- 1Center for Engineering Training and Basic Experimentation, Heilongjiang University of Science and Technology, Harbin, China
- 2Key Laboratory for Photonic and Electronic Bandgap Materials, Ministry of Education and School of Physics and Electronic Engineering, Harbin Normal University, Harbin, China
- 3College of Science, Qiqihar University, Qiqihar, China
CoSe2 is a typical transition metal chalcogenide and an hydrogen evolution reaction hydrogen evolution catalyst due to its efficient and inexpensive characteristics. In this work, CoSe2/CNT composite was synthesized through a one-step solvothermal method. Specifically, a current density of 10 mA cm−2 can be achieved under an overpotential of 163 mV, and the Tafel slope is 69.4 mV dec−1. The reasons for these excellent performances are: 1) the abundant catalytic active sites of CoSe2; 2) the high conductivity of CNTs; and 3) the synergistic effect between CoSe2 and CNTs; iv the solar irradiation excites electrons to join the reaction. This work proposes a new way to reasonably design the high-efficiency and low-cost transition metal chalcogenide hydrogen evolution catalysts.
Introduction
In recent years, people have paid more and more attention to renewable energy, such as bioenergy, hydrogen energy, solar energy, etc., because of the decreasing fossil energy and the increasingly severe environmental pollution. Hydrogen has become the focus of attention because of its high energy density, cleanliness, and renewability (Fan et al., 2019; Gao et al., 2018; Li et al., 2020; Yu, Qu, et al., 2016; Yu, Zhang, et al., 2016). Electrocatalysis decomposes water into hydrogen and oxygen, which might be used to tackle energy and environmental problems. The hydrogen evolution reaction (HER) plays a vital role in electrochemical water splitting and has been widely studied (Cao et al., 2022; Yuanmiao Sun et al., 2020; Ji et al., 2020; Zhao et al., 2022). To date, platinum-based materials have been considered to be the best hydrogen evolution reaction catalysts because they have the smallest overpotential, and the Gibbs free energy (ΔGH) of Pt to adsorb hydrogen is close to zero (Greeley et al., 2006). However, industrial production has been restricted due to few reserves and high prices. Therefore, people are committed to looking for abundant, low-cost, efficient, and stable catalysts to replace the precious metal platinum-based catalysts (Liu et al., 2016; Ye et al., 2016).
In recent years, people have made great efforts to study the catalytic effects of transition metal sulfides, metal oxides, phosphides, and selenides on HER, proving that these materials are promising electrocatalysts that can replace platinum-based materials. Among these materials, the transition metal selenides MoSe2 (Deng et al., 2018), WSe2 (Henckel et al., 2017), NiSe2 (Sun et al., 2018), and CoSe2 (Chen et al., 2016) have good catalytic performance, and low cost, so they get extensive studies. Since CoSe2 has good catalytic activity and durability characteristics, it has been proven to have excellent HER electrocatalytic performance in acidic media (McCarthy et al., 2016; Yang et al., 2021). However, CoSe2 always agglomerate and leads to a lack of active sites in practical applications. Also, its conductivity is not particularly excellent enough. To address these advantages, pure CoSe2 is usually prepared into different morphologies and coupled with some conductive substrates, such as carbon cloth, carbon paper, and carbon nanotubes (Lee et al., 2016; Wang et al., 2015). These strategies favor the catalyst exposing many active sites and improving the overall conductivity, which is vital for HER (Yan et al., 2013). For instance, the co-precipitation and annealing methods have been adopted by Chen et al (Wang et al., 2019) to grow CoSe2 nanosheet arrays on Co-N doped carbon cloth with excellent HER performance. Kang et al. used spray pyrolysis and selenization to make macroporous CoSe2/CNT composite microspheres, which have good electrocatalytic properties in acid electrolyte (Kim et al., 2017). However, the preparation mentioned above process is quite complicated. On the other hand, the solar assistant electrochemical strategy has recently attracted much attention, which can induce photocarrier generation and further boosts the kinetic process (Ma et al., 2022).
In this work, we employ a simple one-step solvothermal method to successfully synthesize the CoSe2/CNT composite, which exposes more active sites, increases the composite’s electrical conductivity, and improves the overall HER catalytic performance of the material, but also prevents CoSe2 crystals from clumping together. Importantly, we found that solar-irradiation can excite electrons during catalysis, which boosted the HER effectively. For these reasons, the CoSe2/CNT composite can drive the current density of 10 mA cm−2 with only 105 mV, superior to the performance of pure CoSe2.
