- 1Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China
- 2Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing, China
- 3State Key Laboratory of Catalysis, Dalian, China
Palladium is a prototypical hydride-forming metal, which can accommodate a large volume of hydrogen through the formation of either interstitial or complex hydrides. Interstitial palladium hydrides, especially those with exceptionally high hydrogen loadings, have attracted considerable interest from the low-energy nuclear reaction (LENR) community, as they have been invoked to explain the anomalous nuclear effects related to the known but controversial Pons-Fleischmann experiment. Complex palladium hydrides also constitute a class of solid-state hydrides that present stoichiometric PdH2, PdH3, or PdH4 units within the crystal structure, but remain unexplored as far as the unusual H/Pd ratio is concerned. This minireview gives a brief introduction to these two types of solid-state palladium hydrides, with the hope of providing some information for materials development relevant to LENR research.
1 Introduction
In March 1989, Stanley Pons and Mattin Fleischmann announced that they had seen signs of room-temperature nuclear fusion when they electrolyzed deuterated lithium hydroxide (LiOD) in heavy water (D2O) using palladium electrodes (Fleischmann et al., 1989). Such a phenomenon, known as cold fusion, raised hopes of a cheap and abundant source of energy, but also triggered strong skepticism because nuclear fusion is thought to happen only at temperatures up to tens of million degrees such as in the Sun. A lack of details in their experiment, on the other hand, frustrated efforts to replicate this work. Despite numerous endeavors from laboratories worldwide, there is, unfortunately, no sufficient evidence to support the existence of cold fusion. Some scholars believed that loading the palladium cathode with plenty of deuterium is a necessary precursor to trigger fusion, under the hypothesis that the deuterium atoms could be squeezed into palladium lattice to help them fuse under normal conditions (Storms, 2002; Storms, 2003). In the 1990s, McKubre (2015), who led one of the largest projects on cold fusion, suggested that only when the palladium cathode was saturated with hydrogen beyond a threshold of H/Pd > 0.875 could the nuclear effect be observed. Such an influence of H/Pd ratio on excess energy production was also reported by Kunimatsu et al. These studies infer that the failure to meet the threshold conditions is a major reason for the unsuccessful replication. However, there are also studies arguing that the factors preventing replication of the Pons-Fleischmann experiment were associated with the properties of the bulk palladium and impurities within the electrolyte, and the nuclear-active environment is complicated that contains certain impurities such as lithium, oxygen, and perhaps no palladium at all (Storms, 2002). It was proposed that the deposition of various impurities makes the surface a complex alloy, which forms a suitable structure capable of storing a large amount of deuterium (Storms, 2003). Unfortunately, neither the structure nor the composition of such alloy hydrides has been identified so far. Almost 30 years after the original event, Google gathered a group of scientists from University of British Columbia, Massachusetts Institute of Technology, University of Maryland, and Lawrence Berkeley National Laboratory to revisit the case of cold fusion (now rebranded as low-energy nuclear reactions, LENR), wherein learning how to create, characterize, and sustain highly hydrided palladium is a priority in their research (Berlinguette et al., 2019). The experimental findings again concluded no credible evidence that cold fusion is possible. Nevertheless, they manifested that there are still some fascinating aspects to the materials science of palladium-hydrogen system, and palladium hydride materials with a larger H/Pd ratio remain interesting in the LENR research.
Apart from the binary or alloy interstitial palladium deuterides that may form in Pons-Fleischmann experiment, we, on the other hand, wonder whether complex palladium deuteride such as Li2PdD2 could form under the electrochemical condition where LiOD, D2O, and Pd are present. From electrochemical point of view, the reaction (1) would need a driving force of ca. −1.91 V at ambient conditions, which is lower than that of the reaction (2).
Despite having unusual H/Pd ratios (H/Pd = 2), such a complex hydride Li2PdD2 has received far less attention in the LENR-related research. Complex palladium hydrides are composed of palladium hydride complex anions and alkali/alkaline earth/rare earth metal cations, wherein a number of H atoms bind to the palladium center covalently to form hydride complexes with a variety of H-coordination modes including PdH2, PdH3, and PdH4 (Yvon and Renaudin, 2006). Very recently, this type of solid-state Pd hydrides has been found highly active in catalyzing semi-hydrogenation of alkylenes to alkenes (Guo et al., 2021), as well as in promoting dinitrogen fixation and ammonia synthesis (Yan et al., 2021).
In this context, a survey of various palladium-based hydrides might provide some implications on the materials exploration for nuclear fusion. Excellent reviews have been published detailing the synthesis, structures, and properties of the sub-stoichiometric PdHx (x < 1), which readers may like to refer to (Flanagan and Oates, 1991; Jewell and Davis, 2006; Setayandeh et al., 2020). The focus of this minireview is on the solid-state palladium hydrides with a H/Pd ratio equal to or higher than 1, which are of fundamental interest in fusion reactions. Herein, the advancements in the research of both interstitial and complex palladium hydrides are briefly accounted, mainly from the aspects of formation, bonding, structure, and stability.
