
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mater. , 13 April 2021
Sec. Colloidal Materials and Interfaces
Volume 8 - 2021 | https://doi.org/10.3389/fmats.2021.618674
This article is part of the Research Topic 2021 Retrospective: Colloidal Materials and Interfaces View all 9 articles
 Tinghua Li1*
Tinghua Li1* Chongjian Dong1
Chongjian Dong1 Yupeng Liu2,3*
Yupeng Liu2,3* Jun Wu1
Jun Wu1 Xia Zhang1
Xia Zhang1 Xiaowei Gong1
Xiaowei Gong1 Wei Zhao1
Wei Zhao1 Daoai Wang2,3*
Daoai Wang2,3* Donglai Zhu1*
Donglai Zhu1*Superhydrophobic and oleophobic surfaces have attracted increasing attention because of their self-cleaning properties. A composite coating composed of anodized titanium and sol-gel (TiAO/SG) was developed and has good superhydrophobic and oleophobic property. The anodized titanium coating was prepared on the titanium substrate and then a sol-gel layer was coated on the surface of the anodized titanium layer to obtain a composite coating with superhydrophobic and oleophobic properties. The adhesion weight of glycerol on the surface of the superhydrophobic titanium wire decreased to 4.8% of that of untreated titanium wire, which showed that the material had good oleophobic property. This new composite coating could achieve self-healing superhydrophobicity by releasing loaded perfluorodenytriethoxysilane to the surface of the coating. Given its superhydrophobicity, self-healing and wear resistance, the TiAO/SG coating was expected to achieve healable self-cleaning protection in titanium devices.
In the past few decades, the superhydrophobic and oleophobic surfaces have attracted sustained attention due to its self-cleaning properties (Liu et al., 2016; Qu et al., 2016; Wen and Guo, 2016; Wang et al., 2017; Li and Guo, 2019; Liu et al., 2019; Shen et al., 2019; Xing et al., 2019; Guo et al., 2020; Naderizadeh et al., 2020). The composite surfaces based on a combination of micro-nano structures and low surface energies were important to obtain superhydrophobic property (Liu et al., 2015; Ciriminna et al., 2016; Latthe et al., 2019; Latthe et al., 2019). Water droplets easily roll on such surfaces at a small angle of inclination and removed the dust from the surface (Bai et al., 2016; Falde et al., 2016; Giacomello et al., 2016; Milionis et al., 2016; Rao et al., 2016; Wen and Guo, 2016; Fu et al., 2017; Long et al., 2017; Alamri et al., 2018; Kim et al., 2018). With the increasing use of titanium in daily life, its superhydrophobic and oleophobic properties have attracted increasing attention. Titanium, as a light metal material, is widely used due to its good mechanical property. It is therefore of great significance to make titanium surfaces that reduce adhesion to common liquids in daily life, such as water and oil (Tang et al., 2016; Zhao et al., 2016; Zhang et al., 2017; Ge et al., 2018; Yeganeh and Mohammadi, 2018; Shen et al., 2019). Surface microstructure and low surface energy were two necessary factors for a surface to have superhydrophobic and oleophobic performance. As an electrochemical method to prepare nanostructures on metal surfaces, anodizing could produce various structures on the surface of aluminum and titanium (Tang et al., 2016; Vilaró et al., 2017; Zhang et al., 2017; Matijosius et al., 2020; Neves et al., 2020). The anodizing method can be used to form nanostructures on the titanium surface, which includes nanotubes, nanowires, etc. (Wang et al., 2010; Wang et al., 2013; Wei et al., 2016; Zhang et al., 2017; Zhang et al., 2018). Low surface energy is another factor to achieve surface superhydrophobic and oleophobic performance. Fluorination, a commonly used method to create a surface with low surface energy, has been used to make superhydrophobic surfaces. However, the low surface energy materials prepared by fluorination are easily damaged and loses superhydrophobic and oleophobic performance. Sol-gel technology, as a coating preparation method, has the advantage of simplicity, controllable composition, and easy fabrication. The low surface energy coating formed by hydrolysis of silanes has good stability due to its high crosslinking density and has been widely applied in various fields (Raimondo et al., 2017; Zhou and Chen, 2018; Jiang et al., 2019; Xie et al., 2019). Organic silicone and fluorine components could be added into sol-gel coating to prepare hydrophobic and oleophobic coating (Bouvet-Marchand et al., 2018).