Experimental section
Chemicals and materials
The used chemicals and materials include analytical grade Carbon nanotube dispersion, Cobalt Chloride Hexahydrate (CoCl2.6H2O, 98%), sodium borohydride (NaBH4, 98%), selenium (Se, 99.9%). It is worth noting that they were used without being purified further.
Synthesis of CoSe2/CNT composite
The CoSe2/CNT method was synthesized based on a classical synthesized method (Yue et al., 2017), wherein the solvent was changed from deionized water to DMF, along with a decreased reaction temperature. First, 671 µL of carbon nanotube solution (41.2 mg ml−1) was dispersed in 20 ml of N, N-dimethylformamide (DMF), and then they were treated with ultrasonic for an hour. Second, 215 mg of selenium powder (Lee et al.) and 128.6 mg of sodium borohydride (NaBH4) were dissolved in 10 ml of DMF and magnetically agitated to form solution A, which was mixed for 30 min after adding the dispersed carbon nanotube solution and 323.18 mg of cobalt chloride (CoCl2•6H2O). Finally, the prepared solution was heated in a 50 ml Teflon-lined autoclave at 200°C for 20 h, and then it was washed with deionized water and ethanol and dried in a vacuum at 60°C for 12 h. CoSe2/CNT composite was obtained. The synthesis of pure CoSe2 is the same as the CoSe2/CNT method.
Preparation of CoSe2/CNT electrode
20 mg CoSe2/CNT was ground in mortar for 1–2 h. Then, to obtain uniformly dispersed ink, the NMP contains the CoSe2/CNT, and 5 wt% of polyvinylidene fluoride (PVDF) (mass ratio: CoSe2/CNT: PVDF is 9:1) was sonicated for 3 h. The 1mg/uL ink was evenly coated on hydrophilic carbon paper (1 cm × 1 cm, load ≈2 mg cm−2) and dried in a vacuum drying oven at 60°C for 12 h.
Characterization
To characterize the morphology and elemental composition of the composite material, we use the scanning electron microscope (SEM) and transmission electron microscope (TEM) equipped with an energy dispersive X-ray spectrometer (EDX). In addition, the crystal structure of the material was characterized by X-ray diffraction (XRD), and the Raman spectrum was measured by a miniature Raman spectrometer (JY; HR800, France) at an excitation wavelength of 488 nm.
Electrochemical experiment
The electrochemical performances such as intrinsic HER activity and durability of the composite were tested by the VMP3 electrochemical workstation (France Bio-logic) in a three-electrode configuration under 0.5 M H2SO4 electrolyte. The electrode we prepared in advance, the saturated calomel electrode (SCE), and the carbon rod was respectively used as the working electrode, the reference electrode, and the counter electrode. The electric potential, which is applied by the reversible hydrogen electrode (RHE), is expressed by formula E(RHE) = E(SCE) + 0.059 V × pH + 0.241 V. The polarization curve is obtained by linear sweep voltammetry (LSV) at a scan rate of 5 mV s−1 in the voltage range of −0.1∼ −0.7 V. The test condition of electrochemical impedance spectroscopy (EIS) is limited to the frequency parameter of 100 mHz–100 kHz under an overpotential of −0.2 V (vs. RHE). Cyclic voltammetry (CV) is used to measure the electric double-layer capacitance (Cdl) at 0.45–0.55 V (vs. RHE) is measured by the CV, in which the scan rate ranges from 20 to 200 mV. When the rated voltage is 0.5 V (vs. RHE), the difference between the anode and cathode current densities (Δj = ja-jc) for different scan rates is calculated to obtain the slope and the half is defined as Cdl. All overpotentials are corrected by 85% ohm potential drop (iR) compensation.