2 Interstitial palladium hydrides
Interstitial hydrides generally derive from hydrogenation of metals or alloys, in which hydrogen atoms randomly occupy the interstitial positions of the metal lattice. Except for an expansion and an occasional distortion of the lattice, hydrogenation usually leaves the metallic matrix intact (Latroche, 2004). They are also known as metallic hydrides considering that the nature of metal-hydrogen bond is generally considered to be metallic. In contrast to the covalent hydrides, metallic hydrides often have non-stoichiometric compositions and disordered structures, which preclude local H configurations and reliable interatomic distances from being determined by conventional experimental methods. Therefore, the bonding properties of metallic hydrides are less well characterized, but can be learned in depth by combing with theoretical calculations. Palladium is a prototypical hydride-forming metal which absorbs hydrogen at ambient conditions, forming fcc palladium hydride limited to a stoichiometry of PdHx (x ≈ 0.7) (Lewis, 1982; Flanagan and Oates, 1991; Klotz and Mattson, 2009). Further absorption of hydrogen to create highly hydrided palladium is challenging as increasing repulsive interactions of Pd-H would diminish the hydrogen capacity (Borgschulte et al., 2020). As a matter of fact, an exponential increase in hydrogen pressure is required to achieve a hydrogen content larger than 0.7. For instance, the equilibrium hydrogen pressure for the formation of the stoichiometric PdH was estimated in the gigapascal (GPa) range. (Lewis, 1982; Berlinguette et al., 2019). There are very few studies that provide convincing experimental evidence for a bulk loading of x ≥ 1 in the binary PdHx (Möller et al., 1982; Fukai and Ōkuma, 1993; Fukai and Ōkuma, 1994), mainly because it is difficult to be synthesized and characterized accurately without specialized laboratory facilities.
High-pressure techniques are often needed to produce and sustain highly hydrided palladium. Recent studies have shown that, under sufficiently high pressures, the palladium matrix is capable of dissolving more hydrogen atoms, reaching a stoichiometry of PdH∼1 at around 2 GPa and room temperature (Brownsberger et al., 2017; Guigue et al., 2020; Geballe et al., 2021). The formed PdH∼1 was suggested to crystallize in the NaCl-typed structure, with hydrogen atoms filling the octahedral sites of the fcc Pd lattice. However, further compression did not change the loading content, even under a hydrogen pressure up to 100 GPa (Guigue et al., 2020). High pressure has also been combined with high temperature to generate metastable forms of palladium hydrides with an H/Pd ratio larger than 1. By heating Pd to 700 °C in a hydrogen atmosphere of 5 GPa, a stoichiometry of PdH∼1.33 was observed in the form of Cu3Au-typed structure with ordered Pd vacancies (Fukai and Ōkuma, 1994). The formation of this superabundant vacancy phase has been confirmed by later studies, but the exact position of the hydrogen atoms is uncertain and presumed to occupy the octahedral sites (Dos Santos et al., 1999). Electrochemical loading is another effective way to produce PdHx. In theory, a modest applied potential rather than high-pressure hydrogen gas is required to achieve a high hydrogen concentration (Maoka and Enyo, 1981; Benck et al., 2019). In a recent study, the effects of Pd cathode thickness, electrolyte type, and temperature on the hydrogen loading content have been systematically investigated (Benck et al., 2019). By using reliable techniques to quantify the H content, the study concluded that the maximum H/Pd ratio achievable under ambient conditions is 0.96 ± 0.02, and it is difficult to reach exceptionally high hydrogen loading levels due to competing hydrogen desorption reactions. A very recent work has claimed the formation of PdHx with x up to 1.97 by means of electrolytic charging at a high negative potential of ca. −2 eV vs. SHE (Fukumuro et al., 2020). Ion implantation of deuterium into palladium was also reported to produce over-stoichiometric palladium deuterides at cryogenic temperatures (Möller et al., 1982; Myers et al., 1991). However, these results have yet to be independently confirmed.
Obviously, the hydrogen content of the known PdHx is much below the number of interstitial sites in the palladium lattice that can be accessible by hydrogen. Higher hydrides are in principle possible through occupation of the tetrahedral other than the octahedral sites. Despite the lack of credible experimental evidence, there are several theoretical studies predicting the stability and structures of highly hydrided palladium such as stoichiometric PdH, PdH2, and PdH3 (Houari et al., 2014; Yang et al., 2017; Long et al., 2018; Liu et al., 2020). The monohydride PdH is considered in either NaCl-typed fcc structure with hydrogen atoms filling all of the octahedral positions, or CaF2-typed fcc structure with hydrogen atoms filling half of the tetrahedral positions. Under high-pressure conditions, the NaCl-typed PdH has been identified as the ground state, consistent with the experimental finding. The hypothetical dihydride PdH2 is also stabilized in a fcc structure with hydrogen atoms occupying all tetrahedral positions. Formation of PdH2 has been calculated to be thermodynamically favorable at hydrogen pressures above 2.1 GPa, but was not observed experimentally even up to 100 GPa (Guigue et al., 2020). When all interstitial sites of Pd lattice are embedded by hydrogen atoms, a fcc PdH3 can be created. However, such a fcc structure was recently suggested to be energetically less stable than a hcp structure (Yang et al., 2017). Moreover, the possibility of synthesizing palladium superhydrides (PdH8, PdH10, PdH12, etc.) has been evaluated using DFT calculations. It is suggested that, by combining pressure and electrochemistry, superhydride PdH10 is likely to be formed in a structure that consists of undissociated H-H pairs (Guan et al., 2021). Another point to be noted here is that the incorporation of hydrogen into Pd lattice may induce superconductivity, and the palladium superhydrides have attracted great interest in the research of high-temperature superconductors (Setayandeh et al., 2020).
