- Pacific Northwest National Laboratory, Richland, WA, United States
Conventional rate theory often uses the mean field concept to describe the effect of inhomogeneous microstructures on the evolution of radiation induced defect and solute/fission product segregation. However, the spatial and temporal evolution of defects and solutes determines the formation and spatial distribution of radiation-induced second phase such as precipitates and gas bubbles/voids, especially in materials with complicated microstructures and subject to high dose radiation. In this work, a microstructure-dependent model of radiation-induced segregation (RIS) has been developed to investigate the effect of inhomogeneous thermodynamic and kinetics properties of defects on diffusion and accumulations of solute A in AB binary alloys. Four independent concentrations: atom A, interstitial A, interstitial B, and vacancy on [A, B] sublattice are used as field variables to describe temporal and spatial distribution and evolution of defects and solute A. The independent concentrations of interstitial A and interstitial B allow to describe their different generation rates, thermodynamic and kinetic properties, and release the assumptions of interstitial generation and sink strength used in the conventional rate theory. Microstructure and concentration dependent chemical potentials of defects are used to calculate the driving forces of defect diffusions. With the model, the effects of defect chemical potentials and mobilities on the RIS in polycrystalline AB model alloys have been simulated. The results demonstrated the model capability in predicting defect evolution in materials with inhomogeneous thermodynamic and kinetic properties of defects. The model can be extended to materials with complicated microstructures such as a wide range of grain size distribution, coating structure and multiphases as well as radiation-induced precipitation subject to severe radiation damage.
Introduction
Radiation-induced segregation (RIS) and precipitation are important material property degradation mechanisms in irradiated materials (Lam et al., 1978; Odette and Lucas, 1986; Farrell et al., 1994; Akamatsu et al., 1995; Lu et al., 2008; Was et al., 2011; Hu et al., 2012; Nastar and Soisson, 2012; Field et al., 2013; Wharry and Was, 2013; Yang et al., 2016). In Ferritic/Martensitic (F/M) steels, experimental results suggested that the RIS of Cr at grain boundaries (GBs) enhances Cr precipitation, which not only led to embrittlement but also altered the corrosion resistance of grain boundaries (Bruemmer et al., 1999; Simonen and Bruemmer, 1999; Nastar and Soisson, 2012). Moreover, experimental data indicated that a number of factors such as temperature, grain boundary structure, and radiation rate might affect whether solutes segregate or deplete on grain boundaries (Damcott et al., 1995; Lu et al., 2008; Was et al., 2011). For instance, the RIS of Cr showed a bell-shape temperature dependence, changing from Cr enrichment to Cr depletion at grain boundaries with increasing temperatures (Wharry and Was, 2013). Low angle grain boundaries (LAGB) exhibited a suppressed RIS response when compared to relatively high angle grain boundaries (HAGB), and at the special Sigma coincident site lattice (CSL) boundaries, the RIS of Cr was also suppressed (Hu et al., 2012; Field et al., 2013).
In different length and time scales, theoretical models including rate theory (Woo and Singh, 1992; Grandjean et al., 1994; Allen and Was, 1998; Golubov et al., 2001; Was, 2016), cluster dynamics (CD) (Faney and Wirth, 2014; Jourdan et al., 2014; Ke et al., 2018), kinetic Monte Carlo (KMC) (Ortiz, 2007; Huang and Marian, 2016), atomistic kinetic Monte Carlo (AKMC) (Senninger et al., 2016; Soisson and Jourdan, 2016), Object kinetic Monte Carlo (OKMC) (Souidi et al., 2006; Dunn et al., 2013), and phase-field approaches (Badillo et al., 2015; Piochaud et al., 2016; Li et al., 2017) have been developed to describe defect evolution and RIS. AKMC takes into account all atoms in the system which could more accurately describe and calculate the effect of defect interaction on defect diffusion and clustering. But it is challenging to apply it in large simulation domains and high radiation doses. Coarse grained methods such as KMC and OKMC only consider defects in the system and are therefore computationally more efficient. However, to describe the complicated thermodynamic and kinetic properties of defects and defect clusters such as the elastic interaction, reaction, and anisotropic mobility requires a large set of model parameters which might be coupled and make the simulation difficult. The mean field approaches such as rate theory and CD treat structural defects such as dislocations, grain boundaries and second phase particle interfaces as sinks. With the effective sink strength of structural defects, the temporal evolution of average defect concentrations over the representative volume can be obtained efficiently by solving one dimensional rate theory (ODE). Spatially dependent rate theory has been developed to consider the effect of free surface on diffusion, trapping and detrapping kinetics of helium in BCC iron foils (Xu and Wirth, 2009; Xu and Wirth, 2010). It is well known that the thermodynamic and kinetic properties of defects on structural defects such as interface and grain boundaries are very different from those inside grains, which may result in inhomogeneous RIS and inhomogeneous precipitation of second phase. But the inhomogeneous thermodynamic and kinetics properties haven’t been considered in the existing rate theory models. Phase-field approach is good at describing spatial and temporal evolution of chemistry and microstructure in a system with inhomogeneous thermodynamic and kinetic properties. Current phase-field models of RIS (Piochaud et al., 2016; Xia et al., 2020) in bi-crystalline structure were extended from the rate theory. Like the rate theory, the sink strength of grain boundaries is derived by assuming that defects at sinks remain thermal equilibrium concentrations. However, this assumption might not be true for a system far from equilibrium. In nuclear materials, especially in materials with high irradiation dose and/or high dose rate, rich microstructure change such as recrystallization and gas bubble evolution can lead the system far from equilibrium.
