- 1Key Laboratory of Interfacial Physics and Technology, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai, China
- 2University of Chinese Academy of Sciences, Beijing, China
- 3Shanghai Synchrotron Radiation Facility, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai, China
As predicted by classical macroscopic theory, the lifetime for nanoscale gas bubbles is extremely short, which causes conflict when detecting stable bulk nanobubbles experimentally in recent years. In fact, the stability of bulk nanobubbles depends on the surrounding liquid environment. Also, the dynamic process of gas in water involves the dissolution, diffusion, release, and transportation of gas as well as the properties of nanobubbles inside. Here, based on previous reports, we introduce the gas transport parameter
Introduction
Long-lived, freely suspended, and spherical nanoscopic bubbles are called bulk nanobubbles (Seddon and Lohse, 2011; Attard, 2014; Zhang and Lohse, 2015; Fang et al., 2020). The applications of bulk nanobubbles have sprung up in the last decade. They exhibit excellent impacts such as in the bioremediation of groundwater pollution and water treatment (Agarwal et al., 2011; Li et al., 2014; Wang et al., 2018; Zhang et al., 2018), mineral separations using froth flotation (Sedlák and Rak, 2014), depletion flocculation for the dewatering of mineral tailings (Ngai et al., 2008), etc. However, the study of their underlying mechanisms is behind that of their applications. There are still many significant questions that need to be answered. For example, their long lifetimes could not be explained by classical theories (Zhang et al., 2008). Other applications, such as the electrification of gas-liquid interfaces (Bunkin et al., 1996), high mass transfer efficiency (Xiao and Xu, 2019), and the tensile strength discrepancy of water between experiment and theory (Mørch, 2007) are also lacking in reasonable explanations.
The basic question is the explanation of the stability of bulk nanobubbles. The diffusive dynamics of a spherical bubble in a liquid is described by the Epstein-Plesset (EP) theory (Plesset and Sadhal, 1982), which predicts that bubbles in solution will shrink or expand, depending on whether the dissolved gas in solution is oversaturated or not. Significantly, the survival time for nano-sized bubbles should be less than 0.02 s, fueling long-standing suspicion over the existence of bulk nanobubbles (Jadhav and Barigou, 2020).
In contrast to the theoretical prediction, numerous experimental results indicated that bulk nanobubbles do exist using dynamic light scattering (Alheshibri et al., 2016) Nanoparticle Tracking Analysis (Oh et al., 2015; Midtvedt et al., 2019). Although many theories reported try to explain the stability of bulk nanobubbles—for example, contaminant mechanism (Sedlák and Rak, 2014; Li et al., 2016; Yasui et al., 2016), dynamic equilibrium theory (Yasui et al., 2016), surface charge adsorption (Millare and Basilia, 2018; Tan et al., 2020), and the high-density mechanism (Zhang et al., 2008; Zhang et al., 2013)—they may partially lift the veil of mystery surrounding bulk nanobubbles under some limited conditions.
Different from surface nanobubbles, bulk nanobubbles usually exist in the bubble suspension, and their stability is easily influenced by the liquid environment surrounding them. It was reported that the storage condition may also influence the lifetime of bulk nanobubbles (Kanematsu et al., 2020). Zhou et al. reported that the high-resolution soft X-ray imaging of a single oxygen nanobubble and its near-edge fine structure absorption spectra of oxygen K-edge were obtained by scanning transmission X-ray microscopy (STXM) (Zhou et al., 2020). During the STXM nanobubble measurement the highly supersaturated liquid environment in which bubbles were sealed in between two Si3N4 membranes. This liquid environment is a confined and closed system in which no gas is exchanged with the external atmosphere. But for an open system, dissolved gas would exchange with the gas inside of the bubble near the gas/water interface and the distance of nanobubbles from the gas/water interface would also influence the stability. The process will involve dissolution, diffusion, release and transport of gas, as well as the density of nanobubbles inside.