Results and discussion
Morpho-structural characterization
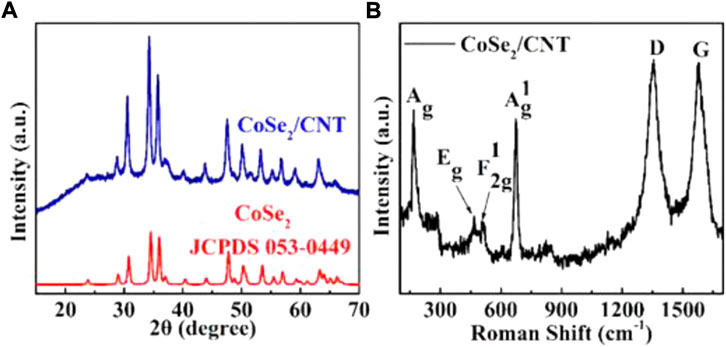
First, Se powder and NaBH4 were added to DMF. Then, after the DMF solutions were stirred evenly, the pre-dispersed mixtures of carbon nanotube solution and CoCl2.6H2O with series mass ratios were dispersed into the above solutions. Finally, the one-step solvothermal method is applied to obtain the CoSe2/CNT composite. In order to analyze the structure and composition, Next, we first performed the XRD test to analyze the structure and composition. As shown in Figure 1A. It can be seen that the diffraction peak at 26° is derived from CNTs. CoSe2/CNTs sample has three prominent diffraction peaks at 30.77°, 34.52°, 35.96°, and there are some small diffraction peaks at 2θ = 28.97°, 47.72°, 50.66°, 53.48°, 55.42°, 56.95°, and 59.27°, corresponding to the orthogonal structure of CoSe2 (PDF card No. 53-449) (101), (111), (120), (011), (211), (130), (031), (221), (131), and (310) crystal planes, respectively (Fan et al., 2019). No impurities were observed, indicating that a higher purity CoSe2/CNTs was synthesized. Furthermore, as shown in Figure 1B, the sharp peak at 173.5 cm−1 corresponds to the Se-Se stretching mode of orthorhombic CoSe2 (o-CoSe2), which proved the successful synthesis, the high purity as well as high crystalline of CoSe2/CNTs. The Raman spectra of CoSe2 show other three typical peaks (Eg,
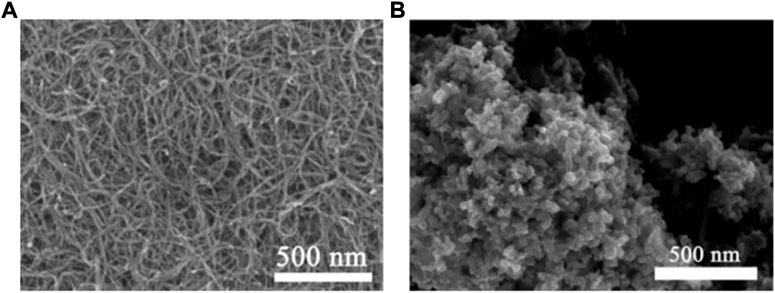
The SEM image shows the morphological information of CNT, pure CoSe2 and CoSe2/CNT. The smooth diameter of the CNT surface is shown in Figure 2A and is about 10–15 nm. In Figure 2B, pure CoSe2 exists in the form of nanoparticles with a size of about 35–50 nm, and the nanoparticles are stacked on each other. It can be seen from Figure 3A that after CoSe2 and CNT are compounded, CoSe2 is more uniformly dispersed, and the size is reduced to about 20–35 nm. This should be attributed to the presence of CNT that allowed CoSe2 to grow on top of them, thereby preventing CoSe2 stacking. Good contact between CoSe2 nanoparticles and CNT and further composite nanostructures can enhance the charge transfer ability of CoSe2. Energy-dispersive X-ray spectroscopy (EDS) analysis is shown in Figure 3B, which indicates that Se is nearly twice that of Co., which conforms to the stoichiometric ratio.
