
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mater., 15 February 2021
Sec. Carbon-Based Materials
Volume 7 - 2020 | https://doi.org/10.3389/fmats.2020.616787
This article is part of the Research Topic2021 Retrospective: Carbon-Based MaterialsView all 6 articles
Population-driven socioeconomic urban expansion, industrialization, and intensified modern agricultural practices are interlinked to environmental challenges culminating in compromised water quality due to pollution by toxic, persistent, and bioaccumulative heavy metal ions, pesticides, nitroaromatics, and other emerging pollutants. Considering the detrimental impact of pollutants on human health and ecosystem, their detection in different media including water is paramount. Notably, electrochemical techniques are more appealing owing to their recognized advantages. This research summarizes and evaluates the most recent advances in the electrochemical sensing of environmental pollutants such as heavy metal ions, pesticides, nitroaromatics, and other distinct emerging contaminants. Besides, the review focuses on the application of electrochemical detection of the selected pollutants through analysis of representative reports in the five years from 2016 to 2020. Therefore, the review is intended to contribute insights and guidelines to contemporary progress in specific electrochemical application practices based on graphene derivatives, toward the aforenamed pollutants. Thus, it focused on sensing methods such as cyclic voltammetry, anodic stripping voltammetry, and electrochemical impedance spectroscopy employing different sensing elements incorporating graphene. Moreover, the review also highlighted graphene synthesis pathways, sensor design strategies, and functionalization. Furthermore, the review showed that there is congruence in the literature that functionalized graphene and its derivatives remain as viable modifiers in electrochemical sensing of pollutants. Nonetheless, the study also appraised the absence of literature reports on electrochemical detection of natural organic matter substances like humic acid and fulvic acid using a graphene-based sensor. In reckoning, current challenges related to graphene synthesis and applicability, envisaged opportunities, and future perspectives are outlined.
Owing to global socioeconomic growth spurred by exponential population rise, water quality has been gradually depreciating. Expansion in the intricately interrelated developmental spheres of urbanization, industrialization, and agriculture has massively contributed to environmental challenges. Furthermore, inadequate treatment of industrial and municipal waste coupled with compromised austerity in regulatory monitoring of effluent has resulted in the deposition of pollutants into the ecosystem. Apart from that, environmental issues including uncapped pollution and diminished groundwater replenishment due to low rainfall have further exacerbated water scarcity and pollution levels (Zhang et al., 2019b; Perreault et al., 2015; Arfin and Rangari 2018; Su et al., 2018; Priya et al., 2018). Among the pollutants which have generated widespread apprehension are heavy metals (HMs) viz mercury, lead, cadmium, pesticides, and emerging chemical pollutants. Even though some HMs are derived from biogeochemical mechanisms, significant HMs in the aquatic media are derived from anthropogenic operations such as fossil fuel combustion, mining processes, incineration, and release of municipal wastewater. Moreover, pesticides have become an integral part of modern extensive agricultural practices and out/indoor domestic health. Meanwhile, personal care products (PCPs) and endocrine disruptive chemicals originating from pharmaceutical and industrial applications are recognized constituents of emerging pollutants (Lingamdinne et al., 2019; Ullah et al., 2018; Sakthinathan and Chen 2015; Sharma and Bhattacharya 2017; Álvarez-Ruiz and Picó 2020).
Inorganic arsenite and HM ions such as mercury, copper, cadmium, and lead have a detrimental impact on the environment besides the health of mankind on account of their toxic nature, persistence in different media, and disposition to biologically accumulate along the trophic system. Human beings become exposed to these metals through consuming contaminated food and portable water. For instance, trivalent arsenic ions are known to cause impairment of major human organs such as the lungs, the liver, and the reproductive system besides weakening the immune system (Molina et al., 2016; Zuo et al., 2019). These pollutants have detrimental effects including damage to body organs and malfunctioning of hormonal systems. Consequently, there is strong motivation to protect ecosystems through environmental analysis and determination of contaminants (Qu et al., 2013; Hou et al., 2018; Liyuan Wang et al., 2013; Lingamdinne and Koduru 2018; Wang et al., 2020). Currently used spectroscopic, chromatographic, and hyphenated techniques are reliable, sensitive, and precise; however, they have inherent shortcomings including prolonging and tedious sample preparatory steps, less economical, utilization of potentially harmful solvents, and the need for trained and certified operators. Inevitably, these approaches become limited for on-site, instantaneous, and in situ analysis (Molina et al., 2016; He et al., 2018a; Huang et al., 2019a; Wen et al., 2018).
Conversely, electrochemical (EC) approaches are hugely acclaimed to be versatile in the detection of variant natural and anthropogenic pollutants such as HM ions, pesticides, and other substances of concern owing to numerous merits comparative to the conventional laboratory-centralized means. Specifically, EC methods are accredited for their superior sensitivity and discriminatory ability, lowly detection limits, and cost-effectual status. Moreover, their facileness in operation, rapid analytical response, absence of sample pretreatment, technically miniaturized devices, and portable state make them amenable for on-site analysis (Silwana et al., 2016; Bansod et al., 2017; Smith et al., 2019).
Again, EC methods are acknowledged for their short response time, simple preparation procedures which are used, and high target specificity even when analyte concentration is extremely low especially in complex matrices; thus, there is a diminished impact from potentially interfering chemicals (Theyagarajan et al., 2020a; Jerome and Sundramoorthy 2020).
Using bare electrodes during the analysis of contaminants is prone to some drawbacks including electrode passivation, high overpotential of analyte reactions, and slow direct electron transfer (Hang et al., 2019; Lee et al., 2018; He et al., 2019a). Notwithstanding, there are strategies to mitigate against such limitations, thus improving the sensitivity and preciseness of electrodes. Electrode modification is one such approach using metal (oxide) NPs, polymers, and other carbonaceous materials. Notably, modification serves to decrease the overpotential of EC reactions and preconcentrating capability for some analytes, and it culminates in the generation of an electrode-modifier interface which ensures the formation of bridges and pathways to enable electron shuttling to ultimately improve signal amplification (Salih et al., 2016; Krishnan et al., 2019; Jerome and Sundramoorthy 2020). Besides, electrode modifying facilitates the simultaneous covalent immobilization and effectual anchoring of biomolecules on the electrode surface. It also promotes accessing of embedded redox-active sites by mediators while it preserves the enzyme’s original nature and activity (Lawal 2018; Kandaswamy Theyagarajan et al., 2020; Murugan et al., 2020). Among the carbon-based materials are carbon nanotubes and graphenic carbon nanomaterials. For instance, graphene-derived nanomaterials are recognized owing to environmentally friendly synthesis methods; for example, during EC synthesis of graphene, nontoxic solvents are used; comparatively, high yield is obtained and minimum residual defects are formed (Nagarajan and Sundramoorthy 2019). In addition, they have a large specific area-to-volume ratio, conductivity, and fast electron transfer kinetics. Therefore, they have attracted global attention; thus, they are explored in different applications. In particular, graphene-derivatized nanomaterials have become entrenched in applications among others, energy harvesting and storage, and environmental analysis as electrochemical sensors or biosensors (Dideikin and Vul’ 2019; Nagarani et al., 2018; Dywili et al., 2019; Li et al., 2015; Gumpu et al., 2017).
This review summarizes and evaluates recent advances in the development and application of EC detection of selected environmental pollutants such as HM ions, pesticides, endocrine disruptors, nitroaromatics, and other pollutants of concern, all based on graphene derivative platforms. Graphene and its derivative synthesis methods and functionalization are briefly outlined. Ultimately, confronting issues, ensuing opportunities, and prospects are further discussed. The review evaluated representative published reports available through the Internet, on the respective subject in the five years from 2016 to 2020, and in the process, more than 200 reports were examined. The review has been organized into sections which include EC techniques, graphene and its derivatives synthesis approaches, and functionalization of graphene-based materials. Further sections focused on application in EC sensing of diverse environmental pollutants, HM ions, pesticides, emerging contaminants, natural organic matter, nitroaromatic compounds, and other pollutants of interest, all with relevant examples to illustrate contemporary progress. Figure 1 summarizes the main pollutants and functionalizing materials associated with graphene in this review. The penultimate part is dedicated to challenges, envisaged opportunities, and future perspectives interrelated with the synthesis and application of graphene originating materials.
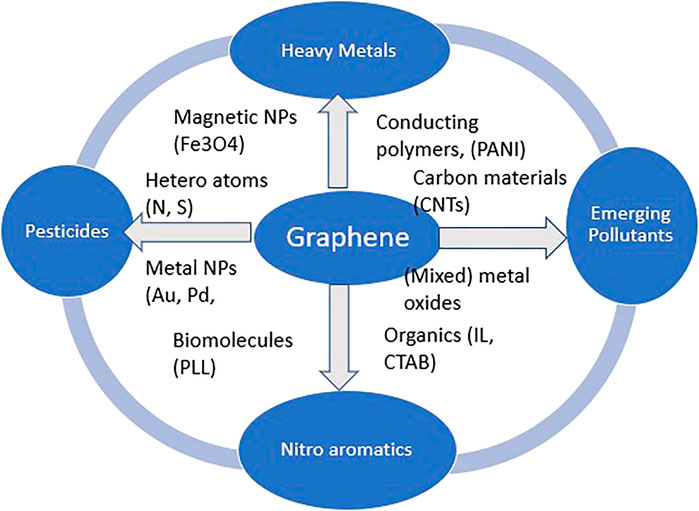
FIGURE 1. Scheme summarizing the main pollutants reviewed and functionalizing materials associated with graphene in this review.
Diverse EC techniques are at the disposal of the scientific research and academic community viz impedimetric and voltammetry. The techniques have gained global recognition owing to their advantages such as high sensitivity, facile operation and procedures, cost-effectiveness, and miniaturizable, portable, and hence potential on-site application. In this part, cyclic voltammetry (CV), anodic stripping voltammetry (ASV), and electrochemical impedance spectroscopy (EIS) are briefly outlined.
Fundamentally, voltammetry pertains to the applying of potential in an EC cell and consequently measuring the resulting current which is controlled by the mass transport. The applied potential progressively changes with the independent time variable while the current response is a function of the potential but has a linear relation with the earmarked analyte concentration in the bulk sample (Yilong et al., 2015; Silwana et al., 2016; Wongkaew et al., 2019; Banerjee et al., 2020). Contemporarily, voltammetry has been used in sync with other innovative strategies including differential pulse, square wave, and anodic stripping with an acclaimed reputation in sensing various analytes.
ASV is a sensing technique which is reliant on a preconcentration stage when the dissolved HM ions drift from the bulky solution to the bare or modified electrode. Depending on an enabling potential difference, the HM ions are electroreduced generating the elemental metal. The neutral metal adsorbates accumulate on the electrode surface leading to the transferring of mobile charges to the surface modifier (Gęca and Korolczuk 2017; Waheed et al., 2018). Uniquely, when a specific anodizing voltage is applied, the elemental metals are dissolved releasing electrons. Simultaneously, the di/trivalent cations diffuse into the electrolyte while generating an intense voltammetric stripping current even in ultralow metal concentration matrices. Significantly, there exists a direct interrelationship between the rate-determining prior concentration step along with the ensuing stripping current (Lee et al., 2017; Waheed et al., 2018; Shtepliuk and Yakimova 2019; Suherman et al., 2017). The basic steps in ASV and relevant data to be extracted are shown in Figure 2. ASV stands out as a technique of choice associated with its unique sensing capability features like discriminant selectivity, rapid detection period, and elevated sensitivity.
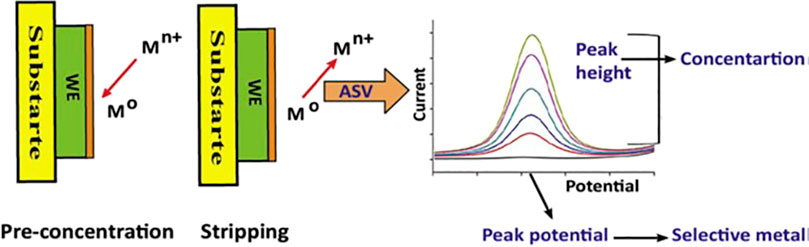
FIGURE 2. A schematic representation of the basic steps of ASV and the information to be obtained. Reproduced with authorization from (Waheed et al., 2018). Copyright 2018, Elsevier.
Several researchers explored ASV realizing incredible results; typically, Wen et al. fabricated and applied a reduced graphene oxide-manganese dioxide (rGO/MnO2) nanocomposite doped with nitrogen for GCE modification. Based on CV, EIS, and ASV in the mercuric determination, the enhanced sensor behavior was on account of synergic impact emanating from obtained nanostructured material and doping. Thus, they reported an elevated sensitivity of 72.16 μA μM−1 and a low detection limit of 0.0414 nM within a linear range of 0.01–0.2 µM (Wen et al., 2018).
In a different report, Chimezie et al. produced an rGO and ex situ synthesized magnetite nanocomposite (rGO/Fe3O4) for refashioning a screen-printed carbon electrode (SPCE) surface and investigated quantification of arsenite through DPASV and EIS technologies. Enhanced perfomance of EC sensor was achieved at a linear range of 2–300 μg L−1 with low detection limit of 0.10 μg L−1 (S/N = 3) compared to reported limit of quantification (0.33 μg L−1). They accounted for the enrichment of performance through synergistically large active surface centers for electron transfer routes (Chimezie et al., 2017).
In other respects, EIS is an accomplished technique undertaken to determine the impedance whose components, frequency-reliant-resistance, and capacitance of the electrode are preceded by perturbating in alternating current mode. Results of experiments are mathematically evaluated with an equivalent electrical circuit (EEC) and reliably provide quantitative electrochemical data on mass transfer hence reaction rates, electrical conductivity, dielectric constant, and direct electron mobility (Jin and Maduraiveeran 2018; Wongkaew et al., 2019). For example, Manavalan et al. fabricated GO-functionalized ZnO nanostars for SPCE modification. They evaluated the altered electrode through CV, DPV, and EIS for methyl parathion (MP) detection (Manavalan et al., 2020). Remarkable sensor behavior was reported giving a low sensing limit of 1.2 nM and superior sensitivity of 16.5 μA μM−1 cm−2 over a linear range from 0.03 to 670 µM. Furthermore, the amplified EC sensor properties were aftermath due to the combinational collaborative effect of the materials used as indicated by the raised peak currents and lower charge transfer resistance. Meanwhile, the proposed sensor’s applicability was assessed in real matrices for MP and it proved a viable candidate. Similarly, Zhou et al. constructed graphene nanoplatelets integrated with a noble metal and Au nanoparticles (GNPs-Au NPs) for refashioning a GCE surface. They investigated the determination of trace level bisphenol A (BPA) using CV, DPV, and EIS strategies and applied the sensor to different water matrices. Their findings demonstrated a low detection capping of 0.027 nM spanning a linear function of 5 × 10−3–100 µM. More so, the improved sensor properties were attributable to increased electrocatalytic behavior due to synergy between the composite materials (Zou et al., 2019).
Sensor platforms intended to ensure sufficient selectivity and high sensitivity apart from having long-term shelf life and short response time. Additionally, they ought to be economic in terms of low power needs and cost-effective. Numerous efforts that are based on material chemistry, target analyte reactivity, sensing mechanism, and other factors have been pursued by the scientific community. In this section, the following are presented: sensor design strategies, the role of nanosized materials, graphene derivative platform synthesis, and lastly the functionalization using diverse materials like metal (oxides) NPs, polymers, and organic and inorganic materials.
The detection behavior of a sensor is responsive to the interaction which occurs at the electrode interface with the targeted chemical species. Primarily, any sensor system has key components like the sensing element, recognition element, and transducer (Jin and Maduraiveeran 2018; Peña-Bahamonde et al., 2018). Thus, the sensor components and interface events influence design strategies. Sensor design strategies are premised on the realization that actual environmental, clinical, food, or security-related samples may be complicated matrices and the earmarked analyte may be present in extremely low concentrations. Therefore, the design promotes superior sensitivity and differentiation and upgraded target specificity while ameliorating nonfouling effects. Notably, some acknowledged design strategies that have been applied solely or in combination include analyte affinity enrichment, enhancement of the surface area, increased catalytic effect, high loading capacity, and amplification of EC signal (Kumar et al., 2019; Hang et al., 2019; Numan et al., 2020; Wongkaew et al., 2019; Moro et al., 2019; Yan et al., 2020a).
The integration of properties of components of sensor configurations has been widely explored with beneficial impact. In one study, Yan et al. constructed a functional sensing configuration deploying Au NPs, ferrocene dendrimer (FcDr), and rGO. The Au NPs/FcDr/rGO nanohybrid was employed to modify GCE and applied in the detection of dichlorvos (Yan, 2020). Remarkably, FcDr is an effectual signaling constituent associated with the strong anchoring on the electrode, electrostatic interaction, and covalent immobilization besides serving as an Fc+2/Fc natural redox probe for EC signal (Yan et al., 2020b). The sensing element components Au NPs have excellent electrical conductivity and improve analyte interaction while rGO nanosheets have a large surface area which is conducive for effective loading and dispersion of metallic NPs onto rGO. Therefore, these components have a synergistic effect stimulating the electron transfer process, hence amplifying the EC signal of the Au NPs/FcDr/rGO/GCE sensor. The proposed sensor attained micromolar detection sensitivity for the pesticide analysis.
Similarly, Malakootian, Hamzeh, and Mahmoudi-Moghaddam investigated the modification of carbon paste electrode (CPE) employing a magnetic FeNi3/CuS/BiOCl NC for concurrent detection of divalent cadmium and lead. The researchers reckoned that the biocompatible and superparamagnetic FeNi3 promotes electrical communication between electroactive species and electrode surface (Malakootian et al., 2020). Meantime, the stable semiconductor BiOCl has high catalytic activity while CuS possesses electrical conductivity qualities like metals. Thus, there was synergistic aftermath that boosted the electron transfer kinetics, ultimately amplifying the sensor signal. Furthermore, through SWASV, the corresponding detection limit of 0.4 μg L−1 and 0.1 μg L−1 for Cd+2 and Pb+2 was reported while the sensor demonstrated viability when its applicability was evaluated in real samples.
Recently, Kaur assembled a novel NC of ErGO-chitosan (CS)-hemoglobin (Hb) coatings (ErGO-CS/Hb) through a green synthesis method prior to modifying a fluorine-tin oxide (FTO) electrode. The ErGO-CS/Hb configured platform was employed in investigating the quantification of MP via SWV and EIS (Kaur et al., 2020). Notably, the immobilization of Hb onto the ErGO-CS matrix ensued a fast charge transfer rate to the embedded electrode center while safeguarding the nativity of the biomolecule. Evidently, there was magnified pesticide affinity attributed to the delocalized π electron system of rGO which initiated robust π-π stacking interaction with MP. Besides, electrostatic interaction and hydrogen bonding promoted firm binding of the pesticide on the modifier surface. The combination of rGO with CS through amide linkage also improved the dispersion during synthesis, producing a stable nanohybrid. Furthermore, rGO possesses a large surface area, superior electrical conductivity, lower background current, and wide EC potential window while Hb has active redox centers and the hydrophobic biopolymer CS endowed with good film-forming ability was synergistically integrated, manifested in excellent sensitivity and selectivity. So, a limit of detection (LOD) of 79.77 nM and sensitivity of 45.77 A cm−2 µM−1 were attained.
Likewise, Zeng et al. devised a nanohybrid involving Pd nanoflower-decorated 3D CNT-graphene nanosheets (GNSs) network assembled from CNTs and GNSs for modification of an SPE. The sensing element PdNFs-CNTs-GNSs/SPE was engaged for simultaneous sensing of nitroaromatics, p-nitrophenol (4-NP), 1,3-dinitrobenzene (DNB), 1-chloro-2,4-dinitrobenzene (Cl-DNB), 2,4-dinitrotoulene (DNT), 1,3,5-trinitrobenzene (TNB), and trinitrotoluene (TNT). Initially, 1D CNTs were embedded into 2D GNSs to form a native 3D architectural framework instrumental for prohibiting CNTs and GNSs from agglomerating during synthesis (Zeng et al., 2019). Besides, the 3D structure has a large surface area plus numerous electrochemically active surface sites conducive for high loading of functional NMs on itself confirmed by PdNFs being finely dispersed on the porous CNTs-GNSs scaffold. Effectively, synergism between active PdNFs and CNTs-GNSs porous 3D network facilitates electron channeling between the electrode and redox species, hence fostering electrical communication. Furthermore, the unique configuration had superior electrocatalytic activity toward the reduction of nitroaromatics. The sensor achieved detection limits in the nanomolar level, and the proposed portable sensor system demonstrated potential for prompt, in situ, and point-of-analysis assessments.
Nanostructured materials have the potential to considerably boost the surface area-to-volume ratio and they are endowed with numerous active centers and hence influence the analytical performance of a sensor.
Nanopillars are sharp spiked nanostructures with high antibacterial efficacy whenever bacteria come into contact. Their potency mode is piercing the bacterial cell wall, thus rupturing and damaging the microorganism, Thus, nanopillars are favorable modifier candidates where antimicrobial surfaces are needed (Bhadra et al., 2018; Chen et al., 2020; Canalejas-Tejero et al., 2018).
The central bore of nanoneedles is a critical feature which is exploited in the medical field. The bore serves as a conduit where the desired molecules pass through. So, nanoneedles are useful for the delivery of bioactive molecules into cells, loading of drugs, and delivery of drugs to specific points (Shende et al., 2018).
Nanorods are 1D structures with a diameter between 1 and 100 nm and they are amenable for use in the sensing and medical field. For example, Hang et al. constructed a hierarchically vertical fluorinated graphene/ZnO nanorods plus nanoseeds platform which was employed for H2O2 determination. The sensor had increased active surface area and inherent self-cleaning and fouling-proof capabilities which enhanced its analytical performance (Hang et al., 2019).
Nanowires (NWs) are “sticks” with a diameter of less than 100 nm and varying lengths. However, when interconnected, NWs form 2D or 3D conductive independent frameworks that are self-supporting but have an excellent specific surface area. Moreover, NWs have the capability of hosting several structural defects; they are corrosion proof to the reaction conditions and provide the direct charge connectivity between underlying active centers and the electrode surface. Ultimately, NWs improve the electrocatalytic activity and promote signal amplification. For instance, Zhuang et al. designed a sensor premised on 3D nickel oxide NPs, PANI nanowires, and GO. The NiO NP/PANINW/GO matrix was used to modify a GCE and applied for the detection of glucose (Zhuang et al., 2016). The sensing platform exhibited superior electrocatalytic activity while its sensitivity was high (376.22 μA μM−1 cm−2) and LOD was in micromolar level. Similarly, nanotubes also serve as conducting channels linking redox sites with the electrode, besides improving the active surface area which forms the bedrock of EC reactions (Kumar et al., 2019; Cho et al., 2020; Zhang et al., 2018a). Theyagarajan et al. fabricated a sensor employing MWCNTs and a functionalized ionic liquid where the horseradish peroxidase was anchored before modifying a GCE (Theyagarajan et al., 2020b). The sensing configuration was evaluated through detection of H2O2 and the corresponding sensitivity and linear range were 55.08 μA μM−1 cm−2 and 0.01–2.07 nM while the LOD was at micromolar level. Furthermore, there were synergism of properties of MWCNTs and functionalized ionic liquid, large surface area, superior electrical conductivity, π–π stacking, covalent immobilization, and accessing of embedded redox sites. Thus, the sensor performance was enhanced.
