- 1Haute Ecole Arc Conservation Restauration, University of Applied Sciences and Arts HES-SO, Neuchâtel, Switzerland
- 2Laboratory of Technologies for Heritage Materials (LATHEMA), Institute of Chemistry, University of Neuchâtel, Neuchâtel, Switzerland
- 3Arc’Antique Research and Conservation–restoration Laboratory, Nantes, France
- 4Department of Chemistry “G. Ciamician”, University of Bologna, Ravenna, Italy
The research study is aimed to design innovative bio-gel formulations able to tackle altered historical metal artworks in a green and sustainable perspective. The target of the research is the removal of undesired or altered materials of both inorganic (i.e., corrosion and tarnishing) and organic nature (i.e., protective coatings). The designed gel systems are initially assessed on mock-ups. Iron-, copper- and silver-based substrates, chosen as mostly present in historical metal collections, are chemically aged to form corrosion on the surface. Alternatively, they are coated with organic protectives (i.e., acrylic and nitrocellulose varnish) that are mostly used for indoor metal care. After multi-modal analytical assessment to check the action and safety of the gels on the metal mock-ups, the developed solutions are applied on real cases, thanks to the strong and fruitful collaboration with curators and conservators of metal artworks.
1 Introduction
Our research topics integrate innovative aspects and inventive interdisciplinary approach at the boundaries between art conservation and natural sciences, with the aim to act against corrosion on metal artefacts (i.e., sculptures, archaeological items, historical collections) or on archaeological organic materials (i.e., waterlogged wood) contaminated with iron corrosion products. Thanks to advanced chemical imaging techniques, we study interactions between metals and their environment, and aspire to propose alternative green approaches in metal conservation.
The phenomenon of surface alteration on metal artworks is a major concern in terms of visual aspect, structural damages, or loss of valuable information about artefact manufacturing, for example, and can be related to corrosion processes occurring. Also, the presence of organic coatings (e.g., wax, acrylic, and nitrocellulose lacquers) applied during past conservation interventions can fail achieving protection through the time and need to be removed (and replaced) for the long-term conservation of metal heritage. Indeed, the exposure of these artefacts to caustic agents (i.e., from environmental acidic components, pollutants, or using inadequate cleaning agents) causes degradation and results, for example, in tarnishing and failures of protective coatings if present (Argyropoulos et al., 2021). Current cleaning methods in metal conservation are mainly mechanical (e.g., scalpel, abrasive paste), physical (e.g., electrochemistry, laser, plasma), or chemical (e.g., solvents, complexing or acidic solutions) procedures, which are either too invasive, difficult to be applied by non-experts, time-consuming and/or toxic.
In response to the potential hazards of traditional cleaning methods employed in metal art conservation, possibly affecting human health, environment, or artworks themselves, research towards safer and more sustainable practices is of high interest (Passaretti et al., 2021). To this purpose, previous studies demonstrated the efficacy and reliability of microorganisms exploited in conservation treatments (Junier and Joseph, 2017). Studies aiming to achieve stabilization of iron and copper archaeological artefacts using biomineralization revealed the additional potential of the selected microorganisms to remove undesired corrosion, achieving the so-called “bio-cleaning” of the metallic substrates (Comensoli et al., 2019). Nevertheless, the use of living organisms is tricky to be implemented in everyday CRs praxis, therefore research is also evaluating the use of metabolites that are extracted from microorganisms, such as siderophore chelators (Albelda-Berenguer et al., 2019; Cuvillier et al., 2023). In line with this bio-derived approach, solvents that can be produced from fermented biomass are also considered in our research in order to replace other harmful solvents (e.g., acetone, white spirit) conventionally used in metal care for instance for the removal of altered protective films (Esson et al., 2018; Prati et al., 2019).