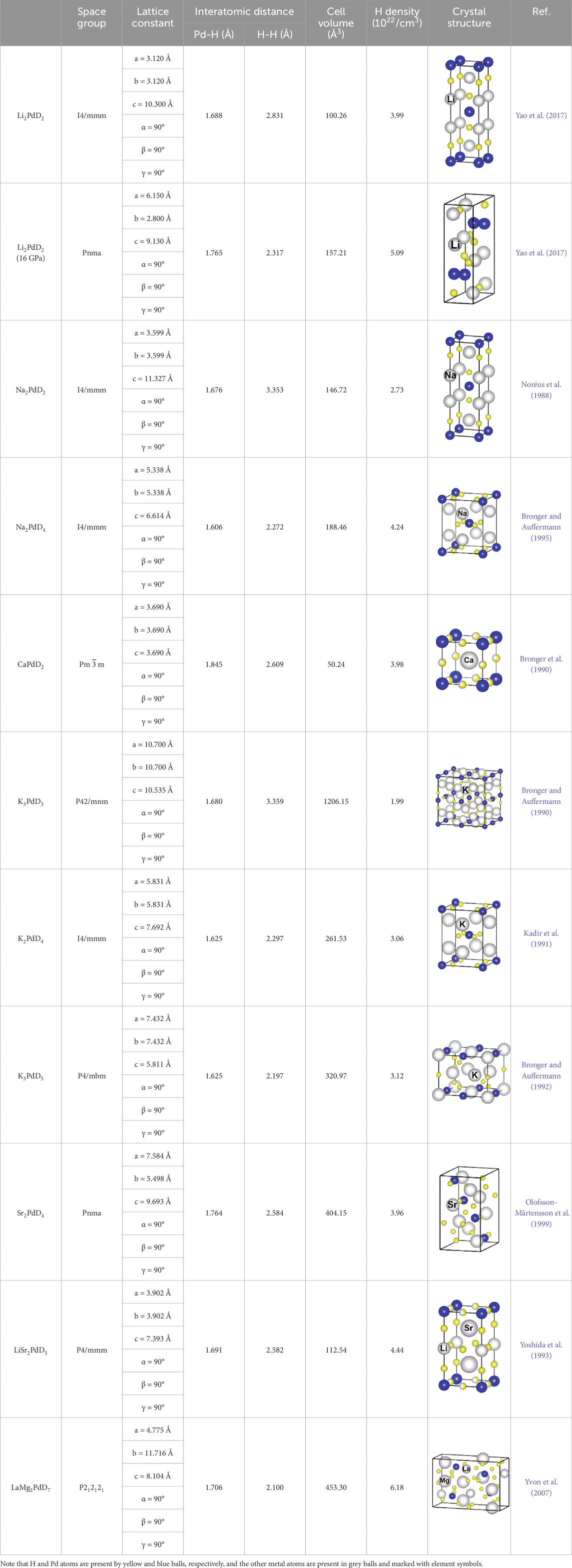
The structural information of some selected PdHx varieties is summarized in Table 1. It is clearly seen that the insertion of hydrogen leads to an expansion of the Pd lattice, and high loading levels may cause lattice distortion relative to the original fcc structure of Pd. For the PdHx (x = 1–3) series with the same fcc structure, there is a decrease in interatomic H-H distance and an increase in Pd-Pd distance as the H/Pd ratio varies from 1 to 3. In the case of fcc PdH3, the interatomic H-H distance is as low as 2.033 Å, and the volumetric density of hydrogen reaches up to 11.6 × 1022 atom/cm3. Besides, the impact of hydrogen insertion on the electronic properties has been evaluated by theoretical simulations. A systematic analysis on the electronic structures of PdHx (x = 1–3) has revealed that the insertion of hydrogen does not alter the metallic character of palladium, but results in a reduction of the density of states (DOS) at the Fermi level (Houari et al., 2014; Yang et al., 2017; Long et al., 2018). It is predicted that PdH2 shows semimetallic properties because the DOS around Fermi level is very close to zero. Moreover, for all the PdHx (x = 1–3) considered, the occupied states below the Fermi level are mainly contributed by the Pd 4d electrons, with a small H 1s component at the deeply lower energy parts.
It is known that the hydrogen absorption properties of palladium can be tuned by alloying with other metal elements (Dekura et al., 2019). To date, a few palladium matrix alloys have been investigated revealing enhanced hydrogen capacity relative to pure Pd, and hence attracted attention in attempting to synthesizing hydrides with a higher hydrogen content. A representative example is the palladium-rhodium (Pd-Rh) alloy system, where the formation of monohydride PdxRh1-xH and dihydride PdxRh1-xH2 has been observed recently (Kuzovnikov and Tkacz, 2017). When compressed in a hydrogen atmosphere, the fcc Pd-Rh alloys can absorb hydrogen and expand unit-cell volume to form fcc monohydrides at around 2 GPa and fcc dihydrides at around 10 GPa. Based on the simulated structures, the hydrogen atoms tend to occupy the octahedral sites for monohydrides and the tetrahedral sites for dihydrides (Yang et al., 2017; Yang et al., 2018). The structural information of two typical examples, i.e., the monohydride Pd0.25Rh0.75H and the dihydride Pd0.25H0.75H2, is listed in Table 1. Palladium-lithium alloy system is also known to present impressive hydrogen capacities, and the formation of nearly stoichiometric LiPdH∼1 has been indicated in early reports either by sintering LiPd alloy under 270 MPa of H2 or by reacting an equimolar mixture of LiH and Pd under 1 MPa of H2 and 340°C (Noréus and Rapp, 1990; Schirber et al., 1991). Recently, high-pressure technique has been applied to the lithium-palladium-hydrogen system, but there is no conclusive evidence for the formation of LiPdHx with x > 1 at pressures up to ∼10 GPa (Liu et al., 2018; Frost et al., 2022). As shown in Table 1, the stoichiometric LiPdH is arranged in a tetragonal structure which consists of planar PdH units separated by Li atoms. The calculations on electronic structure indicated that LiPdH presents metallic behaviors with large electronic states at the Fermi energy.
