- 1State Key Laboratory of Fire Science, University of Science and Technology of China, Hefei, China
- 2State Key Laboratory of Coal Mine Safety Technology, CCTEG Shenyang Research Institute, Fushun, China
In the goaf of the coal mine, there will be some high-temperature points before or during the fire. Under certain conditions, these high-temperature points will radiate heat to the surrounding coal in the form of thermal radiation, which, in turn, may also ignite the coal. Taking this situation into consideration, this study aims to investigate the influence of high-temperature thermal radiation on the transformation characteristics of coal oxidation and spontaneous combustion using the high-temperature thermal radiation method. The results show that an increase in thermal radiation value reduces the ignition time of coal gradually. The peak heat release rate, total heat release, peak smoke release rate, and total smoke release gradually increase. Additionally, the total carbon monoxide release reduces gradually, and the peak carbon dioxide production rate increases gradually. It is worth noting that as the heat radiation value increases, the peak value of CO production rate of lignite and bituminous coal is noted to decrease gradually, whereas that of anthracite increases gradually. The total carbon dioxide emissions of bituminous coal and anthracite increased gradually, whereas the total carbon dioxide emissions of lignite increased firstly and then decreased. This work proposes a novel method to study the coal oxidation and spontaneous combustion by a widely-recognized combustion apparatus.
Introduction
It is well known that there are three main forms of heat transfer: conduction, convection and radiation. For coal, because it is a poor heat conductor, and the space in the goaf of coal mine is relatively closed, the heat is mainly transferred to the surrounding coal in the form of thermal radiation during and after the oxidation and spontaneous combustion of coal (Wen et al., 2017; Wang et al., 2018; Shao et al., 2021; Xiao et al., 2021).
At present, in the field of coal mine safety, there are relatively few reports on the study of thermal radiation characteristics using high temperature thermal radiation method. The related researches mainly focus on the use of cone calorimeter to investigate the effect of typical inorganic salt inhibitors (Guo et al., 2021; Lv et al., 2021; Mohalik et al., 2021; Xi et al., 2021), and analyze the combustibles with fire risk such as underground support wood and conveyor belts. However, as one of the key factors to initiate or promote coal oxidation and spontaneous combustion, there are few reports on the effect of high temperature thermal radiation on the transformation characteristics of coal oxidation and spontaneous combustion (Wang L et al., 2019).
Before or in the process of coal oxidation and spontaneous combustion fire, the local high temperature points will generally appear in the goaf of coal mine. These high temperature points mainly radiate heat to the surrounding coal through thermal radiation, and ignite the coal under certain conditions, which leads to the occurrence of mine fire. In addition, these high temperature points are more likely to cause highly located fire areas, which increases the difficulty of mine fire prevention and control. In view of this, this work uses the method of high temperature thermal radiation to study the influence of high temperature thermal radiation on the transformation characteristics of coal oxidation and spontaneous combustion (Pranda et al., 2001; Liu et al., 2020a).
The cone calorimeter has been widely used to measure the time to ignition (TTI), peak heat release rate (PHRR), total heat release (THR), peak smoke release rate (PSPR), total smoke release (TSR) of materials (Zhang et al., 2004, Brohez, 2005; Chow and Han, 2011; Pretrel et al., 2014; Guo et al., 2018; Wang D et al., 2019; Wang et al., 2020b). Peak carbon monoxide production rate (PCOPR), and carbon monoxide total release (COTY), peak carbon dioxide production rate (PCO2PR) and total carbon dioxide release (CO2TY) have also been used to investigate the combustion behavior of the material (Wang L et al., 2019; Liu et al., 2020b; Shi et al., 2020; Shi et al., 2021). Therefore, in this work, the effects of heat radiation on the transition characteristics of three kinds of coal with different spontaneous combustion tendencies, such as lignite, bitumite and anthracite, have been studied by using the cone calorimeter based on four different heat radiation intensities. And in the end, the influence law of high-temperature thermal radiation on the spontaneous combustion process of coal oxidation has been quantitatively revealed.
Experimental
Materials
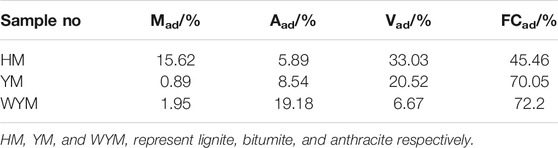
The lignite (named as HM) was obtained from Inner Mongolia Pingzhuang Coal Mine Co. Ltd. The bitumite (denoted as YM) was provided by Shanxi Zhongyang Coal Mine Co. Ltd. The anthracite (indicated as WYM) was acquired from Jincheng Lanyan Coal Mime Co. Ltd. Tables 1 and 2 show the industrial and ultimate analysis data of coal samples.
Instruments and Measurements
The composition analysis of three kinds of coal samples was performed by a 5E-SDLA618 Automatic Industrial Analyzer (Sande Instruments, China). The ultimate analysis of the coal samples was performed by using an Elemental Analyzer (Kaiyuan Instrument, China). The combustion properties of coal samples were investigated via a cone calorimeter (TESTech, Suzhou, China) according to ISO 5660. The specimens with size of 100 × 100 × 3 mm3 were radiated by a heat flux of 15, 25, 35, and 50 kW m−2, and were placed in aluminum foils.
All these procedures were performed three times for each specimen.
Results and Discussion
Time to Ignition
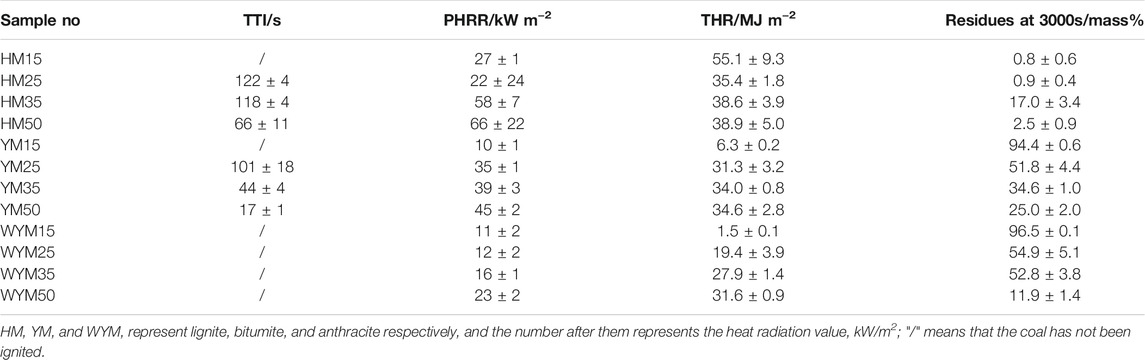
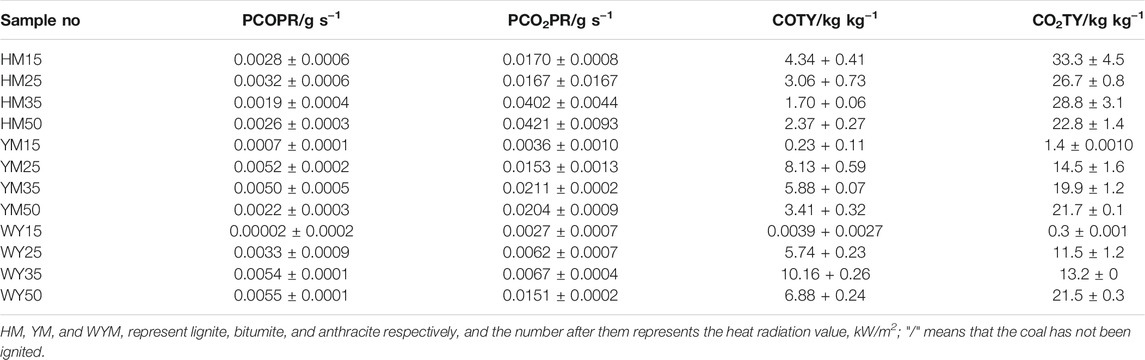
Table 3 presents the TTI of coal sample. As presented in Table 3, TTI of lignite and bituminous coal decreases as the thermal radiation value increases. This shows that under relatively higher thermal radiation, the oxidation degree of coal is accelerated, and the coal is more easily ignited.
