- 1Laboratory of Technologies for Heritage Materials, Institute of Chemistry, University of Neuchâtel, Neuchâtel, Switzerland
- 2Haute Ecole Arc Conservation-restauration, University of Applied Sciences and Arts HES-SO, Neuchâtel, Switzerland
A biopassivation method has been proposed as a new ecological and sustainable solution for preserving copper-based artefacts using selected microorganisms. This technology is based on the natural capacity of some fungi to form copper oxalates on the corroded surface of copper alloys. Existing unstable and pulverulent corrosion products are converted into an insoluble and chemically stable biopatina that provides the treated surfaces with a stabilization of the corrosion process and an aesthetically attractive green color. This treatment allows to stabilize the active corrosion induced for example by copper chlorides and to prevent green staining of surrounding materials (stone pedestal, wall…) due the leaching and loss of pulverulent and water-soluble corrosion products (i.e. copper sulfates). The behavior and performance of the proposed treatment have been followed during natural aging procedures, which have shown that this method stabilizes the corrosion process while having less chromatic variation compared to more traditional protective systems. The application protocol was then validated on real cases such as outdoor sculptures and archaeological objects. Based on these results, a ready-to-use kit is now proposed to conservators.
Introduction
In recent decades, biological methods have been increasingly proposed as ecological and sustainable alternatives for the heritage conservation, in particular for the cleaning of frescoes and ornamental stones (De Muynck et al., 2010; Troiano et al., 2013) Indeed, there is a real interest in biotechnological applications, respectful of the environment (at room pressure and temperature) and using no hazardous substances (Bharde et al., 2006). Designing such alternative methods, particular attention should be then paid to durability, efficiency and toxicity. Metal-microbes interactions are thus widely studied, especially in the bio-alteration of minerals (Bonneville et al., 2009). Furthermore, this ability to accumulate and precipitate metals was successfully applied to remediate to soil pollution or as waste treatment (Gharieb et al., 2004; Gadd, 2010). In addition, advances in corrosion inhibition using microbial films have been illustrated as an innovative strategy to protect metallic substrates (Videla and Herrera, 2005; Kuklinski and Sand, 2014).
For the past fifteen years or so, we have been exploiting the ability of a unique fungal strain to create copper oxalate patinas on archaeological and historical objects made of copper alloys. Just as the “patine du temps”, composed of calcium oxalates, forms on stone monuments, copper oxalates were observed on open air bronze monuments, while it is known that these compounds are not part of the cyclic corrosion occurring on outdoor artefacts (Graedel, 1987). On the contrary, they exhibit high chemical stability and insolubility even in an acidic atmosphere (pH 3). Matte, homogenous and cohesive films are thus created, providing bronze surfaces with a natural-aged appearance and protection against corrosion (Marabelli and Mazzeo, 1993).
Article Types
Methods article.
Materials and Equipment
The biopassivation treatment is proposed as a ready-to-use kit composed of a gelified culture of Beauveria bassiana. To this purpose, the formulation is prepared with the following elements:
• Tube A containing a culture of B. bassiana,
• Tube B containing a nutrient solution,
• Tube C containing a specific thickening agent.
In average, 2.5 L of formulation are enough to cover surface of 1 m2. All kit elements, excepted tube A, can be stored at room temperature. The culture of B. bassiana can be stored at 4°C for over six months. When ready, the whole formulation can be stored at 4°C for over six months. The treatment can be applied between 10 and 30°C and would be optimal at 20°C.
Given prior research illustrated the versatility of gel systems tested, such as carboxycellulose-based gel Metylan, a specific xanthan-type gel is here employed (Domon-Beuret et al., 2015). However, conservators are free to choose other delivery systems according to their own experience. Here, class 2- microorganisms with weak risk are employed in a water solution and therefore the treatment is not considered to be toxic or pathogenic. However, it is possible sensitized persons encountered allergic reactions in case of inhalation or irritation in case of contact with skin. To wear gloves and face masks is recommended for this specific group of persons.
Methods
Objectives
The proposed method was standardized with the aim to create stable and protective biogenic layers on corroded copper and bronze surfaces. Criteria such as efficiency, retreatability and impact on visual appearance were taken into account, adressing issues reported on the commonly used protective systems (Appelbaum, 2012). In order to move towards more sustainable conservation products, green engineering is also considered (Anastas and Zimmerman, 2003). In fact, organic coatings, represented by waxes or acrylic resins together with corrosion inhibitors are applied in a non-selective way without specifc adaption to the metal conservation field, and can be hazardous to health and the environment (Dillmann et al., 2007).