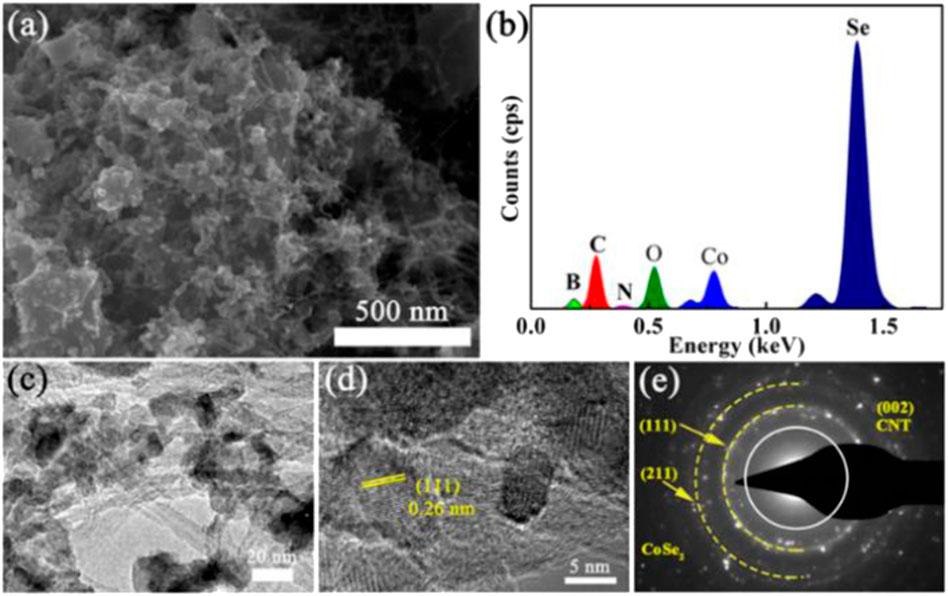
FIGURE 3. (A) SEM image, (B) EDS spectrum, (C) TEM image, (D) HRTEM image, and (E) SAED pattern of CoSe2/CNT composite.
TEM was employed further to study the morphology and structure of CoSe2/CNT. As shown in Figure 3C, CoSe2 nanoparticles were grown dispersed on CNT and have smaller sizes. In addition, the high-resolution TEM (HRTEM) image is shown in Figure 3D, where the lattice spacing is 0.26 nm, which is well to the (111) crystal plane of CoSe2. Please note that the lattice parameter is consistent with the results calculated by the Bragg equation from XRD data. Figure 3E shows the selected area electron diffraction (SAED) image of the CoSe2/CNT composite, in which the yellow dotted line and the white circled area correspond to the (111) and (211) crystal planes of CoSe2 and the (002) crystal plane of CNT, respectively (Shao et al., 2021). The discrete concentric rings prove that the substances in CoSe2/CNT composites are crystalline. These results confirm that CoSe2/CNT composite has been successfully prepared (Zhang et al., 2015).
Electrochemical performances
The electrochemical performance of CoSe2/CNT composites and pure CoSe2 were measured based on the three-electrode system in 0.5 M H2SO4 to study HER performance. As shown in Figure 4, the electrocatalytic performance of pure CoSe2 and CoSe2/CNT composite were compared. The current density of the overpotential of CoSe2/CNT electrode at 163 mV is 10 mA cm−2 and is super than pure CoSe2 powder (189 mV) and the CNT (over 450 mV). The main functions of CNT are to improve the conductivity of the composite material and inhibit the growth of CoSe2. Excited, the HER activity was dramatically enhanced after solar irradiation and only needed 105 mV to drive the current density of 10 mA cm−2. According to the LSV results, we can obtain the Tafel curve and fit the Tafel equation (η = blog |j| + a) by the linear region, where η, j, and b are respectively the overpotential, the current density, and the Tafel slope. The value of the Tafel slope is used to compare the catalytic activity of the catalyst. From Figure 4B, the Tafel slope of the CoSe2/CNT composite is 69.4 mV dec−1, significantly lower than pure CoSe2 (86.3 mV dec−1) and CNT (208 mV dec−1), thus proving that the CoSe2/CNT composite has a faster reaction kinetics.
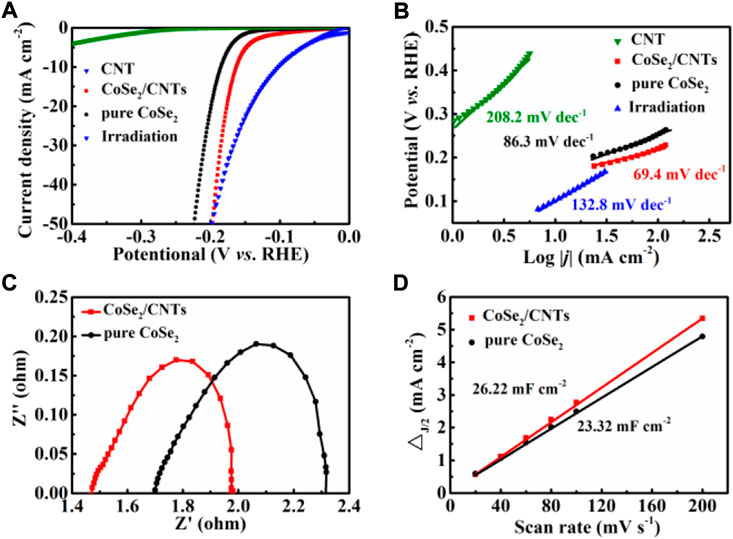
FIGURE 4. (A) Polarization curve, (B) Tafel slopes, (C) electrochemical impedance spectra, and (D) Cdl value of different electrodes.