In this work, we extended the rate theory to considers the effect of the inhomogeneous thermodynamic and kinetic properties of defects on RIS in polycrystalline structures. Defect diffusion is assumed to be driven by chemical potential gradient. The sink strength at grain boundaries that depends on local defect concentrations, diffusivity, and reaction rate, is replaced by local reaction. The model can release the assumptions discussed above. Because the same thermodynamic and kinetic models are used in phase-field models of phase transition the current model can be coupled with phase-field model to study radiation-induced precipitation.
Microstructure-Dependent Rate Theory Model
Description of Chemistry and Defects
We consider an AB binary model alloy and regard atom A as solute atom while atom B as solvent atom. In the conventional rate theory (Was, 2016), three independent variables, i.e., concentrations of atom A, interstitial (A and B) and vacancy (A and B) are used to describe spatial and temporal distributions of solute A and defects. Considering the fact that interstitials A and B might have 1) different generation rates during the cascade; 2) different chemical potentials on sinks, 3) different recombination rates with vacancies; 4) different binding energy with atom A and/or B, four independent variables, i.e., concentrations of atom A, interstitial A, interstitial B, and vacancy are used in the current model. They are denoted by
Chemical Potentials
For a regular solution, the Gibbs free energy can be written as
where
In irradiated materials, the vacancy and interstitial concentrations can be a few orders of magnitude higher than their thermal equilibrium concentrations although their concentrations are still lower compared with the concentrations of alloy atoms. The high defect concentrations affect not only the diffusivities of species, but also chemical potentials of species. If one assumes that 1) A and B form a regular solution; and 2) vacancy and interstitial A and B are dilute defects, the Gibbs free energy of the system can be written as
The concentration
Chemical potentials of species at structural defects such as GBs, interfaces, and surfaces are usually different from those inside grains. The microstructure and/or spatial dependent chemical potential in the polycrystalline AB alloy cause an additional diffusion driving force that affects the fluxes of defects and species, hence, their segregation or depletion, and phase stability. To capture the effect of thermodynamic and kinetic properties of GBs on RIS, we extend the conventional rate theory to a microstructure-dependent rate theory model. The thermodynamic properties of the GBs are expected to closely correlate with the atomic density and composition. van der Waals (1979) showed that the energy of an interface can be described as a function of mass density and its variations within the interface region. Very recently, Kamachali et al. (2019) proposed a density-based thermodynamic model of GBs. The Gibbs free energy of GBs was described by the bulk free energy, atomic density, and gradient coefficient of atomic density. A Gaussian function was used to describe the atomic density change across a GB that evolves with composition. Atomic simulations (Kamachali et al., 2019) in alpha Fe showed that the atomic density of a GB with high symmetry has the Gaussian distribution. We use a shape function of
where
where
The spatial dependent chemical potential
Microstructure-Dependent Rate Theory
The rate theory model of RIS (Was, 2016) has been successfully used in predicting the effects of defect mobility, solute size, and defect-binding energies on solute segregation on surfaces and grain boundaries. However, the effects of grain morphology and inhomogeneous thermodynamic and kinetic properties of defects on RIS were ignored because most modeling was carried out in one dimension. In this work, we extend the rate theory model of RIS into polycrystalline structures. According to the rate theory, the evolution of chemical and defect concentrations is given by:
where
where
where
the evolutions Eqs 5, 6 can be expressed as
In polycrystalline structures the inhomogeneous chemical potential
Assuming that 1) the chemical potentials
With the assumption of
where
If we further assume that
where
In the conventional rate theory, the generation rate of defects
Model Parameters
The normalized time
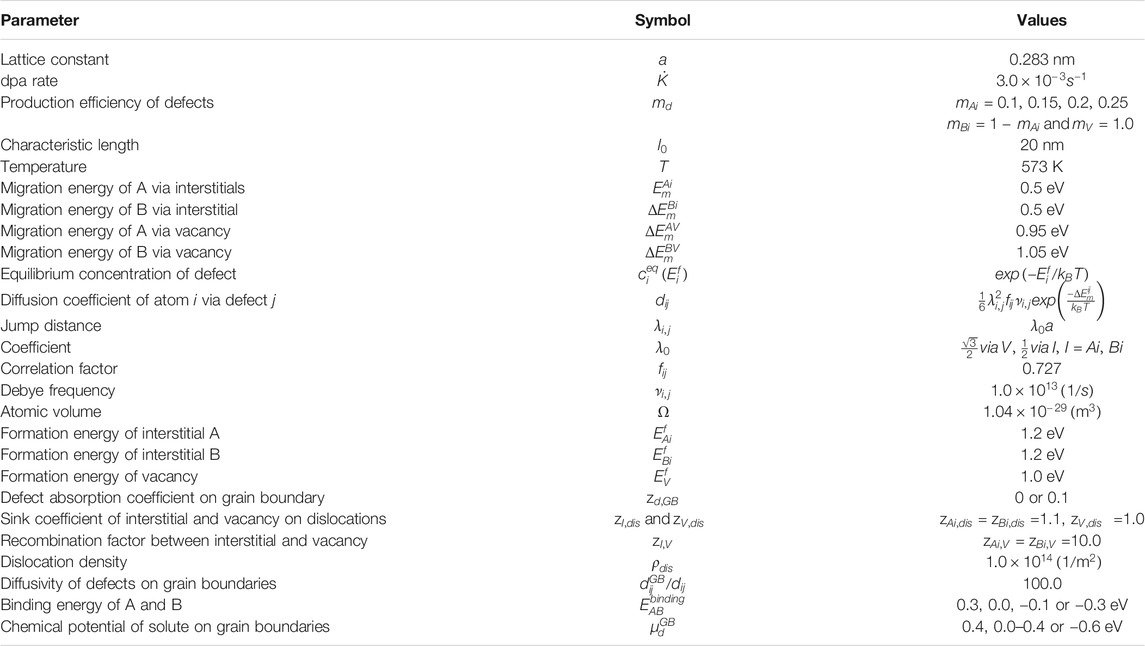
TABLE 1. Parameters for the ABVI model system named by Huang and Marian (2016) and Was (2016).
