
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mater. , 09 February 2021
Sec. Biomaterials
Volume 7 - 2020 | https://doi.org/10.3389/fmats.2020.560372
This article is part of the Research Topic Biomaterials for Cell-Based Therapy View all 10 articles
Neural stem cells (NSCs) show great promise in drug discovery and clinical application. Yet few efforts have been made to optimize biocompatible materials for such cells to be expanded and used in clinical conditions. We have previously demonstrated that NSCs are readily cultured on substrates of certain recombinant spider silk protein without addition of animal- or human-derived components. The question remains however whether this material allows differentiation into functional neurons, and whether such differentiation can take place also when the NSCs are cultured not only upon but also within the biodegradable material. Here we demonstrate that “foam”-like structures generated from recombinant spider silk protein (4RepCT) provided excellent matrices for the generation and multicellular analysis of functional excitatory neurons from NSCs without addition of animal- or human-derived components. NSCs isolated from the cerebral cortices of rat embryos were cultured at either 4RepCT matrices shaped as foam-like structures without coating, or on conventional polystyrene plates coated with poly-L-ornithine and fibronectin. Upon treatment with recombinant proteins including the extracellular signaling factor BMP4 or a combination of BMP4 and the signaling factor Wnt3a, the cortical NSCs cultured in 4RepCT foam-like structures differentiated efficiently into neurons that responded to glutamate receptor agonists, such as AMPA, to the same extent as control cultures. Matrices derived from recombinant spider silk proteins thus provide a functional microenvironment for neural stem cells with little or no animal- or human-derived components and can be employed in the development of new strategies in stem cell research and tissue engineering.
The microenvironment strongly influences stem cell characteristics in various ways. These effects include changes in differentiation-associated gene expression via epigenetic mechanisms, e.g., chromatin modifications, and related changes in transcriptional activity. Several reports from recent years, from our lab and our colleagues, have demonstrated how substrate characteristics such as stiffness and roughness, and incorporation of specific cell binding motifs influence the differentiation of various types of stem cells in a cell context-specific manner (Teixeira et al., 2007; Discher et al., 2009; Teixeira et al., 2009; Teixeira et al., 2010; Blumenthal et al., 2014). Accordingly, a wide range of alternative solutions for optimization of biomaterials for stem cell research and tissue engineering has been tested during recent years.
Spider silk is an interesting biomaterial (Lewis, 2006; Hardy and Scheibel, 2009; Omenetto and Kaplan, 2010; Rising et al., 2011), due to its extreme mechanical properties (Gosline et al., 1999) combined with local tolerance when implanted in living tissues (Vollrath et al., 2002; Allmeling et al., 2008; Fredriksson et al., 2009; Radtke et al., 2011). A recombinant spider silk protein (4RepCT; consisting of four tandem repeats and the C-terminal domain of the major ampullate spidroin 1) that corresponds to approximately 10% of the native spider silk protein, can be readily produced in Escherichia coli (E. coli) and spontaneously assembles into films, foams and fibers (Stark et al., 2007; Widhe et al., 2010). 4RepCT matrices provide excellent substrates for human primary fibroblasts (Widhe et al., 2010) and we have demonstrated that this recombinant spider silk protein provides a suitable substrate also for culturing of rodent cortical neural stem cells (NSCs) as these cells proliferate, survive, retain multipotency, and differentiate into multiple cell types efficiently on film structures derived from 4RepCT without any additional coating or animal-derived components (Lewicka et al., 2012). The analysis of differentiation in our previous study was however based on gene expression and immunocytochemistry, and it is still not known whether the differentiated neurons and astrocytes derived from neural stem cells grown on 4RepCT are functional or as to which degree this can be analyzed. As it has been shown repeatedly that the expression of differentiation markers not necessarily correlate with functionality (Andersson et al., 2011) such functional analysis is required before proceeding with transplantations and in vivo studies in animals.
Production of protein matrices: The recombinant miniature spider silk protein 4RepCT was produced in E. coli and purified as described previously (Hedhammar et al., 2008). A procedure for depletion of lipopolysaccharides (LPS) was also included (Hedhammar et al., 2010). After purification, the protein solution was sterilized by passage through 0.22 μm filter and concentrated to 3 mg/ml by ultrafiltration (Amicon Ultra, Millipore; unless otherwise stated, all general reagents were supplied by Swedish distributors and suppliers in accordance with the national procurement law) before preparation of foam matrices as previously described (Widhe et al., 2010). Matrices were dried over night under sterile conditions at room temperature (RT) and stored in RT until use. The matrices were washed twice with sterile PBS and pre-incubated with serum-free DMEM:F12 media (Sigma-Aldrich) for 1 h at 37°C with 5% CO2 before plating the cells. The matrices were prepared in six-well cell culture polystyrene plates or 35 mm in diameter polystyrene plates (Sarstedt). Surface morphology, thickness, diameter, pore size, scanning electron microscopy (SEM), z-stacks, and in-depth analysis of the 4RepCT foam structure have previously been described elsewhere (Widhe et al., 2010).