Herein, considering the complicated processes above, we mainly focus on the effects of gas transport around nanobubbles at different horizontal positions in a container and combine new inner density data to theoretically analyze the stability of bulk nanobubbles. We numerically simulate how gas diffusion to the atmosphere influences the lifetime of a single bulk nanobubble. On the basis of the diffusion equation, we reconsider the initial condition and derive the relevant equations for a single nanobubble. In addition, the Macleod-Sugden relationship is considered to estimate the gas-liquid interfacial tension in the case of high density inside a single bulk nanobubble. The relevant parameter is varied to demonstrate the robustness of a single nanobubble’s long lifetime in varying conditions. The numerical simulation results show that the lifetime of a single bulk nanobubble lifetime could reach the order of 10–100 s, and approaches to the timescale for experimental observations depending on inner density and gas transport near bulk phase nanobubbles at different horizontal positions in a container.
The Analysis of the Stability of a Single Nanobubble
First, in order to study the effect of gas diffusion to the atmosphere on bulk nanobubble lifetime, we consider the initial condition to consist of a linear concentration profile around a single bulk nanobubble. The schematic diagram of a single bulk nanobubble is shown in Figure 1. The opening system is studied and it is supposed that bulk nanobubbles are stored in an unsealed container. In this system, we presume that a single bulk nanobubble is located in a super-saturated liquid environment and has a high density inside as reported (Zhou et al., 2020). The MacLeod-Sugden relationship for the gas-liquid interfacial tension is adopted. We conduct the numerical simulation to study the distribution of gas concentration and the evolution of the single nanobubble in order to acquire the effect of the diffusion distance and inner density on the lifetime of the nanobubble.
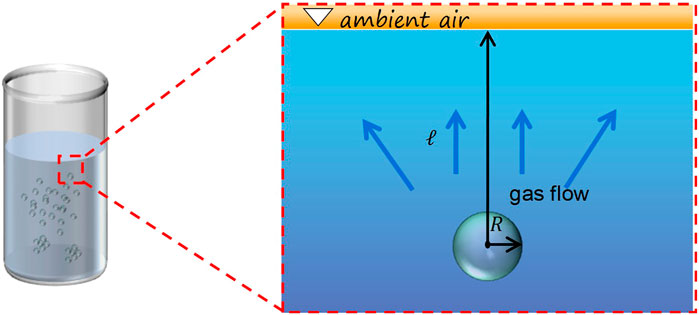
FIGURE 1. The schematic diagram of a single bulk nanobubble stored in an open system in which there is gas exchange between the bubble and the atmosphere.
A key point for the study of bubble diffusion dynamics is to describe the change rate of bubble mass by Fick’s diffusion law (Plesset and Sadhal, 1982):
We now fully describe the boundary conditions for the above differential equation. They are given by Henry’s law and Laplace’s law, which relates the gas concentration in the liquid to the gas pressure outside the liquid near the interfaces:
The survival environment of surface nanobubbles during the STXM nanobubble measurement can help us effectively describe the initial conditions of bulk nanobubbles. Due to the “traffic jam effect” which lowers the diffusion rate of gas or even a chemical equilibrium in a closed system, high-density bubbles are expected to be stable in a high gas concentration environment. The high gas concentration at the nucleation site of bulk nanobubbles indicates that bulk nanobubbles also have the “traffic jam effect” similar to surface nanobubbles. In a closed system, a steady gas-saturated environment can stabilize bulk nanobubbles, but in an open system, the gas diffusion to the atmosphere is inevitable. So we simplify the gas concentration around the mature bulk nanobubble decays radially to
Solving Eqs (2, 3) simultaneously, we first compute the diffusion Eq. 2 numerically. The simulations were done for the initial condition.