Commonly, Tafel slopes of 30, 40 and 120 mV dec−1 correspond to the mechanisms of Tafel, Heyrovsky or Volmer reactions, respectively. Therefore the CoSe2/CNT follows the Volmer-Heyrovsky mechanism, and CoSe2/CNT composites have much better HER performance than pure CoSe2. It shows that the Tafel for CoSe2/CNT composites increases to 132 mV dec−1, thus indicating that the solar irradiation induces a mechanism conversation. This may be derived from the that CoS2 is an electronic type semiconductor, wherein abundant electrons were excited to join the reaction by solar irradiation.
In addition, the EIS shown in Figure 4C further verified the reaction kinetics. It can be observed that the ohmic resistance (Rs), i.e., the distance between the intersection of the EIS and the x-axis in the high-frequency region, is the resistance between the interface contact and the electrolyte. The charge transfer resistance (Rct), i.e., the diameter of the semicircle in the high-frequency region, represents the electrocatalytic kinetics of the interface between the electrocatalyst and the electrolyte. The figure indicates that the Rct of the CoSe2/CNT catalyst is 0.194Ω and lower than that of pure CoSe2 (0.290Ω), indicating that the CoSe2/CNT catalyst has faster reaction kinetics in the HER process. The mass transfer resistance (Rmt) of the electrode is obtained from the semicircle in the low-frequency region, in which the Rmt of the CoSe2/CNT electrode is lower than that of the pure CoSe2 electrodes. Based on that the Tafel value changed from 86.3 to 132.8 mV dec−1, it can be considered that solar irradiation has changed govern process from Tafel to the Volmer-Tafel. This is similar to previous literature that photo-induced carriers generated once the catalysts were irradiated and reasonable to explain that the second react step becomes the rate-determining step (Ma et al., 2022).
Electrochemical double-layer capacitance is usually employed to estimate the electrochemically active specific surface area (ECSA). Figure 5 shows the cyclic voltammetry curves of CoSe2/CNT and pure CoSe2. The linear slope in Figure 4D indicates that the Cdl value is 26.22 mF cm−2, significantly greater than pure CoSe2 (17.39 mF cm−2). It shows that CoSe2/CNT has higher ECSA, electrode material, and electrolysis. The liquid contact interface has more active sites. CoSe2/CNT can adsorb more hydrated protons to form the intermediate Hads. The decreased Rct and increased ECSA of CoSe2/CNT may be derived from the unique structure, which possesses a transfer network for electro and electrolyte and also a space for active center exposure.
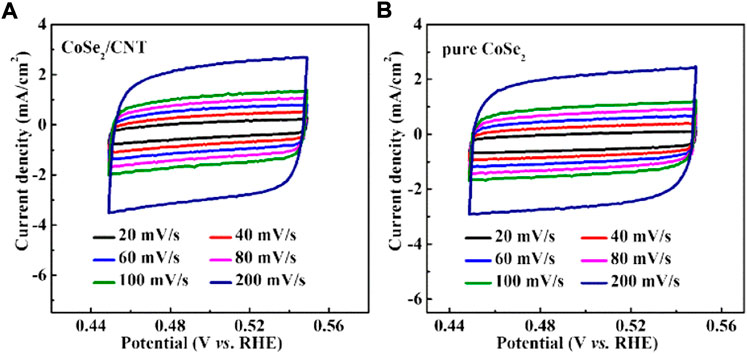
FIGURE 5. CVs were scanned at various rates from 20 to 200 mV s−1: (A) CoSe2/CNT and (B) pure CoSe2.
