
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mater. , 01 February 2022
Sec. Semiconducting Materials and Devices
Volume 9 - 2022 | https://doi.org/10.3389/fmats.2022.845664
This article is part of the Research Topic Micro-Nano Optics and Photocatalysis Materials, Devices, and Applications View all 11 articles
Photocatalysis is regarded as a green technology to degrade organic dyes driven by light energy. The selection of photocatalyst restricts the development of photocatalytic technology. Aluminate is a kind of potential broad-gap semiconductor photocatalyst and also an excellent phosphor substrate materials. The physical and chemical properties of aluminate are strongly dependent on the preparation method. Insight into the influence of synthesis methods on photocatalytic activity of aluminate based photocatalysts is helpful for the development of novel aluminate based photocatalysts. In this paper, the typical synthesis methods of aluminate photocatalysts, ion-doped aluminate based photocatalysts and heterojunction type aluminate photocatalysts, and their photocatalytic activities are reviewed. Based on the energy band theory, the photocatalytic mechanisms of single component aluminate photocatalyst, ion-doped aluminate based photocatalyst, and heterojunction type aluminate photocatalyst were reviewed. The future development of aluminate based photocatalyst will give priority to the salinization of aluminate modified by silver and other metal particles and the photocatalytic application of activated ion modified aluminate based phosphors.
The economic development of all countries in the world has a great impact on the environment, especially human beings’ thirst for necessities such as leather, textiles, medicines, food and so on, and the increasing demand for dyes, thus causing different degrees of pollution to the environment. (Wang et al., 2021a; Piriyanon et al., 2021; Ibrahim et al., 2022; Lahiri et al., 2022). Since water is needed for all kinds of necessities, and these factories are built along rivers, direct discharge of organic dyes into rivers will cause devastating pollution to the environment. This forces mankind to consider the problem of environmental pollution while developing. To deal with the pollution of organic dyes to water resources, the countries all over the world have invested a lot of money to solve this problem. Many mature methods have been developed to solve the problem of organic dye contamination, including: 1) Thermocatalytic technique driven by thermal energy. (Bao et al., 2020; Forouzesh et al., 2021) 2) Electrocatalytic technique driven by electric field or magnetic field. (Li et al., 2017; Szroeder et al., 2019). 3) Piezoelectric catalytic technique driven by mechanical energy. (Cheng et al., 2021a; Cheng et al., 2021b) 4) Biodegradation technique (Ghalei and Handa, 2022; Mathew et al., 2022). 5) Adsorption technique (Gao et al., 2021; Liu et al., 2022). 6) Photocatalytic technique driven by light energy (Pu et al., 2021; Gao et al., 2022; Zhang et al., 2022). 7) Multi - technology hybrid degradation of organic matter. (Wang et al., 2021b). Among these technologies, the catalyst is the key factor affecting the degradation rate of dyes.
Recently, a series of photocatalysts have been developed to degrade organic dyes. There are three main types of photocatalysts (Wang et al., 2017; Wang et al., 2020a; Rajabathar et al., 2020; Shifa Wang et al., 2020; Cheng et al., 2021c; Wang et al., 2021c; Ivashchenko et al., 2021; Kim et al., 2021; Kumar and Luxmi, 2021; Musa et al., 2021; Taazayet et al., 2021): 1) Single-component photocatalysts 2) Photocatalyst of two components 3) Ternary or multi-component photocatalysts. For a long time, single-component photocatalysts have been widely favored by researchers because of their advantages of simple composition and easy synthesis. Spinel aluminate is a kind of such single component photocatalyst, which has wide application prospect in the field of photocatalysis due to its high chemical and thermal stability, high catalytic activity, high specific surface area, and high surface defects and active sites. (Kharlanov et al., 2019; Boudiaf et al., 2020; Chen et al., 2021). Spinel aluminate generally has the structure of MB2O4, A is generally Mg, Ca, Sr, Ba, Co, Ni, Cu, Mn, and other bivalent metal ions, B is Al, Fe, Ga, Cr, and other trivalent metal ions. (Sharma et al., 2014; Sriram et al., 2020). Among these spinel aluminates, MAl2O4 (A = Mg, Sr, and Ba) has attracted extensive attention from researchers due to its excellent physicochemical properties make it can be used as long afterglow phosphor base materials, lightweight helmets, photoelectric devices, microwave dielectric capacitors, and high temperature windows, etc. (Han et al., 2018; Takebuchi et al., 2020; Basyrova et al., 2021; Kiryakov et al., 2021) Simultaneously, MAl2O4 is a kind of environmental friendly material, in the photocatalytic field, especially in the degradation of organic dyes has a good application. (Wang et al., 2019a; Shifa Wang et al., 2020; Liu et al., 2022). Therefore, the work of MAl2O4 and MAl2O4 based photocatalysts in the degradation of organic dyes is reviewed, which has important research significance for the development of new aluminate based photocatalysts.
It is well known that the photocatalytic activity of aluminate based photocatalysts is strongly dependent on the preparation method. Different preparation methods will produce aluminate with different morphology, which may have special defect structure, thus enhancing the photocatalytic activity of aluminate. Ion doping and heterostructure construction will accelerate the transfer and separation of electrons and holes, and improve the photocatalytic activity of the system. Therefore, the influence of ion doping and heterostructure construction on the photocatalytic activity of aluminate photocatalyst should not be underestimated. In this paper, we start from the preparation of aluminate based photocatalysts, reviewed the preparation of single-component aluminate, metal ion doped aluminate and multiple heterojunction aluminate based photocatalysts, and their applications in the field of photocatalysis. Based on electron hole pair transfer, separation and energy band theory, the photocatalytic mechanism of single component aluminate and heterojunction aluminate photocatalysts was reviewed, and which provided technical support for the development of aluminate based photocatalysts.
The photocatalytic activity of catalysts strongly depends on morphology, size, dimension, specific surface area, defec,t and impurity. Ultimately, these parameters affect the electron hole pair transfer and separation efficiency of the photocatalyst, which in turn accelerates the oxidation or reduction capacity of the electrons and holes. The dye is oxidized or reduced to form non-toxic small organic molecules. To regulate these parameters, the special synthesis methods are necessary. Currently, the MAl2O4(M = Mg, Sr, and Ba) based photoatalysts have been synthesized in a number of ways to construct specific defect structures.
Spinel aluminate is a wide-gap semiconductor, such as BeAl2O4 (6.450 eV) (Ching et al., 2001), MgAl2O4 (3.923 eV) (Wang et al., 2019a), CaAl2O4 (7.400 eV) (Moirangthem et al., 2019), SrAl2O4 (3.984 eV) (Shifa Wang et al., 2020), BaAl2O4 (3.910 eV) (Nair and Pillai, 2021), MnAl2O4 (4.030 eV) (Bhavani et al., 2018), FeAl2O4 (1.780 eV) (Mu et al., 2017), CoAl2O4 (1.948 eV) (Gao et al., 2018), NiAl2O4 (3.000 eV) (Chellammal Gayathri et al., 2021), CuAl2O4 (2.920 eV) (Potbhare et al., 2019), and ZnAl2O4 (3.800 eV) (Shang-Pan et al., 2020). Based on the obtained optical band gap value and band theory, the conduction band potential and valence band potential of MAl2O4 were calculated.
Where, X of MAl2O4 was estimated by Eq. 3, Ee is 4.5 eV and Eg is optical band gap value.