3 Complex palladium hydrides
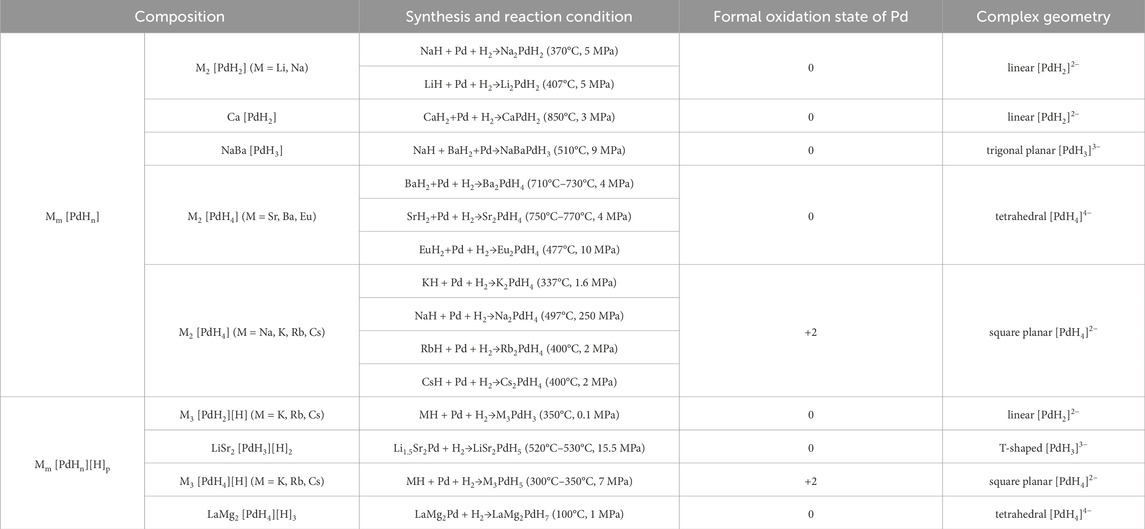
Most of the known metallic hydrides have a hydrogen-to-metal ratio less than two, while in complex metal hydrides, a higher ratio is quite common. Complex metal hydrides represent a class of hydrogen-rich compounds (Yvon, 2003; Yvon and Renaudin, 2006). They derive their name from the presence of covalently bonded metal hydride complexes within the crystal structure that are often centered by d-block metal elements. The complexes are anionic and stabilized by surrounding alkali, alkaline earth, or rare earth metal cations. These hydride compounds, in most cases, contain hydrogen bonded to TM elements only and has the general formula Mm [TMHn] (TM = 3 days, 4 days, or 5 days elements; M = alkali, alkaline-earth, or rare-earth elements), while some also have additional hydride ions coordinated to the metal cations Mδ+, corresponding to the formula Mm [TMHn][H]p. In contrast to the interstitial hydrides mentioned above, complex hydrides have covalent bonded hydrogens within the [TMHn] complexes, and usually exhibit stoichiometric compositions, ordered structures, and non-metallic properties at ambient conditions. Because of their rich composition, diverse structure, flexible bonding environment, and unique configuration chemistry, complex hydrides exhibit a range of fascinating properties and functionalities that can be exploited in the research fields such as hydrogen storge, thermal energy storage, superconductor, optical sensing, and catalysis (Møller et al., 2017; He et al., 2019; Takagi and Orimo, 2020; Wang et al., 2023).
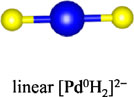
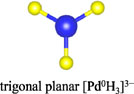
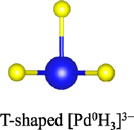
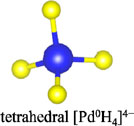
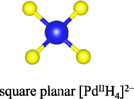
Among the numerous complex metal hydrides reported up to now, the palladium series show variable structural features as palladium element forms hydride complexes with different formal oxidation states and H-coordination geometries, encompassing linear [Pd0H2]2−, trigonal planar or T-shaped [Pd0H3]3−, tetrahedral [Pd0H4]4−, and square planar [PdIIH4]2− (Yvon, 2003; Parker, 2010). Moreover, some of these compounds are capable of incorporating additional ionic hydrides within the lattice, which further enriches their compositions and structures. The general compositions, structures, and synthesis of the known complex palladium hydrides are summarized in Table 2. The most common route for synthesizing the Pd complex hydrides is to react Pd metal with a binary hydride of the alkali, alkaline earth, or rare earth metal element under hydrogen pressures (0.1–250 MPa) and elevated temperatures (100°C–850°C). Exceptions are LiSr2PdH5 and LaMg2PdH7 that derive from hydrogenation of the parent alloys (Yoshida et al., 1993; Yvon et al., 2007). And in general, an increase of hydrogen pressure is required to synthesize complex Pd hydrides with a higher oxidation state. Examples are found for palladium in M3Pd0H3 and M3PdIIH5, which form at 1 and 70 bar, respectively.
