
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mater., 20 March 2023
Sec. Biomaterials and Bio-Inspired Materials
Volume 10 - 2023 | https://doi.org/10.3389/fmats.2023.1137672
This article is part of the Research TopicBiological Stimuli-Responsive Smart MaterialsView all 5 articles
Introduction: Clear aligner treatment (CAT) has become popular over recent years because it is both comfortable and aesthetically acceptable. However, most of patients undergoing orthodontic treatment request dental bleaching. A safe and controlled bleaching treatment at the same time as the clear aligner treatment can save time and improve patient satisfaction with the outcome of the treatment.
Aim: This study was aimed to develop a thermoforming film loaded with hydrogen peroxide as a clear aligner and detect its efficiency on teeth blenching and its influence on shear bonding strength for attachment.
Methods: The thermoforming film loaded with sodium alginate-dopamine/Mesoporous silica nanoparticles compound gel was immersed in 6 wt% hydrogen peroxide solution and the hydrogen peroxide was loaded into mesoporous silica nanoparticle channels by capillary action. Then, a thermoforming film loaded with sustained-release hydrogen peroxide gel was made. Six dentition models were prepared with 90 isolated human premolars and divided into the experiment group, the condition control group and the blank control group, respectively. Then, the experiment group wore the clear aligner made by the thermoforming film loaded with hydrogen peroxide for 40 days; the conditional control group wore the clear aligner made by the ordinary thermoforming film for 40 days; and the blank control group wore no clear aligner. The aligners were updated every 10 days and the color of teeth was measured every 10 days. Tooth color should be determined by specific parameters (L, a* and b*). What’s more, in order to determine the influence of the thermoforming film loaded with sustained-release hydrogen peroxide gel on shear bonding strength for attachment. The shear bonding strength of attachment of isolated premolars were measured.
Results: Isolated premolars treated by bleaching experiments showed an increase in L value (ΔL = 7.76 ± 0.64) and a decrease in both a* (Δa = −0.82 ± 0.12) and b* (Δb = −3.10 ± 0.21) values. However, the isolated premolars in conditional control group and blank control group exhibited that an decrease in L value (ΔLCCG = −0.91 ± 0.24; ΔLBCG = −0.86 ± 0.15)and a increase in both a* (ΔaCCG = 0.19 ± 0.05; ΔaBCG = 0.18 ± 0.04) and b* (ΔbCCG = 0.43 ± 0.11; ΔbBCG = 0.31 ± 0.10) value. While the shear bonding strength for attachment after bleaching was 22.78 ± 2.28 MPa, which had no significant change compared with the shear bonding strength for attachment without bleaching experiment (22.21 ± 2.77 MPa) (p > 0.05).
Conclusion: A thermoforming film featuring the sustained release of hydrogen peroxide had a good bleaching effect on isolated teeth and had no significant influence on the shear bonding strength for attachment.
A previous study demonstrated that nearly 90% of patients undergoing orthodontic treatment request dental bleaching (Levrini et al., 2020). Clear aligner treatment (CAT) has become popular over recent years because it is both comfortable and aesthetically acceptable (Cheng et al., 2022a). Compared with the fixed orthodontics, CAT provides adult patients with a significant range of aesthetic treatments that do not negatively affect their social lives or relationships (Cheng et al., 2022b). However, Zhang demonstrated that there were significant change for the color of teeth after clear aligner treatment (Zhang et al., 2020). Many studies have investigated teeth discoloration. Ghinea et al. claimed that the clinically discernible threshold for tooth color change (ΔE) was 2.7 (Pérez et al., 2019). Zhang carried out clinical experimental studies and reported that the color change after orthodontic treatment was higher than the clinically discernible threshold (Zhang et al., 2020).
Previous studies reported that the combination of orthodontic treatment and teeth bleaching could provide patients with better treatment satisfaction (Paravina et al., 2019; Levrini et al., 2020). According to the guidelines for bleaching treatment provided by the Chinese Stomatological Association (Goldstein and Garber, 1995; Haywood, 2007; Pérez et al., 2019; Society of Prosthodontics and Chinese Stomatological Saaociation, 2021), bleaching treatment can be used to improve the color of teeth following orthodontic treatment. There are three fundamental approaches for bleaching teeth: in-office or power bleaching, at-home or dentist-supervised night-guard bleaching and bleaching with over-the-counter (OTC) products (Alqahtani, 2014; Pereira et al., 2022). In-office bleaching utilizes a high concentration of tooth-whitening agents (containing 25%–40% hydrogen peroxide). Here, the dentist has complete control throughout the procedure and terminate treatment when the desired shade/effect is achieved. At-home or dentist-supervised night-guard bleaching basically involves the use of a low concentration of whitening agent (10%–20% carbamide peroxide, equivalent to 3.5%–6.5% hydrogen peroxide). Significantly, over-the-counter (OTC) bleaching products have increased in popularity over recent years due to its convenience (Alqahtani, 2014; Pereira et al., 2022). These products contain a low concentration of whitening agent (3%–6% hydrogen peroxide) and are self-applied to the teeth via gum shields, strips or paint-on product formats. Researchers have found that low concentrations of hydrogen peroxide (6%) for 4 h per day over a total of 21 can achieve effective bleaching with good stability; furthermore, follow-up studies reported positive social and psychological impact (Briso et al., 2018; Alkahtani et al., 2020). The principle of tooth bleaching is that hyperoxide reacts with chromogenic substances and then eliminates these substances (Kwon and Wertz, 2015). In recent years, it has been reported that a clear aligner can be used as a carrier for tooth bleaching gel (Levrini et al., 2020; Piknjač et al., 2021). Using orthodontic aligners as bleaching trays saves time when compared with the requirement for additional bleaching treatment after orthodontic therapy (Piknjač et al., 2021). Therefore, it is necessary to innovatively explore the application of using an aligner film loaded with hydrogen peroxide sustained-release gel and test its performance.