Validation of the Method
During previous studies, this fungal strain was obtained by isolation from wine fields treated for centuries with copper-based pesticides. Nearly all copper hydroxysulfates or copper hydroxychlorides present were converted to copper oxalates (Mazzeo et al., 2008). The efficacy of this strain was then more deeply studied and the properties (i.e. mechanism of formation and adhesion) of the copper oxalates formed after reactive copper phases and corroded coupons were carefully evaluated (Joseph et al., 2011; Joseph et al., 2012a). An examination of cross sections suggested that the original patina, whether it formed in an urban or marine environment, is completely converted to copper oxalates and this conversion lead to a compact and adherent protective patina of few-microns thickness and composed of copper oxalates (Joseph et al., 2012b). Since 2013, a biopassivation treatment, called biopatina, has been assessed on various natural and artificial patina in urban and marine environments and compared to treatments traditionally used on outdoor bronzes, such as Cosmoloid H80 wax. Two natural exposure sites with a maximum corrosivity class were selected, allowing to evaluate the long-term performance of the treatments studied: an urban-marine environment in Genoa (CNR-ISMAR experimental marine station) and a marine environment in Brest in collaboration with the French Institute of Corrosion. Properties, such as appearance, corrosion inhibition and surface cohesion were assessed with a regular monitoring over a period of 18 months (repeated in the case of the station in Genoa). The results obtained suggest a different behavior of biopatina in comparison with wax, with a lower chromatic variation and better corrosion stabilization and lower degradation than wax after exposure to outdoor aggressive environments (Joseph et al., 2013; Joseph et al., 2014; Albini et al., 2016a). How biopassivation interacts with selected foundry patinas was evaluated as well. Patinated bronze coupons have been treated and exposed on the roof of the University of Neuchatel according to ISO9223 standard during 24 months. After ageing, electrochemical measurements indicates that biopatina-treated areas were stable in terms of corrosiveness while artificial patinas did not arrive yet to such an equilibrium state with the exposure environment (Letardi et al., 2018). Bio-passivation was thus effective in the conversion of incohesive patinas into stable copper oxalates layers, avoiding the leach out of toxic products in the environment (Joseph et al., 2015; Albini et al., 2016b). In parallel with these weathering procedures, an application protocol for archaeological objects was carefully defined and a series of coupons were either treated with biopatina or benzotriazole for comparison. Almost all copper hydroxychlorides were converted into copper oxalates by biopassivation. On the contrary, only few BTA-Cu complexes were formed, failing to provide corrosion inhibition (Albini et al., 2018).
Step-by-Step Procedure
The treatment is carried out in three phases. Prior to intervention, the area to be treated must be cleaned of dust and exogenous deposits (i.e., from pressurized air/water, sandblasting, and cryogenic cleaning) and residual materials from prior treatments removed. All surfaces and material used for the application must be clean with ethanol or isopropyl alcohol 70% (w/w) in deionized water.
Phase 1-Preparation of the Formulation
Put together the tubes A and B in a container (previously cleaned with ethanol 70%). With tweezers (previously cleaned with ethanol 70%), get ride off the white filaments and leave the remaining solution with only whitish flakes. Add then the thickening dose C slowly under strong agitation (sieving the powder can help in this operation to avoid lumps). The gel is ready to be used. For better results, keep the gel some hours at 4°C as it will get thicker. Put the filaments taken off and paper used for cleaning in a plastic waste container and before throwing away, spray ethanol 70% on them (waste treatment to be checked according to each country regulations).
Phase 2-Application
Spray the surface with ethanol 70% (w/w in deionized water) and, if desired, dry with cold air. Apply on the surface the gel with a thickness of 3 mm. May be applied by hand or using plastic spatula. Cover the treated surface with micro-perforated plastic film (and/or wet gauze to avoid extra drying of the gel) and let undisturbed for at least 1 week. No further applications are necessary, and the treatment’s duration is adapted to the thickness of the corrosion layer to be treated. Extra time up to 3 weeks might change the result on thin corrosion layers (less than 10 microns).
In case of outdoor intervention, protect the treated area from sun and rain (i.e. transparent plastic greenhouse, hermetic container). Avoid drying and keep a high humidity atmosphere (min. 60%) could be achieved by water spray or with a humidifier (shut on 1/4 h off).