Table 1 shows the X value, conduction band potential and valence band potential of MAl2O4 photocatalyst. The related energy level diagram of MAl2O4 photocatalyst can be described in Figure 1. As can be seen from Figure 1, the band gap value of MAl2O4 (M = Fe, Co, and Cu) is less than 3, which can easily respond to visible light and degrade organic dyes under visible light conditions (Gholami et al., 2016; Mu et al., 2017; Feng et al., 2021). Other aluminate photocatalysts must adopt special preparation methods to introduce impurities or defects if they are to respond to visible light. Wang et al. (Wang et al., 2019b) used amorphous alumina and α-alumina to modify MnAl2O4 spinel type oxides exhibits high visible light photocatalytic activity. However, these mainly introduce impurities in the form of doping or coupling to enhance the photocatalytic activity of single component aluminate. In particular, the band gap values of MAl2O4 (M = Mg, Sr, and Ba) are close to 4, making it difficult to respond to visible light. Traditional methods such as the solid state reaction method, (Ganesh et al., 2004; Canaza-Mamani et al., 2021), the sol-gel method, (Habibi et al., 2017; Salehabadi et al., 2017), the solvothermal method, (Zhu et al., 2012), the hydrothermal method (Sera et al., 2021), and the coprecipitation method (Zawrah et al., 2007) are difficult to make its have special defect structure. In order to make a single component aluminate photocatalytic activity, special preparation methods must be used to enhance the electron and hole pairs transfer and separation ability. Wang et al. (Wang et al., 2019a) synthesized MgAl2O4 photocatalyst with special defective structure by gamma ray irradiation assisted polyacrylamide gel method, which showed that it had high visible light photocatalytic activity for the degradation of methylene blue. However, the extreme conditions such as high energy, high pressure and high temperature are often needed to prepare aluminate photocatalyst with defective structure by special preparation process, which are very difficult to achieve in general laboratory. Therefore, other means must be found to enhance the photocatalytic activity of single component aluminate photocatalyst.
Ion doping is an effective way to enhance the photocatalytic activity of a single component semiconductor photocatalyst. Generally, ion doping can change the band gap value of a single component semiconductor photocatalyst. For the MAl2O4, doping can choose A site substitution and Al site substitution, A site substitution of ion radius should be close to the A site ion. In the synthesis of dense ceramics, the solid state reaction method is relatively better, and the high temperature, and high pressure conditions are easy to doping ions into the lattice of a single component aluminate. However, due to the small specific surface area and porosity of dense ceramics, the photocatalytic degradation of organic dyes is not favorable, which will greatly limit the application of solid phase reaction method in the synthesis of ion doped aluminate photocatalysts. Alam et al. (Alam et al., 2022) synthesized the Cr3+-doped MgAl2O4 nanoparticles by the solution combustion method exhibits excellent photocatalytic activity against Acid Red-88 (AR-88) dye. Solution combustion method is easy to control the morphology of Cr3+-doped MgAl2O4, adjust the doping ratio, reduce the particle size, resulting in a single component of MgAl2O4 exhibit novel physicochemical properties. Chen et al. (Chen et al., 2009) synthesized MgAl2O4:Eu3+ phosphors by hydrothermal method exhibits high photoluminescence properties. Different morphologies of MgAl2O4:Eu3+ phosphors can be obtained by changing the ratio of precursor salts. The SEM images of MgAl2O4:Eu3+ phosphors as shown in Figure 2. The results further show that it is easy to synthesize different morphologies of ion doped aluminate photocatalysts by hydrothermal method. Wang et al. (Wang et al., 2019c) reported that the Mg1–xCoxAl2O4 photocatalysts synthesized by the irradiation assisted polyacrylamide gel route exhibits high photocatalytic activity. The method can be used to synthesize aluminate photocatalysts with different proportions and morphologies, which is beneficial to improve the photocatalytic activity of single component aluminate photocatalysts.
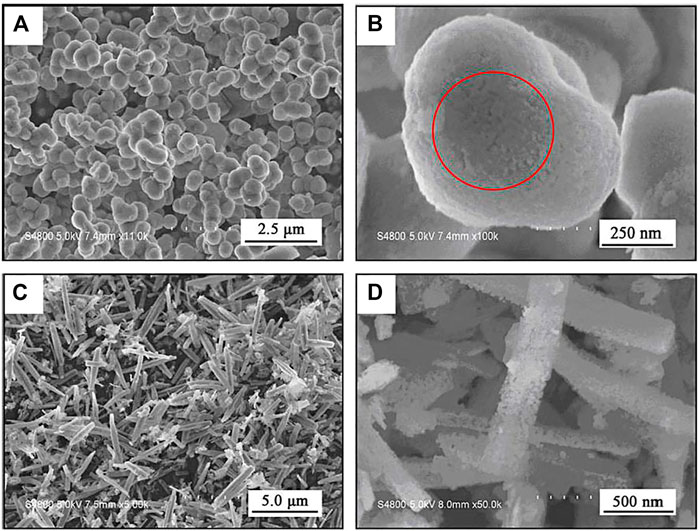
FIGURE 2. SEM images of MgAl2O4:Eu3+ phosphors with different molar ratios of Mg(NO3)2• 6H2O, Al(NO3)3 •9H2O and CO(NH2)2. (A, B) 1:2:10, and (C, D) 1:2:50 (Chen et al., 2009). Adapted from ref. (Chen et al., 2009). Copyright © 2009 Elsevier Inc.
Another way to enhance the photocatalytic activity of semiconductor photocatalysts is to construct multiple photocatalysts with special heterojunction structure. Similarly, the aluminate based phosphors can be used in a similar way to enhance the photoluminescence properties of a single component aluminate (Wang et al., 2020b; Liu et al., 2020). Surface modification of MgAl2O4 with metal particles can enhance its photoluminescence properties due to plasma resonance effect. With the increase of sintering temperature, the metal particles are oxidized, which affects the band gap value of the system. The MgAl2O4: M (M = Mg, Ti, Mn, Co, and Ni) phosphors exhibits wide visible light absorption, suggesting that they have high photocatalytic activity under visible light. Mkhalid et al. (Mkhalid, 2022) synthesized the Ag2O/SrAl2O4/CNT ternary photocatalyst by the sol-gel method exhibits high visible-light-responsive for H2 production. The construction of multiple heterojunctions is beneficial to enhance the electron transport, transfer and separation efficiency of SrAl2O4, and thus improving the photocatalytic activity of the system under visible light irradiation. Sol-gel method has more advantages than solid phase method and coprecipitation method because of its easy composition control and simple synthesis. Hydrothermal method is easy to synthesize the aluminate products with different morphologies, but its application in the construction of multiple heterojunctions is less. Therefore, the sol-gel method is commonly used to synthesize aluminate - based multielement heterostructures.
The photocatalytic activity of aluminate photocatalyst is strongly dependent on the preparation method, ion doping and heterostructure construction.
Due to the large band gap value of MAl2O4(M = Mg, Sr, and Ba) aluminate photocatalyst, there are relatively few studies on its use as photocatalyst alone. Except for MgAl2O4 and BaAl2O4, single component SrAl2O4 has not been used as a photocatalyst to degrade organic dyes. Table 2 shows the photocatalytic activity of MgAl2O4 and BaAl2O4 photocatalyst. Nassar et al. (Nassar et al., 2014) reported the MgAl2O4 photocatalyst prepared by the sol–gel auto combustion method exhibits high photocatalytic activity for the degradation of Reactive Red Me 4BL dye. Qian et al. (Qian et al., 2017) synthesized MgAl2O4 photocatalyst by a simple hydrothermal route exhibits high photocatalytic activity for the degradation of Methylene bule. However, MgAl2O4 synthesized by this method is inadequate in degrading phenol. Li et al. (2011) prepared the mixed amorphous and crystalline MgAl2O4 nanopowders by a simple solution combustion method using glycine and urea as fuel mixtures exhibits high visible light-induced photocatalytic activity for the degradation of Methylene bule. Jiang et al. (2014) synthesized the MgAl2O4 photocatalyst by a sol-gel method exhibits a poor photocatalytic activity for the degradation of various dyes including methyl orange, acid red B and reactive brilliant red K-2G. Parvarinezhad et al. (2019) synthesized the MgAl2O4 nanopowders by one-step solid state reaction method possessed strong light absorption properties in the ultraviolet-visible region. Wang et al. (2019d) synthesized the MgAl2O4 and BaAl2O4 photocatalysts by the polyacrylamide gel method exhibits high photocatalytic activity. The results show that the photocatalytic activity of MAl2O4 synthesized by different preparation methods is different.