Herein, an anodized titanium and sol-gel composite coating with simple preparation, superhydrophobicity, oleophobicity and mechanical stability is developed. The anodized titanium coating was prepared by electrochemical anodizing on the titanium substrate to form a nano-structured surface. Fluorinated sol-gel was prepared by co-hydrolysis of silane and flurosilane, and then the sol-gel was coated on the surface of titanium to form an anodized titanium and sol-gel composite coating. Compared with the untreated titanium surface, the composite coating had larger contact angles with water, hexadecane, propylene glycol, and glycerol. The adhesion weight of glycerol on the surface of the superhydrophobic titanium wire decreased to 4.8% of that of untreated titanium wire. The composite coating had self-healable hydrophobicity and good mechanical strength for sand abrasion. The TIAO/SG composite coatings showed promising applications in self-cleaning titanium devices.
Titanium sheets and wires were purchased from Zhongnuo Advanced Material Technology Co., Ltd. Ammonium fluoride, Methyltriethoxysilane (METS), 3-aminopropyl)triethoxysilane (APTES) and perfluorodenytriethoxysilane (FDTES) were purchased from Shanghai Chemical Regent Co., Ltd. Ethanol, glacial acetic acid, hexadecane, HCl, propylene glycol, and glycerol were purchased from Tianjin Kermel Chemical Regent Co., Ltd. All other chemicals were analytical-grade reagents and were used without further purification.
The titanium sheet was put into the solution of glycol with 0.5% ammonium fluoride and then oxidized for 4 h at 60 V voltage. After the titanium sheet was taken out, it was rinsed with deionization, and then put into the oven at 60°C to obtain a dry anodized titanium coating. The anodizing method of the titanium wire was the same as that of the titanium sheet.
30 ml ethanol, 0.9 ml glacial acetic acid, 2 ml hexanol, 5 ml HCl (0.01 M), 5 ml MTES, 0.5 ml APTES, and 2 ml perfluoroethyltriethoxysilane were added into three bottles at room temperature, and then 0.2 g deionized water was added. After stirring for 24 h, the mixture solvent was aged at room temperature for 7 days. The solution was then diluted with ethanol to obtain the sol-gel treatment solution.
The anodized titanium coating was dipped in the sol-gel treatment solution, dried at room temperature, and then heated in an oven at 180°C for 3 h. The coating was immersed in an ethanol solution of FDTES and dried at room temperature to obtain TiAO/SG coating.
The superhydrophobic surface was treated with atmosphere plasma treatment (Diener Electronic, Germany). The power used was 60 W and the treatment time was 20 s. The etched surface was then placed in an air blast oven at 60°C for 6 h to recover hydrophobic performance. The treatment of etching and healing was repeated for several cycles.
The surface wettability was characterized by the DSA-100 optical contact angle instrument (Kruss, Germany) at room temperature. The volume of tested liquid was 5 μL, and the CA value was automatically obtained by the Laplace young fitting algorithm. The average CA value was calculated by measuring five different positions, and the image was obtained using a digital camera (Sony Co., Ltd., Japan). The surface morphology of the coating was evaluated by the Su 8,020 field emission scanning electron microscope (FE-SEM, Japan) and the Fei TECNAI G2 F30 transmission electron microscope. All the photos were taken by a Canon camera.
Figure 1 shows the preparation process of TiAO/SG coating. In the glycol solution of ammonium fluoride, the inorganic anodized titanium with nanostructure was prepared on titanium substrate by anodizing. Then, the anodized titanium coating was immersed in the sol-gel solution and sol-gel was then coated on its surface. After heat treatment, the sol-gel coating with a highly cross-linked structure formed. The coating was immersed in an ethanol solution of FDTES and dried at room temperature to obtain a healable superhydrophobic composite coating.
Figure 2 shows SEM images of anodized titanium and sol-gel composite coatings with a nano porous structure. Figure 2A shows a scanning electron microscope image of the TiAO/SG coating. The nanostructure of the anodized titanium and sol-gel composite coating is important in the production of the healable superhydrophobic and oleophobic surface. Figure 2B, shows the thickness of the anodization layer at 6.6 μm.
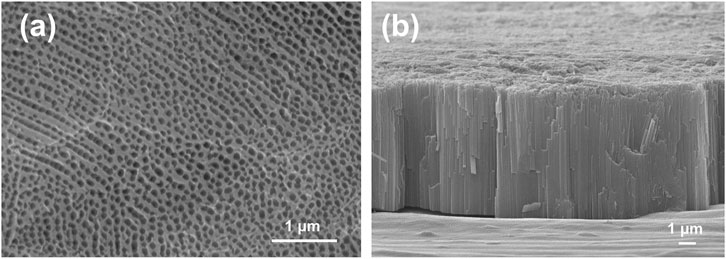
FIGURE 2. (A) Scanning electron microscopy (SEM) image of TiAO/SG coating. (B) The SEM image of the cross section of the anodization layer.