The graphene lineage is composed of the pristine precursor graphene and its derivatives inclusive of GO, rGO, and graphene quantum dots (GQDs). Besides, doped graphene is regarded as an offshoot of that family (Zheng et al., 2017; Smith et al., 2019; Thangamuthu et al., 2019). Graphene is a sole layer of an allotrope of carbon with sp2-hybridized carbon atoms hexagonally arrayed into a two-dimensional (2D) honeycombed entity (Ambrosi et al., 2016; El-Shafai et al., 2018; Beitollahi et al., 2019). For more than a decade, the substantial focus has been directed toward this 2D nanomaterial due to its standout features including excellent chemical inertness and remarkable thermal, optical, mechanical-electrical characteristics (Lee et al., 2019c; Lingamdinne and Koduru 2018; Magesa et al., 2019). Moreover, graphene-derived nanomaterials are conferred with the facility that promotes simple functionalization, superior electron transport capability, and enormous specific surface area-to-volume ratio, besides being biocompatible (Huang et al., 2019b; Kuralay et al., 2016; Kumar et al., 2017; Krishnan et al., 2019). There are diverse pathways for the synthesis of graphene derivatives. Moreover, the derivatives are endowed with peculiar properties which enhance the sensing behavior of composite materials.
Conveniently, pristine graphene synthesis processes as depicted in Figure 3 are segmented as either bottom-up or top to bottom incorporating the Geim-Novoselov pioneered mechanical cleavage of graphite. Notwithstanding that, the technique is roundly recognized as least suitable for scalable commercial graphene production since it is arduous and has inferior reproducibility (Khan et al., 2015; Yin et al., 2015; Molinari and Argurio 2017; Xu et al., 2017; Tiwari et al., 2018). Meanwhile, other respective methods are described in the literature, including defects-generating aqua-chemical exfoliation (Lee et al., 2019b; Lawal 2018), epitaxial forming of graphene on silicon carbide substrate, and chemical vapor deposition (CVD) (Avouris and Dimitrakopoulos 2012; Suvarnaphaet and Pechprasarn 2017; Ahmad et al., 2018), and thermal exfoliation (Mohan et al., 2018; Rowley-Neale et al., 2018). Furthermore, “unzipping” of CNTs is also considered a viable graphene preparation pathway. Comparatively, electrochemical means are preferred owing to their efficacy, rapidness, facile, and environmentally friendly nature (Ambrosi et al., 2016; Sakthinathan et al., 2016). Nonetheless, it has been ascertained that graphene’s applicability is hindered by agglomeration arising from the conjunction of π–π restacking, physical defects, multiplex sheet thickness, and inferior aqueous dispersion (Khan et al., 2015; Silwana et al., 2016; Sturala et al., 2018; Beitollahi et al., 2019; Pei et al., 2020). Consequentially, there has been consensus in exploring mitigatory strategies such as the primal conversion of graphene to functionalities-rich GO.
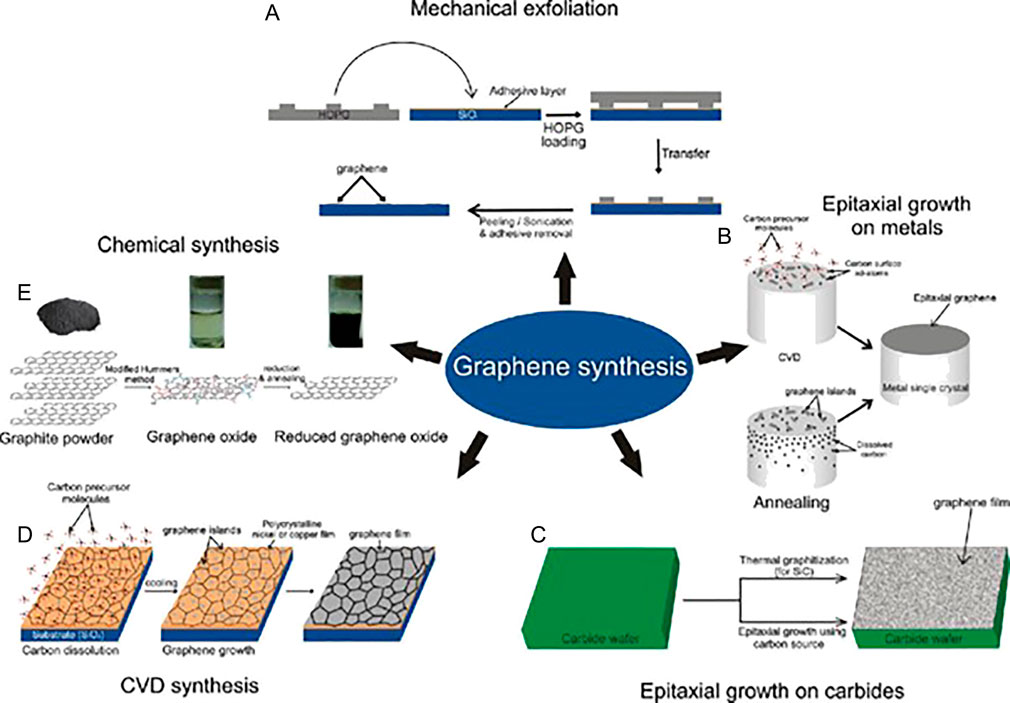
FIGURE 3. Schematic illustration of different graphene synthesis approaches, (A) mechanical exfoliation of graphene flakes, (B) epitaxial graphene growth on metal crystal, (C) epitaxial growth on carbon sources like silicon carbide wafers, (D) CVD method utilizing metal foils to prepare graphene possessing large surface area, and (E) chemical synthesis of graphene from graphite oxide. Reproduced with permission from (Jariwala et al., 2011). Copyright 2011, American Scientific Publishers.
The principal approaches for GO synthesis are based on (improved/modified) Hummers method where pyrolytic graphite precursor is subjected to strongly oxidizing and acidic conditions prior to sonication (Zaaba et al., 2017; Lee et al., 2019c; Eigler and Hirsch 2014; Chang and Baek 2017). The resultant GO is hydrophilic owing to the abundant presence of oxygen functionalities. Accordingly, GO is endowed with improved solvent dispersibility and a predisposition for architectural modulation through functionalizing nanomaterials (Bahadir and Sezgintürk 2016; Muthoosamy and Manickam, 2017). Literature sources abound with detailed properties and synthesis protocols of GO (Mohan et al., 2018; Feicht et al., 2019; Smith et al., 2019; Wongkaew et al., 2019; Turkaslan and Mihrace, 2020). Nevertheless, the prevalence of the functional moieties imparts GO with insulating properties. Therefore, in attempts to circumvent such GO limitation and partially restore native pristine graphene qualities, rGO is prepared instead (He et al., 2018b).
Several methods are at the disposal of researchers for the reduction of GO to rGO to restore certain properties to near pristine graphene. Distinctly, the methods are either thermal or chemical in addition to EC. Significantly, the thermal reduction has concurrent merits of elimination of some oxygen-containing domains, besides stimulating reestablishment of defects through rehybridization of carbon atoms (Dideikin and Vul’, 2019; Oliveira and Morais, 2018). Meanwhile, chemical reduction of GO fosters the worthwhile restoration of prototype graphenic structure, although it introduces structural defects. Apart from that, green and soft reduction technologies continue to be attractive. Relatively, thermal reduction is a preferred method while EC reduction is appealing since it promotes tuning of material properties using green route (Cinti and Arduini, 2017; Su et al., 2018). Figure 4 shows the GO reduction mechanism and the progressive change in oxygen functionalities.
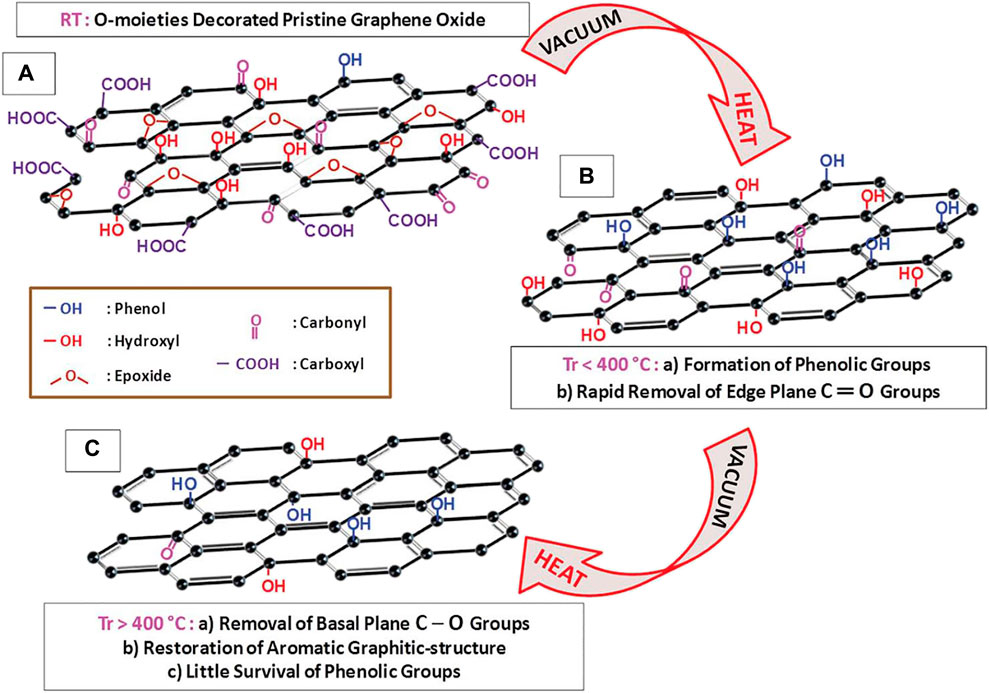
FIGURE 4. Schematic flowchart of GO reduction pathways. Reproduced with consent from (Khan et al., 2015). Copyright 2015, The Royal Society of Chemistry.
As much as the development of N-doped graphene is concerned, prevalent strategies are variant. Among them are CVD with ammonia gas, nitrogen plasma annealing of graphene, heat conditioning of graphite oxide using melamine, electrically huge energy treatment in ammonia, and hydrothermal tempering involving nitrogen in the form of urea (Lei et al., 2017).
Functionalization with the sole purpose of tuning graphene-based nanomaterial chemicophysical characteristics such as reactivity, electrochemical activity or resistivity, surface energy, and electronic structure has been a subject of research interest for decades. Thus, functionalization has been accomplished either via covalent means or noncovalent interactions. Covalent functionalizing entails the incorporation of variant functionalities onto the graphenic basic structure through attaching by primarily covalent bonds. The incorporation of other entities occurs through the nonmetallic heteroatom replacement of carbon, free radical addition to conjugated bonds, and edge oxygenated moieties reacting with other functional materials. Meanwhile, intermolecular forces viz π-anionic attraction, hydrogen bonding, π–π stacking, van der Waals forces, and hydrophobic interaction are the principal contributors toward noncovalent narrative. A distinctive characteristic of the noncovalent functionalization is that it does not interfere with the graphitic structure while entrenching it with functionalities (Georgakilas et al., 2012; Eigler and Hirsch 2014; Xu et al., 2017; Lawal 2018). Furthermore, graphene is adorned with semiconductor properties despite the absence of bandgap energy. The opening of such a null bandgap is a critical feature of graphene derivatization which enhances target analyte detection (Garg et al., 2014; Talirz et al., 2016; Ould Ne et al., 2017; Sturala et al., 2018). Figure 5 shows the absence of an energy bandgap in pristine graphene which becomes adjustable due to functionalization. Ultimately, the resultant altered product has inherent hybrid and synergistic properties of the initial components which are advantageous while concurrently overshadowing the individual original limitations. Among others, these impactful synergies enhance the electrochemical performance of the sensing system.
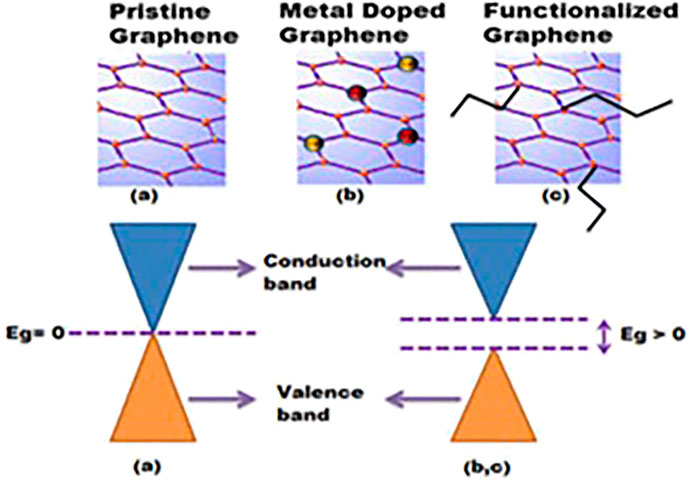
FIGURE 5. Schematic representation showing (A) null bandgap in pristine graphene and (B) and (C) graphene bandgap opened due to doping and functionalization with different materials. Reproduced with permission from (Garg et al., 2014). Copyright 2014, MDPI.
EC methods have typical drawbacks when utilizing bare electrodes as follows: carbon paste (CP) or glassy carbon (GC) electrodes. Typically, high overpotential and inactivation of the electrode due to the deposition of by-products have been confronted. Notwithstanding, modifying electrode surfaces is an acceptable mitigatory means to counter electrode passivation due to accumulating of reaction by-products and raised overpotential (Hanssen et al., 2016; Campuzano et al., 2019). Electrode surface modification has been accomplished by employing diverse materials. Among them, metal/metal oxide nanoparticles, organic materials including ionic liquids, or carbon nanomaterials especially graphene, SWCNT, or MWCNT have been explored. Other than that, inorganic materials, conducting polymers such as polyaniline (PANI) and heteroatoms doping continue to draw considerable attention of the scientific community owing to numerous merits. Specifically, beneficial synergistic effects that enhance sensor characteristics for the detection of analytes have been realized (Sakthinathan and Chen 2015; Si et al., 2018; Venu et al., 2018). Furthermore, the emanating synergic aftermaths hugely enhance structural morphology and photochemical and electrochemical properties, and the impedance of the electrode surface is diminished while improving preferential accumulation and boosting the electron transport process. Ultimately, the sensor performance regarding sensitivity, selectivity, and stability is improved (Silwana et al., 2016; Bollella et al., 2017; Si et al., 2018). Figure 6 shows a schematic illustration depicting electrode modification by chitosan-Fe(OH)3 composite (CFC) nanomaterials and the sensing effect.
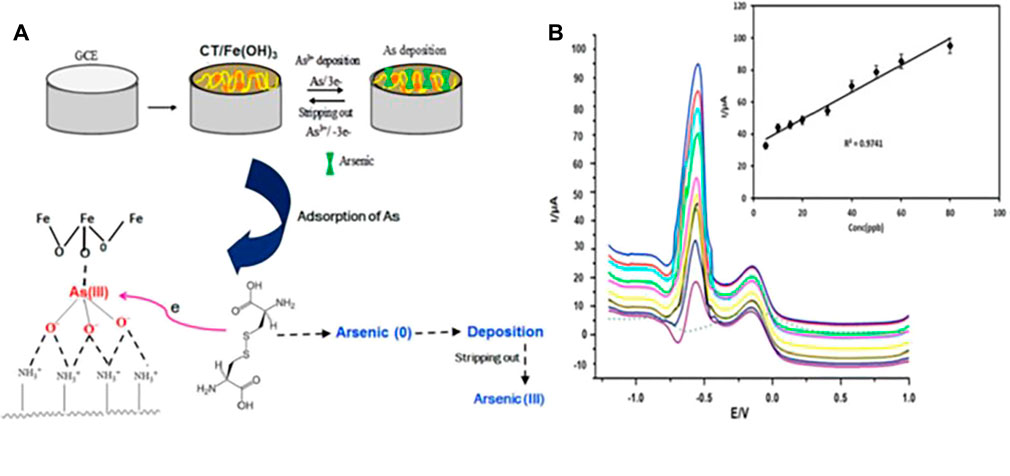
FIGURE 6. Schematic illustration of (A) GC electrode modification by chitosan-Fe(OH)3 composite (CFC) and the As (III) sensing mechanism and (B) ASV signal of the CFC modified electrode for various concentrations of As (III) solutions. Reproduced with permission from (Lalmalsawmi et al., 2020). Copyright 2020, Elsevier.
Uniquely, HM ions can remain in the environment for prolonged timespans since they are not degradable by biological means to form innocuous substances. Furthermore, they are bioavailable and hence have the tendency to be bioaccumulative in the trophic systems and ultimately reach the consumer, the human being. Because of their proclivity to persist in the environment, bioaccumulative disposition, and elevated toxicity (PBT), considerable attention has been directed to HMs based on the detrimental aftermath on ecological systems and the health of the general populace. Even though some HMs such as copper, manganese, nickel, and zinc are an essential trace dietary expectation, exceeding intake levels may have an adverse impact on body functioning. Additionally, when the concentration of some HM ions surpass regulatory limits and there is sufficient exposure to human beings, they may have adverse effects on their health (Stortini et al., 2020). Besides, some HM ions are suspected carcinogens and mutagens. Meanwhile, there have been reports of varying ecological, biota, and human health ramifications due to excessive exposure to some typical metal ions like arsenic, lead, or mercury in parts of the world (Jan et al., 2015; Orr and Bridges 2017; Latif et al., 2018; Pratush 2018; Rehman et al., 2018; Wan et al., 2020). Moreover, their mortal mode is through complications, impairment, or damaging of critical body organs such as liver, reproductive system kidneys, lungs, heart including the central nervous system, and the skin (Zhou et al., 2018; AL-Gahouari et al., 2020; Raril and Manjunatha 2020). Accordingly, for the sake of protecting the environment and for human health safety, timeous detection (and removal) of harmful HM ions surpassing international guideline levels in the media is of critical importance.
Functionalization applying small organic molecules boosts sensor performance. As such, Raril and Manjunatha constructed a novel polyglycine-modified graphene paste electrode (PGMGPE) for simultaneous EC detection of the divalent ions of mercury and lead. The researchers realized the enhancement of redox signal for the HM ions by the polymer-modified electrode attributable to superior electron transfer kinetics, thus affirming that there was considerable electrode surface property change (Raril and Manjunatha 2020). Thereupon, the PGMGPE was assessed and it demonstrated satisfactory electrocatalytic activity, good stability, and wide linearity response coupled with low corresponding Hg (II) and Pb (II) detection limits of 6.6 and 0.8 µM, respectively. Furthermore, they applied the fabricated EC sensor for the determination of the foregoing HM ions in water and blood serum samples where the recovery was commendable.
Numerous research efforts have been explored exploiting the interlinked microporous nature of three-dimensional nanocomposite structures which improves the effectual surface area and reactive sites for collection/accumulation of targeted substances. Likewise, there is a powerful synergism between graphene and the functional material plus smooth charge carrier movement since the interconnected graphenic network provides unimpeded channels enhancing the sensitivity and selectivity of the modified detection system (Yan et al., 2015; Wang et al., 2015; Guan et al., 2018). Distinctively, Shi et al. (2017a) studied a facile electrode alteration based on a 3D graphene network integrated with bismuth nanoparticles toward multiple HMs sensing. Significantly, wide linear ranges of 1–120 μg L−1 for both Cd (II) and Pb (II) with 40–300 μg L−1 for Zn accompanied by LOD of 0.05, 0.02, and 4.0 μg L−1, respectively, were proclaimed. They reckoned that the diminished limits, improved sensitivity, selectivity, and other properties of the proposed sensor were associated with the boosted active metal cations binding sites of the porous graphene framework an enhanced path for charge mobilities and bismuth-graphene intensified conductivity synergy. In another study, Xiao et al. (2016) via a self-assembly method constructed a graphitized mesoporous framework and investigated its potential applicability for on-site sensing of HMs contaminants present in aqueous media. The modified bismuth mesoporous graphene framework Nafion bound (Bi/MGF-Nafion) electrode showed enriched properties such as elevated sensitivity (0.437 and 0.210 μA L µg−1), 0.3 and 0.1 μg L−1 LOD over a broad linear function of 2–70 and 0.5–110 μg L−1 for the divalent cadmium and lead correspondingly. Notably, they deduced that the enhancement in properties was linked to the combinational impact of in situ coated bismuth with adhesive Nafion and the 3D mesoporosity nature, superior electrical conductivity, and raised active surface area of MGF.
Similarly, Al-Gahouari et al. explored the effects of modifying GCE using a 3D nanohybrid of electrochemically (E) rGO combined with MWCNTs. This was done via covalently functionalizing with an amino acid and l-cysteine (ErGO-MWCNTs-L-cys/GCE) and applied the modified electrode in the monodetermination of lead (Pb2+) ions in water (AL-Gahouari et al., 2020). The authors reported a linearity span of 0.2–40 μg L−1 and a calculated sensing limit of 0.1 μg L−1 which was recognized to be lower compared to the international standard quantity for portable water. Additionally, by comparison to the bare electrode, the developed sensor system had an upgrade in the electrochemical conductivity, sensitivity, and selectivity which they attributed to synergistic impact among the components ErGO, MWCNTs, and the amino acid. The CV in Figure 7 validates the proffered deduction while the EIS analysis reveals the combinational effect in lowering the resistance at electrode boundary for electron movement, hence, further affirming that altering electrode surface promoted quick electron relaying by the hybridized material. Correspondingly, Priya et al. designed an HM ion sensor by fashioning the surface of GCE using a nanocomposite material fabricated from GO, the functional group rich amino acid l-cysteine, and a biodegradable but compatible natural biopolymer ĸ-carrageenan (Priya et al., 2018). They studied the feasibility of the sensing system employing SWASV and hence revealed a broad linearity range of 5–50 nM with comparably low LOD of 0.58 and 1.08 nM and remarkable sensitivity of 1.39 and 1.32 μA nM−1 for the divalent Cd and Pb, respectively. Furthermore, they ascribed these enhanced characteristics of the modified sensor to the combinational effects of high surface area and functionalized edges of GO, the chelation capacity of the multifunctional amino acid, and the active adsorptive binding of carrageenan toward the HM ions.