Table 3 shows the crystal structure information of some representative complex palladium hydrides, which is mainly determined by X-ray and neutron powder diffraction on deuterides. As observed, there exists a wide range of crystal structure types, but one common feature is the presence of ordered [PdDn] units separated by a cationic sublattice. For each structure, the Pd-D bond length as well as the shortest D-D distance are stated in Table 3. It is seen that the Pd-D bond lengths range between 1.60 and 1.70 Å, and the D-D distance usually exceeds 2.0 Å with the shortest value occurring in LaMg2PdH7. Unlike the interstitial palladium hydrides, there are few reports about the behavior of complex hydrides under extreme conditions. In a very recent work, the structural characteristics of Li2PdD2 was studied under pressures up to 50 GPa, and a clear phase transition from tetragonal to monoclinic structure was observed at around 10 GPa (Yao et al., 2017). Of particular interest is the high-pressure structure of Li2PdD2, in which the [PdD2] units are connected via extended chains. As a consequence, the H-H distance is significantly reduced, and the hydrogen density increases to a value of 5.09×1022/cm3 which is comparable to that of the monohydride PdH.
The complex hydrides present a mixed ionic-covalent bonding character, as there are not only covalent TM-H interactions within [TMHn] units but also ionic interactions between [TMHn] and surrounding electropositive metals. Assuming the full charge transfer from the surrounding cation matrix, the bonding features of [TMHn] complexes can be simply interpreted based on the conventional electron counting rules and s-p-d hybridization schemes. In terms of the Pd series, there are different bonding configurations including (d10)sp3 for 18-electron [PdH4]4−, (d8)dsp2 for 16-electron [PdH4]2−, (d10)sp2 for 16-electron [PdH3]3−, and (d10)sp for 14-electron [PdH2]2− (Yvon, 2003). To obtain a better description of the bonding nature within the palladium complex, the electronic structure of these hydrides has been calculated (Olofsson-Mårtensson et al., 2000; Orgaz, 2007; Parker, 2010; Kiruthika et al., 2023). One noticeable bonding feature is the existence of substantial covalency between the complex hydrogen ligands and the surrounding metal cations (Olofsson-Mårtensson et al., 2000). Such strong M-H interactions contribute to the stabilization of palladium complex hydrides through distributing electron density away from the Pd center. Besides, the DOS studies suggested that the complex palladium hydrides often show nonmetallic properties with the band gap ranging from 0.36 to 3.03 eV. Notable exceptions are Li2PdH2 and Na2PdH2 that are metallic, at least in certain directions.
4 Conclusion
In this minireview, we describe the compositional and structural characteristics of interstitial and complex Pd hydrides, both of which show promise in accommodating large concentrations of hydrogen. By comparison, interstitial hydrides have been gained much more attention from the LENR community whereas complex hydrides have much less. It is our hope that such a survey of various kinds of solid-state palladium hydrides would contribute to materials exploration for LENR-related research, which in turn would advance the understandings of the possible relevance of palladium hydrides to the nuclear effects. In a broad sense, insights into the fundamentals of palladium hydrides especially those with unusual H/Pd ratios would be instructive to revealing their latent properties and functionalities that can be exploited towards other research fields. As a typical example, available studies have manifested the potential of highly hydrided palladium in high-temperature conductivity (Meninno and Errea, 2023).
Author contributions
QW: Writing–original draft, Writing–review and editing. SZ: Writing–original draft. JG: Writing–original draft. PC: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors are grateful for the financial support from the National Natural Science Foundation of China (22379139, 22202195, and 21988101) and the Liaoning Revitalization Talents Program (XLYC2002076 and XLYC2007173).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Benck, J. D., Jackson, A., Young, D., Rettenwander, D., and Chiang, Y.-M. (2019). Producing high concentrations of hydrogen in palladium via electrochemical insertion from aqueous and solid electrolytes. Chem. Mater. 31, 4234–4245. doi:10.1021/acs.chemmater.9b01243
Berlinguette, C. P., Chiang, Y.-M., Munday, J. N., Schenkel, T., Fork, D. K., Koningstein, R., et al. (2019). Revisiting the cold case of cold fusion. Nature 570, 45–51. doi:10.1038/s41586-019-1256-6
Borgschulte, A., Terreni, J., Billeter, E., Daemen, L., Cheng, Y., Pandey, A., et al. (2020). Inelastic neutron scattering evidence for anomalous H–H distances in metal hydrides. Proc. Natl. Acad. Sci. 117, 4021–4026. doi:10.1073/pnas.1912900117
Bronger, W., and Auffermann, G. (1990). K3PdH3, ein komplexes hydrid mit linearen [PdH22−]-baugruppen. J. Less Common Metals 158, 163–167. doi:10.1016/0022-5088(90)90443-N