Stability is an efficient factor for an ideal HER catalyst. We subsequently measured the stability ability and the morphology and structure conversion information. As shown in Figure 6A, the potential retention was kept around 100%. The corresponding XRD pattern (Figure 6B) is consistent with that before HER, indicating that the structure is maintained well. Figure 6C shows that the sample after HER still expresses particle-like morphology with a size distribution from 20 to 35 nm, similar to pristine CoSe2/CNT catalyst. The SEM image also shows that the spatial structure is kept. These data certify that the CoSe2/CNT has good electrochemical stability under solar irradiation. In addition, combining the corresponding excellent and poor HER performances for CoSe2/CNT catalyst and CNT and structural maintenance of CoSe2, it is evidenced that CoSe2 is the actual catalytic center which follows a behavior of semiconductor-based HER catalyst (Han et al., 2017).
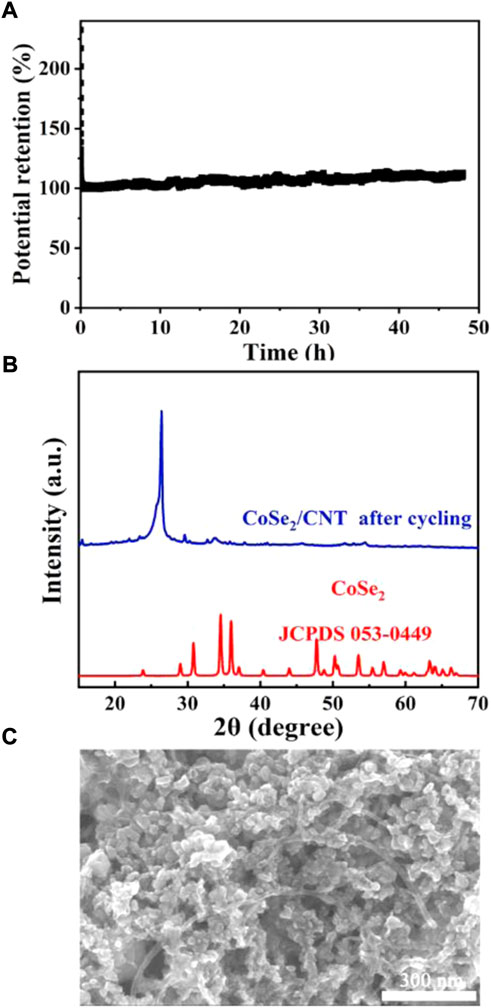
FIGURE 6. (A) Solar-irradiated HER stability data; (B,C) XRD pattern and SEM image of CoSe2/CNTs after HER.
Conclusion
In short, we use a simple one-step solvothermal method to synthesize the CoSe2/CNTs composite material, which has been proved to have excellent photo electrochemical HER performance, with an overpotential of 105 mV at a current density of 10 mA cm−2. These excellent properties are attributed to the smaller CoSe2 nanoparticles, more active sites, the excellent conductivity of CNTs, as well as the ideal photo-electrochemical response. It opens up a new opportunity to develop low-cost, high-efficiency metal compound catalysts.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
ZP: Methodology, Data curation, original draft preparation, writing reviewing. HY: Language help. ZH: Data curation. ZY: Writing–review and editing. PY: Project administration, supervision, funding acquisition, and project guide. XM: Investigation, writing–original draft, data validation.