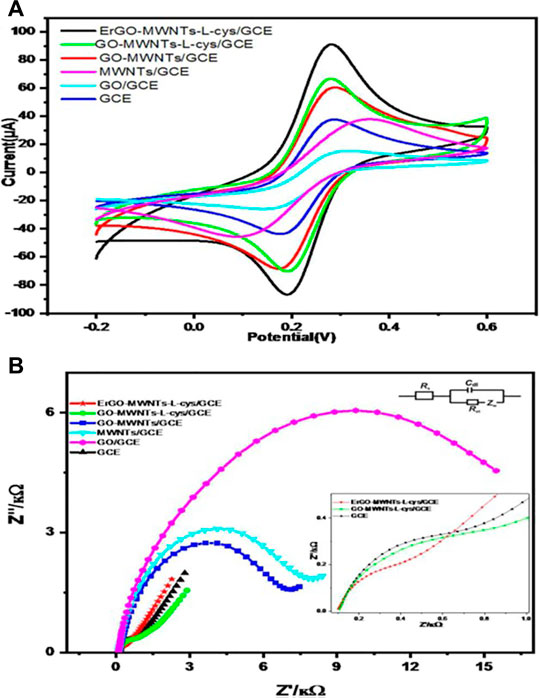
FIGURE 7. CV and EIS images comparing the behavior of bare GCE as well as differently modified electrodes. Reproduced with permission from (AL-Gahouari et al., 2020). Copyright 2020, Frontiers Media S.A.
Recently, Baghayeri et al. investigated a solvothermally produced graphene-zinc metal-organic framework (G/MOF) platform for arsenic (III) determination. The researchers ascribed the enriched As (III) sensing sensitivity to a fruitful synergistic relationship between the mechanically strong large surface area of graphene and the microporous rich network MOF giving a large active surface area. More significantly, they achieved a detection limit of 0.06 ppb which is more than a hundred times less than the internationally set value while a broad linear span of 0.2–25 ppb was realized demonstrating potential applicability for arsenite detection in real samples (Baghayeri et al., 2020).
Heteroatoms including nitrogen, boron, sulfur, or halogen elements have been incorporated into graphene-derived nanocomposites for modification of electrodes with significant amplifying of properties like electric conduction or electrochemical behavior. Among other contributory factors are the influential dopant-to-carbon electron linkage and the emerging of possible reactive sites for heavy metal ions to accumulate on (Shi et al., 2017b; Guan et al., 2018). Therefore, considerable efforts have been focused on doping of variant nonmetal elements into sensing platforms founded on graphene nanomaterials, exploiting the accruing benefits. For example, Lin et al. designed and investigated a nitrogen-doped laser-engraved graphene electrochemical sensor system using polyaniline, PANI, and polyvinylpyrrolidone, PVP, as the N-doping reagents, for concurrent recognition of lead and cadmium ions employing SWASV (Lin et al., 2018). They attained broad linearity relation in the span from 5 to 380 g L−1 and 0.5–380 g L−1 for the corresponding Cd (II) and Pb (II) with LOD of 1.08 and 0.16 g L−1. These authors further deduced that there was an integrated effect between the dopant’s electron affinity to carbon atoms in the structure and the 3D porous nature of piled graphene layers. Essentially, that raised the effective adsorption surface area, expedited the electron transfer, and ultimately enhanced the electrical conduction, the sensitivity, the response range, and the EC performance of the modified electrode. Figure 8 shows a representative diagram of the fabrication pathway for the N@LEG electrodes.
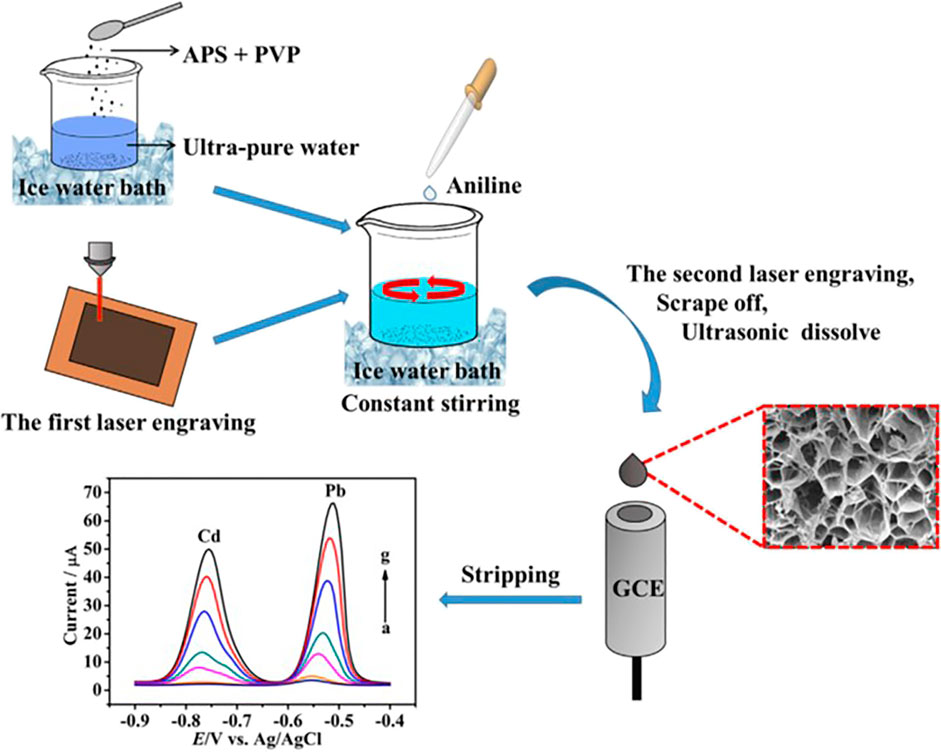
FIGURE 8. Pictorial schematic of the electrode preparation protocol via N@LEG for the dual EC sensing of Cd (II) and Pb (II). Reproduction was consented from (Lin et al., 2018). Copyright 2018, Elsevier.
Similarly, Lei et al. (2017) designed a nitrogen-doped graphene-modified electrode for joint EC detection of copper (II) and lead (II) ions in aqueous media. Their findings indicated that the proposed sensor showed satisfactory quality with respect to reproducibility plus resistance to interference by chemically similar moieties. Besides, they recorded a calibration detection range of 0.05–2.5 µM with a respective LOD of 11 and 5 nM for copper and lead. Significantly, the researchers accounted for the enriched sensitivity based on the electron interaction between the nitrogen lone pairs and the carbon delocalized π system and increased active sites.
On the other hand, boron as a doping agent is incorporated in a special electrochemical analyte sensing material, boron-doped diamond (BDD) which is hugely acknowledged owing to its reduced background current (bio)chemical stability and wide potential window (Marton et al., 2019). Correspondingly, Pei et al. exploited the uniqueness of BDD and designed a functional independent and innovative electrode based on a BDD substrate, by growing graphene homogenously in situ in a vacuum at a raised annealing temperature. The manipulated G/self-standing BDD electrode with minimum defects was studied revealing refined qualities comparative to unaltered GSBDD including a lowly charge transfer resistance (Rct) of the electrode (Pei et al., 2020). They further asserted that the modified electrode attained 0.475 μA L μg−1 cm−2 sensitivity accompanied by a detection limit of 0.21 μg L−1 for lead ions over a spanning linear relation of 1–100 ppb. These boosted properties inclusive of long shelf period were attributed to advantageous collaborative effects of BDD and graphene resulting in increased routes for rapid charge movement. Moreover, this induced an improved active surface area linked to the two-layered graphene manipulation of SBDD, augmented adsorption centers associated with the microporous framework, and upgraded reaction kinetics which is fostered.
Besides nitrogen and boron, fluorine has been recognized as a viable dopant option owing to the higher electronegativity difference with carbon. Such difference potentially prompts increased ionic interaction and spreading of charge densities which stimulate charge transference. Moreover, the presence of the heteroatom causes manipulation of graphene energy gap which ameliorates the conveying of electrons and possible metal-ligand complexing. Subsequently, the synergism promotes the EC performance of the refashioned electrode (Shahzad et al., 2017; Thiruppathi et al., 2017). Accordingly, Thiruppath et al. utilizing CV and SWASV investigated multiple HM ions determination founded on fluorinated GO modified electrode and they reported linear concentration range of 0.6–5.0, 0.3–5.0, and 1.0–6.0 µM for bivalent Cd, Pb, and Hg, respectively. Besides, a recognition limit of 10 nM for both Cd (II) and Pb (II) was submitted and this was confirmation of enrichment of sensor performance shown by peak stripping currents attributable to increased target metal chelation and adsorption centers (Thiruppathi et al., 2017) Table 1. Although pesticides like organochlorine (OCPs) are an integral feature of modern agricultural practices, they have adverse repercussions on human health and the ecosystem owing to their typical characteristics like toxicity. Accordingly, it is paramount to advance environmental protection through monitoring and determinations. Comparably, EC techniques are more appealing credited to their facile status, short response time, cost-effectiveness, and potential on-site application, besides sensitivity and selectivity (Aragay et al., 2012; Koçak et al., 2015; Capoferri et al., 2018; Ren et al., 2019). Because of their predisposition to persist in an ecosystem accompanied by an inclination to be absorbed by aquatic biota consequently, they bioaccumulate and get magnified along the food trophic system. Further, they are lipophilic and cumulatively collected in fatty tissue of organisms; eventually, they may be consumed by human beings through food and water (Fayemi et al., 2016; Hernandez-Vargas et al., 2018; Zamora-Sequeira et al., 2019). OCP such as chlordane, aldrin, lindane, and dieldrin are recognized to be toxic with severe effects on humans and the ecological system. Recent efforts on lindane detection have utilized one-dimensional graphenic carbon in the form of MWCNTs functionalized with either conducting polymer PANI compared to macrosynthetic organic molecule Nylon 6,6 and various metal oxides. The findings of Fayemi et al., based on EC investigations, showed a lowly limit of concentration recognition of 32.0 nM stretching over a linear relationship ranging from 9.90 pM to 5.0 µM. The boosted sensor properties including magnified sensitivity were ascribed to synergy of the integrated properties of the respective nanofibers, MWCNTs, Nylon 6,6, and magnetic material (Fayemi et al., 2016). Even Boke et al. assembled nanocomposites of graphitic carbon nitride (C3N4 NTs) and polyoxometalate (POM; H3PW12O40) and developed molecularly imprinted sensors. Through CV and EIS, they realized a linear concentration span from 1.0 × 10−10 to 1.0 × 10−8 M with a recognition limit of 2.0 × 10−11 M for gamma lindane (Pelin Böke et al., 2020). On the other hand, organophosphate pesticides (OPP) although regulated, excessive use in protecting crops such as vegetables from pests has resulted in contamination of soils and water and eventually food. Furthermore, these pesticides are toxic and reasonably persistent in the ecosystem coupled with being prone to bioaccumulate along the food chain. The major exposure pathways include ingestion of contaminated food and/or water, inhaling sprays, and absorption through the skin while their harmful modal impact is principally nonreversible inhibition of acetylcholinesterase enzyme. Consequently, the neurotransmission activity is hampered with further implications on visual, respiratory, cognitive, sensory, and nervous impairment (Pellicer-Castell et al., 2020; Fu et al., 2019; Zhang et al., 2019c; Sgobbi and Machado 2018; Rajaji et al., 2019; Tian et al., 2018; Heydari et al., 2020). Similarly, carbamates, namely, carbendazim and carbaryl, are used to improve crop production and quality of yields via the elimination of pests. Notwithstanding that, carbamates contaminate ecological systems and are equally harmful to humans and animals on account of their toxicity besides having the same mechanism of disrupting neuronal transmission with detrimental effects including fatalities (Santana et al., 2019; Oliveira et al., 2020; Rahmani et al., 2018). Even though techniques currently being employed including chromatography are accurate and sensitive, they inherently have drawbacks. Among others, the techniques are laboratory-based, arduous sample preparation; they require certified personnel to operate and inappropriate for local analysis. Therefore, it is paramount to instantaneously detect the presence of different pesticides utilizing cost-effectual, portable, elevated sensitive, and selective devices especially within the aquatic media to enable remedial measures to be instituted when their presence exceeds permissible limits (Govindhan et al., 2014; Capoferri et al., 2018; Hernandez-Vargas et al., 2018; Willner and Vikesland 2018). Remarkably, variant innovative electrochemical technologies have been widely employed in sensing the presence of OCPs as reflected. Organophosphorus containing pesticides (OPP) specifically malathion, MP, methyl chlorpyrifos, and diazinon are extensively used for the eradication of crops and plant pests in contemporary agricultural practices. Significant amounts of toxic, persistent, and bioaccumulative OPPs ultimately contaminate soil and aqueous media hence have hazardous effects on nontarget species like people. Therefore, detection of such pesticides in food and water with cost-effective, facile, rapid, and portable devices usable in loco is worthwhile (Sgobbi and Machado 2018; Ramachandran and Dhayabaran, 2019; Bolat and Abaci 2018). Ramachandran and Dhayabaran devised a hybrid chemically polymerized in situ composite of MnO2, polythiophene, and rGO for modifying a GCE intended for sensing MP, an OP categorized pesticide (Ramachandran and Dhayabaran, 2019). Owing to synergistic outcomes among the integrated components including π-π interactive force with MP, enormous effective surface area, satisfactory electrical conductivity, and improved charge transportation, enrichment of EC behavior was realized. The affirmed results were magnified selectiveness, remarkable sensitivity (0.0498 μA μM−1), and low LOD of 5.72 nM as well as long shelf span stability thus showing the sensor’s potential applicability. In parallel, Tan et al. explored the modification of GCE employing nanohybridized rGO and ionic supramolecular arene for MP determination via EIS, DPV, and other techniques (Tan et al., 2019). Significantly, they reported boosted EC performance of the sensor system including a broad linearity dynamic range of 0.001–150 µM in conjunction with an inferior recognition molarity limit of 0.0003 µM, fast response, specificity, and sensitivity relative to beta-cyclodextrin-functionalized rGO-altered GCE. Additionally, they reckoned that the enrichment in EC properties was ascribable to improved supramolecular preciseness of CP5 toward MP and the synergic outcome of the macrocyclic arene such as fascinating function, complexation host-guest relation based on hydrogen bonds, and π-π interactions. Meanwhile, Figure 9 shows the comparative development of β-CD-rGO and CP5-rGO and the sensing of MP. Furthermore, a mechanistic sensing route was proposed involving a nitro reduction in a four-electron pathway. Ultimately, the researchers showed the potential viability of the developed system in actual sample analysis. FIGURE 9. Symbolic diagram of MP, CP5, and β-CD plus the illustrative scheme of CP5-rGO (A) and β-CD-rGO (B) nanomaterials developed for EC detection route of MP. Reproduction was sanctioned from (Tan et al., 2019). Copyright 2019, The Royal Society of Chemistry. Pajooheshpour et al. designed an enzyme-free gold-platinum bimetallic nanocluster template on bovine serum albumin (BSA) before integrating it with graphene nanoribbons (Au-Pt@BSA-GNRs). This nanocomposite was used to modify a GCE for the sole purpose of quantification of diazinon (Pajooheshpour et al., 2018). Their CV and SWASV investigation attained a diazinon detection concentration limit of 0.002 µM, over linear function ranges of 0.01–10.0 and 10.0–170 µM. Besides, other properties of the altered electrode such as reproducibility, accuracy, and stability were improved. The refinement in EC properties was attributable to the synergistic impact of the Au-Pt nanoclusters. Effectively, they reduced the analyte transferring distance, generated quantum effects coupled to greatly functionalized protein, BSA, and multiplexed graphene electrical conduction plus vast specific surface area. Also, the fabricated sensor had satisfactory application potential. In another study on OPP, Yola fabricated hexagonally structured boron nitride quantum dots solely incorporated on GO for manipulation of GCE surface. The innovative BNQDs/GO/GCE manipulated electrode was applied for sensing of MP, diazinon, and chlorpyrifos synchronously (Yola 2019). The researcher asserted improving sensitivity and linear relation span of 1.0 × 10−12 M to 1.0 × 10−8 M and corresponding low LOD for diazinon, chlorpyrifos, and MP were 6.7 × 10−14 M, 3.3 × 10−14 M, and 3.1 × 10−13 M. Remarkably, the magnified EC performance was ascribed to the hybridization synergy of the wide bandgap, electrical conduction, and active surface area of 2D white graphene and novel GO properties. While exploiting GQDs properties such as superior electron transfer, elevated surface area, and remarkable electric conduction, Mehta et al. attained a comparatively lower parathion low LOD of 46 pg L−1 within a very broad linearity calibration span of 0.01–106 ng L−1. These results were established when they explored CV and EIS using an SPCE modified electrode with decorated amine-functionalized nanostructured GQDs. Additionally, they submitted that GQDs were homogeneously distributed on the electrode besides acting dually as an electron-donating component and acceptor which enriched the sensor behavior (Mehta et al., 2017). Rahmani et al. constructed sulfur and nitrogen dually doped (via thiourea) three-dimension graphene decorated with gold nanoparticles composite for electrode modifying hence detection of carbaryl in different matrices. A low LOD of 0.0012 µM was attained within a linear relationship range of 0.004–0.3 µM through EC methods (Rahmani et al., 2018). Moreover, the augmented properties including appropriate lifespan, reproducibility, and satisfactory selectivity were accounted for by the collaborative impact of the hugely porous, enormous particular surface area, and rapid direct electron movement kinetics of 3D graphene evidenced by the amplified anodic peak currents for the altered electrode. Besides, the graphene is interlinked and made functional by the thiourea sulfur and nitrogen atoms relevant for complexation coupled to the excellent electrocatalytic quality of gold nanoparticles. Correspondingly, exploitation of synergistic impacts of noble metal nanoparticles which provide active adsorption centers and excellent electrical conduction, reactivating effect of macromolecule oxime against electrode passivation by the pesticide and doped graphene properties, has been undertaken. Zhang et al. explored the beneficial advantages of the cited materials and fabricated Au NPs-hybridized nitrogen-doped graphene further functionalized with a–SH containing oxime for electrode decoration. They achieved satisfactory sensitivity and reduced dimethoate concentration detection limit of 8.7 × 10−13 M over a broad linearity function range, 1.0 × 10−12 to 4 × 10−8 M, attributable to combinational advantages of the materials (Zhang et al., 2017). Emerging pollutants of concern refer to newly discovered compounds, already recognized chemicals that are still being studied to fully establish their ecological and human health implications particularly in conjunction with those recently found. Furthermore, there may be purely present-day issues cropping up on historically known and accepted “harmless” compounds (Su et al., 2018; Borrull et al., 2019; Álvarez-Ruiz and Picó 2020). Even though natural contaminants are present in ecosystems at diminished concentrations, there is the persistent anthropogenic deposition of these compounds, including pharmaceuticals, personal consumer products, industrial chemicals, pesticides, chemical warfare agents, and illicit drugs. Moreover, several of these emerging pollutants have endocrine disruptive characteristics, hence disturbing the natural hormonal functions. Apart from their ecological and human health implications they also causes different cancers and impairment of reproductive cardiovascular functions on human (Azzouz et al., 2019; Jaffrezic-Renault et al., 2020). Notably, triclosan, parabens (alky) phenols, polychlorinated biphenyls (PCBs), perfluorinated compounds, bisphenol A, phthalates, organo/heavy metals, and other chemicals are among the endocrine interfering category generating distress worldwide (Borrull et al., 2019; Karzi et al., 2019; Álvarez-Ruiz and Picó 2020). While currently used standard techniques for the determination of endocrine interfering chemicals including chromatographic and spectroscopic are reliable and sensitive, they have limitations. Among them are the need for certified personnel to operate the instruments, arduous sample preparation means, and being laboratory-based, thus not applicable for in situ analysis. Given the stated reasons, the detection of emerging pollutants including TCS (alkyl) phenols, PCBs, parabens, and other endocrine interfering chemicals in various media inclusive of water, using enormously sensitive, selective, cost-effective, portable, and fast response devices, remains critical even in present time (Zheng et al., 2018; Du et al., 2019; Moyo et al., 2015; Teixeira et al., 2020; Sk et al., 2019; Butmee et al., 2019). Triclosan (TCS, 5-chloro-2-(2,4-dichlorphenyoxy) phenol) is extensively utilized as a dual preservative and antimicrobial constituent of pharmaceutical, household hygienic goods, and individual care products. Among others are toothpaste, deodorants, soaps, shampoos, lotions, dishwashing detergents, medical disinfectants, and underclothing. Ordinarily, TCS is anthropogenically released into ecological systems and water bodies through wastewater, however ineffectually treated, and it possibly has adverse effects on aquatic biota (Yola et al., 2015; Lehutso et al., 2017; Wu et al., 2017; Zheng et al., 2018; Teixeira et al., 2020). Furthermore, TCS is lipophilic and has been recognized as interfering with endocrine hormonal functioning; hence, it is globally considered a contemporary contaminant to worry about. Apart from that, TCS degradation intermediates and derivatives which include dioxins-like chemicals are potentially harmful owing to their carcinogenic status. Although TCS is regarded as low-level toxic and is regulated by international organizations, its detrimental human impact has been chronicled especially dermal aftermaths as a result of persistent exposure (Jaffrezic-Renault et al., 2020; Du et al., 2019; Moyo et al., 2015; Montaseri and Forbes 2018; Cullinan et al., 2015; Saljooqi et al., 2020). Wu et al. fabricated nanocomposites consisting of palladium metal NPs, graphene, and a cationic polyelectrolyte poly (diallyl dimethyl ammonium chloride) (PDDA-Gr/Pd NPs) in a facile chemical protocol shown in Figure 10. The hybrids were used to refashion the GCE surface and evaluated through CV and DPV for the quantification of TCS. They established a TCS recognition concentration limit of 3.5 nM over a linearity correlation (9.0 nM–20.0 µM), with a remarkable shelf lifetime and satisfactory reproducibility. Particularly, they attributed the enriched sensor performance to the