In case of treatment of small objects, these latter can be stored in hermetic boxes during treatment. The humidity produced will be enough to avoid desiccation.
Check after 72 h that small white spots appeared on the surface of the formulation indicating the treatment works fine. After one week, the whole treated surface should be covered with white snow clusters. The formulation will gradually become green and should be kept wet.
In case white spots are not visible after maximum 72 h (this has been observed with objects highly contaminated with copper chlorides), repeat the application with fresh formulation till the apparition of the white spots.
Phase 3-End of the Treatment
Take off as much as possible of the gel by hand in a plastic waste container and before throwing away, spray ethanol 70% all over (waste treatment to be checked according to each country regulations). Wash the treated surface with pressurized water or alternatively (when water should be avoided) dry with compressed air. Eventual residues of formulation can be eliminated by brushing.
Results and Discussion
Diverse copper-based heritage has been successfully treated with this biogenic patina, preventing further degradation to occur. Additional purposes are also foreseen, such as to level the differently corroded areas with a homogenous visual appearance, to achieve pre-patination of raw metal surfaces intended to be exposed to corrosive environments or to offer decorative patinas.
Such an example is the intervention in Lausanne, Switzerland on 36 bronze sculptures by the artist Sara. H that present foundry patina. This group of sculptures, referred as Légende d’Automne, has been displayed on the Schnetzler promenade since 2015. These artworks comprised 18 stations, each composed of a scene and a lectern. Microcrystalline wax was applied to nine stations, and a double layer of biopassivation and wax was applied to the other stations (Figure 1). This allowed to compare the level of protection offered by bio-based treatment respect to traditional protective systems applied on outdoor bronze artefacts. After three years of outdoor exposure, a diagnostic campaign was carried out with colorimetry and Electrochemical Impedance Spectroscopy (Letardi et al., 2018). All measurements showed the presence of Cu oxalates formed after the biopatina treatment on the surface. A minimal color difference was observed between the two differently treated groups, suggesting that the majority of wax was removed by rain runoff. Similar EIS results were observed for surfaces with wax or biopassivation + wax. However, a better repeatability was obtained with the bio-based method indicating a more homogeneous protection of the surfaces. From this study, biopassivation proved to be an excellent pre-treatment for organic coatings that have usually a short shelf life (i.e. 2 years for microcrystalline waxes), allowing to have an prolonged preservation period on outdoor sculptures.

FIGURE 1. Biopassivation method applied on the Légende d’Automne sculptures (Sara.H, 2015) located in Lausanne, Switzerland, detail of a station: (A) before biopassivation treatment applied (B) right after treatment, and (C) after 3 years of exposure.
In addition, a bronze sculpture by Hugo Siegwart (The wrestler group, 1908) that is exhibited in Lucerne, Switzerland, have been entirely treated by biopassivation. The formation of both copper and tin oxalates was demonstrated on sheltered and unsheltered areas. This intervention allowed the differently corroded parts, such as non-aesthetic drips, to gain a more homogeneous post-treatment surface appearance (Figure 2). The outermost part of the corrosion layer was modified as insoluble compounds, preventing staining of adjacent materials. Additional sculptures were tested using this procedure during professional continuous learning workshops, such as the deer group, located in La Chaux-de-Fonds, Switzerland. Bio-passivation was effective in the conversion of incohesive patinas into stable copper oxalates layers, avoiding the leach out of toxic products in the environment (Figure 3).

FIGURE 2. Biopassivation method applied on the Wrestler group (H. Siegwart, 1908) located in Lucerne, Switzerland: (A). Back of the sculpture with unaesthetical drips visible before treatment and details after treatment from (B) the back and (C) the legs.