The color space theory proposed by the International Commission on Illumination (CIE) reported that tooth color should be determined by specific parameters (L, a* and b*) (Johnston, 2009; Pradhan et al., 2020; Nitu et al., 2022). Parameter L represents brightness, parameters b* represents red and green (a positive value is red and a negative value is green) and parameter c* represents yellow and blue (a positive value is yellow and a negative value is blue). This theory made it possible to study color changes in an objective and quantitative manner. The CIE76 formula for color difference correlates the change of color parameters (L, a*, b*) with the difference of color perceived by human eyes; this formula permits the quantitative study of color change. This formula has been applied to previous studies involving tooth bleaching treatments. To evaluate the effect of bleaching treatment, we selected the classic CIE76 color difference formula to calculate differences in tooth color after bleaching treatment. (Revilla-León et al., 2020; Donmez et al., 2021).
In addition, the attachment was the effective auxiliary device. The attachment was composite resin material bonded on the teeth. Previous studies have reported that bleaching treatment could reduce the shear bonding strength (SBS) of brackets (Carlos et al., 2018; Sadeghian et al., 2021). However, there are few studies on the change of SBS of composite resin attachment under the condition of clear aligner treatment. If the clear aligner with sustained release hydrogen peroxide developed in this study were to be applied in clinical practice, it was necessary to study its influence on the SBS of attachment of isolated teeth, so as to ensure that whether the attachment shedding rate increase or not (Putrino et al., 2022).
To sum up, the purpose of this study was to develop a thermoforming film loaded with sustained-release hydrogen peroxide gel and to test its bleaching effect and determine its influence on the SBS for attachments.
The materials required for the experiment are as follows:Cetyltrimethyl ammonium bromide (CTAB, Analyte Pure, McLean Biochemical Technology Co., LTD., Shanghai), Tetraethyl silicate (TEOS, Maya reagent) Ethanol (Analytical Pure, Fuyu Fine Chemical Co., LTD., Tianjin),hydrochloric acid, sodium hydroxide (Analytical pure, Sinopharm Group), SPLINT film (Round, Thickness 0.8mm, Diameter 120 ± 1mm, Yamahachi Co., Japan), Sodium alginate (Alg, Aladdin Reagent Co., LTD., Shanghai), Dopamine hydrochloride (DA, 98%, McLean Biochemical Technology Co., LTD., Shanghai), ammonia (Analytical Pure, Pengcai Select Chemical Co., LTD., Hebei), Calcium Chloride (Analytical Pure, Aladdin Reagent Co., LTD., Shanghai), Thymol (Sinopsin Chemical Reagent Co., LTD., Shanghai), GRACEY Scraper (Hoverboard, United States of America), Reinforced silicone rubber heavy body impression material (3M Company, United States of America), Silicone rubber lightweight Impression Material (3M Company, United States of America), Lightweight silicone rubber Injection Gun (3M Company, United States of America), Silicone rubber trimming knife (Conte Corporation, United States of America), Dentition mold,Self-setting Resin Powder and Monomer (Rijin Dental Materials Co., LTD., Jiangsu),Acid-resistant Nail Polish (Estee Lauder, US), sodium bicarbonate, Sodium dihydrogen phosphate (Analytically pure, Sinopharm Group), 3M Adper Single Bond2 Adhesive (3M Company, United States of America), Filtek Z350 Nano Filling Resin (3M Company, United States of America),35% phosphoric acid (Gluma, HeraeusKulzerGmbH,Germany).
The instruments required for the experiment are as follows:Magnetic heating Agitator (Kosu Instrument Equipment Co., LTD., Hebei), Muffle Furnace (GW1200, Yagelong Technology Co., LTD., Hebei), Blast Drying Oven (DHG-9030, Hongzhang Technology Co., LTD., Guangdong),Thermostatic water bath, sonic washer, Vita Easyshade Vspectrophotometer (VitaZahnfabrik, BadSackingen,Germany), Scanning electron microscopy (SEM, HITACHI S-4800, Hitachi of Japan),microhardness tester (X-1000 Qinming Optical Instrument Co., LTD., Shanghai),Refrigerator (Gree Electric Appliances Co., LTD., Guangdong),Transmittance fog meter (WGT-S, Shanghai Shenguang Instrument Co., LTD.), parting tool, 30x Magnifying Glass (Solan, Yiwu), Vernier Caliper (Menet, Germany), Photocuring lamp (1,000–200 mW/cm2; Foshan, Guangdong), Fast turbo cell phone, Blue Label Emery Car Needle (TC-21, Mani, Japan), Yellow Label Polishing Needle (T-11EF, Mani, Japan).
The SPLINT films, the commercial name of a kind of thermoforming film with 0.8 mm thickness and 120 ± 1mmdiameter, were choosen. This SPLINT films were made of Glycol terephthalate material. The thermoforming film loaded with hydrogen peroxide sustained-release gel were grown using the following methods (Fattahi et al., 2021). First, mesoporous silica nanoparticles (MSN) were prepared by the cetyltrimethylammonium bromide template method and the alkaline catalysis sol-gel method. Second, 200 mg of dopamine hydrochloride was dissolved in 25 mL of purified water at 25 °C and the pH was adjusted to 10 with Tris buffer salt. We removed the protective film on one side of a thermoforming diaphragm and soaked it in the pre-prepared solution of DA. Then, we added 10 mL of a 40 mg/mL aqueous solution of sodium alginate (ALG) to the MSN and 1mLof ammonia. This mixture was stirred well and allowed to stand for 24 h at 37 °C. Next, 20 mL of 5 wt% calcium chloride solution was added and allowed to stand for 5 h. When the ion cross-linking reaction was complete, the residual calcium chloride solution was aspirated. The thermoforming diaphragm was then soaked in purified water; the purified water was changed once for 3 h until the leaching solution was colorless and transparent. Then, the surface of the diaphragm was cleaned with a large amount of purified water and the protective film on the remaining side was removed. The thermoforming diaphragm was loaded with AlG-DA/MSN composite gel through the above preceding operations. We observed this film under Scanning electron microscopy. The new film was stored at 4 °C and immersed in 6 wt% hydrogen peroxide solution; the hydrogen peroxide was then loaded into the MSN channels by capillary action, thus generating sustained-release hydrogen peroxide film. The thermoforming film loaded with hydrogen peroxide sustained-release gel was then used to determine its effects on the coloration of isolated human teeth and the shear bonding strength for attachment.
