang et al., 2008). The homeostasis of metal ions is maintained via highly regulated processes of uptake, storage, and secretion and is crucial for bacteria because they release several factors such as dehydrogenases, cofactors, and catalysts to participate in various metabolic functions, as well as balancing agents useful for chemicals and DNA-binding proteins (Fenno et al., 2011). Additionally, some bacteria exhibit influx and efflux mechanisms across the membrane for metal ions such as Zn2+ to maintain a stable intracellular ion concentration (Jones et al., 2008). However, ZnONPs with a positive zeta potential may destroy the cell membranes of microbes such as G− E. coli. The reaction of ZnO powder with bacteria may result in a slow release of Zn2+ ions. In terms of the oxidative capacity of ZnO, these Zn2+ ions react with organic functional groups (thioglucosides, carboxyl groups, and hydroxyl groups) and fuse with bacterial cells and membrane proteins. They enter bacterial cells and damage their electron mobility framework, thereby inhibiting the targeted gene expression of enzymes and proteins within the bacteria (Yu et al., 2013). Therefore, irreversible damage occurs to all internal bacterial organelles via the active absorption of Zn2+ ions.
Nevertheless, the extent to which Zn2+ contributes to the antibacterial properties of ZnONPs is debatable. Bellanger et al. presented a comprehensive and systematic study and concluded no quantitative relationship between the Zn2+ ions released and ZnONP antibacterial activity against E. coli MG1655 and metal-resistant Cupriavidus metallidurans CH34 (Bellanger et al., 2015). However, other study conclusions are inconsistent with this mechanism, where they do not necessarily negate the toxic effects of dissolved Zn2+ ions. This can be attributed to the nature of the bacteria themselves when interacting with ZnONPs or Zn2+. Some bacteria are more sensitive than others. Additionally, changes in the culture medium and components used for antibacterial testing are usually unavoidable. Thus, comprehensive studies should be performed promptly to overcome these shortcomings (Figure 2).
5.2 ROS generation
ZnONPs exert remarkable antimicrobial effects via two different mechanisms: the photolytic release of ROS and the induction of ROS production within cells (Wang et al., 2020). Examples include superoxide and H2O2. The generated ROS damages the cells and intracellular components by means of oxidative stress, causing dysfunction that ultimately results in bacterial death (Sultana et al., 2021).
Theoretically, with light energy (hv) greater than the bandgap of ZnO, electrons (ē) and holes (h+) in ZnONPs are separated (Karunakaran et al., 2011). Owing to high oxidizing properties, the photogenerated holes degrade H2O molecules in the atmosphere into hydroxyl radicals (•OH) and protonated hydrogen (H+) in the ZnO suspension. Simultaneously, the photoinduced ē reacts with molecular oxygen through a reduction process, forming superoxide anion radicals (•O2−), which then react with H+ to produce hydroperoxyl radicals (HO2•). The aqueous reaction of •O2− indirectly produces singlet oxygen (1O2), a strong oxidant. In the final step, HO2•, H+, and ē combine to form H2O2 molecules. Each product is considered to have a biochemical induction process in bacteria. •OH radicals are the most reactive and non-selective oxidants that can damage most biomolecules, including carbohydrates, nucleic acids, lipids, and amino acids. •O2 is responsible for phototoxicity and damages the microbial membrane surface via oxidative stress; thus, reducing bacterial cell integrity (Selvam and Sundrarajan 2012). Three types of ROS, ·OH, 1O2, and ·O2−, albeit with varying intensities of ROS, significantly promote lipid peroxidation in the membranes of various biological systems via oxidative stress (Figure 3). Furthermore, H2O2 is an important factor affecting the antibacterial activity of ZnONPs (Sawai, 2003). H2O2 formation on the ZnONP surface is related to oxygen species. H2O2 can penetrate cells with weaker electrostatic interconnections, causing functional impairments in DNA and cellular proteins and molecular death (Hameed et al., 2016). The surface grains and ZnONP size regulate H2O2 levels; smaller ZnONPs usually contain higher H2O2 and oxygen species concentrations on their surfaces, logically exhibiting better antibacterial properties (Reddy et al., 2014). However, whether ROS generation can be triggered by photon energy is debatable. Some studies have provided compelling evidence that ZnO exhibits equivalent antibacterial properties even in the absence of light (Hirota et al., 2010). Thus, the toxicity of ZnO toward bacteria must be because of coexisting mechanisms. A major possibility is that pre-existing oxygen defects in n-type ZnO; thus, leading to ROS generation in electron-rich crystals (Wu and Kao, 2015). Lipovsky et al. showed that ROS generated by ZnONPs exerted a considerable inhibitory effect on the growth of Candida albicans (Lipovsky et al., 2011). However, for a complete understanding, more studies should be performed on oxidative stress mechanisms induced by ROS generation and interactions between fungal cell walls and ZnONPs. Zinc plays structural and catalytic roles in metalloenzymes. Zinc is a cofactor for Cu/Zn superoxide dismutase, an important antioxidant enzyme, and plays a significant role in oxidative stress (Vural et al., 2010).
Here, it must be emphasized that toxicity may be produced against eukaryotic cells. The antifungal mechanisms of ZnONPs are similar to the aforementioned antibacterial mechanisms (Kairyte et al., 2013; Dananjaya et al., 2018). Several studies have already examined the cytotoxicity of ZnONPs against human cells [167, 168] and have attributed the toxic effects to the upregulation of the p53 protein pathway in the presence of ZnONPs, which subsequently induces cell apoptosis. This is a response to the oxidative stress exerted by ROS on DNA. Several authors describe this in conjunction with Zn2+ release as the main driving factor for toxicity in mammalian cells.
5.3 Contact with the cell membrane
Another proposed mechanism is based on physical interactions between ZnONPs and bacterial surfaces. These interactions can be van der Waals forces, electrostatic attraction, hydrophobic interactions, and receptor–ligand interactions, which disrupt the charge balance on the bacterial surface; thus, leading to cell deformation and bacterial destruction.
ZnONPs with a positive zeta potential are adsorbed onto negatively charged bacterial surfaces via electrostatic interactions, whereas those with a negative zeta potential are adsorbed via receptor–ligand interactions (Qi et al., 2017). Although many bacteria contain a net negative charge because of carbonyl groups, ZnONPs maintain their positive charge in an aqueous suspension (around pH 7) (Zhang et al., 2008). These opposing charges result in strong electrostatic interactions, thereby forming a physical bond between the two. Consequently, the cells are damaged, and bacteria die due to apoptosis (Zhang et al., 2007). Biological transmission electron microscopy showed that ZnO adhered to the outer membrane of bacteria; thus, inducing the formation of small indentations, and the released lipopolysaccharides inhibited bacterial growth. Furthermore, ZnO slowly penetrated these small indentations, especially into cytoplasmic and periplasmic spaces, in order to impede cell metabolism and membrane integrity (Zhang et al., 2007; Sirelkhatim et al., 2015).
Endocytosis in bacteria is similar to that occurs in eukaryotic cells, thereby representing another potential transport pathway for NPs (Kamyab et al., 2023). However, no studies have been performed to explore this aspect. Therefore, the most plausible explanation is that bacterial cells exposed to low concentrations of NPs completely disintegrate, lose their lipopolysaccharide coatings, and protrude from the cell surface in the form of vesicles. These protrusions attach to NPs, which are subsequently drawn to and penetrate the cell by electrostatic fields. Additionally, only a few studies have focused on intracellular processes that reduce activity. Further, despite the considerable oxidative stress induced by NPs, studies on their effects on bacterial cell metabolism, protein synthesis, and gene expression are scarce.
Current studies indicate that the three antibacterial mechanisms often coexist and are intertwined; however, their mechanistic analysis has not been thoroughly performed. Therefore, investigating antibacterial mechanisms from molecular biology and genetic perspectives, such as cell repair and protein conversion, is recommended Yao, 2021.