Figure 3A is the XPS spectrum of the TiAO/SG coating surface. When the surface of anodized titanium was coated with sol-gel, the fluorine-containing segments in the sol-gel process easily accumulated on the surface of the coating due to their low surface energy, which made the coating hydrophobic.
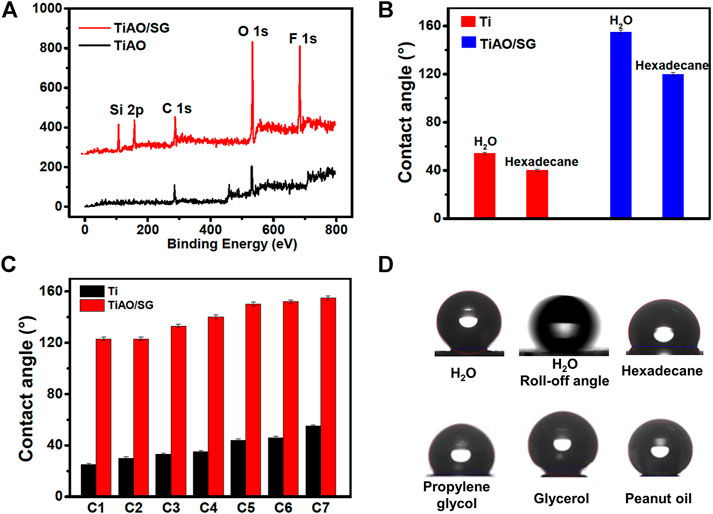
FIGURE 3. (A) XPS spectrum of TiAO/SG coating surface. (B) Contact angle of water and hexadecane on Ti and TiAO/SG coating. (C) The contact angles of different liquids on the surface of Ti and TiAO/SG composite coating. c1. propylene glycol, c7, glycerol. c2-c6, were mixed solvents and the ratio of propylene glycol to glycerol was 2:8, 4:6, 5:5, 6:4, 8:2, respectively. (D) The contact angles and roll-off angle of H2O, hexadecane, propylene glycol, glycerol and peanut oil on the surface of TiAO/SG composite coating.
Figure 3B shows the contact angles of water and n-hexadecane on titanium sheet and TiAO/SG composite coating. It can be seen from the figure that the contact angles of water and n-hexadecane on the surface of untreated titanium sheet were 54° and 40°, respectively. The contact angles on the surface of TiAO/SG coating were 155° and 120° for water and n-hexadecane. The sliding angle of water droplet was 3°. This indicates that the contact angles of water and n-hexadecane on the TiAO/SG coating surface increased. The nanostructure was formed on the surface of the titanium sheet and the low surface energy was provided by the sol-gel coating. The combination of nanostructures and a low surface energy allowed the TiAO/SG composite coating to have good hydrophobicity.
The surface tension of the solid can be calculated by two liquid methods, and the calculation formula was as follows. γsd, γLd, and γ in the formula are the solid dispersion force, liquid dispersion force, and solid surface tension, respectively. The liquid used was water and n-hexadecane. The γL of and water and n-hexadecane were 72.8 mN/m and 27.6, separately. The γLp/γLd of water and n-hexadecane were 2.36 and 0, separately.
The surface energy of the titanium sheet and TiAO/SG composite coating are shown in Table 1. The surface tension of the titanium sheet was 47.1 mN/m, and the surface tension of the TiAO/SG composite coating was 1.861 mN/m. It shows that the surface tension of the titanium sheet obviously decreased after anodizing and sol-gel treatment. The change of the surface tension increased the contact angles of water and hexadecane.