Preparation of an in vitro model of dentition, sustained-release hydrogen peroxide clear aligner and ordinary clear aligner.
In total, 90 extracted premolars were collected from patients attending the Department of Oral Surgery at the Stomatological Hospital of Air Force Medical University with informed consent from patients (age 18–30 years) under a protocol (IACUC-20210760) approved by the Air force medical university. Teeth were collected according to strict inclusion criteria and washed several times with purified water; residual water was dried with filter paper. The teeth were placed in 0.2% thymol solution and stored at 4 °C to await analysis. Subsequently, the extracted premolars were randomly divided into an experimental group, a conditional control group and a blank control group with 30 teeth per group. The isolated teeth were then inserted into a dentition mold (15 teeth per mold). Each group involved two dentition molds; these were fixed with self-setting resin. The teeth were dried with filter paper and then stored in 0.2% thymol solution in a refrigerator at 4 °C for further use.
Inclusion and exclusion criteria 1) The patients were aged 18–30 years and had no systemic disease; 2) The tooth crown was complete without defect or restoration; 3) No surface demineralization, no caries; 4) No enamel cracks, no enamel hypoplasia, no dental fluorosis, no tetracycline teeth; 5) The color of the buccal surface of the teeth was determined by Vita Easyshade V (VitaZahnfabrik, BadSackingen Germany) spectrophotometer, and the color was greater than A3 for three times.
An isolated silicone rubber impression was obtained by a secondary method. Silicone rubber impressions of the experimental group and the control group were delivered to the Invisible Appliance Manufacturing Company (Ailicen Medical Technology Co., LTD., Xi ‘an) in order for a clear aligner to be designed and manufactured. A thermoforming film loaded with hydrogen peroxide sustained-release gel was used to produce clear aligner that was fitted to the isolated dentition mold of the experimental group. An untreated thermoforming film was used to produce invisible appliances that were fitted to the isolated dentition mold of the conditional control group. Attachment bonding was performed on the buccal surface of teeth to simulate clinical practice.
Before bleaching experiments, the surfaces of teeth were divided into mesial and distal regions according to the axial ridge of the central buccal surface. Protective acid-resistant nail polish was then applied to the distal area of each tooth to insulate the influence of bleach on this area of the tooth surface for subsequent (in the part II). The color parameters of each tooth were measured with a Vita Easyshade V spectrophotometer (Vita Zahnfabrik, Bad Sackingen, Germany). Measurements were conducted in a ellipses area on the mesial surface of the premolars, as shown in Figure 1, and three consecutive measurements were made for each tooth, thus allowing calculation of a mean value. Color measurement was performed using the International Commission on illumination (CIE) L*, a* b* chromaticity system and the measured color parameters were given as L, a* and b*.
The dentition in the experimental group was fitted with a sustained-release hydrogen peroxide clear aligner, the dentition in the control group was fitted with an ordinary clear aligner and the dentition in the blank group was used without a clear aligner. The three groups of dentition molds were then placed in the device shown in Figure 2. The three groups of dentition molds were then immersed in artificial saliva which was allowed to flow through the system to simulate the flow rate of human saliva (0.5 mL/min). To simulate the oral environment, the experiment was carried with an electronic thermostat set to a temperature of 37 °C. According to previous relevant studies, the home-use bleaching treatment usually lasted approximately 1 month (do Amaral et al., 2012). In order to ensure the accuracy and credibility of the results, this experiment lasted 40 days considering the low concentration of hydrogen peroxide in this new type film. The aligners were updated every 10 days and the color parameters (L, a* and b*) of teeth was measured every 10 days.
After bleaching experiment, color parameters were determined by the same researcher during the same time period under natural light conditions. A Vita Easyshade V spectrophotometer was used to determine the color parameters on the mesial buccal surface of the three groups of isolated teeth. After measurement, the sustained release hydrogen peroxide clear aligner in the experimental group and the ordinary clear aligner in the control group were replaced. Then, the three groups of detached teeth were placed into the in vitro device for further treatment. The bleaching experiment lasted for 40 days and parameters were measured every 10 days. Changes of color in the isolated teeth were analyzed and the value of ΔL*, Δa*, Δb* and ΔE: ΔL* = L* (post)-L* (pre), Δa* = a* (post)-a* (pre) and Δb* = b* (post)-b* (pre). The color difference for each group of teeth before treatment and at each measurement point was calculated by the international DE76 color difference formula using ΔE as a difference in color:
Data were statistically processed, analyzed and plotted by SPSS version 23.0 statistical software (IBM Corp., Armonk, NY, United States of America) and Graph Pad Prism software (version 8). x ± s was used to describe the measurement data, analysis of variance (ANOVA) was used to compare the mean values of variables between groups, and Tukey’s test was used for multiple comparisons between groups if there were significant differences in the test results. p < 0.5 was considered statistically significant.
The sample was fixed on the base of a mechanical universal testing machine, and the direction of shear force perpendicular was adjusted to the top plane of the attachment. The force was set at a speed of 1 mm/min until the attachment fell off. The maximum shear force (F) (unit: N) and the attachment bonding area (A) (unit: mm2) was recorded. To calculate shear strength, we used the following formula: Shear bond strength
Data were statistically processed, analyzed and plotted by SPSS version 23.0 statistical software (IBM Corp., Armonk, NY, United States of America) and Graph Pad Prism eight software (version 8). x ± s was used to describe the measurement data, unpaired t-test was used for comparison between groups, and p < 0.05 was considered statistically significant.