Results
We consider a model 10at % AB alloy. A denotes the solute atom while B the solvent atom. A phase field model of grain growth was used to generate a polycrystalline structure. The grain boundaries are defined by a shape function
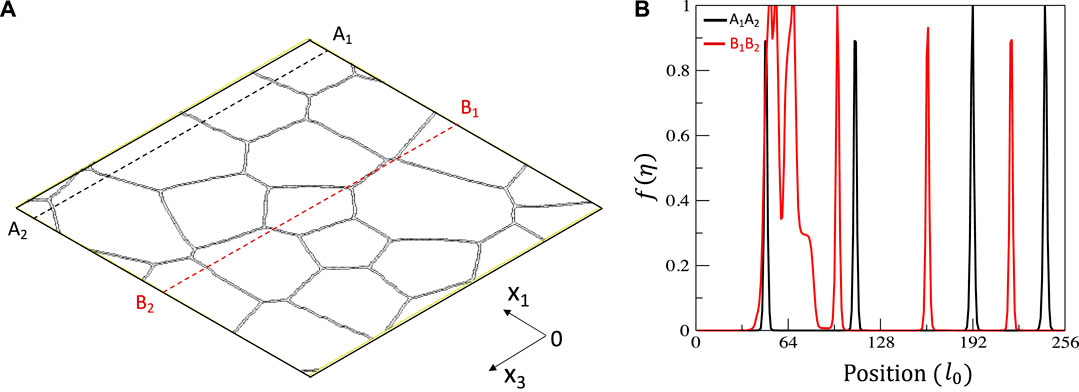
FIGURE 1. (A) the polycrystalline structure used in the simulations and (B) the shape function
Effect of Thermodynamic and Kinetic Properties of Grain Boundaries on Solute Segregation
In the conventional rate theory, the grain boundary is treated as a perfect sink of defects by assuming that defects have their thermal equilibrium concentrations. Current model releases this assumption and uses inhomogeneous chemical potentials (
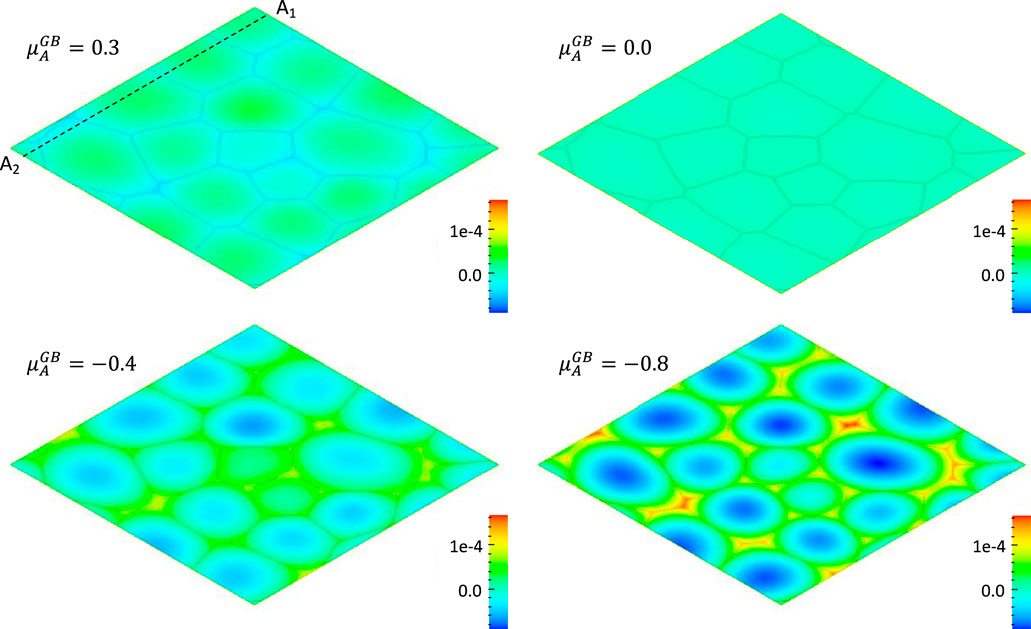
FIGURE 2. Effect of chemical potential
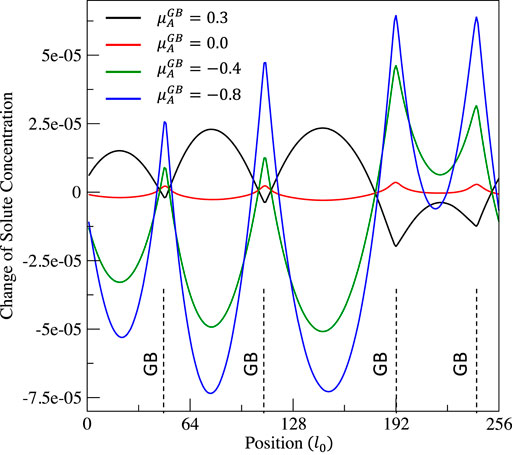
FIGURE 3. Distribution of solute concentration change
From Figures 2, 3, it can be clearly seen that 1) the solute A segregates on grain boundaries when
The equilibrium concentrations of interstitials (
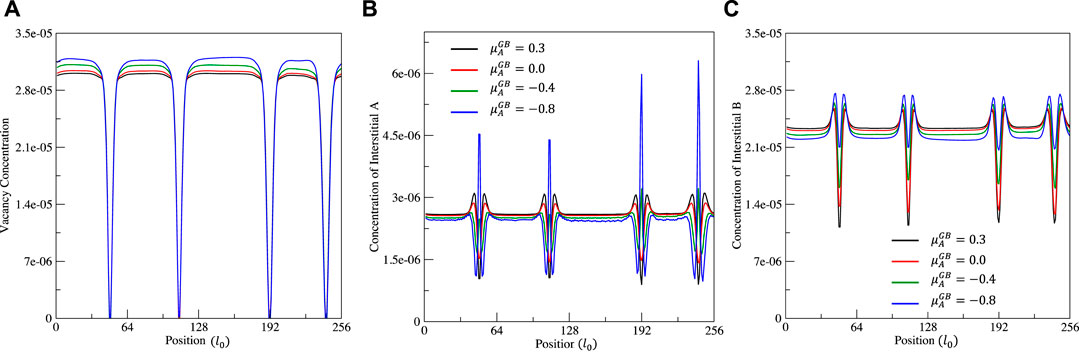
FIGURE 4. Effect of chemical potential
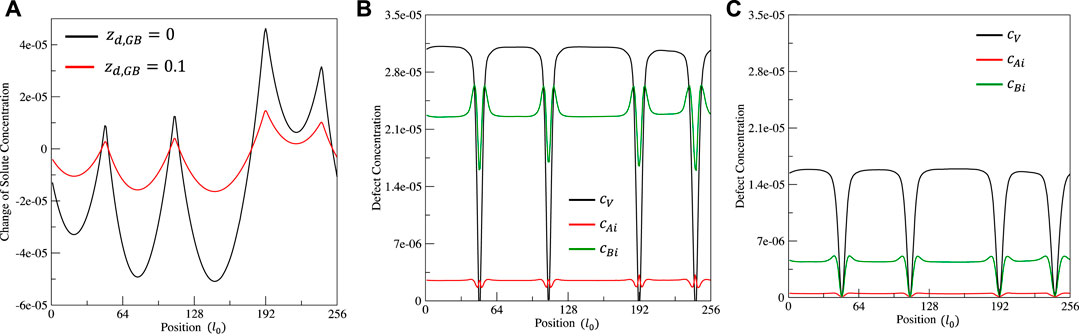
FIGURE 5. Effect of sink term
Effect of Binding Energy
For a regular solution AB, the mixing entropy can be approximated by the ideal solution mixing entropy:
The non-ideality of the solution is represented by the enthalpy of mixing, which, in the quasi-chemical approximation, is given by
where
where
The Gibbs free energy for a regular solution can be written as
where
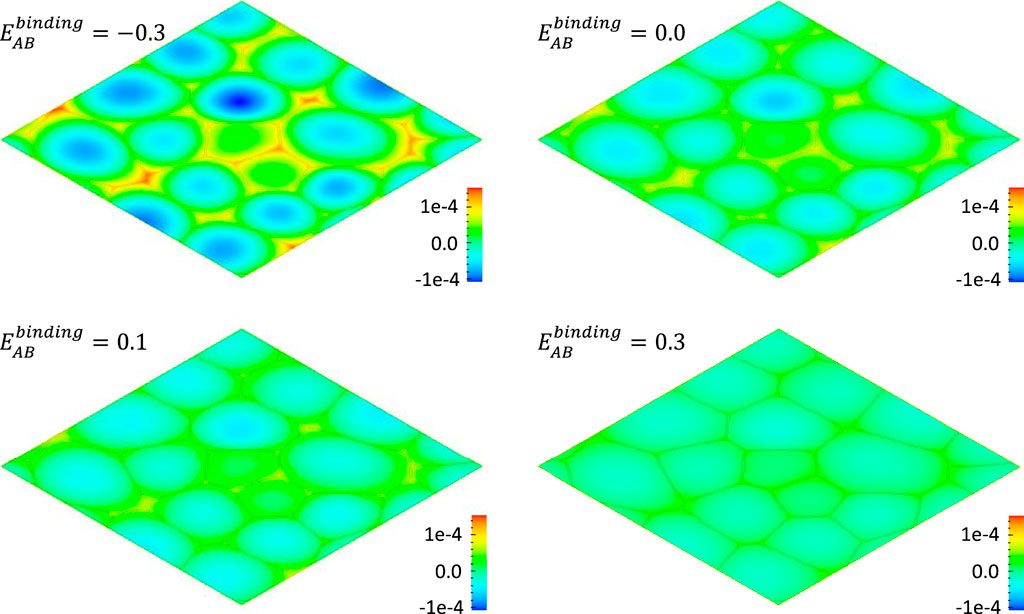
FIGURE 6. Effect of AB binding energy
If the binding among defects is strong, the faster diffusive defect has a drag effect on the slower diffusive defect. For instance, undersize solutes may tightly bind to interstitials forming interstitial-solute complexes that migrate as solute interstitials. In the rate theory (Was, 2016), the binding energy is used to scale the interstitial concentration of the solute which affect the partial diffusivity. The Gibbs free energy of the system with strong defect interaction can be generally written as
The binding of defects
Effect of Defect Generation Efficiency
In conventional rate theory, the generation rate of solute interstitials is assumed to be proportional to its concentration in the alloy i.e.,
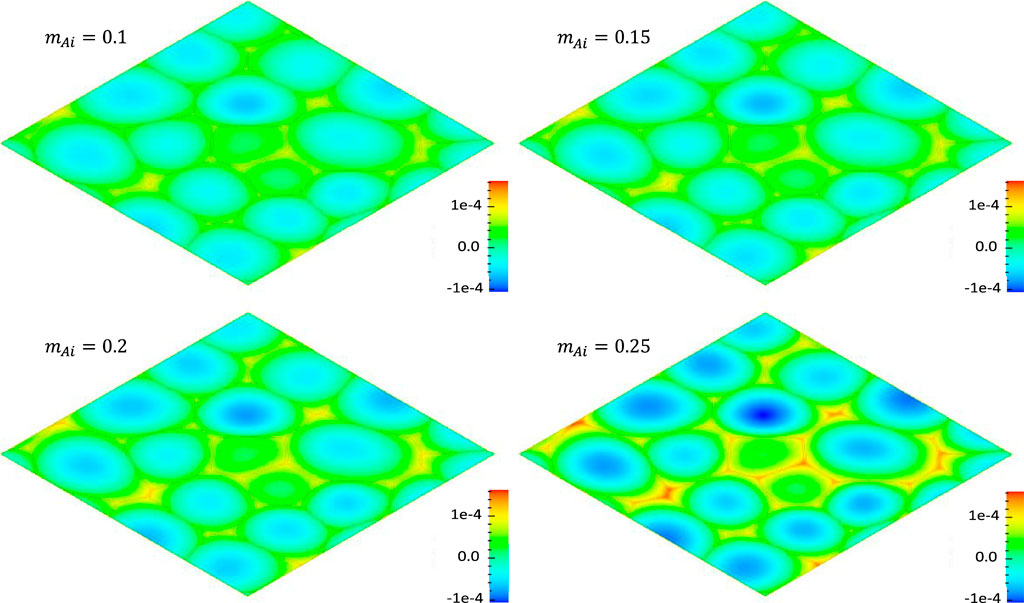
FIGURE 7. Effect of solute interstitial production efficiency
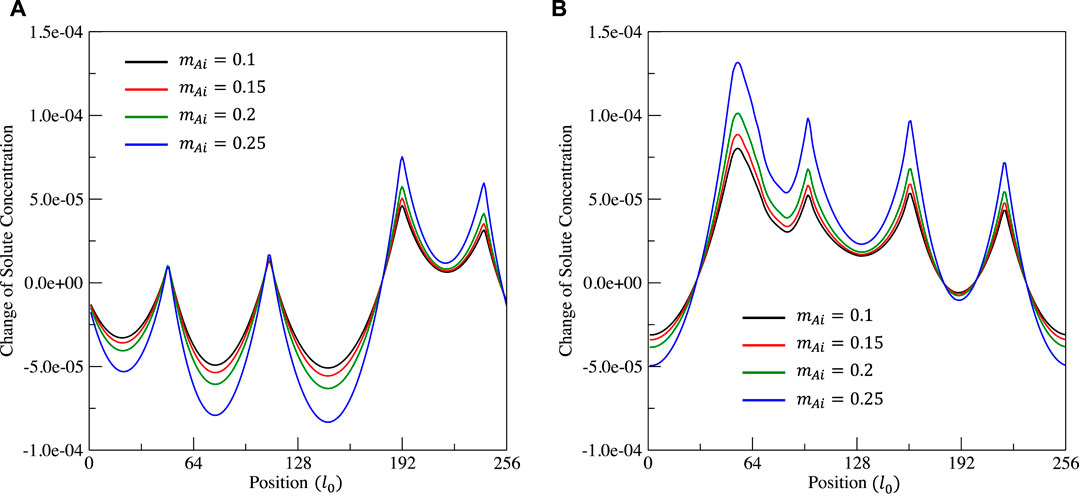
FIGURE 8. Distribution of the solute concentration change ΔcA at time