6 Conclusion and future perspective
This review covers the following four aspects: the advantages of ZnONPs compared with those of antibiotics and other metal ions, traditional and latest preparation methods of ZnONPs, their applications in antibacterial treatments, and their possible antibacterial mechanisms. The study overviews the utility of ZnONPs in various aspects of clinical settings and future life. The current understanding of the antibacterial mechanisms of ZnONPs remains somewhat unclear. Thus, future in-depth investigation into these mechanisms and understanding the principles behind them will pave the way for developing better biomaterials for clinical applications. In the future, we can expect more research and innovation concerning ZnONPs. By developing new preparation methods, optimizing material properties, and exploring new application fields, the performance and efficacy of ZnONPs can be further improved. Additionally, strengthening the existing composites and the integration of ZnONPs with other materials will help open up new application areas. In conclusion, the outlook for ZnONPs is promising. As an important inorganic compound, ZnONPs possess tremendous potential in the fields of energy, electronics, environmental protection, and biomedicine, especially owing to continuous technological advancements and growing demand for environmentally friendly materials. ZnONP applications will make a positive contribution to human societal development.
Author contributions
JN: Writing–original draft. YC: Writing–review and editing. RG: Writing–review and editing. PC: Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Basic Scientifc Research Project of University belongs to Heilongjiang (Grant No. 2021-KYYWFMY-0012). Basic Scientifc Research Project of University belongs to Heilongjiang (Grant No. 2021-KYYWFMY-0059).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdo, A. M., Fouda, A., Eid, A. M., Fahmy, N. M., Elsayed, A. M., Khalil, A. M. A., et al. (2021). Green synthesis of zinc oxide nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and their activity against pathogenic microbes and common house mosquito, Culex pipiens. Materials 14 (22), 6983. doi:10.3390/ma14226983
Agrawal, A., Sharma, R., Sharma, A., Gurjar, K. C., Kumar, S., Chatterjee, S., et al. (2023). Antibacterial and antibiofilm efficacy of green synthesized ZnO nanoparticles using Saraca asoca leaves. Environ. Sci. Pollut. Res. 30 (36), 86328–86337. doi:10.1007/s11356-023-28524-7
Al Abdullah, K., Awad, S., Zaraket, J., and Salame, C. (2017). Synthesis of ZnO nanopowders by using sol-gel and studying their structural and electrical properties at different temperature. Energy Procedia 119, 565–570. doi:10.1016/j.egypro.2017.07.080
Alavi, M., and Nokhodchi, A. (2020). An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 227, 115349. doi:10.1016/j.carbpol.2019.115349
Anan, H. H., Zidan, R. A., Abd EL-Baset, S. A., and Ali, M. M. (2018). Ameliorative effect of zinc oxide nanoparticles on cyclophosphamide induced testicular injury in adult rat. Tissue Cell. 54, 80–93. doi:10.1016/j.tice.2018.08.006
Aouadi, A., Saoud, D. H., Laouini, S. E., Rebiai, A., Achouri, A., Mohammed, H. A., et al. (2023). Synergistic chitin-zinc nanocomposites from shrimp shell waste: characterization, antioxidant, and antibacterial properties. Biomass Convers. Biorefinery. doi:10.1007/s13399-023-05237-y
Arya, S., Mahajan, P., Mahajan, S., Khosla, A., Datt, R., Gupta, V., et al. (2021). Review—influence of processing parameters to control morphology and optical properties of sol-gel synthesized ZnO nanoparticles. ECS J. Solid State Sci. Technol. 10 (2), 023002. doi:10.1149/2162-8777/abe095
Asenjo, A., Oteo-Iglesias, J., and Alós, J.-I. (2021). What’s new in mechanisms of antibiotic resistance in bacteria of clinical origin? Enfermedades Infecc. Microbiol. clinica English39 (6), 291–299. doi:10.1016/j.eimce.2020.02.017
Aydin Sevinç, B., and Hanley, L. (2010). Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomaterials 9999B, 22–31. doi:10.1002/jbm.b.31620
Azzi, M., Mokni, S., Medila, I., Toumi, I., Hasan, G. G., Laouini, S. E., et al. (2024). Optimizing synergies: unraveling the effect of ZnO nanoparticle calcination on in vitro antibacterial potency and photocatalytic efficiency of Cephalexin A study into kinetics and isotherms. Chem. Afr. doi:10.1007/s42250-024-00963-w
Babayevska, N., Przysiecka, Ł., Iatsunskyi, I., Nowaczyk, G., Jarek, M., Janiszewska, E., et al. (2022). ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 12 (1), 8148. doi:10.1038/s41598-022-12134-3
Babitha, N., Priya, L. S., Christy, S. R., Manikandan, A., Dinesh, A., Durka, M., et al. (2019). Enhanced antibacterial activity and photo-catalytic properties of ZnO nanoparticles: pedalium murex plant extract-assisted synthesis. J. Nanosci. Nanotechnol. 19 (5), 2888–2894. doi:10.1166/jnn.2019.16023
Barani, D., Tedjani, M. L., Younes, Z., Meneceur, S., Laouini, S. E., and Hammami, H. (2023). Biomass-mediated synthesis of ZnO and Mg@ZnO nanoparticles for enhancing the degradation of m-toluidine and p-toluidine. Biomass Convers. Biorefinery 13 (8), 7311–7318. doi:10.1007/s13399-022-03411-2
Bashandy, S. A. E., Alaamer, A., Moussa, S. A. A., and Omara, E. A. (2018). Role of zinc oxide nanoparticles in alleviating hepatic fibrosis and nephrotoxicity induced by thioacetamide in rats. Can. J. Physiology Pharmacol. 96 (4), 337–344. doi:10.1139/cjpp-2017-0247
Basnet, P., Inakhunbi Chanu, T., Samanta, D., and Chatterjee, S. (2018). A review on bio-synthesized zinc oxide nanoparticles using plant extracts as reductants and stabilizing agents. J. Photochem. Photobiol. B Biol. 183, 201–221. doi:10.1016/j.jphotobiol.2018.04.036
Bellanger, X., Billard, P., Schneider, R., Balan, L., and Merlin, C. (2015). Stability and toxicity of ZnO quantum dots: interplay between nanoparticles and bacteria. J. Hazard. Mater. 283, 110–116. doi:10.1016/j.jhazmat.2014.09.017
Bharathi, D., Ranjithkumar, R., Chandarshekar, B., and Bhuvaneshwari, V. (2019). Preparation of chitosan coated zinc oxide nanocomposite for enhanced antibacterial and photocatalytic activity: as a bionanocomposite. Int. J. Biol. Macromol. 129, 989–996. doi:10.1016/j.ijbiomac.2019.02.061
Bhowmick, A., Banerjee, S. L., Pramanik, N., Jana, P., Mitra, T., Gnanamani, A., et al. (2018). Organically modified clay supported chitosan/hydroxyapatite-zinc oxide nanocomposites with enhanced mechanical and biological properties for the application in bone tissue engineering. Int. J. Biol. Macromol. 106, 11–19. doi:10.1016/j.ijbiomac.2017.07.168