collaborative impact of the constituent material properties such as the good catalytic activity of Pd NPs and superior graphene conductivity and active surface area which enhanced electron transport speed. Besides, the viability of that sensor system was assessed in actual aqueous media samples (Wu et al., 2017). FIGURE 10. Illustrative scheme of the synthesis method of PDDA-Gr/Pd NPs along with the EC detection of TCS on modified GCE. Reproduced with consent from (Wu et al., 2017). Copyright 2016, Elsevier. In a different investigation, Saljooqi et al. fabricated a magnetic iron oxide (Fe3O4)-functionalized GO before coating with gold and polypyrrole (Fe3O4@Au-PPy/GO) for GCE modification. The modified electrode was used in studying the quantification of TCS through EC techniques such as CV and DPV (Saljooqi, Shamspur, and Mostafavi, 2020). Their findings indicated marked sensitivity, long-term stability in addition to an LOD, and calibration range of 2.5 × 10−9 M and 0.01–1.0 μmol mL−1, respectively. Distinctly, the enhanced performance of this sensor configuration was ascribed to combinational effects of the original properties of the hybrid materials. Meanwhile, Akylidirim assembled a novel NC integrating through a hydrothermal strategy, silver NPs, with graphitic carbon nitride, C3N4, GQDs, and an ionic liquid (IL) (Ag NPs/C3N4 NTs@GQDs/IL) as electrode modifier (Akyıldırım, 2020). The researcher employed the modified GC electrode in the determination of TCS via CV, DPV, and EIS, subsequently reporting a low LOD of 2.0 × 10−12 M and a linear response spanning from 1.0 × 10−12 to 1.0 × 10−8 M. The Ag NPs/C3N4 NTs@GQDs/IL nanohybrid potentially exhibited integral properties emanating from the individual composite materials. These included the large electroactive area and outstanding conductivity of GQDs, the excellent ionic conductivity of IL, electron-conducting pathways of g-C3N4, and the electrocatalytic effect of Ag NPs toward TCS and the aftermath was rapid electron mobility. Comparably, another category of chemicals that were initially considered less harmful as they are employed as additives for preservation and antiseptic purposes is parabens. Particularly, they are used in cosmetics, personal care, and pharmaceutical formulations as well as food and beverage preservatives; hence, they find their way into water sources. Nonetheless, parabens have proven to be disruptive to the human endocrine functional system and dermal problems and may have hazardous environmental implications (Kajornkavinkul et al., 2016; Mendonça et al., 2017; Piovesan et al., 2018; Faradilla Wan Khalid et al., 2019; Muñoz et al., 2020). Piovesan et al. constructed a nanocomposite integrating rGO and gold nanoparticles in a dispersion of chitosan (Au NPs-rGO-CS) toward modifying the GCE surface. CS served concurrently as GO reductant and nanohybrid stabilizer. They applied the adjusted electrode for the detection of methyl paraben (MeP), an endocrine disruptive constituent of PCPs. The CV and EIS results indicated a wider linear calibration range of 0.03–1.30 μmol L−1 with respective limits of quantification and concentration detection of 41.73 and 13.77 μmol L−1 and superior sensitivity. Additionally, the upgraded sensor performance as evidenced by the magnified anodic peak currents was attributed to synergic effects of the integrated components (Piovesan et al., 2018). Mendonca et al. described a solvothermally synthesized ruthenium NPs-functionalized rGO for modifying electrode surface character. The integrated rGO-Ru NPs NC-modified GCE was then evaluated for the quantification of MeP using DPV. Enhanced signal for the altered electrode was observed; hence, an LOD and calibration linear range of 2.40 × 10−7 mol L−1 and 5.00 × 10−7 to 3.00 × 10−6 mol L−1, respectively, were reported. This was due to the synergism as a result of the integration of individual material properties which facilitated rapid electron transfer rate and improved conductivity (Mendonça et al., 2017). Analogously, Khalid et al. designed a cellulose nanocrystal (CNC)-functionalized rGO NC for electrode modification, using a facile protocol of sonication and drop-casting (Faradilla Wan Khalid et al., 2019). The CNC-rGO/SPE was employed for MeP determination through CV, DPV, and EIS. In this instance, the acceptable film-forming qualities and antiadsorption ability of CNC were beneficial as well as the outstanding electrical conductivity of rGO. The synergic interaction enhanced sensing by the configured electrode through inhibiting MeP from adsorbing onto the surface of rGO owing to π-π functioning, improved selectivity, and fast charge transfer. An LOD of 1.0 × 10−4 M with a concentration span of 2 × 10−4 to 9 × 10−4 M was attained. The sensor system showed reusability potential and proved to be a viable option in actual product analysis for MeP. Likewise, Munoz et al. composed different dimensional nanocomposites including rGO decorated with platinum nanoparticles (Pt NPs/rGO) as a surface modifier for GCE. They explored through CV and DPV the detection of MeP and reported magnification of sensor performance such as a low recognition limit of 2.5 µM accompanied by a wider correlation range of 5.0–50 µM and elevated sensitivity of 45 μA μM−1. Meanwhile, they concluded that the platinum-functionalized one dimension CNT was the best sensor platform with LOD and linearity in the nanomolar range (Muñoz et al., 2020). Similarly, Santana and Spinelli exploited the benefits emanating from hybridizing GQDs with a natural polymer CS. They modified GCE and investigated through CV and DPV the determination of TCS and MeP concurrently. Recognition limits of 0.03 μmol L−1 plus 0.04 μmol L−1 for respective TCS and MeP were achieved over a broad functional span of 0.10–10.0 μmol L−1. Moreover, the enrichment of properties was on account of synergism between GQDs and CS (Santana and Spinelli, 2020). Similarly, bisphenol A, BPA (2,2-bis (4-hydroxyphenyl) propane), is a primary ingredient widely employed for the commercial production of macromolecular products viz polycarbonate polymers and epoxy resins. Furthermore, BPA is thus a useful component of packaging containers employed in the food and beverages industries. However, the presence of BPA in different media including water sources has been substantiated while it is partially treated in the water treatment terrain (Zou et al., 2019; Canevari et al., 2019; Yu et al., 2017). BPA has been recognized for its tendency to accumulate and be magnified along the trophic chain besides its persistence within the ecosystem. Recently, BPA has been acknowledged for its adverse impact on the ecology and health of mankind. Specifically, BPA is an endocrine system disrupting compound which imitates the natural estrogens’ hormonal system. Concurrently, the toxic chemical further severely impairs the human nervous function, reproductive system, cardiovascular role apart from being potentially a carcinogen (Sk et al., 2019; Li et al., 2017; Butmee et al., 2019; Pang et al., 2020). Shi et al. devised a single-step EC reduction of GO in which the formed rGO was used to enfold copper (I) oxide, Cu2O. The GCE was modified with Cu2O-rGO nanohybrid and assessed through CV in the determination of BPA (Shi et al., 2017a). When compared to Cu2O/GCE, rGO/GCE, and the bare electrode, Cu2O-rGO/GCE had good electrocatalytic performance, as evidenced by the high anodic currents. A diminished LOD and wider linearity function of 5.3 × 10−8 M and 1 × 10−7 to 8 × 10−5 M respectively were reported. Furthermore, the enriched sensor properties were on account of the synergistic impact of rGO possessing high electroactive and superior conductivity plus the marked catalytic activity of the metal oxide which facilitated electron transport. The sensor configuration was evaluated to be a viable candidate for BPA sensing in aqueous media. Similarly, Canevari et al. fabricated hybridized rGO and carbon NPs material through dispersing GO in alcoholic media with CNPs which simultaneously acted as reductant and attachment link to rGO. The CNPs-rGO hybrids were used in SPCE modification then explored the determination of BPA through DPV and EIS (Canevari et al., 2019). The researchers reported a low LOD of 1 × 10−9 mol L−1, a sensitivity of 189.5 μmol L−1, and a broad linear response spanning from 7.5 × 10−9 to 2.6 × 10−7 mol L−1. The rGO-CNPs-modified electrode showed an elevated electrocatalytic response and reduced charge transfer resistance, thus expediting the unimpeded electron transport from the solution toward the electrode. Significantly, such enriched sensor characteristics were due to the synergy premised on the huge electroactive surface area of CNPs and magnificent electrical conductivity of rGO. Moreover, the sensor design proved to be a potential candidate for BPA sensing in drinking water. Equally, Li et al. assembled a simple GCE modifier integrating reduced graphene oxide, silver metal NPs, and a biopolymer, poly-l-lysine (rGO-Ag NPs/PLL), and then explored the sensor system for BPA detection through CV, DPV, and EIS. Distinctly, a widely varying linear relationship of 1–80 µM with a capped sensing response of 0.54 µM is attained plus satisfactory stability and good specificity. These were attributable to improved electroactive anchoring sites, hydrogen bond linkages, and enhanced electron mobility (Li et al., 2017). Likewise, Butmee et al. fabricated a facile nanocomposite of graphene nanoplatelets functionalized by an ionic liquid (IL-GNPs) as shown in Figure 11. The NC was applied for electrode modification prior to investigating BPA sensing using CV, DPV, and EIS techniques. They reported enrichment of properties including a low LOD of 6.4 nM over a linearity function range of 0.02–5.0 µM besides reasonable stability, and enhanced sensitivity was due to synergistic impact owing to elevated ionic conductivity of IL and large surface area of GNPs leading to fast electron mobility (Butmee et al., 2019). FIGURE 11. Schematic representation of (A) fabrication of IL-GNPs EC sensor and (B) EC oxidation detection mechanism of BPA on IL-GNPs/GCPE. Reprinted with approval from (Butmee et al., 2019). Copyright 2018, Elsevier. Additionally, Table 2 shows further findings of recent research utilizing graphene-derived materials toward sensing of pesticides in addition to other emerging pollutants. TABLE 2. Graphene-based analyte detection techniques, sensing material, emerging organic analytes, and performance parameters. Natural organic matter (NOM) refers to complicated heterogeneous matrices of nonhumic products, humin, humic acid, and fulvic acid other than colloidal matter derived from different hydrological and biogeochemical processes. Moreover, by various means either internal to an ecosystem or exterior like anthropogenic pathways, including microbiological degradation, the formation of natural humic substances principally occurs. Notably, humic acid and fulvic acid have functional component groups viz carboxyl, hydroxyl, phenols, amino acids, among others which influence their behavior in water. Several studies have indicated the variability of NOM in surface waters (Nkambule et al., 2012; Särkkä et al., 2015; Sillanpää et al., 2018; Fakayode et al., 2020). Significantly, NOM in water is by no means solely toxic nor is it a contaminant. Nonetheless, water’s unpalatable organoleptic characteristics inclusive of inferior taste, undesirable color, foul odor, and lack of freshness are attributed to the presence of NOM. Additionally, the organic content is considered responsible for the corresponding and concurrent chemical coagulant expenditure and the regularly undertaken plant backwashing owing to membrane fouling (Moyo et al., 2019; Roosmini et al., 2018; Sarno et al., 2019; Chaukura et al., 2018; Islam et al., 2020). Apart from that, NOM nurtures microbial regrowth and promotes complexation since the terminal functional groups act as ligands which complex with HM ions boosting the bioavailability and toxicity of organometals (Adekunle et al., 2020; Ma et al., 2018; Zhao et al., 2019; Islam et al., 2020). Furthermore, when there is inadequate removal of NOM before either chlorinating or chloramination, it is recognized that the respective chlorine or chlorine dioxide gases react with the organic material forming known mutagenic and carcinogenic disinfectant by-products (DBPs) (Basumallick and Santra 2017; Haarhoff et al., 2010; Nkambule et al., 2009; Ray et al., 2017; Fakayode et al., 2019b; Li et al., 2012; Sillanpää et al., 2018). Besides, the DBPs have other impactful effects, namely, liver damage (Zhang et al., 2019a). In view of the grave challenges stemming from the variability, indeterminate composition, and the recalcitrant predisposition status of NOM, it is accordingly imperative that innovative technologies be modeled to monitor its prevalence (and removal) to inhibit the formation of DBPs right through the water purification terrain (Särkkä et al., 2015; Ye et al., 2018; Pavitt and Tratnyek 2019; Adekunle et al., 2020). Lately, electrochemical strategies have been proposed and gained considerable recognition ascribable to being cost-effective, the potential for actual-time, online analysis, elevated sensitivity, and selectivity. Significant reports in the literature show that considerable attention has been focused on the detection of NOM particularly humic and fulvic acids in aquatic environments using spectroscopic techniques. Nevertheless, much of the electrochemical sensing material has been based on either organic macromolecules or (un)functionalized metal (oxides) nanoparticles (Basumallick and Santra 2017; Ma et al., 2018; Fakayode et al., 2019a; Pavitt and Tratnyek 2019; Adekunle et al., 2020; Fakayode et al., 2020). Nitroaromatics, namely, nitrophenol compounds are key ingredients in agriculture and industry. Notwithstanding, nitroaromatics compounds find their way into the ecology hence, contributing significantly to environmental pollution due to their elevated toxicity (Qin et al., 2019; Li et al., 2019). Nitroaromatics such as DNT, TNP, TNT, and NB are used in chemical explosives and landmines. Explosives residues are recognized to cause harmful effects to the central nervous, cardiovascular, and respiratory systems among others (Wen et al., 2018; Jigyasa and Kaur Rajput, 2018; Ramachandran and Karunkaran Yesodha, 2019). Therefore, it is crucial to protect the environment through timeous, target-specific, simple, sensitive detection means. EC techniques have gained recognition due to analyte specificity, sensitivity, portability, on-site, and real-time determinations. He et al. prepared a sensor for EC determination of nitrophenol based on acetylene black paste and graphene hybrid electrode (GR/ABPE). Through CV, respective linear range and LOD of 20 nM to 8.0 and 8.0 µM were reported (He et al., 2019b). Most recently, Hwa et al. fabricated an EC sensor exploiting halloysite nanotubes (HNTs) and Ag NPs decorated on rGO for modification of GCE. The rGO-HNTs-Ag NPs-configured sensor was examined in the sensing of 4-NP through CV and DPV. Significantly, the researchers achieved a linear response in the concentration range 0.1–363.9 µM, with an LOD of 48.6 nM and sensitivity of 35.25 µAµM−1 cm−2. Additionally, the sensor was found viable when evaluated using different sample matrices (Hwa and Ganguly, 2020). Similarly, Zhang et al. adopted dual heteroatom doping introducing nitrogen and sulfur into graphene nanoribbons prior to base washing (BW) and then modified a GCE. The BW-NS-rGO NRs/GCE sensor was studied through CV, DPV, and EIS in detecting TNT. The sensor platform recorded a wide linear range of 0.0008–5.1 ppm and LOD of 0.1 ppb. The superb sensing performance is attributed to the dual doping of N and S atoms and full exposure of the rich defective active sites of BW-NS-rGO NRs after base washing, and rGO enables target molecules binding besides its high electrical conductivity; thus, signal amplification was realized. The proposed sensing platform was evaluated and exhibited potential for TNT determination in water samples (Zhang et al., 2018b). Li et al. fabricated a core-shell GO@polymerized-Mn-porphyrin (GMPP@AMP) nanocomposite for altering GCE surface and the platform was applied for the determination of NB via CV and DPV. Evidently, there was synergistic impact owing to the prevalence of functionalizing nitrogen-containing moieties, π-π porous conjugated caged metalloporphyrin structure, and GO conductivity expediting the high electron transfer capacity, strong mutual affinity, and increased contact area between NB and the mesoporous polymer enhancing the electrocatalytic activity of the sensor. The reported linear range was 0.04–0.24 mM while LOD was 0.243 × 10−6 M and the evaluated sensor exhibited practical prospects for sensing NB in ecological matrices (Li et al., 2019). Ramachandran, Nair, and Yesodha explored aromatic nitrogen-doped GQDs synthesized via the hydrothermal treatment of precursor PANI. The modified GCE (N-GQD/GCE) was employed for the detection of TNP through DPV and an LOD of 0.2 ppb (1 nM) was revealed. They concluded that the enhanced sensitivity and selectivity emanate from rich N-doped aromatic structure of the N-GQD which promoted closer and selective molecular interactions with nitroaromatics through ring stacking, π-π interaction, or hydrogen bonding which improved the electrical conductivity and electron transferability. Again, the sensor also differentiated various nitroaromatic species, TNP, DNT, and NP DNT (Ramachandran and Karunakaran Yesodha, 2019). Polyphenols including flavonoids, phenolic acids, tannic acid, and polyphenolic amides are abundantly prevalent in fruits, vegetables, beverages, herbs, and spices among others. Further, notable examples viz garlic acid, curcumin, and catechins are readily available. Polyphenols have diverse acclaimed health benefits; however, some may have potential toxicity. Either way, their detection with facile, sensitive, cost-effective, on-site portable devices is worthwhile (Kokulnathan et al., 2018; Ganesh et al., 2019; Manikandan et al., 2019; Ansari and Arvand 2020; Suvina et al., 2020). Manikandan et al. (2019) configured a sensor based on fluorine-doped GO and modified a GCE. The F-GO/GCE sensor was applied for the detection of caffeic acid via CV and DPV and then realized concentration range and LOD of 0.5–100.0 and 0.018 µM, respectively. Fluorine has elevated electron affinity which leads to the formation of fluorine dopant-to-graphenic carbon electron linkages and generation of reactive centers, hence improving electrical communication. Moreover, the applicability of the sensor was judged to be a potential quality control means for the determination of polyphenols in food and beverages industry. Analogously, Kokulnathan et al. constructed a hexamine cobalt (III) coordination complex [Co(NH3)6]+3 grafted onto a rGO NC-modified GCE. The rGO/[Co(NH3)6]+3 chelate was produced through a sonochemical process. The platform was employed via CV, DPV, and EIS for the determination of a flavonoid, morin (MR) (Kokulnathan et al., 2018). They revealed a wide linear range, LOD, and sensitivity of 0.008–72.35 µM, 1.0 nM, and 4.326 μA μM−1 cm−2 correspondingly. Notably, the cobalt hexamine complex has excellent electrocatalytic activity attributed to its heterogeneous electron transferability which enhanced the sensor’s analytical performance. Recently, Ansari and Arvand developed a magnetic bar carbon paste electrode (MBCPE) modified using cobalt ferrite magnetic electrospun nanofibers (NFs) and GO and reconnoitred the determination of rutin, a flavonoid. They applied CV and DPV to investigate the behavior of rutin on the modified MBCPE (Ansari and Arvand, 2020). Significantly, they reported two linear ranges viz 0.001–0.1 nM and 1.0–100 nM besides an extremely low LOD of 0.94 pM. Likewise, they ascribed the enriched sensor characteristics to the spinel ferrite’s superior electrical conductivity due to swinging of electrons between different valence states of the metals and availing surface redox-active sites for adsorption. Catechol (CT) has varied health advantages and is regarded as a principal fragment of tea catechins. However, CT is recognized to have potential toxic and carcinogenic characteristics; thus, its determination has been pursued. Suvina et al. (2020) devised an original CT sensor based on lanthanum cobaltite anchored on graphene nanosheets (LaCo/GNSs) and modified a GCE prior to monophenol determination through CV and EIS. The sensor configuration attained LOD of 1.0 nM, sensitivity of 5.68 μA μM−1 cm−2, and broad linear range of 0.009–132 µM. Distinctly, the sensor components LaCo and GNSs impacted the sensor analytical performance as LaCo has a nanostructured framework with great biochemical compatibility, superior electrical conductivity, large surface area, rapid electron transfer, and short