Neural Stem Cell (NSC) culture: NSCs were harvested from cortices at day E15.5 embryos obtained from pregnant Sprague Dawley rats as described previously (Ilkhanizadeh and Teixeira, 2007; Andersson et al., 2011). Cells were mechanically dissociated followed by culture in serum-free DMEM:F12 media (Gibco) enriched with N2 supplements. After mechanical dissociation, the cells were cultured in serum-free DMEM:F12 media (Gibco) with N2 supplement. The cells were maintained in proliferating, undifferentiated state in this media by adding 10 ng/ml recombinant fibroblast growth factor 2 (FGF2; R&D Systems) every 24th hour and typically passaged once before usage in the experiments. NSCs were then seeded at a density of 10,000 cells/cm2 and allowed to proliferate for 24–72 h to reach appropriate confluency (50–60%) prior to the experiments. The 4RepCT foams covered ∼60% of the surface area of the cell culture dishes before NSCs were applied. NSCs used in positive control experiments were seeded on conventional polystyrene culture plates coated with poly-L-ornithine and fibronectin from bovine plasma (P+F) (Sigma-Aldrich) and uncoated polystyrene dishes served as negative controls to these. Soluble factors were re-supplemented at 24 h and medium replaced at 48 h time intervals. FGF2 was withdrawn from the cell culture media and then replaced with bone morphogenic protein (BMP4; R&D Systems) and/or Wnt3a (R&D Systems) to induce differentiation. All animal experiments were approved by the North Stockholm regional committee for animal research and ethics, Stockholm, Sweden (N284/11).
Immunocytochemistry: After a rinse in Phosphate-Buffered Saline (PBS) (Gibco), the cell cultures were fixed in 10% Formalin (Sigma-Aldrich) for 20 min at RT followed by 3 × 5 min washes with PBS with 0.1% Triton-X 100 (Sigma-Aldrich). Plates were then incubated with the respective primary antibody in PBS with 0.1% Triton-X 100 and 1% bovine serum albumin (BSA; Sigma-Aldrich) overnight at 4°C. The primary antibodies sources and dilutions were as follows: mouse anti-intermediate filament protein nestin (1:500; BD Pharmingen), mouse monoclonal anti-Neuronal Class III B-Tubulin (TuJ1; 1:500; Covance), microtubule associated protein 2 (MAP2; 1:500, Sigma-Aldrich), and rabbit poly-clonal anti-glia fibrillary acidic protein (GFAP; 1:500; DAKO). The cells were subsequently washed 6 × 5 min each with PBS with 0.1% Triton-X 100. Secondary antibodies were incubated in PBS with 0.1% Triton-X 100 and 1% BSA at RT for 1 h. The secondary antibodies were species-specific and labeled with Alexa 594 or Alexa 488 (1:500; Molecular Probes). Lastly, the samples were washed 3 × 5 min each in PBS and mounted with Vectashield (Vector Laboratories, Inc.). Fluorescent images were acquired with Axioskop software using Zeiss Axioskop2 microscope coupled to an MRm (Zeiss).
Calcium imaging: Cells were loaded with the Ca2+sensitive fluorescence indicator Fluo-3/AM (5 μM, Life Technologies) together with 0.1% Pluronic f-127 (Life Technologies) at 37°C for 20 min in their own medium, and then moved to a Krebs-Ringer buffer containing (in mM) 119.0 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1.0 NaH2PO4, 20.0 HEPES (pH 7.4), and 11.0 dextrose. Measurements of intracellular Ca2+ were carried out in Krebs-Ringer buffer with or without Ca2+ at 37°C using a heat-controlled chamber (Warner Instruments) with a cooled EMCCD QuantEM 512:SC camera (Photometrics) mounted on an upright microscope (Zeiss Axio Examiner.D1) equipped with an W-Plan apochromat 20X/1.0 objective (Carl Zeiss). Excitation at 495 nm was assessed with a filter wheel (Sutter Instrument) at sampling frequency 0.5 Hz. MetaFluor (Molecular Devices) was used to control all devices and to analyze acquired images. The response to AMPA and ATP was determined using MATLAB software (The MathWorks Inc.) as described previously (Uhlén, 2004) considering only increases of more than 30% compared with the baseline as positive response. Drugs were bath-applied following previous protocol (Andersson et al., 2011).
Statistical analysis: Statistical analysis and graphs were performed using the software Prism 4 (Graph Pad). When 2 groups were compared, statistical analysis was performed using two-tailed unpaired t-test. When repeated measurements taken at different time points from 2 groups were compared, two-way ANOVA analysis of variance was used. The threshold value for statistical significance (α value) was set at 0.05 (*p < 0.05). Data are presented as the mean ± standard error of the mean (SEM) of independent experiments (3–5 experimental series per condition).