In addition, the combination with gel delivery systems provides a controlled cleaning action, easy clean-up and a significant reduction of active agents quantity, appearing as an attractive alternative approach (Junier and Joseph, 2017; Guilminot, 2023). The HELIX research project seeks to develop environmentally friendly, bio-based and easy-to-use gels for the cleaning of altered historical metal artworks, in particular altered copper-, iron- and silver-based objects. Commercial hydrogels from renewable sources are amended with selected microbial metabolites, which are capable to complex metal ions, thus tackling detrimental corrosion. In parallel, bio-derived organogel formulations are designed for the removal of undesired or altered organic coatings present on metallic artefacts.
2 Formulations
Taking advantage of the capabilities of naturally occurring microorganisms, strategies are thus adopted to remove the degradation products present on the before-mentioned alloys, which are most commonly found in historical metal collections. The microbial mechanisms involved in the uptake of metallic ions and fat degradation are deeply investigated over representative corrosion phases and organic substances. In parallel, to comply with the guidelines when cleaning water-sensitive metallic substrates, bio-derived gel systems that present no toxicity are individuated or specially designed to develop biocleaning methodologies. Hence, different combination of bio-based gels amended with microbial extracts are tested on model metal coupons presenting different types of corrosion and/or organic coatings encountered on contemporary or decorative artworks.
High attention is addressed towards the gel system components (i.e., thickening agents, solvents, and chelators), to select only bio-sourced, biodegradable and non-toxic materials.
In particular, the potentialities of natural iron chelators, siderophores, are presented in this contribution as sustainable and effective alternatives to chelating agents commonly exploited in metal care despite their renowned toxicity, as in the case of sodium salt of ethylenediaminetetraacetic acid (Na2EDTA), (Oviedo and Rodríguez, 2003).
Siderophores are produced by several microorganisms in iron-deficient environments, (Albelda-Berenguer et al., 2019). Interestingly, they also present affinity for other metal ions, including copper. As opposed to traditional chelating agents exploited in metal care, they are bio-sourced, biodegradable, and have a pH close to neutral, thus being less harmful for both the environment and targeted artworks (Fazary et al., 2016). In particular, in this brief review, several cleaning tests carried out using the siderophore deferoxamine (DFO), which is, for example, secreted by the bacterium species Streptomyces pilosus, are reported (Bellotti and Remelli, 2021). In complement, EDDS (Ethylenediamine-N,N′-disuccinic acid) another green chelator, with a molecular structure close to EDTA, was assessed (Tandy et al., 2006).
In parallel bio-solvents (e.g., ethyl lactate, γ-valerolactone), derived from renewable sources and coming from microbial metabolic processes, are explored as an alternative and greener way to remove organic coatings once their protection features start failing. The chosen active agents (i.e., bio-complexing agents and solvents) are finally retained in bio-sourced polymers to provide innovative fully bio-derived gelled systems for the cleaning of historical metal heritage. In particular, the selected thickening agents (i.e., polymers) include agar and polyhydroxybutyrate (PHB), which are obtained from red seaweeds and bacterial activity, respectively. According to the peculiar characteristic of each polymer, agar allowed the preparation of hydrogels, whereas polyhydroxybutyrate was exploited for the design of solvent-based gelled systems.
Some assays carried out on metallic mock-ups and test objects with emblematic alteration features related to corrosion and/or the presence of degraded protective materials are here reported to make the conservation community aware of the reliability and efficiency of HELIX green cleaning gels (Table 1).
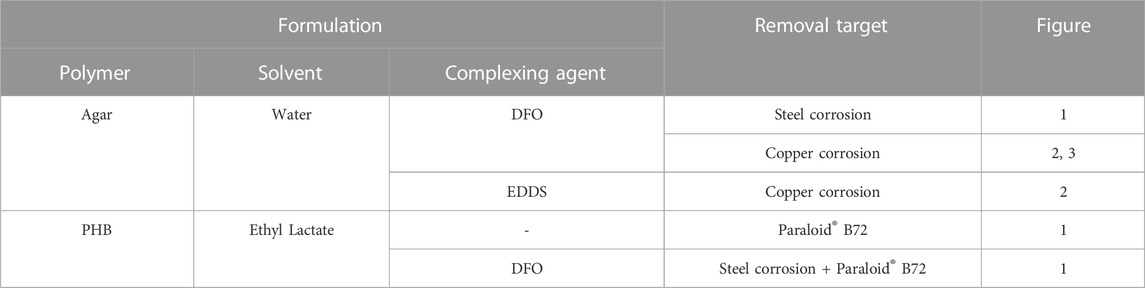
TABLE 1. HELIX gel formulations: list of the green components employed in their design, targets tackled, and figures related to cleaning tests.