How long does the “traffic jam effect” stabilize a single nanobubble according to this description? Eq. 3 directly shows that the time scale of the “traffic jam effect” is related to the gas diffusion distance
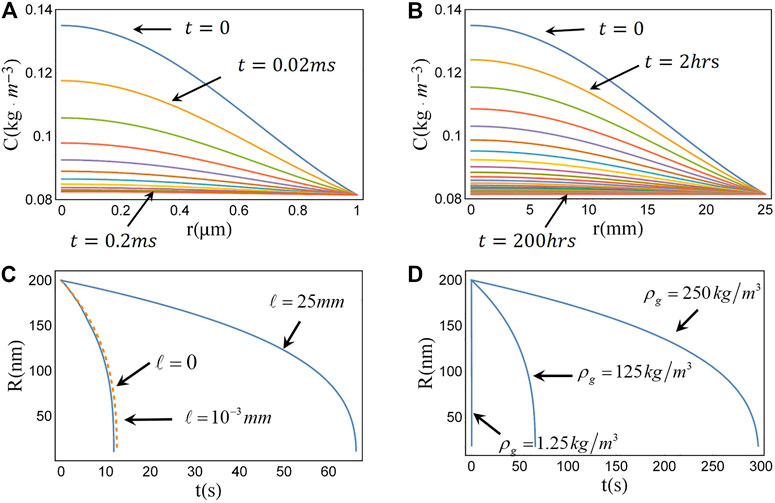
FIGURE 2. Evolution of gas concentration around the single bulk nanobubble and the dissolution process of single bulk nanobubble with different conditions. (A) In the initial moment, the gas is uniformly distributed in the fluid layer with 1 μm. At t = 0.2 ms, the gas concentration reaches an equilibrium. (B) When the liquid layer thickness is 25 mm, the gas diffusion near a single nanobubble reaches equilibrium 200 h later, indicating that the gas near the nanobubble forms a long-time “traffic jam effect”. (C) The dissolution times of bulk nanobubbles at different positions (gas diffusion distance is different) in the container while supposing inner density of bubble was
The time for gas diffusion to reach an equilibrium discussed above is the result of a stationary bubble boundary and the non-zero concentration gradient of the gas near the bubble will cause the bubbles to dissolve. The gas concentration distribution around the nanobubble with the updating radius will also be updated. According to Henry’s law and Laplace’s law, the gradient of the updating gas concentration distribution is higher, and the dissolution rate of the updating nanobubble can be predicted to be accelerated. In the next section, we will solve the evolution equation of the single bulk nanobubble analytically.
According to the diffusion law, the mass that flows out of the bubble per unit time could be
Eq. 4 immediately shows how the inner density and the diffusion flux affect the dissolution rate of a single bulk nanobubble. According to Eq. 2, the concentration gradient
where
It follows from Eq. 6 that nanobubble lifetime depends on the initial radius
First, we focus on the gas diffusion distance
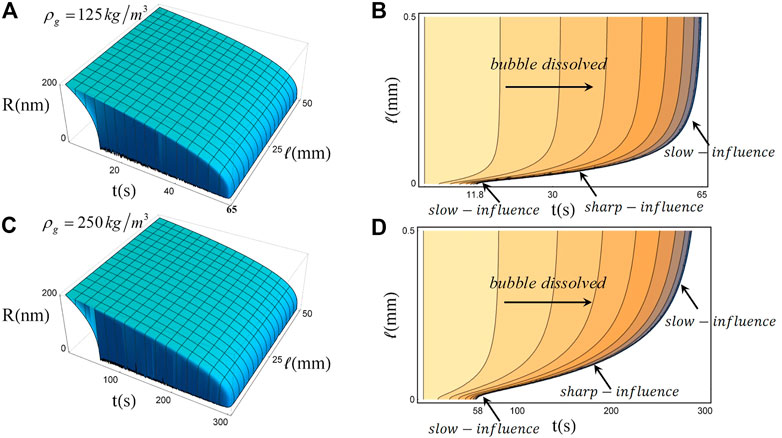
FIGURE 3. Evolution of single bulk nanobubble per unit time. (A) Development of a single bubble with an initial radius of 200 nm (
Then, we further consider the gas density
Next, we further simulate the response of the dissolution process of bulk nanobubbles with internal densities of 125 kg/m3 and 250 kg/m3 to different positions of the single bulk nanobubble (which determines the distance of gas diffusion) as shown in Figures 3A,C. The evolution of nanobubbles with different inner densities at different horizontal positions in the container gives a maximum lifetime of 300 s. The change of the dissolution time of nanobubbles to the gas diffusion distance indicates that the nanobubble near the air/water interface would have a shorter lifetime. It also would provide theoretical guidance for experimental preparation and preservation of bulk phase nanobubbles: 1) requiring a high gas supersaturation environment; 2) avoiding the existence of gas/liquid interfaces.