In control cultures, NSCs derived from rat cortex of embryonic day 15 (E15) were cultured in optimal conditions seeded on polystyrene cell culture plates precoated with poly-L-ornithine and fibronectin as described in Materials and Methods. This protocol is well-established and widely used for over 2 decades, and provides homogenous cultures of multipotent progenitors (i.e. retaining the potency to differentiate into multiple fates) for a large number of passages (Johe et al., 1996; Jepsen et al., 2007; Andersson et al., 2011; Castelo-Branco et al., 2014). The NSCs expand undifferentiated and self-renew in N2 supplemented medium in the presence of FGF2. In contrast, NSCs grow poorly or not at all on uncoated polystyrene plates or plates coated with either poly-L-ornithine or fibronectin (data not shown and Johe et al., 1996; Teixeira et al., 2009; Lewicka et al., 2012).
After initial expansion of the primary cells, we seeded NSCs after the first passage on 4RepCT foam structures and in control conditions, respectively. NSCs seeded on 4RepCT foam structures attached and proliferated 24–72 h after the first passage into 50–60% confluency (Figure 1), indicating that NSC can attach to the 4RepCT protein as previously reported. To investigate if NSCs grown on 4RepCT foam shaped matrices in 3D can differentiate into neurons we exposed the cells to treatment with BMP4 or co-treatment with BMP4 and the signaling factor Wnt3a for 14 days. We have previously shown that BMP4 and BMP4+Wnt3a stimulation induces differentiation of mature, functional glutamate-receptor (GluR)—agonist responsive neuronal cells when NSCs are seeded at relatively high densities (Leao et al., 2010; Andersson et al., 2011). NSCs grown in 3D on 4RepCT foam matrices with pore sizes of around 5–30 µm and exposed to 10 ng/ml BMP4 or BMP4 and Wnt3a every 24 h for 14 days differentiated into neuronal cells positive for the dendritic marker of mature, post-mitotic neurons, microtubule associated protein 2 (MAP2). When analyzing five micrographs per well, we found in average 30 ± 6.4 (BMP4) and 41 ± 12 (BMP4+Wnt3a) MAP2-positive cells in control conditions, and 71 ± 10 (BMP4) and 94 ± 16 (BMP4+Wnt3a) MAP2-positive cells when cultured within the foam 4RepCT (Figure 2). Morphological characteristics of cells grown on 4RepCT foam structures were similar to the poly-L-ornithine and fibronectin-coated polystyrene cell culture plates used as controls (Figure 1). Virtually all MAP2-negative cells were found to stain positive for the astrocytic marker GFAP (Figure 1). NSCs differentiated within 4RepCT 3D foam-like structures thus retained full potential to differentiate into cells expressing neuronal and astrocytic markers, and 4RepCT-derived structures seemed to provide an even better substrate for differentiation than standard culture plates as assessed by MAP2 staining (Figure 2).
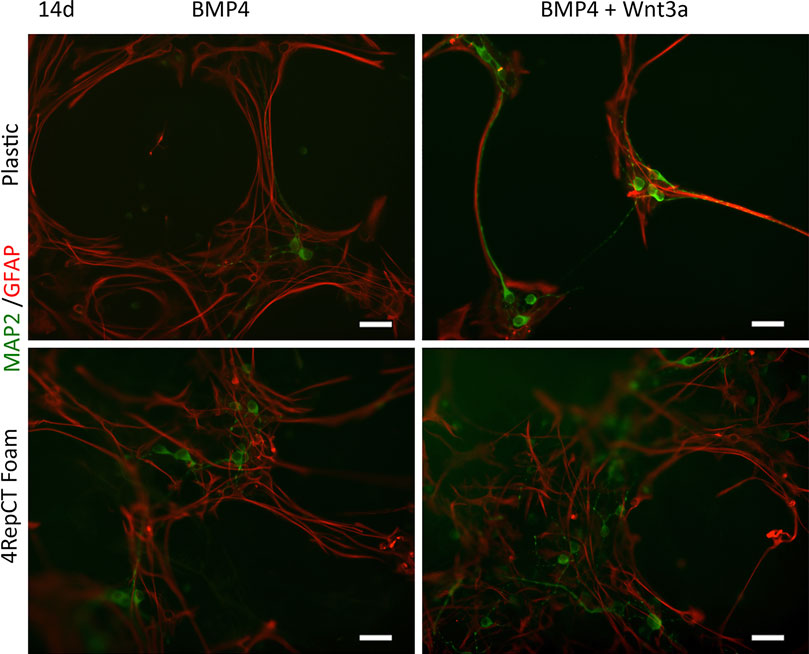
FIGURE 1. NSCs retained their potential to differentiate into mature post-mitotic neuronal cells on 4RepCT-derived foam-like substrates in response to BMP4 and co-treatment of BMP4 and Wnt3a respectively, in comparison to control cultures expanded on conventional (polystyrene) dishes covered with poly-ornithine and fibronectin. Micrographs demonstrating neuronal cells derived from NSCs after treatment with BMP4 only or co-treatment with BMP4 and Wnt3a when grown on control culture plates or 4RepCT. MAP2-staining (green), GFAP-immunoreactivity (red).