Metal ion doping can effectively change the band gap value of aluminate photocatalyst so as to enhance its photocatalytic activity. Different doping ions have different regulation on the band gap of aluminate, so different metal ions doped aluminate show different photocatalytic activity. Table 3 shows the photocatalytic activity of metal ion doped MAl2O4(M = Mg, Sr, and Ba) photocatalyst. Li et al. 2(016) synthesized the Mg1-xZnxAl2O4 spinel nanoparticles by the chemical coprecipitation method have a high photocatalytic activity. The photocatalytic activity of Mg1-xZnxAl2O4 spinel nanoparticles increased with the increasing of x value as shown in Figure 3. When Cu2+ ions were engrafted onto MgAl2O4 nanoparticles by a highly adaptable and energy efficient chemical process, the photocatalytic activity of the system was greatly improved (Mukherjee et al., 2020). The photocatalytic activity of MgAl2O4 can also be improved by introducing Co (Wang et al., 2019c) or Ce (Chen et al., 2019) ions into MgAl2O4. Ce and Mn co-doped MgAl2O4 can further improve the photocatalytic activity of MgAl2O4 (Wang et al., 2019e). Metal ion doping in SrAl2O4 has also been widely used, through Eu2+ and Dy3+ (Park, 2018), Bi (García et al., 2018), Cu (Berlanga et al., 2017), Ce and Mn (Shifa Wang et al., 2020), and rare earth ion (Deepika and Kumar, 2020) doping SrAl2O4, all enhance the photocatalytic activity of SrAl2O4. Similarly, Nd3+ (Mumanga et al., 2021) and Ce and Mn (Wang et al., 2020c) ions are also used in the doping of BaAl2O4, and their photocatalytic activity is greatly improved compared with that of single-phase BaAl2O4.
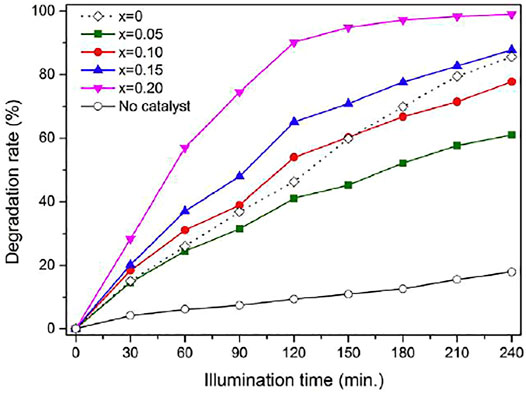
FIGURE 3. Degradation rate curve with x value of Mg1-xZnxAl2O4 spinel nanoparticles (Li et al., 2016). Adapted from ref. (Li et al., 2016). Copyright © 2016 Elsevier Masson SAS.
The construction of multiple heterojunction composite is beneficial to combine the advantages of multiple semiconductor materials and enhance the photocatalytic activity of the system. The MAl2O4 (M = Mg, Sr, and Ba) photocatalyst has a relatively large band gap, which makes it difficult to respond to visible light. Therefore, the semiconductor materials that can respond to visible light are preferentially selected for the construction of heterojunction. Table 4 shows the photocatalytic activity of MAl2O4(M = Mg, Sr, and Ba) based multivariate heterojunction photocatalysts. MgAl2O4 based photocatalyst was constructed by combining various semiconductor materials, and its photocatalytic activity was confirmed to be enhanced (Abbasi Asl et al., 2019; Abbasi Asl et al., 2020; Wang et al., 2021d). Meanwhile, MgAl2O4/CeO2/Mn3O4 ternary heterojunction photocatalyst was constructed by combining CeO2 and Mn3O4, showing high photocatalytic activity for the degradation of methylene blue dye (Li et al., 2021). Aluminate is a very good phosphor base material, the introduction of activated ions will make aluminate luminescence as phosphor. Recently, researchers have found that aluminate activated by metal ions as photocatalysts also have high photocatalytic activity. The photocatalytic activity of SrAl2O4 was greatly enhanced by the construction of multi-component composite SrAl2O4 photocatalysts (García et al., 20162016; Xiao et al., 2018; Liu et al., 2019; Zargoosh and Moradi Aliabadi, 2019; Mavengere and Kim, 2020; Aliabadi et al., 2021). The construction of the heterojunction provides a technical reference for the subsequent study of other aluminate photocatalysts. The photocatalytic activity of aluminate modified by noble metal particles can be greatly improved by plasma resonance effect. However, the modification of Silver particles on the surface of MgAl2O4 easily leads to the hydrolysis of MgAl2O4, which greatly affects the application of MgAl2O4 as a photocatalyst. Zhu et al. (2015) synthesized the Ag/BaAl2O4 photocatalyst shows high photocatalytic activity for the degradation of Gaseous toluene. When the silver content is low, the hydrolysis of BaAl2O4 is inhibited and it can be used as a photocatalyst. Li et al. (2009) constructed the TiO2/BaAl2O4: Eu2+, Dy3+ photocatalyst exhibits high photocatalytic activity. These successful applications provide a new idea for the use of wide-band gap semiconductors as photocatalysts in future research.
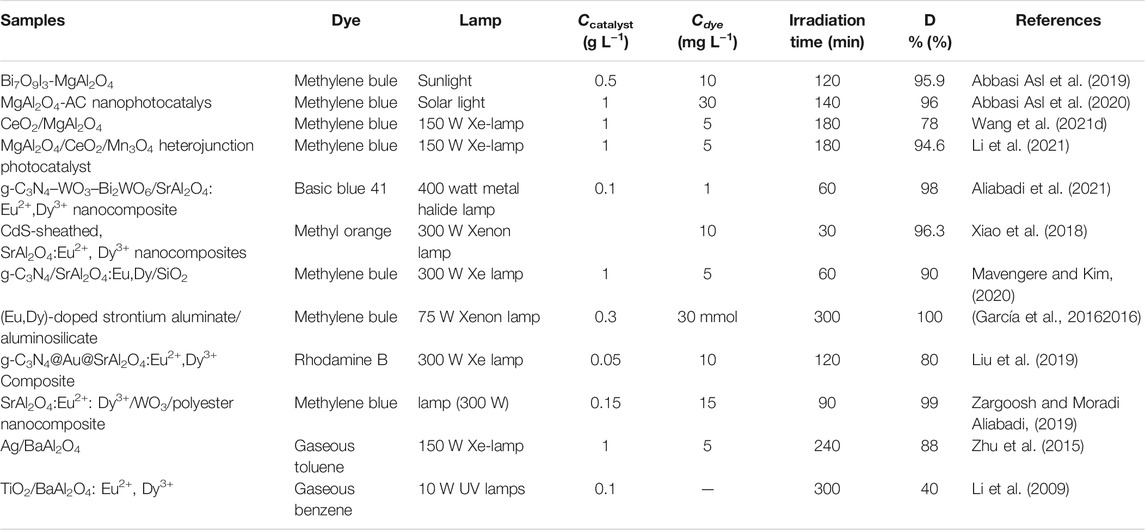
TABLE 4. The photocatalytic activity of MAl2O4(M = Mg, Sr, and Ba) based multivariate heterojunction photocatalysts.
Different types of photocatalysts have slightly different photocatalytic mechanisms. The photocatalytic mechanism of single component aluminate photocatalyst, ion doped aluminate photocatalyst and heterogeneous aluminate photocatalyst was compared and analyzed.
Due to the large band gap value of a single component aluminate, it is difficult to respond directly to visible light. When single component aluminate is synthesized by a special method, it is easy to introduce defects such as oxygen vacancy into the aluminate, so that it has visible photocatalytic activity. Figure 4 shows the photocatalytic mechanism of MgAl2O4 photocatalyst. When a beam of light hits the surface of MgAl2O4, electrons jump from its valence band to the conduction band, and leaving holes in the valence band. It is difficult for electrons to jump directly to the conduction band without the action of defect levels. Therefore, the defect level plays an important role in the whole photocatalytic process. Combined with the band theory analysis, it is found that the degradation of methylene blue dye is difficult to take place in the photosensitization process. Therefore, the whole process is mainly photocatalytic degradation, the valence band electrons and conduction band holes are involved in the reaction, and the final generation of non-toxic and harmless products. The related chemical reactions can be described as follows (Shifa Wang et al., 2020):
(1) The creation of electron hole pairs.