In addition, the effect of gas diffusion distance on a single bulk nanobubble lifetime decreases with the increase of the diffusion distance, until the transport length has no effect obtained from Figures 3B,D: If
Finally, we obtain a possible evolution mechanism of single bulk nanobubble: the high gas concentration around mature nanobubbles leads to a long gas diffusion distance in the open system, which limits the gas diffusion flux near the nanobubbles. The high gas density inside the nanobubbles further limits the diffusion flux by reducing the surface tension at the gas-liquid interface, which is the driving force of nanobubble dissolution. In addition, higher density bubbles require a longer dissolution time at lower gas flux.
In summary, based on our previous experimental results, we simulate the gas flow of a single bulk nanobubble through a liquid to the atmosphere in an open system to study the lifetime of the single bulk nanobubble. In the vicinity of nanobubbles, we find that a nanobubble is not stable, and would dissolve by diffusion. However, due to the influence of high inner density and gas transport on gas diffusion flux near nanobubbles, the time scale of a single nanobubble dissolution is different from that of a free bubble in infinite liquid. The position of the nanobubble in a container determines the gas diffusion distance around the nanobubble. The limited operating distance of gas diffusion makes the nanobubble lifetime differ at different positions in the container. When the density inside the nanobubble is small, the nanobubble lifetime is less affected by the container position.
It should be noted that here we only study the stability of a single nanobubble and do not consider the electrostatic effect and the multi-bubble cluster effect (Du et al., 2020; Jadhav and Barigou, 2021). It may prolong the lifetime of bulk nanobubbles when considering their electrostatic effect and multi-bubble cluster effect in a multi-bubble system because they can reduce the gas flux near the nanobubble and make the nanobubble more stable. Furthermore, it may need to reevaluate the rules on the nanoscale, including the relationship between surface tension and densities of liquid and gas, Henry’s law in the case of the dense gas, and the influence of the structure of water and gas molecules at the interface on the diffusion coefficient. It is also possible that the structure of water and gas molecules at the gas-liquid interface may be different from that in the conventional case when there is a high concentration of gas diffusion layer around the bubble (Nguyen et al., 2020), which may explain the mechanism of mass transfer at the gas-liquid interface at the micro-scale. Further experimental studies, including the influence of electrostatic effect and multi-bubble effect on the existence time of nanobubbles and the analysis of hydrogen bond structure at the bubble/water interface, need to be explored.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
LZ and JH put forward the general idea of the article, YW completed the theoretical calculation of the article and the writing of the main body of the paper, LZ improved the details of the article.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 11874379, 11575281, 11305252, and U1532260), and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. QYZDJ-SSW-SLH019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, A., Ng, W. J., and Liu, Y. (2011). Principle and Applications of Microbubble and Nanobubble Technology for Water Treatment. Chemosphere 84, 1175–1180. doi:10.1016/j.chemosphere.2011.05.054