HM15, YM15, and WYM15 cannot always be ignited when the thermal radiation value is 15 kW/m2. However, the TTI value of HM50 was noted to reduce by 45.9% compared to HM25. TTI of YM25, YM35, and YM50 are 101, 44, and 17 s, respectively. The TTI value of bituminous coal has been found to be always lower than that of lignite at the same thermal radiation value. For example, TTI of YM50 was significantly reduced by 74.2% compared to HM50. The reason is that the moisture content of lignite is significantly higher than that of bituminous coal (Yin et al., 2014). The time of water evaporation of lignite is longer than that of bituminous coal, making lignite more difficult to ignite than bituminous coal (Yashwanth et al., 2016). It is worth noting that no matter what the thermal radiation value increases to, anthracite cannot be ignited, which is deemed consistent with the spontaneous combustion tendency of anthracite.
Heat Release
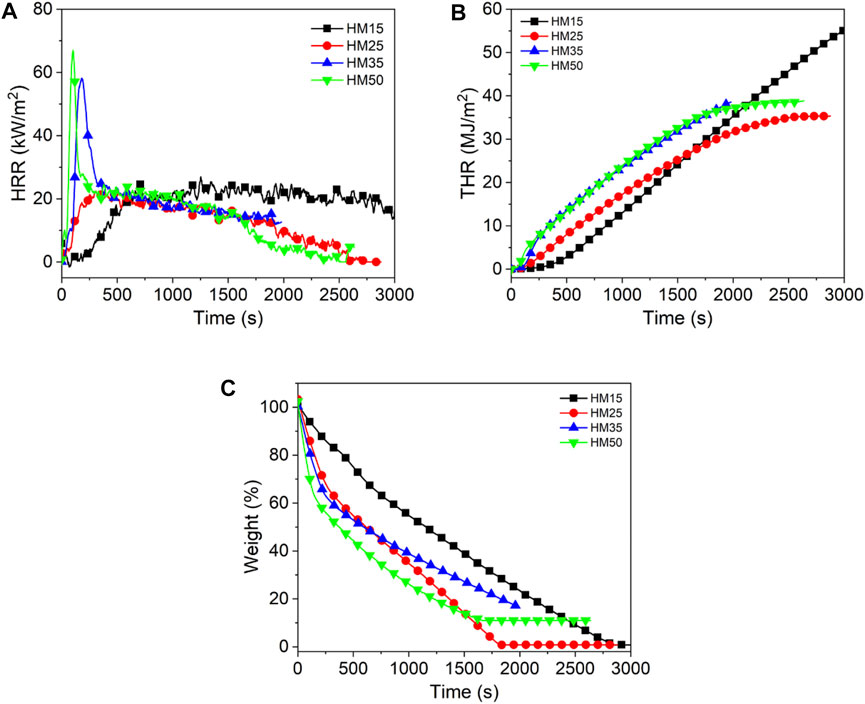
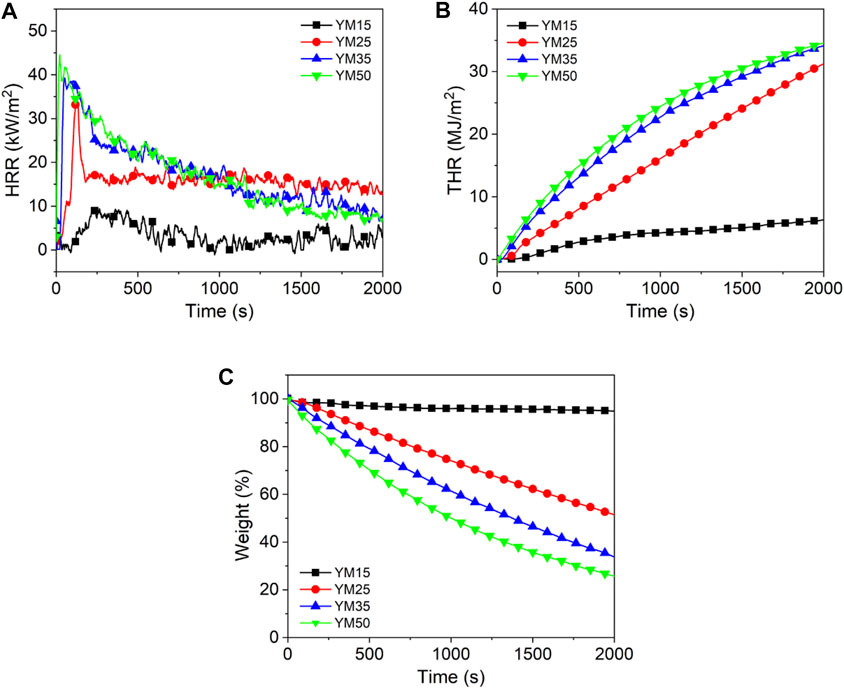
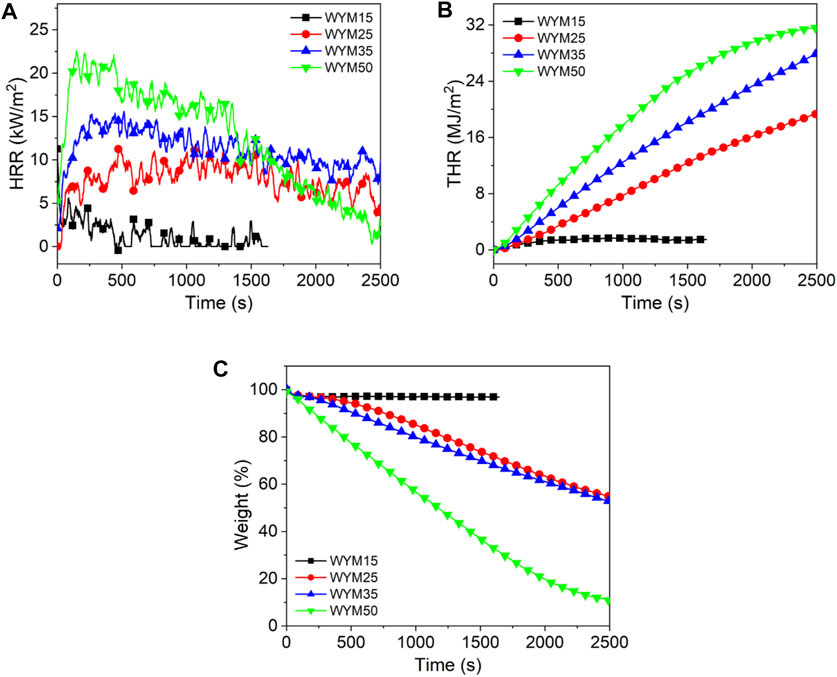
Figures 1–3 show the heat release curves of the coal, while Table 3 summarizes the relevant data.