Figure 3A showed the process of preparation for a thermoforming film loaded with hydrogen peroxide sustained-release gel. MSN were prepared by the CTAB template method, mixed with a certain amount of DA and ALG and then polymerized through the dopamine interface. The crosslinking reaction of ALG ions built a stable interpenetrating network gel on the surface of the thermoforming film. We immersed this film in 6 wt% hydrogen peroxide solution and loaded the hydrogen peroxide into the MSN channels by capillary action. Figure 3B showed the MSN observed at 50000× by SEM. As shown in Figure 3C, the gel was evenly distributed on the unilateral surface of the diaphragm; there were no cracks in the stratification area. Figure 3D showed A large number of MSN were bound within the gel.
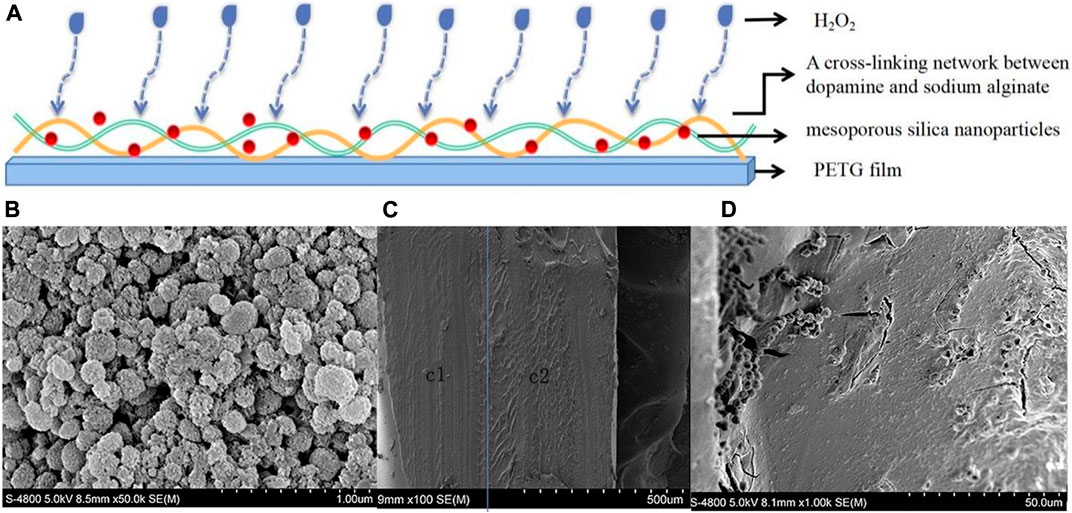
FIGURE 3. (A) Preparation of a thermoforming film loaded with hydrogen peroxide sustained-release gel. (B) MSN observed at 50000× by SEM. (C) The composite gel layer was closely combined with the thermoforming film when observed at 100× by SEM. c1was the thermoforming film; c2 was the composite gel layer. (D) A large number of MSN were bound within the gel when observed at 10000× by SEM.
The L value for extracted teeth in the experiment group increased while the a* and b* values decreased. However, the L value of extracted teeth in the conditional control group and blank control group decreased while the a* and b* values increased (Figure 4).

FIGURE 4. The change in ΔL, Δa and Δb during bleaching for 40 days. (A) The change in ΔL. (B) The change in Δa. (C) The change in Δb. EG refers to Experimental group. CG1refers to Conditional control group. CG2refers to Blank control group.
The values of ΔL, Δa, Δb and ΔE are shown in Table 1; values in the experiment group were significantly different than those in the conditional control group and the blank control group. The value of ΔL and ΔE in the experiment group was positive, while the value of ΔL in the control group was negative; The value of Δa and Δb in the experiment group was negative, while the value of Δa and Δb in the control group was positive. There was significant difference for these values when compared between the experimental group and the other two groups (p < 0.05). For the experimental group, within the first 10 days of bleaching treatment, the color change (ΔE) of isolated teeth changed by more than 4 chromatic aberration units. Subsequently, the rate of tooth color changed more slowly, reaching 8.65 color difference units by 40 days (Figure 5).
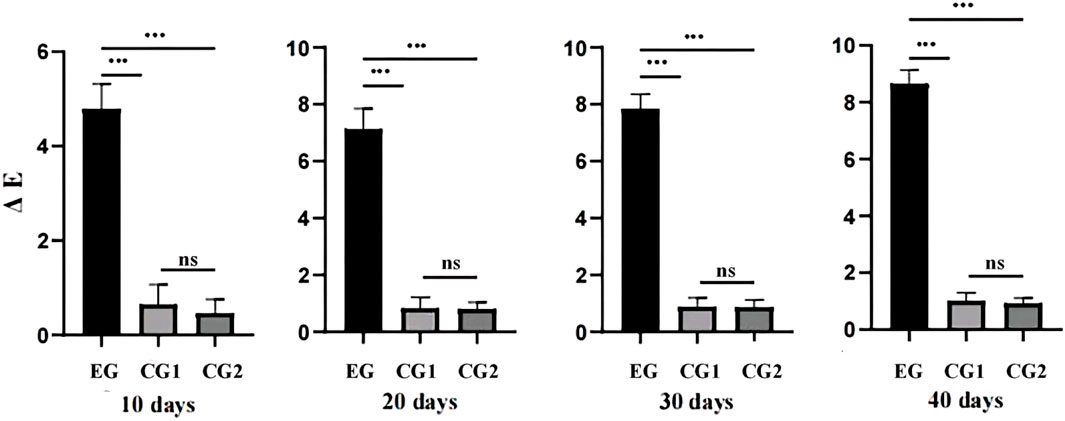
FIGURE 5. The trends for change in color difference (ΔE) in the three groups during bleaching for 40 days. EG refers to Experimental group; CG1refers to Conditional control group; CG2refers to Blank control group.