Borysiewicz, M. A. (2019). ZnO as a functional material, a review. Crystals 9 (10), 505. doi:10.3390/cryst9100505
Bose, S., Fielding, G., Tarafder, S., and Bandyopadhyay, A. (2013). Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 31 (10), 594–605. doi:10.1016/j.tibtech.2013.06.005
Boutalbi, A., Mohammed, H. A., Meneceur, S., Eddine, L. S., Abdullah, J. A. A., Alharthi, F., et al. (2023). Photocatalytic dye degradation efficiency and reusability of potassium polyacrylate hydrogel loaded Ag@ZnO nanocomposite. Transit. Metal. Chem. 48 (5), 353–363. doi:10.1007/s11243-023-00548-5
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Bray, F., Laversanne, M., Weiderpass, E., and Soerjomataram, I. (2021). The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127 (16), 3029–3030. doi:10.1002/cncr.33587
Butt, Z., Aamir, M., Aziz, S., Akhtar, J., Afaq, A., Naseer, S., et al. (2023). Green synthesis of Cu-Mn co-incorporated ZnO nanoparticles for antibacterial and photocatalytic applications. Microsc. Res. Tech. 86 (9), 1132–1143. doi:10.1002/jemt.24386
Cesur, S., Cebeci, S. A., Kavas, G. O., Yılmaz, N., and Buyukkagnici, D. I. (2005). Serum copper and zinc concentrations in patients with chronic hepatitis C. J. Infect. 51 (1), 35–37. doi:10.1016/j.jinf.2004.08.003
Chandra, H., Patel, D., Kumari, P., Jangwan, J. S., and Yadav, S. (2019). Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 102, 212–220. doi:10.1016/j.msec.2019.04.035
Chavali, M. S., and Nikolova, M. P. (2019). Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 1 (6), 607. doi:10.1007/s42452-019-0592-3
Chittofrati, A., and Matijević, E. (1990). Uniform particles of zinc oxide of different morphologies. Colloids Surfaces 48, 65–78. doi:10.1016/0166-6622(90)80219-T
Corl, K. A., Zeba, F., Caffrey, A. R., Hermenau, M., Lopes, V., Phillips, G., et al. (2020). Delay in antibiotic administration is associated with mortality among septic shock patients with Staphylococcus aureus bacteremia. Crit. Care Med. 48 (4), 525–532. doi:10.1097/CCM.0000000000004212
Dananjaya, S. H. S., Kumar, R. S., Yang, M., Nikapitiya, C., Lee, J., and De Zoysa, M. (2018). Synthesis, characterization of ZnO-chitosan nanocomposites and evaluation of its antifungal activity against pathogenic Candida albicans. Int. J. Biol. Macromol. 108, 1281–1288. doi:10.1016/j.ijbiomac.2017.11.046
Das, D., Moniruzzaman, M., Sarbajna, A., and Chakraborty, S. B. (2017). Effect of heavy metals on tissue-specific antioxidant response in Indian major carps. Environ. Sci. Pollut. Res. 24 (22), 18010–18024. doi:10.1007/s11356-017-9415-5
Dawwam, G. E., Al-Shemy, M. T., and El-Demerdash, A. S. (2022). Green synthesis of cellulose nanocrystal/ZnO bio-nanocomposites exerting antibacterial activity and downregulating virulence toxigenic genes of food-poisoning bacteria. Sci. Rep. 12 (1), 16848. doi:10.1038/s41598-022-21087-6
De Matteis, V., Cascione, M., Toma, C., and Leporatti, S. (2018). Silver nanoparticles: synthetic routes, in vitro toxicity and theranostic applications for cancer disease. Nanomaterials 8 (5), 319. doi:10.3390/nano8050319
Dhandapani, K. V., Anbumani, D., Gandhi, A. D., Annamalai, P., Muthuvenkatachalam, B. S., Kavitha, P., et al. (2020). Green route for the synthesis of zinc oxide nanoparticles from Melia azedarach leaf extract and evaluation of their antioxidant and antibacterial activities. Biocatal. Agric. Biotechnol. 24, 101517. doi:10.1016/j.bcab.2020.101517
Diguna, L. J., Fitriani, A. D., Liasari, B. R., Timuda, G. E., Widayatno, W. B., Wismogroho, A. S., et al. (2020). Optical and photodetection properties of ZnO nanoparticles recovered from Zn dross. Crystals 11 (1), 6. doi:10.3390/cryst11010006
Dixit, A., Kumar, N., Kumar, S., and Trigun, V. (2019). Antimicrobial resistance: progress in the decade since emergence of New Delhi metallo-β-lactamase in India. Indian J. community Med. official Publ. Indian Assoc. Prev. and Soc. Med. 44 (1), 4–8. doi:10.4103/ijcm.IJCM_217_18
Doğan, S. Ş., and Kocabaş, A. (2020). Green synthesis of ZnO nanoparticles with Veronica multifida and their antibiofilm activity. Hum. and Exp. Toxicol. 39 (3), 319–327. doi:10.1177/0960327119888270
Dorman, C. J. (2019). DNA supercoiling and transcription in bacteria: a two-way street. BMC Mol. Cell. Biol. 20 (1), 26. doi:10.1186/s12860-019-0211-6
Dulta, K., Koşarsoy Ağçeli, G., Thakur, A., Singh, S., Chauhan, P., and Chauhan, P. K. (2022). Development of alginate-chitosan based coating enriched with ZnO nanoparticles for increasing the shelf life of orange fruits (citrus sinensis L.). J. Polym. Environ. 30 (8), 3293–3306. doi:10.1007/s10924-022-02411-7
Ehsan, S., and Sajjad, M. (2017). Bioinspired synthesis of zinc oxide nanoparticle and its combined efficacy with different antibiotics against multidrug resistant bacteria. J. Biomaterials Nanobiotechnology 08 (02), 159–175. doi:10.4236/jbnb.2017.82011
El-Gendy, A. O., Nawaf, K. T., Ahmed, E., Samir, A., Hamblin, M. R., Hassan, M., et al. (2022). Preparation of zinc oxide nanoparticles using laser-ablation technique: retinal epithelial cell (ARPE-19) biocompatibility and antimicrobial activity when activated with femtosecond laser. J. Photochem. Photobiol. B Biol. 234, 112540. doi:10.1016/j.jphotobiol.2022.112540
Emamifar, A., Kadivar, M., Shahedi, M., and Soleimanian-Zad, S. (2011). Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food control. 22 (3–4), 408–413. doi:10.1016/j.foodcont.2010.09.011
Esmailzadeh, H., Sangpour, P., Shahraz, F., Hejazi, J., and Khaksar, R. (2016). Effect of nanocomposite packaging containing ZnO on growth of Bacillus subtilis and Enterobacter aerogenes. Mater. Sci. Eng. C 58, 1058–1063. doi:10.1016/j.msec.2015.09.078
Eymard-Vernain, E., Luche, S., Rabilloud, T., and Lelong, C. (2018). Impact of nanoparticles on the Bacillus subtilis (3610) competence. Sci. Rep. 8 (1), 2978. doi:10.1038/s41598-018-21402-0
Farhat, N., Ali, A., Bonomo, R. A., and Khan, A. U. (2020). Efflux pumps as interventions to control infection caused by drug-resistance bacteria. Drug Discov. Today 25 (12), 2307–2316. doi:10.1016/j.drudis.2020.09.028
Fenno, L., Yizhar, O., and Deisseroth, K. (2011). The development and application of optogenetics. Annu. Rev. Neurosci. 34 (1), 389–412. doi:10.1146/annurev-neuro-061010-113817
Filius, P. M., and Gyssens, I. C. (2002). Impact of increasing antimicrobial resistance on wound management. Am. J. Clin. Dermatology 3 (1), 1–7. doi:10.2165/00128071-200203010-00001
Franco, M. A., Conti, P. P., Andre, R. S., and Correa, D. S. (2022). A review on chemiresistive ZnO gas sensors. Sensors Actuators Rep. 4, 100100. doi:10.1016/j.snr.2022.100100