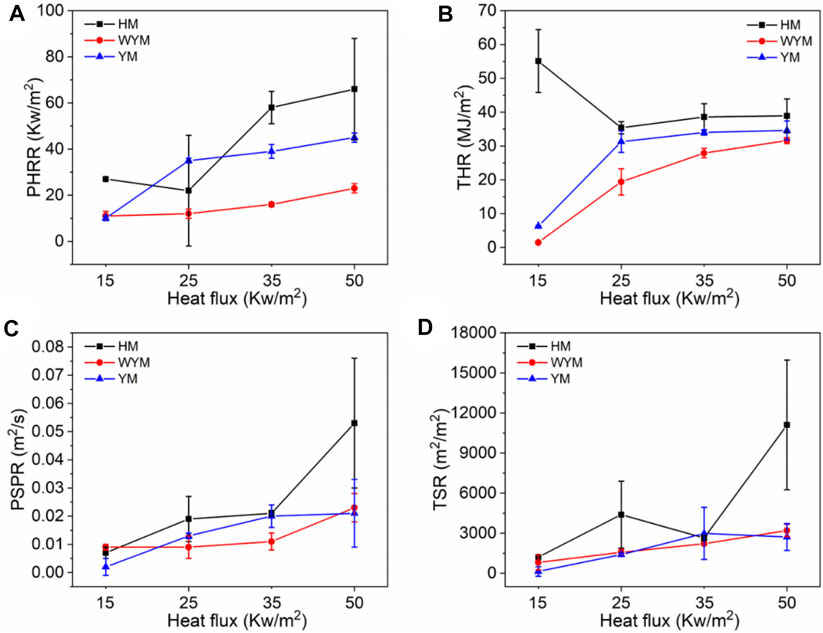
As shown in Figure 1A, the PHRR of lignite increases as the thermal radiation values increase. Especially, when the heat radiation value increases to 50 kW/m2, the PHRR of HM50 is as high as 66 kW/m2, which is 144.4% higher than that of HM15. This is due to an increase in thermal radiation value, and the higher the heating temperature of coal, the more heat it releases. PHRR of YM15, YM25, YM35, and YM50 are 10, 35, 39, and 40 kW/m2, respectively. The PHRR value of bituminous coal increases with an increase in thermal radiation value. Additionally, YM50 offers a significant 300% improvement in PHRR compared to YM15. The PHRR of anthracite increases with an increase in thermal radiation value, which is the same as that of lignite and bituminous coal. For example, the WYM50 has 1.9 times the PHRR of WYM25. It is worth noting that the PHRR of bituminous coal and anthracite is lower than that of lignite at the same thermal radiation value. For example, the PHRR of YM15 and WYM15 are 10 and 11 kW/m2, respectively, which are 63.0 and 59.3% lower than that of HM15. The reason is that the carbon content of bituminous coal and anthracite is higher than that of lignite, resulting in a denser carbon layer during coal combustion, which then effectively prevents the release of heat. Additionally, the PHRR of anthracite decreases compared to bituminous coal. The reason is that bituminous coal has a higher oxygen content than anthracite, making the coal burn more vigorously and release more heat (Riaza et al., 2014).
The THR of lignite increases with an increase in thermal radiation value. For example, the THR value of HM50 is 38.9 MJ/m2, which is 9.9% higher than that of HM25. The THR value of bituminous coal increases with an increase in thermal radiation value. For example, when the thermal radiation value increases to 35 kW/m2, the THR value of YM35 is as high as 34.0 MJ/m2, which is 439.7% higher than that of YM15. The THR values of WYM15, WYM25, WYM35, and WYM50 are 1.5, 19.4, 27.9, and 31.6 MJ/m2, respectively. Consistent with lignite and bituminous coal, the THR of anthracite also increases significantly with an increase in thermal radiation value. In line with previous PHRR case, the THR value of HM50 is 12.4 and 23.1% higher than that of YM50 and WYM50, respectively, at the same thermal radiation value. This further confirms our conjecture that the char residues of bituminous coal and anthracite are denser than those of lignite. Additionally, the THR value of bituminous coal has been found to be higher than that of anthracite under the same thermal radiation value. For example, YM35 has 1.2 times the THR of the WYM35. This confirms our hypothesis that bituminous coal has a higher oxygen content than anthracite, resulting in the complete combustion of bituminous coal and more heat production.
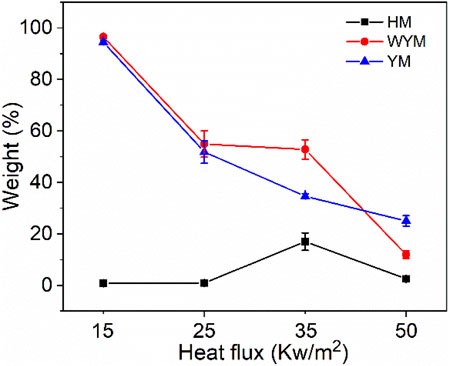
Figure 1C and Figure 3C show the char residue curves of the coal samples, and Table 3 presents the relevant data. With an increase in the thermal radiation value, the char residue of lignite increases gradually. For example, HM50 has 3.1 times more char residue than HM15. However, contrary to lignite, the char residue of bituminous coal decreases gradually with an increase in the thermal radiation value. For example, the char residue of YM50 was significantly reduced by 73.5% compared to YM15. The char residues of WYM15, WYM25, WYM35, and WYM50 were 96.5, 54.9, 54.2, 52.8, and 11.9%, respectively. The char residue of anthracite decreases with an increase in the thermal radiation value. Additionally, the char residues of bituminous coal and anthracite are higher than that of lignite under the same thermal radiation value. For example, the char residue of YM35 is 2.0 times that of HM35, whereas the char residue of WYM35 is 1.5 times that of YM35. These phenomena are attributed to the fact that the fixed carbon content of bituminous coal and anthracite is significantly higher than that of lignite, thus producing more char residue during the combustion process.