The shear bond strength of the experimental group is 22.78 ± 2.28MPa, the shear bond strength of the conditional control group is 22.21 ± 2.77 MPa. Figure 6 shows the distribution of shear bond strength values of the two groups of samples. It can be seen intuitively that the distribution areas of the two groups of measured values are basically the same. For t-test, p > 0.05, there was no significant difference in shear bond strength between the two groups.
This study aimed to develop a thermoforming film loaded with hydrogen peroxide sustained-release gel and determine its bio-compatibility and efficiency on changing the coloration of isolated teeth. Previous studies simply considered aligners as trays for bleaching (Levrini et al., 2020). In this study, we innovatively explored the application of an aligner film loaded gel as a drug carrier. With the development of nanotechnology, nanoscale materials have attracted increasing levels of attention in drug delivery, diagnosis, medical imaging and engineering. MSN with pore sizes ranging from 2 nm to 50 nm are particularly suitable for drug delivery and biomedical applications (Rao et al., 2019; Samyn, 2021). Therefore, MSN was chosen as the main drug carrier in this study. ALG is widely used in hydrogel materials such as drug control carriers because of its good bio-compatibility and mild gelling conditions (Ganesh et al., 2013). However, ALG is associated with poor adsorption performance along with poor drug loading and drug release effects; this ALG needs to be modified for effective clinical application. DA is a popular form of polymer material. As an important component of melanin that is widely distributed in the human body, DA has very high levels of biosafety (Samyn, 2021). More importantly, DA can be deposited on almost any type of substrate with excellent adhesion ability (Samyn, 2021). Therefore, in this study, dopamine was used to modify sodium alginate hydrogel. (Cheng et al., 2023). The gel formed an interpenetrating network and was combined a large number of MSN within a thermoforming film; thus, drug loading function was associated with this new thermoforming film. As shown in Figure 3C, D, the gel was evenly distributed on the unilateral surface of the film, and a large number of MSN were bound in the gel (Addisu et al., 2018). We demonstrated that the aligner film loaded with ALG-DA/MSN gel can realize the slow release of hydrogen peroxide (Fang et al., 2022). What’s more, compared with ordinary aligner film, the elastic modulus of the aligner film loaded with ALG-DA/MSN gel increased, while the tensile strength and elongation at break of that decreased (p < 0.05); Fog increased and light transmittance decreased (p < 0.05); While transmittance is greater than 80%. The results of cytotoxicity evaluation showed that the aligner film loaded with ALG-DA/MSN gel had no significant effect on the proliferation of human gingival fibroblasts (Fang et al., 2022). Therefore, the used of an aligner film loaded with ALG-DA/MSN gel as a drug carrier was clearly feasible.
Jung YS evaluated the efficacy of a brush-off patch containing 3.0% hydrogen peroxide and determined that showed color changes at both 7 and 14 days after patch application (Jung et al., 2019). Levrini L explored the tooth whitening effectiveness of trays with no reservoirs (Invisalign aligners or Vivera retainers used as bleaching trays) and found that the whitening was effective and the patients were completely satisfied with the results (Levrini et al., 2020). It has been reported in the literature that the clinical discrimination threshold of CIE76 color difference is 1.0–3.7 for restorations or intraoral teeth (Johnston and Kao, 1989; Seghi et al., 1989; Pandian et al., 2017). In this study, (Sabouni et al., 2023),we found that within the first 10 days of bleaching treatment, the color change (ΔE) of isolated teeth changed by more than 4 chromatic aberration units; this was higher than the discernibility threshold reported in the literature, thus indicating that the color change of isolated teeth was discernible by eye. Subsequently, the rate of tooth color changed more slowly, reaching 8.65 color difference units at 40 days. The change of tooth color following bleaching was reflected by three aspects: the increase of L value represented an increase of brightness while the decrease of a* and b* values indicated a change of color from blue to green, thus indicating an overall trend for whitening. This is consistent with the results described in previous literature (Jung et al., 2019; Levrini et al., 2020), thus indicating that the sustained release hydrogen peroxide clear aligner has a good bleaching effect on isolated teeth, and the efficiency of bleaching was higher in the early stage of bleaching treatment.
The attachment is an important auxiliary device of the clear aligner, which transfers the orthodontic force from the aligner to the crown and root. These can control the point of action along with the direction and amount of force applied (Yaosen et al., 2021). Attachments come in different shapes that help increase retention and provide better control for teeth movement (Laohachaiaroon et al., 2022). The attachment consists of a composite resin bonded to the tooth surface. Adhesion failure may lead to attachment loss on the tooth surface (Abu Alhaija et al., 2010; Alsaud et al., 2022); this loss may cause significant clinical problems, including prolonged treatment time, increased frequency of review, and affect the prognosis of treatment (Dasy et al., 2015). Previous study has shown that the adhesive strength of brackets decreased, irrespective of whether 25%–35% hydrogen peroxide or 10%–20% urea peroxide gel is used (Lima et al., 2010). However, there are few reports on the change of adhesive strength of composite resin after bleaching on normal enamel surface. Considering the potential effect of bleaching active ingredients on the adhesive adhesion performance, bleaching treatment should be arranged after attachment bonding (Sword and Haywood, 2020). Our results showed that the mean SBS of the attachment treated with the sustained release hydrogen peroxide clear aligner in the experimental group was 22.78 MPa, while that of the control group was 22.21 MPa after application of the conventional clear aligner. Statistical analysis showed that there was no significant difference in SBS between the two groups, indicating that the attachments SBS of the sustained release hydrogen peroxide clear aligner was the same as that of the ordinary clear aligner in the control group. Futhermore, previous studies pointed out that the requirement for brackets in the fixed appliance was 6–8 Mpa (Chu et al., 2015). Bashair A explored the bonding of clear aligner composite attachments to ceramic materials in an vitro study and pointed out that the highest SBS was gained by the interaction of AA with AUB and Filtek Z350 composite was 21.80 ± 3.86 Mpa (Alsaud et al., 2022). Wener Chen pointed out that the SBS of attachment was about 20 Mpa (Chen et al., 2021). Our results in experiment group had not much difference with the relevant results in previous study (Chu et al., 2015; Chen et al., 2021; Alsaud et al., 2022). This phenomenon proved that sustained release hydrogen peroxide bleaching treatment over 40 days would not reduce the SBS of the attachment on the isolated teeth to some extent. The results of this study lay a good foundation for the application of the appliance in clinical practice.