Alheshibri, M., Qian, J., Jehannin, M., and Craig, V. S. J. (2016). A History of Nanobubbles. Langmuir 32, 11086–11100. doi:10.1021/acs.langmuir.6b02489
Attard, P. (2014). The Stability of Nanobubbles. Eur. Phys. J. Spec. Top. 223, 893–914. doi:10.1140/epjst/e2013-01817-0
Bunkin, N. F., Kochergin, A. V., Lobeyev, A. V., Ninham, B. W., and Vinogradova, O. I. (1996). Existence of Charged Submicrobubble Clusters in Polar Liquids as Revealed by Correlation between Optical Cavitation and Electrical Conductivity. Colloids Surf. A Physicochem. Eng. Aspects 110, 207–212. doi:10.1016/0927-7757(95)03422-6
Du, T., Wang, J., Wang, Y., and Huang, C. (2020). A Study of the Collapse Speed of Bubble Clusters. Int. J. Multiphase Flow 129, 103322. doi:10.1016/j.ijmultiphaseflow.2020.103322
Fang, Z., Wang, X., Zhou, L., Zhang, L., and Hu, J. (2020). Formation and Stability of Bulk Nanobubbles by Vibration. Langmuir 36, 2264–2270. doi:10.1021/acs.langmuir.0c00036
Jadhav, A. J., and Barigou, M. (2020). Bulk Nanobubbles or Not Nanobubbles: That Is the Question. Langmuir 36, 1699–1708. doi:10.1021/acs.langmuir.9b03532
Jadhav, A. J., and Barigou, M. (2021). On the Clustering of Bulk Nanobubbles and Their Colloidal Stability. J. Colloid Interf. Sci. 601, 816–824. doi:10.1016/j.jcis.2021.05.154
Kanematsu, W., Tuziuti, T., and Yasui, K. (2020). The Influence of Storage Conditions and Container Materials on the Long Term Stability of Bulk Nanobubbles - Consideration from a Perspective of Interactions between Bubbles and Surroundings. Chem. Eng. Sci. 219, 115594. doi:10.1016/j.ces.2020.115594
Li, H., Hu, L., Song, D., and Lin, F. (2014). Characteristics of Micro-Nano Bubbles and Potential Application in Groundwater Bioremediation. Water Environ. Res. 86, 844–851. doi:10.2175/106143014x14062131177953
Li, M., Tonggu, L., Zhan, X., Mega, T. L., and Wang, L. (2016). Cryo-EM Visualization of Nanobubbles in Aqueous Solutions. Langmuir 32, 11111–11115. doi:10.1021/acs.langmuir.6b00261
Midtvedt, D., Eklund, F., Olsén, E., Midtvedt, B., Swenson, J., and Höök, F. (2019). Size and Refractive index Determination of Subwavelength Particles and Air Bubbles by Holographic Nanoparticle Tracking Analysis. Anal. Chem. 92, 1908–1915. doi:10.1021/acs.analchem.9b04101
Millare, J., and Basilia, B. (2018). Nanobubbles from Ethanol-Water Mixtures: Generation and Solute Effects via Solvent Replacement Method. Chemistryselect 3, 9268–9275. doi:10.1002/slct.201801504
Mørch, K. A. (2007). Reflections on Cavitation Nuclei in Water. Phys. Fluids 19, 1063. doi:10.1063/1.2747210
Ngai, T., Xing, X., and Jin, F. (2008). Depletion Attraction between a Polystyrene Particle and a Hydrophilic Surface in a Pluronic Aqueous Solution. Langmuir 24, 13912–13917. doi:10.1021/la802529q
Nguyen, N. N., Galib, M., and Nguyen, A. V. (2020). Critical Review on Gas Hydrate Formation at Solid Surfaces and in Confined Spaces-Why and How Does Interfacial Regime Matter? Energy Fuels 34, 6751–6760. doi:10.1021/acs.energyfuels.0c01291
Oh, S. H., Han, J. G., and Kim, J.-M. (2015). Long-term Stability of Hydrogen Nanobubble Fuel. Fuel 158, 399–404. doi:10.1016/j.fuel.2015.05.072
Plesset, M. S., and Sadhal, S. S. (1982). On the Stability of Gas Bubbles in Liquid-Gas Solutions. Appl. Scientific Res. 38, 133–141. doi:10.1007/bf00385944
Robert, J. M. P., Reid, C. R., and Sherwood, T. K. (1958). The Properties of Gases and Liquids. Aiche J. 24, 38–40.