Garcia, C. V., Shin, G. H., and Kim, J. T. (2018). Metal oxide-based nanocomposites in food packaging: applications, migration, and regulations. Trends Food Sci. and Technol. 82, 21–31. doi:10.1016/j.tifs.2018.09.021
Garino, N., Limongi, T., Dumontel, B., Canta, M., Racca, L., Laurenti, M., et al. (2019a). A microwave-assisted synthesis of zinc oxide nanocrystals finely tuned for biological applications. Nanomaterials 9 (2), 212. doi:10.3390/nano9020212
Garino, N., Sanvitale, P., Dumontel, B., Laurenti, M., Colilla, M., Izquierdo-Barba, I., et al. (2019b). Zinc oxide nanocrystals as a nanoantibiotic and osteoinductive agent. RSC Adv. 9 (20), 11312–11321. doi:10.1039/C8RA10236H
Gharbi, A. H., Hemmami, H., Laouini, S. E., Amor, I. B., Zeghoud, S., Amor, A. B., et al. (2023). Green synthesis of ZnO@SiO2 nanoparticles using Calligonum comosum L. extract: an efficient approach for organic pollutant degradation in wastewater. Biomass Convers. Biorefinery. doi:10.1007/s13399-023-05063-2
González, S. C. E., Bolaina-Lorenzo, E., Pérez-Trujillo, J. J., Puente-Urbina, B. A., Rodríguez-Fernández, O., Fonseca-García, A., et al. (2021). Antibacterial and anticancer activity of ZnO with different morphologies: a comparative study. 3 Biotech. 11 (2), 68. doi:10.1007/s13205-020-02611-9
Gould, L., Abadir, P., Brem, H., Carter, M., Conner-Kerr, T., Davidson, J., et al. (2015). Chronic wound repair and healing in older adults: current status and future research. J. Am. Geriatrics Soc. 63 (3), 427–438. doi:10.1111/jgs.13332
Gruendner, J., Wolf, N., Tögel, L., Haller, F., Prokosch, H.-U., and Christoph, J. (2020). Integrating genomics and clinical data for statistical analysis by using GEnome MINIng (GEMINI) and fast healthcare interoperability resources (FHIR): system design and implementation. J. Med. Internet Res. 22 (10), e19879. doi:10.2196/19879
Guan, H., Dong, W., Lu, Y., Jiang, M., Zhang, D., Aobuliaximu, Y., et al. (2021). Distribution and antibiotic resistance patterns of pathogenic bacteria in patients with chronic cutaneous wounds in China. Front. Med. 8, 609584. doi:10.3389/fmed.2021.609584
Guerram, A., Laouini, S. E., Mohammed, H. A., Hasan, G. G., Tedjani, M. L., Alharthi, F., et al. (2024). Synergistic performance of ZnO/SnO2 nanocomposites: synthesis, characterization, and applications in photocatalysis and superoxide radical scavenger. J. Clust. Sci. doi:10.1007/s10876-024-02642-9
Guerrero Correa, M., Martínez, F. B., Vidal, C. P., Streitt, C., Escrig, J., and de Dicastillo, C. L. (2020). Antimicrobial metal-based nanoparticles: a review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 11, 1450–1469. doi:10.3762/bjnano.11.129
Gunalan, S., Sivaraj, R., and Rajendran, V. (2012). Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 22 (6), 693–700. doi:10.1016/j.pnsc.2012.11.015
Hameed, A. S. H., Karthikeyan, C., Ahamed, A. P., Thajuddin, N., Alharbi, N. S., Alharbi, S. A., et al. (2016). In vitro antibacterial activity of ZnO and Nd doped ZnO nanoparticles against ESBL producing Escherichia coli and Klebsiella pneumoniae. Sci. Rep. 6 (1), 24312. doi:10.1038/srep24312
Hamza, L., Laouini, S. E., Mohammed, H. A., Meneceur, S., Salmi, C., Alharthi, F., et al. (2024). Biosynthesis of ZnO/Ag nanocomposites heterostructure for efficient photocatalytic degradation of antibiotics and synthetic dyes. Z. fur Phys. Chem. 0. doi:10.1515/zpch-2023-0379
Hatami, Z., Ragheb, E., Jalali, F., Tabrizi, M. A., and Shamsipur, M. (2020). Zinc oxide-gold nanocomposite as a proper platform for label-free DNA biosensor. Bioelectrochemistry 133, 107458. doi:10.1016/j.bioelechem.2020.107458
He, Z., Wang, C., and Chen, C. (2020). Microwave-assisted synthesis and catalytic performance of carbon nanotubes/flower-patterned zinc oxide nanostructures. J. Nanosci. Nanotechnol. 20 (8), 4971–4976. doi:10.1166/jnn.2020.18485
Higashisaka, K., Nagano, K., Yoshioka, Y., and Tsutsumi, Y. (2017). Nano-safety research: examining the associations among the biological effects of nanoparticles and their physicochemical properties and kinetics. Biol. and Pharm. Bull. 40 (3), 243–248. doi:10.1248/bpb.b16-00854
Hirota, K., Sugimoto, M., Kato, M., Tsukagoshi, K., Tanigawa, T., and Sugimoto, H. (2010). Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceram. Int. 36 (2), 497–506. doi:10.1016/j.ceramint.2009.09.026
Hou, Y., Hou, Y., Ren, Y., Shi, Y., Jin, X., Dong, Y., et al. (2020). C. aromaticus leaf extract mediated synthesis of Zinc oxide nanoparticles and their antimicrobial activity towards clinically multidrug-resistant bacteria isolated from pneumonia patients in nursing care. Mater. Res. Express 7 (9), 095015. doi:10.1088/2053-1591/abb427
Hu, Y., Zhang, H. R., Dong, L., Xu, M. R., Zhang, L., Ding, W. P., et al. (2019). Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale 11 (24), 11789–11807. doi:10.1039/C8NR08442D
Huang, P., Ma, K., Cai, X., Huang, D., Yang, X., Ran, J., et al. (2017). Enhanced antibacterial activity and biocompatibility of zinc-incorporated organic-inorganic nanocomposite coatings via electrophoretic deposition. Colloids Surfaces B Biointerfaces 160, 628–638. doi:10.1016/j.colsurfb.2017.10.012
Huang, Z., Zheng, X., Yan, D., Yin, G., Liao, X., Kang, Y., et al. (2008). Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir 24 (8), 4140–4144. doi:10.1021/la7035949
Hussain, S., Khakwani, N., Faiz, Y., Zulfiqar, S., Shafiq, Z., Faiz, F., et al. (2022). Green production and interaction of carboxylated CNTs/biogenic ZnO composite for antibacterial activity. Bioengineering 9 (9), 437. doi:10.3390/bioengineering9090437
Ibrahim, H. M. M. (2015). Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 8 (3), 265–275. doi:10.1016/j.jrras.2015.01.007
Ikram, M., Javed, B., Raja, N. I., and Mashwani, Z.-R. (2021). Biomedical potential of plant-based selenium nanoparticles: a comprehensive review on therapeutic and mechanistic aspects. Int. J. Nanomedicine 16, 249–268. doi:10.2147/IJN.S295053
Iqbal, T., Khan, M. A., and Mahmood, H. (2018). Facile synthesis of ZnO nanosheets: structural, antibacterial and photocatalytic studies. Mater. Lett. 224, 59–63. doi:10.1016/j.matlet.2018.04.078
Ito, A., Ojima, K., Naito, H., Ichinose, N., and Tateishi, T. (2000). Preparation, solubility, and cytocompatibility of zinc-releasing calcium phosphate ceramics. J. Biomed. Mater. Res. 50 (2), 178–183. doi:10.1002/(SICI)1097-4636(200005)50:2<178::AID-JBM12>3.0.CO;2-5
Javidi, S., Mohammadi Nafchi, A., and Moghadam, H. H. (2022). Synergistic effect of nano-ZnO and Mentha piperita essential oil on the moisture sorption isotherm, antibacterial activity, physicochemical, mechanical, and barrier properties of gelatin film. J. Food Meas. Charact. 16 (2), 964–974. doi:10.1007/s11694-021-01217-w