This study provided a good direction for the application of clear aligners in clinical practice. Here, the application of using an aligner film loaded gel as a drug carrier was innovatively explored. Another innovation in this study was that we immersed the orthodontic appliance in artificial saliva to reflect the flow rate of human saliva to simulate the dynamic bleaching process instead of using a static bleaching process. However, there are still several limitations that need to be considered. First, bleaching increased the porosity of the superficial enamel structure and reduced protein concentration and promoted organic matrix degradation (Berger et al., 2010; Monterubbianesi et al., 2021). Whether the thermoforming film loaded with hydrogen peroxide sustained-release gel would affect the surface hardness and surface structure of teeth has yet to be investigated. Second, this study only used isolated teeth as the experimental object. Although the oral environment was simulated, oral experiments using an animal model have yet to be carried out. Thus, the clinical significance of our work has yet to be defined.
For the first time, we report the successfulin developing a thermoforming film loaded with sustained-release hydrogen peroxide gel, crucial for the bleaching treatment during the clear aligner treatment. The wide application of the film in clinic can save patients’ treatment time and improve the satisfaction of treatment. This study developed a new film. The experimental group, conditional control group and blank control group were used to test the bleaching performance of this new type of film, and the influence of this diaphragm on the attachment SBS was analyzed and explored by a slow cutting machine. We demonstrated that the application of using an aligner film loaded gel as a drug carrier was feasible and the thermoforming film featuring the sustained release of hydrogen peroxide had a good bleaching effect on isolated teeth and had no significant influence on the shear bonding strength for attachment. The findings in this study indicate that this new type of film has potential clinical value, which is conducive to our further exploration of this type of film. While the influence of this new film on the enamel structure is uncertain. Ongoing efforts are exploring the influence of the thermoforming film loaded with sustained-release hydrogen peroxide gel on the structure of the tooth surface. It is beneficial to the development of material science in the field of orthodontics to perfect the study of this kind of film.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the ethics committee of Air Force Medical University, China (IACUC-20210760). The patients/participants provided their written informed consent to participate in this study.
YC and SF: conceptualization, methodology, writing of the original draft, investigation, project administration, conduct the experiment and final editing. XlL: supervision, co-project administration, data collection, feedback, and making substantive changes. XL: software, validation, and formal analysis. ZS: visualization and investigation. YM and ZJ: contributed to the conception of the study and revised the manuscript; All authors participated in the distribution of the survey.
This work was supported by grants from the National Clinical Research Center for Oral Diseases (LCA202009); Key research and Development Program of Shaanxi Province (2021SF-050); National Clinical Research Center for Oral Diseases (LCA202202); National Clinical Research Center for Oral Diseases (LCB202209); Key Research and Development Program of Shaanxi Province (2022SF-227); The Central and western Orthodontic research project of youth Clinical Research Fund of Chinese Stomatological Association (CSA-MWO2021–07); College Science and Technology Innovation Plan of Shanxi Education Department (2021 L242).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2023.1137672/full#supplementary-material
Abu Alhaija, E. S., Abu AlReesh, I. A., and AlWahadni, A. M. (2010). Factors affecting the shear bond strength of metal and ceramic brackets bonded to different ceramic surfaces. Eur. J. Orthod. 32 (3), 274–280. doi:10.1093/ejo/cjp098
Addisu, K. D., Hailemeskel, B. Z., Mekuria, S. L., Andrgie, A. T., Lin, Y. C., and Tsai, H. C. (2018). Bioinspired, manganese-chelated alginate-polydopamine nanomaterials for efficient in vivo T1-weighted magnetic resonance imaging. ACS Appl. Mater. interfaces 10 (6), 5147–5160. doi:10.1021/acsami.7b13396
Alkahtani, R., Stone, S., German, M., and Waterhouse, P. (2020). A review on dental whitening. J. Dent. 100, 103423. doi:10.1016/j.jdent.2020.103423
Alqahtani, M. Q. (2014). Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 26 (2), 33–46. doi:10.1016/j.sdentj.2014.02.002
Alsaud, B. A., Hajjaj, M. S., Masoud, A. I., Abou Neel, E. A., Abuelenain, D. A., and Linjawi, A. I. (2022). Bonding of clear aligner composite attachments to ceramic materials: An in vitro study. Mater. (Basel, Switz. 15 (12), 4145. doi:10.3390/ma15124145