Smoke Generation
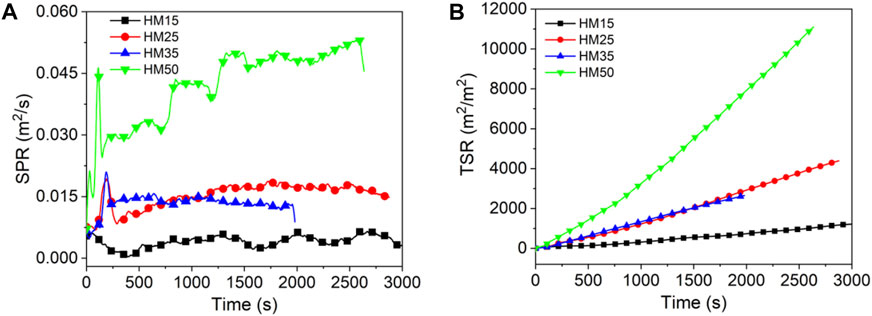
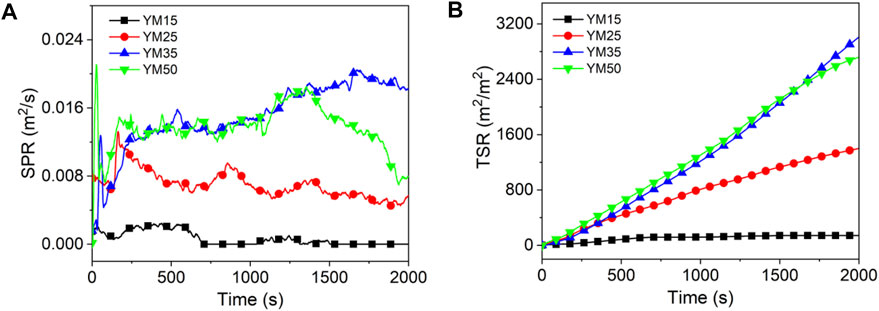
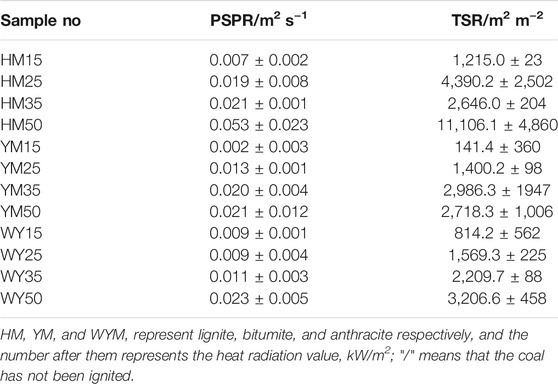
As shown in Figures 4–6 and Table 4, the PSPR of HM15, HM25, HM35, and HM50 values are 0.0007, 0.0019, 0.0021, and 0.0053 m2/s, respectively. Compared with HM15, the PSPR value of HM50 was significantly improved by 657.1%. Simultaneously, the PSPR value of lignite increases with an increase in the thermal radiation value. The changing trend of the PSPR value of bituminous coal is the same as that of lignite, which then increases gradually with an increase in the thermal radiation value. For example, the PSPR value of the YM50 is 10.5 times that of YM15. Consistent with lignite and bituminous coal, the PSPR value of anthracite also increases with an increase in the thermal radiation value. Compared with WYM25, the PSPR value of WYM35 and WYM50 increased by 22.2 and 155.6%, respectively. The PSPR values of bituminous coal and anthracite are lower than that of lignite under the same thermal radiation value. For example, the PSPR value of YM50 and WYM50 was reduced by 60.4 and 56.6%, respectively, compared to HM50.
The total smoke emission of HM15 is 1,215 m2/m2, which is the smallest of all lignite samples. The TSR value of lignite increases with an increase in the thermal radiation value. It is worth noting that the TSR value of HM50 is as high as 11,106.1 m2/m2, which is 9,891 times that of HM15. Consistent with lignite, the TSR value of bituminous coal also increases with increasing thermal emittance. The TSR values of YM15, YM25, YM35, and YM50 are 141.4, 1,400.2, 2,986.3, and 2,718.3 m2/m2, respectively. The changing trend of TSR of anthracite is the same as that of lignite and bituminous coal, which increases gradually as thermal radiation value increases. For example, WYM50 has a 293.9% improvement in TSR compared to WYM15. These phenomena are attributed to the fact that coal burns more intensely at high temperatures and produces a looser carbon layer, leading to more flue gas release. The TSR value of lignite is higher than that of bituminous coal and anthracite under the same thermal radiation value. The reason is that the fixed carbon content of lignite is less, the volatile content is higher, and the carbon layer is relatively loose, which cannot effectively inhibit the release of smoke. The TSR value of YM50 is 15.2% lower than that of WYM50, which is attributed to the lower sulfur content of bituminous coal than anthracite.
Carbon Monoxide and Carbon Dioxide Release
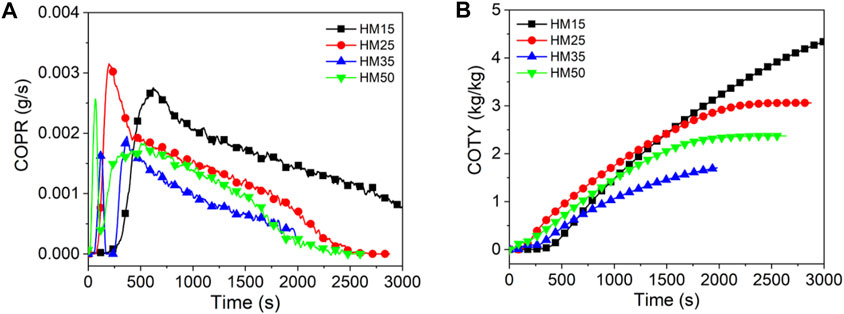
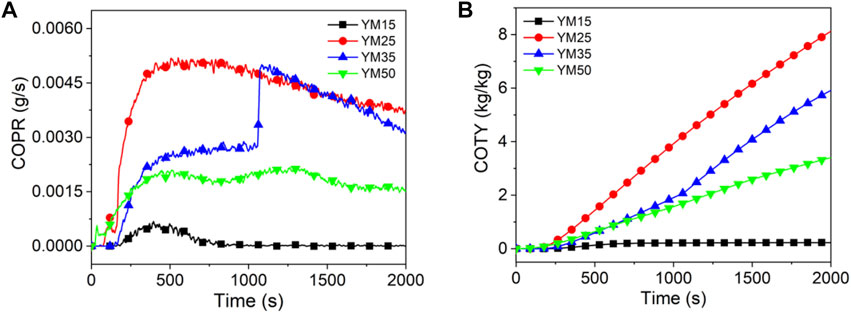
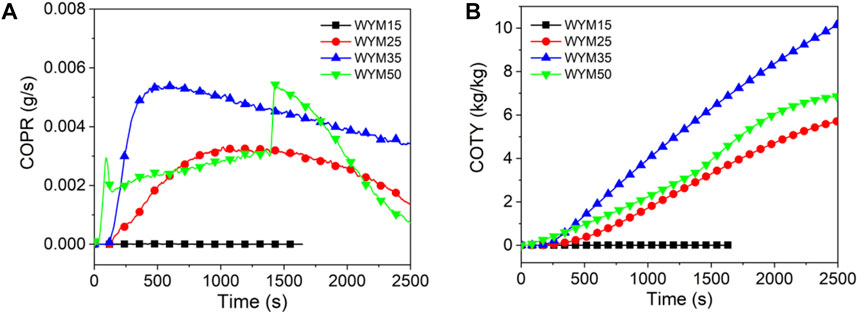
Figures 7–9 show the CO release curves of the coal, and Table 5 presents the relevant data.