To verify the reliability and effectiveness of the developed formulations, micro- and macro-scale analyses were performed on mock-ups and real objects. In particular, appearance modifications were investigated using optical microscopy and colorimetry. Eddy current measurements (thickness measurements) ascertained the removal of corrosion and coating layers. When operating on corrosion removal, confirmation of metal uptake inside the gel matrixes was conducted using X-ray fluorescence (XRF) spectroscopy and Atomic Absorption Spectroscopy (AAS) on the formulations. Finally, Raman and Fourier-Transform Infrared (FTIR) spectroscopies were performed to characterize cleaning targets (i.e., corrosion and organic coatings) before and after cleaning to verify their removal and, in parallel, ascertain the innocuity of the gelled cleaning systems on metal substrates (Figure 1). Analytical details are provided in the related literature (Cuvillier et al., 2022; Cuvillier et al., 2023). The multi-modal assessment, correlated with the characterization (e.g., rheology, cryo-scanning electron microscopy) of developed green gels, allowed to verify the reliability of the innovative systems.
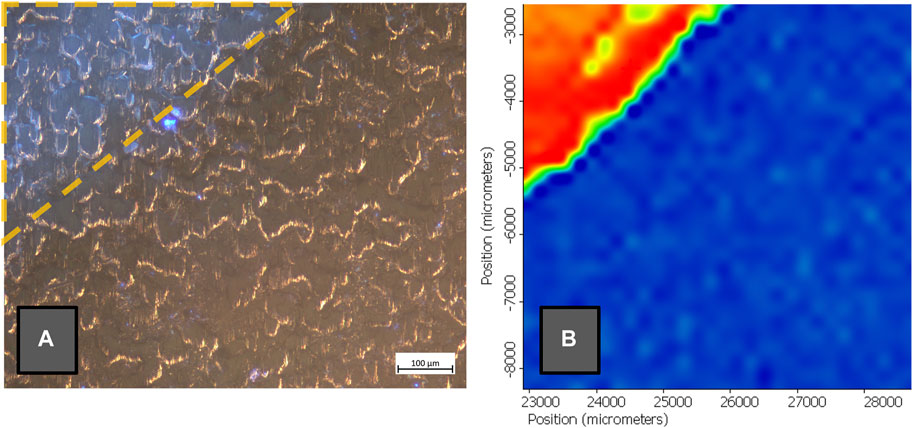
FIGURE 1. (A) Optical microscope observation (×10 original magnification) under UV-LED illumination of a steel coupon coated with Paraloid® B72 and partially cleaned by means of a PHB-EL (7% w/v) organogel applied twice for 5 min (yellow outline) (B) FTIR correlation map performed on the same coupon using a reference spectrum of Paraloid® B72 with red standing for higher correlation and blue lowest correlation.
3 Test systems
As corroded samples, triplicated iron and copper sheets (3 × 3 cm) were chemically aged by immersion in FeCl3 (protocol ASTM G48-11, 2015) and Na2S solutions, respectively. Different application procedures were assessed during pilot studies, concluding that short, repeated contact times (10’) achieved better results than extended contact (Cuvillier et al., 2023). Following cleaning step, subsequent clearance was performed using an ethanol-soaked cotton swab. After testing on samples, most pertinent formulations were assessed on objects belonging to museum collections. When possible, different cleaning formulations were compared on a same artefact. For example, a naturally corroded iron-based mezzaluna knife was treated with a 3%w/v agar hydrogel amended with the studied green chelating agent (6·10–2 M DFO solution) (Figure 2A) and applied by dropping hot liquid gel onto the metallic surface. The success of the complexing reaction is ascertained by the color of the formulation turning to vivid red during application (Figure 2B) (Cuvillier et al., 2022).