Funding
We gratefully acknowledge the support of this research by the National Natural Science Foundation of China (22005078), Research Team Project of Heilongjiang Province (JJ2021TD0011), and the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2020127).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XY declared a shared affiliation with the authors XM to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2022.1005221/full#supplementary-material
References
Cao, J., Zhou, J., Li, M., Chen, J., Zhang, Y., and Liu, X. (2022). Insightful understanding of three-phase interface behaviors in 1T-2H MoS2/CFP electrode for hydrogen evolution improvement. Chin. Chem. Lett. 33 (8), 3745–3751. doi:10.1016/j.cclet.2021.11.007
Chen, P. Z., Xu, K., Tao, S., Zhou, T. P., Tong, Y., Ding, H., et al. (2016). Phase-transformation engineering in cobalt diselenide realizing enhanced catalytic activity for hydrogen evolution in an alkaline medium. Adv. Mat. 28, 7527–7532. doi:10.1002/adma.201601663
Deng, S. J., Yang, F., Zhang, Q. H., Zhong, Y., Zeng, Y. X., Lin, S. W., et al. (2018). Phase modulation of (1T-2H)-MoSe2/TiC-C shell/core arrays via nitrogen doping for highly efficient hydrogen evolution reaction. Adv. Mat. 30, 1802223. doi:10.1002/adma.201802223
Fan, M., Zhang, L., Li, K., Liu, J., Zheng, Y., Zhang, L., et al. (2019). FeS2@C core-shell nanochains as efficient electrocatalysts for hydrogen evolution reaction. ACS Appl. Nano Mat. 2, 3889–3896. doi:10.1021/acsanm.9b00736
Gao, D., Xia, B., Zhu, C., Du, Y., Xi, P., Xue, D., et al. (2018). Activation of the MoSe2 basal plane and Se-edge by B doping for enhanced hydrogen evolution. J. Mat. Chem. A 6, 510–515. doi:10.1039/c7ta09982g
Greeley, J., Jaramillo, T. F., Bonde, J., Chorkendorff, I. B., and Nørskov, J. K. (2006). Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater 5, 909–913. doi:10.1038/nmat1752
Henckel, D. A., Lenz, O., and Cossairt, B. M. (2017). Effect of ligand coverage on hydrogen evolution catalyzed by colloidal WSe2. ACS Catal. 7, 2815–2820. doi:10.1021/acscatal.7b00074
Ji, Y., Xie, J., Yang, Y., Fu, X., Sun, R., and Wong, C. (2020). NiCoP 1D nanothorns grown on 3D hierarchically porous Ni films for high performance hydrogen evolution reaction. Chin. Chem. Lett. 31 (3), 855–858. doi:10.1016/j.cclet.2019.06.021
Kim, J. K., Park, G. D., Kim, J. H., Park, S. K., and Kang, Y. C. (2017). Rational design and synthesis of extremely efficient macroporous CoSe2-CNT composite microspheres for hydrogen evolution reaction. Small 13, 1700068. doi:10.1002/smll.201700068
Lee, C. P., Chen, W. F., Billo, T., Lin, Y. G., Fu, F. Y., Samireddi, S., et al. (2016). Beaded stream-like CoSe2 nanoneedle array for efficient hydrogen evolution electrocatalysis. J. Mat. Chem. A 4, 4553–4561. doi:10.1039/c6ta00464d
Li, S., Que, X., Chen, X., Lin, T., Sheng, L., Peng, J., et al. (2020). One-step synthesis of modified Ti3C2 MXene-supported amorphous molybdenum sulfide electrocatalysts by a facile gamma radiation strategy for efficient hydrogen evolution reaction. ACS Appl. Energy Mat. 3, 10882–10891. doi:10.1021/acsaem.0c01900
Liu, Y. R., Hu, W. H., Li, X., Dong, B., Shang, X., Han, G. Q., et al. (2016). Facile one-pot synthesis of CoS2-MoS2/CNTs as efficient electrocatalyst for hydrogen evolution reaction. Appl. Surf. Sci. 384, 51–57. doi:10.1016/j.apsusc.2016.05.007
Ma, X., Zhang, M., Aza, F., Gao, Q., Xu, Z., Li, L., et al. (2022). Photothermal-effect-promoted interfacial OH− filling and the conversion of carrier type in (Co1−xNix)3C during water oxidation. J. Mat. Chem. A 10 (15), 8258–8267. doi:10.1039/d1ta11015b
McCarthy, C. L., Downes, C. A., Schueller, E. C., Abuyen, K., and Brutchey, R. L. (2016). Method for the solution deposition of phase-pure CoSe2 as an efficient hydrogen evolution reaction electrocatalyst. ACS Energy Lett. 1, 607–611. doi:10.1021/acsenergylett.6b00246