channels. Conversely, graphene is known for its delocalized π electron system, excellent electron communication, numerous structural defects, and high surface area-to-volume ratio. These properties were synergistically integrated attaining increased surface area, increased analyte affinity, and signal amplification. Nitrates are key ingredients of fertilizers which are a backbone of intense farming practices. They are also available as a constituent of the natural nitrogen cycle; however, transcend fertilizer applications cause an imbalance. Consequently, excess nitrates are leached into ecological systems including soil and water sources. Distinctively, excessive exposure to nitrates may pose potential risks to human health on account of potential toxicity. Moreover, nitrates also affect ecological balance in water sources leading to adverse aftermaths on aquatic biota due to eutrophication. On the other hand, sulfates are also valuable constituents of contemporary industrial and agricultural practices. Despite that, they invariably contribute to environmental pollution. Therefore, it is critical to enforce environmental protection and safeguard human health, through EC detection techniques which are more appealing attributable to their simplicity, rapid response, high sensitivity, target specificity, portability, and potential actual on-site analysis (Mahmud et al., 2020; Jiang et al., 2020; Li et al., 2020). Bagheri et al. developed a sensor based on Cu NPs, MWCNTs, and electrochemically prepared rGO where the Cu NPs were deposited on the MWCNTs-rGO matrix to create a platform. The Cu NP/MWCNTs/rGO nanohybrid was utilized in modifying a GCE and then used for concurrent determination of nitrates and nitrites via CV (Bagheri et al., 2017). The authors deduced that the well-dispersed Cu metal on MWCNT-rGO composite contributed to the improvement of the electrochemical activity and the active redox sites. Ultimately, the sensor had high sensitivity and differentiation, while a low LOD of 0.1 µM and a wide linear range of 1–10−3 μM were achieved. Additionally, the sensor was evaluated proving to be a potential candidate for nitrates/nitrites analysis in samples. Correspondingly, Wang, Kim, and Cui prepared a nitrate sensor utilizing polyelectrolytes, positively charged poly(diallyl dimethyl ammonium chloride) (PDDA), and negatively charged poly(sodium 4-styrene sulphonate) (PSS) with self-assembled Cu NPs and graphene NSs (Wang et al., 2018). They modified an electrode surface and then applied the sensor for detection of nitrates through CV attaining sensitivity of 0.2 μA μM−1 and detection limit of 7.89 µM. Furthermore, it was considered that the polyelectrolytes stimulated anchoring the graphene onto the glass substrate and the resultant 3D network actuated enhancement of reactive surface area and ultimately the sensor performance. Again, the practicality of the proposed sensor was examined demonstrating promising viability for the determination of nitrates in real water samples. Yu et al. (2016) designed a sensor based on the electrooxidation of a complex heteropoly blue with poly-L-lysine-functionalized graphene. The platform was employed in modifying GCE prior to investigating the determination of sulfate ions through SW. The determination was undertaken indirectly in the presence of a surfactant cetyltrimethylammonium bromide (CTAB) due to high overpotential. A wide linear range and LOD of 0.8–1,000 and 0.26 µM, respectively, were reported. More so, there was synergism among the blue complex, the surfactant, and graphene which resulted in signal enhancement.Pesticides
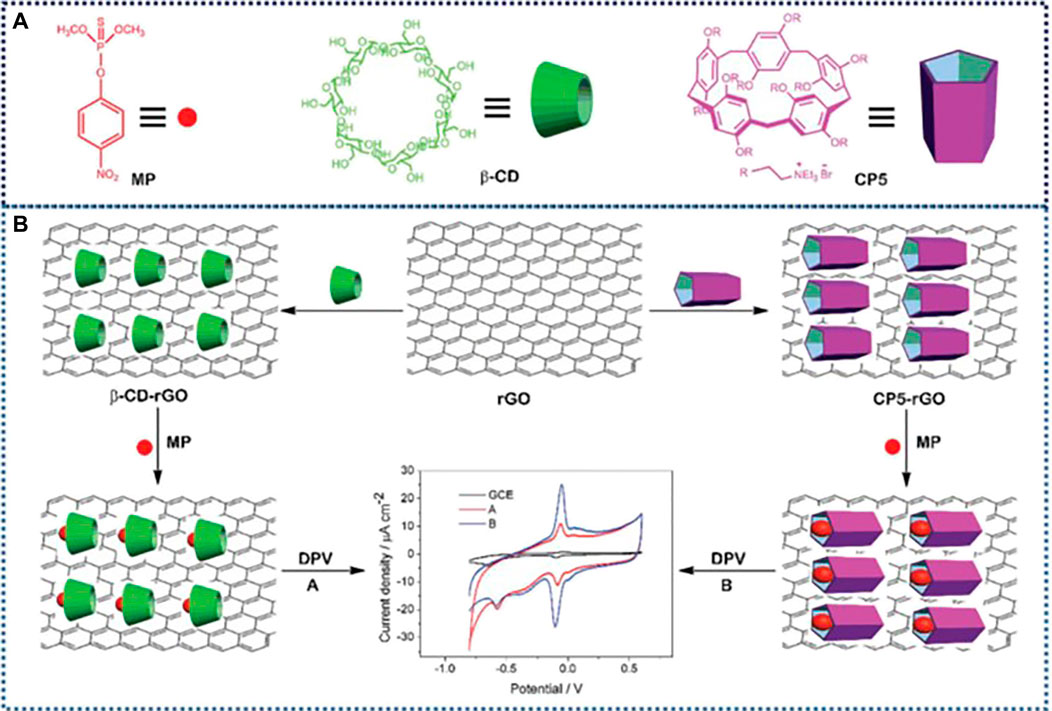
Emerging Pollutants
Triclosan
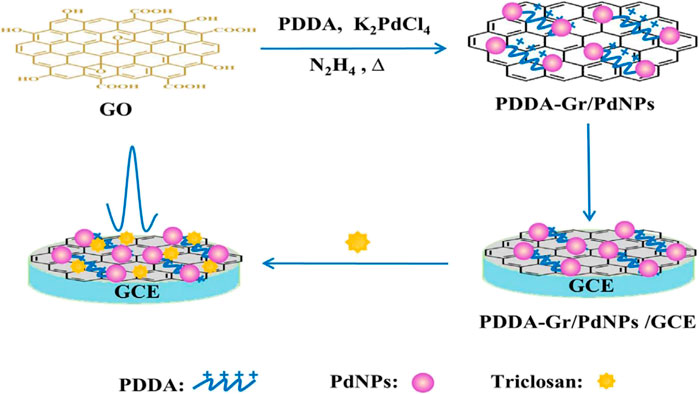
Parabens
Bisphenol A
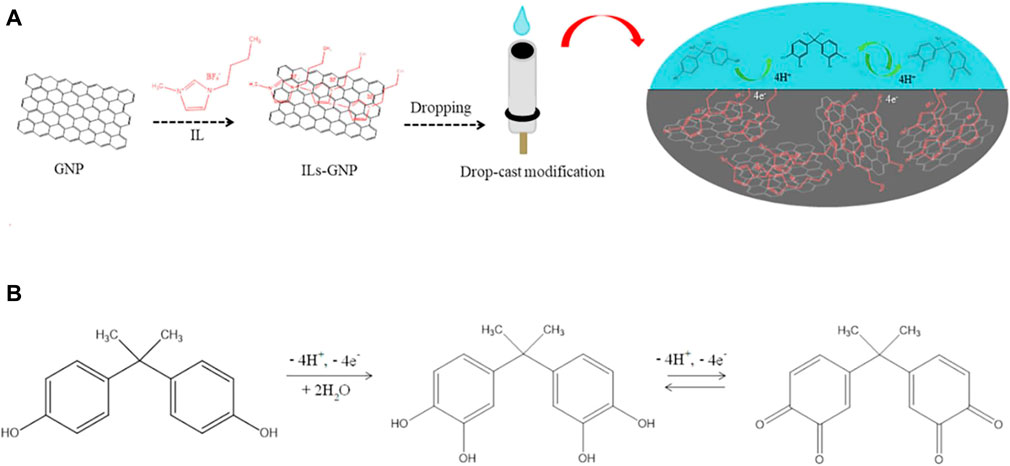
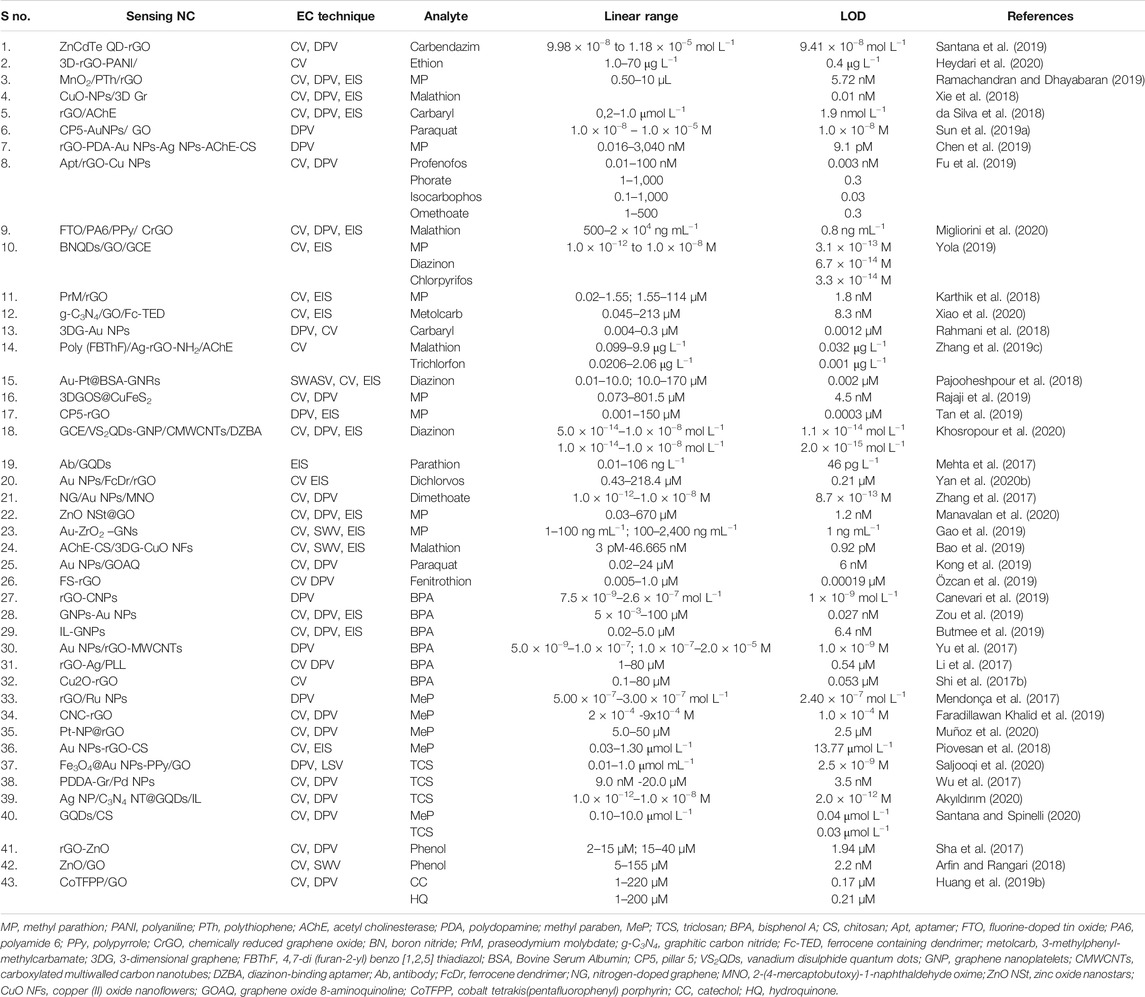
Natural Organic Matter
Nitroaromatics
Polyphenols
Nitrates and Sulfates
Although significant research on graphene synthesis has been undertaken, inherent impediments still exist. Presently, graphene-based nanomaterial preparation methods have prospects for improvement in terms of reproducibility and possible upscaling. The goal remains to synthesize functionalizable graphene-based nanomaterials which would attain preciseness and sensitivity expeditiously though produced through cost-effective means. Furthermore, the fabricated material needs to have prolonged stability and durability under ambient and environmental conditions with the potential to be used again. On the other hand, numerous nanomaterial sensors have been designed, explored, and evaluated at laboratory status but methods for upscaling of nanomaterials to massive production at low cost with satisfactory reproducibility continue to be challenging. Thus, facile but cost-effective graphene-based material synthesis approaches to enable bigger scale production are still an area to pursue (Molina et al., 2016; Nag et al., 2018; Tiwari et al., 2018; Wongkaew et al., 2019).
Utilizing graphene-based material in electrode modulation continues to have drawbacks such as fouling and nonspecific interaction, hence generating other signals. Besides that, agglomeration results in the manifestation of defects particularly when composites are formed integrating organic polymers. Another distinct challenge is the applications in real original matrices which need to be explored further to establish the influence of sample matrix (Sturala et al., 2018; Krishnan et al., 2019; Smith et al., 2019). Currently, the wholesome effects of graphene-derivatized nanomaterials on ecological systems are yet to be fully understood. Moreover, the extent of these graphene-based nanomaterials’ impact on human health is being studied. Besides, understanding the toxicity and persistence, it is prudent to prevent deposition of nanoparticle materials into aquatic media while toxicological impact and scientific knowledge gaps of nanographene and its derivatives on the environment are being reduced. Therefore, the question of the fate and dilemma of bioavailability of nanomaterials to aquatic flora and fauna in the environment needs to be interrogated further (Sharma et al., 2020; Lai et al., 2018; Huang et al., 2019a; Song et al., 2016). Apart from that, the absence of international regulatory framework and proclaimed definitions to guide the evaluation of nanomaterials toxicity on the environment, aquatic biota, and humankind retards progress on assessment. Consequently, there is a need for consensus in establishing such guidelines (Huang et al., 2019b; Song et al., 2016).
Windows of opportunities are present given that it is imperative to continuously improve protocols for graphene synthesis to achieve bulky graphene production. Besides, controlled synthesis is desirable to produce crystals of specific chemicophysical characteristics to prevent restacking of graphene and provide outstanding templates for large-scale preparation. Additionally, modification of graphene surface to mitigate against fouling and nonspecific interactions, improve reusability of materials, and enhance reproducibility and repeatability of synthesis processes requires further studying. Moreover, the assessment of selectivity, stability, and sensitivity of graphene-based nanohybrids in unpleasant conditions and complicated environmental matrices of multiplex analytes presents research possibilities. Beyond this, more research to assure the global population of the biocompatibility, zero environmental persistence, and nontoxicity status of graphene-based NMs is valuable. As such, the perpetual application of graphitic materials would hinge on research to evaluate and repress toxicity, thus guaranteeing the absence of any potential health risk to both humans and the ecosystem (Wang et al., 2020; Peña-Bahamonde et al., 2018; Lee et al., 2019a; Tiwari et al., 2018; Lawal 2018; Cinti and Arduini 2017; Willner and Vikesland 2018).
Critical method refinement for NMs development from 2D to 3D graphenic structures and utilization of 3D printing technology remains very attractive since electrical and electronic characteristics are greatly enhanced with diverse possibilities in applications. Furthermore, there is potential in miniaturization of graphene sensors and fabrication of flexible graphene sensor devices for point of analysis of pollutants. Besides, further exploration of applications of GQDs motivated by their superior properties should be rewarding for the scientific community (Molina et al., 2016; Sturala et al., 2018; Su et al., 2018; Zuo et al., 2019). Therefore, the functionalization of graphene-derived NMs with diverse materials continues to be appealing. Hence, further understanding of the interaction between analytes and hybridized graphene derivatives would be generated. Apart from that, the potential EC detection mechanisms for DBPs-inducing NOM, emerging contaminants of concern, and hazardous pesticides enlist exploration. Moreover, various innovative research initiatives coupling functionalized graphene-derived nanomaterials to different technological dimensions to develop improved and cost-effective devices for point of assessment of contaminants of concern are worthwhile.
This review evaluated recent electrochemical detection of HM ions, pesticides, and emerging pollutants utilizing different electrodes modified with graphene-derived nanocomposite materials. Apart from that, the review summarized the graphene-based materials methods of synthesis and their functionalization. Likewise, the immediate challenges being encountered in the synthesis and application of graphene nanomaterials have been outlined, thus pointing to envisaged opportunities for future research. Among others, massive scale production of graphene nanomaterials remains a dilemma, while the aspects of reproducibility as well as long-term stability of fabricated sensor systems are daunting. Meanwhile, the totality of the impact of nanosized materials including graphene and its offshoots on human health and environment is yet to be fully documented and international guidelines on ecotoxicity need to be established. Virtually, graphene-derived materials including rGO, 3D graphene, GQDs, and doped graphene remain appealing for nanocomposite synthesis and electrode modification. Enhanced sensor configurations culminate in the development of portable sensor devices with the potential for point of analysis and online monitoring of HM ions and organic and emerging pollutants in protecting different media especially food, water, and the environment.
The manuscript was written by CK, while TN, BM, NH, and AA reviewed and edited the manuscript apart from the supervisory functions. Furthermore, TN is the corresponding author on behalf of the authorship. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships or otherwise that could be perceived as a potential conflict of interest.
Financial support from the Institute for Nanotechnology and Water Sustainability (iNanoWS) of the University of South Africa and National Research Foundation (NRF) South Africa is acknowledged and appreciated.
AApt aptamer
Ab antibody
AChE acetyl cholinesterase
ASV anodic stripping voltammetry
BN boron nitride
BPA bisphenol
BSA bovine serum albumin
CA calixarene
2-CBT benzothiazole-2-carboxaldehyde
CC catechol
Cl-DNB 1-chloro-2,4-dinitrobenzene
CMWCNTs carboxylated multiwalled carbon nanotubes
CNT carbon nanotube
CrGO chemically reduced graphene oxide
CoTFPP cobalt tetrakis(pentafluorophenyl) porphyrin
CP5 pillar 5
CS chitosan
CuO NFs copper (II) oxide nanoflowers
3DG 3-dimensional graphene
DNB 1,3-dinitrobenzene
DNT 2,4-dinitrotoulene
DPV differential pulse voltammetry
DZBA diazinon-binding aptamer
EC electrochemical;
FBThF 4,7-di (furan-2-yl) benzo [1,2,5] thiadiazol
Fc-TED ferrocene containing dendrimer
FcDr ferrocene dendrimer
FTO fluorine-doped tin oxide
g-C3N4 graphitic carbon nitride
GE garlic extract
GF graphene flower
GNP graphene nanoplatelets
GOAQ graphene oxide 8-aminoquinoline
GO graphene oxider reduced graphene oxide
GO graphene oxider reduced graphene oxide
GQDs graphene quantum dots
Gr graphene
HM heavy metals
HQ hydroquinone
IL ionic liquid
LEG laser-engraved graphene
metolcarb 3-methylphenyl-methylcarbamate
MeP methyl paraben
MNO 2-(4-mercaptobutoxy)-1-naphthaldehyde oxime
MOF metal-organic framework
MP methyl parathion
NB nitrobenzene
NC nanocomposite
NG nitrogen-doped graphene
NO-Ur nitroso-uracil
4-NP p-nitrophenol
PA6 polyamide
PANI polyaniline
PCPs personal care products
PDA polydopamine
PG polyglycine
PLL poly-l-lysine
6PPy polypyrrole
PrM praseodymium molybdate
PTh polythiophene
PVP polyvinylpyrrolidone
SWV square wave voltammetry
T thiazole derivative
TCS triclosan
TNB 1,3,5-trinitrobenzene
TNT trinitrotoluene
TNP trinitrophenol
TPP tetraphenylporphyrin
VS2QDs vanadium disulphide quantum dots
ZnO NSt zinc oxide nanostars
Adekunle, A. S., Fakayode, O. J., Mamba, B. B., and Nkambule, T. T. I. (2020). Determination of humic acid (HA) and sodium alginate in water using Fe2O3 and CuO nanoparticle-modified glassy carbon electrode. Int. J. Environ. Anal. Chem. 14, 121–137. doi:10.1080/03067319.2020.1726334
Ahmad, H., Fan, M., and Hui, D. (2018). Graphene oxide incorporated functional materials: a review. Compos. B Eng. 145, 270–280. doi:10.1016/j.compositesb.2018.02.006
Akyıldırım, O. (2020). A sensitive voltammetric sensor based on silver nanoparticles/carbon nitride Nanotubes@graphene quantum dots/a novel organic liquid: determination of triclosan in wastewater. Bull. Mater. Sci. 43 (1), 22–25. doi:10.1007/s12034-020-02155-x
AL-Gahouari, H., Theeazen, L., Bodkhe, G., Sayyad, P., Ingle, N., Mahadik, M., et al. (2020). “Electrochemical sensor: L-cysteine induced selectivity enhancement of electrochemically reduced graphene oxide–multiwalled carbon nanotubes hybrid for detection of lead (Pb2+) ions. Front. Mater. 7. doi:10.3389/fmats.2020.00068
Álvarez-Ruiz, R., and Picó, Y. (2020). Analysis of emerging and related pollutants in aquatic biota. Trends Environ. Anal. Chem. 25, 111. doi:10.1016/j.teac.2020.e00082
Ambrosi, A., Chua, C. K., Latiff, N. M., Loo, A. H., Wong, C. H., Eng, A. Y., et al. (2016). Graphene and its electrochemistry - an update. Chem. Soc. Rev. 45 (9), 2458–2493. doi:10.1039/c6cs00136j
Ansari, S. H., and Arvand, H. (2020). A magnetic nanocomposite prepared from electrospun CoFe2O4 nanofibers and graphene oxide as a material for highly sensitive determination of rutin OH. Microchimica Acta. 187, 121. doi:10.1007/s00604-019-4068-3
Aragay, G., Pino, F., and Merkoçi, A. (2012). Nanomaterials for sensing and destroying pesticides. Chem. Rev. 112 (10), 5317–5338. doi:10.1021/cr300020c
Arfin, T., and Rangari, S. N. (2018). Graphene oxide-ZnO nanocomposite modified electrode for the detection of phenol. Anal. Methods. 10 (3), 347–358. doi:10.1039/c7ay02650a
Avouris, P., and Dimitrakopoulos, C. (2012). Graphene: synthesis and applications. Mater. Today. 15 (3), 86–97. doi:10.1016/S1369-7021(12)70044-5
Azzouz, A., Kumar Kailasa, S., Kumar, P., Ballesteros, E., and Kim, K. H. (2019). Advances in functional nanomaterial-based electrochemical techniques for screening of endocrine disrupting chemicals in various sample matrices. Trac. Trends Anal. Chem. 113, 256–279. doi:10.1016/j.trac.2019.02.017
Baghayeri, M., Ghanei-Motlagh, M., Tayebee, R., Fayazi, M., and Narenji, F. (2020). Application of graphene/zinc-based metal-organic framework nanocomposite for electrochemical sensing of as(III) in water resources. Anal. Chim. Acta. 1099, 60–67. doi:10.1016/j.aca.2019.11.045
Bagheri, H., Hajian, A., Rezaei, M., and Shirzadmehr, A. (2017). Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate. J. Hazard Mater. 324, 762–772. doi:10.1016/j.jhazmat.2016.11.055
Bahadir, E. B., and Sezgintürk, M. K. (2016). Applications of graphene in electrochemical sensing and biosensing. Trac. Trends Anal. Chem. 76, 1–14. doi:10.1016/j.trac.2015.07.008
Banerjee, S., McCracken, S., Hossain, M. F., and Slaughter, G. (2020). Electrochemical detection of neurotransmitters. Biosensors. 10 (8). 33. doi:10.3390/bios10080101
Bansod, B., Kumar, T., Thakur, R., Rana, S., and Singh, I. (2017). A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 94 (January), 443–455. doi:10.1016/j.bios.2017.03.031
Bao, J., Huang, T., Wang, Z., Han, Y., Geng, X., Xu, G., et al. (2019). 3D graphene/copper oxide nano-flowers based acetylcholinesterase biosensor for sensitive detection of organophosphate pesticides. Sensor. Actuator. B Chem. 279, 95–101. doi:10.1016/j.snb.2018.09.118
Basumallick, S., and Santra, S. (2017). Monitoring of ppm level humic acid in surface water using ZnO—chitosan nano-composite as fluorescence probe. Appl. Water Sci. 7 (2), 1025–1031. doi:10.1007/s13201-015-0291-1
Beitollahi, H., Safaei, M., and Tajik, S. (2019). Application of graphene and graphene oxide for modification of electrochemical sensors and Biosensors : a review. Int. J. Nano Dimens. (IJND). 10 (2), 125–140. doi:10.18494/sam.2015.1058
Bhadra, C. M., Werner, M., Baulin, V. A., Vi Khanh, T., Kobaisi, M. A., Nguyen, S. H., et al. (2018). Subtle variations in surface properties of black silicon surfaces influence the degree of bactericidal efficiency. Nano-Micro Lett. 10 (2), 1–8. doi:10.1007/s40820-017-0186-9
Bolat, G., and Abaci, S. (2018). Non-enzymatic electrochemical sensing of malathion pesticide in tomato and apple samples based on gold nanoparticles-chitosan-ionic liquid hybrid nanocomposite. Sensors. 18 (3). doi:10.3390/s18030773
Bollella, P., Fusco, G., Tortolini, C., Sanzò, G., Favero, G., Gorton, L., and Antiochia, R. (2017). Beyond graphene: electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 89, 152–166. doi:10.1016/j.bios.2016.03.068
Borrull, J., Colom, A., Fabregas, J., Pocurull, E., and Borrull, F. (2019). A simple, fast method for the analysis of 20 contaminants of emerging concern in river water using large-volume direct injection liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 411 (8), 1601–1610. doi:10.1007/s00216-019-01602-x
Butmee, P., Tumcharern, G., Saejueng, P., Stankovic, D., Ortner, A., Jitcharoen, J., et al. (2019). A direct and sensitive electrochemical sensing platform based on ionic liquid functionalized graphene nanoplatelets for the detection of bisphenol A. J. Electroanal. Chem. 833, 370–379. doi:10.1016/j.jelechem.2018.12.014
Campuzano, S., Pedrero, M., Yanez-Sedeno, P., and Pingarron, J. M. (2019). Antifouling ( bio ) materials for electrochemical ( bio ) sensing. Int. J. Mol. Sci. 20 (423), 1–19. doi:10.3390/ijms20020423
Canalejas-Tejero, V., Hernández, A. L., Casquel, R., Quintero, S. A., Laguna, M. F., and Holgado, M. (2018). Fabrication of Si3N4/SiO2 tiered resonant nanopillars with nickel caps arrays: application for optochemical sensing. Opt. Mater. Express. 8 (4), 1082. doi:10.1364/ome.8.001082
Canevari, T. C., Rossi, M. V., and Alexiou, A. D. P. (2019). Development of an electrochemical sensor of endocrine disruptor bisphenol A by reduced graphene oxide for incorporation of spherical carbon nanoparticles. J. Electroanal. Chem. 832, 24–30. doi:10.1016/j.jelechem.2018.10.044
Capoferri, D., Della Pelle, F., Del Carlo, M., and Compagnone, D. (2018). Affinity sensing strategies for the detection of pesticides in food. Foods. 7 (9), 114. doi:10.3390/foods7090148
Chang, W. D., and Baek, J.-B. (2017). Charge transport in graphene oxide. Nano Today. 17, 38–53. doi:10.1016/j.nantod.2017.10.010
Chaukura, N., Ndlangamandla, N. G., Moyo, W., Titus, A., Msagati, M., Mamba, B. B., et al. (2018). Natural organic matter in aquatic systems - a South African perspective. WaterSA. 44 (4), 624–635. doi:10.4314/wsa.v44i4.11
Chen, K. H., Pan, M. J., Jargalsaikhan, Z., Ishdorj, T. O., and Tseng, F. G. (2020). Development of surface-enhanced Raman scattering (SERS)-Based surface-corrugated nanopillars for biomolecular detection of colorectal cancer. Biosensors. 10 (11), 12–37. doi:10.3390/bios10110163.Tseng
Chen, X., Wang, J., Liu, Z., Li, Y., Huang, J., and Tao, C. A. (2019). One pot fabrication of graphene-bimetallic nanoparticles-based acetylcholinesterase electrochemical biosensor with ultralow detection limit toward methyl parathion. Mater. Res. Express. 6 (10), 39. doi:10.1088/2053-1591/ab3e84
Cheng, Y. M., Huan, B. F., Yin, W., Chang, J. H., Huo, D. Q., Fang, M., et al. (2016). A sensitive electrochemical sensor for lead based on gold nanoparticles/nitrogen-doped graphene composites functionalized with l-cysteine-modified electrode. J. Solid State Electrochem. 20 (2), 327–335. doi:10.1007/s10008-015-3043-0
Chimezie, A. B., Hajian, R., Yusof, N. A., Meng Woi, P., and Shams, N. (2017). Fabrication of reduced graphene oxide-magnetic nanocomposite (RGO–Fe3O4) as an electrochemical sensor for trace determination of as(III) in water resources. J. Electroanal. Chem. 796, 33–42. doi:10.1016/j.jelechem.2017.04.061
Cho, I.-H., Dong, H., and Park, S. (2020). Electrochemical biosensors: perspective on functional nanomaterials for on-site analysis. Biomater. Res. 24 (6), 1–12.