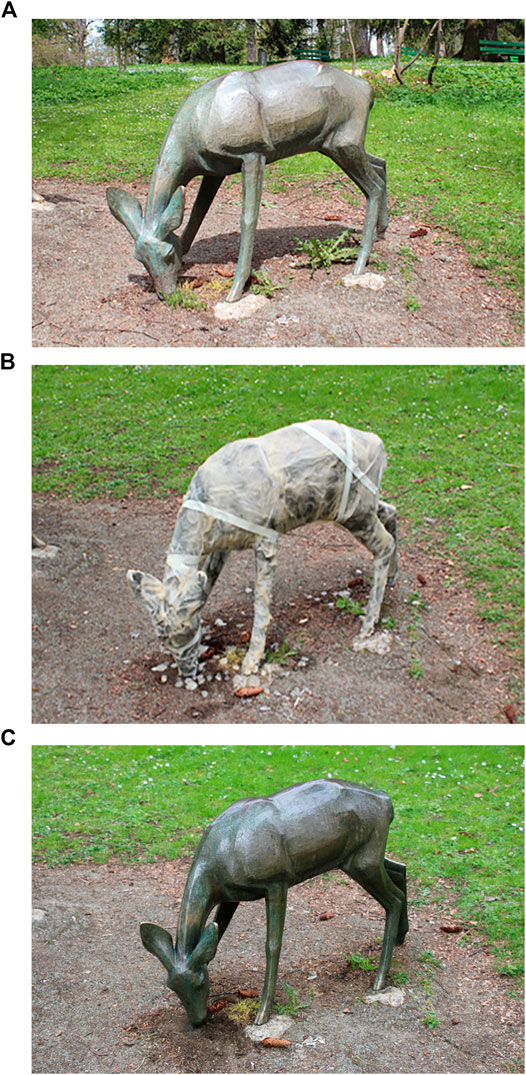
FIGURE 3. Biopassivation method applied on the deer group located in la Chaux-de-Fonds, Switzerland: (A) before (B) during and (C) after treatment.
Biopassivation was also developed for the remediation of active corrosion as an alternative green corrosion inhibitor for copper-based archaeological objects. Artefacts that presented active corrosion induced by chlorides were tested during professional continuous learning workshops and/or in collaboration with different heritage institutions in Switzerland, as for example the Laténium park and museum of Archaeology. Using the same protocol as described above, the behavior of the objects was monitored during treatment. In these occasions, end-users evaluated whether any further damage occurred or novel salt efflorescences appeared in the active corrosion areas. Slight color changes after biopassivation were observed but less than the color shift achieved with organic coatings. In particular, the particulars and decorations on the objects were nicely preserved and sometimes even highlighted. To evaluate the efficiency of the different inhibitors applied, artificial aging procedure simulating an uncontrolled indoor environment was applied on a set of sacrificial objects. After 30 cycles of aging, all items treated with benzotriazole or biopatina displayed a few changes in the visual appearance, while the untreated objects showed corrosion regrowth. These results demonstrated that active corrosion had been inhibited and that biopassivation can be considered as a green alternative corrosion inhibitor. It is worth mention that some peculiar objects from Swiss archaeological collections were examined after a five-years semi air-conditioned storage (uncontrolled, ventilation only, RH = 30–60%). After storage, a visual assessment of the objects allowed to ascertain the stability of the objects treated with the biopassivation method (Figure 4).

FIGURE 4. An Osiris statue of the collections of the Musée d’Ethnographie Neuchâtel, Switzerland, treated with bio-passivation method to stabilize its corrosive layer: (A) before and (B) after treatment, and (C) after 5-years storage in uncontrolled conditions. Courtesy of Emmanuelle Domon Beuret, Laténium park and museum of Archaeology.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
EJ as principal investigator, contributed to conceptualization, formal analysis, validation, writing—original draft preparation, writing—review and editing, supervision, project administration, funding acquisition.
Funding
These works were supported by the European Union, within the VII Framework Program (Contract:BAHAMAS, PIEF-GA-2009-252759, 2010-2012); the Swiss Commission for the Technology and Innovation (Grant Number 14573.2 PFLS-LS); the Swiss National Science Foundation (Grant Numbers PZ00P2_142514, 2013-2016; PP00P2_163653/1, 2016-2020; 205121_188755, 2020-2024); the Gebert Rüf Stiftung (Grant Number 054/12, 2013-2015); the Stiftung für Förderung der Denkmalpflege (Grant New ecological and sustainable solution for protecting architectural metals using an ecologically friendly biological treatment, 2015-2018); and the Réseau de Compétences Design et Arts visuels, University of Applied Sciences Western Switzerland HES-SO (Grant Number 44499, 2015-2018).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author, as head of the Laboratory of Technologies for Heritage Materials (LATHEMA) and Associate Professor at Haute Ecole Arc Conservation-restauration (HE-Arc CR) is thankful to LATHEMA and HE-Arc collaborators for their support in methodology, investigation and data curation on this specific research topic: Monica Albini-PhD student (end 2017), Lucrezia Comensoli-PhD student (end 2018), Emmanuelle Domon Beuret-conservator, HE-Arc research assistant (2013–2014), Wafa Kooli-PhD student (end 2018), Mathilde Monachon-PhD student, Julie Schröter-conservator, HE-Arc research Assistant (2017–2018), Laura Brambilla-Scientific collaborator (2013–2014), Lydia Mathys-Technician. The protocol described here is presented as sent to conservators with the biopatina ready-to-use kit and as reported in annex of the PhD thesis of M. Albini. The author would like to thank Paola Letardi from CNR-ISMAR, Institute of Marine Sciences, Genoa, Italy for her close collaboration with electrochemical measurements and ageing procedures performed in Genoa; Olivier Berger from Art Metal Conservation and Wolf Meyer zu Bargholz as pioneer end-users and useful discussions about the protocol; the French institute of Corrosion, the Swiss National Museum, the Laténium park and museum of Archaeology, the Archaeological service of Bern Canton, the Archaeological Service of Brugg Canton, the Site et Musée romains d’Avenches, Neuchâtel city and La-Chaux-de-Fonds city, as well as the Légende d’Automne Association (Sara.H, DeLaPerouze, Gisela & Edgard Raeber, David Perroud) are thanked for their support.