Sun, Y. Q., Xu, K., Wei, Z. X., Li, H. L., Zhang, T., Li, X. Y., et al. (2018). Strong electronic interaction in dualcationincorporated NiSe 2 nanosheets with lattice distortion for highly efficient overall water splitting. Adv. Mat. 30, 1802121. doi:10.1002/adma.201802121
Wang, K., Xi, D., Zhou, C. J., Shi, Z. Q., Xia, H. Y., Liu, G. W., et al. (2015). CoSe2 necklace-like nanowires supported by carbon fiber paper: A 3D integrated electrode for the hydrogen evolution reaction. J. Mat. Chem. A 3, 9415–9420. doi:10.1039/c5ta01143d
Wang, X. Q., He, J. R., Yu, B., Sun, B. C., Yang, D. X., Zhang, X. J., et al. (2019). CoSe2 nanoparticles embedded MOF-derived Co-N-C nanoflake arrays as efficient and stable electrocatalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 258, 117996. doi:10.1016/j.apcatb.2019.117996
Yan, Y., Ge, X., Liu, Z., Wang, J. Y., Lee, J. M., and Wang, X. (2013). Facile synthesis of low crystalline MoS2 nanosheet-coated CNTs for enhanced hydrogen evolution reaction. Nanoscale 5, 7768–7771. doi:10.1039/c3nr02994h
Yang, S. H., Park, G. D., Kim, J. K., and Kang, Y. C. (2021). New strategy to synthesize optimal cobalt diselenide@hollow mesoporous carbon nanospheres for highly efficient hydrogen evolution reaction. Chem. Eng. J. 424, 130341. doi:10.1016/j.cej.2021.130341
Ye, G., Gong, Y., Lin, J., Li, B., He, Y., Pantelides, S. T., et al. (2016). Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett. 16, 1097–1103. doi:10.1021/acs.nanolett.5b04331
Ye, Z. T., Zhou, L. L., Shao, Y., Yao, X. Z., Ma, L., Wu, H. F., et al. (2021). In situ integration of cobalt diselenide nanoparticles on CNTs realizing durable hydrogen evolution. RSC Adv. 12, 4446–4454. doi:10.1039/d1ra07301j
Yu, B., Qi, F., Zheng, B., Hou, W., Zhang, W., Li, Y., et al. (2018). Self-assembled pearl-bracelet-like CoSe2-SnSe2/CNT hollow architecture as highly efficient electrocatalysts for hydrogen evolution reaction. J. Mat. Chem. A 6 (4), 1655–1662. doi:10.1039/c7ta08955d
Yu, X., Qu, B., Zhao, Y., Li, C., Chen, Y., Sun, C., et al. (2016). Growth of hollow transition metal (Fe, Co, Ni) oxide nanoparticles on graphene sheets through kirkendall effect as anodes for high-performance lithium-ion batteries. Chem. Eur. J. 22 (5), 1638–1645. doi:10.1002/chem.201503897
Yue, H. H., Yu, B., Qi, F., Zhou, J. H., Wang, X. Q., Zheng, B. J., et al. (2017). Interwoven CoSe2/CNTs hybrid as a highly efficient and stable electrocatalyst for hydrogen evolution reaction. Electrochimica Acta 253, 200–207. doi:10.1016/j.electacta.2017.09.066
Zhang, H. X., Yang, B., Wu, X. L., Li, Z. J., Lei, L. C., and Zhang, X. W. (2015). Polymorphic CoSe2 with mixed orthorhombic and cubic phases for highly efficient hydrogen evolution reaction. ACS Appl. Mat. Interfaces 7, 1772–1779. doi:10.1021/am507373g
Keywords: HER, solar-irradiation, CoSe2, CNT, composite
Citation: Pi Z-G, Ye H, Han Z, Yu P, Yin Z and Ma X (2022) Promising CoSe2-CNT composite catalyst for efficient photoelectrochemical hydrogen evolution reaction. Front. Mater. 9:1005221. doi: 10.3389/fmats.2022.1005221
Received: 28 July 2022; Accepted: 08 September 2022;
Published: 10 October 2022.
Edited by:
Wei Feng, Northeast Forestry University, ChinaReviewed by:
Jianlong Wang, Northwest A&F University, ChinaXianbo Yu, Harbin Normal University, China
Bo-Quan Li, Beijing University of Technology, China
Yan Feng, Harbin Engineering University, China
Lei Wang, Heilongjiang University, China
Copyright © 2022 Pi, Ye, Han, Yu, Yin and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Yu, bGF2ZW5kZXJtb29uQDEyNi5jb20=; Zhuoxun Yin, eXp4QHFxaHJ1LmVkdS5jbg==; Xinzhi Ma, bWF4ekBocmJudS5lZHUuY24=
 Zhi-Gang Pi1
Zhi-Gang Pi1 Zuqi Han
Zuqi Han Zhuoxun Yin
Zhuoxun Yin Xinzhi Ma
Xinzhi Ma