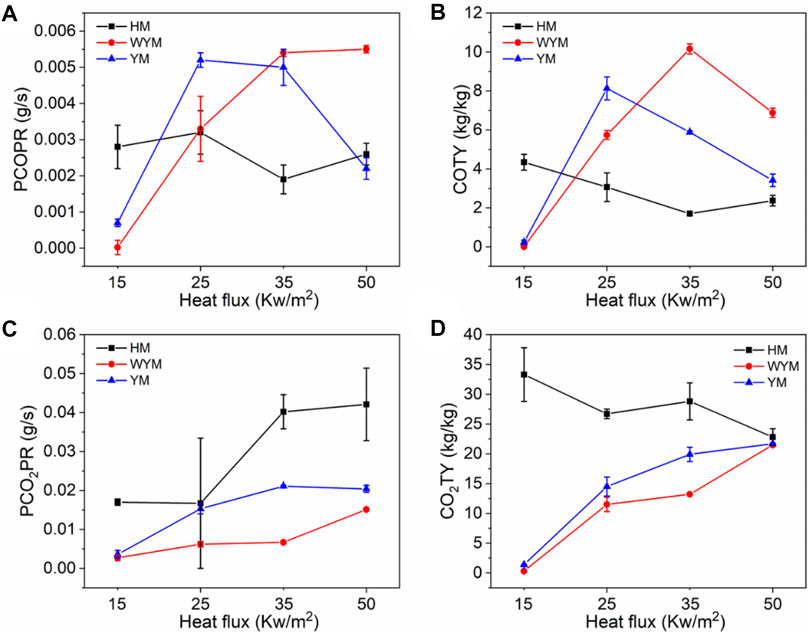
As shown in Figure 7A, there are two peaks in the COPR curve for HM50. The first peak is due to the formation of a protective carbon layer during coal combustion, whereas the second peak is due to the breakdown of the carbon layer via the combustible gas (Yuan et al., 2017). Generally, the PCOPR value of lignite decreases with an increase in the thermal radiation value. The PCOPR values of YM15, YM25, YM35, and YM50 are 7, 52, 50, and 22 g/s, respectively. The PCOPR decreases with an increase in the thermal radiation value. In contrast to lignite and bituminous coal, the PCOPR value of anthracite increases with increasing thermal emittance values. For example, the WYM50 has a PCOPR of 55 g/s, which is 27.5 times that of WYM15. Compared with HM50 and YM50, the PCOPR value of WYM50 increases by 189.5 and 150.0% due to the lower combustion degree and higher fixed carbon content of WYM50 compared with YM50 and HM50, resulting in higher thermal radiation values. Additionally, more CO is produced. Similar to the PCOPR, COTY of lignite decreased with an increase in the thermal radiation value. For example, the COTY value of HM50 is 2.37 kg/kg, which is 45.4% less than that of HM15. The reason is that as the temperature increases, the coal sample burns more thoroughly, thus producing less CO.
As shown in Figure 8B, the COTY value of bituminous coal decreases with increasing heat radiation value. The COTY values of YM15, YM25, YM35, and YM50 are 0.23, 8.13, 5.88, and 3.14 kg/kg, respectively. It is worth noting that the COTY of anthracite increases with an increase in the thermal radiation value, which is contrary to the trend of COTY of lignite and bituminous coal. When the heat radiation value increases to 50 kW/m2, the COTY value of WYM50 is as high as 6.88 kg/kg, which is 1764.5 times that of WYM15. However, anthracite has a higher COTY than lignite and bituminous coal for the same heat radiation values. For example, the COTY value of WYM50 is 190.3 and 101.8% higher than that of HM50 and YM50, respectively. This phenomenon is attributed to the high content of fixed carbon in anthracite, which forms a dense carbon layer during combustion, prevents oxygen from entering the coal sample, and inhibits further coal combustion, thus resulting in incomplete coal combustion (Chavali et al., 2020; Lu et al., 2021; Xu et al., 2020). This, in turn, produces more CO.
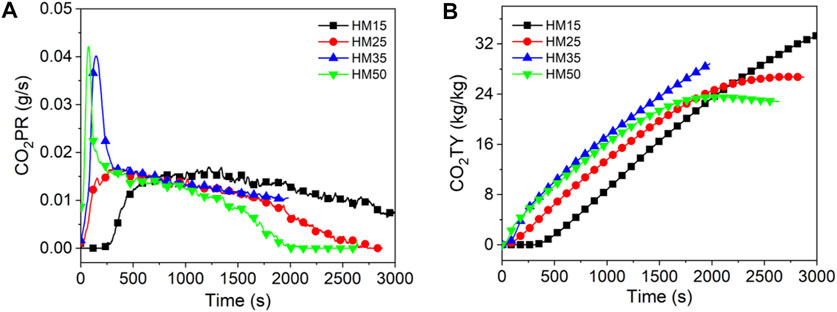
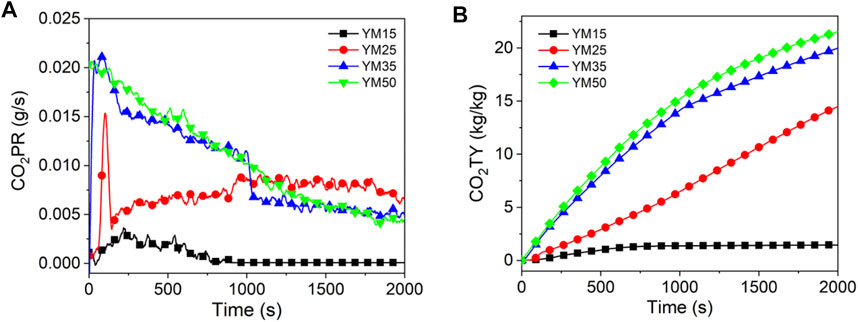
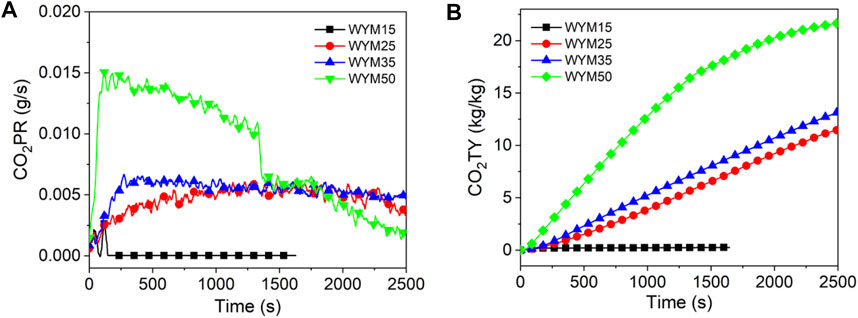
Figures 10–12 show the CO2 release curves of the coal, and Table 5 summarizes the data. The PCO2PR values of HM15, HM25, HM35, and HM50 are 0.0170, 0.0167, 0.0402, and 0.0421 g/s, respectively. Therefore, the PCO2PR value of lignite increases gradually with an increase in the thermal radiation value. Additionally, the PCO2PR value of HM50 increased by 147.6% compared to HM15. As shown in Figure 11A, the PCO2PR value of bituminous coal increases gradually with an increase in the heat radiation value. For example, the PCO2PR value of YM50 is 1.3 times that of YM25. The PCO2PR value of anthracite increases gradually with increasing thermal emissivity values, which is consistent with lignite and bituminous coal. Compared with WYM35, the PCO2PR of WYM50 improves by 62.9%. Additionally, the PCO2PR value of lignite is significantly higher than that of bituminous coal under the same thermal radiation value. For example, the PCO2PR value of HM50 is 106.3% higher than that of YM50. The reason is that the carbon content of lignite is less than that of bituminous coal, making the coal sample burn thoroughly and thus producing more CO2. Under the same thermal radiation value, the PCO2PR of anthracite is noted to be lower than that of bituminous coal. This is attributed to the fact that the carbon content of anthracite is higher than that of bituminous coal; thus, more char residue is produced during the combustion of anthracite, inhibiting further combustion of coal samples.