(2) The production of hydroxyl radicals
(3) The production of superoxide radicals
(4) Dye degradation
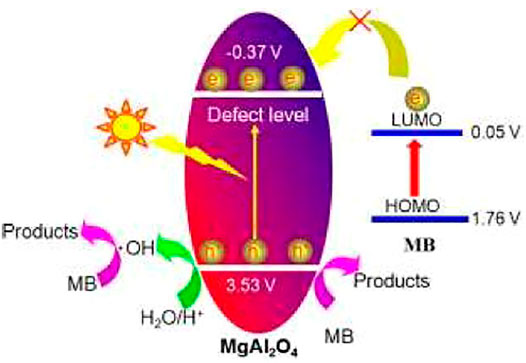
FIGURE 4. Photocatalytic mechanism of MgAl2O4 photocatalyst (Qian et al., 2017). Adapted from ref. (Qian et al., 2017). Copyright © 2017 Royal Society of Chemistry.
During the construction of the multiple heterostructure, the semiconductor material enhancing the photocatalytic activity of visible light is regarded as the defect level, so the other half of the aluminate heterojunction acts as the defect level. However, multiple heterojunction photocatalysts introduce new semiconductor materials and have great influence on the phase purity of the whole system. Therefore, the photocatalytic mechanism is different from that of a single component photocatalyst. Figure 5 shows the photocatalytic mechanism of MgAl2O4/CeO2/Mn3O4 heterojunction photocatalyst. MgAl2O4, CeO2, and Mn3O4 form a double p-n heterojunction structure among each other, which facilitates the transfer and separation of electron hole pairs, thus enhancing the photocatalytic activity of the system. The separation of electron and hole pairs accelerates the oxidation or reduction reactions of each, which then reacts with the dye to produce non-toxic and harmless products.
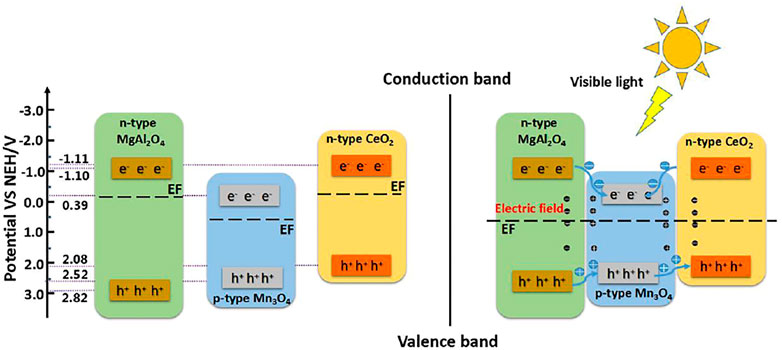
FIGURE 5. Photocatalytic mechanism of MgAl2O4/CeO2/Mn3O4 heterojunction photocatalyst (Li et al., 2021). Adapted from ref. (Li et al., 2021). Copyright © 2020 Elsevier Ltd.
Activated ions induce aluminate luminescence, which is its advantage when used as a photocatalyst. Figure 6 shows the photocatalytic mechanism of CdS-sheathed SrAl2O4: Eu2+, Dy3+ heterojunction photocatalysts. When SrAl2O4: Eu2+, Dy3+ is used as phosphors, the recombination of electron and hole pairs in the system is accelerated. However, when it combines with other semiconductor materials to form heterojunction photocatalyst, the energy absorbed by it can promote the electron transition in the whole system, thus accelerating the separation of electron and hole pairs in the system. The non-meeting of electrons and holes on CdS causes each to react with the dye to form CO2, H2O and other small molecular organics.
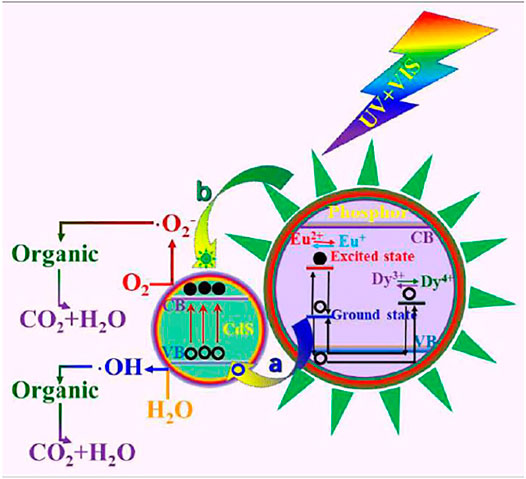
FIGURE 6. Photocatalytic mechanism of CdS-sheathed SrAl2O4: Eu2+, Dy3+ heterojunction photocatalysts (Xiao et al., 2018). Adapted from ref. (Xiao et al., 2018). Copyright © 2018 Royal Society of Chemistry.
Aluminate based photocatalyst is a kind of photocatalyst developed rapidly in recent years. Despite it have a very large band gap, researchers have always found ways to promote the transfer and separation of electrons and hole pairs, and thereby improving their photocatalytic activity. For the single-component aluminate photocatalysts, defects are introduced to provide defect levels to promote electron transition to aluminate conduction band under extreme conditions such as high temperature and high pressure, so as to enhance the photocatalytic activity of visible light. Similarly, impurity ions can be introduced into the lattice of aluminate by ion doping to improve the migration and separation efficiency of electron hole pairs and improve the photocatalytic activity of aluminate. The construction of special heterojunction structure is a simple method with relatively mature technology. By introducing other semiconductor materials with excellent performance, the construction of multiple heterojunction aluminate based photocatalyst has become a hot research field.
There are seven development trends of aluminate based photocatalysts in the future 1) The hydrolysis of aluminate is still one of the key problems to be solved. When silver particles are used to modify aluminate photocatalyst, aluminate will produce different degree of hydrolysis, which has been a difficult problem for researchers. The development of new synthetic pathways may solve this problem. 2) New application of aluminate based heterojunction phosphors in photocatalysis. When aluminate phosphor is combined with other semiconductor photocatalysts, the photocatalytic activity of the whole system will be greatly improved. However, the research of aluminate based phosphor in the field of photocatalysis is still in its infancy, and further research is needed. 3) The photocatalytic mechanism needs further study. The newly developed aluminate photocatalyst will face the problem that the existing mechanism cannot explain, so it is necessary to develop a new explanation mechanism. 4) The effect of different morphologies of aluminate photocatalysts on photocatalytic activity needs further study. The specific surface area of aluminate photocatalysts with different morphologies was different, and their degradation activity to dyes was obviously different. 5) The photocatalytic activity of aluminate photocatalyst modified by lanthanide metal particles is worth further study. There is no evidence that the modification of aluminate photocatalyst by lanthanide metal particles will lead to hydrolysis, so it is also worth studying. 6) Modification of aluminate photocatalyst by organic macromolecular network. The modification of aluminate photocatalyst by organic macromolecular network is beneficial to provide electron transport carrier for aluminate, thus enhancing the photocatalytic activity of aluminate. 7) New applications of photocatalysts are worth exploring. Novel photocatalysts may induce new interpretation mechanisms, thus promoting the application of these photocatalysts in new fields.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by thethe Provincial Natural Science Key Foundation of Anhui University (KJ 2019A0522, KJ 2021A0668), General Project of AnhuiProvince (1908085ME150), and Industry-University Cooperation Collaborative Education Projects by Ministry of Education of China (202102227037, 202102538045)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbasi Asl, E., Haghighi, M., and Talati, A. (2020). Enhanced Simulated Sunlight-Driven Magnetic MgAl2O4-AC Nanophotocatalyst for Efficient Degradation of Organic Dyes. Separat. Purif. Technol. 251, 117003. doi:10.1016/j.seppur.2020.117003