Jiang, J., Pi, J., and Cai, J. (2018). The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorganic Chem. Appl. 2018, 1–18. doi:10.1155/2018/1062562
Jones, L. C., Timmie Topoleski, L. D., and Tsao, A. K. (2017). “Biomaterials in orthopaedic implants,” in Mechanical testing of orthopaedic implants (Elsevier), 17–32. doi:10.1016/B978-0-08-100286-5.00002-0
Jones, N., Ray, B., Ranjit, K. T., and Manna, A. C. (2008). Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 279 (1), 71–76. doi:10.1111/j.1574-6968.2007.01012.x
Kadhum, Q., Sahan, K., Ali, R. A., Mahdi, R. J., Kassim, N. A., Jassim, A., et al. (2015). Synthesis of zinc oxide nanoparticles via sol-gel route and their characterization. Nanosci. Nanotechnol. 5, 1–6. doi:10.5923/j.nn.20150501.01
Kairyte, K., Kadys, A., and Luksiene, Z. (2013). Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. B Biol. 128, 78–84. doi:10.1016/j.jphotobiol.2013.07.017
Kakiuchi, K., Hosono, E., Kimura, T., Imai, H., and Fujihara, S. (2006). Fabrication of mesoporous ZnO nanosheets from precursor templates grown in aqueous solutions. J. Sol-Gel Sci. Technol. 39 (1), 63–72. doi:10.1007/s10971-006-6321-6
Kamyab, H., Chelliapan, S., Hayder, G., Yusuf, M., Taheri, M. M., Rezania, S., et al. (2023). Exploring the potential of metal and metal oxide nanomaterials for sustainable water and wastewater treatment: a review of their antimicrobial properties. Chemosphere 335, 139103. doi:10.1016/j.chemosphere.2023.139103
Kao, Y.-Y., Chen, Y.-C., Cheng, T.-J., Chiung, Y.-M., and Liu, P.-S. (2012). Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol. Sci. 125 (2), 462–472. doi:10.1093/toxsci/kfr319
Karunakaran, C., Rajeswari, V., and Gomathisankar, P. (2011). Enhanced photocatalytic and antibacterial activities of sol–gel synthesized ZnO and Ag-ZnO. Mater. Sci. Semicond. Process. 14 (2), 133–138. doi:10.1016/j.mssp.2011.01.017
Khan, M. M., Matussin, S. N., and Rahman, A. (2022). Recent progress of phytogenic synthesis of ZnO, SnO2, and CeO2 nanomaterials. Bioprocess Biosyst. Eng. 45 (4), 619–645. doi:10.1007/s00449-022-02713-z
Khanam, S. (2020). Toxicological effect of zinc on liver of broiler chicks. Egypt. Liver J. 10 (1), 21. doi:10.1186/s43066-020-00028-w
Khiralla, G. M., and El-Deeb, B. A. (2015). Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT - Food Sci. Technol. 63 (2), 1001–1007. doi:10.1016/j.lwt.2015.03.086
Kielbik, P., Kaszewski, J., Rosowska, J., Wolska, E., Witkowski, B. S., Gralak, M. A., et al. (2017). Biodegradation of the ZnO:Eu nanoparticles in the tissues of adult mouse after alimentary application. Nanomedicine Nanotechnol. Biol. Med. 13 (3), 843–852. doi:10.1016/j.nano.2016.11.002
Kim, A. (2015). Mitochondria in cancer energy metabolism: culprits or bystanders? Toxicol. Res. 31 (4), 323–330. doi:10.5487/TR.2015.31.4.323
Kim, J. S., Kuk, E., Yu, K. N., Kim, J.-H., Park, S. J., Lee, H. J., et al. (2007). Antimicrobial effects of silver nanoparticles. Nanomedicine Nanotechnol. Biol. Med. 3 (1), 95–101. doi:10.1016/j.nano.2006.12.001
Kir, I., Laouini, S. E., Meneceur, S., Bouafia, A., and Mohammed, H. A. M. (2023). Biosynthesis and characterization of novel nanocomposite ZnO/BaMg2 efficiency for high-speed adsorption of AZO dye. Biomass Convers. Biorefinery 14, 19045–19054. doi:10.1007/s13399-023-03985-5
Król, A., Pomastowski, P., Rafińska, K., Railean-Plugaru, V., and Buszewski, B. (2017). Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 249, 37–52. doi:10.1016/j.cis.2017.07.033
Lallo da Silva, B., Caetano, B. L., Chiari-Andréo, B. G., Pietro, R. C. L. R., and Chiavacci, L. A. (2019). Increased antibacterial activity of ZnO nanoparticles: influence of size and surface modification. Colloids Surfaces B Biointerfaces 177, 440–447. doi:10.1016/j.colsurfb.2019.02.013
Laurenti, M., and Cauda, V. (2017). ZnO nanostructures for tissue engineering applications. Nanomaterials 7 (11), 374. doi:10.3390/nano7110374
Lee, N.-Y., Ko, W.-C., and Hsueh, P.-R. (2019). Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front. Pharmacol. 10, 1153. doi:10.3389/fphar.2019.01153
Li, J., Zheng, L.-F., Zhang, K.-F., Feng, X.-Q., Su, Z.-X., and Ma, J.-T. (2008). Synthesis of Ag modified vanadium oxide nanotubes and their antibacterial properties. Mater. Res. Bull. 43 (10), 2810–2817. doi:10.1016/j.materresbull.2007.10.046
Liang, Y., Li, W., Wang, X., Zhou, R., and Ding, H. (2022). TiO2–ZnO/Au ternary heterojunction nanocomposite: excellent antibacterial property and visible-light photocatalytic hydrogen production efficiency. Ceram. Int. 48 (2), 2826–2832. doi:10.1016/j.ceramint.2021.10.072
Liao, C., Li, Y., and Tjong, S. (2019). Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 20 (2), 449. doi:10.3390/ijms20020449
Lin, M.-H., Wang, Y. H., Kuo, C. H., Ou, S. F., Huang, P. Z., Song, T. Y., et al. (2021). Hybrid ZnO/chitosan antimicrobial coatings with enhanced mechanical and bioactive properties for titanium implants. Carbohydr. Polym. 257, 117639. doi:10.1016/j.carbpol.2021.117639
Lipovsky, A., Nitzan, Y., Gedanken, A., and Lubart, R. (2011). Antifungal activity of ZnO nanoparticles—the role of ROS mediated cell injury. Nanotechnology 22 (10), 105101. doi:10.1088/0957-4484/22/10/105101
Lipsky, B. A., Berendt, A. R., Cornia, P. B., Pile, J. C., Peters, E. J. G., Armstrong, D. G., et al. (2012). 2012 infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infectionsa. Clin. Infect. Dis. 54 (12), e132–e173. doi:10.1093/cid/cis346
Liu, J., Wang, Y., Ma, J., Peng, Y., and Wang, A. (2019). A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloys Compd. 783, 898–918. doi:10.1016/j.jallcom.2018.12.330
Look, D. C. (2001). Recent advances in ZnO materials and devices. Mater. Sci. Eng. B 80 (1–3), 383–387. doi:10.1016/S0921-5107(00)00604-8
Mahmoud, U. T., Darwish, M. H. A., Ali, F. A. Z., Amen, O. A., Mahmoud, M. A. M., Ahmed, O. B., et al. (2021). Zinc oxide nanoparticles prevent multidrug resistant Staphylococcus-induced footpad dermatitis in broilers. Avian Pathol. 50 (3), 214–226. doi:10.1080/03079457.2021.1875123
McQuillan, J. S., Groenaga Infante, H., Stokes, E., and Shaw, A. M. (2012). Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology 6 (8), 857–866. doi:10.3109/17435390.2011.626532
Meneceur, S., Bouafia, A., Laouini, S. E., Mohammed, H. A., Daoudi, H., Hasan, G. G., et al. (2023). High-efficiency photocatalytic degradation of antibiotics and molecular docking study to treat the omicron variant of COVID-19 infection using biosynthesized ZnO@Fe3O4 nanocomposites. Phys. Scr. 98 (11), 115926. doi:10.1088/1402-4896/acff2d