Figure 10B shows the curve of CO2TY from lignite. As shown in Figure 10B, the CO2TY value of lignite decreases with increasing thermal radiation values. When the heat radiation value increases to 50 kW/m2, the CO2TY value of HM50 decreases to 22.8 kg/kg. In contrast to lignite, the CO2TY value of bituminous coal increases with increasing thermal emissivities. For example, the CO2TY of YM50 is as high as 21.7 kg/kg, which is 15.5 times higher than that of YM15. As shown in Figure 12B, the CO2TY of anthracite increases with an increase in the thermal radiation value. The CO2TY values of WYM15, WYM25, WYM35, and WYM50 are 0.3, 11.5, 13.2, and 21.5 kg/kg, respectively. However, under the same thermal radiation value, the CO2TY value of lignite is higher than that of bituminous coal and anthracite. For example, the CO2TY value of HM15 is 23.8 and 111 times that of YM15 and WYM15, respectively. This is due to the low carbon content of lignite, which makes the carbon layer produced more loose and failure to effectively suppress the CO2 release during combustion. Additionally, the CO2TY value of WYM50 is smaller than that of YM50. The reason is that the high content of anthracite produces a more continuous and dense carbon layer, which effectively prevents oxygen from entering the coal sample, inhibits the violent combustion of coal to a certain extent, and reduces the gas released.
Analysis of Char Residue After Coal Combustion
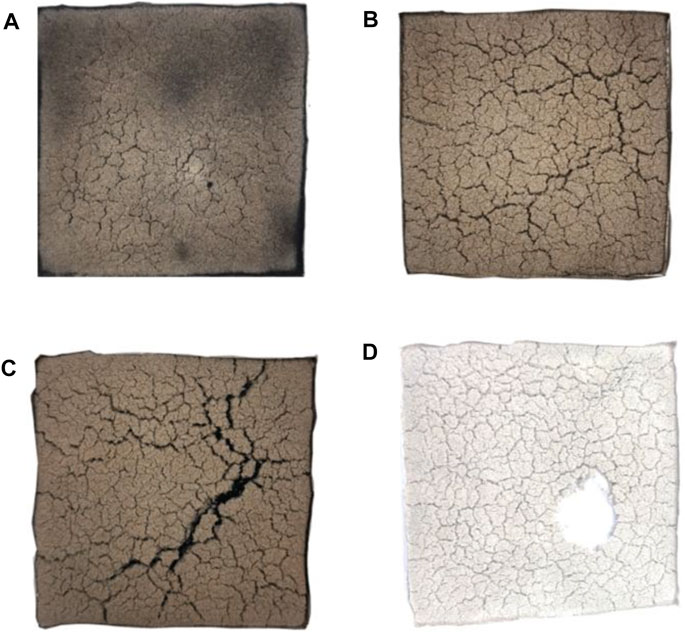
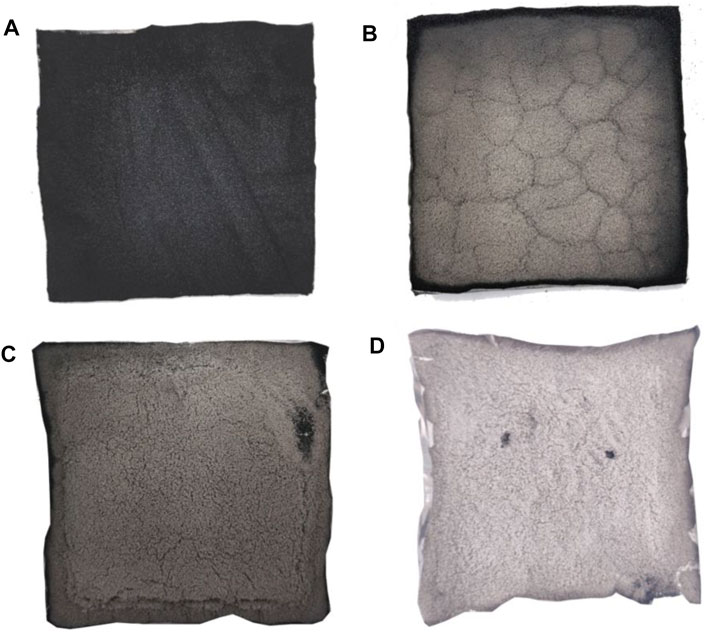
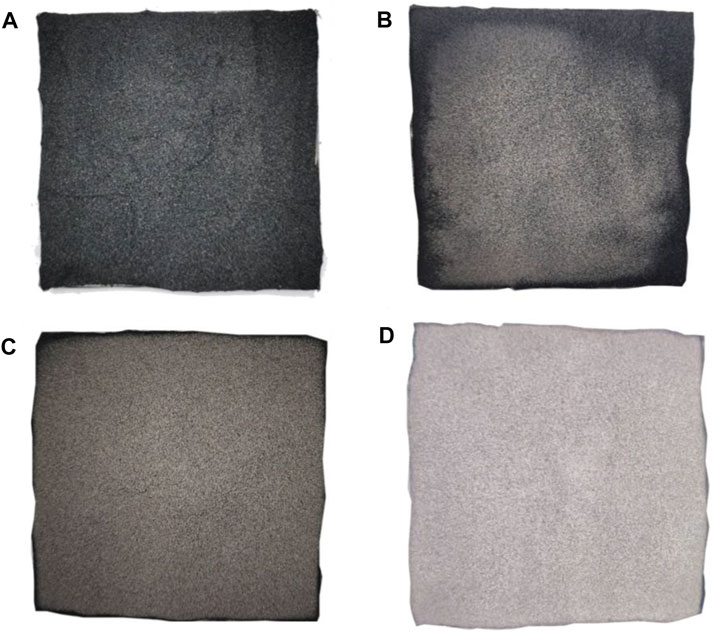
Figures 13–15 show the electronic photos of the char residue of the coal sample.
As shown in these figures, with an increase in the thermal radiation value, the cracks of the char residue of the lignite coal sample become more. Especially, the char residue of HM50 has many cracks and holes, which is not conducive for blocking the release of heat and toxic flue gas. The char residue of bituminous and anthracite coal samples becomes discontinuous with an increase in the thermal radiation value, which is consistent with lignite. However, the char residue of bituminous and anthracite coal samples is denser than that of lignite, resulting in significantly less heat and flue gas production of bituminous and anthracite coal than that of lignite (Deng et al., 2014; Deng et al., 2017; Deng et al., 2018; Cheng et al., 2021). Additionally, the char residue of anthracite is more compact and continuous than bituminous coal. It is also more conducive to inhibit the release of heat and toxic smoke.