Abbasi Asl, E., Haghighi, M., and Talati, A. (2019). Sono-solvothermal Fabrication of Flowerlike Bi7O9I3-MgAl2O4 P-N Nano-Heterostructure Photocatalyst with Enhanced Solar-Light-Driven Degradation of Methylene Blue. Solar Energy 184, 426–439. doi:10.1016/j.solener.2019.04.012
Alam, M. W., Kumar, V. G. D., Ravikumar, C. R., Prashantha, S. C., Murthy, H. C. A., and Kumar, M. R. A. (2022). Chromium (III) Doped Polycrystalline MgAl2O4 Nanoparticles for Photocatalytic and Supercapacitor Applications. J. Phys. Chem. Sol. 161, 110491. doi:10.1016/j.jpcs.2021.110491
Aliabadi, H. M., Zargoosh, K., Afshari, M., Dinari, M., and Maleki, M. H. (2021). Synthesis of a Luminescent G-C3n4-WO3-Bi2WO6/SrAl2O4:Eu2+,Dy3+ Nanocomposite as a Double Z-Scheme Sunlight Activable Photocatalyst. New J. Chem. 45 (10), 4843–4853. doi:10.1039/D0NJ05529H
Bao, H., Zhu, S., Zhou, L., Fu, H., Zhang, H., and Cai, W. (2020). Mars-van-Krevelen Mechanism-Based Blackening of Nano-Sized white Semiconducting Oxides for Synergetic Solar Photo-Thermocatalytic Degradation of Dye Pollutants. Nanoscale 12 (6), 4030–4039. doi:10.1039/C9NR09534A
Basyrova, L., Bukina, V., Balabanov, S., Belyaev, A., Drobotenko, V., Dymshits, O., et al. (2021). Synthesis, Structure and Spectroscopy of Fe2+:MgAl2O4 Transparent Ceramics and Glass-Ceramics. J. Lumin. 236, 118090. doi:10.1016/j.jlumin.2021.118090
Berlanga, A., Garcia-Diaz, R., Garcia, C. R., Oliva, J., Romero, M. T., and Diaz-Torres, L. A. (2017). Photocatalytic Activity of SrAl2O4:xCu Powders under Solar Irradiation. Nhc 16, 63–66. doi:10.4028/www.scientific.net/nhc.16.63
Bhavani, P., Manikandan, A., Jaganathan, S. K., Shankar, S., and Antony, S. A. (2018). Enhanced Catalytic Activity, Facile Synthesis and Characterization Studies of Spinel Mn-Co Aluminate Nano-Catalysts. j nanosci nanotechnol 18 (2), 1388–1395. doi:10.1166/jnn.2018.14112
Boudiaf, S., Nasrallah, N., Mellal, M., Belabed, C., Belhamdi, B., Meziani, D., et al. (2020). Synthesis and Characterization of Semiconductor CoAl2O4 for Optical and Dielectric Studies: Application to Photodegradation of Organic Pollutants under Visible Light. Optik 219, 165038. doi:10.1016/j.ijleo.2020.165038
Canaza-Mamani, E. A., Cano, N. F., Mosqueira-Yauri, J., Rao, T. K. G., Aynaya-Cahui, S. C., Gonsalez-Vasquez, A. J., et al. (2021). TL and EPR Characteristics of SrAl2O4 Phosphor Prepared by Solid‐state Reaction Method. J. Lumin., 118585. doi:10.1016/j.jlumin.2021.118585
Chellammal Gayathri, R., Elakkiya, V., and Sumathi, S. (2021). Effect of Method of Preparation on the Photocatalytic Activity of NiAl2O4. Inorg. Chem. Commun. 129, 108634. doi:10.1016/j.inoche.2021.108634
Chen, C., Li, Q., Zhang, Q., Li, Y., Wei, Y., and Wang, S. (2019). “Artificial Neural Network Algorithm for Predict the Photocatalytic Activity of the Mn Co-doped MgAl2O4: Ce Composite Photocatalyst,” in 2019 IEEE International Conference on Signal, Information and Data Processing (ICSIDP), Chongqing, China, 11-13 Dec. 2019 (IEEE), 1–5.
Chen, W., Huang, J., He, Z.-C., Ji, X., Zhang, Y.-F., Sun, H.-L., et al. (2021). Accelerated Photocatalytic Degradation of Tetracycline Hydrochloride over CuAl2O4/g-C3n4 P-N Heterojunctions under Visible Light Irradiation. Separat. Purif. Technol. 277, 119461. doi:10.1016/j.seppur.2021.119461
Chen, X. Y., Ma, C., Zhang, Z. J., and Li, X. X. (2009). Structure and Photoluminescence Study of Porous Red-Emitting MgAl2O4:Eu3+ Phosphor. Microporous mesoporous Mater. 123 (1-3), 202–208. doi:10.1016/j.micromeso.2009.04.002
Cheng, T., Gao, H., Li, R., Wang, S., Yi, Z., and Yang, H. (2021). Flexoelectricity-induced Enhancement in Carrier Separation and Photocatalytic Activity of a Photocatalyst. Appl. Surf. Sci. 566, 150669. doi:10.1016/j.apsusc.2021.150669
Cheng, T., Gao, H., Sun, X., Xian, T., Wang, S., Yi, Z., et al. (2021). An Excellent Z-Scheme Ag2MoO4/Bi4Ti3O12 Heterojunction Photocatalyst: Construction Strategy and Application in Environmental Purification. Adv. Powder Technol. 32 (3), 951–962. doi:10.1016/j.apt.2021.01.039
Cheng, T., Gao, W., Gao, H., Wang, S., Yi, Z., Wang, X., et al. (2021). Piezocatalytic Degradation of Methylene Blue, Tetrabromobisphenol A and Tetracycline Hydrochloride Using Bi4Ti3O12 with Different Morphologies. Mater. Res. Bull. 141, 111350. doi:10.1016/j.materresbull.2021.111350
Ching, W. Y., Xu, Y.-N., and Brickeen, B. K. (2001). Comparative Study of the Electronic Structure of Two Laser Crystals: BeAl2O4andLiYF4. Phys. Rev. B 63 (11), 115101. doi:10.1103/PhysRevB.63.115101
Deepika, P., and Kumar, C. A. (2020). Photocatalytic Properties of Persistent Luminescent Rare Earth Doped SrAl2O4 Phosphor under Solar Radiation. Nanosystems-Physics Chem. Maths. 11 (5), 590–594.