Effect of Defect Diffusivity on Solute Segregation
In the rate theory of RIS, the inhomogeneity of chemical potential in polycrystalline structures is ignored i.e.,
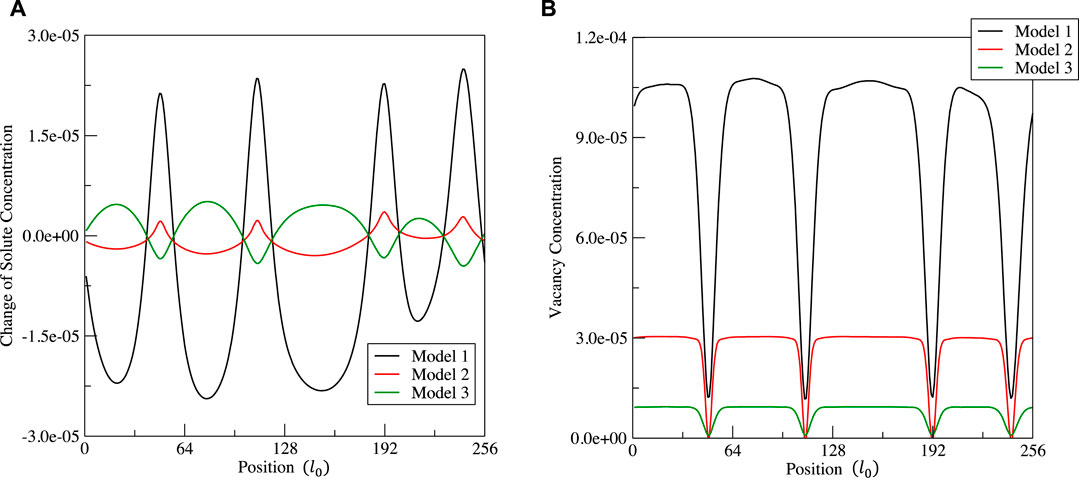
FIGURE 9. Distribution of the solute concentration change
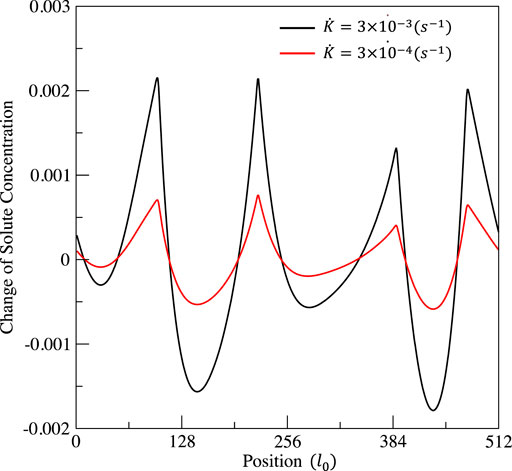
FIGURE 10. Distribution of the solute concentration change ΔcA at time
Conclusion and Discussion
In this work the conventional rate theory of RIS has been extended by taking into account inhomogeneous thermodynamic and kinetics properties of defects. The developed model has the following features: 1) concentrations of solute and solvent interstitials are treated as independent variables. In conventional rate theory the total concentration of solute and solvent interstitials is treated as an independent variable and the fraction of the solute interstitial in the total interstitial concentration is assumed to be the same as the solute concentration in the alloy. The independent solute and solvent interstitial concentrations enable one to describe different thermodynamic and kinetic properties of solute and solvent interstitials including the chemical potentials and diffusivities on grain boundaries, production efficiency and binding energy among defects. 2) thermodynamic and kinetic properties of defects in a polycrystalline structure are expressed to be spatial or microstructure dependent, which enables one to examine the effect of thermodynamic and kinetic properties of defects on RIS at grain boundaries. 3) the assumption that grain boundaries are perfect sink of defects is released. Defect concentrations on grain boundaries are determined by their chemical potentials, recombination and absorption rates. The defect absorption refers to the scenario that defects are consumed by grain growth or migrate to free surface along grain boundaries. With the model, the effect of structural and concentration dependent thermodynamic and kinetic properties on RIS were simulated. For the given kinetic properties of defects inside grains and on grain boundaries, the results indicate that 1) vacancy depletion on grain boundaries is always observed and the relative kinetic property of
The simulations demonstrate that developed model extends and strengthens the capability of the conventional rate theory of RIS. However, the inhomogeneous thermodynamic and kinetic properties add challenges in simulations. For example, to capture the thickness of grain boundaries about few nanometers we have to use a small grid size