Influence of Different Thermal Radiation Values on the Transition Characteristics of Coal Oxidation and Spontaneous Combustion
To better analyze how high-temperature thermal radiation affects coal oxidation and spontaneous combustion transformation characteristics, we have drawn the changing trend of coal char residue and heat and flue gas production with the thermal radiation value from Figures 16–18. As shown in Figure 16, the char residue of all coal samples decreases with an increase in the thermal radiation value, indicating that the coal samples burn more thoroughly at high temperatures. The char residue of lignite is less than that of bituminous coal and anthracite due to its significantly lower carbon content and complete combustion. Anthracite has a higher char residue than bituminous coal, meaning that the carbon layer of anthracite is denser than that of bituminous coal. Generally, the heat and flue gas emissions (PHRR, THR, PSPR, TSR, PCO2PR, and CO2TY) of all coal samples increase gradually with an increase in thermal radiation value. This shows that the coal sample is more prone to spontaneous combustion and ignition when the thermal radiation value increases. Additionally, at higher thermal radiation values, the carbon layer formed via coal oxidation and spontaneous combustion is more likely to show more cracks, which is not conducive to inhibiting the release of heat and toxic smoke (Wang D et al., 2019). Few CO is produced because coal burns more thoroughly at higher radiant heat values. Therefore, the PCOPR and COTY values of the coal sample decrease with increasing thermal radiation values. Except for PCOPR and COTY, the heat and flue gas emissions of lignite are higher than those of anthracite and bituminous coal because the carbon content of lignite is less than that of anthracite and bituminous coal, resulting in a loose carbon layer during combustion, which cannot effectively prevent oxygen from entering the coal sample and make the coal sample burn more thoroughly. Therefore, the PCOPR and COTY values of lignite are lower than those of anthracite and bituminous coal. However, the heat and flue gas emissions of anthracite have been determined to be lower than those of bituminous coal. The reason is that the carbon layer of anthracite is denser and more continuous than that of bituminous coal, which effectively prevents the release of heat and toxic flue gas. Additionally, the anthracite’s oxygen content and combustion degree are lower than those of bituminous coal. Therefore, the smoke generated is also low.
Conclusion
The effect of high-temperature thermal radiation on the oxidation and spontaneous combustion of coal has been investigated using a cone calorimeter. As per the findings of this study, it was determined that the TTI values of lignite and bituminous coal decrease with increasing thermal radiation values. This shows that under relatively higher thermal radiation, the oxidation degree of coal is accelerated, and the coal with spontaneous combustion tendency of Grade Ⅰ and Ⅱ is more likely to be ignited. It is worth noting that anthracite cannot be ignited by the thermal radiation value of less than or equal to 50 kW/m2, which is consistent with the spontaneous combustion tendency of anthracite. Since the carbon content of bituminous coal and anthracite is higher than that of lignite, a denser carbon layer is produced during coal combustion, effectively preventing heat release. Additionally, compared with bituminous coal, the PHRR value of anthracite has a decreasing trend. The reason is that bituminous coal has a higher oxygen content than anthracite, making the coal burn more vigorously and release more heat. Due to the low fixed carbon content and high volatile matter content of lignite, the carbon layer formed is relatively loose, which cannot effectively inhibit the release of smoke. Therefore, under the same heat radiation value, the TSR of lignite is higher than that of bituminous coal and anthracite. There are two peaks in the CO production rate curve of HM50. The first peak is due to the formation of a protective carbon layer during coal combustion. The second peak is due to the breakdown of the carbon layer via the combustible gas. Anthracite has a high content of fixed carbon and forms a dense carbon layer during combustion. Thus, it prevents oxygen from entering the coal sample and inhibits further coal combustion, resulting in incomplete coal combustion and more CO. The carbon content of lignite is low, which makes the carbon layer produced in the combustion process loose and cannot effectively inhibit the release of CO. Thus, the carbon layer produced in the combustion process is loose under the same thermal radiation value. Therefore, the total CO2 released from lignite is significantly larger than that from bituminous coal and anthracite.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
YH performed the project administration and carefully made the investigation. LW made the investigation and wrote the original draft, provided the methodology and formal analysis. WH revised the manuscript. All authors checked the manuscript.
Funding
This research was funded by the Natural Science Foundation of China (Grant No. 51774182), the National Science and Technology Major Project (2016ZX05045006-004), the Science and Technology Innovation Fund of CCTEG (2018MS014), and the Opening Research Fund of State Key Laboratory of Coal Mine Safety Technology (Grant Nos. SKLCMST101, SKLCMST103).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Brohez, S. (2005). Uncertainty Analysis of Heat Release Rate Measurement from Oxygen Consumption Calorimetry. Fire Mater. 29 (6), 383–394. doi:10.1002/fam.895
Chavali, K. S., Pethsangave, D. A., Patankar, K. C., Khose, R. V., Wadekar, P. H., Maiti, S., et al. (2020). Graphene-based Intumescent Flame Retardant on Cotton Fabric. J. Mater. Sci. 55 (29), 14197–14210. doi:10.1007/s10853-020-04989-6
Cheng, X. W., Zhang, W., Wu, Y. X., Ma, Y. D., Xu, J. T., and Guan, J. P. (2021). Borate Functionalized Caramel as Effective Intumescent Flame Retardant for Wool Fabric. Polym. Degrad. Stab. 186, 109469. doi:10.1016/j.polymdegradstab.2020.109469
Chow, W. K., and Han, S. S. (2011). Heat Release Rate Calculation in Oxygen Consumption Calorimetry. Appl. Therm. Eng. 31 (2-3), 304–310. doi:10.1016/j.applthermaleng.2010.09.010
Deng, C.-L., Du, S.-L., Zhao, J., Shen, Z.-Q., Deng, C., and Wang, Y.-Z. (2014). An Intumescent Flame Retardant Polypropylene System with Simultaneously Improved Flame Retardancy and Water Resistance. Polym. Degrad. Stab. 108, 97–107. doi:10.1016/j.polymdegradstab.2014.06.008
Deng, C., Yin, H., Li, R.-M., Huang, S.-C., Schartel, B., and Wang, Y.-Z. (2017). Modes of Action of a Mono-Component Intumescent Flame Retardant MAPP in Polyethylene-Octene Elastomer. Polym. Degrad. Stab. 138, 142–150. doi:10.1016/j.polymdegradstab.2017.03.006
Deng, H.-m., Xu, J.-y., Li, X.-y., Ye, Y.-l., Chen, H.-q., Chen, S.-y., et al. (2018). The Synergistic Action between Anhydride Grafted Carbon Fiber and Intumescent Flame Retardant Enhances Flame Retardancy and Mechanical Properties of Polypropylene Composites. Sci. Technol. Adv. Mater. 19 (1), 718–731. doi:10.1080/14686996.2018.1528567