Feng, H., He, C., Ma, G., and Zhiani, R. (2021). Imidazolium Ionic Liquid Functionalized Nano Dendritic CuAl2O4 for Visible Light-Driven Photocatalytic Degradation of Dye Pollutant. Inorg. Chem. Commun. 132, 108818. doi:10.1016/j.inoche.2021.108818
Forouzesh, M., Ebadi, A., and Abedini, F. (2021). Thermocatalytic Persulfate Activation for Metronidazole Removal in the Continuous Operation. Separat. Purif. Technol. 258, 118055. doi:10.1016/j.seppur.2020.118055
Ganesh, I., Srinivas, B., Johnson, R., Saha, B. P., and Mahajan, Y. R. (2004). Microwave Assisted Solid State Reaction Synthesis of MgAl2O4 Spinel Powders. J. Eur. Ceram. Soc. 24 (2), 201–207. doi:10.1016/S0955-2219(03)00602-2
Gao, H. J., Wang, S. F., Fang, L. M., Sun, G. A., Chen, X. P., Tang, S. N., et al. (2021). Nanostructured Spinel-type M(M = Mg, Co, Zn)Cr2O4 Oxides: Novel Adsorbents for Aqueous Congo Red Removal. Mater. Today Chem. 22, 100593. doi:10.1016/j.mtchem.2021.100593
Gao, H., Yang, H., Wang, S., Li, D., Wang, F., Fang, L., et al. (2018). A New Route for the Preparation of CoAl2O4 Nanoblue Pigments with High Uniformity and its Optical Properties. J. Sol-gel Sci. Technol. 86 (1), 206–216. doi:10.1007/s10971-018-4609-y
Gao, H., Yu, C., Wang, Y., Wang, S., Yang, H., Wang, F., et al. (2022). A Novel Photoluminescence Phenomenon in a SrMoO4/SrWO4 Micro/nano Heterojunction Phosphors Obtained by the Polyacrylamide Gel Method Combined with Low Temperature Calcination Technology. J. Lumin. 243, 118660. doi:10.1016/j.jlumin.2021.118660
García, C. R., Diaz-Torres, L. A., Oliva, J., Romero, M. T., and Salas, P. (20162016). Photocatalytic Activity and Optical Properties of Blue Persistent Phosphors under UV and Solar Irradiation. Int. J. Photoenergy 2016, 1–8. doi:10.1155/2016/1303247
García, C. R., Oliva, J., Arroyo, A., Garcia-Lobato, M. A., Gomez-Solis, C., and Torres, L. A. D. (2018). Photocatalytic Activity of Bismuth Doped SrAl2O4 Ceramic Powders. J. Photochem. Photobiol. A: Chem. 351, 245–252. doi:10.1016/j.jphotochem.2017.10.039
Ghalei, S., and Handa, H. (2022). A Review on Antibacterial Silk Fibroin-Based Biomaterials: Current State and Prospects. Mater. Today Chem. 23, 100673. doi:10.1016/j.mtchem.2021.100673
Gholami, T., Salavati-Niasari, M., and Varshoy, S. (2016). Investigation of the Electrochemical Hydrogen Storage and Photocatalytic Properties of CoAl2O4 Pigment: Green Synthesis and Characterization. Int. J. Hydrogen Energ. 41 (22), 9418–9426. doi:10.1016/j.ijhydene.2016.03.144
Habibi, N., Wang, Y., Arandiyan, H., and Rezaei, M. (2017). Low-temperature Synthesis of Mesoporous Nanocrystalline Magnesium Aluminate (MgAl2O4) Spinel with High Surface Area Using a Novel Modified Sol-Gel Method. Adv. Powder Technol. 28 (4), 1249–1257. doi:10.1016/j.apt.2017.02.012
Han, M., Wang, Z., Xu, Y., Wu, R., Jiao, S., Chen, Y., et al. (2018). Physical Properties of MgAl 2 O 4 , CoAl 2 O 4 , NiAl 2 O 4 , CuAl 2 O 4 , and ZnAl 2 O 4 Spinels Synthesized by a Solution Combustion Method. Mater. Chem. Phys. 215, 251–258. doi:10.1016/j.matchemphys.2018.05.029
Ibrahim, Y., Meslam, M., Eid, K., Salah, B., Abdullah, A. M., Ozoemena, K. I., et al. (2022). A Review of MXenes as Emergent Materials for Dye Removal from Wastewater. Separat. Purif. Technol. 282, 120083. doi:10.1016/j.seppur.2021.120083
Ivashchenko, A. N., Tedeeva, M. A., Kartavova, K. E., Aimaletdinov, T. R., Pribytkov, P. V., and Kustov, A. L. (2021). Oxidative Dehydrogenation of Ethane in the Presence of СО2 on CrOx/SiO2 Catalysts. Russ. J. Phys. Chem. 95 (12), 2417–2421. doi:10.1134/S0036024421120104
Jiang, Y. Y., Zhang, J. W., Hu, Z. Q., and Liu, J. X. (2014). Synthesis and Visible Light Photocatalytic Activity of Spinel MAl2O4 (M=Mg, Zn, Cu). Amm 455, 99–105. doi:10.4028/www.scientific.net/AMM.455.99
Kharlanov, A. N., Pankina, G. V., and Lunin, V. V. (2019). Physicochemical Properties of Potassium-Promoted Fe-Containing Catalysts for the Hydrogenation of CO over Magnesium Aluminum Spinels: IR Spectroscopy. Russ. J. Phys. Chem. 93 (12), 2356–2364. doi:10.1134/S0036024419120136
Kim, K. O., Evdokimenko, N. D., Pribytkov, P. V., Tedeeva, M. A., Borkov, S. A., and Kustov, A. L. (2021). Synthesis of Methanol from СО2 on Cu-Zn/xAl2O3-(1 - x)SiO2 Catalysts. Effect of Support Composition. Russ. J. Phys. Chem. 95 (12), 2422–2425. doi:10.1134/S0036024421120128
Kiryakov, A. N., Zatsepin, A. F., and Osipov, V. V. (2021). Optical Properties of Polyvalent Iron Ions and Anti-site Defects in Transparent MgAl2O4 Ceramics. J. Lumin. 239, 118390. doi:10.1016/j.jlumin.2021.118390
Kumar, A., and Luxmi, V. (2021). Development of an Efficient Eco-Friendly Photo-Catalyst Using Agro-Waste Turmeric Leaves and its Characterizations. Optik 242, 167057. doi:10.1016/j.ijleo.2021.167057
Lahiri, S. K., Zhang, C., Sillanpää, M., and Liu, L. (2022). Nanoporous NiO@SiO2 Photo-Catalyst Prepared by Ion-Exchange Method for Fast Elimination of Reactive Dyes from Wastewater. Mater. Today Chem. 23, 100677. doi:10.1016/j.mtchem.2021.100677
Li, F.-t., Zhao, Y., Liu, Y., Hao, Y.-j., Liu, R.-h., and Zhao, D.-s. (2011). Solution Combustion Synthesis and Visible Light-Induced Photocatalytic Activity of Mixed Amorphous and Crystalline MgAl2O4 Nanopowders. Chem. Eng. J. 173 (3), 750–759. doi:10.1016/j.cej.2011.08.043
Li, H., Liu, Y., Tang, J., and Deng, Y. (2016). Synthesis, Characterization and Photocatalytic Properties of Mg1−xZnxAl2O4 Spinel Nanoparticles. Solid State Sci. 58, 14–21. doi:10.1016/j.solidstatesciences.2016.05.007
Li, J., Wang, S., Sun, G., Gao, H., Yu, X., Tang, S., et al. (2021). Facile Preparation of MgAl2O4/CeO2/Mn3O4 Heterojunction Photocatalyst and Enhanced Photocatalytic Activity. Mater. Today Chem. 19, 100390. doi:10.1016/j.mtchem.2020.100390
Li, S., Wang, W., Chen, Y., Zhang, L., Guo, J., and Gong, M. (2009). Fabrication and Characterization of TiO2/BaAl2O4: Eu2+, Dy3+ and its Photocatalytic Performance towards Oxidation of Gaseous Benzene. Catal. Commun. 10 (7), 1048–1051. doi:10.1016/j.catcom.2008.12.064
Li, X.-Y., Xu, J., Cheng, J.-P., Feng, L., Shi, Y.-F., and Ji, J. (2017). TiO2-SiO2/GAC Particles for Enhanced Electrocatalytic Removal of Acid orange 7 (AO7) Dyeing Wastewater in a Three-Dimensional Electrochemical Reactor. Separat. Purif. Technol. 187, 303–310. doi:10.1016/j.seppur.2017.06.058
Liu, H., Wang, S., Gao, H., Yang, H., Wang, F., Chen, X., et al. (2022). A Simple Polyacrylamide Gel Route for the Synthesis of MgAl2O4 Nanoparticles with Different Metal Sources as an Efficient Adsorbent: Neural Network Algorithm Simulation, Equilibrium, Kinetics and Thermodynamic Studies. Separat. Purif. Technol. 281, 119855. doi:10.1016/j.seppur.2021.119855