The characteristic length
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
SH: Conceptualization, Model development, Writing-original draft, review, and editing. YL: Code development, Simulation, Visualization, Writing-original draft, review, and editing. DB: Conceptualization, Review and editing, Project supervision. DS: Conceptualization, Review and editing, Funding acquisition, Project administration.
Funding
This research was supported by the National Nuclear Security Administration of the U.S. Department of Energy through the Tritium Technology Program at Pacific Northwest National Laboratory. Computation was performed using Environmental Molecular Sciences Laboratory (EMSL) computing resources at Pacific Northwest National Laboratory.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Admal, N. C., Po, G., and Marian, J. (2018). A Unified Framework for Polycrystal Plasticity with Grain Boundary Evolution. Int. J. Plasticity 106, 1–30. doi:10.1016/j.ijplas.2018.01.014
Akamatsu, M., Van Duysen, J. C., Pareige, P., and Auger, P. (1995). Experimental Evidence of Several Contributions to the Radiation Damage in Ferritic Alloys. J. Nucl. Mater. 225, 192–195. doi:10.1016/0022-3115(95)00028-3
Allen, T. R., and Was, G. S. (1998). Modeling Radiation-Induced Segregation in Austenitic Fe-Cr-Ni Alloys. Acta Materialia 46 (10), 3679–3691. doi:10.1016/s1359-6454(98)00019-6
Badillo, A., Bellon, P., and Averback, R. S. (2015). A Phase Field Model for Segregation and Precipitation Induced by Irradiation in Alloys. Model. Simulation Mater. Sci. Eng. 23 (3). doi:10.1088/0965-0393/23/3/035008
Bruemmer, S. M., Simonen, E. P., Scott, P. M., Andresen, P. L., Was, G. S., and Nelson, J. L. (1999). Radiation-induced Material Changes and Susceptibility to Intergranular Failure of Light-Water-Reactor Core Internals. J. Nucl. Mater. 274 (3), 299–314. doi:10.1016/s0022-3115(99)00075-6
Chakraborty, P., Zhang, Y., and Tonks, M. R. (2016). Multi-scale Modeling of Microstructure Dependent Intergranular Brittle Fracture Using a Quantitative Phase-Field Based Method. Comput. Mater. Sci. 113, 38–52. doi:10.1016/j.commatsci.2015.11.010
Damcott, D. L., Allen, T. R., and Was, G. S. (1995). Dependence of Radiation-Induced Segregation on Dose, Temperature and Alloy Composition in Austenitic Alloys. J. Nucl. Mater. 225, 97–107. doi:10.1016/0022-3115(94)00690-3