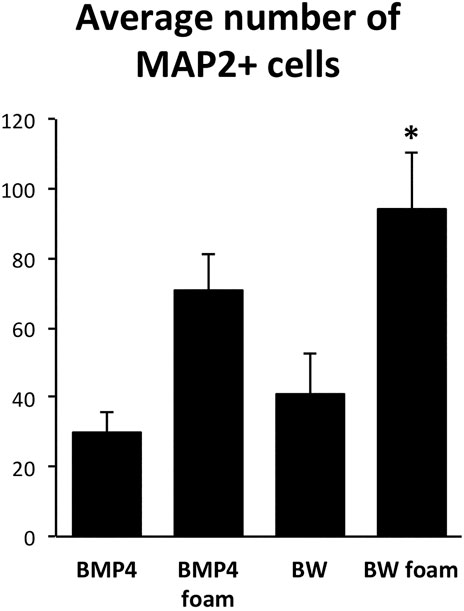
FIGURE 2. Quantifications of the number of MAP2-positive cells after BMP4 or co-treatment with BMP4 and Wnt3a (BW) of control cultures expanded on conventional (polystyrene) dishes covered with poly-ornithine and fibronectin or 4RepCT-derived foam matrix. Y axis represents average number of MAP2-positive cells in 5 micrographs similar to those shown in Figure 1 from three independent experiments in each condition. The number of MAP2-positive cells in the BW group cultured on the 4RepCT foam matrix was significantly higher than in any other condition. *p < 0.05, error bars represent SEM (standard error mean).
BMP4 and Wnt3a are required for proper development of neurons of the hippocampal formation, a structure of the forebrain essential for learning, and mature hippocampal neurons express receptors for the excitatory neurotransmitter glutamate (Lee et al., 2000; Sur and Rubenstein, 2005). We therefore decided to investigate the functionality of BMP4 vs. BMP4+Wnt3a induced neurons grown in foam 4RepCT matrices by examining their responsiveness to specific glutamate receptor and calcium (Ca2+) signaling agonists. Calcium signaling was measured in NSCs differentiated for 14 days and loaded with the Ca2+ sensitive fluorophore Fluo-3/AM.
All cells examined in BMP4 or BMP4+Wnt3a-treated cultures were first shown to respond to KCl depolarization with a rapid increase in the intracellular Ca2+ concentration (Supporting Information), indicating that the cultures were functional and healthy. A major advantage of measuring the calcium responses compared to, e.g., electrophysiological analysis (Leao et al., 2010; Tang-Schomer et al., 2014; Chwalek et al., 2015) is that a large number of cells can be analyzed simultaneously and thus populations of different cell types identified. To investigate the functional identity of the cells, we used an established protocol to discriminate between neurons and astrocytes in the BMP4 and BMP4+Wnt3a-treated cells. Astrocytes express mainly the metabotropic P2Y purinoceptors whereas mature neurons express the ionotropic P2X purinoceptors, plus a small population of P2Y receptors that do not affect intracellular Ca2+ but mediate slow changes in membrane potential (Illes and Ribeiro, 2004; Rowe et al., 2005; Andersson et al., 2011). Neurons should therefore respond to ATP in the presence, but not absence, of extracellular Ca2+, whereas astrocytes should respond to ATP in both conditions (Rowe et al., 2005; Lin et al., 2007). Cells were further characterized functionally by treatment with the selective ionotropic glutamate receptor (GluR) agonist AMPA, which, in the presence of extracellular Ca2+, should evoke a transient response in neurons but not in astrocytes. Stimulation of BMP4 and BMP4+Wnt3a-treated cultures with ATP or AMPA in an extracellular medium with or without Ca2+ confirmed our previous observations that the NSC-derived cells can be divided into several subsets depending on their distinct Ca2+ response patterns. Hence, one subset of cells responded to AMPA and ATP, respectively, with a transient Ca2+ increase in the presence but not absence of extracellular Ca2+, other cells responded to both AMPA and ATP in the presence of Ca2+, while another subset of cells only responded to ATP but not AMPA under both conditions. Examples of response curves are depicted in Figure 3. Supporting Information contains four micrograph time-lapse movies of around 90 s each representing one c:a 60–70 min experiment in each condition demonstrating the calcium response to ATP, AMPA, and KCl similar to the results from each condition shown in Figure 3.