Bronger, W., and Auffermann, G. (1992). Darstellung und struktur ternärer palladiumhydride A3PdH5 mit A=K, Rb und Cs. J. Alloys Compd. 187, 81–85. doi:10.1016/0925-8388(92)90523-C
Bronger, W., and Auffermann, G. (1995). High pressure synthesis and structure of Na2PdH4. J. Alloys Compd. 228, 119–121. doi:10.1016/0925-8388(95)01670-8
Bronger, W., Jansen, K., and Müller, P. (1990). CaPdH2, ein ternäres hydrid mit perowskitverwandter struktur. J. Less Common Metals 161, 299–302. doi:10.1016/0022-5088(90)90040-Q
Brownsberger, K., Ahart, M., Somayazulu, M., Park, C., Gramsch, S. A., and Hemley, R. J. (2017). X-ray diffraction, lattice structure, and equation of state of PdHx and PdDx to megabar pressures. J. Phys. Chem. C 121, 27327–27331. doi:10.1021/acs.jpcc.7b09290
Dekura, S., Kobayashi, H., Kusada, K., and Kitagawa, H. (2019). Hydrogen in palladium and storage properties of related nanomaterials: size, shape, alloying, and metal-organic framework coating effects. ChemPhysChem 20, 1158–1176. doi:10.1002/cphc.201900109
Dos Santos, D., Miraglia, S., and Fruchart, D. (1999). A high pressure investigation of Pd and the Pd–H system. J. Alloys Compd. 291, 1–5. doi:10.1016/s0925-8388(99)00281-9
Flanagan, T. B., and Oates, W. (1991). The palladium-hydrogen system. Annu. Rev. Mater. Sci. 21, 269–304. doi:10.1146/annurev.ms.21.080191.001413
Fleischmann, M., Pons, S., and Hawkins, M. (1989). Electrochemically induced nuclear fusion of deuterium. J. Electroanal. Chem. Interfacial Electrochem 261, 301–308. doi:10.1016/0022-0728(89)80006-3
Frost, M., McBride, E. E., Smith, J. S., and Glenzer, S. H. (2022). The high-pressure lithium–palladium and lithium–palladium–hydrogen systems. Sci. Rep. 12, 12341. doi:10.1038/s41598-022-16694-2
Fukai, Y., and Ōkuma, N. (1994). Formation of superabundant vacancies in Pd hydride under high hydrogen pressures. Phys. Rev. Lett. 73, 1640–1643. doi:10.1103/physrevlett.73.1640
Fukai, Y. F. Y., and Ōkuma, N. Ō. N. (1993). Evidence of copious vacancy formation in Ni and Pd under a high hydrogen pressure. Jpn. J. Appl. Phys. 32, L1256–L1259. doi:10.1143/jjap.32.l1256
Fukumuro, N., Fukai, Y., Sugimoto, H., Ishii, Y., Saitoh, H., and Yae, S. (2020). Superstoichiometric hydride PdHx≤ 2 formed by electrochemical synthesis: dissolution as molecular H2 proposed. J. Alloys Compd. 825, 153830. doi:10.1016/j.jallcom.2020.153830
Geballe, Z. M., Somayazulu, M., Armanet, N., Mishra, A. K., Ahart, M., and Hemley, R. J. (2021). High-pressure synthesis and thermodynamic stability of PdH1±ε up to 8 GPa. Phys. Rev. B 103, 024515. doi:10.1103/physrevb.103.024515
Guan, P.-W., Hemley, R. J., and Viswanathan, V. (2021). Combining pressure and electrochemistry to synthesize superhydrides. Proc. Natl. Acad. Sci. 118, 70118. doi:10.1073/pnas.2110470118
Guigue, B., Geneste, G., Leridon, B., and Loubeyre, P. (2020). An x-ray study of palladium hydrides up to 100 GPa: synthesis and isotopic effects. J. Appl. Phys. 127, 075901. doi:10.1063/1.5138697
Guo, Q., Chen, R., Guo, J., Qin, C., Xiong, Z., Yan, H., et al. (2021). Enabling semihydrogenation of alkynes to alkenes by using a calcium palladium complex hydride. J. Am. Chem. Soc. 143, 20891–20897. doi:10.1021/jacs.1c09489
Häglund, J., Guillermet, A. F., Grimvall, G., and Körling, M. (1993). Theory of bonding in transition-metal carbides and nitrides. Phys. Rev. B 48, 11685–11691. doi:10.1103/PhysRevB.48.11685
He, T., Cao, H., and Chen, P. (2019). Complex hydrides for energy storage, conversion, and utilization. Adv. Mater. 31, 1902757. doi:10.1002/adma.201902757
Houari, A., Matar, S. F., and Eyert, V. (2014). Electronic structure and crystal phase stability of palladium hydrides. J. Appl. Phys. 116, 173706. doi:10.1063/1.4901004
Jewell, L. L., and Davis, B. H. (2006). Review of absorption and adsorption in the hydrogen–palladium system. Appl. Catal. A-GEN 310, 1–15. doi:10.1016/j.apcata.2006.05.012
Kadir, K., Kritikos, M., Noréus, D., and Andresen, A. (1991). Metallic properties in the series K2Pd(II)H4, Na2Pd(0)H2 and Li2Pd(0)H2 correlated with the stabilization of a formally zero-valent palladium-hydrogen complex. J. Less Common Metals 172, 36–41. doi:10.1016/0022-5088(91)90430-C
Kiruthika, S., Sundar, P., and Ravindran, P. (2023). Structural phase stability and thermodynamical properties of transition metal complex hydrides Na2MgTMH7 (TM= Sc− Cu) for hydrogen storage applications. J. Solid State Chem. 321, 123867. doi:10.1016/j.jssc.2023.123867
Klotz, E., and Mattson, B. (2009). Hydrogen and palladium foil: two classroom demonstrations. J. Chem. Educ. 86, 465. doi:10.1021/ed086p465
Kuzovnikov, M., and Tkacz, M. (2017). Dihydride formation in the palladium–rhodium alloys under high hydrogen pressure. Int. J. Hydrogen Energy 42, 340–346. doi:10.1016/j.ijhydene.2016.11.133
Latroche, M. (2004). Structural and thermodynamic properties of metallic hydrides used for energy storage. J. Phys. Chem. Solids 65, 517–522. doi:10.1016/j.jpcs.2003.08.037
Lewis, F. (1982). “The palladium-hydrogen system,” in Platinum met. Rev. (London, United Kingdom: Johnson Matthey Press), 20–27.