As shown in Figure 3C, the contact angles of propylene glycol and glycerol on the TiAO/SG composite coating are 123° and 155°, respectively. For mixed liquid, the TiAO/SG composite coating also has good repellency. The c2-c6 were a mixed solvent and the ratio of glycerol to propanediol was 2:8, 4:6, 5:5, 6:4, and 8:2, respectively. It could be seen from the Figure 3C that solvents with different mixing ratios had different contact angles on the surface of the composite coating. The contact angles of mixed solvent with the ratio of glycerol to propanediol of 2:8, 4:6, 5:5, 6:4, 8:2 was 123°, 133°, 140°, 150°, 152°, respectively. With the increase of glycerol content in the mixed solvents, the contact angle of the mixed solvents on the surface of the TiAO/SG composite coating increased gradually from 123° to 152°. This was consistent with the wettability of propylene glycol and glycerol on the surface of the TiAO/SG coating. Compared with titanium substrate, the repelling performance of the TiAO/SG coating with propylene glycol and glycerol increased significantly. The contact angle of propylene glycol on this surface increased from 25° to 123°, and that of glycerol on this surface increased from 55° to 155°. Figure 3D shows the contact angles and slide angle of H2O, hexadecane, propylene glycol, glycerol, and peanut oil on the surface of the TiAO/SG composite coating. The contact angle of peanut oil on the TiAO/SG composite coating was 133° which indicated that titanium sheets treated with anodized titanium and sol-gel coating had oleophobicity.
The natural surface released a self-healing substance to keep it superhydrophobic, for example the lotus leaves released a wax-like substance. For the surface of artificial materials, superhydrophobicity could be permanently destroyed, when the superhydrophobic substances were lost in the process of use. For the artificial surface, it was important to release low surface energy substances and to keep its self-healable superhydrophobicity. A schematic of the plasma treatment and healing of the superhydrophobic coating is shown in Figure 4A. As shown in Figure 4B, the coating loaded with FDTES lost superhydrophobicity after being treated with plasma. Its contact angle decreased from 155° to 20°. When the surface was heated at 60°C for 6 h, its superhydrophobicity recovered and the WCA increased to 153°. The surface maintained its superhydrophobicity after five plasma etching/healing cycles (Figures 4C,D).
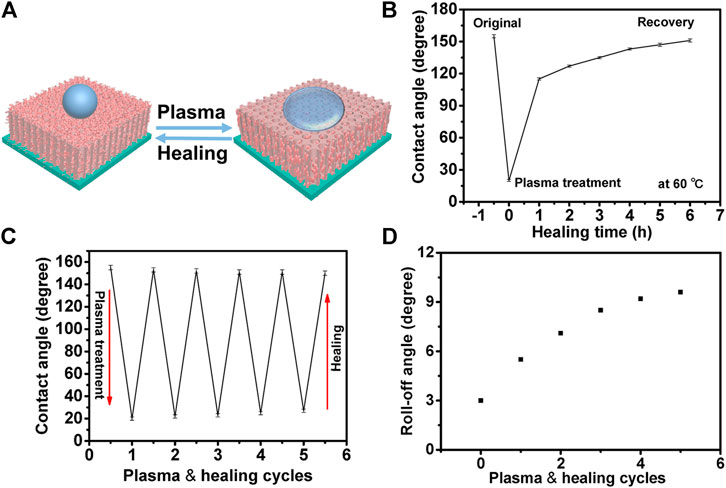
FIGURE 4. (A) Schematic of plasma treatment and healing of the superhydrophobic coating. (B) Static water contact angles of a plasma treated surface with the healing time at 60 °C. (C) Static water contact angles after five plasma-etching/healing cycles. (D) Roll-off angles with five plasma-etching/healing cycles.
It was obvious that FDTES loaded in nanotubes migrated to the coating surface in the process of healing. The fluorine content on the surface decreased after plasma treatment and lead to a change from being superhydrophobic to hydrophilic. When the FDTES filled in the pores that gradually migrated to the surface, the coating restored its superhydrophobicity. This change could also be characterized by the surface element composition in Figure 5. When the surface was treated with plasma, its fluorine element on the surface decreased, and the surface showed hydrophilicity. However, when the surface was heated at 60°C for 6 h, the fluorine element concentration increased, and the superhydrophobicity of the surface recovered. The surface maintained its superhydrophobicity after five plasma etching/healing cycles.
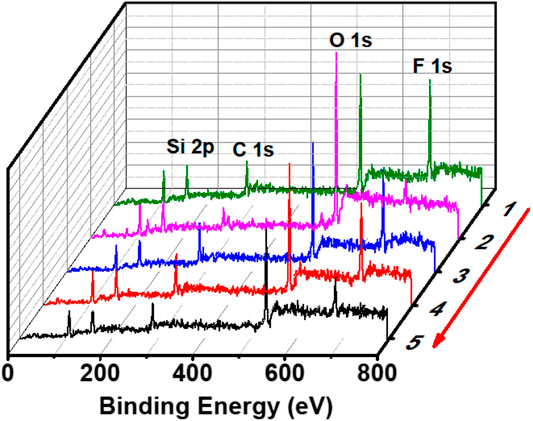
FIGURE 5. X-ray photoelectron spectra of (1) superhydrophobic coating, (2) after plasma treatment, (3) one plasma-etching/healing cycle, (4) three plasma-etching/healing cycles and (5) five plasma-etching/healing cycles.