Duh, T. S., Kai, J. J., Chen, F. R., and Wang, L. H. (2001). Numerical Simulation Modeling on the Effects of Grain Boundary Misorientation on Radiation-Induced Solute Segregation in 304 Austenitic Stainless Steels. J. Nucl. Mater. 294 (3), 267–273. doi:10.1016/s0022-3115(01)00493-7
Dunn, A. Y., Capolungo, L., Martinez, E., and Cherkaoui, M. (2013). Spatially Resolved Stochastic Cluster Dynamics for Radiation Damage Evolution in Nanostructured Metals. J. Nucl. Mater. 443 (1-3), 128–139. doi:10.1016/j.jnucmat.2013.07.009
Faney, T., and Wirth, B. D. (2014). Spatially Dependent Cluster Dynamics Modeling of Microstructure Evolution in Low Energy Helium Irradiated Tungsten. Model. Simulation Mater. Sci. Eng. 22 (6). doi:10.1088/0965-0393/22/6/065010
Farrell, K., Mahmood, S. T., Stoller, R. E., and Mansur, L. K. (1994). An Evaluation of Low Temperature Radiation Embrittlement Mechanisms in Ferritic Alloys. J. Nucl. Mater. 210 (3), 268–281. doi:10.1016/0022-3115(94)90181-3
Field, K. G., Barnard, L. M., Parish, C. M., Busby, J. T., Morgan, D., and Allen, T. R. (2013). Dependence on Grain Boundary Structure of Radiation Induced Segregation in a 9 wt.% Cr Model Ferritic/martensitic Steel. J. Nucl. Mater. 435 (1-3), 172–180. doi:10.1016/j.jnucmat.2012.12.026
Golubov, S. I., Ovcharenko, A. M., Barashev, A. V., and Singh, B. N. (2001). Grouping Method for the Approximate Solution of a Kinetic Equation Describing the Evolution of Point-Defect Clusters. Philos. Mag. A 81 (3), 643–658. doi:10.1080/01418610108212164
Grandjean, Y., Bellon, P., and Martin, G. (1994). Kinetic Model for Equilibrium and Nonequilibrium Segregation in Concentrated Alloys under Irradiation. Phys. Rev. B 50 (6), 4228–4231. doi:10.1103/physrevb.50.4228
Hu, R., Smith, G. D. W., and Marquis, E. A. (2012). Atom Probe Study of Radiation Induced Grain Boundary Segregation/depletion in a Fe-12%Cr Alloy. Prog. Nucl. Energ 57, 14–19. doi:10.1016/j.pnucene.2011.10.011
Hu, S., Burkes, D. E., Lavender, C. A., Senor, D. J., Setyawan, W., and Xu, Z. (2016). Formation Mechanism of Gas Bubble Superlattice in UMo Metal Fuels: Phase-Field Modeling Investigation. J. Nucl. Mater. 479, 202–215. doi:10.1016/j.jnucmat.2016.07.012
Hu, S., and Henager, C. H. (2009). Phase-field Modeling of Void Lattice Formation under Irradiation. J. Nucl. Mater. 394 (2), 155–159. doi:10.1016/j.jnucmat.2009.09.002
Huang, C. H., and Marian, J. (2016). A Generalized Ising Model for Studying Alloy Evolution under Irradiation and its Use in Kinetic Monte Carlo Simulations. J. Phys-condens Mat 28 (42). doi:10.1088/0953-8984/28/42/425201