Liu, X., Chen, X., Li, Y., Wu, B., Luo, X., Ouyang, S., et al. (2019). A G-C3N4@Au@SrAl2O4:Eu2+,Dy3+ Composite as an Efficient Plasmonic Photocatalyst for Round-The-Clock Environmental Purification and Hydrogen Evolution. J. Mater. Chem. A. 7 (32), 19173–19186. doi:10.1039/C9TA06423K
Liu, X., Wang, S., Yu, X., Tang, S., Fang, L., and Lei, L. (2020). Fabrication and Photoluminescence Properties of MgAl2O4: Mg Phosphors. Chin. J. Mater. Res. 34 (10), 784–792. doi:10.11901/1005.3093.2020.072
Mathew, M., Rad, M. A., Mata, J. P., Mahmodi, H., Kabakova, I. V., Raston, C. L., et al. (2022). Hyperbranched Polymers Tune the Physicochemical, Mechanical, and Biomedical Properties of Alginate Hydrogels. Mater. Today Chem. 23, 100656. doi:10.1016/j.mtchem.2021.100656
Mavengere, S., and Kim, J.-S. (2020). Photocatalytic Properties of G-C3n4-Supported on the SrAl2O4:Eu,Dy/SiO2. Coatings 10 (10), 917. doi:10.3390/coatings10100917
Mkhalid, I. A. (2022). Hydrogen Evolution over Sol-Gel Prepared Visible-Light-Responsive Ag2O/SrAl2O4/CNT Ternary Photocatalyst. Ceramics Int. 48 (2), 1542–1549. doi:10.1016/j.ceramint.2021.09.233
Moirangthem, N. S., Lisham, P. C., Barua, A. G., and Gartia, R. K. (2019). Electron Life Time (τ) in Trap Levels of Dy3+ Activated Calcium Aluminate: Implications in TL Dosimetry. J. Phys. Conf. Ser. 1330 (1), 012009. doi:10.1088/1742-6596/1330/1/012009
Mu, H.-Y., Li, F.-T., An, X.-T., Liu, R.-H., Li, Y.-L., Qian, X., et al. (2017). One-step Synthesis, Electronic Structure, and Photocatalytic Activity of Earth-Abundant Visible-Light-Driven FeAl2O4. Phys. Chem. Chem. Phys. 19 (14), 9392–9401. doi:10.1039/C7CP01007A
Mukherjee, A., Adak, M. K., Dhak, P., and Dhak, D. (2020). A Simple Chemical Method for the Synthesis of Cu2+ Engrafted MgAl2O4 Nanoparticles: Efficient Fluoride Adsorbents, Photocatalyst and Latent Fingerprint Detection. J. Environ. Sci. 88, 301–315. doi:10.1016/j.jes.2019.09.004
Mumanga, T. J., Díaz-Torres, L. A., and Gómez-Solís, C. (2021). Nd3+ Doped BaAl2O4 for Enhanced Photocatalytic Degradation of Methylene Blue. Mater. Lett. 292, 129664. doi:10.1016/j.matlet.2021.129664
Musa, M. A., Xu, D., Sun, F., Shao, H., Dong, X., Azis, R. A. S., et al. (2021). Electrospun ZnFe2O4/Al: ZnFe2O4 Nanofibers for Degradation of RhB via Visible Light Photocatalysis and Photo-Fenton Processes. J. Mater. Sci. Mater. Electron., 1–11. doi:10.1007/s10854-021-07443-8
Nair, I. I. J., and Pillai, R. K. V. (2021). “Growth of BaAl2O4 Nanoparticles by Auto-Ignition Combustion Method and its Characterization,” in AIP Conference Proceedings 2379, 030006, International Conference on Recent Trends in Theoretical and Applied Physics, 12 July 2021 (AIP Publishing).
Nassar, M. Y., Ahmed, I. S., and Samir, I. (2014). A Novel Synthetic Route for Magnesium Aluminate (MgAl2O4) Nanoparticles Using Sol-Gel Auto Combustion Method and Their Photocatalytic Properties. Spectrochimica Acta A: Mol. Biomol. Spectrosc. 131, 329–334. doi:10.1016/j.saa.2014.04.040
Park, B.-G. (2018). Characteristics of Eu2+, Dy3+-Doped SrAl2O4 Synthesized by Hydrothermal Reaction and its Photocatalytic Properties. Msce 06 (02), 12–21. doi:10.4236/msce.2018.62002
Parvarinezhad, S., Salehi, M., and Khademinia, S. (2019). Solid State Synthesis of MgAl2O4 Nanomaterials and Solar Light-Induced Photocatalytic Removal of Malachite Green. Int. J. Nano Dimension 10 (1), 89–104. Available at: http://www.iaujournals.ir/article_660987.html?lang=en.
Piriyanon, J., Takhai, P., Patta, S., Chankhanittha, T., Senasu, T., Nijpanich, S., et al. (2021). Performance of Sunlight Responsive WO3/AgBr Heterojunction Photocatalyst toward Degradation of Rhodamine B Dye and Ofloxacin Antibiotic. Opt. Mater. 121, 111573. doi:10.1016/j.optmat.2021.111573
Potbhare, A. K., Chauke, P. B., Zahra, S., Sonkusare, V., Bagade, R., Ummekar, M., et al. (2019). Microwave-mediated Fabrication of Mesoporous Bi-doped CuAl2O4 Nanocomposites for Antioxidant and Antibacterial Performances. Mater. Today Proc. 15, 454–463. doi:10.1016/j.matpr.2019.04.107
Pu, X., Wang, C., Chen, X., Jin, J., Li, W., and Chen, F. (2021). Synthesis and Photocatalytic Degradation of Water to Produce Hydrogen from Novel Cerium Dioxide and Silver-Doped Cerium Dioxide Fiber Membranes by the Electrospinning Method. Front. Mater. 8, 414. doi:10.3389/fmats.2021.776817
Qian, X., Li, B., Mu, H.-y., Ren, J., Liu, Y., Hao, Y.-j., et al. (2017). Deep Insight into the Photocatalytic Activity and Electronic Structure of Amorphous Earth-Abundant MgAl2O4. Inorg. Chem. Front. 4 (11), 1832–1840. doi:10.1039/C7QI00478H
Rajabathar, J. R., Arunachalam, P., Issa, Z. A., and Ahmed M, T. (2020). Synthesis and Characterization of Novel Metal Chalcogenide Modified Ni-Co-MnO2 Nanofibers Rolled with Graphene Based Visible Light Active Catalyst for nitro Phenol Degradation. Optik 224, 165538. doi:10.1016/j.ijleo.2020.165538
Salehabadi, A., Salavati-Niasari, M., Sarrami, F., and Karton, A. (2017). Sol-Gel Auto-Combustion Synthesis and Physicochemical Properties of BaAl2O4 Nanoparticles; Electrochemical Hydrogen Storage Performance and Density Functional Theory. Renew. Energ. 114, 1419–1426. doi:10.1016/j.renene.2017.07.119
Sera, M., Yamamoto, M., Tomita, K., Yabara, Y., Izawa, S., Hiramoto, M., et al. (2021). Morphology Control and Synthesis of Afterglow Materials with a SrAl2O4 Framework Synthesized by Surfactant-Template and Hydrothermal Methods. Chem. Phys. Lett. 780, 138916. doi:10.1016/j.cplett.2021.138916
Shang-Pan, H., Zhi-Qiang, W., Xiao-Juan, W., and Ji-Wen, S. (2020). Optical Properties of Cr Doped ZnAl2O4 Nanoparticles with Spinel Structure Synthesized by Hydrothermal Method. Mater. Res. Express 7 (1), 015025. doi:10.1088/2053-1591/ab6125
Sharma, S. K., Gourier, D., Viana, B., Maldiney, T., Teston, E., Scherman, D., et al. (2014). Persistent Luminescence of AB2O4:Cr3+ (A=Zn, Mg, B=Ga, Al) Spinels: New Biomarkers for In Vivo Imaging. Opt. Mater. 36 (11), 1901–1906. doi:10.1016/j.optmat.2014.06.020
Shifa Wang, S., Gao, H., Sun, G., Wang, Y., Fang, L., Yang, L., et al. (2020). Synthesis of Visible-Light-Driven SrAl2O4-Based Photocatalysts Using Surface Modification and Ion Doping. Russ. J. Phys. Chem. 94, 1234–1247. doi:10.1134/S003602442006031X
Sriram, B., Baby, J. N., Wang, S.-F., George, M., Joseph, X. B., and Tsai, J.-T. (2020). Surface Engineering of Three-dimensional-like Hybrid AB2O4 (AB = Zn, Co, and Mn) Wrapped on Sulfur-Doped Reduced Graphene Oxide: Investigation of the Role of an Electrocatalyst for Clioquinol Detection. ACS Appl. Electron. Mater. 3 (1), 362–372. doi:10.1021/acsaelm.0c00906