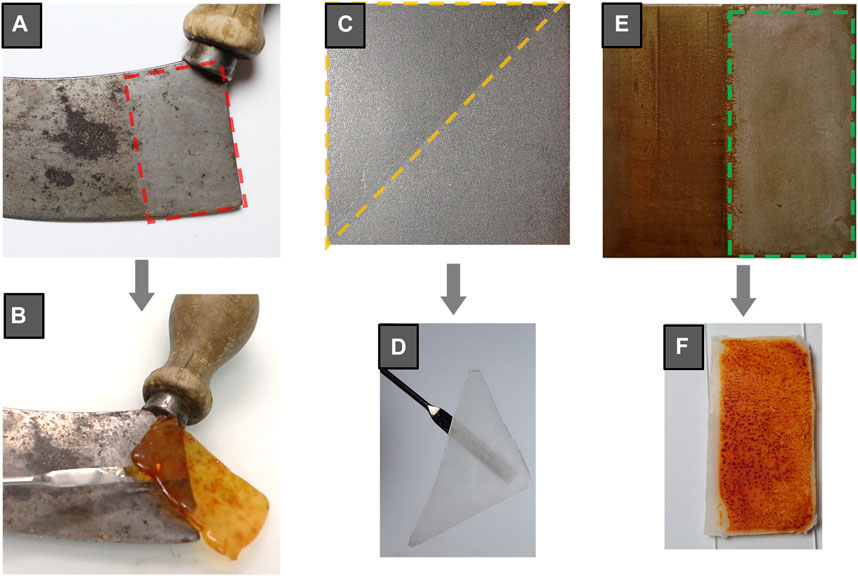
FIGURE 2. (A) Corroded iron-based mezzaluna knife partly cleaned with an agar gel (3% w/v) prepared with a 6▫10–2 M deferoxamine solution (DFO) (region outlined in red) and (B) photography during removal of the cleaning agar-DFO gel after 15 min of application (C) Steel sample coated with Paraloid® B72, removed by means of a PHB-EL (7% w/v) organogel applied twice for 5 min (region outlined in yellow) (D) photography of the cleaning PHB-EL gel after 5 min of application (E) Steel coupon chemically corroded (ASTM G48-11, 2015) and coated with Paraloid® B72 half-treated (region outlined in green) with a PHB-EL gel amended with DFO by two applications of 30 min (F) photography of the cleaning PHB-EL-DFO gel after 30 min of application.
Tests were carried out on copper-based alloys: a naturally corroded brass orthodox polyptych (Figure 3). The efficiency of the same siderophore (DFO at 3·10–2 M) was compared to Na2EDTA and EDDS at the same concentration amended in 3% w/v agar gels. DFO-agar gel 10-min application was repeated 6 times and a unique 10-min application was performed for EDTA and EDDS. On copper-alloys, siderophores offer a progressive and smooth cleaning, whereas EDDS yields a similar efficiency and action as EDTA, but without resorting to hazardous chemicals. On a silver-based pocket-watch displaying blueish copper corrosion stains (Figure 4), a 3·10–2 M DFO amended agar gel (3% w/v) was applied for 20 min.

FIGURE 3. Tarnished brass polyptych from the Grand Patrimoine Loire-Atlantique collections (France). (A) Graphical overview of the areas (outlined in white) differently cleaned using 3% w/v agar gels loaded with 3▫10–2 M DFO (top), EDTA (center), and EDDS (bottom), and (B) close-ups of the areas after cleaning (application of 6 × 10 min for DFO and 1 × 10 min for other chelators).

FIGURE 4. Silver-based pocket watch from the collections of the Historical Museum of Lausanne (Switzerland) (A) before and (B) after removal of blue copper corrosion stains applying an 3% w/v agar-DFO (3▫10–2 M) hydrogel for 20 min.