Jiang, C., Swaminathan, N., Deng, J., Morgan, D., and Szlufarska, I. (2014). Effect of Grain Boundary Stresses on Sink Strength. Mater. Res. Lett. 2 (2), 100–106. doi:10.1080/21663831.2013.871588
Jourdan, T., Bencteux, G., and Adjanor, G. (2014). Efficient Simulation of Kinetics of Radiation Induced Defects: A Cluster Dynamics Approach. J. Nucl. Mater. 444 (1-3), 298–313. doi:10.1016/j.jnucmat.2013.10.009
Hirth, J. P., Lothe, J., and Anderson, P. M. (1968). Theory of Dislocation. New York: McGraw-Hill Book Company.
Kamachali, R. D., da Silva, A. K., McEniry, E., Ponge, D., Gault, B., Neugebauer, J., et al. (2019). Segregation–Assisted Spinodal and Transient Spinodal Phase Separation at Grain Boundaries. npj Comput Mater 6, 191 (2020). https://doi.org/10.1038/s41524-020-00456-7
Ke, J.-H., Ke, H., Odette, G. R., and Morgan, D. (2018). Cluster Dynamics Modeling of Mn-Ni-Si Precipitates in Ferritic-Martensitic Steel under Irradiation. J. Nucl. Mater. 498, 83–88. doi:10.1016/j.jnucmat.2017.10.008
Kuksenko, V., Pareige, C., and Pareige, P. (2012). Intra Granular Precipitation and Grain Boundary Segregation under Neutron Irradiation in a Low Purity Fe-Cr Based Alloy. J. Nucl. Mater. 425 (1), 125–129. doi:10.1016/j.jnucmat.2011.10.031
Lam, N. Q., Okamoto, P. R., Wiedersich, H., and Taylor, A. (1978). Radiation-Induced Solute Segregation and Precipitation in Alloys. Mta 9 (12), 1707–1714. doi:10.1007/bf02663400
Li, B. Y., Hu, S. Y., Li, C. L., Li, Q. L., Chen, J., Shu, G. G., et al. (2017). Simulations of Irradiated-Enhanced Segregation and Phase Separation in Fe-Cu-Mn Alloys. Model. Simulation Mater. Sci. Eng. 25 (6). doi:10.1088/1361-651x/aa7197
Lu, Z., Faulkner, R. G., Was, G., and Wirth, B. D. (2008). Irradiation-induced Grain Boundary Chromium Microchemistry in High Alloy Ferritic Steels. Scripta Materialia 58 (10), 878–881. doi:10.1016/j.scriptamat.2008.01.004
Messina, L., Nastar, M., Garnier, T., Domain, C., and Olsson, P. (2014). Exact Ab Initio Transport Coefficients in Bcc Fe-X (X=Cr, Cu, Mn, Ni, P, Si) Dilute Alloys. Phys. Rev. B 90 (10). doi:10.1103/physrevb.90.104203
Nastar, M., and Soisson, F. (2012). “Radiation-Induced Segregation,” in Comprehensive Nuclear Materials. Editor R. J. M. Konings (Oxford: Elsevier), 471–496. doi:10.1016/b978-0-08-056033-5.00035-5
Odette, G. R., and Lucas, G. E. (1986). Irradiation Embrittlement of Reactor Pressure Vessel Steels: Mechanisms, Models, and Data Correlations. Editor L. E. Steele (West Conshohocken, PA: ASTM International), 206–241.