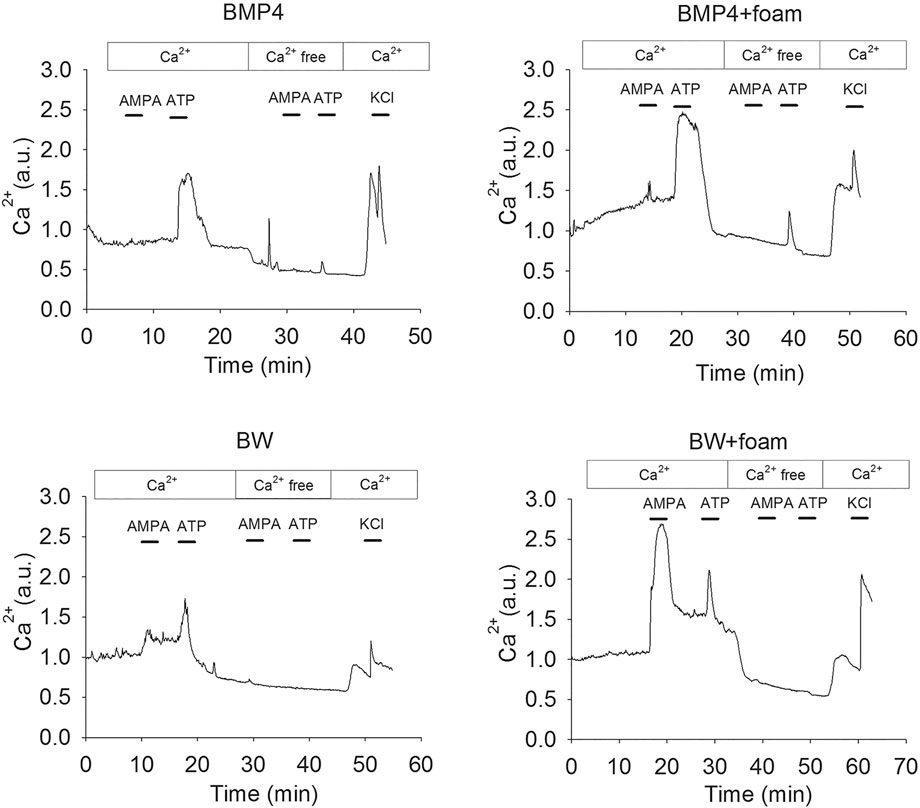
FIGURE 3. Representative single cell Ca2+ response traces after treatment of NSC cultured in control conditions as defined above or 4RepCT-derived foam structures with BMP4 and BMP4+Wnt3a (BW).
We next performed a quantitative analysis of the multirecordings (>30 cells/recording) of BMP4- and BMP4+Wnt3a-treated NSC-cultures and to which extent ATP-responsive cells also responded to AMPA in the presence or absence of Ca2+ in three different experiments for each condition. The total number of responsive cells as analyzed by ATP-stimulation were between on average 65–95% (Figure 4B), of which some also responded in Ca2+-zero conditions, and thus presumably were astrocytes. When analyzing the fraction of cells responding to the glutamate receptor agonist AMPA in the presence of Ca2+ specifically, we found that stimulation of cultures co-treated with BMP4+Wnt3a resulted in a transient Ca2+ increase in on average 25% of cells grown in 3D 4RepCT foam matrices compared to an average of 10–15% in other conditions (Figure 4A). As expected, there were only a very low number of cells responding to AMPA in zero Ca2+ conditions (Figure 4A).
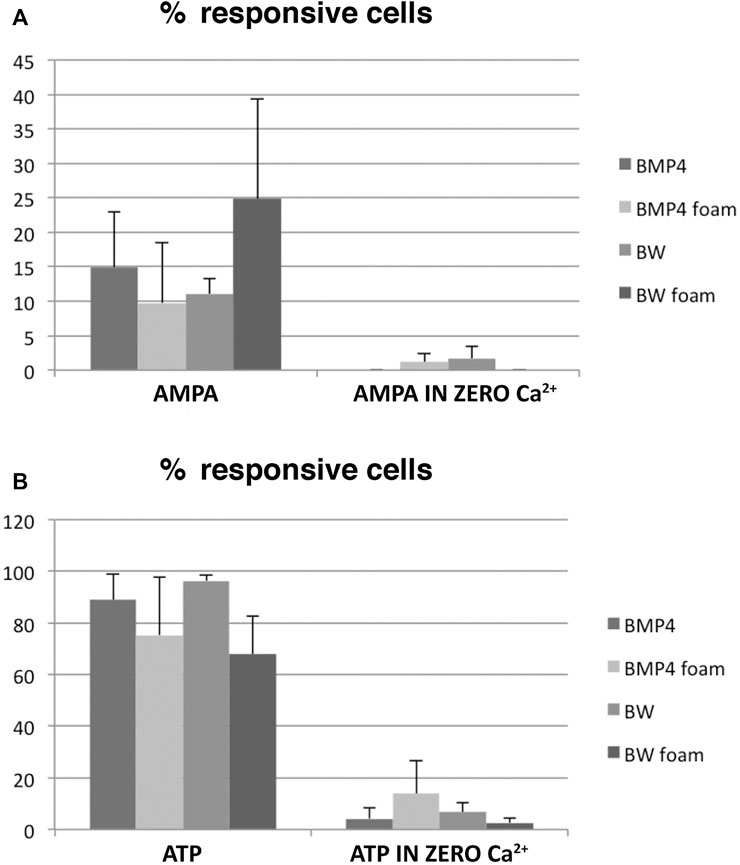
FIGURE 4. Quantifications of the Ca2+ imaging experiments. A large variability was noted, but a substantial fraction of AMPA-responsive neurons was present in the BMP4+Wnt3a (BW)-treated cultures grown in 3D 4RepCT-derived matrices. Each bar represents the means ± SEM (standard error mean; n > 30 cells per condition) of single cell Ca2+ responses as illustrated in Figure 3 from n = 3 independent experimental series. Statistical analysis was performed using two-tailed unpaired t-test after two-way ANOVA analysis of variance was used. The threshold value for statistical significance (α value) was set at 0.05 (*p < 0.05). Data are presented as the mean ± standard error of the mean (SEM) of independent experiments (3–5 experimental series per condition). Please see Materials and Methods section for detailed description of the statistical analysis.