Seddon, J. R. T., and Lohse, D. (2011). Nanobubbles and Micropancakes: Gaseous Domains on Immersed Substrates. J. Phys. Condens. Matter 23, 133001. doi:10.1088/0953-8984/23/13/133001
Sedlák, M., and Rak, D. (2014). On the Origin of Mesoscale Structures in Aqueous Solutions of Tertiary Butyl Alcohol: The Mystery Resolved. J. Phys. Chem. B 118, 2726–2737. doi:10.1021/jp500953m
Tan, B. H., An, H., and Ohl, C.-D. (2020). How Bulk Nanobubbles Might Survive. Phys. Rev. Lett. 124, 134503. doi:10.1103/physrevlett.124.134503
Wang, L., Miao, X., Ali, J., Lyu, T., and Pan, G. (2018). Quantification of Oxygen Nanobubbles in Particulate Matters and Potential Applications in Remediation of Anaerobic Environment. ACS Omega 3, 10624–10630. doi:10.1021/acsomega.8b00784
Weijs, J. H., and Lohse, D. (2013). Why Surface Nanobubbles Live for Hours. Phys. Rev. Lett. 110, 054501. doi:10.1103/PhysRevLett.110.054501
Xiao, W., and Xu, G. (2019). Mass Transfer of Nanobubble Aeration and its Effect on Biofilm Growth: Microbial Activity and Structural Properties. Sci. Total Environ. 703, 134976. doi:10.1016/j.scitotenv.2019.134976
Yasui, K., Tuziuti, T., Kanematsu, W., and Kato, K. (2016). Dynamic Equilibrium Model for a Bulk Nanobubble and a Microbubble Partly Covered with Hydrophobic Material. Langmuir 32, 11101–11110. doi:10.1021/acs.langmuir.5b04703
Zhang, X., and Lohse, D. (2015). Surface Nanobubbles and Nanodroplets. Rev. Mod. Phys. 87, 981–1035. doi:10.1103/RevModPhys.87.981
Zhang, L., Chen, H., Li, Z., Fang, H., and Hu, J. (2008). Long Lifetime of Nanobubbles Due to High Inner Density. Sci. China Ser. G-phys. Mech. Astron. 51, 219–224. doi:10.1007/s11433-008-0026-5
Zhang, M., Tu, Y.-s., and Fang, H.-p. (2013). Concentration of Nitrogen Molecules Needed by Nitrogen Nanobubbles Existing in Bulk Water. Appl. Math. Mech. Engl. Ed. 34, 1433–1438. doi:10.1007/s10483-013-1757-x
Zhang, H., Lyu, T., Bi, L., Tempero, G., Hamilton, D. P., and Pan, G. (2018). Combating Hypoxia/anoxia at Sediment-Water Interfaces: A Preliminary Study of Oxygen Nanobubble Modified clay Materials. Sci. Total Environ. 637-638, 550–560. doi:10.1016/j.scitotenv.2018.04.284
Keywords: bulk nanobubble, gas transport, long lifetime, high inner density, diffusion equation analysis
Citation: Wang Y, Zhou L, Hu J and Zhang L (2022) Theoretical Analysis on the Stability of Single Bulk Nanobubble. Front. Mater. 9:824283. doi: 10.3389/fmats.2022.824283
Received: 29 November 2021; Accepted: 14 February 2022;
Published: 07 April 2022.
Edited by:
Zhiyong Gao, Central South University, ChinaReviewed by:
Xianren Zhang, Beijing University of Chemical Technology, ChinaFederico Rossi, University of Siena, Italy
Copyright © 2022 Wang, Zhou, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Hu, aHVqdW5Ac2luYXAuYWMuY24=; Lijuan Zhang, emhhbmdsaWp1YW5AempsYWIub3JnLmNu
 Yao Wang
Yao Wang Limin Zhou3
Limin Zhou3 Lijuan Zhang
Lijuan Zhang