Liu, W., Wang, E., Chen, G., Zhu, X., Zhang, Y., Sheng, Y., et al. (2018). Absence of superconductivity in LiPdHx. Philos. Mag. 98, 623–631. doi:10.1080/14786435.2017.1411624
Liu, Z., Ahuja, R., Li, H., and Luo, W. (2020). Mechanical and electronic properties of van der Waals layered hcp PdH2. Sci. Rep. 10, 8037. doi:10.1038/s41598-020-61385-5
Long, D., Li, M., Meng, D., He, Y., Yoon, I. T., Ahuja, R., et al. (2018). Accounting for the thermo-stability of PdHx (x= 1–3) by density functional theory. Int. J. Hydrogen Energy 43, 18372–18381. doi:10.1016/j.ijhydene.2018.08.030
Maoka, T., and Enyo, M. (1981). Hydrogen absorption by palladium electrode polarized in sulfuric acid solution containing surface active substances—I. The cathodic region. Electrochim. Acta 26, 607–614. doi:10.1016/0013-4686(81)80027-8
McKubre, M. C. H. (2015). Cold fusion: comments on the state of scientific proof. Curr. Sci. 108, 495–498.
Meninno, A., and Errea, I. (2023). Ab initio study of metastable occupation of tetrahedral sites in palladium hydrides and its impact on superconductivity. Phys. Rev. B 107, 024504. doi:10.1103/PhysRevB.107.024504
Møller, K. T., Sheppard, D., Ravnsbæk, D. B., Buckley, C. E., Akiba, E., Li, H.-W., et al. (2017). Complex metal hydrides for hydrogen, thermal and electrochemical energy storage. Energies 10, 1645. doi:10.3390/en10101645
Möller, W., Besenbacher, F., and Bottiger, J. (1982). Saturation and isotope mixing during low-temperature implantations of hydrogen into metals. Appl. Phys. A 27, 19–29. doi:10.1007/BF01197542
Myers, S., Richards, P., Follstaedt, D., and Schirber, J. (1991). Superstoichiometry, accelerated diffusion, and nuclear reactions in deuterium-implanted palladium. Phys. Rev. B 43, 9503–9510. doi:10.1103/physrevb.43.9503
Noréus, D., and Rapp, Ö. (1990). Absence of superconductivity above 4 K in LiPdHx. Phys. Rev. B 42, 10730–10731. doi:10.1103/physrevb.42.10730
Noréus, D., Törnroos, K., Börje, A., Szabo, T., Bronger, W., Spittank, H., et al. (1988). Na2PdH2, a hydride with a novel linear [PdH2] complex. J. Less Common Metals 139, 233–239. doi:10.1016/0022-5088(88)90004-5
Olofsson-Mårtensson, M., Häussermann, U., Tomkinson, J., and Noréus, D. (2000). Stabilization of electron-dense Palladium− Hydrido complexes in solid-state hydrides. J. Am. Chem. Soc. 122, 6960–6970. doi:10.1021/ja994089o
Olofsson-Mårtensson, M., Kritikos, M., and Noréus, D. (1999). A novel tetrahedral formally zerovalent-palladium hydrido complex stabilized by divalent alkaline earth counterions. J. Am. Chem. Soc. 121, 10908–10912. doi:10.1021/ja991047r
Orgaz, E. (2007). Electronic structure of the complex alkali-transition-metal ternary hydrides A2TH4 (A= Na, K; T= Pd, Pt). Phys. Rev. B 76, 153105. doi:10.1103/PhysRevB.76.153105
Owen, E., and Yates, E. (1933). XLI. Precision measurements of crystal parameters. Lond. Edinb. Dublin Philosophical Mag. J. Sci. 15, 472–488. doi:10.1080/14786443309462199
Parker, S. F. (2010). Spectroscopy and bonding in ternary metal hydride complexes—potential hydrogen storage media. Coord. Chem. Rev. 254, 215–234. doi:10.1016/j.ccr.2009.06.016
Schirber, J., and Morosin, B. (1975). Lattice constants of β−PdHx and β−PdDx with x near 1.0. Phys. Rev. B 12, 117–118. doi:10.1103/PhysRevB.12.117
Schirber, J., Overmyer, D., Baughman, R., and Morosin, B. (1991). Search for high temperature superconductivity in LiPdHx. Phys. C. Supercond. 172, 465–466. doi:10.1016/0921-4534(91)90214-j
Setayandeh, S., Webb, C., and Gray, E. M. (2020). Electron and phonon band structures of palladium and palladium hydride: a review. Prog. Solid State Chem. 60, 100285. doi:10.1016/j.progsolidstchem.2020.100285
Storms, E. (2002). Cold fusion: an objective assessment. Sci. Direct Work. Pap. Available at: https://www.lenr-canr.org/acrobat/StormsEcoldfusiond.pdf.
Storms, E. (2003). A student’s guide to cold fusion. https://lenr-canr.org/acrobat/StormsEastudentsg.pdf.