A substantial variation in responses between the different experiments precluded any statistical analysis, even if the number of experiments would have been increased, but our results clearly demonstrates that the biodegradable, recombinant spider silk substrate 4RepCT permits differentiation by BMP and Wnt3a into functional, glutamate receptor-responsive neurons to an extent at least as efficient as the quintessential NSC culture method.
Most approaches in cell therapy of the central nervous system (CNS) have hitherto been based on delivery of relatively homogenous cell populations to brain structures with limited number of cell types, i.e. substantia nigra or the striatum. However, most parts of the CNS are highly organized with specific functional centers consisting of a larger number of populations with mixed cell types (Zeisel et al., 2015), and due to this highly organized anatomy, 3D-approaches are not only recommended, it is required for continued progress in applied stem cell research and regenerative medicine (Poli et al., 2019). Recombinant spider silk provide several potential advantages over other materials used as matrices and/or over the natural material: 1) it displays mechanical integrity and can be processed into different 3D structures (Stark et al., 2007; Widhe et al., 2010), 2) the production is scalable (Tokareva et al., 2013), 3) the sequence of the proteins can easily be modified to contain also cell binding motifs Wohlrab et al., 2012), 4) recombinant spider silk fibers evoke little inflammatory reaction and are gradually degraded when implanted subcutaneously (Fredriksson et al., 2009; Lucke et al., 2018 and references therein), and 5) the material is chemically defined and of non-animal origin, and thus a possible substrate for long-term, xeno-free cell culture.
Our system has some differences and advantages compared to most published protocols hitherto. First, as previously described, NSCs cultured and differentiated on 4RepCT do not require any additional coating, allowing a xeno-free substrate for organoid cultures as mentioned. In addition, the 4RepCT substrate is transparent allowing analysis of morphological details. Due to this transparency, we could perform calcium analysis after agonist stimulation, and demonstrate the overall efficiency of the differentiation and nature of the differentiated cells and structures of a large proportion of the cells in the cultures. Furthermore, in contrast to many but not all other protocols, our rodent cells have been defined as multipotent and able to differentiate into neurons, astrocytes, and oligodendrocytes in clonal conditions (Jepsen et al., 2007; Castelo-Branco et al., 2014) as well as mesenchymal fates (Andersson et al., 2011) allowing a strict control of the development of the tissue-like structures. Lastly, as the spider silk substrate consists of recombinant protein, it opens up for various possibilities of developing simple protocols for growing neural organoids in GMP-grade environment in the near future.
Here we employed 4RepCT foams in NSCs differentiation in order to successfully identify a defined culture system for NSCs. The 4RepCT foam structure supports NSC attachment, survival, and differentiation into functional neurons in pseudo-3D, and 4RepCT 3D foam implantation has already been successfully applied in animal studies (Fredriksson et al., 2009) as well as transplantation of the NSCs described here (Lundberg et al., 2012), suggesting it to be a promising candidate for future studies of NSC differentiation and formation into functional circuits in vitro and for tests for use of NSCs in cell therapy in various animal models of neurological disease in vivo.
Foam-like structures generated from biodegradable, recombinant spider silk protein (4RepCT) permit differentiation of neural stem cells into functional, AMPA-responsive neuronal circuits without animal- or human-derived components. We propose that matrices generated from recombinant spider silk protein are suitable for development of applications in stem cell research, tissue engineering, and regenerative medicine.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by North Stockholm regional committee for animal research and ethics, #284/11.
ML, Neural stem cell culture, immunohistochemistry, calcium imaging, draft development. PR, Calcium imaging, analysis. JL, Neural stem cell culture, analysis, manuscript production. PU, Calcium imaging, supervision, analysis, figure production. AR, Biomaterial generation, biomaterial expertise, manuscript finalisation. OH, Project design, supervision, figure production, manuscript finalisation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank the Hermanson lab for valuable discussions and comments on this manuscript. We thank Spiber Technologies AB for providing the 4RepCT matrices. This work was funded by grants from CIMED, StratRegen (SFO to KI), Karolinska Institutets Forskningsstiftelser (2011FoBi023) (to AR), VR-MH (Project Grants to PU, AR, and OH), Karolinska Institutet (TEMA), the Swedish Childhood Cancer Foundation (BCF), the Swedish Cancer Society, and Vinnova (to OH). A previous version of this manuscript has been released as a pre-print at bioRxiv (Lewicka et al., 2019).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2020.560372/full#supplementary-material.
NSCs, neural stem cells; BMP4, Bone Morphogenic protein 4; TuJ1, Neuronal class III B‐tubulin; P, poly‐L‐ornithine; F, fibronectin; ECM, extracellular matrix; 3D, three dimensional; BW, BMP4+Wnt3a.