Cinti, S., and Arduini, F. (2017). Graphene-based screen-printed electrochemical (Bio)Sensors and their applications: efforts and criticisms. Biosens. Bioelectron. 89, 107–122. doi:10.1016/j.bios.2016.07.005
Cui, X., Fang, X., Zhao, H., Li, Z., and Ren, H. (2018). Fabrication of thiazole derivatives functionalized graphene decorated with fluorine, chlorine and iodine@SnO2 nanoparticles for highly sensitive detection of heavy metal ions. Colloid. Surface. Physicochem. Eng. Aspect. 546, 153–162. doi:10.1016/j.colsurfa.2018.03.004
Cullinan, M. P., Palmer, J. E., Carle, A. D., West, M. J., Westerman, B., and Seymour, G. J. (2015). The influence of a triclosan toothpaste on adverse events in patients with cardiovascular disease over 5-years. Sci. Total Environ. 508, 546–552. doi:10.1016/j.scitotenv.2014.11.052
da Silva, M. K. L., Vanzela, H. C., Defavari, L. M., and Cesarino, I. (2018). Determination of carbamate pesticide in food using a biosensor based on reduced graphene oxide and acetylcholinesterase enzyme. Sensor. Actuator. B Chem. 277, 555–561. doi:10.1016/j.snb.2018.09.051
Dahaghin, Z., Kilmartin, P. A., and Hassan, Z. M. (2018). Simultaneous determination of lead(II) and cadmium(II) at a glassy carbon electrode modified with GO@Fe3O4@benzothiazole-2-carboxaldehyde using square wave anodic stripping voltammetry. J. Mol. Liq. 249, 1125–1132. doi:10.1016/j.molliq.2017.11.114
Dideikin, A. T., and Vul’, A. Y. (2019). Graphene oxide and derivatives: the place in graphene family. Front. Phys. 6 (JAN). doi:10.3389/fphy.2018.00149
Dong, S., Wang, Z., Asif, M., Wang, H., Yang, Y., Hu, Y., et al. (2017). Inkjet printing synthesis of sandwiched structured ionic liquid–carbon nanotube-graphene film: toward disposable electrode for sensitive heavy metal detection in environmental water samples. Ind. Eng. Chem. Res. 56 (7), 1696–1703. doi:10.1021/acs.iecr.6b04251
Du, F., Zeng, Q., Lai, Z., Cheng, Z., and Ruan, G. (2019). Silicon doped graphene quantum dots combined with ruthenium(III) ions as a fluorescent probe for turn-on detection of triclosan. New J. Chem. 43 (33), 12907–12915. doi:10.1039/c9nj03046h
Dywili, N. R., Ntziouni, A., Ikpo, C., Ndipingwi, M., Hlongwa, N. W., Yonkeu, A. L. D., et al. (2019). Graphene oxide decorated nanometal-poly(anilino-dodecylbenzene sulfonic acid) for application in high performance supercapacitors. Micromachines. 10 (2), 114. doi:10.3390/mi10020115
Eigler, S., and Hirsch, A. (2014). Chemistry with graphene and graphene oxide-challenges for synthetic chemists. Angew Chem. Int. Ed. Engl. 53 (30), 7720–7738. doi:10.1002/anie.201402780
El-Shafai, L., Nagi, M., El-Khouly, M. E., El-Kemary, M., Ramadan, M. S., and Masoud, M. S. (2018). Graphene oxide-metal oxide nanocomposites: fabrication, characterization and removal of cationic rhodamine B dye. RSC Adv. 8 (24), 13323–13332. doi:10.1039/c8ra00977e
Fakayode, O. J., Williams, S., Saheed, A. S., and Nkambule, T. T. I. (2020). Detection of humic acid in water using flat-sheet and folded-rod viscous alkaline glucose syrups. Analyst. 145 (7), 2682–2691. doi:10.1039/c9an02083g
Fakayode, O. J., Abolanle, S. A., and Nkambule, T. T. I. (2019b). Detection of low-level humic acid in water using room temperature-synthesized copper (I) oxide colloids. MRS Commun. 1, 113. doi:10.1557/mrc.2019.128-6
Fakayode, O. J., Adekunle, A. S., and Nkambule, T. T. I. (2019a). Detection of low-level humic acid in water using room temperature-synthesized copper (I) oxide colloids. MRS Commun. 1, 27. doi:10.1557/mrc.2019.128.-6
Faradilla Wan Khalid, W. E., Mohamad Nasir, M. A., Jasmani, L., and Lee, Y. H. (2019). A new sensor for methyl paraben using an electrode made of a cellulose nanocrystal–reduced graphene oxide nanocomposite. Sensors. 19 (12), 33. doi:10.3390/s19122726
Fayemi, O. E., Abolanle, S. A., and Ebenso, E. E. (2016). A sensor for the determination of lindane using PANI/Zn, Fe (III) oxides and Nylon 6, 6/MWCNT/Zn, Fe (III) oxides nanofibers modified glassy carbon electrode. J. Nanomater. 39, 1–10. doi:10.1155/2016/4049730
Feicht, P., Biskupek, J., Gorelik, T. E., Renner, J., Halbig, C. E., Maranska, M., Puchtler, F., et al. (2019). “Brodie’s or Hummers’ Method : oxidation conditions determine the structure of graphene oxide. Chem. Eur J. 25, 8955–8959. doi:10.1002/chem.201901499
Fu, J., An, X., Yao, Y., Guo, Y., and Sun, X. (2019). Electrochemical aptasensor based on one step Co-electrodeposition of aptamer and GO-CuNPs nanocomposite for organophosphorus pesticide detection. Sensor. Actuator. B Chem. 287, 503–509. doi:10.1016/j.snb.2019.02.057
Ganesh, H. V. S., Patel, B. R., Fini, H., Kerman, A. M., and Kerman, K. (2019). Electrochemical detection of gallic acid-capped gold nanoparticles using a multiwalled carbon nanotube-reduced graphene oxide nanocomposite electrode. Anal. Chem. 91, 10116–10124. doi:10.1021/acs.analchem.9b02132.M
Gao, N., He, C., Ma, M., Cai, Z., Zhou, Y., Chang, G., Wang, X., and He, Y. (2019). Electrochemical Co-deposition synthesis of Au-ZrO2-Graphene nanocomposite for a nonenzymatic methyl parathion sensor. Anal. Chim. Acta. 1072, 25–34. doi:10.1016/j.aca.2019.04.043
Garg, R., Dutta, N. K., and Choudhury, N. R. (2014). Work function engineering of graphene. Nanomaterials. 4 (2), 267–300. doi:10.3390/nano4020267
Gęca, I., and Korolczuk, M. (2017). Anodic stripping voltammetry following double deposition and stripping steps: application of a new approach in the course of lead ion determination. Talanta. 171, 321–326. doi:10.1016/j.talanta.2017.05.008
Georgakilas, V., Otyepka, M., Bourlinos, A. B., Chandra, V., Kim, N., Kemp, K., et al. (2012). Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 112 (11), 6156–6214. doi:10.1021/cr3000412
Göde, C., Mehmet, L. Y., Yılmaz, A., Atar, N., and Wang, S. (2017). A novel electrochemical sensor based on calixarene functionalized reduced graphene oxide: application to simultaneous determination of Fe(III), Cd(II) and Pb(II) ions. J. Colloid Interface Sci. 508, 525–531. doi:10.1016/j.jcis.2017.08.086
Govindhan, M., Adhikari, B.-R., and Chen, A. (2014). RSC advances nanomaterials-based electrochemical detection of chemical contaminants. RSC Adv. 4, 63741–63760. doi:10.1039/c4ra10399h
Guan, L. Z., Zhao, L., Wan, Y. J., and Tang, L. C. (2018). Three-dimensional graphene-based polymer nanocomposites: preparation, properties and applications. Nanoscale. 10 (31), 14788–14811. doi:10.1039/c8nr03044h
Gumpu, M. B., Veerapandian, M., Krishnan, U. M., and Rayappan, J. B. (2017). Simultaneous electrochemical detection of Cd(II), Pb(II), as(III) and Hg(II) ions using ruthenium(II)-textured graphene oxide nanocomposite. Talanta. 162, 574–582. doi:10.1016/j.talanta.2016.10.076.2016
Guo, Z., Li, D. D., Luo, X. K., Zhao, Q. N., Li, M. M., Zhao, Y. T., et al. (2017). Simultaneous determination of trace Cd(II), Pb(II) and Cu(II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-l-lysine nanocomposite modified glassy carbon electrode. J. Colloid Interface Sci. 490, 11–22. doi:10.1016/j.jcis.2016.11.006
Haarhoff, J., Kubare, M., Mamba, B., Krause, R., Nkambule, T., Matsebula, B., and Menge, J. (2010). NOM characterization and removal at six southern african water treatment plants. Drink. Water Eng. Sci. 3 (1), 53–61. doi:10.5194/dwes-3-53-2010
Hang, T., Xiao, S., Cheng, Y., Li, X., Guo, C., Gen, H., et al. (2019). Hierarchical graphene/nanorods-based H2O2 electrochemical sensor with self-cleaning and anti-biofouling properties. Sensor. Actuator. B Chem. 289, 15–23. doi:10.1016/j.snb.2019.03.038.2018
Hanssen, B. L., Siraj, S., and Wong, D. K. Y. (2016). Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 35 (1), 1–28. doi:10.1515/revac-2015-0008
He, B., Shen, X. F., Nie, J., Wang, X. L., Liu, F. M., Yin, W., et al. (2018a). Electrochemical sensor using graphene/Fe3O4 nanosheets functionalized with garlic extract for the detection of lead ion. J. Solid State Electrochem. 22 (11), 3515–3525. doi:10.1007/s10008-018-4041-9
He, Q., Tian, Y., Wu, Y., Liu, J., Li, G., Deng, P., et al. (2019b). Electrochemical sensor for rapid and sensitive detection of tryptophan by a Cu2O nanoparticles-coated reduced graphene oxide nanocomposite. Biomolecules. 9, 176. doi:10.3390/biom9050176
He, Q., Tian, Y., Wu, Y., Liu, J., Li, G., and Deng, P. (2019a). Facile and ultrasensitive determination of 4-nitrophenol based on acetylene black paste and graphene hybrid electrode. Nanomaterials. 9 (429), 1–16. doi:10.3390/nano9030429
He, Q., Wu, Y., Tian, Y., Li, G., Liu, J., Deng, P., and Chen, D. (2018b). Facile electrochemical sensor for nanomolar rutin detection based on magnetite nanoparticles and reduced graphene oxide decorated electrode. Nanomaterials. 9, 115. doi:10.3390/nano9010115
Hernandez-Vargas, M., GustavoSosa-Hernández, J. E., Saldarriaga-Hernandez, S., Angel, M., Villalba-Rodríguez, A., Parra-Saldivar, R., et al. (2018). Electrochemical biosensors: a solution to pollution detection with reference to environmental contaminants. Biosensors. 8 (2), 1–21. doi:10.3390/bios8020029
Heydari, M., Saraji, M., and Jafari, M. T. (2020). Electrochemically prepared three-dimensional reduced graphene oxide-polyaniline nanocomposite as a solid-phase microextraction coating for ethion determination. Talanta. 209, 120576. doi:10.1016/j.talanta.2019.120576.2019
Hou, H., Zeinu, K. M., Gao, S., Liu, B., Yang, J., and Hu, J. (2018). Recent advances and perspective on design and synthesis of electrode materials for electrochemical sensing of heavy metals. Energy & Environmental Materials. 1 (3), 113–131. doi:10.1002/eem2.12011
Huang, D. L., Wang, J., Fan, C., Ali, A., Guo, H. S., Xiao, Y., et al. (2019a). Correction to: synergistic effect of a cobalt fluoroporphyrin and graphene oxide on the simultaneous voltammetric determination of catechol and hydroquinone. Microchim. Acta. 186 (6), 186. doi:10.1007/S00604-019-3417-6
Huang, H., Su, S., Wu, N., Wan, H., Wan, S., Bi, H., and Sun, L. (2019b). Graphene-based sensors for human health monitoring. Front. Chem. 7, 1–26. doi:10.3389/fchem.2019.00399
Hwa, K. Y., Kanna Sharma, T. S., and Ganguly, A. (2020). Design strategy of RGO-HNT-AgNPs based hybrid nanocomposite with enhanced performance for electrochemical detection of 4-nitrophenol. Inorg. Chem. Front. 7 (10), 1981–1994. doi:10.1039/d0qi00006j
Islam, M. A., Morton, D. W., Johnson, B. B., and Angove, M. J. (2020). Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: a review. Separ. Purif. Technol. 247, 116949. doi:10.1016/j.seppur.2020.116949
Jaffrezic-Renault, N., Jing, K., Tan, D., and Guo, Z. (2020). New trends in the electrochemical detection of endocrine disruptors in complex media. Anal. Bioanal. Chem. 7, 114. doi:10.1007/s00216-020-02516-9
Jan, A. T., Azam, M., Siddiqui, K., Ali, A., Choi, I., and Haq, Q. M. 2015). Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 16, 29592–29630. doi:10.3390/ijms161226183
Jariwala, D., Srivastava, A., and Ajayan, P. M. (2011). Graphene synthesis and band gap opening. J. Nanosci. Nanotechnol. 11 (8), 6621–6641. doi:10.1166/jnn.2011.5001
Jerome, R., and Sundramoorthy, A. K. (2020). Preparation of hexagonal boron nitride doped graphene film modified sensor for selective electrochemical detection of nicotine in tobacco sample. Anal. Chim. Acta. 1132, 110–120. doi:10.1016/j.aca.2020.07.060
Jiang, C., He, Y., and Liu, Y. (2020). Recent advances in sensors for electrochemical analysis of nitrate in food and environmental matrices. Analyst. 145, 5400–5413. doi:10.1039/d0an00823k
Jigyasa, C., and Kaur Rajput, J. (2018). ‘ON-OFF’ novel fluorescent chemosensors based on nanoaggregates of triaryl imidazoles for superselective detection of nitro-explosive trinitrophenol in multiple solvent systems. Sensor. Actuator. B Chem. 259: 990–1005. doi:10.1016/j.snb.2017.12.145
Jin, W., and Maduraiveeran, G. (2018). Nanomaterial-based environmental sensing platforms using state-of-the-art electroanalytical strategies. J. Anal. Sci. Technol. 9 (18), 1–11. doi:10.1186/s40543-018-0150-4
Kajornkavinkul, S., Punrat, E., Siangproh, W., Rodthongkum, N., Praphairaksit, N., and Chailapakul, O. (2016). Graphene/polyvinylpyrrolidone/polyaniline nanocomposite-modified electrode for simultaneous determination of parabens by high performance liquid chromatography. Talanta. 148, 655–660. doi:10.1016/j.talanta.2015.05.044
Karthik, R., Vinoth Kumar, J., Shen, M. C., Kokulnathan, T., Chen, T. W., Sakthinathan, S., et al. (2018). Development of novel 3D flower-like praseodymium molybdate decorated reduced graphene oxide: an efficient and selective electrocatalyst for the detection of acetylcholinesterase inhibitor methyl parathion. Sensor. Actuator. B Chem. 270, 353–361. doi:10.1016/j.snb.2018.05.054.2017
Karzi, V., Tzatzarakis, M., Katsikantami, I., Stavroulaki, A., Alegakis, A., Vakonaki, E., et al. (2019). Investigating exposure to endocrine disruptors via hair analysis of pregnant women. Environ. Res. 178, 108692. doi:10.1016/j.envres.2019.108692
Kaur, R., Rana, S., Lalit, K., Singh, P., and Kaur, K. (2020). Electrochemical detection of methyl parathion via a novel biosensor tailored on highly biocompatible electrochemically reduced graphene oxide-chitosan-hemoglobin coatings. Biosens. Bioelectron. 167, 112486. doi:10.1016/j.bios.2020.112486
Khan, M., Nawaz Tahir, M., Farooq Adil, S., Ullah Khan, H., Rafiq, M., Siddiqui, H., et al. (2015). Graphene based metal and metal oxide nanocomposites: synthesis, properties and their applications. J. Mater. Chem. 3 (37), 18753–18808. doi:10.1039/c5ta02240a
Khosropour, H., Rezaei, B., Rezaei, P., and Ensafi, A. A. (2020). Ultrasensitive voltammetric and impedimetric aptasensor for diazinon pesticide detection by VS2 quantum dots-graphene nanoplatelets/carboxylated multiwalled carbon nanotubes as a new group nanocomposite for signal enrichment. Anal. Chim. Acta. 1111, 92–102. doi:10.1016/j.aca.2020.03.047
Kokulnathan, T., Sakthinathan, S., and Chen, S-M. (2018). Hexammine cobalt (III) coordination complex grafted reduced graphene oxide composite for sensitive and selective electrochemical. Inorg. Chem. Front. 5, 1145–1155. doi:10.1039/c8qi00055g
Kong, F. Y., Li, R. F., Yao, L., Wang, Z. X., Li, H. Y., Wang, W. J., and Wang, W. (2019). A novel electrochemical sensor based on Au nanoparticles/8-aminoquinoline functionalized graphene oxide nanocomposite for paraquat detection. Nanotechnology. 30 (28), 285502. doi:10.1088/1361-6528/ab10ac
Koçak, B., Er, E., and Çelikkan, H. (2015). Stripping voltammetric analysis of dicofol on graphene-modified glassy carbon electrode. Ionics. 21 (8), 2337–2344. doi:10.1007/s11581-015-1407-1
Krishnan, S. K., Singh, E., Singh, P., Meyyappan, M., and Singh Nalwa, H. (2019). A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 9 (16), 8778–8781. doi:10.1039/c8ra09577a
Kumar, A., Purohit, B., Maurya, P. K., Pandey, L. M., and Chandra, P. (2019). Engineered nanomaterial assisted signal-amplification. Electroanalysis. 31, 1615–1629. doi:10.1002/elan.201900216
Kumar, V., Kim, K. H., Park, J. W., Hong, J., and Kumar, S. (2017). Graphene and its nanocomposites as a platform for environmental applications. Chem. Eng. J. 315, 210–232. doi:10.1016/j.cej.2017.01.008
Kuralay, F., Tunç, S., Bozduman, F., Oksuz, L., and Oksuz, A. U. (2016). Biosensing applications of titanium dioxide coated graphene modified disposable electrodes. Talanta. 160, 325–331. doi:10.1016/j.talanta.2016.07.037
Lai, R. W. S., Yeung, K. W. Y., Yung, M. M. N., Djurišić, A. B., Giesy, J. P., and Kenneth, M. Y. (2018). Regulation of engineered nanomaterials: current challenges, insights and future directions. Environ. Sci. Pollut. Res. Int. 25 (4), 3060–3077. doi:10.1007/s11356-017-9489-0
Lalmalsawmi, J., Tiwari, D., and Dong, J. (2020). Role of nanocomposite materials in the development of electrochemical sensors for arsenic: past, present and future. J. Electroanal. Chem. 877, 114630. doi:10.1016/j.jelechem.2020.114630.Kim
Latif, A., Bilal, M., Asghar, W., Azeem, M., Ahmad, M. I., Abbas, A., et al. (2018). Heavy metal accumulation in vegetables and assessment of their potential health risk. J. Environ. Anal. 5 (1), 1–7. doi:10.4172/2380-2391.1000234
Lawal, A. T. (2018). Progress in utilisation of graphene for electrochemical biosensors. Biosens. Bioelectron. 106, 149–178. doi:10.1016/j.bios.2018.01.030
Lee, C.-S., Su, H., and Kim, T. H. (2018). One-step electrochemical fabrication of reduced graphene oxide/gold nanoparticles nanocomposite-modified electrode for simultaneous detection of dopamine, ascorbic acid, and uric acid. Nanomaterials. 8 (17), 112. doi:10.3390/nano8010017
Lee, C. W., Suh, J. M., and Jang, H. W. (2019a). Chemical sensors based on two-dimensional (2D) materials for selective detection of ions and molecules in liquid. Front. Chem. 7, 1–21. doi:10.3389/fchem.2019.00708
Lee, J. H., Park, S. J., and Choi, J. W. (2019c). Electrical property of graphene and its application to electrochemical biosensing. Nanomaterials. 9 (2), 7. doi:10.3390/nano9020297
Lee, J-H., Park, S.-J., and Choi, J.-W. (2019b). Electrical property of graphene and its application to electrochemical biosensing. Nanomaterials. 9, 297–315. doi:10.3390/nano9020297
Lee, K. Y., Ambrosi, A., and Martin, P. (2017). 3D-Printed metal electrodes for heavy metals detection by anodic stripping voltammetry. Electroanalysis. 29 (11), 2444–2453. doi:10.1002/elan.201700388
Lehutso, R. F., Daso, A. P., and Okonkwo, J. O. (2017). Occurrence and environmental levels of triclosan and triclocarban in selected wastewater treatment plants in gauteng Province, South Africa. Emerging Contaminants. 3 (3), 107–114. doi:10.1016/j.emcon.2017.07.001
Lei, C., Zhang, S., and Zhao, S. (2017). Synthesis of N-doped graphene for simultaneous electrochemical detection of lead and copper in water. Int. J. Electrochem. Sci. 12 (6), 4856–4866. doi:10.20964/2017.06.03
Li, D., Tan, W., Li, Z., Xu, X., and Wang, C. (2020). “Application of graphene-based materials for detection of nitrate and nitrite in water — a review. Sensors. 20 (54), 1–35
Li, F., Jiang, X., Zhao, J., and Zhang, S. (2015). “Graphene Oxide : a promising nanomaterial for energy and environmental applications. Nanomater. Energy. 16, 488–515. doi:10.1016/j.nanoen.2015.07.014
Li, H., Huang, C., Li, Y., and Yang, W. (2019). Electrocatalytic reduction of trace nitrobenzene using a graphene-oxide@polymerized-manganese-porphyrin composite. RSC Adv. 9, 22523–22530. doi:10.1039/c9ra02932j
Li, J., Zhang, S., Chen, C., Zhao, G., Yang, X., Li, J., and Wang, X. (2012). Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces. 4, 4991–5000. doi:10.1021/am301358b
Li, Y., Wang, H., Yan, B., and Zhang, H. (2017). An electrochemical sensor for the determination of bisphenol A using glassy carbon electrode modified with reduced graphene oxide-silver/poly-L-lysine nanocomposites. J. Electroanal. Chem. 805, 39–46. doi:10.1016/j.jelechem.2017.10.022