Szroeder, P., Sahalianov, I., Radchenko, T., Tatarenko, V., and Prylutskyy, Y. (2019). The Strain- and Impurity-dependent Electron States and Catalytic Activity of Graphene in a Static Magnetic Field. Opt. Mater. 96, 109284. doi:10.1016/j.optmat.2019.109284
Taazayet, W. B., Zouari, I. M., Hosni, N., Dkhil, B., and Mliki, N. T. (2021). Facile Synthesis of Pure BiFeO3 and Bi2Fe4O9 Nanostructures with Enhanced Photocatalytic Activity. J. Mater. Sci. Mater. Electron., 1–16. doi:10.1007/s10854-021-07459-0
Takebuchi, Y., Fukushima, H., Nakauchi, D., Kato, T., Kawaguchi, N., and Yanagida, T. (2020). Scintillation and Dosimetric Properties of Ce-Doped MgAl2O4 Single Crystals. J. Lumin. 223, 117139. doi:10.1016/j.jlumin.2020.117139
Wang, S., Chen, C., Li, Y., Zhang, Q., Li, Y., and Gao, H. (2019). Synergistic Effects of Optical and Photoluminescence Properties, Charge Transfer, and Photocatalytic Activity in MgAl2O4:Ce and Mn-Codoped MgAl2O4:Ce Phosphors. J. Elec Materi 48 (10), 6675–6685. doi:10.1007/s11664-019-07479-x
Wang, S., Gao, H., Chen, C., Wei, Y., and Zhao, X. (2019). Irradiation Assisted Polyacrylamide Gel Route for the Synthesize of the Mg1-xCoxAl2O4 Nano-Photocatalysts and its Optical and Photocatalytic Performances. J. Sol-gel Sci. Technol. 92 (1), 186–199. doi:10.1007/s10971-019-05062-8
Wang, S., Gao, H., Fang, L., Hu, Q., Sun, G., Chen, X., et al. (2021). Synthesis of Novel CQDs/CeO2/SrFe12O19 Magnetic Separation Photocatalysts and Synergic Adsorption-Photocatalytic Degradation Effect for Methylene Blue Dye Removal. Chem. Eng. J. Adv. 6, 100089. doi:10.1016/j.ceja.2021.100089
Wang, S., Gao, H., Fang, L., Wei, Y., Li, Y., and Lei, L. (2019). Synthesis and Characterization of BaAl2O4 Catalyst and its Photocatalytic Activity towards Degradation of Methylene Blue Dye. Z. für Physikalische Chem. 233 (8), 1161–1181. doi:10.1515/zpch-2018-1308
Wang, S., Gao, H., Li, J., Wang, Y., Chen, C., Yu, X., et al. (2021). Comparative Study of the Photoluminescence Performance and Photocatalytic Activity of CeO2/MgAl2O4 Composite Materials with an N-N Heterojunction Prepared by One-step Synthesis and Two-step Synthesis Methods. J. Phys. Chem. Sol. 150, 109891. doi:10.1016/j.jpcs.2020.109891
Wang, S., Gao, H., Wei, Y., Li, Y., Yang, X., Fang, L., et al. (2019). Insight into the Optical, Color, Photoluminescence Properties, and Photocatalytic Activity of the N-O and C-O Functional Groups Decorating Spinel Type Magnesium Aluminate. CrystEngComm 21 (2), 263–277. doi:10.1039/c8ce01474d
Wang, S., Gao, H., Yu, H., Li, P., Li, Y., Chen, C., et al. (2020). Optical and Photoluminescence Properties of the MgAl2O4:M (M = Ti, Mn, Co, Ni) Phosphors: Calcination Behavior and Photoluminescence Mechanism. Trans. Indian Ceram. Soc. 79 (4), 221–231. doi:10.1080/0371750X.2020.1817789
Wang, S., Gao, H., Yu, X., Tang, S., Wang, Y., Fang, L., et al. (2020). Nanostructured SrTiO3 with Different Morphologies Achieved by mineral Acid-Assisted Hydrothermal Method with Enhanced Optical, Electrochemical, and Photocatalytic Performances. J. Mater. Sci. Mater. Electron. 31 (20), 17736–17754. doi:10.1007/s10854-020-04328-0
Wang, S., Li, D., Yang, C., Sun, G., Zhang, J., Xia, Y., et al. (2017). A Novel Method for the Synthesize of Nanostructured MgFe2O4 Photocatalysts. J. Sol-gel Sci. Technol. 84 (1), 169–179. doi:10.1007/s10971-017-4471-3
Wang, S., Wang, Y., Gao, H., Li, J., Fang, L., Yu, X., et al. (2020). Synthesis and Characterization of BaAl2O4: Ce and Mn-Ce- Co-doped BaAl2O4 Composite Materials by a Modified Polyacrylamide Gel Method and Prediction of Photocatalytic Activity Using Artificial Neural Network (ANN) Algorithm. Optik 221, 165363. doi:10.1016/j.ijleo.2020.165363
Wang, S., Wei, X., Gao, H., and Wei, Y. (2019). Effect of Amorphous Alumina and α-alumina on Optical, Color, Fluorescence Properties and Photocatalytic Activity of the MnAl2O4 Spinel Oxides. Optik 185, 301–310. doi:10.1016/j.ijleo.2019.03.147
Wang, Y., Gao, H., Wang, S., Fang, L., Chen, X., Yu, C., et al. (2021). Facile Synthesis of BaMoO4 and BaMoO4/BaWO4 Heterostructures with Type -I Band Arrangement and Enhanced Photoluminescence Properties. Adv. Powder Technol. 32 (11), 4186–4197. doi:10.1016/j.apt.2021.09.028
Wang, Y., Sun, X., Xian, T., Liu, G., and Yang, H. (2021). Photocatalytic Purification of Simulated Dye Wastewater in Different pH Environments by Using BaTiO3/Bi2WO6 Heterojunction Photocatalysts. Opt. Mater. 113, 110853. doi:10.1016/j.optmat.2021.110853
Xiao, Y., Luo, B., Cheng, B., Huang, Q., Ye, Y., Fang, L., et al. (2018). Enhanced Visible Light Catalysis Activity of CdS-Sheathed SrAl2O4:Eu2+,Dy3+ Nanocomposites. Dalton Trans. 47 (24), 7941–7948. doi:10.1039/C8DT01151F
Zargoosh, K., and Moradi Aliabadi, H. (2019). SrAl2O4:Eu2+: Dy3+/WO3/Polyester Nanocomposite as a Highly Efficient and Environmentally Friendly Photocatalyst for Removal of Dyes from Industrial Wastes. Environ. Nanotechnology, Monit. Manage. 12, 100273. doi:10.1016/j.enmm.2019.100273
Zawrah, M. F., Hamaad, H., and Meky, S. (2007). Synthesis and Characterization of Nano MgAl2O4 Spinel by the Co-precipitated Method. Ceramics Int. 33 (6), 969–978. doi:10.1016/j.ceramint.2006.02.015
Zhang, M., Chen, X., Zu, M., Tang, Y., Liu, C., Li, W., et al. (2022). Synthesis of Fibrous Micro-nano Hierarchical Porous Cerium Dioxide Materials by the Impregnation and thermal Decomposition Method and Is Enhanced Photocatalytic Activity. Front. Mater. 8, 502. doi:10.3389/fmats.2021.775027
Zhu, Z., Li, X., Li, H., Li, Y., Sun, C., and Cao, Y. (2012). Synthesis and Characterization of BaAl2O4 Nanorods by a Facile Solvothermal Method. Mater. Lett. 86, 1–4. doi:10.1016/j.matlet.2012.06.093
Keywords: photocatalysis, aluminate, heterojunction, photocatalytic mechanism, photocatalytic application
Citation: Han X, Sun M, Chai X, Li J, Wu Y and Sun W (2022) Progress in Synthesis and Photocatalytic Activity of MAl2O4(M=Mg, Sr, Ba) Based Photocatalysts. Front. Mater. 9:845664. doi: 10.3389/fmats.2022.845664
Received: 30 December 2021; Accepted: 17 January 2022;
Published: 01 February 2022.
Edited by:
Zao Yi, Southwest University of Science and Technology, ChinaReviewed by:
Fang Leiming, China Academy of Engineering Physics, ChinaCopyright © 2022 Han, Sun, Chai, Li, Wu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meijuan Sun, aGhqMjAwNjA2QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.