Berger, S. B., Cavalli, V., Martin, A. A., Soares, L. E., Arruda, M. A., Brancalion, M. L., et al. (2010). Effects of combined use of light irradiation and 35% hydrogen peroxide for dental bleaching on human enamel mineral content. Photomed. laser Surg. 28 (4), 533–538. doi:10.1089/pho.2009.2506
Briso, A., Silva, Ú., Souza, M., Rahal, V., Jardim Júnior, E. G., and Cintra, L. (2018). A clinical, randomized study on the influence of dental whitening on Streptococcus mutans population. Aust. Dent. J. 63 (1), 94–98. doi:10.1111/adj.12569
Carlos, L. N., Jorge, O. S., Paranhos, L. R., de Freitas Vincenti, S. A., and de Carvalho Panzeri Pires-de-Souza, F. (2018). The effect of different bleaching treatments and thermal-mechanical cycling on the shear bond strength of orthodontic brackets. Turkish J. Orthod. 31 (4), 110–116. doi:10.5152/TurkJOrthod.2018.17055
Chen, W., Qian, L., Qian, Y., Zhang, Z., and Wen, X. (2021). Comparative study of three composite materials in bonding attachments for clear aligners. Orthod. craniofacial Res. 24 (4), 520–527. doi:10.1111/ocr.12465
Cheng, M., Cui, Y., Guo, Y., Zhao, P., Wang, J., Zhang, R., et al. (2023). Design of carboxymethyl chitosan-reinforced pH-responsive hydrogels for on-demand release of carvacrol and simulation of release kinetics. Food Chem. 405, 134856. doi:10.1016/j.foodchem.2022.134856
Cheng, Y., Gao, J., Fang, S., Wang, W., Ma, Y., and Jin, Z. (2022). Torque movement of the upper anterior teeth using a clear aligner in cases of extraction: A finite element study. Prog. Orthod. 23 (1), 26. doi:10.1186/s40510-022-00421-8
Cheng, Y., Liu, X., Chen, X., Li, X., Fang, S., Wang, W., et al. (2022). The three-dimensional displacement tendency of teeth depending on incisor torque compensation with clear aligners of different thicknesses in cases of extraction: A finite element study. BMC oral health 22 (1), 499. doi:10.1186/s12903-022-02521-7
Chu, K., Wang, H., Zheng, Z., and Li, Q. (2015). Comparative study of three bonding methods in attaching removable thermoplastic appliances. Hua Xi Kou Qiang Yi Xue Za Zhi 33, 497–499. doi:10.7518/hxkq.2015.05.012
Dasy, H., Dasy, A., Asatrian, G., Rózsa, N., Lee, H. F., and Kwak, J. H. (2015). Effects of variable attachment shapes and aligner material on aligner retention. Angle Orthod. 85 (6), 934–940. doi:10.2319/091014-637.1
do Amaral, F. L., Sasaki, R. T., da Silva, T. C., França, F. M., Flório, F. M., and Basting, R. T. (2012). The effects of home-use and in-office bleaching treatments on calcium and phosphorus concentrations in tooth enamel: An in vivo study. J. Am. Dent. Assoc. 143 (6), 580–586. doi:10.14219/jada.archive.2012.0236
Donmez, M. B., Olcay, E. O., and Demirel, M. (2021). Influence of coloring liquid immersion on flexural strength, Vickers hardness, and color of zirconia. J. Prosthet. Dent. 126 (4), 589. doi:10.1016/j.prosdent.2020.11.020
Fang, S., Liu, C., Liu, Q., and etal, (2022). A study on the mechanical properties and biological properties of a novel slow-release hydrogen peroxide invisible appliance materials. J. Pract. Stomatol. 38, 3. doi:10.3969/j.issn.1001-3733.2022.03.002
Fattahi, M., Ezzatzadeh, E., Jalilian, R., and Taheri, A. (2021). Micro solid phase extraction of cadmium and lead on a new ion-imprinted hierarchical mesoporous polymer via dual-template method in river water and fish muscles: Optimization by experimental design. J. Hazard. Mater. 403, 123716. doi:10.1016/j.jhazmat.2020.123716
Ganesh, N., Hanna, C., Nair, S. V., and Nair, L. S. (2013). Enzymatically cross-linked alginic-hyaluronic acid composite hydrogels as cell delivery vehicles. Int. J. Biol. Macromol. 55, 289–294. doi:10.1016/j.ijbiomac.2012.12.045
Haywood, V. B. (2007). Tooth whitening: Indications and outcomes of nightguard vital bleaching. Germany: Quintessence.
Johnston, W. M. (2009). Color measurement in dentistry. J. Dent. 37 (1), e2–e6. doi:10.1016/j.jdent.2009.03.011
Johnston, W. M., and Kao, E. C. (1989). Assessment of appearance match by visual observation and clinical colorimetry. J. Dent. Res. 68 (5), 819–822. doi:10.1177/00220345890680051301
Jung, Y. S., Jo, H. Y., Ahn, J. H., Kim, J. Y., Jin, M. U., Cho, M. J., et al. (2019). In vivo and in vitro assessment of the bleaching effectiveness of a brush-off patch containing 3.0% hydrogen peroxide. Clin. oral Investig. 23 (6), 2667–2673. doi:10.1007/s00784-018-2675-8
Kwon, S. R., and Wertz, P. W. (2015). Review of the mechanism of tooth whitening. J. esthetic Restor. Dent. official Publ. Am. Acad. Esthetic Dent. 27 (5), 240–257. doi:10.1111/jerd.12152
Laohachaiaroon, P., Samruajbenjakun, B., and Chaichanasiri, E. (2022). Initial displacement and stress distribution of upper central incisor extrusion with clear aligners and various shapes of composite attachments using the finite element method. Dent. J. 10 (6), 114. doi:10.3390/dj10060114
Levrini, L., Paracchini, L., Bakaj, R., Diaconu, A., and Cortese, S. (2020). Dental bleaching during orthodontic treatment with aligners. Int. J. esthetic Dent. 15 (1), 44–54.
Lima, A. F., Fonseca, F. M., Cavalcanti, A. N., Aguiar, F. H., and Marchi, G. M. (2010). Effect of the diffusion of bleaching agents through enamel on dentin bonding at different depths. Am. J. Dent. 23 (2), 113–115.