The superhydrophobic surface could be damaged by mechanical friction during usage. Sand abrasion was used to evaluate the wear resistance of the coating. 50 g sand grains flowed out of the funnel and impacted the superhydrophobicity surface with an inclination of 45°. The falling height of sand was 30 cm (Figure 6A). The surface of the coating remained superhydrophobicity after sand abrasion, and no obvious structure damage was found (Figure 6B).
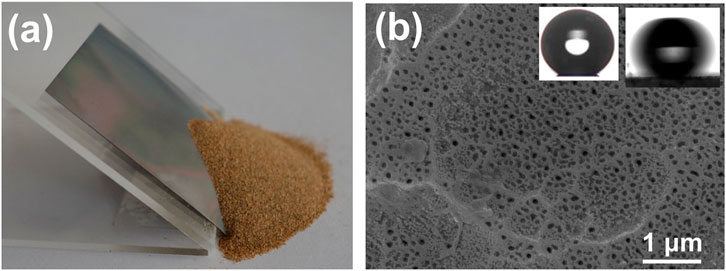
FIGURE 6. Wear resistance evaluated by sand abrasion. (A) Photograph of sand grains impinged on the tilted 45° coating from a height of 30 cm. (B) SEM image of the surface after sand abrasion. The inset was a water contact angle and roll-off angle of the coating after sand abrasion.
Figure 7A is the optical picture of the titanium wire and glycerol. It can be seen from Figure 7B that when the untreated titanium wire was immersed in glycerol, there was an obvious adhesion on its surface. When the surface of the titanium wire was anodized and treated with a sol-gel coating, no obvious adhesion to glycerol was found, which indicates that the TiAO/SG composite coating has hydrophobicity. It can be seen from Figure 7C that the weight of the adhered glycerol to the untreated titanium wire is 0.1827 g. After the anodization and sol-gel treatment, the weight of adhered glycerol to treated titanium wire reduced to 0.0087 g. The amount of adhesion of the glycerol on the surface of the superhydrophobic wire decreased to 4.8% of that of the untreated titanium wire, indicating that it reduced the adhesion of glycerol (Supplementary Videos S1, S2). After anodizing, the surface of the titanium wire produced a nanostructure and the combination of the nanostructure and low surface energy allowed the TiAO/SG composite coating to have superhydrophobicity.
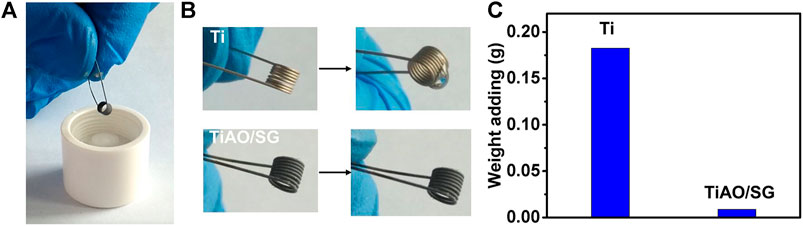
FIGURE 7. (A) Optical picture of titanium wire and glycerol. (B) Optical picture of adhesion of untreated titanium wire and titanium wire with TiAO/SG composite coating to glycerol. (C) Weight adding of untreated titanium wire and titanium wire with TiAO/SG composite coating to glycerol.
A superhydrophobic and oleophobic surface, based on anodized titanium and a sol-gel composite coating, was provided. By fabricating the sol-gel coating on the surface of the anodized titanium, a superhydrophobic and oleophobic coating was obtained. The adhesion amount of glycerol on the surface of a superhydrophobic titanium wire decreased to 4.8% of that of untreated titanium wire. The TiAO/SG composite coating had self-healable superhydrophobicity. The new TiAO/SG composite coating with a simple structure, low cost, wear resistance, and self-healing superhydrophobicity has broad application prospects in self-cleaning titanium devices.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
TL: Investigation, Formal analysis, Data curation, Writing-original draft, Visualization. CD Formal analysis, Writing-review and editing. YL: Conceptualization, Methodology, Supervision. JW: Methodology, Validation. XZ: Conceptualization, Validation. XG: Software, Resources. WZ: Software, Conceptualization. DW: Conceptualization, Validation, Supervision. DZ: Conceptualization, Methodology, Supervision.