Guo, H., Lyon, R. E., and Safronava, N. (2018). Accuracy of Heat-Release Rate Measured in Microscale Combustion Calorimetry. J. Test. Eval. 46 (3), 20160651–20161098. doi:10.1520/Jte20160651
Guo, S. L., Yan, Z., Yuan, S. J., and Geng, W. L. (2021). Inhibitory Effect and Mechanism of L-Ascorbic Acid Combined with tea Polyphenols on Coal Spontaneous Combustion. Energy 229, 120651. doi:10.1016/j.energy.2021.120651
Liu, C., Fu, L., Yang, J., Zhang, S., Shi, Y., Yang, F., et al. (2020a). A Novel Understanding of Combustion Behavior of Coals by Cone Calorimeter. J. Therm. Anal. Calorim. 143 (1), 139–150. doi:10.1007/s10973-019-09250-0
Liu, C., Wu, W., Shi, Y., Yang, F., Liu, M., Chen, Z., et al. (2020b). Creating MXene/reduced Graphene Oxide Hybrid towards Highly Fire Safe Thermoplastic Polyurethane Nanocomposites. Composites B: Eng. 203, 108486. doi:10.1016/j.compositesb.2020.108486
Lu, W. M., Ye, J. W., Zhu, L. H., Jin, Z. F., and Matsumoto, Y. (2021). Intumescent Flame Retardant Mechanism of Lignosulfonate as a Char Forming Agent in Rigid Polyurethane Foam. Polymers 13 (10), 1585. doi:10.3390/Polym13101585
Lv, H. P., Li, B., Deng, J., Ye, L. L., Gao, W., Shu, C. M., et al. (2021). A Novel Methodology for Evaluating the Inhibitory Effect of Chloride Salts on the Ignition Risk of Coal Spontaneous Combustion. Energy 231, 121093. doi:10.1016/J.Energy.2021.121093
Mohalik, N. K., Ray, S. K., Mishra, D., Pandey, J. K., Mondal, S., Khan, A. M., et al. (2021). Prevention and Control of Spontaneous Combustion/fire in Coal Stockpiles of Power Plants Using Firefighting Chemicals. Int. J. Coal Preparation Utilization 1, 1964489. doi:10.1080/19392699.2021.1964489
Pranda, P., Prandová, K., Hlavacek, V., and Yang, F. (2001). Combustion of Fly-Ash Carbon. Fuel Process. Technol., 72(3), 227–233. doi:10.1016/S0378-3820(01)00189-8
Pretrel, H., Le Saux, W., and Audouin, L. (2014). Experimental Determination of Fire Heat Release Rate with OC and CDG Calorimetry for Ventilated Compartments Fire Scenario. Fire Mater. 38 (4), 474–506. doi:10.1002/fam.2193
Riaza, J., Khatami, R., Levendis, Y. A., Álvarez, L., Gil, M. V., Pevida, C., et al. (2014). Single Particle Ignition and Combustion of Anthracite, Semi-anthracite and Bituminous Coals in Air and Simulated Oxy-Fuel Conditions. Combustion and Flame 161 (4), 1096–1108. doi:10.1016/j.combustflame.2013.10.004
Shao, H., Lu, Q., and Jiang, S. (2021). Effect of Frequency Conversion Ventilation on Coal Spontaneous Combustion. Combustion Sci. Technol. 193 (10), 1766–1781. doi:10.1080/00102202.2020.1713769
Shi, Y., Liu, C., Duan, Z., Yu, B., Liu, M., and Song, P. (2020). Interface Engineering of MXene towards Super-tough and strong Polymer Nanocomposites with High Ductility and Excellent Fire Safety. Chem. Eng. J. 399, 125829. doi:10.1016/j.cej.2020.125829
Shi, Y., Liu, C., Fu, L., Feng, Y., Lv, Y., Wang, Z., et al. (2021). Highly Efficient MXene/Nano-Cu Smoke Suppressant towards Reducing Fire Hazards of Thermoplastic Polyurethane. Composites A: Appl. Sci. Manufacturing 150, 106600. doi:10.1016/j.compositesa.2021.106600
Wang, D., Feng, X. M., Zhang, L. P., Li, M., Liu, M. M., Tian, A. L., et al. (2019). Cyclotriphosphazene-bridged Periodic Mesoporous Organosilica-Integrated Cellulose Nanofiber Anisotropic Foam with Highly Flame-Retardant and Thermally Insulating Properties. Chem. Eng. J. 375, 121933. doi:10.1016/J.Cej.2019.121933
Wang, D., Peng, H., Wu, Y., Zhang, L., Li, M., Liu, M., et al. (2020a). Bioinspired Lamellar Barriers for Significantly Improving the Flame-Retardant Properties of Nanocellulose Composites. ACS Sustain. Chem. Eng. 8 (11), 4331–4336. doi:10.1021/acssuschemeng.9b07745
Wang, D., Peng, H. Y., Yu, B., Zhou, K. Q., Pan, H. F., Zhang, L. P., et al. (2020b). Biomimetic Structural Cellulose Nanofiber Aerogels with Exceptional Mechanical, Flame-Retardant and thermal-insulating Properties. Chem. Eng. J. 389, 124449. doi:10.1016/J.Cej.2020.124449
Wang, L., Tawiah, B., Shi, Y., Cai, S., Rao, X., Liu, C., et al. (2019). Highly Effective Flame-Retardant Rigid Polyurethane Foams: Fabrication and Applications in Inhibition of Coal Combustion. Polymers 11 (11), 1776. doi:10.3390/polym11111776
Wang, Y., Li, X., and Guo, Z. (2018). Prediction of Self-Ignition Fire Propagation and Coal Loss in an Inclined Seam. Heat Trans. Res. 49 (9), 827–845. doi:10.1615/HeatTransRes.2018020029
Wen, H., Yu, Z., Fan, S., Zhai, X., and Liu, W. (2017). Prediction of Spontaneous Combustion Potential of Coal in the Gob Area Using CO Extreme Concentration: A Case Study. Combustion Sci. Technol. 189 (10), 1713–1727. doi:10.1080/00102202.2017.1327430
Xi, X., Jiang, S. G., Yin, C. C., and Wu, Z. Y. (2021). Experimental Investigation on Cement-Based Foam Developed to Prevent Spontaneous Combustion of Coal by Plugging Air Leakage. Fuel 301, 121091. doi:10.1016/J.Fuel.2021.121091
Xiao, Y., Zhang, H.-M., Yin, L., Shu, C.-M., and Li, Q.-W. (2021). Spontaneous Combustion Risk of Coal-Based Activated Carbon. Combustion Sci. Technol. 1, 1933961. doi:10.1080/00102202.2021.1933961
Xu, B., Shao, L. S., Wang, J. Y., Liu, Y. T., and Qian, L. J. (2020). Enhancement of the Intumescent Flame Retardant Efficiency in Polypropylene by Synergistic Charring Effect of a Hypophosphite/cyclotetrasiloxane Bi-group Compound. Polym. Degrad. Stab. 181, 109281. doi:10.1016/j.polymdegradstab.2020.109281
Yashwanth, B. L., Shotorban, B., Mahalingam, S., Lautenberger, C. W., and Weise, D. R. (2016). A Numerical Investigation of the Influence of Radiation and Moisture Content on Pyrolysis and Ignition of a Leaf-like Fuel Element. Combustion and Flame 163, 301–316. doi:10.1016/j.combustflame.2015.10.006
Yin, P., Liu, N., Chen, H., Lozano, J. S., and Shan, Y. (2014). New Correlation between Ignition Time and Moisture Content for Pine Needles Attacked by Firebrands. Fire Technol. 50 (1), 79–91. doi:10.1007/s10694-012-0272-y
Yuan, B., Fan, A., Yang, M., Chen, X., Hu, Y., Bao, C., et al. (2017). The Effects of Graphene on the Flammability and Fire Behavior of Intumescent Flame Retardant Polypropylene Composites at Different Flame Scenarios. Polym. Degrad. Stab. 143, 42–56. doi:10.1016/j.polymdegradstab.2017.06.015
Keywords: thermal radiation, coal, oxidation and spontaneous combustion, transition characteristics, mine fire
Citation: Wang L, Hu W and Hu Y (2021) Influence of High Temperature Thermal Radiation on the Transition Characteristics of Coal Oxidation and Spontaneous Combustion. Front. Mater. 8:778485. doi: 10.3389/fmats.2021.778485
Received: 17 September 2021; Accepted: 29 November 2021;
Published: 13 December 2021.
Edited by:
Wei Wu, City University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Dong Wang, Jiangnan University, ChinaSiqi Huo, Zhejiang University, China
Jian Zhang, Henan Polytechnic University, China
Copyright © 2021 Wang, Hu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Hu, eXVhbmh1QHVzdGMuZWR1LmNu
 Liancong Wang
Liancong Wang Weizhao Hu
Weizhao Hu Yuan Hu1*
Yuan Hu1*