Mishra, P. K., Mishra, H., Ekielski, A., Talegaonkar, S., and Vaidya, B. (2017). Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov. Today 22 (12), 1825–1834. doi:10.1016/j.drudis.2017.08.006
Mohamed, A. R., Gowdhami, B., Mohamed, M. S., Archunan, G., and Suganthy, N. S. (2019a). Anticancer potential of zinc oxide nanoparticles against cervical carcinoma cells synthesized via biogenic route using aqueous extract of Gracilaria edulis. Mater. Sci. Eng. C 103, 109840. doi:10.1016/j.msec.2019.109840
Mohamed, L. A., El Hindawy, M. M., Alagawany, M., Salah, A. S., and El Sayed, S. A. A. (2019b). Effect of low or high CP diet with cold pressed oil supplementation on growth, immunity and antioxidant indices of growing quail. J. Animal Physiology Animal Nutr. 103 (5), 1380–1387. doi:10.1111/jpn.13121
Mohd Yusof, H., Abdul Rahman, N., Mohamad, R., Hasanah Zaidan, U., and Samsudin, A. A. (2021). Antibacterial potential of biosynthesized zinc oxide nanoparticles against poultry-associated foodborne pathogens: an in vitro study. Animals 11 (7), 2093. doi:10.3390/ani11072093
Mohd Yusof, H., Mohamad, R., Zaidan, U. H., and Abdul Rahman, N. A. (2019). Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J. Animal Sci. Biotechnol. 10 (1), 57. doi:10.1186/s40104-019-0368-z
Morton, L. M., and Phillips, T. J. (2016). Wound healing and treating wounds. J. Am. Acad. Dermatology 74 (4), 589–605. doi:10.1016/j.jaad.2015.08.068
Mousa, H. M., Abdal-hay, A., Bartnikowski, M., Mohamed, I. M. A., Yasin, A. S., Ivanovski, S., et al. (2018). A multifunctional zinc oxide/poly(lactic acid) nanocomposite layer coated on magnesium alloys for controlled degradation and antibacterial function. ACS Biomaterials Sci. and Eng. 4 (6), 2169–2180. doi:10.1021/acsbiomaterials.8b00277
Murugan, R., and Ramakrishna, S. (2004). Bioresorbable composite bone paste using polysaccharide based nano hydroxyapatite. Biomaterials 25 (17), 3829–3835. doi:10.1016/j.biomaterials.2003.10.016
Nabavi, S., Habtemariam, S., Daglia, M., Braidy, N., Loizzo, M., Tundis, R., et al. (2015). Neuroprotective effects of ginkgolide B against ischemic stroke: a review of current literature. Curr. Top. Med. Chem. 15 (21), 2222–2232. doi:10.2174/1568026615666150610142647
Nath, J., Dror, I., Landa, P., Vanek, T., Kaplan-Ashiri, I., and Berkowitz, B. (2018). Synthesis and characterization of isotopically-labeled silver, copper and zinc oxide nanoparticles for tracing studies in plants. Environ. Pollut. 242, 1827–1837. doi:10.1016/j.envpol.2018.07.084
Nikaido, H. (2009). Multidrug resistance in bacteria. Annu. Rev. Biochem. 78 (1), 119–146. doi:10.1146/annurev.biochem.78.082907.145923
Noman, M. T., Petru, M., Militký, J., Azeem, M., and Ashraf, M. A. (2019). One-pot sonochemical synthesis of ZnO nanoparticles for photocatalytic applications, modelling and optimization. Materials 13 (1), 14. doi:10.3390/ma13010014
Oberdörster, G., Oberdörster, E., and Oberdörster, J. (2005). Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 113 (7), 823–839. doi:10.1289/ehp.7339
Osborn, J. F., and Weiss, T. (1978). Hydroxylapatite ceramics--a bone-like biomaterial. Preliminary report. Schweiz. Monatsschrift fur Zahnheilkd. = Rev. Mens. suisse d’odonto-stomatologie 88 (10), 1166–1172.
Parveen, K., Banse, V., and Ledwani, L. (2016). Green synthesis of nanoparticles: their advantages and disadvantages. doi:10.1063/1.4945168020048
Pelgrift, R. Y., and Friedman, A. J. (2013). Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 65 (13–14), 1803–1815. doi:10.1016/j.addr.2013.07.011
Phan, D.-N., Rebia, R. A., Saito, Y., Kharaghani, D., Khatri, M., Tanaka, T., et al. (2020). Zinc oxide nanoparticles attached to polyacrylonitrile nanofibers with hinokitiol as gluing agent for synergistic antibacterial activities and effective dye removal. J. Industrial Eng. Chem. 85, 258–268. doi:10.1016/j.jiec.2020.02.008
Powers, J. G., Higham, C., Broussard, K., and Phillips, T. J. (2016). Wound healing and treating wounds. J. Am. Acad. Dermatology 74 (4), 607–625. doi:10.1016/j.jaad.2015.08.070
Puca, V., Marulli, R. Z., Grande, R., Vitale, I., Niro, A., Molinaro, G., et al. (2021). Microbial species isolated from infected wounds and antimicrobial resistance analysis: data emerging from a three-years retrospective study. Antibiotics 10 (10), 1162. doi:10.3390/antibiotics10101162
Qi, K., Cheng, B., Yu, J., and Ho, W. (2017). Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 727, 792–820. doi:10.1016/j.jallcom.2017.08.142
Qin, X., Zhang, J., Wang, B., Xu, G., Yang, X., Zou, Z., et al. (2021). Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 17 (12), 4266–4285. doi:10.1080/15548627.2021.1911016
Qiu, Y., Yang, J., Chen, Y., Yang, J., Zhu, Q., Zhu, C., et al. (2021). Microbiological profiles and antimicrobial resistance patterns of pediatric bloodstream pathogens in China, 2016–2018. Eur. J. Clin. Microbiol. and Infect. Dis. 40 (4), 739–749. doi:10.1007/s10096-020-04069-2
Rabiee, N., Bagherzadeh, M., Ghadiri, A. M., Kiani, M., Aldhaher, A., Ramakrishna, S., et al. (2020). Green synthesis of ZnO NPs via Salvia hispanica: evaluation of potential antioxidant, antibacterial, mammalian cell viability, H1N1 influenza virus inhibition and photocatalytic activities. J. Biomed. Nanotechnol. 16 (4), 456–466. doi:10.1166/jbn.2020.2916
Rajeshkumar, S., Kumar, S. V., Ramaiah, A., Agarwal, H., Lakshmi, T., and Roopan, S. M. (2018). Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme Microb. Technol. 117, 91–95. doi:10.1016/j.enzmictec.2018.06.009
Ramesh, M., Anbuvannan, M., and Viruthagiri, G. (2015). Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 136, 864–870. doi:10.1016/j.saa.2014.09.105
Raouf Hosseini, M., and Nasiri Sarvi, M. (2015). Recent achievements in the microbial synthesis of semiconductor metal sulfide nanoparticles. Mater. Sci. Semicond. Process. 40, 293–301. doi:10.1016/j.mssp.2015.06.003
Ratan, Z. A., Mashrur, F. R., Chhoan, A. P., Shahriar, S. M., Haidere, M. F., Runa, N. J., et al. (2021). Silver nanoparticles as potential antiviral agents. Pharmaceutics 13 (12), 2034. doi:10.3390/pharmaceutics13122034
Reddy, K. M., Feris, K., Bell, J., Wingett, D. G., Hanley, C., and Punnoose, A. (2007). Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 90 (21), 2139021–2139023. doi:10.1063/1.2742324
Reddy, L. S., Nisha, M. M., Joice, M., and Shilpa, P. N. (2014). Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 52 (11), 1388–1397. doi:10.3109/13880209.2014.893001