Thanks for the financial support of the Major Science and Technology Project of China National Tobacco Corporation (110202001013(XX-09)) and the Key Scientific Research Project of China Tobacco Yunnan Industrial Co., Ltd (2019XY02). Taishan Scholars of Shandong province (No. ts20190965), NSFC (No.51722510, 51905518).
Authors TL, CD, JW, XZ, XG, WZ and DZ were employed by company Technical Center of China Tobacco Yunnan Industrial Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank and acknowledge the financial support from the Major Science and Technology Project of China National Tobacco Corporation [110202001013(XX-09)] and the Key Scientific Research Project of China Tobacco Yunnan Industrial Co., Ltd [2019XY02]. Taishan Scholars of Shandong province (No. ts20190965), NSFC (No.51722510, 51905518).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2021.618674/full#supplementary-material.
Alamri, H., Al-Shahrani, A., Bovero, E., Khaldi, T., Alabedi, G., Obaid, W., et al. (2018). Self-cleaning superhydrophobic epoxy coating based on fibrous silica-coated iron oxide magnetic nanoparticles. J. Colloid Interf. Sci. 513, 349–356. doi:10.1016/j.jcis.2017.11.042
Bai, X., Xue, C.-H., and Jia, S.-T. (2016). Surfaces with sustainable superhydrophobicity upon mechanical abrasion. ACS Appl. Mater. Inter. 8 (41), 28171–28179. doi:10.1021/acsami.6b08672
Bouvet-Marchand, A., Graillot, A., Abel, M., Koudia, M., Boutevin, G., Loubat, C., et al. (2018). Distribution of fluoroalkylsilanes in hydrophobic hybrid sol-gel coatings obtained by co-condensation. J. Mater. Chem. A. 6 (48), 24899–24910. doi:10.1039/c8ta10191d
Ciriminna, R., Fidalgo, A., Palmisano, G., Ilharco, L. M., and Pagliaro, M. (2016). Silica-based sol–gel coatings: a critical perspective from a practical viewpoint. Beverly, MA, USA: Biobased and Environmental Benign Coatings, 149–159.
Falde, E. J., Yohe, S. T., Colson, Y. L., and Grinstaff, M. W. (2016). Superhydrophobic materials for biomedical applications. Biomaterials 104, 87–103. doi:10.1016/j.biomaterials.2016.06.050
Fu, Y., Jiang, J., Zhang, Q., Zhan, X., and Chen, F. (2017). Robust liquid-repellent coatings based on polymer nanoparticles with excellent self-cleaning and antibacterial performances. J. Mater. Chem. A. 5 (1), 275–284. doi:10.1039/c6ta06481g
Ge, B., Han, L., Liang, X., Li, F., Pu, X., Zhu, X., et al. (2018). Fabrication of superhydrophobic Cu-BiOBr surface for oil/water separation and water soluble pollutants degradation. Appl. Surf. Sci. 462, 583–589. doi:10.1016/j.apsusc.2018.08.174
Giacomello, A., Schimmele, L., Dietrich, S., and Tasinkevych, M. (2016). Perpetual superhydrophobicity. Soft Matter 12 (43), 8927–8934. doi:10.1039/c6sm01727d
Guo, W. W., Wang, X., Huang, J. L., Zhou, Y. F., Cai, W., Wang, J. L., et al. (2020). Construction of durable flame-retardant and robust superhydrophobic coatings on cotton fabrics for water-oil separation application. Chem. Eng. J. 398, 15. doi:10.1016/j.cej.2020.125661
Jiang, C., Liu, W., Sun, Y., Liu, C., Yang, M., and Wang, Z. (2019). Fabrication of durable superhydrophobic and superoleophilic cotton fabric with fluorinated silica sol via sol-gel process. J. Appl. Polym. Sci. 136 (4), 9. doi:10.1002/app.47005
Kim, Y. S., Shang, M., Kang, S., Karsseboom, J. J., and Niu, J. (2018). Strong hydrophobic coating by conducting a new hierarchical architecture. J. Phys. Chem. C 122 (8), 4628–4634. doi:10.1021/acs.jpcc.7b11144
Latthe, S. S., Sutar, R. S., Bhosale, A. K., Nagappan, S., Ha, C.-S., Sadasivuni, K. K., et al. (2019). Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog. Org. Coat. 137, 105373. doi:10.1016/j.porgcoat.2019.105373
Latthe, S. S., Sutar, R. S., Kodag, V. S., Bhosale, A. K., Kumar, A. M., Kumar Sadasivuni, K., et al. (2019). Self - cleaning superhydrophobic coatings: potential industrial applications. Prog. Org. Coat. 128, 52–58. doi:10.1016/j.porgcoat.2018.12.008
Li, Z., and Guo, Z. (2019). Bioinspired surfaces with wettability for antifouling application. Nanoscale 11 (47), 22636–22663. doi:10.1039/c9nr05870b