References
Albini, M., Chiavari, C., Bernardi, E., Martini, C., Mathys, L., Letardi, P., et al. (2016a). “Influence of outdoor bronze alloying elements on the performances of a biological treatment,” in Metal 2016 Proceedings of the Interim meeting of the ICOM-CC metals working group. Editors R. Menon, C. Chemelllo, and A. Pandya (New Delhi, India: International Council of Museums – Committee for Conservation (ICOM-CC) and Indira Gandhi National Centre for the Arts (IGNCA))), 306–313.
Albini, M., Comensoli, L., Brambilla, L., Domon Beuret, E., Kooli, W., Mathys, L., et al. (2016b). Innovative biological approaches for metal conservation. Mater. Corros. 67, 200–206. doi:10.1016/j.corsci.2018.08.020
Albini, M., Letardi, P., Mathys, L., Brambilla, L., Schröter, J., Junier, P., et al. (2018). Comparison of a bio-based corrosion inhibitor versus benzotriazole on corroded copper surfaces. Corros. Sci. 143, 84–92. doi:10.3390/coatings10030217
Anastas, P. T., and Zimmerman, J. B. (2003). Design through the 12 principles of green engineering. Environ. Sci. Technol. 37, 94A–101A. doi:10.1021/es032373g
Appelbaum, B. (2012). Conservation treatment methodology. London: Routledge. doi:10.4324/9780080561042
Bharde, A., Rautaray, D., Bansal, V., Ahmad, A., Sarkar, I., Yusuf, S. M., et al. (2006). Extracellular biosynthesis of magnetite using fungi. Small. 2, 135–141. doi:10.1002/smll.200500180
Bonneville, S., Smits, M. M., Brown, A., Harrington, J., Leake, J. R., Brydson, R., et al. (2009). Plant-driven fungal weathering: early stages of mineral alteration at the nanometer scale. Geology. 37, 615–618. doi:10.1007/978-3-642-27833-4_5400-1
De Muynck, W., De Belie, N., and Verstraete, W. (2010). Microbial carbonate precipitation in construction materials: a review. Ecol. Eng. 36, 118–136. doi:10.1016/j.ecoleng.2009.02.006
Dillmann, P., Béranger, G., Piccardo, P., and Matthiesen, H. (2007). Corrosion of metallic heritage artefacts: investigation, conservation and prediction of long term behaviour. Cambridge England: Woodhead Publishing and Maney Publishing. doi:10.1080/13505033.2016.1182776
Domon-Beuret, E., Mathys, L., Brambilla, l., Albini, M., Cevey, C., Bertholon, R., et al. (2015). Biopatines: des champignons au service des alliages cuivreux. Cahier Technique de l'ARAAFU 22, XXVIIIe Journées des restaurateurs en archéologie, Arles.
Gadd, G. M. (2010). Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology (Reading, Engl). 156, 609–643. doi:10.1099/mic.0.037143-0
Gharieb, M. M., Ali, M. I., and el-Shoura, A. A. (2004). Transformation of copper oxychloride fungicide into copper oxalate by tolerant fungi and the effect of nitrogen source on tolerance. Biodegradation. 15, 49–57. doi:10.1023/b:biod.0000009962.48723.df
Graedel, T. E. (1987). Copper patinas formed in the atmosphere--II. A qualitative assessment of mechanisms. Corrosion Sci. 27, 721.