Monterubbianesi, R., Tosco, V., Bellezze, T., Giuliani, G., Özcan, M., Putignano, A., et al. (2021). A comparative evaluation of nanohydroxyapatite-enriched hydrogen peroxide home bleaching system on color, hardness and microstructure of dental enamel. Mater. (Basel, Switz. 14 (11), 3072. doi:10.3390/ma14113072
Nitu, S., Milea, M. S., Boran, S., Mosoarca, G., Zamfir, A. D., Popa, S., et al. (2022). Experimental and computational study of novel pyrazole azo dyes as colored materials for light color paints. Mater. (Basel, Switz. 15 (16), 5507. doi:10.3390/ma15165507
Pandian, A., Ranganathan, S., and Padmanabhan, S. (2017). Enamel color changes following orthodontic treatment. Indian J. Dent. Res. official Publ. Indian Soc. Dent. Res. 28 (3), 330–336. doi:10.4103/ijdr.IJDR_404_15
Paravina, R. D., Pérez, M. M., and Ghinea, R. (2019). Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J. esthetic Restor. Dent. official Publ. Am. Acad. Esthetic Dent. 31 (2), 103–112. doi:10.1111/jerd.12465
Pereira, R., Silveira, J., Dias, S., Cardoso, A., Mata, A., and Marques, D. (2022). Bleaching efficacy and quality of life of different bleaching techniques - randomized controlled trial. Clin. oral Investig. 26 (12), 7167–7177. doi:10.1007/s00784-022-04678-5
Pérez, M. M., Herrera, L. J., Carrillo, F., Pecho, O. E., Dudea, D., Gasparik, C., et al. (2019). Whiteness difference thresholds in dentistry. Dent. Mater. official Publ. Acad. Dent. Mater. 35 (2), 292–297. doi:10.1016/j.dental.2018.11.022
Piknjač, A., Soldo, M., Illeš, D., and Knezović Zlatarić, D. (2021). Patients' assessments of tooth sensitivity increase one day following different whitening treatments. Acta stomatol. Croat. 55 (3), 280–290. doi:10.15644/asc55/3/5
Pradhan, M. K., Rao, T. L., Goutam, U. K., and Dash, S. (2020). Probing structural and photophysical features of Eu3+ activated NaCdPO4 orthophosphate phosphor. Spectrochimica acta. Part A, Mol. Biomol. Spectrosc. 240, 118593. doi:10.1016/j.saa.2020.118593
Putrino, A., Abed, M. R., and Lilli, C. (2022). Clear aligners with differentiated thickness and without attachments - a case report. J. Clin. Exp. Dent. 14 (6), e514–e519. doi:10.4317/jced.59618
Rao, Z., Liu, S., Wu, R., Wang, G., Sun, Z., Bai, L., et al. (2019). Fabrication of dual network self-healing alginate/guar gum hydrogels based on polydopamine-type microcapsules from mesoporous silica nanoparticles. Int. J. Biol. Macromol. 129, 916–926. doi:10.1016/j.ijbiomac.2019.02.089
Revilla-León, M., Umorin, M., Özcan, M., and Piedra-Cascón, W. (2020). Color dimensions of additive manufactured interim restorative dental material. J. Prosthet. Dent. 123 (5), 754–760. doi:10.1016/j.prosdent.2019.06.001
Sabouni, W., Muthuswamy Pandian, S., Vaid, N. R., and Adel, S. M. (2023). Distalization using efficient attachment protocol in clear aligner therapy-A case report. Clin. case Rep. 11 (1), e6854. doi:10.1002/ccr3.6854
Sadeghian, S., Garavand, S., and Davoudi, A. (2021). Effect of different bleaching treatment protocols on shear bond strength of bonded orthodontic brackets with no-primer adhesive resin. J. Orthod. Sci. 10, 11. doi:10.4103/jos.JOS_5_19
Samyn, P. (2021). A platform for functionalization of cellulose, chitin/chitosan, alginate with polydopamine: A review on fundamentals and technical applications. Int. J. Biol. Macromol. 178, 71–93. doi:10.1016/j.ijbiomac.2021.02.091
Seghi, R. R., Hewlett, E. R., and Kim, J. (1989). Visual and instrumental colorimetric assessments of small color differences on translucent dental porcelain. J. Dent. Res. 68 (12), 1760–1764. doi:10.1177/00220345890680120801
Society of Prosthodontics, Chinese Stomatological Saaociation (2021). Guideline of tooth bleaching technology. Chin. J. Stomatol. 56, 1191–1193. doi:10.3760/cma.j.cn112144-20210903-00395
Sword, R. J., and Haywood, V. B. (2020). Teeth bleaching efficacy during clear aligner orthodontic treatment. Compend. continuing Educ. Dent. (Jamesburg, N. J. 1995) 41 (5), e11–e16.
Yaosen, C., Mohamed, A. M., Jinbo, W., Ziwei, Z., Al-Balaa, M., and Yan, Y. (2021). Risk factors of composite attachment loss in orthodontic patients during orthodontic clear aligner therapy: A prospective study. BioMed Res. Int. 2021, 1–6. doi:10.1155/2021/6620377
Keywords: dental blenching, materials, hydrogen peroxide, sustained release, attachment, shear bonding strength
Citation: Cheng Y, Fang S, Liu X, Li X, Song Z, Ma Y and Jin Z (2023) Development and performance of a clear aligner film loaded with sustained release hydrogen peroxide gel. Front. Mater. 10:1137672. doi: 10.3389/fmats.2023.1137672
Received: 04 January 2023; Accepted: 09 March 2023;
Published: 20 March 2023.
Edited by:
Farooq Sher, Nottingham Trent University, United KingdomReviewed by:
Lizie Daniela Tentler Prola, Independent researcher, Greenville, NC, United StatesCopyright © 2023 Cheng, Fang, Liu, Li, Song, Ma and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuolin Jin, enVvbGluakAxNjMuY29t; Yanning Ma, Ymx1ZXNreWFiYzEwMDdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.