Liu, S., Liu, X., Latthe, S. S., Gao, L., An, S., Yoon, S. S., et al. (2015). Self-cleaning transparent superhydrophobic coatings through simple sol-gel processing of fluoroalkylsilane. Appl. Surf. Sci. 351, 897–903. doi:10.1016/j.apsusc.2015.06.016
Liu, Y.-P., Liu, H.-F., Feng, Y.-G., Liu, Z.-L., Hu, H.-Y., Yu, B., et al. (2016). A nanotubular coating with both high transparency and healable superhydrophobic self-cleaning properties. RSC Adv. 6 (26), 21362–21366. doi:10.1039/c5ra26977f
Liu, Y., Zheng, Y., Li, T., Wang, D., and Zhou, F. (2019). Water-solid triboelectrification with self-repairable surfaces for water-flow energy harvesting. Nano Energy 61, 454–461. doi:10.1016/j.nanoen.2019.05.007
Long, M., Peng, S., Deng, W., Yang, X., Miao, K., Wen, N., et al. (2017). Robust and thermal-healing superhydrophobic surfaces by spin-coating of polydimethylsiloxane. J. Colloid Interf. Sci. 508, 18–27. doi:10.1016/j.jcis.2017.08.027
Matijosius, T., Pivoriunas, A., Cebatariuniene, A., Tunaitis, V., Staisiunas, L., Stalnionis, G., et al. (2020). Friction reduction using Nanothin Titanium layers on anodized aluminum as potential bioceramic material. Ceramics Int. 46 (10), 15581–15593.
Milionis, A., Loth, E., and Bayer, I. S. (2016). Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interf. Sci. 229, 57–79. doi:10.1016/j.cis.2015.12.007
Naderizadeh, S., Dante, S., Picone, P., di Carlo, M., Carzino, R., Athanassiou, A., et al. (2020). Bioresin-based superhydrophobic coatings with reduced bacterial adhesion. J. Colloid Interf. Sci. 574, 20–32. doi:10.1016/j.jcis.2020.04.031
Neves, J. C., Mohallem, N. D. S., and Viana, M. M. (2020). Polydimethylsiloxanes-modified TiO2 coatings: the role of structural, morphological and optical characteristics in a self-cleaning surface. Ceramics Int. 46 (8), 11606–11616. doi:10.1016/j.ceramint.2020.01.190
Qu, M., Liu, S., He, J., Yu, C., Liu, X., Yao, Y., et al. (2016). Bioinspired fabrication of mechanically durable superhydrophobic materials with abrasion-enhanced properties. RSC Adv. 6 (96), 93403–93409. doi:10.1039/c6ra18327a
Raimondo, M., Veronesi, F., Boveri, G., Guarini, G., Motta, A., and Zanoni, R. (2017). Superhydrophobic properties induced by sol-gel routes on copper surfaces. Appl. Surf. Sci. 422, 1022–1029. doi:10.1016/j.apsusc.2017.05.257
Rao, Q., Chen, K., and Wang, C. (2016). Facile preparation of self-healing waterborne superhydrophobic coatings based on fluoroalkyl silane-loaded microcapsules. RSC Adv. 6 (59), 53949–53954. doi:10.1039/c6ra09582h
Shen, J., Ming, P., Zhang, X., Yan, L., Zheng, X., and Wang, W. (2019). Broad spectrum anti-fouling, photocatalytic antibacterial and superamphiphobic coating fabricated by composite electrodeposition process. J. Electrochem. Soc. 166 (16), E564–E575. doi:10.1149/2.0101916jes
Tang, M.-K., Huang, X.-J., Li, X.-W., Huang, Z.-Y., Zhang, S.-M., and Zhang, Q.-X. (2016). Fabrication of superhydrophobic surface with superior anticorrosion and great mechanical stability on AA7075 Al alloy via a convenient and efficient approach. Mat Express 6 (2), 101–115. doi:10.1166/mex.2016.1293
Vilaró, I., Yagüe, J. L., and Borrós, S. (2017). Superhydrophobic copper surfaces with anticorrosion properties fabricated by solventless CVD methods. ACS Appl. Mater. Inter. 9 (1), 1057–1065. doi:10.1021/acsami.6b12119
Wang, D., Wang, X., Liu, X., and Zhou, F. (2010). Engineering a titanium surface with controllable oleophobicity and switchable oil adhesion. J. Phys. Chem. C 114 (21), 9938–9944. doi:10.1021/jp1023185
Wang, D., Zhang, L., Lee, W., Knez, M., and Liu, L. (2013). Novel three-dimensional nanoporous alumina as a template for hierarchical TiO2Nanotube Arrays. Small 9 (7), 1025–1029. doi:10.1002/smll.201201784
Wang, Q., Yu, M. G., Chen, G. X., Chen, Q. F., and Tai, J. L. (2017). Facile fabrication of superhydrophobic/superoleophilic cotton for highly efficient oil/water separation. BioResources 12 (1), 643–654.