Lin, X., Lu, Z., Dai, W., Liu, B., Zhang, Y., Li, J., and Ye, J. (2018). Laser engraved nitrogen-doped graphene sensor for the simultaneous determination of Cd(II) and Pb(II). J. Electroanal. Chem. 828, 41–49. doi:10.1016/j.jelechem.2018.09.016
Lingamdinne, L. P., and Reddy Koduru, J. (2018). Magnetic graphene oxide composites are the solutions for sustainable remediation of ecosystems. Environ. Anal. Ecol. Stud. 2, 2–5. doi:10.31031/EAES.2018.02.000528
Lingamdinne, L. P., Koduru, J. R., and Karri, R. R. (20192018). A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. J. Environ. Manag. 231, 622–634. doi:10.1016/j.jenvman.2018.10.063
Liu, F. M., Zhang, Y., Yin, W., Chang, J. H., Huo, D. Q., He, Bin., et al. (2017). A high–selectivity electrochemical sensor for ultra-trace lead (II) detection based on a nanocomposite consisting of nitrogen-doped graphene/gold nanoparticles functionalized with ETBD and Fe3O4@TiO2 core–shell nanoparticles. Sensor. Actuator. B Chem. 242, 889–896. doi:10.1016/j.snb.2016.09.167
Liu, X., Li, Z., Ding, R., Ren, B., and Li, Y. (2016). A nanocarbon paste electrode modified with nitrogen-doped graphene for square wave anodic stripping voltammetric determination of trace lead and cadmium. Microchimica Acta. 183 (2), 709–714. doi:10.1007/s00604-015-1713-3
Ma, C., Chen, M., Liu, H., Wu, K., He, H., and Wang, K. (2018). A rapid method for the detection of humic acid based on the poly(thymine)-templated copper nanoparticles. Chin. Chem. Lett. 29 (1), 136–138. doi:10.1016/j.cclet.2017.09.012
Magesa, F., Wu, Y., Tian, Y., Mary Vianney, J., Buza, J., He, Q., et al. (2019). Graphene and graphene like 2D graphitic carbon nitride: electrochemical detection of food colorants and toxic substances in environment. Trends Environ. Anal. Chem. 23, e00064. doi:10.1016/j.teac.2019.e00064
Mahmud, M. A. P., Ejeian, F., Azadi, S., Myers, M., Pejcic, B., Abbassi, R., et al. (2020). Recent progress in sensing nitrate , nitrite , Phosphate , and ammonium in aquatic environment. Chemosphere. 259, 127492. doi:10.1016/j.chemosphere.2020.127492
Malakootian, M., Hamzeh, S., and Mahmoudi-moghaddam, H. (2020). A new electrochemical sensor for simultaneous determination of Cd ( II ) and Pb ( II ) using FeNi3/CuS/BiOCl : RSM optimization. Microchem. J. 158, 1–10. doi:10.1016/j.microc.2020.105194
Manavalan, S., Veerakumar, P., Chen, S. M., and King, C. (2020). Three-dimensional zinc oxide nanostars anchored on graphene oxide for voltammetric determination of methyl parathion. Microchimica Acta. 187 (1), 33. doi:10.1007/s00604-019-4031-3
Manikandan, V. S., Sidhureddy, B., Thiruppathi, A. R., and Chen, A. (2019). Sensitive electrochemical detection of caffeic acid in wine based on fluorine-doped graphene oxide. Sensors. 19 (1604), 1–16. doi:10.3390/s19071604
Marton, M., Michniak, P., Behul, M., Rehacek, V., Vojs Stanova, A., Redhammer, R., et al. (2019). Bismuth modified boron doped diamond electrode for simultaneous determination of Zn, Cd and Pb ions by square wave anodic stripping voltammetry: influence of boron concentration and surface morphology. Vacuum. 167, 182–188. doi:10.1016/j.vacuum.2019.06.012
Mehta, J., Bhardwaj, N., Bhardwaj, S. K., Tuteja, S. K., Vinayak, P., Paul, A. K., et al. (2017). Graphene quantum dot modified screen printed immunosensor for the determination of parathion. Anal. Biochem. 523, 1–9. doi:10.1016/j.ab.2017.01.026
Mendonça, C. D., Prado, T. M., Cincotto, F. H., Verbinnen, R. T., and Sergio, A. S. M (2017). Methylparaben quantification via electrochemical sensor based on reduced graphene oxide decorated with ruthenium nanoparticles. Sensor. Actuator. B Chem. 251, 739–745. doi:10.1016/j.snb.2017.05.083
Migliorini, F. L., Sanfelice, R. C., Mercante, L. A., Facure, M. H. M., and Correa, D. S. (2020). Electrochemical sensor based on polyamide 6/polypyrrole electrospun nanofibers coated with reduced graphene oxide for malathion pesticide detection. Mater. Res. Express. 7 (1). doi:10.1088/2053-1591/ab5744
Mohan, V. B., Lau, K. T., Hui, D., and Bhattacharyya, D. (2018). Graphene-based materials and their composites: a review on production, applications and product limitations. Compos. B Eng. 142, 200–220. doi:10.1016/j.compositesb.2018.01.013
Molina, J., Cases, F., and Moretto, L. M. (2016). Graphene-based materials for the electrochemical determination of hazardous ions. Anal. Chim. Acta. 946, 9–39. doi:10.1016/j.aca.2016.10.019
Molinari, R., and Argurio, P. (2017). Arsenic removal from water by coupling Photocatalysis and complexation-ultrafiltration processes: a Preliminary study. Water Res. 109, 327–336. doi:10.1016/j.watres.2016.11.054
Montaseri, H., and Forbes, P. B. C. (2018). A triclosan turn-ON fluorescence sensor based on thiol-capped core/shell quantum dots. Spectrochim. Acta Mol. Biomol. Spectrosc. 204, 370–379. doi:10.1016/j.saa.2018.06.043
Moro, G., De Wael, K., and Maria Moretto, L. (2019). Electrochemistry challenges in the electrochemical (bio) sensing of nonelectroactive food and environmental contaminants. Current Opinion in Electrochemistry. 16, 57–65. doi:10.1016/j.coelec.2019.04.019
Moyo, M., Florence, L. R., and Okonkwo, J. O. (2015). Improved electro-oxidation of triclosan at nano-zinc oxide-multiwalled carbon nanotube modified glassy carbon electrode. Sensor. Actuator. B Chem. 209, 898–905. doi:10.1016/j.snb.2014.12.059
Moyo, W., Chaukura, N., Titus, A., Msagati, M., Mamba, B. B., Heijman, S. G. J., and Nkambule, T. T. I. (2019). The properties and removal efficacies of natural organic matter fractions by South African drinking water treatment plants. J. Environ. Chem. Eng. 7 (3), 103101. doi:10.1016/j.jece.2019.103101
Muñoz, J., Álvarez-Prada, I., Lopez-Lopez, E., Escriche, L., Romero, N., Sala, X., et al. (2020). Synthesis of 0D to 3D hybrid-carbon nanomaterials carrying platinum(0) nanoparticles: towards the electrocatalytic determination of methylparabens at ultra-trace levels. Sensor. Actuator. B Chem. 305, 127467. doi:10.1016/j.snb.2019.127467
Murugan, C., Murugan, N., Sundramoorthy, A. K., and Sundaramurthy, A. (2020). “Gradient triple-layered ZnS/ZnO/Ta2O5−SiO2 core−shell nanoparticles for enzyme-based electrochemical detection of cancer biomarkers. ACS Appl. Nano Mater. 3, 8461–8471. doi:10.1021/acsanm.0c01949
Muthoosamy, K., and Manickam, S. (2017). State of the art and recent advances in the ultrasound-assisted synthesis, exfoliation and functionalization of graphene derivatives. Ultrason. Sonochem. 39, 478–493. doi:10.1016/j.ultsonch.2017.05.019
Nag, A., Mitra, A., and Mukhopadhyay, S. C. (2018). Graphene and its sensor-based applications: a review. Sens. Actuators, A. 270, 177–194. doi:10.1016/j.sna.2017.12.028
Nagarajan, R. D., and Sundramoorthy, A. K. (2019). One-pot electrosynthesis of silver nanorods/graphene nanocomposite using 4-sulphocalix [4] arene for selective detection of oxalic acid. Sensor. Actuator. B Chem. 301, 127132. doi:10.1016/j.snb.2019.127132
Nagarani, S., Sasikala, G., Satheesh, K., Yuvaraj, M., and Jayavel, R. (2018). Synthesis and characterization of binary transition metal oxide/reduced graphene oxide nanocomposites and its enhanced electrochemical properties for supercapacitor applications. J. Mater. Sci. Mater. Electron. 29 (14), 11738–11748. doi:10.1007/s10854-018-9272-0
Nkambule, T. I., Krause, R. W., Mamba, B. B., and Haarhoff, J. (2009). Removal of natural organic matter from water using ion-exchange resins and cyclodextrin Polyurethanes. Phys. Chem. Earth. 34 (13–16), 812–818. doi:10.1016/j.pce.2009.07.013
Nkambule, T. I., Krause, R. W. M., Haarhoff, J., and Mamba, B. B. (2012). Natural organic matter (NOM) in South African waters: NOM characterisation using combined assessment techniques. WaterSA. 38 (5), 697–706. doi:10.4314/wsa.v38i5.7
Numan, A., Gill, A. A. S., Rafique, S., Guduri, M., Zhan, Y., Maddiboyina, B., et al. (2020). Rationally engineered nanosensors: a novel strategy for the detection of heavy metal ions in the environment. J. Hazard Mater. 7, 124493. doi:10.1016/j.jhazmat.2020.124493
Oliveira, T. M. B. F., and Morais, S. (2018). New generation of electrochemical sensors based on multi-walled carbon nanotubes. Appl. Sci. 8 (10), 5–7. doi:10.3390/app8101925
Oliveira, T. M. B. F., Ribeiro, F. W. P., Sousa, C. P., Salazar-Banda, G. R., Pedro de Lima-Neto, A. N. C., and Morais, S. (2020). Current overview and perspectives on carbon-based (Bio)Sensors for carbamate pesticides electroanalysis. Trac. Trends Anal. Chem. 124, 115779. doi:10.1016/j.trac.2019.115779
Orr, S. E., and Bridges, C. C. (2017). Chronic kidney disease and exposure to nephrotoxic metals. Int. J. Mol. Sci. 18 (1039), 1–35. doi:10.3390/ijms18051039
Ould Ne, M. L., Abbassi, A., El hachimi, A. G., Benyoussef, A., Ez-Zahraouy, H., and El Kenz, A. (2017). Electronic optical, properties and widening band gap of graphene with Ge doping. Opt. Quant. Electron. 49 (6), 1–13. doi:10.1007/s11082-017-1024-5
Özcan, A., Topçuoğulları, D., and Atılır Özcan, A. (2019). Fenitrothion sensing with reduced graphene oxide decorated fumed silica nanocomposite modified glassy carbon electrode. Sensor. Actuator. B Chem. 284, 179–185. doi:10.1016/j.snb.2018.12.122
Pajooheshpour, N., Rezaei, M., Ali, H., Afkhami, A., Sillanpää, M., Arduini, F., et al. (2018). Protein templated Au–Pt nanoclusters-graphene nanoribbons as a high performance sensing layer for the electrochemical determination of diazinon. Sensor. Actuator. B Chem. 275, 180–189. doi:10.1016/j.snb.2018.08.014
Pandey, S. K., Sachan, S., and Singh, S. K. (2019). Ultra-trace sensing of cadmium and lead by square wave anodic stripping voltammetry using ionic liquid modified graphene oxide. Mater. Sci. Energy Technol. 2 (3), 667–675. doi:10.1016/j.mset.2019.09.004
Pang, Y. H., Huang, Y. Y., Wang, L., Shen, X. F., and Wang, Y. Y. (2020). Determination of bisphenol A and bisphenol S by a covalent organic framework electrochemical sensor. Environ. Pollut. 263, 114616. doi:10.1016/j.envpol.2020.114616
Pavitt, A. S., and Tratnyek, P. G. (2019). Electrochemical characterization of natural organic matter by direct voltammetry in an aprotic solvent. Environ. Sci.: Processes Impacts. 21 (10), 1664–1683. doi:10.1039/c9em00313d
Pei, J., Xiang, Y., Zhang, Z., Zhang, J., Wei, S., and Boukherroub, R. (2020). In-Situ graphene modified self-supported boron-doped diamond electrode for Pb(II) electrochemical detection in seawater. Appl. Surf. Sci. 527, 146761. doi:10.1016/j.apsusc.2020.146761
Pelin Böke, C., Karaman, O., Medetalibeyoglu, H., Karaman, C., Atar, N., and Lütfi Yola, M. (2020). A new approach for electrochemical detection of organochlorine compound lindane: development of molecular imprinting polymer with polyoxometalate/carbon nitride nanotubes composite and validation. Microchem. J. 157, 105012. doi:10.1016/j.microc.2020.105012
Pellicer-Castell, E., Belenguer-Sapiña, C., Amorós, P., El Haskouri, J., Herrero-Martínez, J. M., Mauri-Aucejo, A. R., et al. (2020). Comparison of silica-based materials for organophosphorus pesticides sampling and occupational risk assessment. Anal. Chim. Acta. 1110, 26–34. doi:10.1016/j.aca.2020.03.008
Peña-Bahamonde, J., Nguyen, H. N., Fanourakis, S. K., and Rodrigues, D. F. (2018). Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 16 (1), 75–17. doi:10.1186/s12951-018-0400-z
Perreault, F., Fonseca de Faria, A., and Elimelech, M. (2015). Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 44 (16), 5861–5896. doi:10.1039/c5cs00021a
Piovesan, J. V., Santana, E. R., and Spinelli, A. (2018). Reduced graphene oxide/gold nanoparticles nanocomposite-modified glassy carbon electrode for determination of endocrine disruptor methylparaben. J. Electroanal. Chem. 813, 163–170. doi:10.1016/j.jelechem.2018.02.025
Pratush, A., Kumar, A., and Hu, Z. (2018). Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: a review. Int. Microbiol. 21, 97–106. doi:10.1007/s10123-018-0012-3
Priya, T., Dhanalakshmi, N., Thennarasu, S., and Thinakaran, N. (2018). A novel voltammetric sensor for the simultaneous detection of Cd. Carbohydr. Polym. 182, 199–206. doi:10.1016/j.carbpol.2017.11.0172017
Qin, L., Zeng, G., Cui, L., Huang, D., Zhang, C., Cheng, M., et al. (2019). Science of the total environment synthetic strategies and application of gold-based nanocatalysts for nitroaromatics reduction. Sci. Total Environ. 652, 93–116. doi:10.1016/j.scitotenv.2018.10.215
Qu, X., Alvarez, P. J. J., and Qilin, Li. (2013). Applications of Nanotechnology in water and wastewater treatment. Water Res. 47 (12), 3931–3946. doi:10.1016/j.watres.2012.09.058
Rahmani, T.., Bagheri, H.., Behbahani, M., Ali, H., and Afkhami, A. (2018). Modified 3D graphene-Au as a novel sensing layer for direct and sensitive electrochemical determination of carbaryl pesticide in fruit, vegetable, and water samples. Food Anal. Methods. 11 (11), 3005–3014. doi:10.1007/s12161-018-1280-4
Rajaji, U., Murugan, K., Shen, M., Govindasamy, M., Chen, T. W., Lin, P. H., et al. (2019). Graphene oxide encapsulated 3D porous chalcopyrite (CuFeS2) nanocomposite as an emerging electrocatalyst for agro-hazardous (methyl Paraoxon) detection in vegetables. Compos. B Eng. 160, 268–276. doi:10.1016/j.compositesb.2018.10.042
Ramachandran, A., and Karunakaran Yesodha, S. (2019). Polyaniline-derived nitrogen-doped graphene quantum dots for the ultratrace level electrochemical detection of trinitrophenol and the effective differentiation of nitroaromatics: structure matters. ACS Sustain. Chem. Eng. 7, 6732–6743. doi:10.1021/acssuschemeng.8b05996
Ramachandran, T., and Dhayabaran, V. (2019). Utilization of a MnO2/polythiophene/RGO nanocomposite modified glassy carbon electrode as an electrochemical sensor for methyl parathion. J. Mater. Sci. Mater. Electron. 30 (13), 12315–12327. doi:10.1007/s10854-019-01590-9
Raril, C., and Manjunatha, J. G. (2020). Fabrication of novel polymer-modified graphene-based electrochemical sensor for the determination of mercury and lead ions in water and biological samples. J. Anal. Sci. Technol. 11 (1). doi:10.1186/s40543-019-0194-0
Ray, S. K., Majumder, C., and Saha, P. (2017). Functionalized reduced graphene oxide (FRGO) for removal of fulvic acid contaminant. RSC Adv. 7 (35), 21768–21779. doi:10.1039/c7ra01069a
Rehman, K., Fatima, F., Waheed, I., and Akash, M. S. H. 20182017). Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 119, 157–184. doi:10.1002/jcb.26234
Ren, H., Zhang, Y., Liu, L., Li, Y., Wang, D., Zhang, R., et al. (2019). Synthesis of hollow Mo2C/carbon spheres, and their application to simultaneous electrochemical detection of hydroquinone, catechol, and resorcinol. Microchimica Acta. 186 (5). doi:10.1007/s00604-019-3432-7
Roosmini, D., Notodarmojo, S., and Sururi, M. R. (2018). The characteristic of natural organic matter (NOM) of water from cikapundung river Pond. IOP Conf. Ser. Earth Environ. Sci. 160 (1). doi:10.1088/1755-1315/160/1/012021
Rowley-Neale, S., Samuel, J., Randviir, E. P., AhmedAbo Dena, S., and Banks, C. E. (2018). An overview of recent applications of reduced graphene oxide as a Basis of electroanalytical sensing platforms. Appl. Mater. Today. 10, 218–226. doi:10.1016/j.apmt.2017.11.010
Ruengpirasiri, P., Punrat, E., Chailapakul, O., and Chuanuwatanakul, S. (2017). Graphene oxide-modified electrode coated with in-Situ antimony film for the simultaneous determination of heavy metals by sequential injection-anodic stripping voltammetry. Electroanalysis. 29 (4), 1022–1030. doi:10.1002/elan.201600568
Sakthinathan, S., and Chen, S. M. (2015). Graphene supported nanocomposite for electrochemical detection of pollutant materials: a short review. Int. J. Electrochem. Sci. 10 (8), 6527–6536. doi:10.21741/9781644900956-8
Sakthinathan, S., Kubendhiran, S., Shen, M., and Tamizhdurai, P. (2016). Reduced graphene oxide/gold tetraphenyl porphyrin (RGO/Au-TPP) nanocomposite as an ultrasensitive amperometric sensor for environmentally toxic hydrazine. RSC Adv. 6 (61), 56375–56383. doi:10.1039/c6ra09129f.Chen
Salih, E., Mekawy, M., and Hassan, R. Y. A. (2016). Synthesis , characterization and electrochemical-sensor applications of zinc oxide/graphene oxide nanocomposite. J. Nanostruct. Chem. 6 (2), 137–144. doi:10.1007/s40097-016-0188-z
Saljooqi, A., Shamspur, T., and Ali, M. (2020). A sensitive electrochemical sensor based on graphene oxide nanosheets decorated by Fe3O4@Au nanostructure stabilized on polypyrrole for efficient triclosan sensing. Electroanalysis. 32 (6), 1297–1303. doi:10.1002/elan.201900634
Santana, E. R., and Spinelli, A. (2020). Electrode modified with graphene quantum dots supported in chitosan for electrochemical methods and non-linear deconvolution of spectra for spectrometric methods: approaches for simultaneous determination of triclosan and methylparaben. Mikrochim. Acta. 187 (4), 250–312. doi:10.1007/s00604-020-04225-7
Santana, P., Lima, J., Santana, T., Santos, L., Matos, C., da Costa, L., et al. (2019). Semiconductor nanocrystals-reduced graphene composites for the electrochemical detection of carbendazim. J. Braz. Chem. Soc. 30 (6), 1302–1308. doi:10.21577/0103-5053.20190026
Särkkä, H., Vepsäläinen, M., and Sillanpää, M. (2015). Natural organic matter (NOM) removal by electrochemical methods - a review. J. Electroanal. Chem. 755, 100–108. doi:10.1016/j.jelechem.2015.07.029
Sarno, M., Scudieri, C., and Ponticorvo, E. (2019). Flower-like AuPtPd-Fe3O4 nanocatalyst for electrochemical removal of humic acids and Cr(VI). Chem. Eng. Trans. 73, 229–234. doi:10.3303/CET1973039
Sgobbi, L. F., and Machado, S. A. S. (20182017). Functionalized Polyacrylamide as an acetylcholinesterase-inspired Biomimetic device for electrochemical sensing of organophosphorus pesticides. Biosens. Bioelectron. 100, 290. doi:10.1016/j.bios.2017.09.019
Sha, R., Kumar Puttapati, S., VSS Srikanth, V., and Badhulika, S. (2017). Ultra-sensitive phenol sensor based on overcoming surface fouling of reduced graphene oxide-zinc oxide composite electrode. J. Electroanal. Chem. 785, 26–32. doi:10.1016/j.jelechem.2016.12.001
Shahzad, F., Zaidi, S. A., and Koo, C. M. (2017). Synthesis of multifunctional electrically tunable fluorine-doped reduced graphene oxide at low temperatures. ACS Appl. Mater. Interfaces. 9 (28), 24179–24189. doi:10.1021/acsami.7b05021
Sharma, P., Nain, R., Chaudhary, S., and Kumar, R. (2020). Architectonical frameworks of porous graphene: a potential stratagem to combat nanocontamination in the environment. Environ. Nanotechnol. Monitor. Manag. 14, 100298. doi:10.1016/j.enmm.2020.100298
Sharma, S., and Bhattacharya, A. (2017). Drinking water contamination and treatment techniques. Appl. Water Sci. 7 (3), 1043–1067. doi:10.1007/s13201-016-0455-7
Shende, P., Sardesai, M., and Gaud, R. S. (2018). “Micro to nanoneedles: a trend of modernized transepidermal drug delivery system.” artificial cells. Nanomed. Biotechnol. 46 (1), 19–25. doi:10.1080/21691401.2017.1304409
Shi, L., Li, Y., Rong, X., Wang, Y., and Ding, S. (2017a). Facile fabrication of a novel 3D graphene framework/Bi nanoparticle film for ultrasensitive electrochemical assays of heavy metal ions. Anal. Chim. Acta. 968, 21–29. doi:10.1016/j.aca.2017.03.013