Onsager, L. (1931). Reciprocal Relations in Irreversible Processes. I. Phys. Rev. 37 (4), 405–426. doi:10.1103/physrev.37.405
Ortiz, M. J. C. C. J. (2007). Simulation of Defect Evolution in Irradiated Materials: Role of Intracascade Clustering and Correlated Recombination Phys. Rev. B 75, 184101. doi:10.1103/PhysRevB.75.184101
Piochaud, J. B., Nastar, M., Soisson, F., Thuinet, L., and Legris, A. (2016). Atomic-based Phase-Field Method for the Modeling of Radiation Induced Segregation in Fe-Cr. Comput. Mater. Sci. 122, 249–262. doi:10.1016/j.commatsci.2016.05.021
Senninger, O., Soisson, F., Martínez, E., Nastar, M., Fu, C.-C., and Bréchet, Y. (2016). Modeling Radiation Induced Segregation in Iron-Chromium Alloys. Acta Materialia 103, 1–11. doi:10.1016/j.actamat.2015.09.058
Simonen, E. P., and Bruemmer, S. M. (1999). Radiation-induced Grain Boundary Segregation in Austenitic Stainless Steels. Mater. Sci. Forum 294-2, 755–758. doi:10.4028/www.scientific.net/MSF.294-296.755
Soisson, F., and Jourdan, T. (2016). Radiation-accelerated Precipitation in Fe-Cr Alloys. Acta Materialia 103, 870–881. doi:10.1016/j.actamat.2015.11.001
Souidi, C. S. B. A., Domain, C., Terentyev, D., Malerba, L., Calder, A. F., Bacon, D. J., et al. (2006). Dependence of Radiation Damage Accumulation in Iron on Underlying Models of Displacement Cascades and Subsequent Defect Migration. J. Nucl. Mater. 355 (1–3), 89–103. doi:10.1016/j.jnucmat.2006.04.009
Tonks, M. R., Gaston, D., Millett, P. C., Andrs, D., and Talbot, P. (2012). An Object-Oriented Finite Element Framework for Multiphysics Phase Field Simulations. Comput. Mater. Sci. 51 (1), 20–29. doi:10.1016/j.commatsci.2011.07.028
Tschopp, M. A., Horstemeyer, M. F., Gao, F., Sun, X., and Khaleel, M. (2011). Energetic Driving Force for Preferential Binding of Self-Interstitial Atoms to Fe Grain Boundaries over Vacancies. Scripta Materialia 64 (9), 908–911. doi:10.1016/j.scriptamat.2011.01.031
van der Waals, J. D. (1979). The Thermodynamic Theory of Capillarity under the Hypothesis of a Continuous Variation of Density. J. Stat. Phys. 20 (2), 200–244. doi:10.1007/bf01011514
Was, G. S., Wharry, J. P., Frisbie, B., Wirth, B. D., Morgan, D., Tucker, J. D., et al. (2011). Assessment of Radiation-Induced Segregation Mechanisms in Austenitic and Ferritic-Martensitic Alloys. J. Nucl. Mater. 411 (1-3), 41–50. doi:10.1016/j.jnucmat.2011.01.031
Wharry, J. P., and Was, G. S. (2013). A Systematic Study of Radiation-Induced Segregation in Ferritic-Martensitic Alloys. J. Nucl. Mater. 442 (1-3), 7–16. doi:10.1016/j.jnucmat.2013.07.071
Wharry, J. P., and Was, G. S. (2014). The Mechanism of Radiation-Induced Segregation in Ferritic-Martensitic Alloys. Acta Materialia 65, 42–55. doi:10.1016/j.actamat.2013.09.049
Woo, C. H., and Singh, B. N. (1992). Production Bias Due to Clustering of Point Defects in Irradiation-Induced Cascades. Philos. Mag. A 65 (4), 889–912. doi:10.1080/01418619208205596
Xia, L. D., Ji, Y. Z., Liu, W. B., Chen, H., Yang, Z. G., Zhang, C., et al. (2020). Radiation Induced Grain Boundary Segregation in Ferritic/martensitic Steels. Nucl. Eng. Technol. 52 (1), 148–154. doi:10.1016/j.net.2019.07.009
Xu, D. H., and Wirth, B. D. (2010). Modeling Spatially Dependent Kinetics of Helium Desorption in BCC Iron Following He Ion Implantation. J. Nucl. Mater. 403 (1-3), 184–190. doi:10.1016/j.jnucmat.2010.06.025
Xu, D., and Wirth, B. D. (2009). Spatially Dependent Rate Theory Modeling of Thermal Desorption Spectrometry of Helium-Implanted Iron. Fusion Sci. Technol. 56 (2), 1064–1068. doi:10.13182/fst09-a9052
Yang, Y., Field, K. G., Allen, T. R., and Busby, J. T. (2016). Roles of Vacancy/interstitial Diffusion and Segregation in the Microchemistry at Grain Boundaries of Irradiated Fe-Cr-Ni Alloys. J. Nucl. Mater. 473, 35–53. doi:10.1016/j.jnucmat.2016.02.007
Zhang, Y. X., Schwen, D., and Bai, X. M. (2017). Molecular Dynamics Simulations of Concentration- Dependent Defect Production in Fe-Cr and Fe-Cu Alloys. J. Appl. Phys. 122 (22). doi:10.1063/1.5008757
Keywords: microstructure, rate theory, radiation-induced segregation, binary alloy, polycrystalline
Citation: Hu S, Li Y, Burkes D and Senor DJ (2021) Microstructure-Dependent Rate Theory Model of Radiation-Induced Segregation in Binary Alloys. Front. Mater. 8:682686. doi: 10.3389/fmats.2021.682686
Received: 19 March 2021; Accepted: 03 May 2021;
Published: 31 May 2021.
Edited by:
Shijun Zhao, City University of Hong Kong, ChinaReviewed by:
Linyun Liang, Beihang University, ChinaDavid Simeone, Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), France
Cong Dai, Canadian Nuclear Laboratories (CNL), Canada
Copyright © 2021 Hu, Li, Burkes and Senor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenyang Hu, c2hlbnlhbmcuaHVAcG5ubC5nb3Y=
 Shenyang Hu*
Shenyang Hu* Yulan Li
Yulan Li