Takagi, S., and Orimo, S.-i. (2020). New functionalities of hydride complexes with high hydrogen coordination. J. Phys. Soc. Jpn. 89, 051010. doi:10.7566/JPSJ.89.051010
Wang, Q., Guo, J., and Chen, P. (2023). Complex transition metal hydrides for heterogeneous catalysis. Chem. Catal. 3, 100524. doi:10.1016/j.checat.2023.100524
Yan, H., Gao, W., Wang, Q., Guan, Y., Feng, S., Wu, H., et al. (2021). Lithium palladium hydride promotes chemical looping ammonia synthesis mediated by lithium imide and hydride. J. Phys. Chem. C 125, 6716–6722. doi:10.1021/acs.jpcc.1c01230
Yang, X., Cao, Y., Fan, C., Liang, X., and Li, H. (2018). Mechanical stability and formation analysis of Pd/Rh dihydride alloys under pressure. Solid State Commun. 277, 33–38. doi:10.1016/j.ssc.2018.04.010
Yang, X., Li, H., Ahuja, R., Kang, T., and Luo, W. (2017). Formation and electronic properties of palladium hydrides and palladium-rhodium dihydride alloys under pressure. Sci. Rep. 7, 3520. doi:10.1038/s41598-017-02617-z
Yao, Y., Stavrou, E., Goncharov, A. F., Majumdar, A., Wang, H., Prakapenka, V. B., et al. (2017). High-pressure phase transition of alkali metal–transition metal deuteride Li2PdD2. J. Chem. Phys. 146, 234506. doi:10.1063/1.4986245
Yoshida, M., Yvon, K., and Fischer, P. (1993). LiSr2PdH5, the first mixed alkali-alkaline earth transition metal hydride. J. Alloys Compd. 194, L11–L13. doi:10.1016/0925-8388(93)90635-Z
Yvon, K. (2003). Hydrogen in novel solid-state metal hydrides. Z Krist. Cryst. Mater 218, 108–116. doi:10.1524/zkri.218.2.108.20672
Yvon, K., Rapin, J.-P., Penin, N., Ma, Z., and Chou, M. (2007). LaMg2PdH7, a new complex metal hydride containing tetrahedral [PdH4] 4− anions. J. Alloys Compd. 446, 34–38. doi:10.1016/j.jallcom.2006.11.209
Keywords: interstitial palladium hydrides, complex palladium hydrides, H/Pd ratio, Pd electrode, low-energy nuclear fusion
Citation: Wang Q, Zhang S, Guo J and Chen P (2024) Advances in highly hydrided palladium. Front. Mater. 11:1365526. doi: 10.3389/fmats.2024.1365526
Received: 04 January 2024; Accepted: 08 February 2024;
Published: 22 February 2024.
Edited by:
Dimiter Alexandrov, Lakehead University, CanadaReviewed by:
Riccardo Checchetto, University of Trento, ItalyCopyright © 2024 Wang, Zhang, Guo and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Chen, cGNoZW5AZGljcC5hYy5jbg==
†These authors have contributed equally to this work
 Qianru Wang
Qianru Wang Shengyuan Zhang1,2†
Shengyuan Zhang1,2†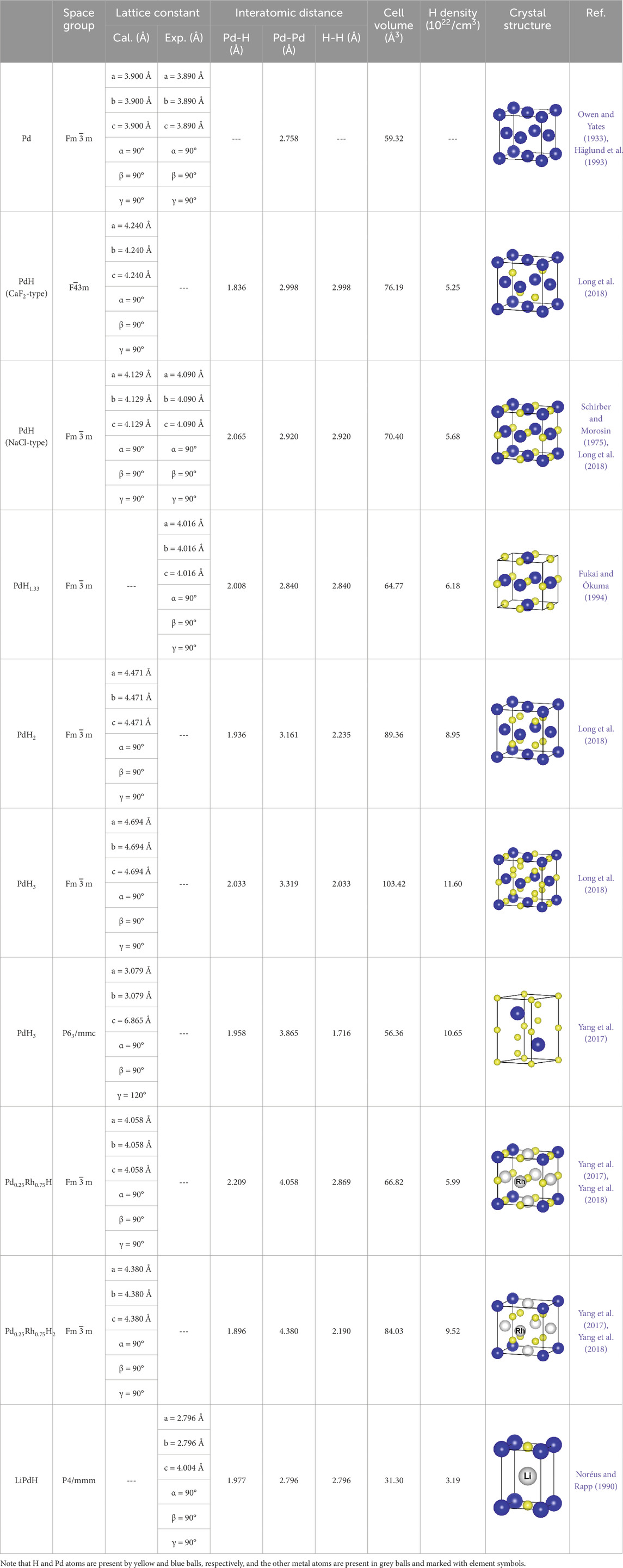
 .
.