Shi, R., Liang, J., Zhao, Z., Liu, A., and Tian, Y. (2017b). An electrochemical bisphenol A sensor based on one step electrochemical reduction of cuprous oxide wrapped graphene oxide nanoparticles modified electrode. Talanta. 169, 37–43. doi:10.1016/j.talanta.2017.03.042
Shtepliuk, I., and Yakimova, R. (2019). Interaction of epitaxial graphene with heavy metals: towards novel sensing platform. Nanotechnology. 30 (29, 294002). doi:10.1088/1361-6528/ab1546
Si, Y., Liu, J., Chen, Y., Miao, X., Ye, F., Liu, Z., et al. (2018). RGO/AuNPs/Tetraphenylporphyrin nanoconjugate-based electrochemical sensor for highly sensitive detection of cadmium ions. Anal. Methods. 10 (29), 3631–3636. doi:10.1039/c8ay01020j
Sillanpää, M., Chaker Ncibi, M., Matilainen, A., and Vepsäläinen, M. (2018). Removal of natural organic matter in drinking water treatment by coagulation: a comprehensive review. Chemosphere. 190, 54–71. doi:10.1016/j.chemosphere.2017.09.113
Silwana, B., Van Der Horst, C., Iwuoha, E., and Somerset, V. (2016). A Brief review on recent developments of electrochemical sensors in environmental application for PGMs. J. Environ. Sci. Health. A Tox. Hazard Subst. Environ. Eng. 51 (14), 1233–1247. doi:10.1080/10934529.2016.1212562
Sk, S., Rahaman, A., Shahadat, M., Basu, S., Shaikh, Z. A., and Wazed Ali, S. (2019). Polyaniline/carbon nanotube-graphite modified electrode sensor for detection of bisphenol A. Ionics. 25 (6), 2857–2864. doi:10.1007/s11581-018-2807-9
Smith, A. T., Anna, M. L. C., Songshan, Z., Bin, L., and Luyi, S. (2019). Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 1 (1), 31–47. doi:10.1016/j.nanoms.2019.02.004
Song, Y., Luo, Y., Zhu, C., Li, H., Du, D., and Lin, Y. (2016). Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 76, 195–212. doi:10.1016/j.bios.2015.07.002
Stortini, A. M., Antonietta Baldo, M., Moro, G., Polo, F., and Maria Moretto, L. (2020). Bio- and biomimetic receptors for electrochemical sensing of heavy metal ions. Sensors. 20, 1–29. doi:10.3390/s20236800
Sturala, J., Jan, L., Martin, P., and Sofer, Z. (2018). Chemistry of graphene derivatives: synthesis, applications, and perspectives. Chem. Eur J. 24 (23), 5992–6006. doi:10.1002/chem.201704192
Su, S., Chen, S., and Fan, C. (2018). Recent advances in two-dimensional nanomaterials-based electrochemical sensors for environmental analysis. Green Energy and Environment. 3 (2), 97–106. doi:10.1016/j.gee.2017.08.005
Suherman, A. L., Tanner, E. E. L., and Compton, R. G. (2017). Recent developments in inorganic Hg2+ detection by voltammetry. Trac. Trends Anal. Chem. 94, 161–172. doi:10.1016/j.trac.2017.07.020
Sun, J., Guo, F., Shi, Q., Wu, H., Sun, Y., Chen, M., and Diao, G. (2019a). Electrochemical detection of paraquat based on silver nanoparticles/water-soluble pillar[5]Arene functionalized graphene oxide modified glassy carbon electrode. J. Electroanal. Chem. 847 (May), 113221. doi:10.1016/j.jelechem.2019.113221
Sun, Y. F., Jian-Wang, Y., Pei, H., Yang, M., and Xing, J. H. (2019b). Highly sensitive electrochemical detection of Pb(II) based on excellent adsorption and surface Ni(II)/Ni(III) cycle of porous flower-like NiO/RGO nanocomposite. Sensor. Actuator. B Chem. 292, 136–147. doi:10.1016/j.snb.2019.04.131Li
Suvarnaphaet, P., and Pechprasarn, S. (2017). Graphene-based materials for biosensors: a review. Sensors. 17 (10), 33. doi:10.3390/s17102161
Suvina, V., Kokulnathan, T., Wang, T. J., and Balakrishna, R. G. (2020). Lanthanum cobaltite supported on graphene nanosheets for non-enzymatic electrochemical determination of catechol. Mikrochim. Acta. 187 (189), 189–197. doi:10.1007/s00604-020-4165-3
Talirz, L., Ruffieux, P., and Fasel, R. (2016). On-surface synthesis of atomically precise graphene nanoribbons. Adv Mater Weinheim. 28 (29), 6222–6231. doi:10.1002/adma.201505738
Tan, X., Liu, Y., Zhang, T., Luo, S., Liu, X., Tian, H., et al. (2019). Ultrasensitive electrochemical detection of methyl parathion pesticide based on cationic water-soluble pillar[5]Arene and reduced graphene nanocomposite. RSC Adv. 9 (1), 345–353. doi:10.1039/C8RA08555B
Teixeira, P. R., Machado, T. R., Machado, F., Sodré, F. F., Silva, J. G., Neto, B. A. D., et al. (2020). Au nanoparticle-poly(ionic liquid) nanocomposite electrode for the voltammetric detection of triclosan in lake water and toothpaste samples. Microchem. J. 152, 104421. doi:10.1016/j.microc.2019.104421
Thangamuthu, M., Hsieh, K. Yu., Kumar, P. V., and Guan, Y. (2019). Graphene- and graphene oxide-based nanocomposite platforms for electrochemical biosensing applications. Int. J. Mol. Sci. 20 (12), 1–25. doi:10.3390/ijms20122975
Theyagarajan, K., Elancheziyan, M., Aayushi, P. S., and Thenmozhi, K. (2020a). Facile strategy for immobilizing horseradish peroxidase on a novel acetate functionalized ionic liquid/MWCNT matrix for electrochemical biosensing. Int. J. Biol. Macromol. 163, 358–365. doi:10.1016/j.ijbiomac.2020.07.005
Theyagarajan, K., Yadav, S., Satija, J., Thenmozhi, K., and Senthilkumar, S. (2020b). Gold nanoparticle-redox ionic liquid based nanoconjugated matrix as a novel multifunctional biosensing interface. ACS Biomater. Sci. Eng. 6, 6076–6085. doi:10.1021/acsbiomaterials.0c00807
Thiruppathi, A. R., Sidhureddy, B., Keeler, W., and Chen, A. (2017). Facile one-pot synthesis of fluorinated graphene oxide for electrochemical sensing of heavy metal ions. Electrochem. Commun. 76, 42–46. doi:10.1016/j.elecom.2017.01.015
Tian, B., Kou, Y., Jiang, X., Lu, J., Xue, Y., Wang, M., and Tan, L. (2020). Ultrasensitive determination of mercury ions using a glassy carbon electrode modified with nanocomposites consisting of conductive polymer and amino-functionalized graphene quantum dots. Mikrochim. Acta. 187 (4), 210. doi:10.1007/s00604-020-4191-1
Tian, X., Liu, L., Li, Y., Yang, C., Zhou, Z., Nie, Y., et al. (2018). Nonenzymatic electrochemical sensor based on CuO–TiO2 for sensitive and selective detection of methyl parathion pesticide in ground water. Sensor. Actuator. B Chem. 256, 135–142. doi:10.1016/j.snb.2017.10.066
Tiwari, S. K., Raghvendra, K. M., Sung Kyu, H., and Andrzej, H. (2018). Evolution of graphene oxide and graphene: from imagination to industrialization. ChemNanoMat. 4 (7), 598–620. doi:10.1002/cnma.201800089
Turkaslan, B. E., and Mihrace, S. A. (2020). Optimizing parameters of graphene derivatives synthesis by modified improved Hummers. Math. Methods Appl. Sci. 7, 13. doi:10.1002/mma.6704
Ullah, N., Mansha, M., Khan, I., and Qurashi, A. (2018). Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: recent advances and challenges. Trac. Trends Anal. Chem. 100, 155–166. doi:10.1016/j.trac.2018.01.002
Venu, M., Venkateswarlu, S., Reddy, Y. V. M., Seshadri Reddy, A., Gupta, V. K., Yoon, M., and Madhavi, G. (2018). Highly sensitive electrochemical sensor for anticancer drug by a zirconia nanoparticle-decorated reduced graphene oxide nanocomposite. ACS Omega. 3 (11), 14597–14605. doi:10.1021/acsomega.8b02129
Waheed, A., Mansha, M., and Ullah, N. (2018). Nanomaterials-based electrochemical detection of heavy metals in water: current status, challenges and future direction. Trac. Trends Anal. Chem. 105, 37–51. doi:10.1016/j.trac.2018.04.012
Wan, X., Lei, M., and Chen, T. (2020). Review on remediation technologies for arsenic- contaminated soil. Front. Environ. Sci. Eng. 14 (2), 1–14
Wang, H., Yuan, X., Zeng, G., Wu, Y., Liu, Y., Jiang, Q., and Gu, S. (2015). Three dimensional graphene based materials: synthesis and applications from energy storage and conversion to electrochemical sensor and environmental remediation. Adv. Colloid Interface Sci. 221, 41–59. doi:10.1016/j.cis.2015.04.005
Wang, L., Peng, X., Fu, H., Huang, C., Li, Y., and Liu, Z. (2020). Recent advances in the development of electrochemical aptasensors for detection of heavy metals in food. Biosens. Bioelectron. 147, 111777. doi:10.1016/j.bios.2019.111777
Wang, L., Kim, J., and Cui, T. (2018). Self-assembled graphene and copper nanoparticles composite sensor for nitrate determination. Microsyst. Technol. 24 (9), 3623–3630. doi:10.1007/s00542-018-3792-7
Wang, Y., Zhao, S., Li, M., Li, W., Zhao, Y., Qi, J., and Cui, X. (2017). Graphene quantum dots decorated graphene as an enhanced sensing platform for sensitive and selective detection of copper(II). J. Electroanal. Chem. 797, 113–120. doi:10.1016/j.jelechem.2017.05.031
Wen, G. L., Zhao, W., Chen, X., Jia, Q., Wang, Y., Zhang, Y., et al. (2018). N-doped reduced graphene oxide/MnO2 nanocomposite for electrochemical detection of Hg2+ by square wave stripping voltammetry. Electrochim. Acta. 291, 95–102. doi:10.1016/j.electacta.2018.08.121
Willner, M. R., and Vikesland, P. J. (2018). Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 16, 95. doi:10.1186/s12951-018-0419-1.-16
Wongkaew, N., Simsek, M., Griesche, C., Baeumner, A. J., and Baeumner, (2019). Functional nanomaterials and nanostructures enhancing electrochemical biosensors and lab-on-a-chip performances: recent progress, applications, and future perspective. Chem. Rev. 119 (1), 120–194. doi:10.1021/acs.chemrev.8b00172
Wu, T., Li, T., Liu, Z., Guo, Y., and Dong, C. (2017). Electrochemical sensor for sensitive detection of triclosan based on graphene/palladium nanoparticles hybrids. Talanta. 164, 556–562. doi:10.1016/j.talanta.2016.12.027
Xiao, F., Li, H., Yan, X., Yan, L., Zhang, X., Wang, M., et al. (2020). Graphitic carbon nitride/graphene oxide(g-C3N4/GO) nanocomposites covalently linked with ferrocene containing dendrimer for ultrasensitive detection of pesticide. Anal. Chim. Acta. 1103, 84–96. doi:10.1016/j.aca.2019.12.066
Xiao, L., Wang, B., Ji, L., Wang, F., Yuan, Q., Hu, G., et al. (2016). An efficient electrochemical sensor based on three-dimensionally interconnected mesoporous graphene framework for simultaneous determination of Cd(II) and Pb(II). Electrochim. Acta. 222, 1371–1377. doi:10.1016/j.electacta.2016.11.113
Xie, Y., Yu, Y., Lu, L., Xue, M., Gong, L., Huang, X., et al. (2018). CuO nanoparticles decorated 3D graphene nanocomposite as non-enzymatic electrochemical sensing platform for malathion detection. J. Electroanal. Chem. 812, 82–89. doi:10.1016/j.jelechem.2018.01.043
Xu, J., Wang, Y., and Hu, S. (2017). Nanocomposites of graphene and graphene oxides: synthesis, molecular functionalization and application in electrochemical sensors and biosensors. A review. Microchimica Acta. 184 (1), 1–44. doi:10.1007/s00604-016-2007-0
Yan, L., Yan, X., Li, H., Zhang, X., Wang, M., Fu, S., et al. (2020a). Reduced graphene oxide nanosheets and gold nanoparticles covalently linked to ferrocene-terminated dendrimer to construct electrochemical sensor with dual signal amplification strategy for ultra-sensitive detection of pesticide in vegetable. Microchem. J. 157, 105016. doi:10.1016/j.microc.2020.105016
Yan, L., Yan, X., Li, H., Zhang, X., Wang, M., Fu, S., et al. (2020b). Reduced graphene oxide nanosheets and gold nanoparticles covalently linked to ferrocene-terminated dendrimer to construct electrochemical sensor with dual signal amplification strategy for ultra-sensitive detection of pesticide in vegetable. Microchem. J. 157, 105016. doi:10.1016/j.microc.2020.105016
Yan, Z., Yao, W., Hu, L., Liu, D., Wang, C., and Lee, C. S. (2015). Progress in the preparation and application of three-dimensional graphene-based porous nanocomposites. Nanoscale. 7 (13), 5563–5577. doi:10.1039/c5nr00030k
Ye, T., Chen, W., Xu, H., Geng, N., and Cai, Y. (2018). Preparation of TiO2/graphene composite with appropriate N-doping ratio for humic acid removal. J. Mater. Sci. 53 (1), 613–625. doi:10.1007/s10853-017-1509-4
Yilong, Z., Zhao, D., and Li, D. (2015). Electrochemical and other methods for detection and determination of dissolved Nitrite : a review. Int. J. Electrochem. Sci. 10, 1144–1168. doi:10.3403/00082406u
Yin, P. T., Shah, S., Chhowalla, M., and Lee, K. B. (2015). Design, synthesis, and characterization of graphene-nanoparticle hybrid materials for bioapplications. Chem. Rev. 115 (7), 2483–2531. doi:10.1021/cr500537t
Yola, M. L., Atar, N., Eren, T., Karimi-Maleh, H., and Wang, S. (2015). Sensitive and selective determination of aqueous triclosan based on gold nanoparticles on polyoxometalate/reduced graphene oxide nanohybrid. RSC Adv. 5 (81), 65953–65962. doi:10.1039/c5ra07443f
Yola, M. L. (2019). Electrochemical activity enhancement of monodisperse boron nitride quantum dots on graphene oxide: its application for simultaneous detection of organophosphate pesticides in real samples. J. Mol. Liq. 277, 50–57. doi:10.1016/j.molliq.2018.12.084
Yu, H., Xiao, F., Chen, X. X., Jin, L., Gao, X. L., Xu, N., et al. (2017). Electrochemical determination of bisphenol A on a glassy carbon electrode modified with gold nanoparticles loaded on reduced graphene oxide-multi walled carbon nanotubes composite. Chin. J. Anal. Chem. 45 (5), 713–720. doi:10.1016/S1872-2040(17)61014-4
Yu, L., Xu, Q., Jin, D., Zhang, Q., Mao, A., Shu, Y., et al. (2016). Highly sensitive electrochemical determination of sulfate in PM2.5 based on the formation of heteropoly blue at poly-l-lysine-functionalized graphene modified glassy carbon electrode in the presence of cetyltrimethylammonium bromide. Chem. Eng. J. 294, 122–131. doi:10.1016/j.cej.2016.02.063
Zaaba, N. I., Foo, K. L., Hashim, U., Tan, S. J., Liu, Wei. Wen., and Voon, C. H. (2017). Synthesis of graphene oxide using modified Hummers method: solvent influence. Procedia Eng. 184, 469–477. doi:10.1016/j.proeng.2017.04.118
Zamora-Sequeira, R., Starbird-Pérez, R., Rojas-Carillo, O., and Vargas-Villalobos, S. (2019). What are the main sensor methods for quantifying pesticides in agricultural activities? A review. Molecules. 24 (14), 1–26. doi:10.3390/molecules24142659
Zeng, W., Manoj, D., Sun, H., Yi, R., Huang, X., and Sun, Y. (2019). One-pot synthesis of high-density Pd nanoflowers decorated 3D carbon nanotube-graphene network modified on printed electrode as portable electrochemical sensing platform for sensitive detection of nitroaromatic explosives. J. Electroanal. Chem. 833, 527–535. doi:10.1016/j.jelechem.2018.12.028
Zhang, P., Sun, T., Rong, S., Zeng, D., Yu, H., Zhang, Z., et al. (2019a). A sensitive amperometric AChE-biosensor for organophosphate pesticides detection based on conjugated polymer and Ag-rGO-NH. Bioelectrochemistry. 127, 163–170. doi:10.1016/j.bioelechem.2019.02.003
Zhang, R., Zhang, C., Zheng, F., Li, X., and Sun, C.-L. (2018a). Nitrogen and sulfur Co-doped graphene nanoribbons : a novel metal-free catalyst for high performance electrochemical detection of 2,4,6-trinitrotoluene (TNT). Carbon. 126, 328–337. doi:10.1016/j.carbon.2017.10.042
Zhang, W., Wang, L., Yang, Y., Paul, G., and Teng, K. S. (2019b). Recent AdVances on electrochemical sensors for the detection of organic disinfection byproducts in water. ACS Sens. 4 (5), 1138–1150. doi:10.1021/acssensors.9b00272
Zhang, Y., Huan, B., He, B., Chang, J., Huo, D. Q., Tian, C., et al. (2017). Electrochemical biomimetic sensor based on oxime group-functionalized gold nanoparticles and nitrogen-doped graphene composites for highly selective and sensitive dimethoate determination. J. Solid State Electrochem. 21 (7), 2117–2128. doi:10.1007/s10008-017-3560-0FaHouXia
Zhang, Y. N., Niu, Q., Gu, X., Yang, N., and Zhao, G. (2019c). Recent progress on carbon nanomaterials for the electrochemical detection and removal of environmental pollutants. Nanoscale. 11 (25), 11992–12014. doi:10.1039/c9nr02935d
Zhang, Y., Chu, G., Guo, Y., Zhao, W., Yang, Q., and Sun, X. (2018b). An electrochemical biosensor based on Au nanoparticles decorated reduced graphene oxide for sensitively detecting of Hg2+. J. Electroanal. Chem. 824, 201–206. doi:10.1016/j.jelechem.2018.07.053
Zhang, Z., Fu, X., Li, K., Liu, R., Peng, D., He, L., et al. (2016). One-step fabrication of electrochemical biosensor based on DNA-modified three-dimensional reduced graphene oxide and chitosan nanocomposite for highly sensitive detection of Hg(II). Sensor. Actuator. B Chem. 225, 453–462. doi:10.1016/j.snb.2015.11.091
Zhao, G., Wang, H., Liu, G., Wang, Z., and Cheng, J. (2017). Simultaneous determination of trace Cd(II) and Pb(II) based on Bi/Nafion/Reduced graphene oxide-gold nanoparticle nanocomposite film-modified glassy carbon electrode by one-step electrodeposition. Ionics. 23 (3), 767–777. doi:10.1007/s11581-016-1843-6
Zhao, T., Fang, M., Tang, Z., Zhao, X., Xie, F., Wu, F., et al. (2019). Effects of fulvic acid on aggregation, sedimentation, and adsorption of Fe3O4 magnetic nanoparticles. Environ. Sci. Pollut. Control Ser. 26 (21), 21463–21474. doi:10.1007/s11356-019-05441-2
Zheng, J., Zhang, M., Yang, L., Xu, J., Hu, S., Hayat, T., et al. (2018). Fabrication of one dimensional CNTs/Fe3O4@PPy/Pd magnetic composites for the accumulation and electrochemical detection of triclosan. J. Electroanal. Chem. 818, 97–105. doi:10.1016/j.jelechem.2018.04.026
Zheng, J., Ma, J., Wang, Z., Xu, S., David Waite, T., and Wu, Z. (2017). Contaminant removal from source waters using cathodic electrochemical membrane filtration: mechanisms and implications. Environ. Sci. Technol. 51 (5), 2757–2765. doi:10.1021/acs.est.6b05625
Zhou, S. F., Xiao, J., Fan, H. L., Huang, J., and Liu, Y. Q. (2018). Enhanced electrochemical performance for sensing Pb(II) based on graphene oxide incorporated mesoporous MnFe2O4 nanocomposites. J. Alloys Compd. 747, 447–454. doi:10.1016/j.jallcom.2018.03.037Han
Zhuang, X., Tian, C., Luan, F., Wu, X., and Chen, L. (2016). One-step electrochemical fabrication of a nickel oxide nanoparticle/polyaniline nanowire/graphene oxide hybrid on a glassy carbon electrode for use as a non-enzymatic glucose biosensor. RSC Adv. 6 (95), 92541–92546. doi:10.1039/c6ra14970g
Zou, J., Guo, Q., Teng, J., Qi, L., Jiang, X. Y., Jiao, F. P., and Jin, G. Y. (2019). Highly sensitive detection of bisphenol A in real water samples based on in-situ assembled graphene nanoplatelets and gold nanoparticles composite. Microchem. J. 145, 693–702. doi:10.1016/j.microc.2018.11.040
Keywords: cyclic voltammetry, electrochemical detection, emerging pollutants, functionalization, graphene derivatives, reduced graphene oxide, heavy metal ions, pesticides
Citation: Kumunda C, Adekunle AS, Mamba BB, Hlongwa NW and Nkambule TTI (2021) Electrochemical Detection of Environmental Pollutants Based on Graphene Derivatives: A Review. Front. Mater. 7:616787. doi: 10.3389/fmats.2020.616787
Received: 13 October 2020; Accepted: 28 December 2020;
Published: 15 February 2021.
Edited by:
Ashok K. Sundramoorthy, SRM Institute of Science and Technology, IndiaReviewed by:
Tu Binh Minh, VNU University of Science, VietnamCopyright © 2021 Kumunda, Adekunle, Mamba, Hlongwa and Nkambule. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thabo T. I. Nkambule, bmthbWJ0dEB1bmlzYS5hYy56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.