Allmeling, C., Jokuszies, A., Reimers, K., Kall, S., Choi, C. Y., Brandes, G., et al. (2008). Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Prolif. 41, 408–420. doi:10.1111/j.1365-2184.2008.00534.x
Andersson, T., Duckworth, J. K., Fritz, N., Lewicka, M., Södersten, E., Uhlén, P., et al. (2011). Noggin and Wnt3a enable BMP4-dependent differentiation of telencephalic stem cells into GluR-agonist responsive neurons. Mol. Cell. Neurosci. 47, 10–18. doi:10.1016/j.mcn.2011.01.006
Blumenthal, N. R., Hermanson, O., Heimrich, B., and Shastri, V. P. (2014). Stochastic nanoroughness modulates neuron-astrocyte interactions and function via mechanosensing cation channels. Proc. Natl. Acad. Sci. USA 111, 16124–16129. doi:10.1073/pnas.1412740111
Castelo-Branço, G., Lilja, T., Wallenborg, K., Falcão, A. M., Marques, S. C., Gracias, A., et al. (2014). Neural stem cell differentiation is dictated by distinct actions of nuclear receptor corepressors and histone deacetylases. Stem Cell Reports 3, 502–515. doi:10.1016/j.stemcr.2014.07.008
Chwalek, K., Tang-Schomer, M. D., Omenetto, F. G., and Kaplan, D. L. (2015). In vitro bioengineered model of cortical brain tissue. Nat. Protoc. 10, 1362–1373. doi:10.1038/nprot.2015.091
Discher, D. E., Mooney, D. J., and Zandstra, P. W. (2009). Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677. doi:10.1126/science.1171643
Fredriksson, C., Hedhammar, M., Feinstein, R., Nordling, K., Kratz, G., Johansson, J., et al. (2009). Tissue response to subcutaneously implanted recombinant spider silk: an in vivo study. Materials 2, 1908–1922. doi:10.3390/ma2041908
Gosline, J. M., Guerette, P. A., Ortlepp, C. S., and Savage, K. N. (1999). The mechanical design of spider silks: from fibroin sequence to mechanical function. J. Exp. Biol. 202, 3295–3303.
Hardy, J. G., and Scheibel, T. R. (2009). Silk-inspired polymers and proteins. Biochem. Soc. Trans. 37, 677–681. doi:10.1042/BST0370677
Hedhammar, M., Bramfeldt, H., Baris, T., Widhe, M., Askarieh, G., Nordling, K., et al. (2010). Sterilized recombinant spider silk fibers of low pyrogenicity. Biomacromolecules 11, 953–959. doi:10.1021/bm9014039
Hedhammar, M., Rising, A., Grip, S., Martinez, A. S., Nordling, K., Casals, C., et al. (2008). Structural properties of recombinant nonrepetitive and repetitive parts of major ampullate spidroin 1 from Euprosthenops australis: implications for fiber formation. Biochemistry 47, 3407–3417. doi:10.1021/bi702432y
Illes, P., and Ribeiro, J. A. (2004). Neuronal P2 receptors of the central nervous system. Curr. Top. Med. Chem. 4, 831–838. doi:10.2174/1568026043451032
Ilkhanizadeh, S., and Teixeira, A. I. (2007). Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 28, 3936–3943. doi:10.1016/j.biomaterials.2007.05.018
Jepsen, K., Solum, D., Zhou, T., McEvilly, R. J., Kim, H. J., Glass, C. K., et al. (2007). SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature 450, 415–419. doi:10.1038/nature06270
Johe, K. K., Hazel, T. G., Muller, T., Dugich-Djordjevic, M. M., and McKay, R. D. (1996). Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 10, 3129–3140. doi:10.1101/gad.10.24.3129
Leão, R. N., Reis, A., Emirandetti, A., Lewicka, M., Hermanson, O., and Fisahn, A. (2010). A voltage-sensitive dye-based assay for the identification of differentiated neurons derived from embryonic neural stem cell cultures. PloS One 5, e13833. doi:10.1371/journal.pone.0013833
Lee, S. M., Tole, S., Grove, E., and McMahon, A. P. (2000). A local Wnt-3a signal is required for development of the mammalian hippocampus. Development 127, 457–467.
Lewicka, M., Hermanson, O., and Rising, A. U. (2012). Recombinant spider silk matrices for neural stem cell cultures. Biomaterials 33, 7712–7717. doi:10.1016/j.biomaterials.2012.07.021
Lewicka, M., Rebellato, P., Lewicki, J., Uhlén, P., Rising, A., and Hermanson, O. (2019). Recombinant spider silk protein matrices facilitate multi-analysis of calcuim-signaling in neural stem cell-derived AMPA-responsive neurons. bioRxiv 579, 292.