Wei, N., Liu, Y., Zhang, T., Liang, J., and Wang, D. (2016). Hydrogenated TiO2 nanotube arrays with enhanced photoelectrochemical property for photocathodic protection under visible light. Mater. Lett. 185, 81–84. doi:10.1016/j.matlet.2016.08.109
Wen, Q., and Guo, Z. (2016). Recent advances in the fabrication of superhydrophobic surfaces. Chem. Lett. 45 (10), 1134–1149. doi:10.1246/cl.160621
Xie, A., Cui, J., Chen, Y., Lang, J., Li, C., Yan, Y., et al. (2019). One-step facile fabrication of sustainable cellulose membrane with superhydrophobicity via a sol-gel strategy for efficient oil/water separation. Surf. Coat. Technology 361, 19–26. doi:10.1016/j.surfcoat.2019.01.040
Xing, W., Li, Z., Yang, H. U., Li, X. L., Wang, X. Y., and Li, N. (2019). Anti-icing aluminum alloy surface with multi-level micro-nano textures constructed by picosecond laser. Mater. Des. 183, 9. doi:10.1016/j.matdes.2019.108156
Yeganeh, M., and Mohammadi, N. (2018). Superhydrophobic surface of Mg alloys: a review. J. Magnesium Alloys 6 (1), 59–70. doi:10.1016/j.jma.2018.02.001
Zhang, C. L., Zhang, F., Song, L., Zeng, R. C., Li, S. Q., and Han, E. H. (2017). Corrosion resistance of a superhydrophobic surface on micro-arc oxidation coated Mg-Li-Ca alloy. J. Alloys Compounds 728, 815–826. doi:10.1016/j.jallcom.2017.08.159
Zhang, J., Ur Rahman, Z., Zheng, Y., Zhu, C., Tian, M., and Wang, D. (2018). Nanoflower like SnO2-TiO2 nanotubes composite photoelectrode for efficient photocathodic protection of 304 stainless steel. Appl. Surf. Sci. 457, 516–521. doi:10.1016/j.apsusc.2018.06.307
Zhang, T., Rahman, Z. U., Wei, N., Liu, Y., Liang, J., and Wang, D. (2017). In situ growth of single-crystal TiO2 nanorod arrays on Ti substrate: controllable synthesis and photoelectro-chemical water splitting. Nano Res. 10 (3), 1021–1032. doi:10.1007/s12274-016-1361-x
Zhao, G., Xue, Y., Huang, Y., Ye, Y., Walsh, F. C., Chen, J., et al. (2016). One-step electrodeposition of a self-cleaning and corrosion resistant Ni/WS2superhydrophobic surface. RSC Adv. 6 (64), 59104–59112. doi:10.1039/c6ra07899k
Keywords: anodized titanium, sol-gel, composite coating, superhydrophobicity, self-healing
Citation: Li T, Dong C, Liu Y, Wu J, Zhang X, Gong X, Zhao W, Wang D and Zhu D (2021) An Anodized Titanium/Sol-Gel Composite Coating with Self-Healable Superhydrophobic and Oleophobic Property. Front. Mater. 8:618674. doi: 10.3389/fmats.2021.618674
Received: 19 October 2020; Accepted: 18 February 2021;
Published: 13 April 2021.
Edited by:
P. Davide Cozzoli, University of Salento, ItalyReviewed by:
Piotr Warszynski, Polish Academy of Sciences, PolandCopyright © 2021 Li, Dong, Liu, Wu, Zhang, Gong, Zhao, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tinghua Li, dGluZ2h1YV9saUBhbGl5dW4uY29t; Yupeng Liu, bHlwNjA2QDE2My5jb20=; Daoai Wang, d2FuZ2RhQGxpY3AuY2FzLmNu; Donglai Zhu, emh1ZGxAeW56eS10b2JhY2NvLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.