On the other side, HELIX cleaning organogels are designed in order to remove undesired or altered organic coatings, employing exclusively biopolymers (i.e., polyhydroxybutyrate) and bio-solvents (i.e., ethyl lactate) in the formulation. Assays were carried out on mild steel coupons coated with Paraloid® B72 (10% w/v in ethyl acetate) and brass sheets coated with the nitrocellulose lacquer Zaponlack and finally artificially aged for 3 months in a climatic chamber. The gel employed for coating removal was formed by the biopolymer PHB loaded with ethyl lactate. The system was applied twice for 5 min, ending the cleaning procedure by gentle dry cotton swabbing for a complete removal of any possible residue (e.g., swollen coating, residual solvent). The gelled system (Figure 2D) showed compelling results on both lacquers, and Figure 2C displays the successful outcome obtained on steel mock-ups coated with Paraloid® B72.
Following these remarkable outcomes, pioneering research is addressed to design green gels capable to bio-clean simultaneously undesired corrosion and organic coatings. Promising evidence is already obtained and reported in Figure 2E in the case of concurrent removal of iron corrosion products and Paraloid® B72 present on mild steel coupons by means of a PHB gel englobing both DFO and ethyl lactate (Figure 2F) as active agents in the cleaning process.
4 Conclusive remarks
HELIX formulations come as great green alternatives in metal care praxis, providing satisfactory cleaning performances and concurrently keeping high attention towards the green chemistry principles (Anastas and Williamson, 1998). Various microbial pathways are currently under study to assess their potentialities to clean other metallic substrates, for instance silver-alloys, which inherent antibiotic nature makes it challenging (Chernousova and Epple, 2013). A close collaboration with conservators appears fundamental to verify the reliability on real artworks and bring innovation out of the research laboratory. As confirmed by a worldwide survey conducted by the authors, despite the global trend towards sustainability, the exploitation of biologically derived gelling agents, organic solvents or chelating agents is extremely rare in metal heritage conservation. It appears at a challenge for future heritage generations to get trained in sustainability approaches and global wellbeing. Therefore, current research initiatives are conducted integrating the preparation of education modules about sustainability in conservation cursus as well as life-long learning workshops proposed to conservation professionals.
Author contributions
AP: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing–original draft. LC: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing–original draft. EG: Resources, Supervision, Validation, Writing–review and editing. GS: Resources, Supervision, Validation, Writing–review and editing. EJ: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The HELIX (Investigating metal bioremediation for the preservation of historical metal artworks) project 2020-2024, is funded by the Swiss National Science Foundation (SNSF), grant number 205121_188755.
Acknowledgments
The authors acknowledge Professor Stephan von Reuss for his co-supervision of the PhD theses within the HELIX project, Lidia Mathys for laboratory technical support as well as Claude-Alain Künzi from the Historical Museum of Lausanne and Claire De Lalande from Grand Patrimoine Loire Atlantique for the access to their collections.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GM declared a shared affiliation with the author GS to the handling editor at time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albelda-Berenguer, M., Monachon, M., and Joseph, E. (2019). Siderophores: from natural roles to potential applications. Adv. Appl. Microbiol. 106, 193–225. doi:10.1016/bs.aambs.2018.12.001
Anastas, P. T., and Williamson, T. C. (1998). Green chemistry: Frontiers in benign chemical syntheses and processes. Oxford, UK: Oxford University Press.
Argyropoulos, V., Boyatzis, S. C., Giannoulaki, M., Guilminot, E., and Zacharopoulou, A. (2021). “Organic green corrosion inhibitors derived from natural and/or biological sources for conservation of metals cultural heritage,” in Microorganisms in the deterioration and preservation of cultural heritage (Berlin, Germany: Springer International Publishing), 341–367.