Joseph, E., Cario, S., Simon, A., Wörle, M., Mazzeo, R., Junier, P., et al. (2012a). Protection of metal artifacts with the formation of metal-oxalates complexes by Beauveria bassiana. Front. Microbiol. 2, 270. doi:10.3389/fmicb.2011.00270
Joseph, E., Simon, A., Prati, S., Wörle, M., Job, D., and Mazzeo, R. (2011). Development of an analytical procedure for evaluation of the protective behaviour of innovative fungal patinas on archaeological and artistic metal artefacts. Anal. Bioanal. Chem. 399, 2899–2907. doi:10.1007/s00216-010-4279-2
Joseph, E., Albini, M., Letardi, P., Domon-Beuret, E., Brambilla, L., Mathys, L., et al. (2014). “BIOPATINAS: innovative biological patinas for copper-based artefacts,” in Outdoor metallic sculpture from the XIXth to the beginning of the XXth century: identification, conservation, restoration. Champ-sur-Marne: SFICC). Editors A. Texier, and A. Azéma, 154–162.
Joseph, E., Junier, P., Albini, M., Letardi, P., Beuret, E. D., Brambilla, L., et al. (2015). “Biologically induced patina for metal built heritage,” in Metalli in architettura, 31 convegno scienza e beni culturali. Editors G. Biscontin, G. Driussi, and M. Bressanone (Venezia, Italy: ARCADIA ricerche srl), 273–282.
Joseph, E., Letardi, P., Comensoli, L., Simon, A., Junier, P., Job, D., et al. (2013). “Assessment of a biological approach for the protection of copper alloys artefacts,” in METAL2013 Proceedings of the Interim Meeting of the ICOM-CC metal working group. Editors E. Hyslop, V. Gonzalez, L. Troalen, and L. Wilson (Edinburgh, Scotland: Historic Scotland and International Council of Museums), 203–208.
Joseph, E., Simon, A., Mazzeo, R., Job, D., and Wörle, M. (2012b). Spectroscopic characterization of an innovative biological treatment for corroded metal artefacts. J. Raman Spectrosc. 43, 1612–1616. doi:10.1002/jrs.4164
Kuklinski, A., and Sand, W. (2014). “Microbiologically influenced corrosion inhibition,” in Encyclopedia of applied electrochemistry. Editors G. Kreysa, K.-I. Ota, and R. F. Savinell (New York, NY: Springer), 1290–1297.
Letardi, P., Albini, M., and Joseph, E. (2018). EIS measurements for treatments testing: the case of a bio-based method applied on outdoor bronze statues in Switzerland. doi:10.14293/S2199-1006.1.SOR-
Marabelli, M., and Mazzeo, R. (1993). La corrosione dei bronzi esposti all'aperto: problemi di caratterizzazione. La Metallurgia Italiana. 85, 247–254.
Mazzeo, R., Bittner, S., Job, D., Farron, G., Fontinha, R., Joseph, E., et al. (2008). “Development and evaluation of new treatments for outdoor bronze monuments,” in Conservation science 2007. Editor J. Townsend (London: Archetype publications Ltd.), 40–48. doi:10.13140/RG.2.1.1430.0009
Troiano, F., Gulotta, D., Balloi, A., Polo, A., Toniolo, L., Lombardi, E., et al. (2013). Successful combination of chemical and biological treatments for the cleaning of stone artworks. Int. Biodeterior. Biodegrad. 85, 294–304. doi:10.3389/fmicb.2014.00155
Keywords: heritage, bronze, copper alloys, corrosion, green method, biotechnology
Citation: Joseph E (2021) Biopassivation Method for the Preservation of Copper and Bronze Artefacts. Front. Mater. 7:613169. doi: 10.3389/fmats.2020.613169
Received: 01 October 2020; Accepted: 18 December 2020;
Published: 27 January 2021.
Edited by:
Elwira Sieniawska, Medical University of Lublin, PolandReviewed by:
Alessio Mezzi, Consiglio Nazionale delle Ricerche Italiano (CNR), ItalyFahe Cao, Sun Yat-Sen University, China
Copyright © 2021 Joseph. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edith Joseph, ZWRpdGguam9zZXBoQHVuaW5lLmNo, ZWRpdGguam9zZXBoQGhlLWFyYy5jaA==
 Edith Joseph
Edith Joseph