Sabir, S., Arshad, M., and Chaudhari, S. K. (2014). Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci. World J. 2014, 1–8. doi:10.1155/2014/925494
Saha, R. K., Debanath, M. K., Paul, B., Medhi, S., and Saikia, E. (2020). Antibacterial and nonlinear dynamical analysis of flower and hexagon-shaped ZnO microstructures. Sci. Rep. 10 (1), 2598. doi:10.1038/s41598-020-59534-x
Sakthivel, S., Dhanapal, A. R., Paulraj, L. P., Gurusamy, A., Venkidasamy, B., Thiruvengadam, M., et al. (2022). Antibacterial activity of seed aqueous extract of Citrus limon (L.) mediated synthesis ZnO NPs: an impact on Zebrafish (Danio rerio) caudal fin development. Heliyon 8 (9), e10406. doi:10.1016/j.heliyon.2022.e10406
Saleh, T. A. (2020). Nanomaterials: classification, properties, and environmental toxicities. Environ. Technol. and Innovation 20, 101067. doi:10.1016/j.eti.2020.101067
Sana, S. S., Kumbhakar, D. V., Pasha, A., Pawar, S. C., Grace, A. N., Singh, R. P., et al. (2020). Crotalaria verrucosa leaf extract mediated synthesis of zinc oxide nanoparticles: assessment of antimicrobial and anticancer activity. Molecules 25 (21), 4896. doi:10.3390/molecules25214896
Sasani Ghamsari, M., Alamdari, S., Han, W., and Park, H.-H. (2016). Impact of nanostructured thin ZnO film in ultraviolet protection. Int. J. Nanomedicine 12, 207–216. doi:10.2147/IJN.S118637
Sawai, J. (2003). Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 54 (2), 177–182. doi:10.1016/S0167-7012(03)00037-X
Şeker, Ş., Elçin, A. E., Yumak, T., Sınağ, A., and Elçin, Y. M. (2014). In vitro cytotoxicity of hydrothermally synthesized ZnO nanoparticles on human periodontal ligament fibroblast and mouse dermal fibroblast cells. Toxicol. Vitro 28 (8), 1349–1358. doi:10.1016/j.tiv.2014.06.016
Selvam, S., and Sundrarajan, M. (2012). Functionalization of cotton fabric with PVP/ZnO nanoparticles for improved reactive dyeability and antibacterial activity. Carbohydr. Polym. 87 (2), 1419–1424. doi:10.1016/j.carbpol.2011.09.025
Serouti, A., Eddine, L. S., Meneceur, S., Hasan, G. G., Mohammed, H. A., Salmi, C., et al. (2024). Biogenic ZnO/CuO/Fe2O3 nanocomposite: a groundbreaking approach for enhanced degradation capabilities and reusability in dye removal applications. Arabian J. Sci. Eng. 49 (1), 753–764. doi:10.1007/s13369-023-08495-0
Seyfi, M., Fattah-alhosseini, A., Pajohi-Alamoti, M., and Nikoomanzari, E. (2021). Effect of ZnO nanoparticles addition to PEO coatings on AZ31B Mg alloy: antibacterial effect and corrosion behavior of coatings in Ringer’s physiological solution. J. Asian Ceram. Soc. 9 (3), 1114–1127. doi:10.1080/21870764.2021.1940728
Shaba, E. Y., Jacob, J. O., Tijani, J. O., and Suleiman, M. A. T. (2021). A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 11 (2), 48. doi:10.1007/s13201-021-01370-z
Shakerimoghaddam, A., Razavi, D., Rahvar, F., Khurshid, M., Ostadkelayeh, S. M., Esmaeili, S. A., et al. (2020). Evaluate the effect of zinc oxide and silver nanoparticles on biofilm and icaA gene expression in methicillin-resistant Staphylococcus aureus isolated from burn wound infection. J. Burn Care and Res. 41 (6), 1253–1259. doi:10.1093/jbcr/iraa085
Shaw, J. J. P., Boyer, T. L., Venner, E., Beck, P. J., Slamowitz, T., Caste, T., et al. (2020). Inhibition of lysosomal function mitigates protective mitophagy and augments ceramide nanoliposome–induced cell death in head and neck squamous cell carcinoma. Mol. Cancer Ther. 19 (12), 2621–2633. doi:10.1158/1535-7163.MCT-20-0182
Shi, L.-E., Li, Z.-H., Zheng, W., Zhao, Y.-F., Jin, Y.-F., and Tang, Z.-X. (2014). Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review. Food Addit. and Contam. Part A 31 (2), 173–186. doi:10.1080/19440049.2013.865147
Shim, K., Abdellatif, M., Choi, E., and Kim, D. (2017). Nanostructured ZnO films on stainless steel are highly safe and effective for antimicrobial applications. Appl. Microbiol. Biotechnol. 101 (7), 2801–2809. doi:10.1007/s00253-017-8099-6
Shrestha, B. K., Shrestha, S., Tiwari, A. P., Kim, J. I., Ko, S. W., Kim, H. J., et al. (2017). Bio-inspired hybrid scaffold of zinc oxide-functionalized multi-wall carbon nanotubes reinforced polyurethane nanofibers for bone tissue engineering. Mater. and Des. 133, 69–81. doi:10.1016/j.matdes.2017.07.049
Siddiqi, K. S., ur Rahman, A., and Tajuddin and Husen, A. (2018). Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 13 (1), 141. doi:10.1186/s11671-018-2532-3
Singh, A., Gautam, P. K., Verma, A., Singh, V., Shivapriya, P. M., Shivalkar, S., et al. (2020). Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: a review. Biotechnol. Rep. 25, e00427. doi:10.1016/j.btre.2020.e00427
Sirelkhatim, A., Mahmud, S., Seeni, A., Kaus, N. H. M., Ann, L. C., Bakhori, S. K. M., et al. (2015). Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 7 (3), 219–242. doi:10.1007/s40820-015-0040-x
Steffy, K., Shanthi, G., Maroky, A. S., and Selvakumar, S. (2018). Potential bactericidal activity of S. nux-vomica–ZnO nanocomposite against multidrug-resistant bacterial pathogens and wound-healing properties. J. Trace Elem. Med. Biol. 50, 229–239. doi:10.1016/j.jtemb.2018.07.009
Su, Y., Cockerill, I., Zheng, Y., Tang, L., Qin, Y.-X., and Zhu, D. (2019). Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 4, 196–206. doi:10.1016/j.bioactmat.2019.05.001
Sulak, M. (2021). Preparation of G-CuO NPs and G-ZnO NPs with mallow leaves, investigation of their antibacterial behavior and synthesis of bis(indolyl)methane compounds under solvent-free microwave assisted dry milling conditions using G-CuO NPs as a catalyst. Turkish J. Chem. 45 (5), 1517–1532. doi:10.3906/kim-2105-31
Sultana, A., Zare, M., Luo, H., and Ramakrishna, S. (2021). Surface engineering strategies to enhance the in situ performance of medical devices including atomic scale engineering. Int. J. Mol. Sci. 22 (21), 11788. doi:10.3390/ijms222111788
Sun, L., Guan, J., Xu, Q., Yang, X., Wang, J., and Hu, X. (2018). Synthesis and applications of molecularly imprinted polymers modified TiO2 nanomaterials: a review. Polymers 10 (11), 1248. doi:10.3390/polym10111248
Świątek, Z. M., Woźnicka, O., and Bednarska, A. J. (2020). Unravelling the ZnO-NPs mechanistic pathway: cellular changes and altered morphology in the gastrointestinal tract of the earthworm Eisenia andrei. Ecotoxicol. Environ. Saf. 196, 110532. doi:10.1016/j.ecoenv.2020.110532
Tabet, A., Meneceur, S., Laouini, S. E., Salmi, C., Mohammed, H. A., Kir, I., et al. (2024). One post biosynthesis of novel ternary nanocomposite ZnO/CuO/Cu2MgO3 for enhancing photocatalytic degradation of Bromocresol green in wastewater. J. Clust. Sci. 35 (3), 765–777. doi:10.1007/s10876-023-02519-3