Bellotti, D., and Remelli, M. (2021). Deferoxamine B: a natural, excellent and versatile metal chelator. Molecules 26 (11), 3255. doi:10.3390/molecules26113255
Chernousova, S., and Epple, M. (2013). Silver as antibacterial agent: ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 52 (6), 1636–1653. doi:10.1002/anie.201205923
Comensoli, L., Kooli, W., Monachon, M., Albini, M., Junier, P., and Joseph, E. (2019). “The potential of microorganisms for the conservation-restoration of iron artworks,” in Proceedings of the Metal 2019, 9th interim meeting of the ICOM-CC metals working group, Neuchâtel, Switzerland, September 2019, 2–6.
Cuvillier, L., Passaretti, A., Guilminot, E., and Joseph, E. (2023). Testing of the siderophore deferoxamine amended in hydrogels for the cleaning of iron corrosion. Eur. Phys. J. Plus 138 (6), 569. doi:10.1140/epjp/s13360-023-04159-y
Cuvillier, L., Passaretti, A., Raimon, A., Dupuy, V., Guilminot, E., and Joseph, E. (2022). “Exploiting biologically synthetized chelators in conservation: gel-based bio-cleaning of corroded iron heritage objects,” in Proceedings of the Metal 2022, proceedings of the interim meeting of the ICOM-CC metals working group, ICOM-CC, Helsinki, Finland, September, 2022 (The National Museum of Finland).
Esson, J. M., Scott, R., and Hayes, C. J. (2018). Chemistry and art: removal of graffiti ink from P.aints grounded in a real-life scenario. J. Chem. Educ. 95 (3), 400–402. doi:10.1021/acs.jchemed.7b00536
Fazary, A. E., Al-Shihri, A. S., Alfaifi, M. Y., Saleh, K. A., Alshehri, M. A., Elbehairi, S. E., et al. (2016). Microbial production of four biodegradable siderophores under submerged fermentation. Int. J. Biol. Macromol. 88, 527–541. doi:10.1016/j.ijbiomac.2016.03.011
Guilminot, E. (2023). The use of hydrogels in the treatment of metal cultural heritage objects. Gels 9 (3), 191. doi:10.3390/gels9030191
Junier, P., and Joseph, E. (2017). Microbial biotechnology approaches to mitigating the deterioration of construction and heritage materials. Microb. Biotechnol. 10 (5), 1145–1148. doi:10.1111/1751-7915.12795
Oviedo, C., and Rodríguez, J. (2003). EDTA: THE CHELATING AGENT UNDER ENVIRONMENTAL SCRUTINY. Quimica Nova 26 (6), 901–905. doi:10.1590/s0100-40422003000600020
Passaretti, A., Cuvillier, L., Sciutto, G., Guilminot, E., and Joseph, E. (2021). Biologically derived gels for the cleaning of historical and artistic metal heritage. Appl. Sci. 11 (8), 3405. doi:10.3390/app11083405
Prati, S., Sciutto, G., Volpi, F., Rehorn, C., Vurro, R., Blümich, B., et al. (2019). Cleaning oil paintings: NMR relaxometry and SPME to evaluate the effects of green solvents and innovative green gels. New J. Chem. 43 (21), 8229–8238. doi:10.1039/c9nj00186g
Keywords: biocleaning, green chemistry, metabolites, bio-solvents, gels, historical metal heritage
Citation: Passaretti A, Cuvillier L, Guilminot E, Sciutto G and Joseph E (2023) Biocleaning of historical metal artworks: innovative green gels amended with microbial derivatives. Front. Mater. 10:1277972. doi: 10.3389/fmats.2023.1277972
Received: 15 August 2023; Accepted: 17 November 2023;
Published: 08 December 2023.
Edited by:
Cristina Cicero, University of Rome Tor Vergata, ItalyReviewed by:
Giulia Masi, University of Bologna, ItalyPilar Bosch-Roig, Universitat Politècnica de València, Spain
Copyright © 2023 Passaretti, Cuvillier, Guilminot, Sciutto and Joseph. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edith Joseph, ZWRpdGguam9zZXBoQGhlLWFyYy5jaA==
†These authors have contributed equally to this work and share first authorship
 Arianna Passaretti1,2†
Arianna Passaretti1,2† Giorgia Sciutto
Giorgia Sciutto Edith Joseph
Edith Joseph