Lewis, R. V. (2006). Spider silk: ancient ideas for new biomaterials. Chem. Rev. 106, 3762–3774. doi:10.1021/cr010194g
Lin, J. H., Takano, T., Arcuino, G., Wang, X., Hu, F., Darzynkiewicz, Z., et al. (2007). Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev. Biol. 302, 356–366. doi:10.1016/j.ydbio.2006.09.017
Lucke, M., Mottas, I., Herbst, T., Hotz, C., Römer, L., Schierling, M., et al. (2018). Engineered hybrid spider silk particles as delivery system for peptide vaccines. Biomaterials 172, 105–115. doi:10.1016/j.biomaterials.2018.04.008
Lundberg, J., Södersten, E., Sundström, E., Le Blanc, K., Andersson, T., Hermanson, O., et al. (2012). Targeted intra-arterial transplantation of stem cells to the injured CNS is more effective than intravenous administration: engraftment is dependent on cell type and adhesion molecule expression. Cell Transpl. 21, 333–343. doi:10.3727/096368911X576036
Omenetto, F. G., and Kaplan, D. L. (2010). New opportunities for an ancient material. Science 329, 528–531. doi:10.1126/science.1188936
Poli, D., Magliaro, C., and Ahluwalia, A. (2019). Experimental and computational methods for the study of cerebral organoids: a review. Front. Neurosci. 13, 162. doi:10.3389/fnins.2019.00162
Radtke, C., Allmeling, C., Waldmann, K. H., Reimers, K., Thies, K., Schenk, H. C., et al. (2011). Spider silk constructs enhance axonal regeneration and remyelination in long nerve defects in sheep. PloS One 6, e16990. doi:10.1371/journal.pone.0016990
Rising, A., Widhe, M., Johansson, J., and Hedhammar, M. (2011). Spider silk proteins: recent advances in recombinant production, structure-function relationships and biomedical applications. Cell. Mol. Life Sci. 68, 169–184. doi:10.1007/s00018-010-0462-z
Rowe, E. W., Jeftinija, D. M., Jeftinija, K., and Jeftinija, S. (2005). Development of functional neurons from postnatal stem cells in vitro. Stem Cell. 23, 1044–1049. doi:10.1634/stemcells.2005-0037
Stark, M., Grip, S., Rising, A., Hedhammar, M., Engström, W., Hjälm, G., et al. (2007). Macroscopic fibers self-assembled from recombinant miniature spider silk proteins. Biomacromolecules 8, 1695–1701. doi:10.1021/bm070049y
Sur, M., and Rubenstein, J. L. (2005). Patterning and plasticity of the cerebral cortex. Science 310, 805–810. doi:10.1126/science.1112070
Tang-Schomer, M. D., White, J. D., Tien, L. W., Schmitt, L. I., Valentin, T. M., Graziano, D. J., et al. (2014). Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. USA 111, 13811–13816. doi:10.1073/pnas.1324214111
Teixeira, A. I., Duckworth, J. K., and Hermanson, O. (2007). Getting the right stuff: controlling neural stem cell state and fate in vivo and in vitro with biomaterials. Cell Res. 17, 56–61. doi:10.1038/sj.cr.7310141
Teixeira, A. I., Ilkhanizadeh, S., Wigenius, J. A., Duckworth, J. K., Inganäs, O., and Hermanson, O. (2009). The promotion of neuronal maturation on soft substrates. Biomaterials 30, 4567–4572. doi:10.1016/j.biomaterials.2009.05.013
Teixeira, A. I., Hermanson, O., and Werner, C. (2010). Designing and engineering stem cell niches. MRS Bull. 35, 591–596. doi:10.1038/sj.cr.7310141
Tokareva, O., Michalczechen-Lacerda, V. A., Rech, E. L., and Kaplan, D. L. (2013). Recombinant DNA production of spider silk proteins. Microb. Biotechnol. 6, 651–663. doi:10.1111/1751-7915.12081
Uhlén, P. (2004). Spectral analysis of calcium oscillations. Sci. STKE 2004 (258), pl15. doi:10.1126/stke.2582004pl15
Vollrath, F., Barth, P., Basedow, A., Engström, W., and List, H. (2002). Local tolerance to spider silks and protein polymers in vivo. In Vivo 16, 229–234. doi:10.3390/ma2041908
Widhe, M., Bysell, H., Nystedt, S., Schenning, I., Malmsten, M., Johansson, J., et al. (2010). Recombinant spider silk as matrices for cell culture. Biomaterials 31, 9575–9585. doi:10.1016/j.biomaterials.2010.08.061
Wohlrab, S., Müller, S., Schmidt, A., Neubauer, S., Kessler, H., Leal-Egaña, A., et al. (2012). Cell adhesion and proliferation on RGD-modified recombinant spider silk proteins. Biomaterials 33, 6650–6659. doi:10.1016/j.biomaterials.2012.05.069
Keywords: neural stem cell, biomaterial, spider silk, 3D cultures, scaffold
Citation: Lewicka M, Rebellato P, Lewicki J, Uhlén P, Rising A and Hermanson O (2021) Recombinant Spider Silk Protein Matrices Facilitate Differentiation of Neural Stem Cells Into Mature and Functional Neurons. Front. Mater. 7:560372. doi: 10.3389/fmats.2020.560372
Received: 08 May 2020; Accepted: 29 December 2020;
Published: 09 February 2021.
Edited by:
Liyuan Zhang, Harvard University, United StatesReviewed by:
John George Hardy, Lancaster University, United KingdomCopyright © 2021 Lewicka, Rebellato, Lewicki, Uhlén, Rising and Hermanson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ola Hermanson, b2xhLmhlcm1hbnNvbkBraS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.