Tănase, M. A., Marinescu, M., Oancea, P., Răducan, A., Mihaescu, C. I., Alexandrescu, E., et al. (2021). Antibacterial and photocatalytic properties of ZnO nanoparticles obtained from chemical versus Saponaria officinalis extract-mediated synthesis. Molecules 26 (7), 2072. doi:10.3390/molecules26072072
Teow, S.-Y., Wong, M., Yap, H.-Y., Peh, S.-C., and Shameli, K. (2018). Bactericidal properties of plants-derived metal and metal oxide nanoparticles (NPs). Molecules 23 (6), 1366. doi:10.3390/molecules23061366
Thakkar, K. N., Mhatre, S. S., and Parikh, R. Y. (2010). Biological synthesis of metallic nanoparticles. Nanomedicine Nanotechnol. Biol. Med. 6 (2), 257–262. doi:10.1016/j.nano.2009.07.002
Vural, H., Demirin, H., Kara, Y., Eren, I., and Delibas, N. (2010). Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. J. Trace Elem. Med. Biol. 24 (3), 169–173. doi:10.1016/j.jtemb.2010.02.002
Wahab, R., Siddiqui, M. A., Saquib, Q., Dwivedi, S., Ahmad, J., Musarrat, J., et al. (2014). ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surfaces B Biointerfaces 117, 267–276. doi:10.1016/j.colsurfb.2014.02.038
Wang, G., Tang, K., Meng, Z., Liu, P., Mo, S., Mehrjou, B., et al. (2020). A quantitative bacteria monitoring and killing platform based on electron transfer from bacteria to a semiconductor. Adv. Mater. 32 (39), e2003616. doi:10.1002/adma.202003616
Wang, L., Hu, C., and Shao, L. (2017a). The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomedicine 12, 1227–1249. doi:10.2147/IJN.S121956
Wang, Y., Ma, J., Xu, Q., and Zhang, J. (2017b). Fabrication of antibacterial casein-based ZnO nanocomposite for flexible coatings. Mater. and Des. 113, 240–245. doi:10.1016/j.matdes.2016.09.082
Wang, Y., Zhang, C., Bi, S., and Luo, G. (2010). Preparation of ZnO nanoparticles using the direct precipitation method in a membrane dispersion micro-structured reactor. Powder Technol. 202 (1–3), 130–136. doi:10.1016/j.powtec.2010.04.027
Wangoye, K., Mwesigye, J., Tungotyo, M., and Twinomujuni Samba, S. (2022). Chronic wound isolates and their minimum inhibitory concentrations against third generation cephalosporins at a tertiary hospital in Uganda. Sci. Rep. 12 (1), 1195. doi:10.1038/s41598-021-04722-6
Wiesmann, N., Tremel, W., and Brieger, J. (2020). Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J. Mater. Chem. B 8 (23), 4973–4989. doi:10.1039/D0TB00739K
Wojnarowicz, J., Chudoba, T., and Lojkowski, W. (2020). A review of microwave synthesis of zinc oxide nanomaterials: reactants, process parameters and morphologies. Nanomaterials 10 (6), 1086. doi:10.3390/nano10061086
Wu, J. M., and Kao, W. T. (2015). Heterojunction nanowires of AgxZnxO–ZnO photocatalytic and antibacterial activities under visible-light and dark conditions. J. Phys. Chem. C 119 (3), 1433–1441. doi:10.1021/jp510259j
Wu, S., Xu, J., Zou, L., Luo, S., Yao, R., Zheng, B., et al. (2021). Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 12 (1), 3303. doi:10.1038/s41467-021-23069-0
Xie, B., Guo, R. s., Yang, X. w., Wan, L., Yao, W. y., Lai, Q., et al. (2020). Epidemiology and drug resistance analysis of mixed infection in orthopedic surgical sites. Surg. Infect. 21 (5), 465–471. doi:10.1089/sur.2019.276
Xiong, H. (2013). ZnO nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 25 (37), 5329–5335. doi:10.1002/adma.201301732
Xu, J.-W., Cui, Z.-M., Liu, Z.-Q., Xu, F., Chen, Y.-S., and Luo, Y.-L. (2019). Organic–inorganic nanohybrid electrochemical sensors from multi-walled carbon nanotubes decorated with zinc oxide nanoparticles and in-situ wrapped with poly(2-methacryloyloxyethyl ferrocenecarboxylate) for detection of the content of food additives. Nanomaterials 9 (10), 1388. doi:10.3390/nano9101388
Yavaş, A., Güler, S., Onak, G., Erol, M., Torman Kayalar, M., Karaman, O., et al. (2022). Li-doped ZnO nanowires on flexible carbon fibers as highly efficient hybrid antibacterial structures. J. Alloys Compd. 891, 162010. doi:10.1016/j.jallcom.2021.162010
Yu, J., Tahmasebi, A., Han, Y., Yin, F., and Li, X. (2013). A review on water in low rank coals: the existence, interaction with coal structure and effects on coal utilization. Fuel Process. Technol. 106, 9–20. doi:10.1016/j.fuproc.2012.09.051
Zeng, J., Ren, L., Yuan, Y., Wang, Y., Zhao, J., Zeng, R., et al. (2013). Short-term effect of magnesium implantation on the osteomyelitis modeled animals induced by Staphylococcus aureus. J. Mater. Sci. Mater. Med. 24 (10), 2405–2416. doi:10.1007/s10856-013-4982-6
Zhang, H., Chen, W.-G., Li, Y.-Q., and Song, Z.-H. (2018). Gas sensing performances of ZnO hierarchical structures for detecting dissolved gases in transformer oil: a mini review. Front. Chem. 6, 508. doi:10.3389/fchem.2018.00508
Zhang, H.-J., Xiong, H.-M., Ren, Q.-G., Xia, Y.-Y., and Kong, J.-L. (2012). ZnO@silica core–shell nanoparticles with remarkable luminescence and stability in cell imaging. J. Mater. Chem. 22 (26), 13159. doi:10.1039/c2jm30855j
Zhang, J., Li, Y., Zhang, B., Wang, H., Xin, Q., and Song, A. (2015). Flexible indium–gallium–zinc–oxide Schottky diode operating beyond 2.45 GHz. Nat. Commun. 6 (1), 7561. doi:10.1038/ncomms8561
Zhang, L., Ding, Y., Povey, M., and York, D. (2008). ZnO nanofluids – a potential antibacterial agent. Prog. Nat. Sci. 18 (8), 939–944. doi:10.1016/j.pnsc.2008.01.026
Zhang, L., Jiang, Y., Ding, Y., Povey, M., and York, D. (2007). Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 9 (3), 479–489. doi:10.1007/s11051-006-9150-1
Zhao, C., Zhang, X., and Zheng, Y. (2018). Biosynthesis of polyphenols functionalized ZnO nanoparticles: characterization and their effect on human pancreatic cancer cell line. J. Photochem. Photobiol. B Biol. 183, 142–146. doi:10.1016/j.jphotobiol.2018.04.031
Zhao, R., Liang, H., Clarke, E., Jackson, C., and Xue, M. (2016). Inflammation in chronic wounds. Int. J. Mol. Sci. 17 (12), 2085. doi:10.3390/ijms17122085
Keywords: bacteria, zinc oxide nanoparticles, antibacterial mechanisms, material synthesis, green synthesis
Citation: Nan J, Chu Y, Guo R and Chen P (2024) Research on the antibacterial properties of nanoscale zinc oxide particles comprehensive review. Front. Mater. 11:1449614. doi: 10.3389/fmats.2024.1449614
Received: 15 June 2024; Accepted: 13 August 2024;
Published: 23 September 2024.
Edited by:
Jiajun Fan, University of York, United KingdomReviewed by:
Laouini Salah Eddine, El-Oued University, AlgeriaRene García Contreras, National Autonomous University of Mexico, Mexico
Copyright © 2024 Nan, Chu, Guo and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhui Chu, Y2h1eWFuaHVpQG1kam11LmVkdS5jbg==
 Jiahe Nan
Jiahe Nan Yanhui Chu
Yanhui Chu Ran Guo
Ran Guo Peijian Chen
Peijian Chen